Abstract
During obstructive cholestasis, increased concentrations of bile acids activate ERK1/2 in hepatocytes, which up-regulates early growth response factor 1, a key regulator of proinflammatory cytokines, such as macrophage inflammatory protein 2 (MIP-2), which, in turn, exacerbates cholestatic liver injury. Recent studies have indicated that IL-17A contributes to hepatic inflammation during obstructive cholestasis, suggesting that bile acids and IL-17A may interact to regulate hepatic inflammatory responses. We treated mice with an IL-17A neutralizing antibody or control IgG and subjected them to bile duct ligation. Neutralization of IL-17A prevented up-regulation of proinflammatory cytokines, hepatic neutrophil accumulation, and liver injury, indicating an important role for IL-17A in neutrophilic inflammation during cholestasis. Treatment of primary mouse hepatocytes with taurocholic acid (TCA) increased the expression of MIP-2. Co-treatment with IL-17A synergistically enhanced up-regulation of MIP-2 by TCA. In contrast to MIP-2, IL-17A did not affect up-regulation of Egr-1 by TCA, indicating that IL-17A does not affect bile acid–induced activation of signaling pathways upstream of early growth response factor 1. In addition, bile acids increased expression of IL-23, a key regulator of IL-17A production in hepatocytes in vitro and in vivo. Collectively, these data identify bile acids as novel triggers of the IL-23/IL-17A axis and suggest that IL-17A promotes hepatic inflammation during cholestasis by synergistically enhancing bile acid–induced production of proinflammatory cytokines by hepatocytes.
Cholestatic liver diseases have many causes, such as familial genetic disorders, xenobiotic exposure, autoimmune disease, and tumors that disrupt bile flow.1 A common feature of most types of cholestatic liver disease is an elevation in proinflammatory cytokines and hepatic accumulation of immune cells.2–4 Studies in animal models have identified several immune cell types and immunomodulatory cytokines that are essential for the pathogenesis of cholestatic liver diseases.3,5,6 The mechanisms that regulate hepatic inflammation during cholestasis, however, are not fully understood. Elucidation of these pathways could provide important insight pertinent to the development of therapeutics for cholestatic liver disease. Our recent studies indicate an important role for bile acids in the development of inflammation during cholestasis.
In obstructive cholestasis, elevated biliary pressure disrupts the integrity of intrahepatic bile ducts, resulting in leakage of bile into the liver.7 As a result, concentrations of bile acids, in particular taurine and glycine conjugates, increase in the liver and serum in patients and in animal models.8,9 During bile duct ligation (BDL) in mice, serum concentrations of bile acids exceed 3 mmol/L, with the highest fraction of bile acids being conjugated with taurine.8 Modest increases in bile acid concentrations modulate metabolic pathways in hepatocytes that promote detoxification of bile acids.10 However, pathologic concentrations of bile acids trigger a strong inflammatory response in hepatocytes characterized by induction of diverse classes of cytokines that promote infiltration of inflammatory cells, including neutrophils, macrophages, and lymphocytes.11,12 Accumulating neutrophils extravasate from sinusoids and adhere to hepatocytes, which stimulates their activation. The activated neutrophils release toxic mediators that exacerbate hepatocyte injury during cholestasis.2
We recently identified a bile acid–activated signaling network that elicits the production of proinflammatory cytokines by hepatocytes. In these studies, pathologic concentrations of taurocholic acid (TCA) activated ERK1/2 and up-regulated the transcription factor early growth response factor 1 (Egr-1) in hepatocytes.11–13 Egr-1 then regulated the production of several cytokines, chemokines, and adhesion molecules that are crucial for neutrophil accumulation and activation during cholestasis.11,12 In addition, bile acids increased the expression of many proinflammatory mediators in an Egr-1–independent manner, suggesting that bile acids promote inflammation by multiple mechanisms.11 Collectively, these studies indicated that bile acids are central mediators of hepatic inflammation during cholestasis.
It was recently reported that the cytokine IL-17A contributes to the hepatic production of proinflammatory mediators and the progression of fibrosis in BDL mice.14 IL-17A is the best-characterized member of the IL-17 cytokine family and is primarily produced and secreted by CD4+ T helper (Th) 17 cells.15 Differentiation of murine naïve T cells into Th17 effector cells is mediated by the cytokine transforming growth factor-β with either IL-6 or IL-21.15,16 In addition, IL-23 is required for the maintenance and stabilization of Th17 cells.17 IL-17A signals via its receptor complex, IL-17RA and IL-17RC, as a homodimer or as a heterodimer with IL-17F.16 Through adaptor proteins, IL-17A elicits the production of chemokines and cytokines that promote neutrophil recruitment and, thus, an immune reaction to extracellular pathogens.18,19 It was reported that IL-17A-positive cells are increased in the livers of patients with primary biliary cirrhosis.20 Similarly, it was demonstrated that IL-17A levels are increased in mice subjected to BDL.14 In this study, liver injury and fibrosis were reduced in IL-17RA knockout mice 3 weeks after surgery. Furthermore, there were reduced levels of the cytokines tumor necrosis factor α, IL-1β, and IL-6, indicating an important role for IL-17A in the development of liver disease during cholestasis.14
Considering the importance of bile acids and IL-17A to hepatic inflammation during cholestasis, a potential link between these inflammatory mediators in the pathogenesis of hepatocellular injury during cholestasis was investigated. Accordingly, we tested the hypothesis that bile acids and the IL-17 signaling axis interact to promote hepatic inflammation during cholestasis.
Materials and Methods
Animals
Studies were performed on 8- to 10-week-old male C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME). All the mice were maintained on a 12-hour light/dark cycle under controlled temperature (18°C to −21°C) and humidity. Food (Rodent Chow; Harlan-Teklad, Madison, WI) and tap water were allowed ad libitum. For bile acid feeding, mice were given an AIN-93M diet supplemented with 0.3% cholic acid or an AIN-93M diet alone (Dyets Inc., Bethlehem, PA) for 1 week. All the procedures on the animals were performed in accordance with the Guide for the Care and Use of Laboratory Animals promulgated by the NIH.
Bile Duct Ligation
Mice were treated with 100 μg of anti–IL-17A antibody (clone 50104; R&D systems, Minneapolis, MN, and Bio X Cell, West Lebanon, NH) or control IgG2A (clone 54447; R&D systems and Bio X Cell) by i.p. injection 1 hour before surgery.21 BDL was then performed on mice as described previously.13 The mice were given an additional 50 μg of anti–IL-17A antibody or control IgG on days 3 and 6 after surgery. Nine days after surgery, liver and blood samples were collected from the mice for analysis.
Hepatocyte Isolation
Hepatocytes were isolated from mice by collagenase perfusion as described previously.13 Hepatocytes were plated in Williams medium E (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO) and penicillin-streptomycin (Sigma-Aldrich). After 2 hours, hepatocytes were washed with 1× PBS and were cultured in serum-free Williams medium E. Hepatocytes were treated with 10 ng/mL of recombinant mouse IL-17A (R&D Systems), 100 nmol/L wortmannin (Cayman Chemical Co., Ann Arbor, MI), 30 μmol/L SP600125 (Cayman Chemical Co.), or 10 μmol/L SB203580 (Cayman Chemical Co.) in the presence or absence of 200 μmol/L TCA (Sigma-Aldrich) as indicated.
Serum Chemical Analysis
Levels of serum alanine aminotransferase activity (Thermo Scientific, Middletown, VA), total serum bilirubin (Pointe Scientific Inc., Canton, MI), serum alkaline phosphatase (Pointe Scientific Inc.), and total serum bile acids (colorimetric total bile acids assay kit; Bio-Quant, San Diego, CA) were measured using commercially available kits per the manufacturer's instructions.
Protein Analysis
IL-23 and macrophage inflammatory protein 2 (MIP-2) proteins were quantified in culture medium by enzyme-linked immunosorbent assay (ELISA) (BioLegend, San Diego, CA). Total protein was collected from hepatocytes lyzed with radioimmunoprecipitation assay buffer. Protein was separated on a 4% to 20% polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA). Membranes were incubated with either anti–phospho-AKT (Thr308), anti-AKT, anti–phospho-JNK (Thr183/Tyr185), anti-JNK, anti–phospho-p38 (Thr180/Tyr182), or anti-p38 antibodies (Cell Signaling Technology Inc., Danvers, MA). Membranes were then incubated with the appropriate secondary antibody conjugated with horseradish peroxidase. Protein bands were detected using an electrochemiluminescence detection kit (GE Healthcare, Buckinghamshire, UK).
Immunofluorescence
Liver pieces were frozen in isopentane cooled in liquid nitrogen for 8 minutes. Sections of frozen liver were cut and fixed in 4% formalin for 10 minutes. The sections were incubated with a rabbit polyclonal anti–IL-23A antibody (Abnova Corp., Walnut, CA), a rat anti-mouse CD68 antibody (AbD Serotec, Raleigh, NC), or a rat anti-mouse F4/80 antibody (AbD Serotec) in 10% goat serum diluted with PBS for 3 hours. The sections were then incubated with the appropriate secondary antibody conjugated with Alexa Fluor 594 (Invitrogen). CD68 and F4/80 immunostaining were quantified and described previously.22
Immunohistochemical Analysis
Paraffin-embedded liver sections were incubated with rabbit anti-rat polymorphonuclear leukocyte antibody (dilution 1:100) for 1 hour or rabbit anti–α-smooth muscle actin (α-SMA) antibody (dilution 1:100; Abcam Inc., Cambridge, MA) overnight at 4°C. Neutrophils were quantified by counting the number of positively stained cells in twenty 200× fields per tissue section. The analysis was performed in a blinded manner.
Real-Time PCR
TRIzol reagent (Sigma-Aldrich) was used to isolate RNA. Contaminating DNA was removed by using the TURBO DNA-free kit (Ambion, Austin, TX). cDNA was synthesized as previously described.13 Relative mRNA levels were measured using fast SYBR green reagent (Applied Biosystems, Foster City, CA) on a 7500 fast real-time PCR system (Applied Biosystems). Primer sequences are shown in Table 1. Primers used for these studies were intron spanning.
Table 1.
Primer Pair Sequences
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| MIP2 | 5′-CCTCAACGGAAGAACCAAAGAG-3′ | 5′-CTCAGACAGCGAGGCACATC-3′ |
| IL17A | 5′-CCGCAATGAAGACCCTGATAGA-3′ | 5′-TCATGTGGTGGTCCAGCTTTC-3′ |
| IL23 | 5′-GTGACCCACAAGGACTCAAGGA-3′ | 5′-AGCAGGCTCCCCTTTGAAGAT-3′ |
| Cxcl5 | 5′-GCTGGCATTTCTGTTGCTGTT-3′ | 5′-CGGTTAAGCAAACACAACGCA-3′ |
| Ccl7 | 5′-AAGATCCCCAAGAGGAATCTCA-3′ | 5′-CAGACTTCCATGCCCTTCTTT-3′ |
| PAI1 | 5′-AGTCTTTCCGACCAAGAGCA-3′ | 5′-ATCACTTGCCCCATGAAGAG-3′ |
| RPL13a | 5′-ACAAGAAAAAGCGGATGGTG-3′ | 5′-TTCTCCTCCAGAGTGGCTGT-3′ |
| ICAM1 | 5′-AACAGTTCACCTGCACGGAC-3′ | 5′-GTCACCGTTGTGATCCCTG-3′ |
| Egr1 | 5′-TGGGATAACTCGTCTCCACC-3′ | 5′-GAGCGAACAACCCTATGAGC-3′ |
| αSMA | 5′-GTTCAGTGGTGCCTCTGTCA-3′ | 5′-ACTGGGACGACATGGAAAAG-3′ |
| Col1a1 | 5′-TAGGCCATTGTGTATGCAGC-3′ | 5′-ACATGTTCAGCTTTGTGGACC-3′ |
Statistics
Data are expressed as means ± SEM. When two or more groups were analyzed, a two-way analysis of variance was performed. Comparison between groups was performed by using the Holm-Sidak method. The criterion for significance was a 95% confidence, with P < 0.05.
Results
Neutralization of IL-17A Reduces Neutrophil Accumulation and Liver Injury after BDL
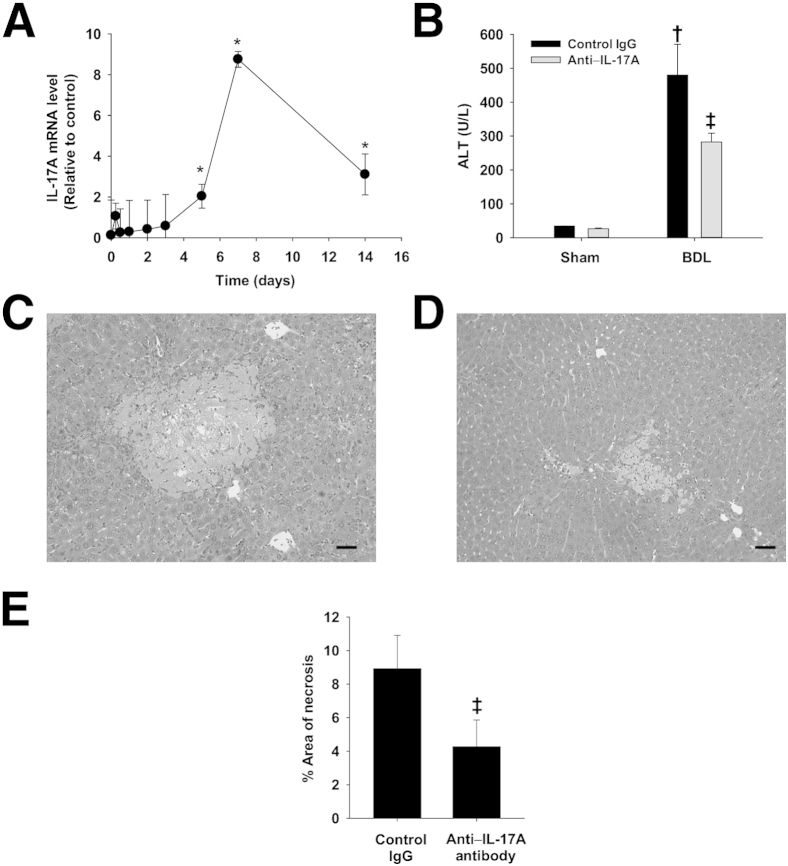
It was previously shown that neutrophils contribute to hepatic injury after BDL.2 Given that IL-17A is instrumental in driving neutrophilic inflammation, we characterized the expression of IL-17A in liver over time after BDL. IL-17A mRNA levels did not increase until 5 days after BDL (Figure 1A). This increase in expression peaked on day 7 and remained elevated at 14 days. Next, we determined the role of IL-17A in the development of hepatocellular injury and neutrophilic inflammation after BDL. Mice were treated with either an anti–IL-17A antibody or control IgG. The mice were then subjected to BDL or sham operation for 3 or 9 days. BDL caused an increase in serum alanine aminotransferase activity, which was reduced in mice treated with anti–IL-17A antibody (Figure 1B). Furthermore, neutralization of IL-17A decreased the area of liver necrosis in BDL mice (Figure 1, C–E). Neutralization of IL-17A did not affect hepatocellular injury 3 days after surgery (data not shown), a time point before up-regulation of IL-17A (Figure 1A).
Figure 1.
Role of IL-17A in the development of hepatocellular injury after BDL. A: IL-17A mRNA levels were quantified by real-time PCR in the livers of mice subjected to BDL for the indicated times.∗P < 0.05 versus day 0. Mice were treated with either an anti–IL-17A antibody or isotype control. The mice were then subjected to BDL or sham operation. Alanine aminotransferase (ALT) activity (B) and percentage area of liver necrosis (C–E) were quantified 9 days after surgery. Scale bars: 100 μm. †P < 0.05 versus sham-operated mice; ‡P < 0.05 versus isotype control BDL mice. Data are given as means ± SEM.
To ascertain whether blocking IL-17A specifically decreased neutrophil-mediated inflammation or instead caused a reduction in cholestasis in general, the effect of anti–IL-17A on markers of cholestasis was investigated. Levels of alkaline phosphatase, total bilirubin, and total bile acids were increased in the serum of mice 9 days after BDL (Table 2). Neutralization of IL-17A did not affect the increase in these markers of cholestasis (Table 2).
Table 2.
Neutralization of IL-17A Does Not Affect Markers of Cholestasis
| Measurement | Control IgG sham | Anti–IL-17A sham | Control IgG BDL | Anti–IL-17A BDL |
|---|---|---|---|---|
| Alkaline phosphatase (U/L) | 146.7 ± 2.2 | 155.4 ± 14.3 | 973.6 ± 142.5∗ | 916.6 ± 51.9∗ |
| Bilirubin (mg/dL) | 0.1 ± 0.1 | 0.07 ± 0.04 | 16.8 ± 1.2∗ | 17.4 ± 1.0∗ |
| Serum bile acid (μmol/L) | 13.6 ± 1.5 | 11.5 ± 0.5 | 274.3 ± 7.6∗ | 277.0 ± 10.9∗ |
Data are given as means ± SEM. The mice were treated with 100 μg of anti–IL-17A antibody or control IgG. After 24 hours, the mice were subjected to BDL or sham surgery. The mice were further treated with 50 μg of anti–IL-17A antibody or control IgG 3 and 6 days after surgery. The mice were sacrificed 9 days after surgery. Alkaline phosphatase, bilirubin, and total bile acid levels were measured using commercially available kits.
Significantly different from sham-operated mice (P < 0.05).
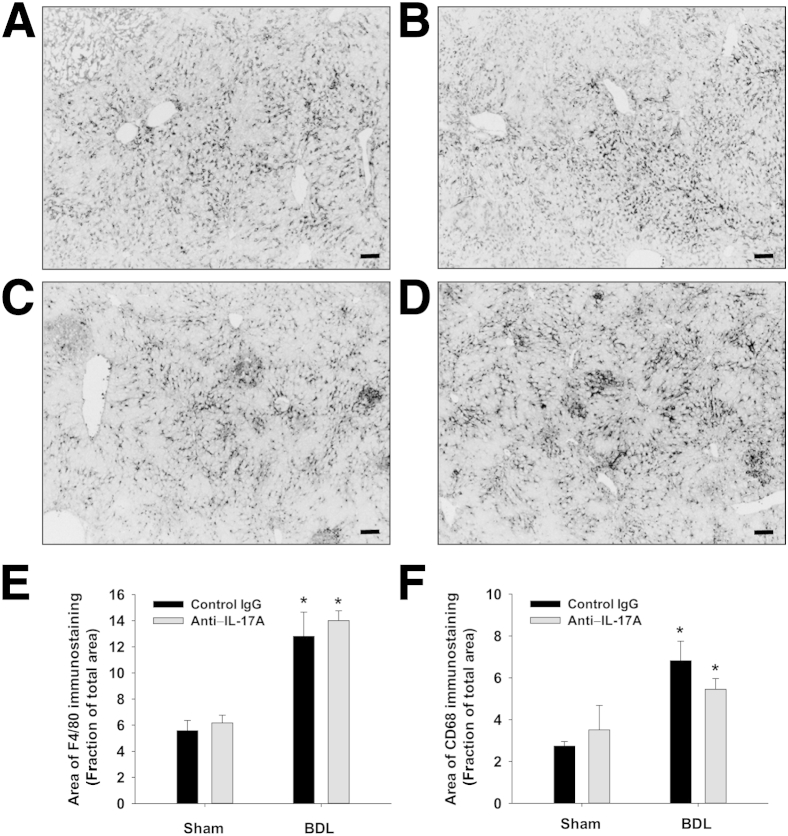
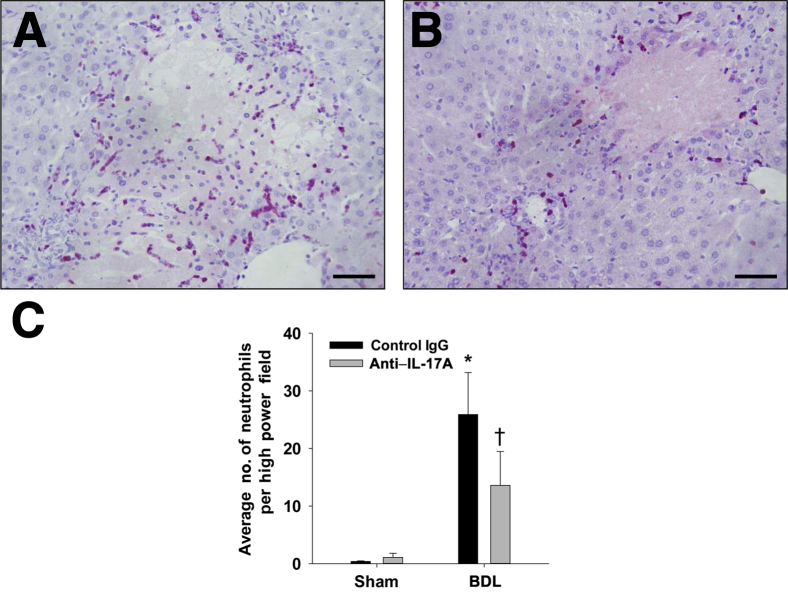
Next, we tested the hypothesis that neutralization of IL-17A prevents accumulation of inflammatory cells in the liver after BDL. Macrophages were detected in the liver by immunohistochemical (IHC) staining for F4/80 and CD68 (Figure 2, A–D). F4/80- and CD68-positive macrophages increased in the livers of BDL mice treated with control IgG (Figure 2, E and F). Neutralization of IL-17A, however, did not affect the accumulation of macrophages in the liver (Figure 2, E and F). In addition, neutrophil numbers increased in the livers of BDL mice treated with control IgG (Figure 3). In contrast to macrophages, neutralization of IL-17A decreased neutrophil numbers after BDL (Figure 3).
Figure 2.
Effect of IL-17A neutralization on hepatic accumulation of macrophages in the liver after BDL. Mice were treated with either an anti–IL-17A antibody or isotype control and then were subjected to BDL or sham operation. Nine days after surgery, F4/80-positive macrophages were identified in the liver by immunostaining in control IgG BDL mice (A) and in anti–IL-17A antibody BDL mice (B). In addition, isotype control BDL livers (C) and anti–IL-17A antibody BDL livers (D) were stained for CD68-positive macrophages. Scale bars: 100 μm. E and F: The area of positive staining was then quantified in sections of liver and expressed as a fraction of the total area. ∗P < 0.05 compared with sham-operated control mice. Data are given as means ± SEM.
Figure 3.
Neutralization of IL-17A decreased hepatic neutrophil numbers after BDL. Neutrophils were stained in sections of liver by IHC staining. A: Section of liver from a mouse treated with isotype control and subjected to BDL. B: Section of liver from a mouse treated with anti–IL-17A antibody and subjected to BDL. Positive cells appear red in the photomicrographs. Scale bars: 50 μm. C: Neutrophils were counted in sections of liver. ∗P < 0.05 versus sham-operated mice; †P < 0.05 versus isotype control–treated mice subjected to BDL. Data are given as means ± SEM.
Cytokine Gene Expression Decreases in Anti–IL-17A–Treated BDL Mice
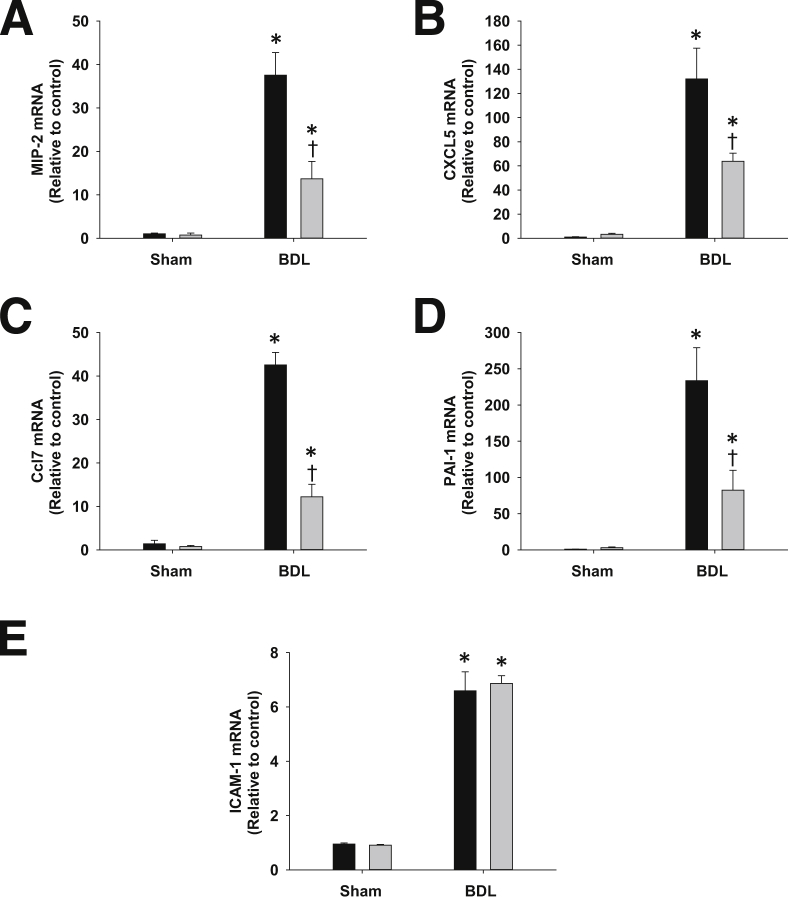
Because IL-17A neutralization decreased hepatic accumulation of neutrophils, we investigated the expression of cytokines and adhesion molecules on neutralization of IL-17A in BDL mice. Hepatic mRNA levels of MIP-2, Cxcl5, Ccl7, plasminogen activator inhibitor-1, and intercellular adhesion molecule-1 (ICAM-1) were increased in mice treated with control IgG and subjected to BDL for 9 days (Figure 4). Neutralization of IL-17A in BDL mice attenuated the increase in MIP-2, Cxcl5, Ccl7, and plasminogen activator inhibitor-1 levels (Figure 4, A–D). In contrast, ICAM-1 mRNA levels were unaffected by IL-17A neutralization (Figure 4E).
Figure 4.
Neutralization of IL-17A decreased the expression of pro-inflammatory mediators after BDL. Mice were treated with anti–IL-17A antibody (gray bars) or control IgG (black bars) and were subjected to BDL or sham surgery as detailed in Materials and Methods. Nine days after surgery, mRNA levels of the proinflammatory mediators MIP-2 (A), Cxcl5 (B), Ccl7 (C), plasminogen activator inhibitor-1 (PAI-1) (D), and ICAM-1 (E) were measured by real-time PCR. ∗P < 0.05 versus sham-operated mice; †P < 0.05 versus BDL mice treated with control IgG. Data are given as means ± SEM.
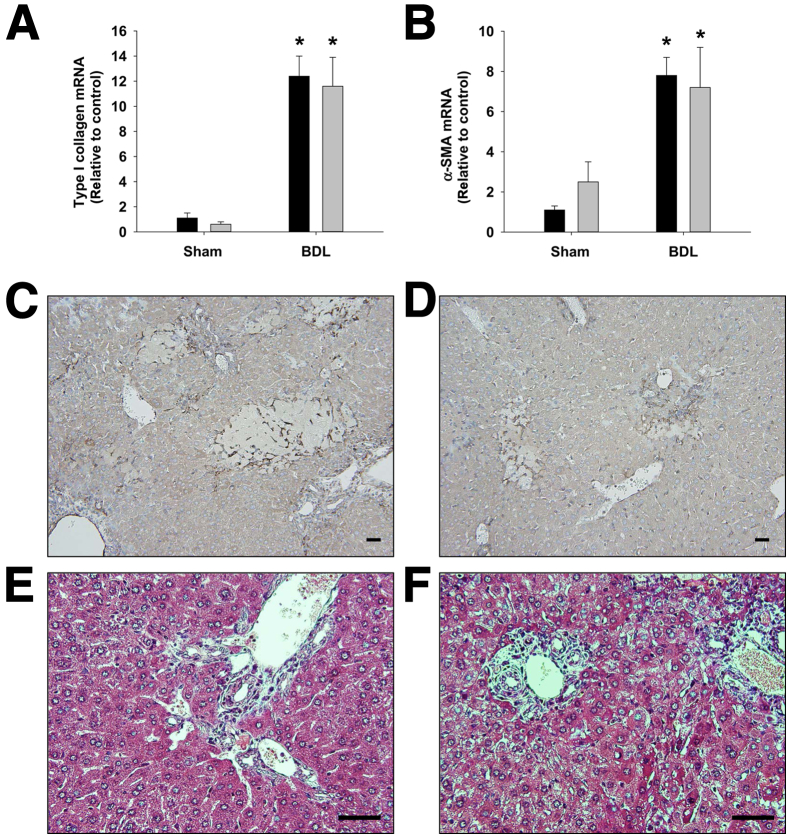
α-SMA and Type I Collagen Levels Are Unaffected by Neutralization of IL-17A
α-SMA and type I collagen mRNA levels were increased to a similar extent in BDL mice treated with either control IgG or anti–IL-17A (Figure 5, A and B). Similar to the mRNA levels, α-SMA protein levels, detected by IHC analysis, and collagen protein levels, detected by trichrome staining, were not different between control IgG–treated mice and anti–IL-17A–treated mice subjected to BDL (Figure 5, C–F).
Figure 5.
Neutralization of IL-17A did not affect liver fibrosis 9 days after BDL. Mice were treated with anti–IL-17A antibody (gray bars) or control IgG (black bars) and were subjected to BDL or sham surgery as detailed in Materials and Methods. Nine days after surgery, mRNA levels of type I collagen (A) and α-SMA (B) were quantified. Data are given as means ± SEM. ∗P < 0.05 versus sham-operated mice. α-SMA protein was detected in the livers of BDL mice treated with control IgG (C) or anti–IL-17A (D) by IHC analysis. Positive staining appears dark brown. Sections of liver from BDL mice treated with control IgG (E) or anti–IL-17A (F) were stained with Masson trichrome. Scale bars: 50 μm.
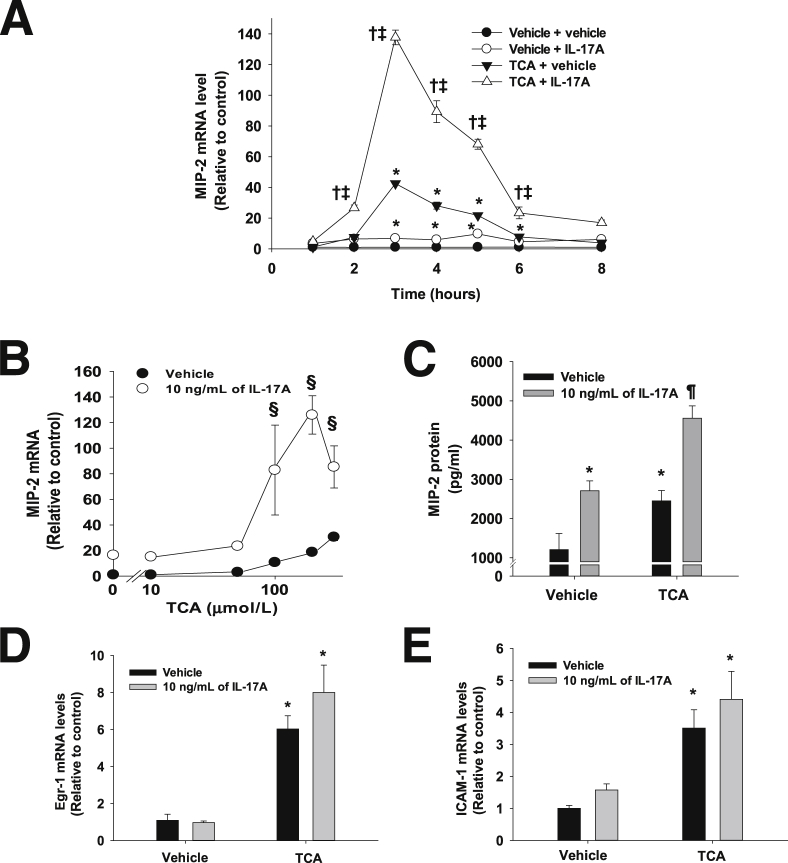
IL-17A Synergistically Enhances TCA-Induced Up-Regulation of MIP-2 mRNA in Primary Mouse Hepatocytes
We previously demonstrated that TCA promotes proinflammatory mediator production by hepatocytes, which is important for inflammation in the liver during cholestasis.11–13 Considering that IL-17A is also important for inflammation during cholestasis (Figures 3 and 4), we investigated whether IL-17A affects bile acid–induced up-regulation of inflammatory mediators by hepatocytes. We, therefore, treated hepatocytes with IL-17A in the presence or absence of 200 μmol/L TCA. IL-17A and TCA alone increased MIP-2 gene expression in primary mouse hepatocytes (Figure 6A). Co-treatment with IL-17A and TCA synergistically enhanced the induction of MIP-2 mRNA compared with treatment with IL-17A or TCA alone (Figure 6, A and B). MIP-2 protein concentrations were increased in the medium from hepatocytes treated with either IL-17A or TCA (Figure 6C). Similar to MIP-2 mRNA levels, co-treatment with IL-17A and TCA substantially increased MIP-2 protein levels compared with hepatocytes treated with either IL-17A or TCA alone (Figure 6C). We previously demonstrated that up-regulation of MIP-2 in bile acid–treated hepatocytes is Egr-1 dependent.11 Therefore, we determined the effect of IL-17A on up-regulation of Egr-1 by bile acids. As expected, TCA increased Egr-1 mRNA levels in hepatocytes (Figure 6D). IL-17A did not affect up-regulation of Egr-1 by TCA (Figure 6D).
Figure 6.
Effect of IL-17A on up-regulation of inflammatory mediators by TCA. A: Primary mouse hepatocytes were treated with 10 ng/mL of IL-17A. The cells were then treated with 200 μmol/L TCA for the indicated times. MIP-2 mRNA levels were measured by real-time PCR. ∗P < 0.05 versus vehicle-treated hepatocytes, †P < 0.05 versus TCA-treated hepatocytes, and ‡P < 0.05 versus IL-17A–treated hepatocytes. B: Primary mouse hepatocytes were treated with 10 ng/mL of IL-17A for 16 hours. The cells were then treated with the indicated concentrations of TCA. MIP-2 mRNA levels were measured by real-time PCR. §P < 0.05 versus hepatocytes treated with vehicle and TCA. C: For quantification of MIP-2 protein, hepatocytes were treated with 10 ng/mL of IL-17A. The cells were then treated with 200 μmol/L TCA for 12 hours. MIP-2 protein was quantified in the medium by ELISA. ∗P < 0.05 versus vehicle-treated hepatocytes; ¶P < 0.05 versus hepatocytes treated with either TCA or IL-17A alone. Primary mouse hepatocytes were treated with 10 ng/mL of IL-17A followed by treatment with 200 μmol/L TCA. Egr-1 (D) and ICAM-1 (E) mRNA levels were measured by real-time PCR. ∗P < 0.05 versus vehicle-treated hepatocytes. Data are given as means ± SEM.
Unlike MIP-2, neutralization of IL-17A in mice did not affect up-regulation of ICAM-1 in the liver after BDL (Figure 4E). Accordingly, we determined whether IL-17A would affect up-regulation of ICAM-1 in bile acid–treated hepatocytes. Consistent with in vivo (Figure 4E), IL-17A did not enhance TCA-induced up-regulation of ICAM-1 (Figure 6E).
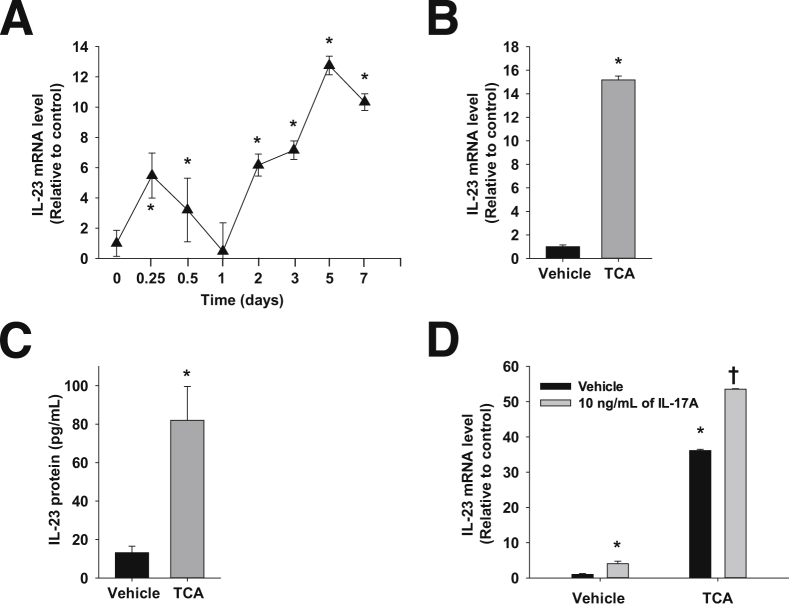
Up-Regulation of IL-23 mRNA and Protein in Bile Acid–Treated Primary Mouse Hepatocytes
Because IL-23 promotes IL-17A production, IL-23 gene expression was measured in wild-type mice subjected to BDL over a 14-day period. IL-23 gene expression was biphasic, with an initial peak at 6 hours followed by a second peak at 5 days (Figure 7A). Previous studies have demonstrated that serum and liver bile acid concentrations8 followed a similar time course as IL-23 mRNA levels, thereby suggesting that bile acids may contribute to up-regulation of IL-23 after BDL. Therefore, we tested the hypothesis that TCA increases IL-23 production by primary mouse hepatocytes. Treatment of hepatocytes with 200 μmol/L TCA increased IL-23 mRNA and protein levels (Figure 7, B and C). Similar to its effects on MIP-2, IL-17A enhanced up-regulation of IL-23 in hepatocytes by TCA (Figure 7D).
Figure 7.
Up-regulation of IL-23 in the liver and primary mouse hepatocytes. A: Mice were subjected to BDL or sham operation. IL-23 mRNA levels were measured in the liver. ∗P < 0.05 versus day 0. Primary mouse hepatocytes were treated with 200 μmol/L TCA. IL-23 mRNA (B) and (C) protein levels were measured. ∗P < 0.05 versus vehicle-treated hepatocytes. D: Hepatocytes were treated with 10 ng/mL of IL-17A followed by treatment with 200 μmol/L TCA. IL-23 mRNA levels were measured by real-time PCR. ∗P < 0.05 versus vehicle-treated hepatocytes; †P < 0.05 versus hepatocytes treated with either IL-17A or TCA. Data are given as means ± SEM.
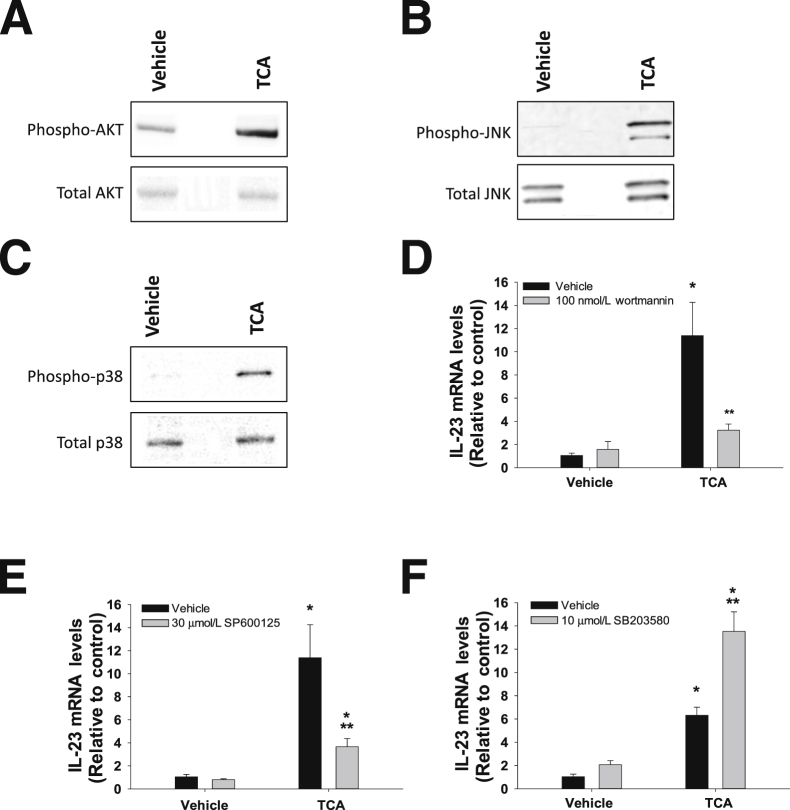
Signal Transduction Pathways Involved in IL-23 Induction by TCA
In primary mouse hepatocytes, 200 μmol/L TCA activated AKT (Figure 8A), c-Jun N-terminal kinases (JNK) (Figure 8B), and p38 mitogen-activated kinase (p38) (Figure 8C). Inhibition of PI3K/AKT or JNK signaling by wortmannin or SP600125, respectively, attenuated up-regulation of IL-23 by TCA (Figure 8, D and E). Inhibition of p38 by SB203580, however, enhanced IL-23 induction by TCA (Figure 8F).
Figure 8.
Role of signal transduction pathways in the up-regulation of IL-23 by TCA. Hepatocytes were treated with 200 μmol/L TCA for 30 minutes. Western blot was used to detect phospho-AKT and total AKT (A), phospho-JNK and total JNK (B), and phospho-p38 and total p38 (C). Hepatocytes were treated with 100 nmol/L wortmannin (D), 30 μmol/L SP600125 (E), or 10 μmol/L SB203580 (F) with or without 200 μmol/L TCA. IL-23 mRNA levels were measured by real-time PCR. ∗P < 0.05 versus vehicle treated hepatocytes; ∗∗P < 0.05 versus hepatocytes treated with TCA in the absence of inhibitor. Data are given as means ± SEM.
Bile Acid Feeding Promotes Hepatic IL-23 Expression
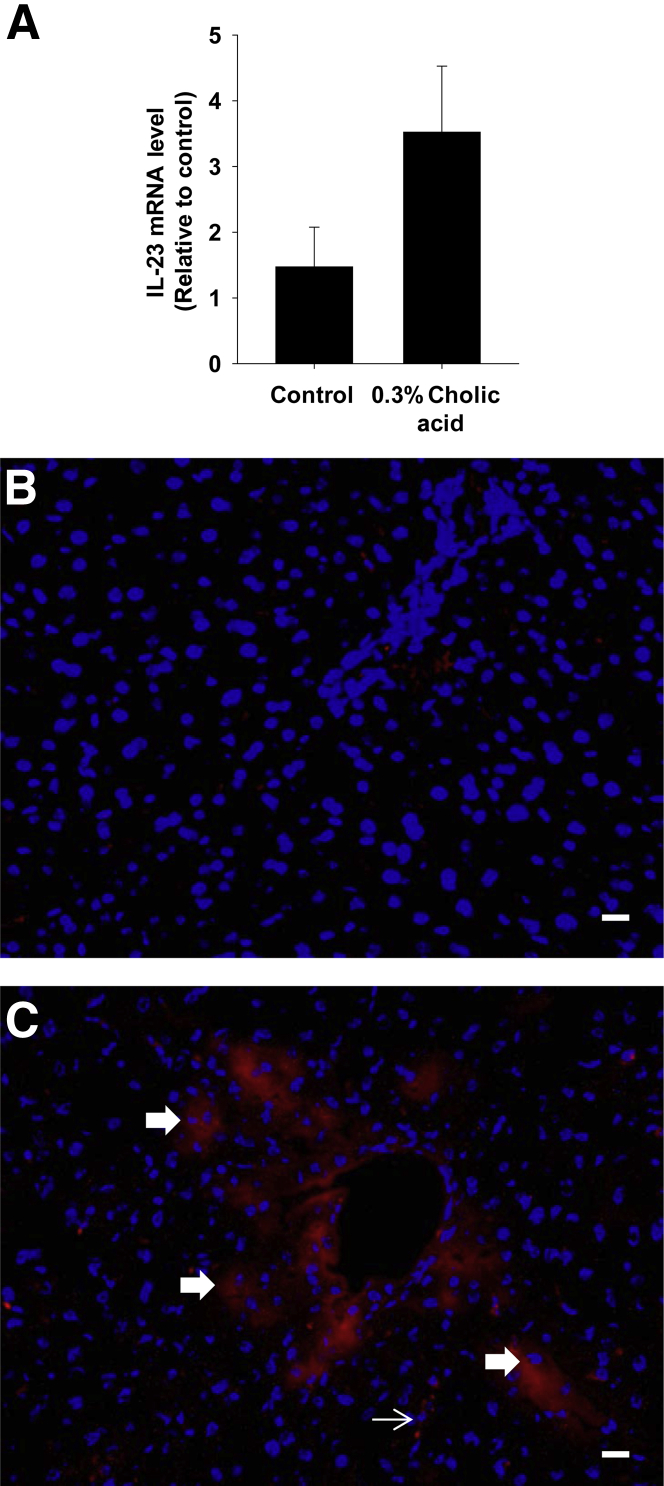
To determine whether bile acids increase IL-23 expression in vivo, mice were fed a diet containing 0.3% cholic acid or a control diet for 1 week. This diet increased serum bile acid concentrations, including TCA, by 1 week of feeding.23 IL-23 mRNA levels were increased in the livers of mice fed the 0.3% cholic acid diet (Figure 9A). IHC staining for IL-23 in the liver demonstrated increased IL-23 protein levels in hepatocytes and sinusoidal cells (Figure 9, B and C).
Figure 9.
Up-regulation of IL-23 in the liver after bile acid feeding. Mice were given either an AIN-93M diet supplemented with 0.3% cholic or an AIN-93M diet alone. A: IL-23 mRNA was measured in the liver by real-time PCR. Data are given as means ± SEM. IHC staining was used to detect IL-23 in sections of liver from mice fed an AIN-93M control diet (B) or an AIN-93M diet supplemented with 0.3% cholic acid (C). Positive staining for IL-23 appears red in the photomicrographs. The thick arrows indicate IL-23 staining in hepatocytes. The thin arrow indicates the area of IL-23 staining in the sinusoid. Scale bars: 50 μm. DAPI, which appears blue in the photomicrographs, was used to stain nuclei.
Discussion
IL-17A Contributes to Neutrophil-Dependent Liver Injury during Obstructive Cholestasis
During obstructive cholestasis, neutrophils accumulate in the liver, become activated, and exacerbate hepatocellular injury.2 A recent study demonstrated that IL-17RA null mice had reduced liver injury 3 weeks after BDL, suggesting that IL-17A signaling is a prerequisite for hepatic neutrophil accumulation during obstructive cholestasis.14 In support of this, the present study demonstrated that neutralization of IL-17A reduced neutrophil numbers in the liver after BDL (Figure 3), which was associated with a reduction in alanine aminotransferase levels and the area of hepatic necrosis (Figure 1, B–E). Hepatic neutrophil accumulation depends on up-regulation of various inflammatory cytokines and adhesion molecules.3,5,6 In the present studies, neutralization of IL-17A attenuated up-regulation of several proinflammatory cytokines, including MIP-2, plasminogen activator inhibitor-1, Ccl7, and Cxcl5 (Figure 4, A–D), suggesting that IL-17A promotes hepatic neutrophil accumulation through up-regulation of proinflammatory mediators that stimulate neutrophil migration to the liver during obstructive cholestasis. Unlike a previous report, however, we did not see a reduction in markers of liver fibrosis (Figure 5). One possible explanation for these disparate findings is that we investigated a much earlier time point after BDL (ie, 9 days versus 3 weeks in the previous study).14
IL-17A Synergistically Enhances Up-Regulation of Inflammatory Mediators in Bile Acid–Treated Hepatocytes
We recently demonstrated that bile acids up-regulate several cytokines, chemokines, and adhesion molecules in hepatocytes in an Egr-1–dependent manner.11 In addition, we demonstrated that this process is important for neutrophil-dependent liver injury during cholestasis. The present studies demonstrate that IL-17A is also required for up-regulation of inflammatory cytokines and neutrophil-dependent liver injury similar to bile acids. In many cell types, IL-17A alone is not a potent inflammatory cytokine.24 However, IL-17A modifies the inflammatory response produced by other cytokines, such as tumor necrosis factor α and IL-6. For example, IL-17A synergistically enhances up-regulation of proinflammatory mediators by tumor necrosis factor α or IL-6 in alveolar type II cells, endothelial cells, mesangial cells, astrocytes, and fibroblasts.25–29 This finding suggests that IL-17A may interact with other inflammatory mediators, such as bile acids, to produce inflammation during cholestasis. The present results demonstrate that IL-17A and TCA do interact to promote robust production of inflammatory cytokines by hepatocytes. IL-17A alone modestly increased MIP-2; however, MIP-2 production by hepatocytes was markedly increased in the presence of IL-17A and TCA (Figure 6, A–C). Bile acids activate ERK1/2 in hepatocytes, which stimulate up-regulation of Egr-1. Egr-1 then regulates production of MIP-2. Interestingly, IL-17A did not affect up-regulation of Egr-1 (Figure 6D), suggesting that IL-17A does not enhance up-regulation of MIP-2 by modulating bile acid signaling upstream of Egr-1.
The synergistic enhancement of MIP-2 expression by IL-17A and TCA may not only be important for obstructive cholestasis but could be critical in other liver diseases, including primary biliary cirrhosis and alcoholic hepatitis, where IL-17A and bile acid concentrations are increased.30–32 In these diseases, IL-17A may promote modest hepatic inflammation during the early stages. When these diseases become severe and hepatic bile acid concentrations increase, however, the interaction between IL-17A and bile acids could severely exacerbate hepatic inflammation and disease progression.
Bile acids may not only modify the response of hepatocytes to IL-17A but may also alter the response of other cell types, in particular Kupffer cells. Studies have shown that IL-17A stimulates the production of inflammatory mediators by Kupffer cells.14 In contrast to hepatocytes, however, bile acids inhibit the production of inflammatory cytokines by Kupffer cells.33,34 It has been proposed that this occurs through activation of the bile acid receptor, TGR5.34 Accordingly, it is possible that bile acids may inhibit IL-17A–induced production of inflammatory cytokines by Kupffer cells during obstructive cholestasis, which may explain the lack of tumor necrosis factor α production by hepatic macrophages during obstructive cholestasis, although concentrations of the macrophage activators IL-17A and lipopolysaccharide are increased.3 A similar effect could occur with other cell types that express TGR5, including bile duct epithelial cells and sinusoidal endothelial cells.34,35 The present in vitro data demonstrate that hepatocytes express higher levels of inflammatory mediators in the presence of bile acids and IL-17A, suggesting that in liver diseases, such as cholestasis, where bile acid concentrations are increased, the production of inflammatory mediators may shift from prototypical inflammatory cells toward hepatocytes. This type of inflammation may be more resistant to commonly used anti-inflammatory drugs, such as glucocorticoids, which may explain the resistance of many types of cholestatic liver disease to anti-inflammatory therapies.36
Bile Acids Are Novel Inducers of IL-23 by Hepatocytes
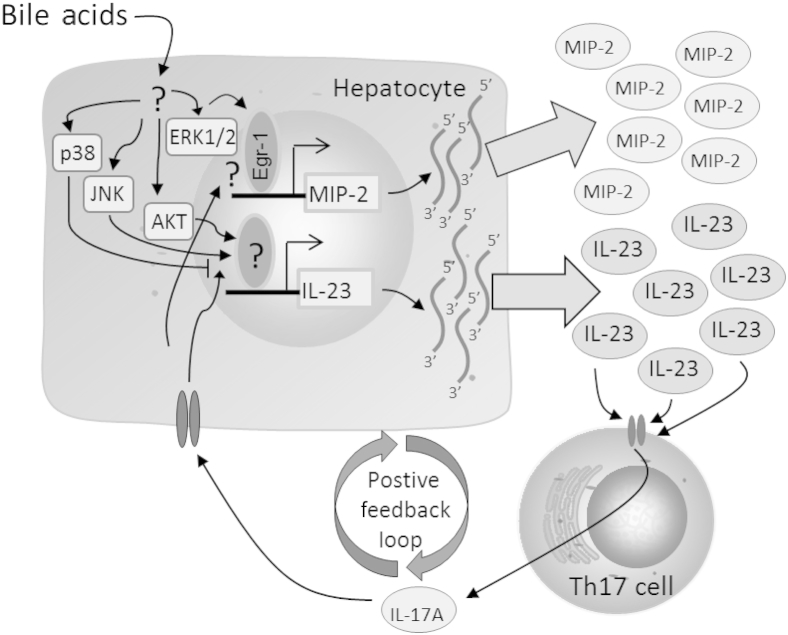
IL-23 is critical for the maintenance and stabilization of CD4+ Th17 cells into effector Th cells.15 After BDL, IL-23 concentrations are increased, and this increase is required for the production of IL-17A (Figure 7A).14 Interestingly, the present studies demonstrated that the time course of IL-23 up-regulation in the liver after BDL was biphasic and identical to the time course of bile acid concentration changes in the serum and liver (Figure 7A),8 suggesting that bile acids may be important for up-regulation of IL-23 in the liver. In support of this, TCA increased expression of IL-23 in primary mouse hepatocytes (Figure 7, B and C), and IL-23 protein levels were increased in hepatocytes in mice fed a diet containing 0.3% cholic acid (Figure 9). Furthermore, up-regulation of IL-23 by TCA required JNK and AKT (Figure 8), indicating that bile acids could sustain and exacerbate a Th17 response by stimulating the production of IL-23, a process that was AKT and JNK dependent (Figure 8, D and E). Similar to its effects on MIP-2, IL-17A enhanced the TCA-induced production of IL-23 by hepatocytes (Figure 7D), which could initiate a positive feedback loop whereby bile acids stimulate the production of IL-23 by hepatocytes, which enhances IL-17A production by Th17 cells. IL-17A then enhances the bile acid–induced production of IL-23 and other inflammatory mediators by hepatocytes. This process further enhances IL-17A production and, ultimately, IL-23 and other inflammatory cytokines that exacerbates hepatic inflammation and injury. This process is illustrated in Figure 10.
Figure 10.
Proposed mechanism of the interaction between bile acids and the IL-23/IL-17A axis. Bile acids up-regulate MIP-2 and other cytokines in hepatocytes by an Egr-1–dependent mechanism. Bile acids also up-regulate IL-23 in hepatocytes through AKT and JNK activation. IL-23 maintains Th17 cellular expansion and promotes the production of IL-17A. Through an unknown signaling cascade, IL-17A synergistically enhances TCA-induced production of MIP-2 and IL-23 by hepatocytes. Enhanced production of IL-23 leads to the formation of a positive feedback loop, which further amplifies inflammation during cholestasis.
The present results demonstrate that IL-17A synergistically enhances the bile acid–induced production of inflammatory mediators by hepatocytes. Furthermore, we demonstrated for the first time that bile acids stimulate hepatocytes to produce IL-23. Although these studies suggest that the inhibition of IL-17A in patients with cholestatic liver disease may be a logical therapeutic option, systemic inhibition of IL-17A could negatively impact the ability of patients to clear bacterial infections, which may be particularly dangerous in patients with cholestasis who are at risk for infections. Accordingly, elucidation of the signaling mechanisms involved in the interaction between IL-17A and bile acids may provide important insight into specifically targeting the pathways involved in hepatic inflammation during cholestasis without affecting the host defense against invading pathogens.
Acknowledgments
We thank Sophia Kaska, Aaron Pace, and Kara Kelly for their excellent technical work.
Footnotes
This study was supported by NIH grants DK073566 (B.L.C) and ES017537 (J.P.L.). K.M.O. was supported by NIH training grant T32ES007255.
References
- 1.Li M.K., Crawford J.M. The pathology of cholestasis. Semin Liver Dis. 2004;24:21–42. doi: 10.1055/s-2004-823099. [DOI] [PubMed] [Google Scholar]
- 2.Gujral J.S., Farhood A., Bajt M.L., Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–363. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- 3.Gehring S., Dickson E.M., San Martin M.E., van Rooijen N., Papa E.F., Harty M.W., Tracy T.F., Jr., Gregory S.H. Kupffer cells abrogate cholestatic liver injury in mice. Gastroenterology. 2006;130:810–822. doi: 10.1053/j.gastro.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Georgiev P., Jochum W., Heinrich S., Jang J.H., Nocito A., Dahm F., Clavien P.A. Characterization of time-related changes after experimental bile duct ligation. Br J Surg. 2008;95:646–656. doi: 10.1002/bjs.6050. [DOI] [PubMed] [Google Scholar]
- 5.Gujral J.S., Liu J., Farhood A., Hinson J.A., Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G499–G507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- 6.Wintermeyer P., Cheng C.W., Gehring S., Hoffman B.L., Holub M., Brossay L., Gregory S.H. Invariant natural killer T cells suppress the neutrophil inflammatory response in a mouse model of cholestatic liver damage. Gastroenterology. 2009;136:1048–1059. doi: 10.1053/j.gastro.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fickert P., Zollner G., Fuchsbichler A., Stumptner C., Weiglein A.H., Lammert F., Marschall H.U., Tsybrovskyy O., Zatloukal K., Denk H., Trauner M. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238–1251. doi: 10.1053/gast.2002.35948. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y., Hong J.Y., Rockwell C.E., Copple B.L., Jaeschke H., Klaassen C.D. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 2012;32:58–69. doi: 10.1111/j.1478-3231.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trottier J., Bialek A., Caron P., Straka R.J., Heathcote J., Milkiewicz P., Barbier O. Metabolomic profiling of 17 bile acids in serum from patients with primary biliary cirrhosis and primary sclerosing cholangitis: a pilot study. Digest Liver Dis. 2012;44:303–310. doi: 10.1016/j.dld.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 10.Wang H., Chen J., Hollister K., Sowers L.C., Forman B.M. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 11.Allen K., Jaeschke H., Copple B.L. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178:175–186. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen K., Kim N.D., Moon J.O., Copple B.L. Upregulation of early growth response factor-1 by bile acids requires mitogen-activated protein kinase signaling. Toxicol Appl Pharmacol. 2010;243:63–67. doi: 10.1016/j.taap.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim N.D., Moon J.O., Slitt A.L., Copple B.L. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci. 2006;90:586–595. doi: 10.1093/toxsci/kfj111. [DOI] [PubMed] [Google Scholar]
- 14.Meng F., Wang K., Aoyama T., Grivennikov S.I., Paik Y., Scholten D., Cong M., Iwaisako K., Liu X., Zhang M., Osterreicher C.H., Stickel F., Ley K., Brenner D.A., Kisseleva T. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765–776.e1-3. doi: 10.1053/j.gastro.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 16.Gaffen S.L. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stritesky G.L., Yeh N., Kaplan M.H. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol. 2008;181:5948–5955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang S.H., Park H., Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281:35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 19.Schwandner R., Yamaguchi K., Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med. 2000;191:1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan R.Y., Salunga T.L., Tsuneyama K., Lian Z.X., Yang G.X., Hsu W., Moritoki Y., Ansari A.A., Kemper C., Price J., Atkinson J.P., Coppel R.L., Gershwin M.E. Hepatic IL-17 responses in human and murine primary biliary cirrhosis. J Autoimmun. 2009;32:43–51. doi: 10.1016/j.jaut.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi M., Higuchi S., Mizuno K., Tsuneyama K., Fukami T., Nakajima M., Yokoi T. Interleukin-17 is involved in alpha-naphthylisothiocyanate-induced liver injury in mice. Toxicology. 2010;275:50–57. doi: 10.1016/j.tox.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Copple B.L., Kaska S., Wentling C. Hypoxia-inducible factor activation in myeloid cells contributes to the development of liver fibrosis in cholestatic mice. J Pharmacol Exp Ther. 2012;341:307–316. doi: 10.1124/jpet.111.189340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song P., Zhang Y., Klaassen C.D. Dose-response of five bile acids on serum and liver bile acid concentrations and hepatotoxicty in mice. Toxicol Sci. 2011;123:359–367. doi: 10.1093/toxsci/kfr177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maione F., Paschalidis N., Mascolo N., Dufton N., Perretti M., D'Acquisto F. Interleukin 17 sustains rather than induces inflammation. Biochem Pharmacol. 2009;77:878–887. doi: 10.1016/j.bcp.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y., Mei J., Gonzales L., Yang G., Dai N., Wang P., Zhang P., Favara M., Malcolm K.C., Guttentag S., Worthen G.S. IL-17A and TNF-alpha exert synergistic effects on expression of CXCL5 by alveolar type II cells in vivo and in vitro. J Immunol. 2011;186:3197–3205. doi: 10.4049/jimmunol.1002016. [DOI] [PubMed] [Google Scholar]
- 26.Griffin G.K., Newton G., Tarrio M.L., Bu D.X., Maganto-Garcia E., Azcutia V., Alcaide P., Grabie N., Luscinskas F.W., Croce K.J., Lichtman A.H. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188:6287–6299. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iyoda M., Shibata T., Kawaguchi M., Hizawa N., Yamaoka T., Kokubu F., Akizawa T. IL-17A and IL-17F stimulate chemokines via MAPK pathways (ERK1/2 and p38 but not JNK) in mouse cultured mesangial cells: synergy with TNF-alpha and IL-1beta. Am J Physiol Renal Physiol. 2010;298:F779–F787. doi: 10.1152/ajprenal.00198.2009. [DOI] [PubMed] [Google Scholar]
- 28.Ma X., Reynolds S.L., Baker B.J., Li X., Benveniste E.N., Qin H. IL-17 enhancement of the IL-6 signaling cascade in astrocytes. J Immunol. 2010;184:4898–4906. doi: 10.4049/jimmunol.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogura H., Murakami M., Okuyama Y., Tsuruoka M., Kitabayashi C., Kanamoto M., Nishihara M., Iwakura Y., Hirano T. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29:628–636. doi: 10.1016/j.immuni.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Harada K., Shimoda S., Sato Y., Isse K., Ikeda H., Nakanuma Y. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin Exp Immunol. 2009;157:261–270. doi: 10.1111/j.1365-2249.2009.03947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takiguchi S., Koga A. Effects of bile acids and endotoxin on the function and morphology of cultured hamster Kupffer cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;54:303–311. doi: 10.1007/BF02899227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemmers A., Moreno C., Gustot T., Marechal R., Degre D., Demetter P., de Nadai P., Geerts A., Quertinmont E., Vercruysse V., Le Moine O., Deviere J. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646–657. doi: 10.1002/hep.22680. [DOI] [PubMed] [Google Scholar]
- 33.Sheen-Chen S.M., Chau P., Harris H.W. Obstructive jaundice alters Kupffer cell function independent of bacterial translocation. J Surg Res. 1998;80:205–209. doi: 10.1006/jsre.1998.5467. [DOI] [PubMed] [Google Scholar]
- 34.Keitel V., Donner M., Winandy S., Kubitz R., Haussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 35.Keitel V., Reinehr R., Gatsios P., Rupprecht C., Görg B., Selbach O., Häussinger D., Kubitz R. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45:695–704. doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- 36.Silveira M.G., Lindor K.D. Treatment of primary biliary cirrhosis: therapy with choleretic and immunosuppressive agents. Clin Liver Dis. 2008;12:425–443. doi: 10.1016/j.cld.2008.02.008. x-xi. [DOI] [PubMed] [Google Scholar]