Abstract
A number of disease-associated PrP forms characterized by abnormally short proteinase K–resistant fragments (atypical PrPres) were recently described in prion diseases. The relationship between atypical PrPres and PrPSc, and their role in etiology of prion diseases, remains unknown. We examined the relationship between PrPSc and atypical PrPres, a form characterized by short C-terminal proteinase K–resistant fragments, in a prion strain of synthetic origin. We found that the two forms exhibit distinct neuronal tropism, deposition patterns, and degree of pathological lesions. Immunostaining of brain regions demonstrated a partial overlap in anatomic involvement of the two forms and revealed the sites of their selective deposition. The experiments on amplification in vitro suggested that distinct neuronal tropism is attributed to differences in replication requirements, such as preferences for different cellular cofactors and PrPC glycoforms. Remarkably, deposition of atypical PrPres alone was not associated with notable pathological lesions, suggesting that it was not neurotoxic, but yet transmissible. Unlike PrPSc, atypical PrPres did not show significant perineuronal, vascular, or perivascular immunoreactivity. However, both forms showed substantial synaptic immunoreactivity. Considering that atypical PrPres is not associated with substantial lesions, this result suggests that not all synaptic disease–related PrP states are neurotoxic. The current work provides important new insight into our understanding of the structure–pathogenicity relationships of transmissible PrP states.
Prion diseases are a family of transmissible, neurodegenerative maladies associated with misfolding and aggregation of a soluble, cellular isoform of a prion protein (PrPC) into an abnormal, proteolytically resistant, β-sheet–rich isoform (PrPSc).1 In classical PrPSc, the region encompassing approximately residues 90 to 231 is resistant to proteinase K (PK) digestion and sufficient for prion infectivity.2 In the past decade, a number of disease-associated PrP forms characterized by abnormally short PK-resistant fragments were described in human and animal prion diseases (reviewed in Tranulis et al3). C-terminal PK-resistant fragments encompassing residues 154/156 to 231 and 162/167 to 231 were found in a majority of individuals with sporadic Creutzfeldt-Jakob disease (sCJD).4 A novel sporadic prion disease referred to as variable protease-sensitive prionopathy was also accompanied by accumulation of several atypical PrP fragments of variable length that were PK resistant.5 In addition, abnormal PK-resistant fragments were described in atypical bovine spongiform encephalopathy (BSE), which is believed to be sporadic in origin,6 and in ovine scrapie.7,8
With the discovery of atypical, disease-associated, PK-resistant PrP fragments (referred to as atypical PrPres) in prion diseases of sporadic origin, a number of questions have been raised. What is a relationship between atypical PrPres and PrPSc? Are atypical PrPres neurotoxic and transmissible? Do they play any role in the etiology of sporadic prion diseases? Although understanding these topics is important for developing adequate screening and diagnostic tools, addressing these questions has been difficult because self-propagating PrP states are often present in mixtures, their properties continue to evolve during serial passages, and because of the challenges in modeling diseases of sporadic origin in animal models.9,10
In previous studies, transmissible prion diseases were induced by inoculating amyloid fibrils produced in vitro from recombinant PrP.11–16 In wild-type animals, the diseases triggered by fibrils were characterized by a long, clinically silent stage and accumulation of atypical PrPres products,14,15 the features that appeared to be common with prion diseases of sporadic origin. Remarkably, atypical PrPres fragments found in animals inoculated with fibrils were very similar to the C-terminal PK-resistant fragments found in patients with sCJD or atypical BSE.4,6 Although atypical PrPres and PrPSc were found to replicate independently of each other,15,17 the dynamics in appearance of the two forms suggested that atypical PrPres was a precursor of PrPSc.15 Thus, these studies on synthetic prions uncovered one of the plausible pathways by which PrPSc can emerge.
The current study took advantage of the previously established system for generating transmissible disease de novo for examining the relationship between pathogenic features of the two alternative self-replicating PrP states. Using synthetic Syrian hamster strain S05, we show that atypical PrPres and classical PrPSc exhibit distinct neuronal tropism, deposition patterns, and significantly different severity of pathological lesions. The results of experiments on amplification in vitro suggested that distinct neuronal tropism could be in part due to differences in replication requirements of the two states, such as preferences for different cellular cofactors and PrPC glycoforms. Remarkably, deposition of atypical PrPres alone was not associated with notable pathological lesions, suggesting that this form was not neurotoxic, but yet transmissible. The current work provides important new insight into our understanding of the structure–pathogenicity relationship of transmissible PrP states.
Materials and Methods
Ethics Statement
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore (Assurance Number A32000-01; Permit Number: 0309001).
Bioassay
Weanling Golden Syrian hamsters (all males) were inoculated intracerebrally under 2% O2/4 MAC (minimum alveolar concentration) isoflurane anesthesia. In the current study, animals 1 through 5 or 6 through 8 received 50 μL of 10% brain homogenate (BH) inoculum prepared from animal 4 or 7, respectively, from the first passage of the synthetic strain S05.15 After inoculation, hamsters were observed daily for disease using a blinded scoring protocol. Hamsters were euthanized as they approached the terminal stage of the disease. Animals that did not develop clinical signs or those that did not progress to the terminal stage were euthanized at 664 days post inoculation (dpi).
For control experiments, animals received 50 μL of 10% BH inoculum prepared using animals of 661 days of age (first passage), or 10% BH inoculum prepared using animals inoculated with age-matched control brain materials and euthanized at 661 dpi (second passage). In addition, another control animal group received 50 μL of 0.5 mg/mL recombinant α-PrP.
Histopathological Studies
Formalin-fixed brain halves divided at the midline (hemisphere) were processed for H&E stain, as well as for immunohistochemistry for PrP, using the mouse monoclonal anti-PrP antibody 3F4 (1:1000; Covance, Berkeley, CA), SAF-84 (1:1000; Cayman Chemical, Ann Arbor, MI), and anti–glial fibrillar acidic protein (GFAP; 1:3000; Dako, Glostrup, Denmark). Blocks were treated in formic acid (96%) before embedding in paraffin. For detection of disease-associated PrP, we applied a pretreatment of 30 minutes hydrated autoclaving at 121°C followed by 5 minutes in 96% formic acid. We evaluated all tissues for the presence of inflammation and PrP immunoreactivity, and the brain for the presence of spongiform changes and degree of gliosis. The degree of spongiform changes, neuronal loss, and gliosis, and intensity of PrP immunostaining were semiquantitatively evaluated (0, none; 1, mild; 2, moderate; and 3, severe), as previously described,13 in the following anatomical regions: frontal cortex, hippocampus, caudate-putamen, thalamus, brainstem, and cerebellum. Lesion profiles were obtained for each animal and for animal groups by averaging scores of spongiform change, neuronal loss, and gliosis for each anatomical region.
Proteinase K Digestion
Brains were collected aseptically and cut in half with disposable scalpels. One half was used to prepare 10% BH in PBS or conversion buffer as described elsewhere,13 whereas the second half was stored at −80°C for future analysis or fixed in formalin for histopathology. For the PK digestion of BH in sarcosyl, an aliquot of 10% BH was mixed with an equal volume of 4% sarcosyl in PBS, supplemented with 50 mmol/L Tris (pH 7.5), and digested with 20 μg/mL PK for 30 minutes at 37°C with 1000 rpm shaking using a DELFIA Plateshake plate shaker (PerkinElmer Wallac, Yurku, Finland) placed in a 37°C incubator. PK digestion was stopped by adding SDS sample buffer and heating the samples for 10 minutes in a boiling water bath. After loading onto NuPAGE 12% Bis-Tris gels and transfer to polyvinylidene difluoride membrane, PrP was detected with 3F4 (epitope 109–112) or SAF-84 (epitope 160–70) antibody, as indicated. Western blot images were collected using FluorChem M system (ProteinSimple, Santa Clara, CA), and density profiles were generated using AlphaView SA software version 3.4.0 (ProteinSimple).
Protein Misfolding Cyclic Amplification with Beads
Ten percent normal brain homogenate (NBH) from healthy hamsters was prepared as described before14 and used as a substrate for protein misfolding cyclic amplification with beads (PMCAb) assay.18 The sonication program consisted of 20 seconds sonication pulses delivered at 170 W energy output applied every 20 minutes during a 24-hour period. For each subsequent round, 10 μL of the reaction from the previous round was added to 90 μL of fresh substrate. Each PMCAb reaction was performed in the presence of three 3/32-inch Teflon beads (AmazonSupply, Seattle, WA).
To produce partially deglycosylated substrate, 10% NBH from healthy hamsters prepared for PMCAb (see above) was treated with PNGase F (glycerol-free; New England BioLabs, Ipswich, MA) as follows. After pre-clearance of NBH at 500 × g for 2 minutes, 1500 U/mL PNGase F was added to the supernatant, the reaction was incubated on a rotator at 37°C for 5 hours, and then, partially deglycosylated NBH was used as a substrate in PMCAb. The PMCAb format that utilizes partially deglycosylated NBH is referred to as dgPMCAb. To prepare the RNA-depleted dgPMCA substrate, 100 μg/mL RNase A (Cat. No. R4875; Sigma-Aldrich, St. Louis, MO) was added to the NBH following PNGase F treatment, and the reaction was incubated on a rotator at 37°C for 1 hour as previously described.19
To analyze production of PK-resistant PrP material in PMCAb, 10 μL of sample were supplemented with 5 μL of SDS and 5 μL of PK, to a final concentration of 0.25% SDS and 50 μg/mL PK, followed by incubation at 37°C for 1 hour. The digestion was terminated by addition of SDS–sample buffer and heating the samples for 10 minutes in a boiling water bath. Samples were loaded onto NuPAGE 12% Bis-Tris gels, transferred to polyvinylidene difluoride membrane, and probed with SAF-84 antibody.
Results
Atypical PrPres and PrPSc Display Different in Vitro Replication Requirements
Eight animals were examined from the second passage of the synthetic strain S05 that was produced by inoculating recombinant PrP amyloid fibrils.15 Depending on the extent of the disease progression, the eight animals could be divided into three groups. Animals 1 and 2 (group 1) did not show any clinical signs of the disease (Table 1). Animals 3, 4, and 5 (group 2) developed clinical signs, but did not reach the terminal stage of the disease and were euthanized due to old age (Table 1). Animals 6, 7, and 8 (group 3) developed clinical signs and progressed to the terminal stage (Table 1).
Table 1.
Incubation Time to Disease for Individual Animals
| Animal No. | Incubation time (days)∗ | Euthanized on day |
|---|---|---|
| 1 | No clinical signs† | 664 |
| 2 | No clinical signs† | 664 |
| 3 | 505 | 664‡ |
| 4 | 505 | 664‡ |
| 5 | 393 | 664‡ |
| 6 | 463 | 588 |
| 7 | 463 | 588 |
| 8 | 463 | 664 |
Incubation time to first clinical signs.
No clinical signs for as late as 664 days after inoculation.
Animals did not reach terminal stage and were euthanized due to old age.
To detect disease-associated PrP forms, we took advantage of previous studies that showed that PrPSc could be detected by both 3F4 (epitope 109–112) and SAF-84 (epitope 160–170) antibodies, whereas atypical PrPres reacts only with SAF-84.14,15,17 Previously, we showed that PK treatment of atypical PrPres produces three major bands of approximately 23, 16, and 13 kDa, which correspond to di-, mono-, and unglycosylated glycoforms of PK-resistant fragments approximately encompassing residues 152/153 to 231, respectively (Figure 1A).15,17 Because of the overlap between the di- and monoglycosylated atypical PrPres with the mono- and unglycosylated PrPSc, respectively, the PK-resistant profiles of brain materials containing mixtures of atypical PrPres and PrPSc consist of four bands (Figure 1A).
Figure 1.
Biochemical analysis of brain material. A: Schematic representation of the PK-resistance profiles showing an overlap between atypical PrPres (gray boxes) and PrPSc (black boxes). B: PK-resistance profiles for individual animals obtained by densitometry of Western blots stained with SAF-84. Four peaks at 30, 23, 16, and 13 kDa represent six PK-resistant products. C: Western blots of brain material stained with SAF-84 and 3F4. D: Analysis of replication requirements. PMCAb and dgPMCAb reactions were seeded with 103-fold diluted brain material containing a mixture of atypical PrPres and PrPSc, and subjected to three serial rounds in NBH (lanes 3 to 5), RNA-depleted NBH (lanes 6 to 8), NBH pretreated with PNGase F (lanes 9 to 11), and RNA-depleted NBH pretreated with PNGase F (lanes 12 to 14). Western blot was stained with SAF-84 antibody. E: Western blots of the following control groups stained with SAF-84: animals euthanized at 661 days of age (lanes 1 to 3); animals inoculated with brain material of aged animals and euthanized at 559 to 661 dpi (lanes 4 to 6); second passage of brain materials of aged animals and euthanized at 526 to 664 dpi (lanes 7 to 9); and animals inoculated with recombinant α-PrP and euthanized at 664 dpi (lanes 10 to 12). Brain material from a second passage of S05 is shown as a reference.
As judged from the PK-resistance profiles, atypical PrPres was predominant in animals 1 and 2, whereas brain material from animals 3 through 8 showed mixtures of atypical and PrPSc (Figure 1C). Consistent with the previous study, atypical PrPres preferred monoglycosylated PrPC, whereas PrPSc was predominantly diglycosylated15 (Figure 1C). Although animals of all three groups had similar amounts of atypical PrPres (Figure 1C), animals in group 1 (animals 1 and 2) had very minor amounts of PrPSc, animals in group 2 (3, 4, and 5) intermediate, and animals in group 3 (6, 7, and 8) large amounts of PrPSc (Figure 1, B and C).
The following animal groups were examined as negative controls: uninfected animals euthanized at 661 days of age (n = 3); animals inoculated with brain material of aged animals and euthanized at 559 to 661 dpi (n = 15); second passage of brain materials of aged animals euthanized at 526 to 664 dpi (n = 8); and animals inoculated with recombinant α-PrP and euthanized at 664 dpi (n = 8). None of the animals from the control groups developed any clinical signs nor did they show any PK resistant products on Western blot analysis (Figure 1E). These control experiments are in good agreement with the results of several independent negative-control experiments reported in our previous studies, in which atypical PrPres and PrPSc could be found only in animals inoculated with recombinant PrP fibrils.13,15
Experiments using modified PMCAb that used partially deglycosylated PrPC as a substrate revealed that atypical PrPres and PrPSc were different, not only with respect to the length of their PK-resistant cores, but also with respect to in vitro amplification requirements. PrPSc could be steadily amplified only in NBH containing RNA, but not in RNA-depleted NBH (Figure 1D). In contrast to PrPSc, amplification of atypical PrPres was found to be steady regardless of RNA presence. However, unlike PrPSc, atypical PrPres could be amplified only in the modified PMCAb referred to as dgPMCAb that used partially deglycosylated PrPC as a substrate (Figure 1D). The differences in amplification requirements argue that atypical PrPres and PrPSc are two alternative self-propagating states.
The Extent of Brain Lesions Correlates with the Amount of PrPSc But not Atypical PrPres
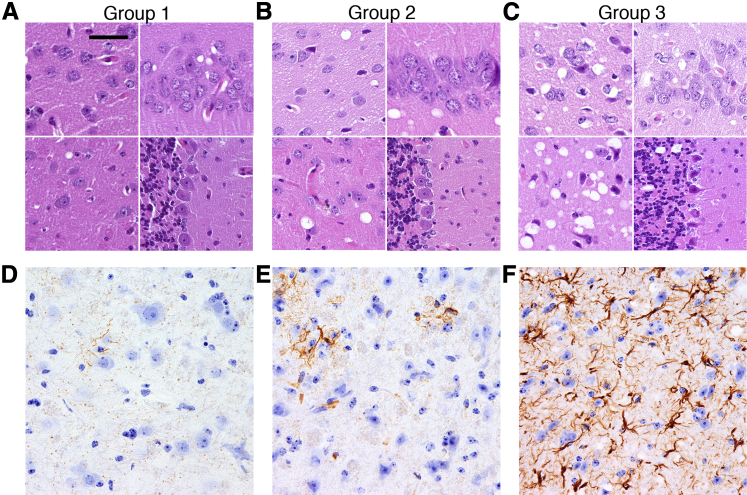
Histopathological evaluation revealed spongiform degeneration and reactive astrogliosis without any signs of inflammatory infiltration in all animals. However, the extent of lesions was significantly different between the three groups. In group 1, spongiform changes and reactive gliosis were very mild (Figure 2, A and D) and largely restricted to the hippocampus and brainstem (Figure 3A). One animal in this group showed mild lesions in the thalamus (Figures 2A and 3A). Minor lesions in animals 1 and 2 were consistent with the lack of clinical symptoms in this group (Table 1). By contrast, all three animals in group 3 showed prominent lesions in the thalamus, caudate-putamen, and brainstem, and variable involvement of the cortex, hippocampus, and cerebellum (Figures 2, C and F, and 3A). Animals in group 2 showed intermediate levels of brain lesions, with moderate lesioning in hippocampus, the caudate-putamen, thalamus, brainstem, and cortex (Figures 2, B and E, and 3A). Overall, histopathological assays revealed strong correlation between the extent of lesions and the amounts of PrPSc, but not atypical PrPres.
Figure 2.
Histopathological analysis of the three groups of animals. A–C: Spongiform change in the frontal cortex (top left images), hippocampus (top right images), thalamus (bottom left images), and cerebellum (bottom right images). Brain slices were stained with H&E. D–F: Reactive astrogliosis in the thalamus detected by immunostaining for GFAP. Scale bar: 20 μm (A–C); 15 μm in (D–F).
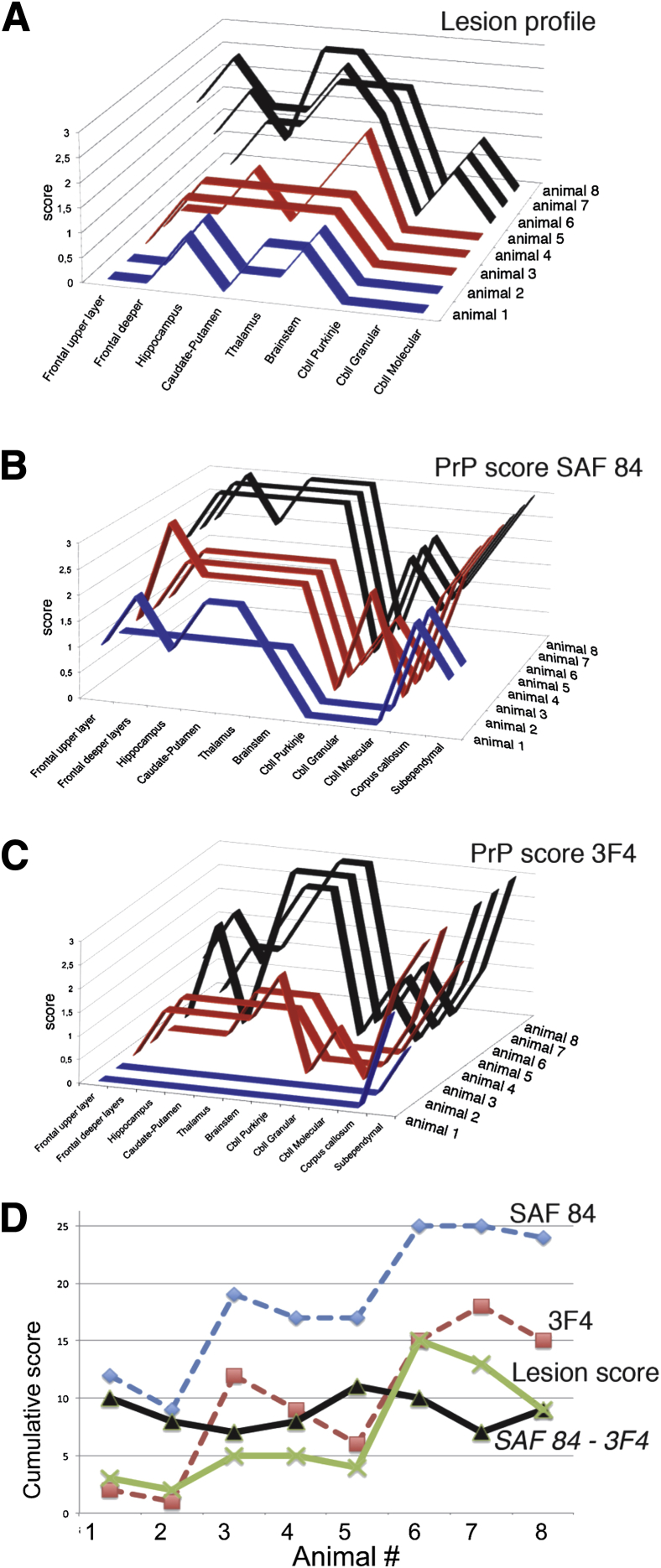
Figure 3.
Comparison of lesion profiles and immunoreactivity scores. Individual lesion profiles (A) and the PrP immunoreactivity scores for SAF-84 (B) and 3F4 (C) antibodies for animals in group 1 (blue), group 2 (red), and group 3 (black). D: Cumulative lesion score (green) and immunoreactivity scores (SAF-84, blue; 3F4, red) of all examined anatomic regions for each animal. The subtraction of cumulative 3F4 from SAF-84 immunoreactivity scores reflects relative amounts of atypical PrPres (black).
Atypical PrPres and PrPSc Displayed Distinct Deposition Patterns
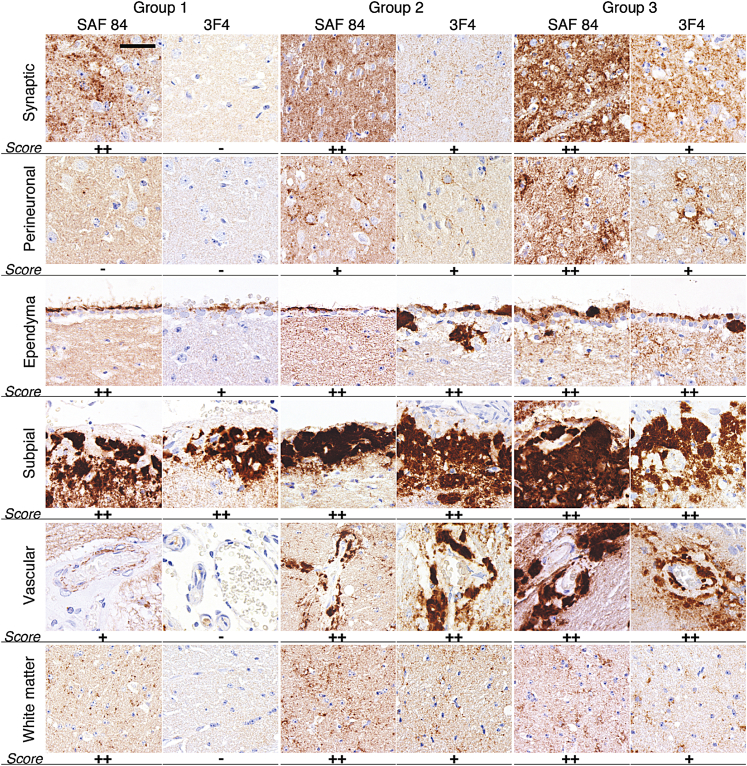
PrP immunostaining performed with 3F4 and SAF-84 antibody revealed the following types of PrP deposits: diffuse/synaptic fine deposits, perineuronally accentuated deposits with somewhat coarser granules surrounding neuronal perikarya, dot-like deposits in the white matter (mainly in the cross sections of the corpus callosum), small granular accumulations on the ependyma, perivascular PrP aggregates seen also in the vessel walls, and large plaques and amorphous deposits in the subpial and subependymal regions (Figure 4). Overall, SAF-84 showed more widespread distribution and labeled more types of PrP deposits than 3F4, which was consistent with the fact that SAF-84 stains both atypical and PrPSc, whereas 3F4 reacts only with PrPSc.
Figure 4.
Analysis of PrP immunoreactivity in the three groups of animals. Representative images of synaptic and perineuronal PrP deposition in thalamus, ependymal, subpial, and vascular plaques, and the white matter dot-like PrP deposition in the corpus callosum. Relative PrP deposition scores are shown: none (−), mild/focal (+), or prominent (++). Brain slices were immunostained using SAF-84 or 3F4 as indicated. Scale bar = 30 μm for all panels.
Striking differences in staining patterns between animals of the three groups were observed (Figures 3, B and C, and 4). Because animals in group 1 showed predominantly atypical PrPres, detailed comparison of group 1 with groups 2 and 3 revealed differences between the deposition patterns of atypical PrPres and PrPSc. Lack of perineuronal immunoreactivity, and vascular and perivascular deposits in group 1 suggests that unlike PrPSc, atypical PrPres does not have significant affinity to these sites (Figure 4). In group 1, diffuse synaptic staining in various regions and dot-like deposits in the white matter were detected with SAF-84, but not 3F4; therefore, these deposits have to be attributed to atypical PrPres (Figure 4). This observation argues that atypical PrPres, like PrPSc, can form synaptic and dot-like types of deposits. In group 1, subpial and ependymal PrP deposits were detected with both SAF-84 and with 3F4, suggesting that these deposits were formed by both atypical and PrPSc (Figure 4). Subpial and ependymal reactivity detected by 3F4 in the group could be attributed to minor amounts of PrPSc found in this group (Figure 1C).
When compared to group 1, animals in groups 2 and 3 showed progressive increases in the amount and intensity of diffuse/synaptic deposits and subpial and subependymal plaques (Figure 3, B and C). In addition, perineuronal deposits and perivascular PrP aggregates appeared in animals in group 2, and become more pronounced in animals in group 3. Because the amount of atypical PrPres was similar for all three groups (Figures 1B and 3D), these changes have to be attributed to the accumulation of PrPSc.
Atypical PrPres and PrPSc Have Distinct Neuronal Tropism
Scoring of PrP immunoreactivity using both SAF-84 and 3F4 antibodies revealed progressive increases in the deposition of total PrP in most of the brain regions from group 1 to group 2 and, to a larger extent, group 3, with the exception of the cerebellum Purkinje layer (Figure 3, B–D). Such dynamics correlated well with an increase in the combined amounts of atypical PrPres and PrPSc as analyzed by Western blot (Figure 1C).
The differences in the neuronal tropism between atypical PrPres and PrPSc could be illustrated by a comparison of SAF-84 scoring in group 1, where predominantly atypical PrPres was found, with that of groups 2 and 3. The cerebellum granular layer showed negative immunoreactivity for SAF-84 in group 1, but a notable increase in groups 2 and 3. This result suggests that atypical PrPres does not target the cerebellum. On the other hand, the corpus callosum showed relatively high scores for SAF-84 staining in group 1; this score did not increase in groups 2 and 3 despite dramatic increases in the amounts of PrPSc in these groups. This result indicates that atypical PrPres, but not PrPSc, had high affinity for the corpus callosum. Comparison of 3F4 to SAF-84 scoring in the groups that contained mixtures of the two forms (groups 2 and 3) provided additional information regarding differential neuronal tropism of atypical PrPres and PrPSc. Substantially lower scoring by 3F4 than SAF-84 antibody in the frontal upper and deeper layers and hippocampus within each of the two groups suggests that these regions might prefer atypical PrPres over PrPSc. In summary, although deposition of atypical PrPres and PrPSc were found to overlap in most brain regions, the cerebellum had a high affinity for PrPSc, whereas the corpus callosum and, to a lesser extent, the frontal upper and deeper layers and hippocampus appeared to have high selectivity for atypical PrPres.
Discussion
In the current study, two alternative transmissible PrP states, referred to as atypical PrPres and classical PrPSc, were found to display partially overlapping neuronal tropisms, and distinct deposition patterns and pathological lesion profiles within the same host. The lesion profile and PrP deposition patterns in animals with predominantly atypical PrPres were found to be remarkably distinct from those with abundant PrPSc. The extent of brain lesions was found to correlate well with the amounts of PrPSc, but not atypical PrPres, in brain. Indeed, accumulation of atypical PrPres alone did not lead to the significant vacuolation and reactive gliosis that are typically associated with transmissible spongiform encephalopathies. Moreover, animals in group 1 were asymptomatic. By contrast, the animals in group 2 with an intermediate level of PrPSc developed clinical signs, but did not reach the terminal stage of the disease for up to 664 days, when they had to be euthanized due to old age. The animals in group 3 with the largest amounts of PrPSc progressed to the terminal stage of the diseases. As judged from the length and position of the PK-resistant region, the atypical PrPres described here is similar to several PK-resistant PrP fragments previously observed in human and animal transmissible spongiform encephalopathies, including sCJD, atypical BSE, and scrapie.4,6,7
Analysis of region-specific PrP immunostaining using 3F4 and SAF-84 demonstrated a partial overlap in the anatomical involvement of PrPSc and atypical PrPres and, revealed the sites that were selectively targeted by PrPSc or atypical PrPres. Indeed, the cerebellum accumulated predominantly PrPSc, whereas the corpus callosum and, to a lesser extent, the frontal upper and deeper layers and hippocampus displayed high selectivity for atypical PrPres. Differences in neuronal tropism could be attributed to the differences in the biochemical requirements for replication of the two forms. Consistent with the previous studies, the PMCAb assay revealed that PrPSc preferred diglycosylated PrPC as a substrate, whereas atypical PrPres favored mono- and unglycosylated PrPC molecules. Taking into account that the ratio of PrPC glycoforms varies in different brain regions and cell types,20–22 the current work supports the hypothesis that different brain regions/cell types give selective advantage to either PrPSc or atypical PrPres, depending on the relative ratio of un-, mono-, and diglycosylated PrPC. Moreover, differences in the cofactor requirements might also contribute to selective replication of the two forms by different brain regions. Regardless of whether RNA plays a role in prion replication in animal brain, the difference in RNA dependency of PMCAb amplification highlighted distinct cofactor requirements between atypical PrPres and PrPSc.
On the basis of the finding that a strain with longer incubation times exhibited more widespread PrPSc distribution than a strain with short incubation times, previous studies suggested that the differences in strain-specific PrPSc distribution in brain anatomic regions could be attributed to differences in incubation times to the disease.23 In the current study, the animals in group 3 were euthanized at the same or earlier time points than the animals in group 1, yet they showed more widespread PrPSc distribution in comparison to atypical PrPres. This result argues that the differences in neuronal tropism between atypical and PrPSc were not due to differences in animal age.
PrPSc and atypical PrPres were found to display distinct deposition patterns. Both states were found to form synaptic, subpial, and ependymal deposits. However, unlike PrPSc, atypical PrPres did not show significant perineuronal, vascular, or perivascular immunoreactivity. In humans, molecular subtypes (strains) of sCJD are characterized by different anatomic involvement (ie, lesion profiles) as well as distinct immunostaining patterns for disease-associated PrPs.24–26 Although diffuse/synaptic types of deposits appeared in all sCJD subtypes, plaques or perineuronal immunodeposits were more characteristic of cases with type 2 disease–associated PrP. Because both synaptic and perineuronal accumulation of the disease-associated PrP are considered to impact interneuronal information processing, these depositions are likely to lead to synaptic/neuronal dysfunction, and the differences in their distribution and appearance might result in variable expression of clinical symptoms. Absence of clinical disease and perineuronal PrP immunoreactivity in group 1 suggests that this type of deposition is most likely associated with the neuronal dysfunction. On the other hand, animals in group 1 showed substantial synaptic immunoreactivity. This observation suggests that the synaptic form of atypical PrPres might not be as toxic as the synaptic deposition of PrPSc. Reduced toxicity could be due to differences in the molecular structures of PrPSc and atypical PrPres and/or absence of the N-terminals and the conserved hydrophobic domains in atypical PrPres. Interestingly, in previous studies of human prion diseases, anti-PrP antibodies that recognize the N-terminal epitopes were found to detect fewer synaptic deposits than those that react with the central region of PrP.27 Taking into account previous studies on familial forms of prion disease, PrP deposits with amyloid tinctorial properties may not necessarily be a reliable marker of TSE transmissibility.28 On the other hand, PrP deposits that lack obvious amyloid tinctorial properties and are localized in the vicinity of synapses (ie, strategic localization for causing dysfunction) might represent a transmissible PrP state, but display little if any neurotoxic effect. In summary, the current study indicates that not all disease-related PrP forms that accumulate in the vicinity of synapses are neurotoxic.
In previous studies on generating transmissible prion diseases with recombinant PrP fibrils, we proposed that i) atypical PrPres was the first product of PrPC misfolding triggered by recombinant fibrils; ii) deposition of atypical PrPres represented a clinically silent stage; and iii) atypical PrPres gave rise to authentic PrPSc, which eventually outcompeted atypical PrPres.14,15 We also showed that despite transmission within the same host, several serial passages were required to stabilize a strain-specific disease phenotype of a synthetic strain in a manner similar to the phenomenon of prion strain adaptation that accompanies cross-species transmission.29
Parallel studies on mouse synthetic prions revealed that a range of transmissible PrPSc states and disease phenotypes can be generated in mice by inoculating recombinant PrP fibrils produced in vitro under different solvent conditions.12,30 In a manner similar to evolution of hamster strains, biochemical and biological properties of synthetic mouse strains were found to change gradually on serial passaging, indicative of prion strain adaptation.16,31 Remarkably, serial passaging led to a convergence of independently produced mouse strains into a common type.16 Similarly, after several serial passages, three hamster strains of synthetic origin, SSLOW, LOTSS, and S05, also showed very similar physical and biological properties (unpublished observations). Competitive selection between alternative self-replicating states was proposed as a possible mechanism responsible for convergence of strain phenotypes and adaptation to a particular replication environment.16
The current work provides new insight into the evolution of transmissible prion states. First, different self-propagated states were found to display partially overlapping, yet different neuronal tropism and deposition patterns. Distinct neuronal tropism could be attributed to differences in replication requirements for the two PrP states. Considering that replacement of atypical PrPres by PrPSc requires several serial passages, these results could also explain previously observed findings that serial transmission of synthetic prions is accompanied by an apparent change in neurotropism and involvement of new brain regions.29 Second, deposition of atypical PrPres alone was found to result in minimal lesions, which is consistent with our previous studies that atypical PrPres can propagate and transmit silently.15 Third, the current findings illustrate that atypical PrPres and PrPSc prefer different biochemical environments consistent with the idea that these two forms are structurally different. Previously, we proposed that atypical PrPres occasionally produces PrPSc in seeding events that appeared to be relatively rare and stochastic, and were described by a deformed templating mechanism.15,32 As soon as the first PrPSc particles were generated, the two self-propagating PrP states replicated in parallel and competed for the same substrate. This model explains why full transition from atypical PrPres to PrPSc might take several years, a time period that in rodents is equivalent to several serial passages. In addition to competition for a substrate, atypical PrPres and PrPSc molecules might also compete for certain synaptic localizations and replication sites. The outcome of the competition appears to depend on PrPC glycosylation status and the immediate biochemical environment. A possible therapeutic intervention could involve interference with PrPSc replication by an alternative abnormal state that is considerably less toxic than PrPSc, but capable of self-replicating. Interestingly, a recent study revealed the coexistence of atypical PrP conformations and PrPSc in a patient with sCJD with a prolonged clinical course.33 This finding supports the concept of possible beneficial effects of alternative self-replicating PrP states. It would be interesting to determine in future studies whether atypical PrPres states can interfere with replication of PrPSc in vivo and whether this approach could be used as a therapeutic approach for slowing down or blocking disease progression.
Acknowledgment
We thank Pamela Wright for editing the manuscript.
Footnotes
Supported by NIH grants NS045585 and NS074998 (I.V.B.).
G.G.K. and N.M. contributed equally to this work.
References
- 1.Prusiner S.B. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prusiner S.B., Groth D.F., Bolton D.C., Kent S.B., Hood L.E. Purification and structural studies of a major scrapie prion protein. Cell. 1984;38:127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- 3.Tranulis M.A., Benestad S.L., Baron T., Kretzchmar H. Atypical prion diseases in humans and animals. Top Curr Chem. 2011;305:23–50. doi: 10.1007/128_2011_161. [DOI] [PubMed] [Google Scholar]
- 4.Zou W.Q., Capellari S., Parchi P., Sy M.S., Gambetti P., Chen S.G. Identification of novel proteinase K-resistant C-terminal fragments of PrP in Creutzfeldt-Jakob disease. J Biol Chem. 2003;278:40429–40436. doi: 10.1074/jbc.M308550200. [DOI] [PubMed] [Google Scholar]
- 5.Zou W.Q., Puoti G., Xiao X., Yuan J., Qing L., Cali I., Shimoji M., Langeveld J.P., Castellani R., Notari S., Crain B., Schmidt R.E., Geschwind M., DeArmond S.J., Cairns N.J., Dickson D., Honig L., Torres J.M., Mastrianni J., Capellari S., Giaccone G., Belay E.D., Schonberger L.B., Cohen M., Perry G., Kong Q., Parchi P., Tagliavini F., Gambetti P. Variably protease-sensitive prionopathy: a new sporadic disease of the prion protein. Ann Neurol. 2010;68:162–172. doi: 10.1002/ana.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biacabe A.G., Jacobs J.G., Bencsik A., Langeveld J.P., Baron T.G. H-type bovine spongiform encephalopathy: complex molecular features and similarities with human prion diseases. Prion. 2007;1:61–68. doi: 10.4161/pri.1.1.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron T., Bencsik A., Vulin J., Biacabe A.G., Morignat E., Verchere J., Betemps D. A C-terminal protease-resistant prion fragment distinguishes ovine “CH1641-like” scrapie from bovine classical and L-type BSE in ovine transgenic mice. PLOS Pathog. 2008;4:e1000137. doi: 10.1371/journal.ppat.1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benestad S.L., Arsac J.N., Goldmann W., Noremark M. Atypical/Nor98 scrapie: properties of the agent, genetics, and epidemiology. Vet Res. 2008;39:19. doi: 10.1051/vetres:2007056. [DOI] [PubMed] [Google Scholar]
- 9.Baron T., Vulin J., Biacabe A.G., Lakhdar L., Verchere J., Torres J.M., Bencsik A. Emergence of classical BSE strain properties during serial passages of H-BSE in wild-type mice. PLoS One. 2011;6:e15839. doi: 10.1371/journal.pone.0015839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comoy E.E., Casalone C., Lescoutra-Etchegaray N., Zanusso G., Freire S., Marcé D., Auvré F., Ruchoux M.M., Ferrari S., Monaco S., Salès N., Caramelli M., Leboulch P., Brown P., Lasmézas C.I., Deslys J.P. Atypical BSE (BASE) transmitted from asymptomatic aging cattle to a primate. PloS ONE. 2008;3:e3017. doi: 10.1371/journal.pone.0003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legname G., Baskakov I.V., Nguyen H.O.B., Riesner D., Cohen F.E., DeArmond S.J., Prusiner S.B. Synthetic mammalian prions. Science. 2004;305:673–676. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- 12.Colby D.W., Wain R., Baskakov I.V., Legname G., Palmer C.G., Nguyen H.O., Lemus A., Cohen F.E., DeArmond S.J., Prusiner S.B. Protease-sensitive synthetic prions. PLoS Pathog. 2010;6:e1000736. doi: 10.1371/journal.ppat.1000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makarava N., Kovacs G.G., Bocharova O.V., Savtchenko R., Alexeeva I., Budka H., Rohwer R.G., Baskakov I.V. Recombinant prion protein induces a new transmissible prion disease in wild type animals. Acta Neuropathol. 2010;119:177–187. doi: 10.1007/s00401-009-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makarava N., Kovacs G.G., Savtchenko R., Alexeeva I., Budka H., Rohwer R.G., Baskakov I.V. Genesis of mammalian prions: from non-infectious amyloid fibrils to a transmissible prion disease. PLoS Pathog. 2011;7:e1002419. doi: 10.1371/journal.ppat.1002419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makarava N., Kovacs G.G., Savtchenko R., Alexeeva I., Ostapchenko V.G., Budka H., Rohwer R.G., Baskakov I.V. A new mechanism for transmissible prion diseases. J Neurosci. 2012;32:7345–7355. doi: 10.1523/JNEUROSCI.6351-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghaemmaghami S., Colby D.W., Nquyen H.O., Hayashi S., Oehler A., DeArmond S., Prusiner S.B. Convergent replication of mouse synthetic prion strains. Am J Pathol. 2013;182:866–874. doi: 10.1016/j.ajpath.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makarava N., Savtchenko R., Baskakov I.V. Selective amplification of classical and atypical prions using modified protein misfolding cyclic amplification. J Biol Chem. 2013;288:33–41. doi: 10.1074/jbc.M112.419531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Montalban N., Makarava N., Ostapchenko V.G., Savtchenko R., Alexeeva I., Rohwer R.G., Baskakov I.V. Highly efficient protein misfolding cyclic amplification. PLoS Pathog. 2011;7:e1001277. doi: 10.1371/journal.ppat.1001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Montalban N., Makarava N., Savtchenko R., Baskakov I.V. Relationship between conformational stability and amplification efficiency of prions. Biochemistry. 2011;50:7933–7940. doi: 10.1021/bi200950v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeArmond S.J., Qiu Y., Sànchez H., Spilman P.R., Ninchak-Casey A., Alonso D., Daggett V. PrP C glycoform heterogeneity as a function of brain region: implications for selective targeting of neurons by prion strains. J Neuropathol Exp Neurol. 1999;58:1000–1009. doi: 10.1097/00005072-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Monnet C., Marthiens V., Enslen H., Frobert Y., Sobel A., Mege R.M. Heterogeneity and regulation of cellular prion protein glycoforms in neuronal cell lines. Eur J Neurosci. 2003;18:542–548. doi: 10.1046/j.1460-9568.2003.02777.x. [DOI] [PubMed] [Google Scholar]
- 22.Beringue V., Mallinson G., Kaisar M., Tayebi M., Sattar Z., Jackson G., Anstee D., Collinge J., Hawke S. Regional heterogeneity of cellular prion protein isoforms in the mouse brain. Brain. 2003;126(Pt 9):2065–2073. doi: 10.1093/brain/awg205. [DOI] [PubMed] [Google Scholar]
- 23.Ayers J.I., Kincaid A.E., Bartz J.C. Prion strain targeting independent of strain-specific neuronal tropism. J Virol. 2009;83:81–87. doi: 10.1128/JVI.01745-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parchi P., Strammiello R., Giese A., Kretzchmar H. Phenotypic variability of sporadic human prion disease and its molecular basis: past, present, and future. Acta Neuropathol. 2011;121:91–112. doi: 10.1007/s00401-010-0779-6. [DOI] [PubMed] [Google Scholar]
- 25.Head M.W., Ironside J.W. Review: Creutzfeldt-Jakob disease: prion protein type, disease phenotype and agent strain. Neuropath Appl Neurobiol. 2012;38:296–310. doi: 10.1111/j.1365-2990.2012.01265.x. [DOI] [PubMed] [Google Scholar]
- 26.Hill A.F., Joiner S., Wadsworth J.D.F., Sidle K.C.L., Bell J.E., Budka H., Ironside J.W., Collinge J. Molecular classification of sporadic Creutzfeldt-Jakob disease. Brain. 2003;126:1333–1346. doi: 10.1093/brain/awg125. [DOI] [PubMed] [Google Scholar]
- 27.Kovács G.G., Head M.W., Hegyi I., Bunn T.J., Flicker H., Hainfellner J.A., McCardle L., László L., Jarius C., Ironside J.W., Budka H. Immunohistochemistry for the prion protein: comparison of different monoclonal antibodies in human prion disease subtypes. Brain Pathol. 2002;12:1–11. doi: 10.1111/j.1750-3639.2002.tb00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piccardo P., Manson J.C., King D., Ghetti B., Barron R.M. Accumulation of prion protein in the brain that is not associated with transmissible disease. Proc Natl Acad Sci U S A. 2007;104:4712–4717. doi: 10.1073/pnas.0609241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makarava N., Kovacs G.G., Savtchenko R., Alexeeva I., Budka H., Rohwer R.G., Baskakov I.V. Stabilization of a prion strain of synthetic origin requires multiple serial passages. J Biol Chem. 2012;287:30205–30214. doi: 10.1074/jbc.M112.392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colby D.W., Giles K., Legname G., Wille H., Baskakov I.V., DeArmond S.J., Prusiner S.B. Design and construction of diverse mammalian prion strains. Proc Natl Acad Sci U S A. 2009;106:20417–20422. doi: 10.1073/pnas.0910350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghaemmaghami S., Watts J.C., Nquyen H.O., Hayashi S., DeArmond S.J., Prusiner S.B. Conformational transformation and selection of synthetic prion strains. J Mol Biol. 2011;413:527–542. doi: 10.1016/j.jmb.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makarava N., Baskakov I.V. Genesis of transmissible protein states vie deformed templating. Prion. 2012;6:252–255. doi: 10.4161/pri.19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigues-Martinez A.B., de Munain A.L., Ferrer I., Zarranz J.J., Atares B., Villagra N.T., Arteagoitia J.M., Garrido J.M., Juste R.A. Coexistence of protease sensitive and resistant prion protein in 129VV homozygous sporadic Creutzfeldt-Jakob disease: a case report. J Med Case Rep. 2012;6:348. doi: 10.1186/1752-1947-6-348. [DOI] [PMC free article] [PubMed] [Google Scholar]