Summary
Spine-related muscle pain can affect muscle strength and motor unit activity. This study was undertaken to investigate whether surface electromyographic (sEMG) recordings performed during relaxation and maximal contraction reveal differences in the activity of muscles with or without trigger points (TRPs). We also analyzed the possible coexistence of characteristic spontaneous activity in needle electromyographic (eEMG) recordings with the presence of TRPs.
Thirty patients with non-specific cervical and back pain were evaluated using clinical, neuroimaging and electroneurographic examinations. Muscle pain was measured using a visual analog scale (VAS), and strength using Lovett’s scale; trigger points were detected by palpation. EMG was used to examine motor unit activity.
Trigger points were found mainly in the trapezius muscles in thirteen patients. Their presence was accompanied by increased pain intensity, decreased muscle strength, increased resting sEMG amplitude, and decreased sEMG amplitude during muscle contraction. eEMG revealed characteristic asynchronous discharges in TRPs. The results of EMG examinations point to a complexity of muscle pain that depends on progression of the myofascial syndrome
Keywords: electromyography , muscle strength , myofascial pain syndrome , non-specific cervical and back pain , pain profile , trigger points
Introduction
Cervical and low back pain syndromes are a major epidemiological problem, prompting numerous clinical interventions. The pain, which is very often localized in one or many groups of muscles, is sometimes the first symptom of spine disease, but it can also be reported as the consequence of long-lasting disorders such as radiculopathies ( 1 ) . The most well known – but not the only ( 2 , 3 ) – spine-related pathology is myofascial pain syndrome ( 4 ) . Anatomical and functional disturbances in the bones, ligaments, nerves, vessels and muscles that make up paraspinal and spinal structures are generally indicated as the source of this syndrome ( 5 ) . Its symptoms are ascertained using different clinical diagnostic tools.
The standard clinical diagnosis of muscle pain is based mainly on assessment of pain intensity using visual analog scale (VAS) scores ( 6 , 7 ) . The palpation test allows evaluation of muscle tenderness and in cases of myofascial pain syndrome localizes the active trigger points (TRPs) typical of this pathology ( 8 ) . Studies on muscle strength with Lovett’s scale were not previously performed in detail ( 9 ) . On the basis of our own observations, it can be difficult to ascertain precisely the range of muscle pathology and its progression using the clinical methods mentioned above.
Neurophysiological studies have provided data on electromyographic (EMG) tests used as an objective tool for assessing the activity of muscles, especially those with active TRPs. The local twitch response was described as a characteristic phenomenon in elementary EMG recordings ( 10 ) . Most commonly, invasive elementary (needle) EMG (eEMG) was used, although surface EMG (sEMG) recording has become a popular examination for evaluation of the results of conservative treatment in patients with myofascial pain ( 11 ) . Previous needle EMG recordings have shown different patterns of spontaneous activity in muscles with active TRPs confirmed through palpation ( 12 , 13 ) . Proper interpretation of their results can influence the treatment strategy. Surface EMG recordings in patients with muscle pain have been performed mainly during voluntary muscle contraction, with little attention being paid to recordings during the resting state ( 14 ) . There arises the question: can non-invasive sEMG recording be as useful as eEMG as a tool for identifying muscles with active TRPs? The pathological symptom of muscle dysfunction is a decrease in the bioelectrical activity of motor units due to the pain appearing during stretch ( 15 , 16 ) . Comparison of EMG recordings during maximal contraction with pain profile evaluated with VAS would likely confirm this relationship. On the other hand, Svensson et al. ( 17 ) , studying resting activity of muscles, did not find a clear relationship with coexisting pain symptoms. However, these data came only from experimental tests on healthy subjects.
The aim of this study was to evaluate whether the subjective sensation of muscle pain reported by patients with non-specific cervical and low back pain influences muscle strength. On the basis of results of EMG examinations, we addressed the question of whether muscle pain changes the activity of motor units. This study was also undertaken to evaluate whether sEMG recording performed during the “relaxation state” and during “maximal contraction” shows different pathological activity in muscles with or without active TRPs. We also investigated the possible coexistence of characteristic spontaneous activity in eEMG recordings with the presence of TRPs detected by means of palpation.
Materials and methods
Patients
Thirty patients aged from 34 to 67 years (23 women and 7 men) with cervical and low back pain were enrolled in this study. They were diagnosed in the Department and Clinic for Physiotherapy, Rheumatology and Rehabilitation at the Wiktor Dega Clinical Orthopedic and Rehabilitation Hospital (outpatient ward). Long-lasting (more than 12 weeks) muscle pain caused by spine overloading, established through the patients’ personal medical histories, was the main inclusion criterion. In general, patients reported symmetrical pain limited to cervical and lumbar areas without radiation to the extremities. Patients with spine injuries or systemic diseases as a source of pain, detected through clinical and neuroimaging examinations as well as standard laboratory tests, were excluded from this study. Patients with radiculopathies, as shown by the results of clinical and electroneurographic (ENG) examinations, were also excluded. For comparison, 30 healthy volunteers (control group) aged from 19 to 54 years (14 women and 16 men) were also examined in the same way as the patients.
Clinical evaluation
Standard neurological examinations were performed, including evaluation of tendon and periosteal reflexes, Spurling’s and Lasegue’s tests and assessment of sensory perception ( 18 ) .
Painful muscles were identified in all patients. Pain intensity was estimated by VAS score ( 6 ) . Patients were asked to describe their pain as transient or chronic. Palpation examinations were performed bilaterally in the trapezius, gluteus medius, tensor fasciae latae and lumbar (L) erector spinae muscles in order evaluate muscle tenderness and locate active TRPs. The strength of painful muscles was tested using Lovett’s scale ( 19 ) .
Neurophysiological evaluation
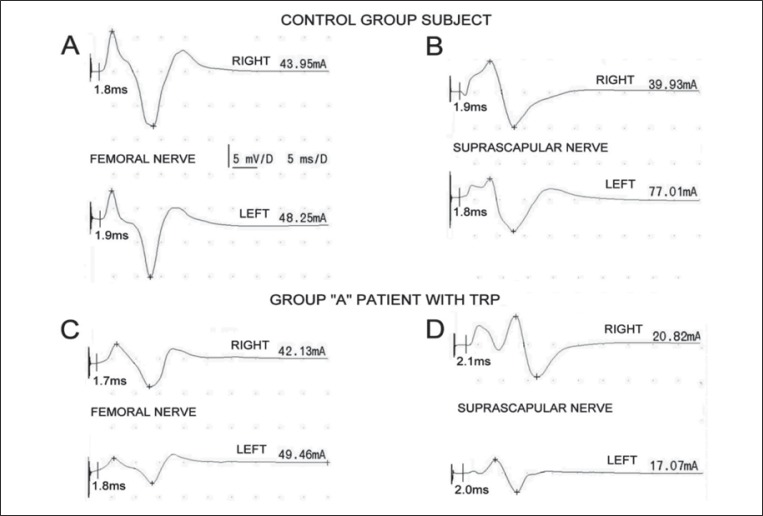
A Keypoint System (Medtronic A/S, Skovlunde, Denmark) was used for the neurophysiological testing. ENG examinations of impulse transmission in peripheral parts of motor fibers within suprascapular and femoral nerves were performed in all participants in order to exclude consequences of radiculopathies ( Fig. 1 ). Nerve branches were stimulated bilaterally with electrical pulses over the skin at the Erb’s point and inguen. Rectangular stimuli with a duration of 0.2 ms were delivered using bipolar electrodes at 1 Hz frequency, while their intensity ranged from 0 to 80 mA. Recordings of M-wave-evoked potentials were performed from the infraspinatus muscle and rectus femoris muscle with pairs of standard electrodes placed over their bellies and distal tendons. The time base was set to 5 ms/D and the amplification to 5000 μV/D during recordings. M-wave amplitudes, latencies and corresponding conduction velocities were calculated and compared with reference values ascertained in similar studies performed in the group of healthy volunteers. In all examinations of all subjects, these parameters were included in the ranges 3000-10000 μV, 1.7–2.0 ms and 54.2–48.2 m/s, respectively. The threshold for activation of all motor fibers during electrical stimulation ranged from 16 mA to 80 mA ( 20 , 21 ) . Stimulation studies were performed to confirm that patients presented only non-specific cervical and low back pain.
Figure 1 .
ENG recordings of M-waves performed following stimulation of femoral and suprascapular nerves. Examples obtained in one of the healthy volunteers from the control group are shown in A and B while analogous recordings from one of the patients with detected trigger points are shown in parts C and D, respectively. Abbreviation: TRP=trigger point.
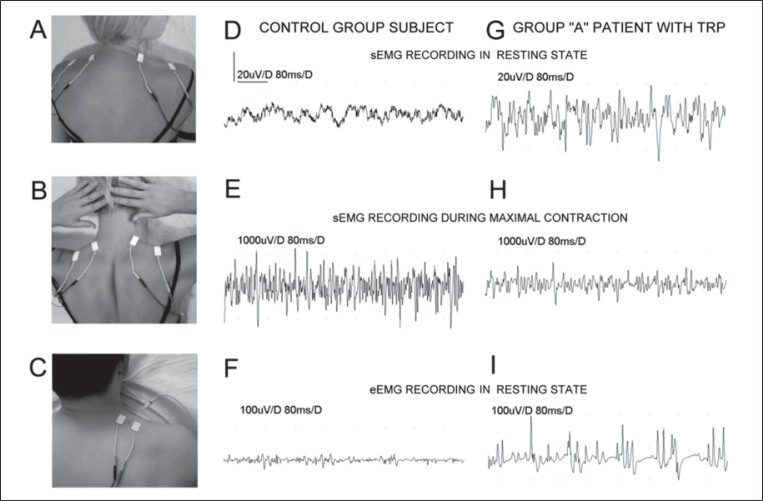
Global surface EMG examinations of the trapezius, gluteus medius, tensor fasciae latae and L erector spinae muscles were performed bilaterally according to standard procedures ( 20 – 24 ) both in the resting state and during maximal stretch lasting 5 seconds. They were performed, by all subjects, in comfortable prone or supine positions. Pairs of bipolar standard electrodes placed on the skin over the muscle belly and its tendon were used as shown in figure 2A and B for trapezius muscle examination. The parameter of average amplitude was analyzed. Recordings were performed with the time base set to 80 ms/D and amplification from 20 μV to 1000 μV/D, respectively. 10 Hz upper and 10 kHz lower filters were used.
Figure 2 .
Bilateral location of surface electrodes during sEMG recordings in the resting state (A), sEMG during maximal contraction of muscles (B) and resting needle eEMG recordings (C). Examples of recordings performed during different stages of the neurophysiological examination in one of the healthy volunteers are shown in D-F, and in one of the group A patients in G-I. Note the different amplification of recordings.
Needle EMG examinations were performed in the resting state in muscles with localized active TRPs and randomly in chronic painful muscles without TRPs. Figure 2C shows an example of the examination setup for eEMG recordings from the trapezius muscle. Only the amplitude, duration and frequency (synchronous or asynchronous firings) of the spontaneous potentials recorded during the resting state were analyzed. Recordings were performed with the time base setting ranging from 80 ms/D to 800 ms/D and the amplification set to between 100 μV/D and 500 μV/D. The same amplifier noise filters as for sEMG recordings were used. Trigger points were explored by insertion of the needle electrode to a depth of up to 25 mm from the muscle surface where the highest amplitudes of the spontaneous potentials were recorded. During eEMG recordings it was assumed that the spontaneous activity typical of the muscles with active TRPs would not show the synchronyous pattern typical of high-frequency firing, and that it would have a different morphology from end-plate potentials, denervation potentials (both fibrillations and positive sharp waves), fasciculations and pseudomyotonic discharges ( 20 ) .
Needle EMG was not performed in healthy volunteers or in patients with transient pain in muscles showing no TRPs. The muscles explored in the volunteers were the same as those studied in the patients, using the same EMG settings. Subjects performed sEMG recordings only during the resting state and at maximal contractions of the examined muscles.
Study design
All the patients and healthy volunteers were examined once. Analysis of each patient’s personal medical history (including clinical evaluation and neuroimaging analysis) and qualification for the study was performed by a rehabilitation doctor. A physiotherapist performed the muscle palpation, VAS and Lovett’s tests, while EMG and ENG recordings were performed by a neurophysiologist. The neurophysiologist did not know which group (healthy volunteer or patient) the subject belonged to (single-blinded trial). Muscles with active TRPs showing the greatest amplitude in resting sEMG recordings were randomly examined unilaterally by eEMG. Patients without ascertained active TRPs were examined with all sets of sEMG recordings but occasionally (n=7), control needle insertions were also performed.
Statistical analysis
The level of significance was accepted at p=0.05. Descriptive analysis included counting the mean value and its standard deviation. The normality distribution was assessed using the Shapiro-Wilk test. Wilcoxon’s test was used to compare data obtained in the patients (divided into three groups) with the reference values recorded in the group of healthy volunteers. To look for significant differences in data obtained from the analyzed groups, we applied the non-parametric Kruskall-Wallis test (index p t <0.05) with multiple comparisons (Dunn’s test).
The study complied with the ethical principles of the Helsinki declaration. It was also approved by the Bioethics Committee of the Poznań University of Medical Sciences.
Results
No adverse effects, reported either by volunteers or by patients, were noted by the researchers during the conducted studies. Neurological examinations excluded radiculopathy as the reason for the muscle pain. No abnormal tendon and periosteal reflexes, disturbances in sensory perception, or positive Spurling’s and Lasegue’s tests were found in any of the subjects included in this study.
ENG examinations confirmed that patients presented only non-specific cervical and low back pain. Figure 1A -D shows examples of M-wave recordings obtained in one of the healthy subjects and in one of the examined patients with ascertained TRPs. No changes in motor fiber transmission were observed in any of the patients (n=30) or healthy volunteers (n=30) when evoked potential amplitudes and latencies were compared.
The results of all the clinical studies performed in the control group of healthy volunteers provided the reference values for the same examinations performed in the patients. On the basis of the results of palpation tests for TRP detection and evaluation of pain duration, the patients were divided into three groups A, B, C ( Table I ).
Table I .
Comparison of pain intensity, occurrence of active trigger points and muscle strength evaluation in normal subjects and patients with myofascial pain syndrome
| Test |
Control group n=30 (mean±SD) |
Group A n=13 (mean±SD) |
Group B n=11 (mean±SD) |
Group C n=6 (mean±SD) |
||||
|---|---|---|---|---|---|---|---|---|
| VAS scale (0–100) | 0.5±0.2 | 6±1.5 * | 5.6±2.1 * | 4.8±1.5 * | ||||
| Duration of pain | no pain | chronic (constant) | chronic (constant) | transient | ||||
| Number of active trigger points in palpation study |
Right side 0 |
Left side 0 |
Right side 48 |
Left side 39 |
Right side 0 |
Left side 0 |
Right side 0 |
Left side 0 |
| Lovett’s scale (0–5) | Right side | Left side | Right side | Left side | Right side | Left side | Right side | Left side |
| Trapezius muscle | 4.8±0.5 | 4.7±0.5 | 3.5±0.5 * | 3.6±0.5 * | 3.6±0.5 * | 3.5±0.5 * | 3.2±0.8 * | 3.5±0.5 * |
| Gluteus medius muscle | 4.7±0.4 | 4.6±0.5 | 4.1±0.7 * | 4.2±0.6 * | 4.1±0.5 * | 4.2±0.6 * | 4.0±0.4 * | 3.8±0.4 * |
| Tensor fasciae latae muscle | 4.9±0.6 | 4.7±0.5 | 4.2±0.7 * | 4.2±0.6 * | 4.1±0.5 * | 4.1±0.5 * | 3.8±0.8 * | 3.8±0.8 * |
| L erector spinae muscles | 4.7±0.5 | 3.9±0.8 * | 4.0±0.6 * | 4.0±0.9 * | ||||
Asterisks indicate significant differences in parameters found in patients and normal subjects at p=0.05.
Pain intensity, as indicated by mean VAS score, was found to be 6 in group A, 5.6 in group B and 4.8 in group C. The patients in groups A and B reported the pain duration as chronic (constant) while the group C patients reported it as transient. Active TRPs were identified only in the 13 subjects making up group A, even though palpation studies were performed in all the patients (n=30). In all the patients, muscle strength was significantly (p=0.05) decreased in the trapezius, L erector spinae, gluteus medius and tensor fasciae latae muscles. No significant differences between groups of patients (A-C) were observed during evaluation of this parameter. Similarly, in all the patients from groups A-C, examination of muscle strength did not show significant differences between the right and left side.
The greatest pathological changes in resting EMG recordings were found, in most examined muscles, in the patients included in group A (p=0.05 compared to the reference values) (see recordings in figure 2D and G ). They were detected bilaterally ( Table II ). The mean value of the resting amplitude in the group B patients was increased only unilaterally in the trapezius (27 μV versus 14 μV in the control group), gluteus medius (16 μV vs 9 μV) and tensor fasciae latae (17 μV vs 9 μV) muscles, but to a lesser degree than observed in the group A patients. No changes in sEMG amplitude recorded in the resting state were found in examined muscles of patients from group C.
Table II .
Comparison of sEMG values recorded in the resting state and during maximal contraction in healthy subjects versus patients with myofascial pain syndrome
| Examined muscles Amplitude (μV) |
Control group n=30 (mean±SD) |
Group A n=13 (mean±SD) |
Group B n=11 (mean±SD) |
Group C n=6 (mean±SD) |
||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Right side | Left side | Right side | Left side | Right side | Left side | Right side | Left side | |
| Resting state | ||||||||
| Trapezius | 17±7 | 14±5 | 27±8 * | 33±8 * | 20±11 | 27±7 * | 18±3 | 19±5 |
| Gluteus medius | 9±3 | 10±4 | 20±14 * | 12±5 | 16±7 * | 11±4 | 13±6 | 11±2 |
| Tensor fasciae latae | 9±3 | 9±3 | 23±13 * | 17±8 * | 17±7 * | 12±4 | 12±4 | 12±4 |
| L erector spinae | 10±2 | 10±4 | 30±18 * | 25±15 * | 13±6 | 13±5 | 12±7 | 13±5 |
| Maximal contraction | ||||||||
| Trapezius | 1606±952 | 1626±963 | 723±238 * | 736±261 * | 645±298 * | 736±353 * | 733±175 * | 700±126 * |
| Gluteus medius | 886±313 | 830±260 | 405±230 * | 470±215 * | 764±238 | 518±279 * | 458±225 * | 450±226 * |
| Tensor fasciae latae | 1046±375 | 1023±414 | 630±306 * | 606±225 * | 673±297 * | 664±254 * | 917±366 | 567±197 * |
| L erector spinae | 1233±547 | 1253±497 | 593±274 * | 611±295 * | 664±418 * | 614±414 * | 583±214 * | 1000±335 |
Asterisks indicate significant differences in parameters found in patients and normal subjects at p=0.05.
Mean values of amplitudes from EMG recordings performed during maximal contractions were decreased bilaterally in patients from group A compared with the reference values (p=0.05) (see examples E and H in figure 2 ). These significant changes were also detected in group B, with the exception of the right gluteus medius muscle. The least changes were observed in group C, in the right tensor fasciae latae and left L erector spinae muscles.
In all 30 patients four painful muscles were examined bilaterally by means of palpation. As shown in Table III , which refers to the 13 group A patients, although 104 muscles were tested, TRPs that could also be found on the contralateral side were present in only 87. The TRPs were mainly localized in the gluteus medius and L erector spinae muscles, on both sides in equal proportions (13 to 13). A right lateralization of TRP incidence was found in the trapezius (11 to 6) and tensor fasciae latae (11 to 7) muscles. Taking into account the largest amplitude values in sEMG recordings during the resting state (a difference of at least 15 μV compared to reference values), 13 muscles were chosen for needle EMG examinations. These were performed most frequently in the trapezius (see recording I in figure 2 ) and L erector spinae muscles on both sides.
Table III .
Data on detection of active trigger points in the group A patients (n=13)
| Examined muscle | Number of TRPs | Number of spontaneous firings in eEMG | ||
|---|---|---|---|---|
|
| ||||
| Right side | Left side | Right side | Left side | |
| Trapezius | 11 | 6 | 3 | 3 |
| Gluteus medius | 13 | 13 | 1 | 0 |
| Tensor fasciae latae | 11 | 7 | 1 | 0 |
| L erector spinae | 13 | 13 | 3 | 2 |
The data in Table IV (over) compare, between the three groups of patients and the normal subjects, the mean values of amplitudes from sEMG recordings. The Kruskall-Wallis tests show that recordings performed bilaterally in patients were statistically different from those performed in normal subjects both in the resting state and during maximal contractions.
Table IV .
Results from EMG recordings performed in three groups of patients and in a control group of healthy volunteers: comparison of significant differences
| Amplitude in EMG recordings | p t (Kruskal-Wallis test) | |||
|---|---|---|---|---|
|
| ||||
| Right side | Left side | |||
| In the resting state | During maximal contraction | In the resting state | During maximal contraction | |
| L erector spinae | 0.0002 * | 0.0001 * | 0.0001 * | 0.0001 * |
| A≠N * | A≠N * | A≠N * | A≠N * | |
| B=N | B≠N * | B=N | B≠N * | |
| C=N | C≠N * | C=N | C=N | |
| Gluteus medius | 0.0001 * | 0.0001 * | 0.0390 * | 0.0001 * |
| A≠N * | A≠N * | A=N | A≠N * | |
| B≠N * | B=N | B=N | B≠N * | |
| C=N | C≠N * | C=N | C≠N * | |
| Tensor fasciae latae | 0.0002 * | 0.0003 * | 0.0008 * | 0.0002 * |
| A≠N * | A≠N * | A≠N * | A≠N * | |
| B≠N * | B≠N * | B=N | B≠N * | |
| C=N | C=N | C=N | C≠N * | |
| Trapezius | 0.0024 * | 0.0001 * | 0.0001 * | 0.0001 * |
| A≠N * | A≠N * | A≠N * | A≠N * | |
| B=N | B≠N * | B≠N * | B≠N * | |
| C=N | C≠N * | C=N | C≠N * | |
Probabilities of p t in Kruskall-Wallis test (statistically significant changes at p t <0.05 marked with asterisks) are shown. Index p t <0.05 for Dunn’s test is shown using the symbol ≠.
The results of the Dunn’s test analysis show that mean resting sEMG recordings from the trapezius, L erector spinae and gluteus medius muscles differed most frequently between the group A patients and the healthy volunteers. The group B patients did not show such frequent differences, while no differences at all were observed in the group C patients. The same statistical method showed that the results of EMG recordings during maximal contractions analyzed between the various groups of patients and normal subjects were usually different.
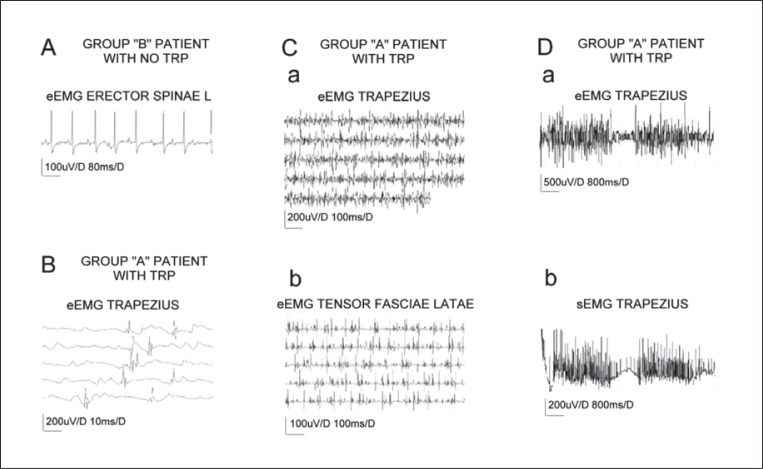
As can be seen in the examples in figure 3 , the recordings of spontaneous activity from muscles with TRPs were exclusively asynchronous (n=13, Ca and Cb), with a mean frequency of 28 Hz (Da and Db). They consisted of mainly polyphasic potentials with a duration of 16.4 ms (index 4.1, B), and average amplitude of 362 μV. This kind of asynchronous firing can easily be distinguished from the synchronous motor end-plate potentials recorded in muscles of group B patients, in which TRPs were not found ( Fig. 3A ). The phenomenon of asynchronous activity does not seem to be restricted to the trapezius muscle ( Fig. 3Ca ); indeed, it could also be observed in other muscles with TRPs found in the same patient ( Fig. 3Cb ). This asynchronous firing was transient and the recording sometimes (7/13 cases) showed it to be interrupted by a period of “bioelectrical silence” (see eEMG recording in figure 3Da ). Sometimes firings could be also observed in simultaneous sEMG recordings ( Fig. 3Db ) when the recording setup was similar to the one presented in figure 2C .
Figure 3 .
Examples of eEMG (A-Ca, b and Da) and sEMG (Db) recordings performed in the resting state in group A patients (B-D) in muscles with trigger points (TRPs) and in one of the group B patients (A) in whom no TRP was found.
Discussion
Available data on myofascial pain syndrome indicate that this phenomenon exists in many disorders which originate in the spine ( 11 ) . Tempomandibular joint disorders also play a significant role in its etiology ( 25 , 26 ) . More than 20 years ago, the incidence of the syndrome in patients was estimated to range between 30% and 55%, according to observations of general practitioners and dentists ( 27 , 28 ) . The leading symptom observed in these cases was the presence of TRPs, whose frequency of occurrence was reported by Fishbain et al. ( 29 ) to be 85%. Gerwin ( 30 ) reported a 74% incidence of TRPs in patients with pain symptoms of different etiology. In our study of 30 patients with cervical and low back pain, TRPs were found in 13 cases (43%), which is similar to the rates reported by Friction et al. ( 27 ) and Skootsky et al. ( 28 ) . In our group A patients, TRPs were repeatedly found in the gluteus medius, L erector spinae, trapezius and tensor fasciae latae muscles, with an incidence of location similar to that reported by Myburgh et al. ( 8 ) .
The presence of TRPs in group A patients was also accompanied by a relatively high pain sensation on the VAS (mean value of 6). This suggests a progression of muscle pathology; similarly, a coexistence of TRPs with pain was also frequently found in the study by Itoh et al. ( 31 ) . Duyur Cakit et al. ( 32 ) underlined that in patients with cervical myofascial pain this sensation is related to the incidence of active TRPs. In the present study, the group B and C patients had a lower pain score and TRPs were not localized through palpation.
Our results show that the decreased strength of the examined muscles lay, in all patients, within a similar range (from 3.5 to 4.2 measured on the Lovett’s scale). This method of the muscle strength assessment, although commonly used in clinical practice ( 23 ) , is not usually applied directly to patients with generalized spine-related myofascial pain syndrome. The existence of trigger points in the group A patients did not significantly influence their muscle strength. It was emphasized by Geisser et al. ( 33 ) and Descarreaux et al. ( 34 ) that the pain sensation in a particular muscle group can limit its strength during maximal contraction. The above observation can be confirmed by results found in the patients from our groups B and C.
Surface EMG recording has generally been used for the assessment of pathological muscle activity when stretching is sustained, mainly in cases of coexisting pain ( 15 , 35 ) . Simons et al. ( 36 ) provided precise descriptions of evaluation of muscle activity in the resting state with surface EMG recordings showing a spontaneous electrical activity phenomenon. In other studies, needle EMG examinations were usually used for assessment of characteristic spontaneous firings in TRPs whose location was primarily identified by palpation ( 6 , 12 , 13 ) .
The results of the present study proved the applicability of the sEMG method for evaluation of the increased amplitude in recordings performed in a majority of patients with clinically evaluated TRPs. It is noteworthy that the resting EMG amplitude value in these cases was greater than 20 to 25 μV, depending on the tested muscle. Data in Table II also indicate that the amplitude increase in resting EMG was accompanied by a decrease of the same parameter in EMG recordings during maximal contractions. This phenomenon, observed in the same muscles, was detected mainly in group A patients. On the basis of the results of our study it can be assumed that sEMG recording performed in the resting state constitutes a specific marker for detection of muscles with active TRPs. Such recordings could constitute a non-invasive screening tool for exploring muscles with active TRPs.
Needle EMG is considered an invasive diagnostic method whose applicability as a diagnostic procedure for myofascial TRP evaluation has not been confirmed by some authors ( 37 ) . In this study it was accepted that the characteristic recording from one active TRP could be deemed representative of all other bilateral TRPs in the same patient. The study by Hong and Simons ( 38 ) also failed to indicate major differences in the morphology of needle EMG recordings performed in a single patient. In order to avoid numerous needle EMG recordings from the 87 TRPs in the group A patients ( Table III ), only 13 muscles were explored in the 13 patients belonging to this group.
The spontaneous activity recorded by needle electrodes in active TRPs has been described in different ways. It was compared to the motor end-plate potential whose first inflection is negative followed by positive ( 6 , 10 , 13 , 36 ) . Hubbard and Berkoff ( 12 ) tried to compare it to fibrillation potentials coexisting with denervation of motor units. Morphologically, these are characterized by high amplitude and short duration when their first inflection is positive followed by negative ( 20 , 39 , 40 ) . These differences in morphology were discussed by Travell and Simons ( 11 ) who underlined more synchronyous than asynchronyous firing properties. Considering the eEMG recordings described in our study spontaneous firings in TRPs cannot be identified with the commonly known pathological source-related potentials. Audette et al. ( 41 ) , exploring TRPs in the quadratus lumborum and levator scapulae muscles, found the spontaneous activity to be similar to fasciculations but more complex, like that found in the recordings performed in our group A patients. In all 13 group A cases, these examinations always presented an asynchronous firing pattern. The parameters (amplitudes, durations and frequencies) presented in the results section seem to be different from the values reported by other authors ( 11 ) . On the other hand, their frequent location in trapezius and L erector spinae muscles overlap with the observations of Chung et al. ( 13 ) on common sites of active TRPs in certain groups of muscles.
The data in Table IV raise an interesting hypothesis about the possibility of evolution of the pathological changes in muscles from a subclinical form to the fully developed myofascial pain syndrome. Complex neurophysiological and clinical diagnostic examinations may also explain the possible etiology of non-specific cervical and back pain arising from active trigger points.
References
- 1. Cannon DE , Dillingham TR , Miao H , Andary MT , Pezzin LE . Musculoskeletal disorders in referrals for suspected cervical radiculopathy . Arch Phys Med Rehabil . 2007 ; 88 : 1256 – 1259 . doi: 10.1016/j.apmr.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 2. Facco E , Ceccherelli F . Myofascial pain mimicking radicular syndromes . Acta Neurochir . 2005 ; 92 : 147 – 150 . doi: 10.1007/3-211-27458-8_32. [DOI] [PubMed] [Google Scholar]
- 3. Huguenin LK . Myofascial trigger points: the current evidence . Phys Ther Sport . 2004 ; 5 : 2 – 12 . [Google Scholar]
- 4. Lavelle ED , Lavelle W , Smith HS . Myofascial trigger points . Anesthesiol Clin . 2007 ; 25 : 841 – 851 . doi: 10.1016/j.anclin.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 5. Graff-Radford SB . Myofascial pain: diagnosis and management . Curr Pain Headache Rep . 2004 ; 8 : 463 – 467 . doi: 10.1007/s11916-004-0068-y. [DOI] [PubMed] [Google Scholar]
- 6. Kostopoulos D , Nelson AJ , Ingber RS , Larkin RW . Reduction of spontaneous electrical activity and pain perception of trigger points in the upper trapezius muscle through trigger point compression and passive stretching . Journal of Musculoskeletal Pain . 2008 ; 16 : 266 – 278 . [Google Scholar]
- 7. Kaya A , Kamanli A , Ardicoglu O , Ozgocmen S , Ozkurt-Zengin F , Bayik Y . Direct current therapy with/without lido-caine ionthophoresis in myofascial pain syndrome . Bratisl Lek Listy . 2009 ; 110 : 185 – 191 . [PubMed] [Google Scholar]
- 8. Myburgh C , Larsen AH , Hartvigsen J . A systematic, critical review of manual palpation for identifying myofascial trigger points: evidence and clinical significance . Arch Phys Med Rehabil . 2008 ; 89 : 1169 – 1176 . doi: 10.1016/j.apmr.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 9. Andersen LL , Nielsen PK , Søgaard K , Andersen CH , Skotte J , Sjøgaard G . Torque-EMG velocity relationship in female workers with chronic neck muscle pain . J Biomech . 2008 ; 41 : 2029 – 2035 . doi: 10.1016/j.jbiomech.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 10. Rivner MH . The neurophysiology of myofascial pain syndrome . Curr Pain Headache Rep . 2001 ; 5 : 432 – 440 . doi: 10.1007/s11916-001-0054-6. [DOI] [PubMed] [Google Scholar]
- 11. Travell JG , Simons DG . The Trigger Point Manual . Baltimore: Williams & Wilkins ; 1999 . Myofascial Pain and Dysfunction. [Google Scholar]
- 12. Hubbard DR , Berkoff GM . Myofascial trigger points show spontaneous needle EMG activity . Spine . 1993 ; 18 : 1803 – 1807 . doi: 10.1097/00007632-199310000-00015. [DOI] [PubMed] [Google Scholar]
- 13. Chung JW , Ohrbach R , McCall WD ., Jr Characteristics of electrical activity in trapezius muscles with myofascial pain . Clin Neurophysiol . 2006 ; 117 : 2459 – 2466 . doi: 10.1016/j.clinph.2006.07.310. [DOI] [PubMed] [Google Scholar]
- 14. Gemmell H , Bagust J . Can surface electromyography differentiate muscle activity between upper trapezius muscles with active versus latent trigger points? A cross-sectional study . Clinical Chiropractic . 2009 ; 12 : 67 – 73 . [Google Scholar]
- 15. Farina D , Arendt-Nielsen L , Graven-Nielsen T . Experimental muscle pain reduces initial motor discharge rates during sustained submaximal contractions . J Appl Physiol . 2005 ; 98 : 999 – 1005 . doi: 10.1152/japplphysiol.01059.2004. [DOI] [PubMed] [Google Scholar]
- 16. Vasseljen O , Westgaard RH . A case-control study of trapezius muscle activity in office and manual workers with shoulder and neck pain and symptom-free controls . Int Arch Occup Environ Health . 1995 ; 67 : 11 – 18 . doi: 10.1007/BF00383127. [DOI] [PubMed] [Google Scholar]
- 17. Svensson P , Graven-Nielsen T , Matre D , Arendt-Nielsen L . Experimental muscle pain does not cause long-lasting increases in resting electromyographic activity . Muscle Nerve . 1998 ; 21 : 1382 – 1389 . doi: 10.1002/(sici)1097-4598(199811)21:11<1382::aid-mus4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 18. Buckup K . Clinical Tests for the Musculoskeletal System . Stuttgart, New York: Thieme ; 2004 . [Google Scholar]
- 19. Cuthbert SC , Goodheart GJ ., Jr On the reliability and validity of manual muscle testing: a literature review . Chiropr Osteopat . 2007 ; 15 : 4 . doi: 10.1186/1746-1340-15-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kimura J . Electrodiagnosis in Diseases of Nerve and Muscle Principles and Practise . New York : Oxford University Press ; 2001 . [Google Scholar]
- 21. DeLisa JA . Manual of Nerve Conduction Velocity and Clinical Neurophysiology . New York : Raven Press ; 1994 . [Google Scholar]
- 22. Clark BC , Manini TM , Mayer JM , Ploutz-Snyder LL , Graves JE . Electromyographic activity of the lumbar and hip extensors during dynamic trunk extension exercise . Arch Phys Med Rehabil . 2002 ; 83 : 1547 – 1552 . doi: 10.1053/apmr.2002.34828. [DOI] [PubMed] [Google Scholar]
- 23. Lisinski P , Huber J , Samborski W , Witkowska A . Neurophysiological assessment of the electrostimulation procedures used in stroke patients during rehabilitation . Int J Artif Organs . 2008 ; 31 : 76 – 86 . doi: 10.1177/039139880803100111. [DOI] [PubMed] [Google Scholar]
- 24. Albani G , Cimolin V , Galli M , et al. Use of surface EMG for evaluation of upper limb spasticity during botulin toxin therapy in stroke patients . Funct Neurol . 2010 ; 25 : 103 – 107 . [PubMed] [Google Scholar]
- 25. Okeson JP . Orofacial Pains. The Clinical Management of Orofacial Pain. Chicago: Quintessence Publishing Co Inc ; 1995. [Google Scholar]
- 26. Wolfe F . Fibromyalgia. In: Sessle BJ, Bryant PS, Dionne RA, editors. Tempomandibular Disorders and Related Pain Conditions Progress in Pain Research and Management. Seattle: IASP Press ; 1995 . [Google Scholar]
- 27. Fricton JR , Kroening R , Haley D , Siegert R . Myofascial pain syndrome of the head and neck: A review of clinical characteristics of 164 patients . Oral Surg Oral Med Oral Pathol . 1985 ; 60 : 615 – 623 . doi: 10.1016/0030-4220(85)90364-0. [DOI] [PubMed] [Google Scholar]
- 28. Skootsky SA , Jaeger B , Oye RK . Prevalence of myofascial pain in general internal medicine practice . West J Med . 1989 ; 151 : 157 – 160 . [PMC free article] [PubMed] [Google Scholar]
- 29. Fishbain DA , Goldberg M , Meagher BR , Steele R , Rosomoff H . Male and female chronic pain patients categorized by DSM-III psychiatric diagnostic criteria . Pain . 1986 ; 26 : 181 – 197 . doi: 10.1016/0304-3959(86)90074-6. [DOI] [PubMed] [Google Scholar]
- 30. Gerwin RD . A study of 96 subjects examined both for fibromyalgia and myofascial pain . Journal of Musculoskeletal Pain . 1995 ; 3 : 121 – 125 . [Google Scholar]
- 31. Itoh K , Katsumu Y , Kitakoji H . Trigger point acupuncture treatment of chronic low back pain in elderly patients – a blinded RCT . Acupunct Med . 2004 ; 22 : 170 – 177 . doi: 10.1136/aim.22.4.170. [DOI] [PubMed] [Google Scholar]
- 32. Duyur Cakit B , Genç H , Altuntas V , Erdem HR . Disability and related factors in patients with chronic cervical myofascial pain. Clin Rheumatol . 2009 ; 28 : 647 – 654 . doi: 10.1007/s10067-009-1116-0. [DOI] [PubMed] [Google Scholar]
- 33. Geisser ME , Haig AJ , Wallbom AS , Wiggert EA . Pain-related fear, lumbar flexion, and dynamic EMG among persons with chronic musculoskeletal low back pain . Clin J Pain . 2004 ; 20 : 61 – 69 . doi: 10.1097/00002508-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 34. Descarreaux M , Lalonde C , Normand MC . Isometric force parameters and trunk muscle recruitment strategies in population with low back pain . J Manipulative Physiol Ther . 2007 ; 30 : 91 – 97 . doi: 10.1016/j.jmpt.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 35. Østensvik T , Veiersted KB , Nilsen P . A method to quantify frequency and duration of sustained low-level muscle activity as a risk factor for musculoskeletal discomfort . J Electromyogr Kinesiol . 2009 ; 19 : 283 – 294 . doi: 10.1016/j.jelekin.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 36. Simons DG , Hong CZ , Simons LS . Endplate potentials are common to midfiber myofacial trigger points . Am J Phys Med Rehabil . 2002 ; 81 : 212 – 222 . doi: 10.1097/00002060-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 37. Durette MR , Rodriquez AA , Agre JC , Silverman JL . Needle electromyographic evaluation of patients with myofascial or fibromyalgic pain . Am J Phys Med Rehabil . 1991 ; 70 : 154 – 156 . doi: 10.1097/00002060-199106000-00009. [DOI] [PubMed] [Google Scholar]
- 38. Hong CZ , Simons DG . Pathophysiologic and electrophysiologic mechanisms of myofascial trigger points . Arch Phys Med Rehabil . 1998 ; 79 : 863 – 872 . doi: 10.1016/s0003-9993(98)90371-9. [DOI] [PubMed] [Google Scholar]
- 39. Johnson EW , Pease WS . Practical Electromyography. Baltimore: William & Wilkins; 1997. [Google Scholar]
- 40. Preston DC , Shapiro BE . Electromyography and Neuro-muscular Disorders: Clinical-Electrophysiologic Correlations . Philadelphia: Elsevier, Butterworth-Heinemann; 2005 . [Google Scholar]
- 41. Audette JF , Wang F , Smith H . Bilateral activation of motor unit potentials with unilateral needle stimulation of active myofascial trigger points . Am J Phys Med Rehabil . 2004 ; 83 : 368 – 374 . doi: 10.1097/01.phm.0000118037.61143.7c. [DOI] [PubMed] [Google Scholar]