Summary
Antiplatelets, antihypertensives, and statins might reduce the severity of the event or improve outcome in patients who, despite prior medical treatment, have a stroke.
We evaluated, in patients who had an ischemic stroke, the effect, on stroke severity and outcome, of prior treatment with antiplatelets, antihypertensives, and statins, used either alone or in a three-drug combination.
Stroke in Italy and Related Impact on Outcome (SIRIO) was a prospective, nationwide, multicenter, hospital-based, observational study that included patients aged ≥18 years with acute ischemic stroke.
We studied 2,529 acute ischemic stroke patients from the SIRIO population: 887 were antiplatelet users, 1,497 antihypertensive users, 231 statin users, and 138 three-drug combination users prior to the index event. The adjusted logistic regression analysis showed an association between prior treatment with statins and good functional outcome at discharge, while prior treatment with antiplatelets, antihypertensives or the three-drug combination did not influence severity or outcome. The absolute probability of a good functional outcome was 46.3% (95% CI: 40.3%–53.2%) in statin users and 36.7% (95% CI: 34.7%–38.7%) in non-users of statins; the absolute risk difference was 9.6% (95% CI: 2.9%–16.4%; p=0.004).
Prior treatment with antiplatelets, antihypertensives, or the three-drug combination did not influence stroke severity or outcome, while prior treatment with statins did not influence stroke severity but was associated with a better functional outcome.
Keywords: antihypertensives , antiplatelets , statins , stroke
Introduction
Antiplatelets, antihypertensives and statins contribute to reducing the occurrence of ischemic stroke ( 1 ) . A possible additional benefit of treatment with these agents might be reduced severity of the event in those patients who, despite preventive treatments, develop a stroke. An association has been reported between prior use of statins and better functional outcome or reduced mortality after stroke ( 2 – 11 ) . As regards antiplatelets, the evidence is more controversial since studies evaluating their prior use reported favorable, unfavorable, and neutral effects on functional outcome ( 12 – 16 ) . Likewise, preventive treatment with several antihypertensive agents was associated with reduced stroke severity in some studies, but this finding was not confirmed in others ( 12 , 17 – 20 ) . The role of the three-drug combination of an antiplatelet agent, an antihypertensive agent, and a statin has been less extensively investigated, in spite of its possible association with a greater reduction in stroke severity ( 21 ) .
We prospectively evaluated, in patients who suffered an ischemic stroke, the effect of prior use of antiplatelets, antihypertensives, and statins (used either singly or as a three-drug combination) on stroke severity and outcome.
Materials and methods
Study design and inclusion criteria
Stroke in Italy and Related Impact on Outcome (SIRIO) was a prospective, nationwide, multicenter, hospital-based, observational study performed in accordance with the Declaration of Helsinki. Each participating center recorded all consecutive incident cases of stroke admitted in a four-month period in 2005 (the centers began this recording only after receiving local ethics committee approval) ( 22 , 23 ) . Patients with transient ischemic attack and intracerebral or subarachnoid hemorrhage were excluded from the study. Men and women ≥18 years of age, with an acute ischemic stroke confirmed by brain computed tomography and/or magnetic resonance imaging and with a neurological deficit scored ≥1 on the National Institutes of Health Stroke Scale (NIHSS) were included ( 24 ) . Informed consent to data handling, given by the patients or by their legal representatives, was mandatory for inclusion. For the purpose of the present study, demographic data, risk factors, and prior drug treatments were considered.
Definition of risk factors
Arterial hypertension was defined as a systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg based on the average of two measurements, a history of arterial hypertension, or current use of anti-hypertensives ( 25 ) . Diabetes mellitus was defined as a fasting glucose level ≥7 mmol/L, a non-fasting glucose level ≥11.1 mmol/L, a history of diabetes, or the use of oral antidiabetics or insulin ( 26 ) . Hypercholesterolemia was defined as a total blood cholesterol level ≥240 mg/dL or a history of treated hypercholesterolemia ( 27 ) . Previous stroke or transient ischemic attack were diagnosed in the presence of a positive history. Coronary heart disease was diagnosed in the presence of a history of acute myocardial infarction or of unstable or stable angina. Peripheral arterial disease was diagnosed in the presence of a history of intermittent claudication, ultrasonography documentation, an ankle-brachial index ≤0.90, or previous arterial surgery. Atrial fibrillation was diagnosed in the presence of electrocardiographic evidence of the arrhythmia. Patients reporting current use of any type of tobacco (i.e. during the past year) were categorized as cigarette smokers. The criterion for alcohol abuse was the reported consumption of ≥4 drinks per day ( 28 ) . Obesity was classified in the presence of obesity class I–III (body mass index ≥30.0) ( 29 ) .
Assessment of prior treatments
Prior treatments were determined by means of a standardized questionnaire interview of the patients, their family and/or general practitioner, in order to assess user or non-user status. Drugs were recorded using the standardized Anatomical Therapeutic Chemical Classification System (ATCCS) of the World Health Organization Collaborating Centre for Drug Statistics Methodology ( 30 ) . Patients were classed as antiplatelet users if they were taking aspirin, ticlopidine, clopidogrel, aspirin plus extended-release dipyridamole, or aspirin plus ticlopidine or clopidogrel, and as non-users in the absence of any of the above reported treatments. Patients were classed as antihypertensive users if they were taking at least one drug included as an antihypertensive agent in the ATCCS, and as non-users in the absence of any antihypertensive treatment. Patients were grouped as statin users if they were taking at least one drug included in the ATCCS as a statin and as non-users in the absence of any treatment with statins. Patients taking an association of an antiplatelet, an antihypertensive, and a statin were classed as three-drug combination users while patients not taking any of the three drug treatments, or taking one or two of them were considered three-drug combination non-users.
Outcomes
Assessment of the severity of the ischemic stroke was based on the NIHSS score on admission ( 24 ) . Functional outcome was measured by the modified Rankin Scale (mRS) score at discharge ( 31 ) . All deaths occurring during hospitalization and within the one-year follow up were recorded. For the purpose of the present study patients with an NIHSS score <8 on admission were classed as patients with mild stroke ( 32 ) and those with an mRS score <2 at discharge as patients with a good functional outcome (deceased patients were scored 6) ( 33 ) .
Statistical analysis
Continuous variables were expressed as means ± standard deviation (SD) and categorical variables as contingency tables. NIHSS and mRS scores were handled as non-continuous variables and expressed as median values with interquartile range (IQR). Comparisons were performed using Student’s t-test, the Mann-Whitney test, or χ 2 test when appropriate. Logistic regression analysis was used to determine the association between stroke severity on admission or functional outcome at discharge and prior treatment. Logistic analyses were adjusted for age, sex, and risk factors, including arterial hypertension, diabetes mellitus, previous stroke or transient ischemic attack, atrial fibrillation, coronary heart disease, peripheral arterial disease, cigarette smoking, alcohol abuse, and obesity. Logistic analysis for functional outcome at discharge was also adjusted for baseline NIHSS score. Results were reported as odds ratios (OR) and 95% confidence intervals (CI). Two-sided p values less than 0.05 were considered to indicate significance. All analyses were performed using the SPSS statistical software (version 15).
Results
We studied 2,529 patients (mean age±SD 71.4±12.3 years) with an acute ischemic stroke, 1,444 men and 1,085 women; women were older than men (mean age±SD 73.9±12.9 vs 69.5±11.5 years; p<0.001); in 1,909 patients the index stroke was a first-ever stroke (75.4%) while 620 patients (24.6%) reported a previous stroke or a previous transient ischemic attack. The median NIHSS score on admission was 6 (IQR: 3–9) while the median mRS score at discharge (not available for 12 patients) was 3 (IQR: 1–6). The mean length of hospital stay was 13.6±12.5 days.
A total of 887 patients (35.1%) were antiplatelet users before the index stroke; the proportion of antiplatelet users was similar in men and women (p=0.221). Of these, 685 were on aspirin, 166 on ticlopidine, 20 on clopidogrel, 10 on aspirin plus clopidogrel, and six on aspirin plus ticlopidine.
The antihypertensive users numbered 1,497 (59.2%), receiving a total of 1,926 medications; in this case, the proportion of women was greater (62.3% vs 56.9%; p=0.006). The statin users numbered 231 (9.1%), and the proportions of men and women were similar (p=0.091). The three-drug combination users numbered 138 (5.4%); in this case, the proportion of women was lower than that of men (4.1% vs 6.5%; p=0.014).
Antiplatelet and antihypertensive users as compared to non-users had higher median NIHSS scores on admission (6, IQR: 3–11 vs 5, IQR: 3–9, p<0.001; and 6, IQR: 3–10 vs 5, IQR: 3–9, p=0.006, respectively) and higher mRS scores at discharge (in both cases, 3, IQR: 1–4 vs 2, IQR: 1–4, p<0.001), while statin users had lower median scores on the NIHSS and mRS scales with respect to non-users (4, IQR: 3–9 vs 5, IQR: 3–10, p=0.037; and 2, IQR: 1–4 vs 3, IQR: 1–4, p=0.016). Median NIHSS and mRS scores were similar in the three-drug combination users and non-users (4, IQR: 2–9 vs 5, IQR: 3–9, p=0.708; and 2, IQR: 1–4 vs 2, IQR: 1–4, p=0.776).
As reported in Table I , higher proportions of antiplatelet and antihypertensive users, with respect to non-users, had arterial hypertension, diabetes mellitus, hypercholesterolemia, previous stroke or transient ischemic attack, atrial fibrillation, coronary heart disease, and peripheral arterial disease, while lower proportions reported cigarette smoking and alcohol abuse. Moreover, the antihypertensive users with respect to the non-users, also had a higher proportion of subjects with obesity. The group of statin users had, compared with the non-users, higher proportions of subjects with arterial hypertension, diabetes mellitus, hypercholesterolemia, previous stroke or transient ischemic attack, coronary heart disease, peripheral arterial disease, and obesity.
Table 1 .
Distribution of risk factors by prior treatment
| Antiplatelets | Antihypertensives | Statins | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| % yes | % no | p | % yes | % no | p | % yes | % no | p | |
| Mean age±SD | 74.7±9.7 | 69.0±13.3 | <0.001 | 73.7±10.0 | 68.0±14.5 | <0.001 | 70.0±8.9 | 71.5±12.7 | 0.020 |
| Arterial hypertension | 81.7 | 62.5 | <0.001 | 92.7 | 36.3 | <0.001 | 84.7 | 68.4 | <0.001 |
| Diabetes mellitus | 29.6 | 22.9 | <0.001 | 28.1 | 20.6 | <0.001 | 30.6 | 24.3 | 0.036 |
| Hypercholesterolemia | 33.8 | 26.7 | <0.001 | 32.4 | 24.0 | <0.001 | 81.6 | 23.5 | <0.001 |
| Previous stroke/TIA | 41.4 | 11.3 | <0.001 | 28.1 | 19.6 | <0.001 | 32.6 | 23.7 | 0.003 |
| Atrial fibrillation | 25.7 | 11.4 | <0.001 | 26.2 | 12.4 | <0.001 | 17.3 | 20.9 | 0.197 |
| CHD | 33.7 | 8.3 | <0.001 | 25.1 | 10.2 | <0.001 | 47.8 | 16.0 | <0.001 |
| PAD | 11.8 | 3.4 | <0.001 | 8.2 | 5.9 | 0.030 | 11.5 | 6.8 | 0.009 |
| Cigarette smoking | 16.0 | 27.4 | <0.001 | 15.5 | 31.6 | <0.001 | 17.5 | 22.4 | 0.092 |
| Alcohol abuse | 5.4 | 9.8 | <0.001 | 6.1 | 10.9 | <0.001 | 6.3 | 8.2 | 0.304 |
| Obesity | 12.3 | 11.1 | 0.405 | 12.7 | 9.5 | 0.016 | 17.9 | 10.5 | 0.001 |
Abbreviations: TIA=transient ischemic attack; CHD=coronary heart disease; PAD=peripheral arterial disease.
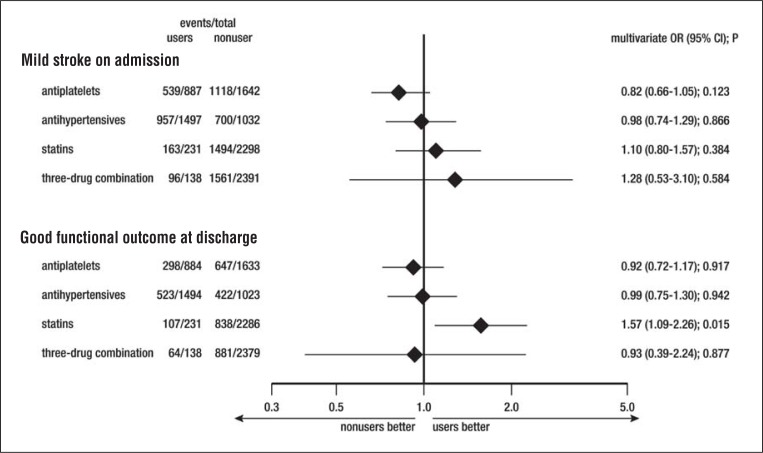
In the logistic regression analysis no effect of any pre-event treatment on the probability of mild stroke on admission was detectable, whereas there did emerge an association between good functional outcome at discharge and statin pre-treatment (OR: 1.57; 95% CI: 1.09–2.26; p=0.015; Fig. 1 ). Figure 2 shows the distribution of mRS scores in statin users and non-users. The absolute probability of a good functional outcome was 46.3% (95% CI: 40.3%–53.2%) in the statin users and 36.7% (95% CI: 34.7%–38.7%) in the non-users; the absolute risk difference was 9.6% (95% CI: 2.9%–16.4%; p=0.004).
Figure 1 .
Logistic regression analysis of the probability of a mild stroke on admission (National Institutes of Stroke Scale score <8 on admission) and a good functional outcome at discharge (modified Rankin Scale score <2 at discharge), adjusted for age, gender, and risk factors, and referring to mRS score at discharge also adjusted for baseline NIHSS.
Figure 2 .
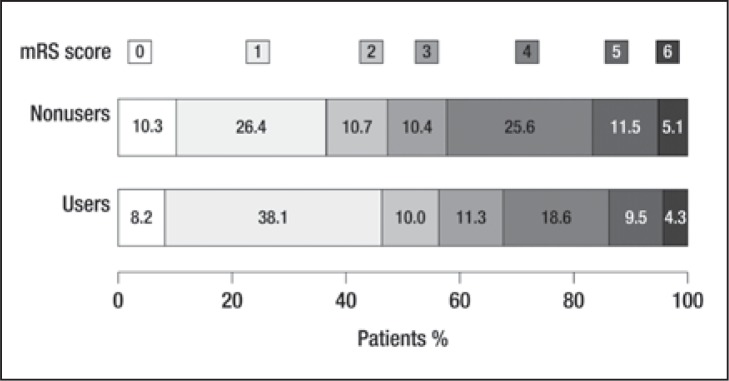
Proportions of statin users and non-users according to the modified Rankin Scale (mRS) score at discharge.
Discussion
In patients who suffered an acute ischemic stroke, prior use of statins was associated with a better functional outcome at discharge with respect to patients who were not treated with statins, while prior use of antiplatelets, or antihypertensives, or of the three-drug combination did not influence stroke severity or outcome. Data were collected from a prospective, nationwide, multicenter, hospital-based study, which included a high number of patients with an acute ischemic stroke ( 22 , 23 ) . One of the main strengths of this study was the possibility of investigating the effects of the different treatments both on stroke severity on admission and on functional outcome at discharge in the same cohort of patients. To date, most of the available data on the possible effects of the different treatment options have been obtained from studies using different cohorts of patients and having a retrospective design. Moreover, the main endpoint was stroke severity in some studies and functional outcome in others, whereas only a few studies concurrently considered both stroke severity on admission and functional outcome ( 7 , 9 , 16 ) . We assessed stroke severity and functional outcome using validated instruments, namely the NIHSS and the mRS. Most of the available studies cannot be compared because they differ in the selected scales, or in the chosen NIHSS and mRS cut-off values.
As expected, medically treated patients had a worse profile in terms of risk factors compared with untreated patients ( 34 ) . Antiplatelet and antihypertensive users with respect to non-users had more severe strokes on admission and a poorer prognosis that mostly depended on their worse profile in terms of risk factors, since data were not confirmed on the multivariate analysis. It follows that when evaluating the effects of prior treatment on stroke severity and outcome proper consideration should be given to the presence of atherothrombotic risk factors.
In agreement with data from the International Stroke Trial, which found no evidence of any association between previous aspirin treatment and baseline stroke severity ( 14 ) , we found that prior use of antiplatelets did not affect stroke severity on admission. The results of our study also showed that antiplatelets did not affect stroke outcome at discharge. In addition, the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial showed that disability after stroke was not different between patients treated with aspirin plus extended release dipyridamole versus clopidogrel ( 12 ) . However, the results of the PRoFESS trial are not comparable with ours since in that trial disability was evaluated after a recurrent stroke, whereas the majority of our patients (75.4%) were first-ever stroke patients. Moreover, we compared active treatments with no treatment, while the PRoFESS trial compared two different antiplatelet treatments.
Prior use of antihypertensives was not associated with reduced stroke severity on admission or with improved outcome at discharge. Conversely, a previous study showed that prior use of antihypertensives was associated with reduced severity of the incident ischemic stroke ( 19 ) ; however, those results might have been biased by the small number of patients included (n=170). Another study found that prior use of angiotensin-converting enzyme inhibitors was associated with a reduced risk of severe stroke ( 18 ) , while a further study did not find any difference in stroke severity between angiotensin II increasers such as thiazide diuretics, calcium channel blockers and angiotensin receptor blockers, and angiotensin II suppressors such as angiotensin-converting enzyme inhibitors and β-blockers ( 19 ) . A PRoFESS substudy showed that treatment with an angiotensin receptor blocker in patients with ischemic stroke was not associated with reduced disability in the presence of a recurrent event ( 12 ) . Those results, too, cannot be compared with ours, given that all the patients in the PRoFESS trial were in secondary prevention while the placebo group received the best available treatment, which included antihypertensives when required, and hence did not correspond to our untreated patients ( 12 ) .
There are several studies in which prior use of statins was shown to be associated with better functional outcome or reduced mortality after an ischemic stroke ( 2 – 11 ) , and in which their discontinuation at stroke onset was associated with a worse outcome as compared to that in patients who continued using them ( 35 ) . In more recent studies, pre-event use of statins has been found to be associated with reduced mortality and disability in patients with intracerebral hemorrhage ( 36 – 38 ) . Remarkably, we found that statin users, compared with non-users, had an improved functional outcome at discharge in the absence of any effect on stroke severity at onset. Our results are similar to those reported by the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial ( 5 ) which showed that atorvastatin was associated with a trend favoring a reduction in the severity of outcome of ischemic strokes, with no difference in neurological severity at onset as defined by the NIHSS score. However, the SPARCL trial was designed for secondary stroke prevention while our findings mostly refer to patients with a first-ever ischemic stroke and strengthen the evidence that statin use may improve stroke outcome. Moreover, while the results of the SPARCL trial refer only to atorvastatin, our findings apply to treatment with statins in general since the subjects in our study were under different statins taken at different doses. The benefits of statin treatment on stroke outcome may be attributed to their pleiotropic effects rather than to their cholesterol and LDL-cholesterol lowering effects ( 21 , 39 , 40 ) . We did not analyze data referring to post-event use of statins and consequently cannot draw any conclusion about the effect on outcome induced by their continuation. However, in the absence of any effect associated with statins on NIHSS scores and in the presence of a favorable effect on mRS scores, we may suppose that their post-event use might have contributed to the improved outcome.
To our knowledge, only one retrospective study has evaluated the possible benefits of prior treatment with the association of an antiplatelet agent, an angiotensin-converting enzyme inhibitor, and a statin after an acute ischemic stroke. It found that the 20 patients who were taking the three treatments had a shorter hospital stay and a better functional outcome compared with the untreated patients ( 21 ) . At variance with this, our results suggest that prior three-drug combination treatment was not independently associated with reduced stroke severity on admission, or with better outcome at discharge. Differences in endpoint definitions might explain the different results. Besides, in our opinion, the benefit obtained with the three-drug combination is probably attributable to statin use alone and for that same reason the three-drug combination was not superior to the use of statins alone.
Our study had some limitations. We did not collect data on continuation, dose, or compliance for each treatment used prior to stroke onset and the patients were not randomized. Moreover, as shown by the mean baseline NIHSS scores, our patients mostly suffered a mild to moderate stroke and consequently our results cannot be generalized to the whole population of patients with acute ischemic stroke. The low proportion of statin users (9.1%) could represent a further limitation.
In conclusion, we found that statins may be beneficial not only to prevent stroke but also to improve the outcome after the event in those patients who, despite treatment, develop a stroke. For this reason, to avoid any underestimation of the benefits of statin use, in future trials of secondary prevention it might be useful to include the functional outcome of the incident stroke among the pre-specified endpoints.
Conversely, antiplatelets, antihypertensives, and the three-drug combination treatment did not appear to contribute to the reduction of stroke severity or to the improvement of outcome, although the specific potential of the different antihypertensive medications needs further evaluation.
Acknowledgments
This study was supported by an unconditional grant from Sanofi-Aventis (Italy) and Bristol-Myers Squibb (Italy). The sponsors had no role in the study design, in the collection, analysis, and interpretation of the data, in the writing of the report, or in the decision to submit the article for publication.
The participating centers are listed in the appendix .
APPENDIX
Participating centers
Clinica neurologica, Università degli Studi di Chieti, Chieti: Marchionno L; Divisione di neurologia, Ospedale San Salvatore, L’Aquila: Mearelli S; Clinica neurologica, Università degli Studi di L’Aquila, L’Aquila: Marini C; Unità operativa di neurologia, Ospedale G. Mazzini, Teramo: Bernardini C, Sgriccia S; Unità operativa di neurologia, Ospedale San Carlo, Potenza: Paciello M; Unità operativa di neurologia, Ospedale Bianchi Melacrino Morelli, Reggio Calabria: Aguglia U; Unità operativa di neurologia, Vibo Valentia: Consoli D, Galati F; Unità operativa complessa di neurologia e stroke unit - Azienda ospedaliera G. Rummo, Benevento: Feleppa M, Apice G; Unità operativa di neurologia, Ospedale San Sebastiano, Caserta: Di Lauro A; Unità operativa di neurologia, Ospedale Loreto Nuovo, Napoli: De Falco FA, Ranieri F; Stroke unit, Ospedale C.T.O., Napoli: Ronga B; Unità operativa di neurologia, Ospedale San Giovanni di Dio, Salerno: De Divitiis M; Unità operativa di medicina fisica e riabilitativa, Policlinico di Bologna, Bologna: Gualandi G; Unità operativa di neurologia, Ospedale Ramazzini, Carpi: Baratti M, Vaghi L; Unità operativa di neurologia, Ospedale Bufalini-Marconi-Angioloni, Cesena: Rasi F; Unità operativa di neurologia, Ospedale Morgagni - Pierantoni, Forlì: Galletti G, Neri W; Clinica neurologica, Università degli Studi di Modena e Reggio Emilia: Cavazzuti M, Zini A; Unità operativa di neurologia, Azienda ospedaliero-universitaria di Parma, Parma: Scoditti U, Antonelli MY; Unità operativa di neurologia, Ospedale di Ravenna: Bianchedi G; Unità di trattamento neurovascolare, Università degli Studi La Sapienza, Policlinico Umberto I, Roma: Puca E, Durastanti L; Clinica Neurologica, Policlinico A. Gemelli, Roma: Tonali PA; Università degli Studi Tor Vergata, Roma: Diomedi M, Leone G; Clinica neurologica, Università degli Studi La Sapienza, Roma: Amabile GA, Pauri F; Dipartimento di neurologia, Università degli studi Campus Biomedico e Ospedale Fatebenefratelli Isola Tiberina, Roma: Vernieri F, Altamura C; Stroke unit, Ospedale Sant’Andrea, Roma: Rasura M, Patella R; Dipartimento di neuroscienze, Ospedale San Filippo Neri, Roma: Fiume Garelli F; Stroke unit, Dipartimento di scienze neurologiche, Università degli Studi La Sapienza, Roma: Di Piero V, Rocco A; Stroke Unit, Dipartimento di neuroscienze, oftalmologia e genetica, Università degli Studi di Genova: Gandolfo C, Reale N; Dipartimento di neurologia, Ospedale Padre Antero Micone, Genova: Tanganelli P; Dipartimento testa-collo, Unità operativa di neurologia, Imperia: Serrati C, Zat C; Unità operativa di neurologia, Ospedale Sant’Andrea, La Spezia: Tartaglione A, Vivarelli F; Stroke unit, Spedali Civili di Brescia, Brescia: Magoni M; Stroke unit, Azienda ospedaliera Sant’Anna, Como: Arnaboldi M, Vidale S; Unità operativa di neurologia, Ospedale Valduce, Como: Guidotti M; Unità operativa di neurologia, Ospedale Maggiore, Crema: Riccardi T, Brusaferri F; Istituti Ospitalieri, Cremona: Bettoni L; Unità operativa di neurologia, Ospedale civile, Desio: Colombo A, Bordo BM; Unità operativa di neurologia, Ospedale G. Salvini, Garbagnate (MI): Cittani D; Unità operativa di neurologia, Ospedale di Lecco, Lecco: Scaccabarozzi C, Agostoni E; Unità operativa di neurologia, Ospedale Civile, Legnano (MI): Calloni MV, Giorgetti A; Unità operativa, Ospedale Maggiore, Lodi: Faggi L; Servizio di neurologia, Ospedale di Magenta: Romorini A, Alberti G; Stroke unit, Ospedale di Merate: De Capitani E; Stroke unit, IRCCS Istituto Auxologico Italiano, Milano: Stramba Badiale M; Unità operativa di neurologia, Ospedale Sacco, Milano: Gambaro P; Ospedale San Carlo Borromeo, Milano: Bassi P; Stroke unit, Ospedale Niguarda Ca’ Granda, Milano: Santilli IM, Gatti A; Dipartimento di neurologia e neuroriabilitazione, IRCCS San Raffaele, Milano: Roveri L, Ghidinelli C; Clinica neurologica, Ospedale San Gerardo dei Tintori, Monza: Ferrarese C; Stroke unit, IRCCS C. Mondino, Pavia: Marcheselli S, Tosi P; Unità operativa di neurologia, IRCCS Humanitas, Rozzano: Merlo P, Stival B; IRCCS Policlinico San Donato, Milano: Meola G, Bet L; Divisione di medicina generale, Ospedale Civile, Vigevano: Attardo-Parrinello G, Franceschetti B; Unità operativa di neurologia, Ospedale civile, Vimercate: Crespi V, Bazzi P; Unità operativa di neurologia, Ospedale di Melegnano, Melegnano: Marsile C, Molini GE; Unità operativa di neurologia, Ospedale civile, Voghera: Borutti G, Dallocchio C; Dipartimento di neurologia, Ospedale di Gallarate, Gallarate: Zarcone D; Unità operativa di neurologia, INRCA IRCCS, Ancona: Scarpino O; Clinica neurologica, Ospedali riuniti, Ancona: Silvestrini M, Provinciali L; Unità operativa di neurologia, Ospedale Mazzoni, Ascoli Piceno: Ragno M, Scarcella M; Unità operativa di neurologia, Ospedale di Fabriano, Fabriano: Curzi NM; Unità operativa di neurologia, Ospedale civile, Jesi: Giaccaglini E; Struttura complessa di neurologia, Ospedale civile, San Benedetto del Tronto: Curatola L, Gobbato R; Unità operativa di neurologia, Ospedale di Senigallia, Senigallia: Del Pesce M, Massi G; Unità operativa di neurologia, Ospedale San Lazzaro, Alba: Asteggiano G, Giraudo M; Unità operativa di neurologia, Ospedale Santi Antonio e Biagio e Cesare Arrigo, Alessandria: Barletta L; Unità operativa di neurologia, Ospedale civile, Asti: Troni W; Unità operativa di neurologia, Ospedale Santa Croce e Carle, Cuneo: Gerbino Promis P, Grasso MF; Dipartimento di neurologia, Università degli Studi di Novara, Novara: Botto E, Reggiani M; Unità operativa di neurologia, Ospedale San Giacomo, Novi Ligure: Cattaneo S; Unità operativa di neurologia, Ospedale San Luigi, Orbassano: Sciolla R, Melis F; Unità operativa di neurologia, Ospedale Santissima Annunziata, Savigliano: Seliak D; Unità operativa di neurologia, Ospedale Mauriziano Umberto I, Torino: Gionco M; Unità operativa di neurologia, Ospedale San Giovanni Bosco, Torino: Ravetti C; Unità operativa di neurofisiopatologia, Policlinico di Bari, Bari: Federico F; Unità operativa di neurologia, Ospedale Miulli, Casamassima: Lastilla M; Unità operativa di neurologia, Ospedali riuniti, Foggia: Terracciano ME, Mundi C; Unità operativa di neurologia, Ospedale G. Brotzu, Cagliari: Melis M; Unità operativa di neurologia, Ospedale A. Segni, Ozieri: Traccis S, Sini M; Clinica neurologica, Università degli Studi di Sassari: Pirisi A, Piras MP; Unità operativa di neurologia, Ospedale di Caltagirone: La Greca F; Unità operativa di neurologia, Ospedale Sant’Elia, Caltanissetta: Giglia L, Randisi MG; Unità operativa di neuropatologia, Ospedale G. Martino, Messina: Messina C; Clinica neurologica, Policlinico universitario di Messina: Musolino R, Calabrò RS; Clinica neurologica, Ospedale D’Anna, Palermo: Monaco P; Unità operativa di neurologia, Ospedale R. Guzzardi, Vittoria: Iemolo F, Giordano A; Clinica neurologica, Azienda ospedaliero-universitaria Careggi, Firenze: Inzitari D; Unità operativa di neurologia, Ospedale civile, Lucca: Giraldi C, Renna M; Clinica neurologica, Università degli Studi di Pisa: Orlandi G; Unità operativa di neurologia, Ospedale Misericordia, Grosseto: Brescia A; Unità operativa di neurologia, Ospedale Santa Maria del Carmine, Rovereto: Rossi G; Unità operativa di neurologia, Ospedale Santa Chiara, Trento: Orrico D; Centro ictus, Ospedale civile, Città della Pieve: Celani MG, Righetti E; Stroke unit, Ospedale di Perugia, Perugia: Agnelli G; Unità operativa di neurologia, Ospedale civile, Terni: Neri G, Costantini F; Dipartimento di neurologia e stroke unit, Ospedale regionale, Aosta: Bottacchi E, Giardini G; Unità operativa di neurologia, Ospedale civile, Dolo: Pellegrini A, De Zanche A; Unità operativa di neurologia, Ospedale civile, Mestre: Pistollato G, Bozzato G; Unità operativa di neurologia, Ospedale di Mirano: Fattorello Salimbeni C, Menegazzo E; Dipartimento di neurologia, Università degli Studi di Padova: Meneghetti G, Santarello G; Stroke unit, Ospedale civile, Portogruaro: D’Anna S; Unità operativa di neurologia, Ospedale civile, Rovigo: De Grandis D; Unità operativa di neurologia, Azienda Ospedaliera, Verona: Bovi P, Tomelleri GP.
References
- 1. Sacco RL , Adams R , Albers G , et al. American Heart Association; American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline . Stroke . 2006 ; 37 : 577 – 617 . doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 2. Alvarez-Sabín J , Huertas R , Quintana M , et al. Prior statin use may be associated with improved stroke outcome after tissue plasminogen activator . Stroke . 2007 ; 38 : 1076 – 1078 . doi: 10.1161/01.STR.0000258075.58283.8f. [DOI] [PubMed] [Google Scholar]
- 3. Arboix A , García-Eroles L , Oliveres M , Targa C , Balcells M , Massons J . Pretreatment with statins improves early outcome in patients with first-ever ischaemic stroke: a pleiotropic effect of statins or a beneficial effect of hypercholesterolemia? . BMC Neurol . 2010 ; 10 : 47 . doi: 10.1186/1471-2377-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deplanque D , Masse I , Lefebvre C , Libersa C , Leys D , Bordet R . Prior TIA, lipid-lowering drug use, and physical activity decrease ischemic stroke severity . Neurology . 2006 ; 67 : 1403 – 1410 . doi: 10.1212/01.wnl.0000240057.71766.71. [DOI] [PubMed] [Google Scholar]
- 5. Goldstein LB , Amarenco P , Zivin J , et al. Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators. Statin treatment and stroke outcome in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial . Stroke . 2009 ; 40 : 3526 – 3531 . doi: 10.1161/STROKEAHA.109.557330. [DOI] [PubMed] [Google Scholar]
- 6. Greisenegger S , Müllner M , Tentschert S , Lang W , Lalouschek W . Effect of pretreatment with statins on the severity of acute ischemic cerebrovascular events . J Neurol Sci . 2004 ; 221 : 5 – 10 . doi: 10.1016/j.jns.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 7. Elkind MS , Flint AC , Sciacca RR , Sacco RL . Lipid-lowering agent use at ischemic stroke onset is associated with decreased mortality . Neurology . 2005 ; 65 : 253 – 258 . doi: 10.1212/01.wnl.0000171746.63844.6a. [DOI] [PubMed] [Google Scholar]
- 8. Jonsson N , Asplund K . Does pretreatment with statins improve clinical outcome after stroke? A pilot case-referent study . Stroke . 2001 ; 32 : 1112 – 1115 . doi: 10.1161/01.str.32.5.1112. [DOI] [PubMed] [Google Scholar]
- 9. Martí-Fàbregas J , Gomis M , Arboix A , et al. Favorable outcome of ischemic stroke in patients pretreated with statins . Stroke . 2004 ; 35 : 1117 – 1121 . doi: 10.1161/01.STR.0000125863.93921.3f. [DOI] [PubMed] [Google Scholar]
- 10. Moonis M , Kane K , Schwiderski U , Sandage BW , Fisher M . HMG-CoA reductase inhibitors improve acute ischemic stroke outcome . Stroke . 2005 ; 36 : 1298 – 1300 . doi: 10.1161/01.STR.0000165920.67784.58. [DOI] [PubMed] [Google Scholar]
- 11. Reeves MJ , Gargano JW , Luo Z , Mullard AJ , Jacobs BS , Majid A , Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators Effect of pretreatment with statins on ischemic stroke outcomes . Stroke . 2008 ; 39 : 1779 – 1785 . doi: 10.1161/STROKEAHA.107.501700. [DOI] [PubMed] [Google Scholar]
- 12. Diener H-C , Sacco RL , Yusuf S , et al. Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) study group Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study . Lancet Neurol . 2008 ; 7 : 875 – 884 . doi: 10.1016/S1474-4422(08)70198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Keyser J , Herroelen L , De Klippel N . Early outcome in acute ischemic stroke is not influenced by the prophylactic use of low-dose aspirin . J Neurol Sci . 1997 ; 145 : 93 – 96 . doi: 10.1016/s0022-510x(96)00250-x. [DOI] [PubMed] [Google Scholar]
- 14. Ricci S , Lewis S , Sandercock P , IST Collaborative Group Previous use of aspirin and baseline stroke severity: an analysis of 17,850 patients in the International Stroke Trial . Stroke . 2006 ; 37 : 1737 – 1740 . doi: 10.1161/01.STR.0000226740.29910.91. [DOI] [PubMed] [Google Scholar]
- 15. Ovbiagele B , Buck BH , Liebeskind DS , et al. Prior antiplatelet use and infarct volume in ischemic stroke . J Neurol Sci . 2008 ; 264 : 140 – 144 . doi: 10.1016/j.jns.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 16. Wilterdink JL , Bendixen B , Adams HP , Jr , Woolson RF , Clarke WR , Hansen MD . Effect of prior aspirin use on stroke severity in the trial of Org 10172 in acute stroke treatment (TOAST) . Stroke . 2001 ; 32 : 2836 – 2840 . doi: 10.1161/hs1201.099384. [DOI] [PubMed] [Google Scholar]
- 17. Bosch J , Yusuf S , Pogue J , et al. HOPE Investigators Heart outcomes prevention evaluation. Use of ramipril in preventing stroke: double blind randomised trial . BMJ . 2002 ; 324 : 699 – 702 . doi: 10.1136/bmj.324.7339.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chitravas N , Dewey HM , Nicol MB , Harding DL , Pearce DC , Thrift AG . Is prestroke use of angiotensin-converting enzyme inhibitors associated with better outcome? . Neurology . 2007 ; 68 : 1687 – 1693 . doi: 10.1212/01.wnl.0000261914.18101.60. [DOI] [PubMed] [Google Scholar]
- 19. Ovbiagele B , Kidwell CS , Starkman S , et al. Angiotensin 2 type 2 receptor activity and ischemic stroke severity . Neurology . 2005 ; 65 : 851 – 854 . doi: 10.1212/01.wnl.0000175984.29283.6d. [DOI] [PubMed] [Google Scholar]
- 20. Selim M , Savitz S , Linfante I , Caplan L , Schlaug G . Effect of pre-stroke use of ACE inhibitors on ischemic stroke severity . BMC Neurol . 2005 ; 5 : 10 . doi: 10.1186/1471-2377-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar S , Savitz S , Schlaug G , Caplan L , Selim M . Antiplatelets, ACE inhibitors, and statins combination reduces stroke severity and tissue at risk . Neurology . 2006 ; 66 : 1153 – 1158 . doi: 10.1212/01.wnl.0000208406.45440.84. [DOI] [PubMed] [Google Scholar]
- 22. Toso V , Carolei A , Gensini GF , et al. SIRIO Study Investigators The Stroke in Italy and Related Impact on Outcome (SIRIO) study: design and baseline data . Neurol Sci . 2006 ; 27 ( Suppl 3 ): S263 – 267 . doi: 10.1007/s10072-006-0633-8. [DOI] [PubMed] [Google Scholar]
- 23. Sacco S , Toni D , Bignamini AA , et al. Acute stroke admission and diagnostic procedures according to hour and day of onset: the SIRIO collaborative data . Eur Neurol . 2009 ; 61 : 100 – 106 . doi: 10.1159/000177942. [DOI] [PubMed] [Google Scholar]
- 24. Brott T , Adams HP , Jr , Olinger CP , et al. Measurements of acute cerebral infarction: a clinical examination scale . Stroke . 1989 ; 20 : 864 – 870 . doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 25. Chobanian AV , Bakris GL , Black HR , et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure . Hypertension . 2003 ; 42 : 1206 – 1252 . doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 26. World Health Organization Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia [ http://www.who.int/diabetes/publications/en/]
- 27. National Institutes of Health . Adult Treatment Panel III. Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Bethesda, MD : National Institutes of Health ; 2002 . [Google Scholar]
- 28. Ruitenberg A , van Swieten JC , Witteman JC , et al. Alcohol consumption and risk of dementia: the Rotterdam Study . Lancet . 2002 ; 359 : 281 – 286 . doi: 10.1016/S0140-6736(02)07493-7. [DOI] [PubMed] [Google Scholar]
- 29. World Health Organization . Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation on Obesity . Geneva : WHO ; 2000 . [PubMed] [Google Scholar]
- 30. World Health Organization WHO Collaborating Centre for Drugs Statistics Methodology [ http://www.whocc.no/atcddd/]
- 31. van Swieten JC , Koudstaal PJ , Visser MC , Schouten HJ , van Gijn J . Interobserver agreement for the assessment of handicap in stroke patients . Stroke . 1988 ; 19 : 604 – 607 . doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 32. Wahlgren N , Ahmed N , Dávalos A , et al. SITS-MOST investigators Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study . Lancet . 2007 ; 369 : 275 – 282 . doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 33. Hacke W , Kaste M , Fieschi C , et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators . Lancet . 1998 ; 352 : 1245 – 1251 . doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 34. Sacco S , Stracci F , Cerone D , Ricci S , Carolei A . Epidemiology of stroke in Italy . Int J Stroke . 2011 ; 6 : 219 – 227 . doi: 10.1111/j.1747-4949.2011.00594.x. [DOI] [PubMed] [Google Scholar]
- 35. Endres M , Laufs U . Discontinuation of statin treatment in stroke patients . Stroke . 2006 ; 37 : 2640 – 2643 . doi: 10.1161/01.STR.0000240690.69406.28. [DOI] [PubMed] [Google Scholar]
- 36. FitzMaurice E , Wendell L , Snider R , et al. Effect of statins on intracerebral hemorrhage outcome and recurrence . Stroke . 2008 ; 39 : 2151 – 2154 . doi: 10.1161/STROKEAHA.107.508861. [DOI] [PubMed] [Google Scholar]
- 37. Leker RR , Khoury ST , Rafaeli G , Shwartz R , Eichel R , Tanne D , NASIS Investigators Prior use of statins improves outcome in patients with intracerebral hemorrhage: prospective data from the National Acute Stroke Israeli Surveys (NASIS) . Stroke . 2009 ; 40 : 2581 – 2584 . doi: 10.1161/STROKEAHA.108.546259. [DOI] [PubMed] [Google Scholar]
- 38. Naval NS , Abdelhak TA , Zeballos P , Urrunaga N , Mirski MA , Carhuapoma JR . Prior statin use reduces mortality in intracerebral hemorrhage . Neurocrit Care . 2008 ; 8 : 6 – 12 . doi: 10.1007/s12028-007-0080-2. [DOI] [PubMed] [Google Scholar]
- 39. Calabrò P , Yeh ET . The pleiotropic effects of statins . Curr Opin Cardiol . 2005 ; 20 : 541 – 546 . doi: 10.1097/01.hco.0000181482.99067.bf. [DOI] [PubMed] [Google Scholar]
- 40. Sacco S , Cerone D , Carolei A . Gender and stroke: acute phase treatment and prevention . Funct Neurol . 2009 ; 24 : 45 – 52 . [PubMed] [Google Scholar]