Abstract
Cerebrospinal fluid (CSF) flow dynamics, which supposedly have a strong relationship with chronic cerebrospinal venous insufficiency (CCSVI), might be expected to be affected in multiple sclerosis (MS) patients. In this study, CSF flow at the level of the cerebral aqueduct was evaluated quantitatively by phase contrast magnetic resonance imaging (PC-MRI) to determine whether CSF flow dynamics are affected in MS patients. We studied 40 MS patients and 40 healthy controls using PC-MRI. We found significantly higher caudocranial (p=0.010) and craniocaudal CSF flow volumes (p=0.015) and stroke volume (p=0.010) in the MS patients compared with the controls. These findings may support the venous occlusion theory, but may also be explained by atrophy-dependent ventricular dilatation independent of the venous theory in MS patients.
Keywords: cerebrospinal fluid , chronic cerebrospinal venous insufficiency , magnetic resonance imaging , multiple sclerosis
Introduction
Cerebrospinal fluid (CSF) is mainly produced by the choroid plexus (80–90%), but also most likely by the parenchyma of the brain and spinal cord (10–20%). CSF flows through the ventricular system and then into the subarachnoid space. According to the most widely accepted theory, CSF absorption occurs mainly through the arachnoid villi into the venous system. However, a new theory ( 1 ) suggests that absorption of CSF also occurs through capillaries within the brain parenchyma. Recent studies investigating venous flow abnormalities in patients with multiple sclerosis (MS) reveal a strong relationship between MS and the venous system ( 2 – 5 ) . Theoretically, CSF circulation should be affected by venous obstruction. Venous obstructions causing blood flow insufficiency have been shown to cause hydrocephaly ( 6 , 7 ) . A study performed by Zamboni et al. ( 8 ) revealed that CSF flow is affected in MS patients with chronic cerebrospinal venous insufficiency (CCSVI). In another study, no alteration was observed in aqueductal CSF flow in MS patients ( 9 ) .
In the present study, CSF flow at the level of the cerebral aqueduct was evaluated quantitatively by phase contrast magnetic resonance imaging (PC-MRI) to determine whether CSF flow dynamics are affected in MS patients.
Materials and methods
MS patients and control group
Between March and August 2010, 40 patients diagnosed with MS according to the Poser ( 10 ) and McDonald ( 11 ) criteria [32 with relapsing-remitting MS (RRMS) and 8 with secondary progressive MS (SPMS)] were recruited consecutively from the Neurology Department at the Haseki Research and Education Hospital in Istanbul and enrolled in this study. All the patients were on disease-modifying therapy. The inclusion criteria were as follows: a RR or SP disease course; an expanded disability status scale (EDSS) ( 12 ) score between 1 and 8.5; age between 18 and 58 years; disease duration between 4 and 240 months; no history of any other neurological disease; and current remission status of the disease. Exclusion criteria were: acute relapse or steroid treatment within the 30 days preceding study entry and pre-existing medical conditions associated with brain pathologies, such as neurodegenerative disorders, alcohol abuse, and other conditions. The control group consisted of 40 neurologically normal individuals who were sex-matched and age-matched (±2 years) with the MS patients.
Informed consent was obtained from the patients after ethics committee approval had been obtained and detailed information about the study had been provided.
PC-MRI technique
MR images were acquired using a 1.5-T unit (Philips Achieva, Best, The Netherlands) with an 8-channel head coil. All cases were first evaluated using routine axial T1- and T2-weighted turbo spin-echo (TSE) cranial sequences. For quantitative investigation of CSF flow, PC-MRI was performed in the axial plane, which was perpendicular to the cerebral aqueduct ( Fig. 1 ). For axial plane images ( Fig. 2 ), the following parameters were used: TR=24 ms; TE=14 ms; cross-section thickness = 4 mm; number of signal averages (NSA) = 2; flip angle = 15°; field of view (FOV) = 100 mm × 100 mm; matrix = 260×182; and velocity encoding (V enc ) = 20 cm/s. These parameters generated 12 time points per cardiac cycle. Caudocranial direction denoted positive and craniocaudal direction negative flow. Cardiac triggering was performed retrospectively using finger plethysmography.
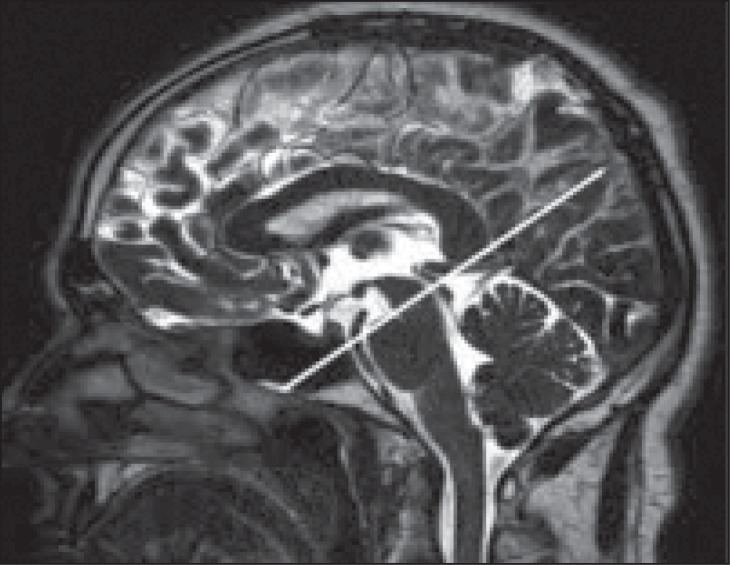
Figure 1 .
PC-MR image in the axial plane positioned perpendicular to the cerebral aqueduct, which is necessary for measurement of CSF flow.
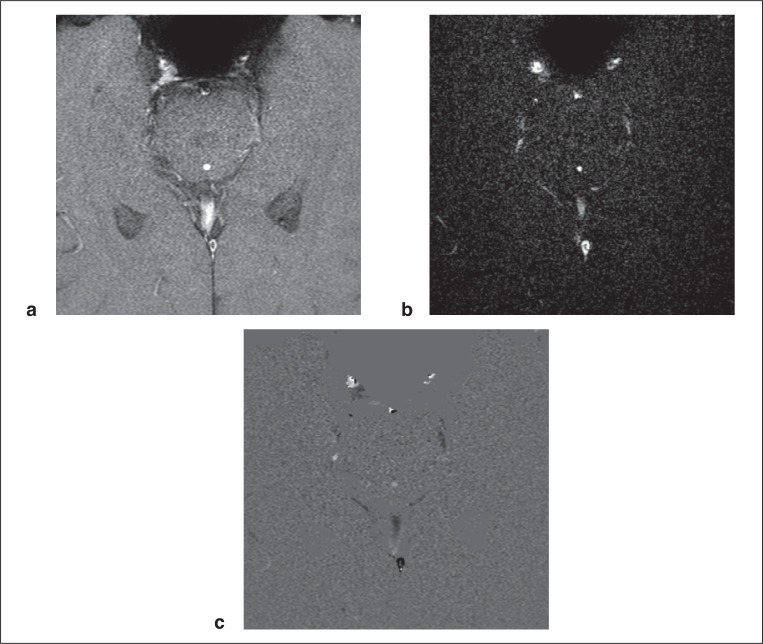
Figure 2 .
Rephase (a), magnitude (b), and phase (c) images perpendicular to the ampulla region of the cerebral aqueduct.
Imaging analysis
All MRI images were evaluated by two radiologists blinded to the study subjects’ clinical conditions (Y.G. and S.A., with 5 and 10 years’ experience, respectively). The findings of these two radiologists were not significantly different.
Quantitative analysis of CSF flow was performed using “2D Q-flow” phase contrast MR angiography software on the Philips MR workstation (Philips, Best, The Netherlands) with axial plane images obtained at the level of the cerebral aqueduct. First, phase-contrast images were transferred to the 2D Q-Flow software. After sufficient magnification of images (always using a magnification ratio of 400%), a region of interest (ROI) was placed manually to completely cover the cerebral aqueduct in every image ( Fig. 3 ). Thereafter, graphs of flow-time, peak velocity-time, mean velocity-time and maximum velocity-time were obtained for one cardiac beat with 2D Q-Flow. The maximum velocity-time graph is shown in figure 4 .
Figure 3 .
ROI placement on the cerebral aqueduct, after sufficient magnification of phase-contrast images with the axial plane perpendicular to cerebral aqueduct.
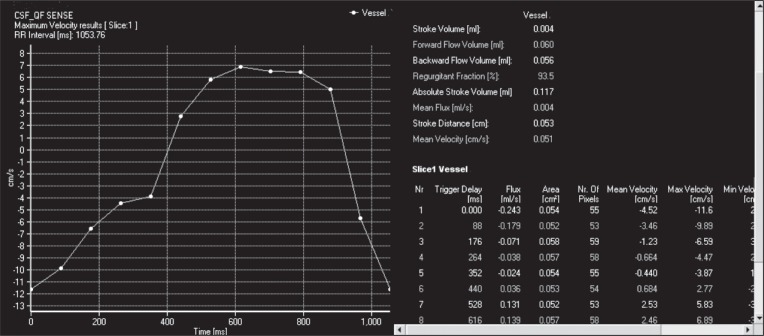
Figure 4 .
Maximum velocity-time graph following ROI placement on the cerebral aqueduct in phase-contrast images obtained [positive values (diastolic) show CSF flow velocity in the caudocranial direction and negative values (systolic) CSF flow velocity in the craniocaudal direction] and CSF flow data [stroke volume (ml), forward flow volume (ml), backward flow volume (ml), regurgitant fraction (%), absolute stroke volume (ml), mean flux (ml/s), stroke distance (cm) and mean velocity (cm/s)] calculated for one cardiac beat by 2D-Q Flow software.
With the exception of peak velocity-time, in all the graphs, positive values (diastolic) indicate caudocranial CSF flow and negative values (systolic) craniocaudal CSF flow. RR intervals are also indicated. Furthermore, on the right-hand side of the graphs, CSF flow data (calculated by the software for one cardiac beat) are given. These data are:
– “stroke volume (ml/min)”: elsewhere ( 13 ) , this parameter is calculated as the mean of the absolute value of the caudocranial and craniocaudal CSF flow volumes, but in our study, it was defined as net CSF flow volume (i.e. the difference between “forward flow volume” and “backward flow volume”);
– “forward flow volume (ml/min)”: caudocranial CSF flow volume during diastole;
– “backward flow volume (ml/min)”: craniocaudal CSF flow volume during systole;
– “regurgitant fraction (%)”: the ratio of the smaller to the larger value, between the “backward flow volume” and “forward flow volume”, however, in the present study this parameter was defined as the ratio of the caudocranial CSF flow volume to the craniocaudal CSF flow volume;
– absolute stroke volume (ml/min)”: the sum of “backward flow volume” and “forward flow volume”, meaning total CSF volume passing through the cerebral aqueduct;
– “mean flux (ml/s)”: mean CSF flow volume per second;
– “mean velocity (cm/s)”: mean CSF flow velocity [positive values (diastolic) show caudocranial CSF flow, negative values (systolic) craniocaudal CSF flow] ( Fig. 4 ).
As indicated above, in this study, stroke volume is defined as net CSF flow volume, i.e. the difference between the caudocranial and craniocaudal flow volumes. If the net CSF flow was directed towards the third ventricle, the net CSF flow volume values were evaluated as positive. Conversely, if the net CSF flow was directed towards the fourth ventricle, the net CSF flow volume values were deemed to be negative.
The MS group and control group data were compared. The data of the RRMS group were compared with those recorded in the control-rr subgroup; in addition, the data of the SPMS group were compared with those recorded in the control-sp subgroup, even though the number of patients included in this subgroup analysis was not sufficient to draw any conclusions.
For this purpose, 32 of the 40 control group subjects were selected as control-rr and the remaining 8 subjects as control-sp; this was done taking great care to ensure sample size, age and sex matching of the MS and relative control groups.
In addition, the RRMS patients were compared with the SPMS patients in relation to the following parameters: age, heart rate, aqueductal area, mean velocity, peak systolic and diastolic velocity, regurgitant fraction, caudocranial and craniocaudal flow volumes, stroke volume, net CSF flow volume and net CSF flow direction. The comparison of the two MS subgroups also included the parameters disease duration and EDSS scores. We also analysed convenient parameters in ml/min in order to study the effect, on the results, of cardiac beat differences between the subjects.
Statistical analyses
The Kolmogorov-Smirnov test was used to test the equality of the distribution of variables, unless stated otherwise. Groups were also compared for net CSF flow direction and sex using Pearson’s chi-squared or Fisher’s exact test statistic. Other differences between groups were assessed using Student’s t-test or the Mann-Whitney U-test, as appropriate. A p-value <0.05 was considered to indicate statistical significance in all analyses.
Results
The various between-group comparisons revealed no statistically significant differences in distribution of sex or net CSF flow direction (i.e. distribution of subjects between caudocranial and craniocaudal direction) ( Table I ).
Table I .
Between-group comparisons for distribution of sex and net CSF flow direction.
| Sex | Net CSF flow dırectıon | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Male | Female | p-value | Caudocranıal | Cranıocaudal | p-value | ||
| GROUP (n:80) | MS (n:40) | 16 | 24 | 1.000 | 18 | 22 | 0.251 |
| CONTROL (n:40) | 16 | 24 | 13 | 27 | |||
| MS TYPE (n:40) | RRMS (n:32) | 11 | 21 | 0.229 | 12 | 20 | 0.110 |
| SPMS (n:8) | 5 | 3 | 6 | 2 | |||
| RRMS & CONTROL-rr (n:64) | RRMS (n:32) | 11 | 21 | 1.000 | 12 | 20 | 0.424 |
| CONTROL-rr (n:32) | 11 | 21 | 9 | 23 | |||
| SPMS & CONTROL-sp (n:16) | SPMS (n:8) | 5 | 3 | 1.000 | 6 | 2 | 0.608 |
| CONTROL-sp (n:8) | 5 | 3 | 4 | 4 | |||
Abbreviations: MS=multiple sclerosis; RRMS=relapsing-remitting multiple sclerosis; SPMS=secondary progressive multiple sclerosis; CSF=cerebrospinal fluid.
Comparison of parameters in MS patients versus control group
Mean velocity was significantly different between the MS patients and the control group (p=0.046). Mean velocity was 0.017 cm/s in the MS patients (caudocranial direction) versus −0.055 cm/s in the controls (craniocaudal direction). Caudocranial flow volume (ml/min) (p=0.010), craniocaudal flow volume (ml/min) (p=0.015) and stroke volume (ml/min) (p=0.010) were found to be significantly higher in the MS patients than in the control group. The other parameters compared showed no statistically significant difference between these two groups ( Table II ).
Table II .
Between-group comparisons of the parameters age, heart rate, aqueductal area, mean velocity, peak systolic and diastolic velocity, regurgitant fraction, caudocranial and craniocaudal flow volumes, stroke volume, net CSF flow volume.
| Parameters | MS & CONTROL | RRMS & CONTROL-rr | SPMS & CONTROL-sp | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| MS (n:40) | CONTROL (n:40) | RRMS (n:32) | CONTROL- rr (n:32) | SPMS (n:8) | CONTROL- sp (n:8) | |||||
|
| ||||||||||
| Median | Median | p-value | Median | Median | p-value | Median | Median | p-value | ||
| Mean | Mean | Mean | Mean | Mean | Mean | |||||
| ±SD | ±SD | ±SD | ±SD | ±SD | ±SD | |||||
| Age | years | 36.5 | 37.0 | 0.919 | 35.0 | 36.0 | 0.923 | 44.0 | 45.5 | 1.000 |
| 37.6 | 37.8 | 36.2 | 36.4 | 43.1 | 43.1 | |||||
| ±10.2 | ±10.2 | ±10.3 | ±10.3 | ±7.9 | ±8.0 | |||||
| Heart rate | beats/min | 70.0 | 68.5 | 0.409 | 69.5 | 70.5 | 0.499 | 75.5 | 64.5 | 0.563 |
| 73.2 | 70.4 | 73.6 | 70.9 | 71.6 | 68.3 | |||||
| ±15.8 | ±14.3 | ±16.6 | ±14.6 | ±12.8 | ±13.9 | |||||
| Aqueductal area | mm 2 | 4.5 | 5.2 | 0.507 | 4.5 | 5.2 | 0.710 | 4.4 | 5.7 | 0.494 |
| 4.8 | 5.1 | 4.8 | 4.9 | 5.1 | 5.7 | |||||
| ±1.6 | ±1.5 | ±1.7 | ±1.4 | ±1.5 | ±1.9 | |||||
| Mean velocity | cm/s | −0.002 | −0.076 | 0.046 | −0.004 | −0.096 | 0.099 | 0.123 | 0.004 | 0.294 |
| 0.017 | −0.055 | −0.003 | −0.070 | 0.095 | 0.007 | |||||
| ±0.183 | ±0.126 | ±0.183 | ±0.133 | ±0.169 | ±0.068 | |||||
| Peak systolic velocity | cm/s | −9.9 | −8.8 | 0.413 | −10.3 | −9.0 | 0.398 | −8.2 | −7.8 | 0.834 |
| −10.2 | −9.3 | −10.6 | −9.6 | −8.4 | −8.1 | |||||
| ±4.0 | ±2.7 | ±4.1 | ±2.8 | ±2.8 | ±1.8 | |||||
| Peak diastolic velocity | cm/s | 8.2 | 7.7 | 0.086 | 8.9 | 7.7 | 0.065 | 7.6 | 7.9 | 0.674 |
| 9.1 | 7.9 | 9.4 | 8.0 | 7.7 | 7.7 | |||||
| ±3.6 | ±2.4 | ±3.7 | ±2.5 | ±2.5 | ±2.5 | |||||
| Regurgitant fraction | %/beat | 98.1 | 93.5 | 0.388 | 97.8 | 90.6 | 0.655 | 111.0 | 100.1 | 0.058 |
| 98.3 | 95.0 | 96.1 | 94.1 | 106.9 | 98.7 | |||||
| ±17.5 | ±15.7 | ±18.3 | ±17.1 | ±10.9 | ±7.8 | |||||
| Caudocranial flow volume | μl/beat | 43.00 | 36.00 | 0.061 | 44.00 | 34.50 | 0.034 | 39.00 | 41.00 | 0.753 |
| 48.45 | 36.30 | 50.25 | 34.38 | 41.25 | 44.00 | |||||
| ±28.85 | ±17.38 | ±31.02 | ±15.12 | ±17.39 | ±24.20 | |||||
| ml/min | 3.11 | 2.47 | 0.010 | 3.32 | 2.38 | 0.014 | 2.90 | 2.74 | 0.753 | |
| 3.33 | 2.43 | 3.40 | 2.34 | 3.04 | 2.76 | |||||
| ±1.84 | ±1.01 | ±1.91 | ±0.93 | ±1.60 | ±1.29 | |||||
| Craniocaudal flow volume | μl/beat | −45.50 | −39.00 | 0.137 | −47.50 | −36.00 | 0.062 | −33.50 | −40.50 | 0.674 |
| −48.68 | −38.58 | −50.84 | −37.09 | −40.00 | −44.50 | |||||
| ±27.81 | ±17.59 | ±29.24 | ±15.53 | ±20.41 | ±24.59 | |||||
| ml/min | −3.24 | −2.68 | 0.015 | −3.38 | −2.62 | 0.014 | −2.75 | −2.71 | 0.834 | |
| −3.34 | −2.57 | −3.44 | −2.51 | −2.94 | −2.79 | |||||
| ±1.74 | ±1.01 | ±1.74 | ±0.93 | ±1.80 | ±1.33 | |||||
| Stroke volume | μl/beat | 45.00 | 36.25 | 0.103 | 47.00 | 35.00 | 0.046 | 36.25 | 41.00 | 0.713 |
| 48.56 | 37.38 | 50.55 | 35.64 | 40.63 | 44.31 | |||||
| ±28.17 | ±17.26 | ±29.98 | ±15.02 | ±18.71 | ±24.33 | |||||
| ml/min | 3.11 | 2.64 | 0.010 | 3.27 | 2.63 | 0.013 | 2.90 | 2.73 | 0.753 | |
| 3.33 | 2.49 | 3.42 | 2.42 | 2.99 | 2.78 | |||||
| ±1.78 | ±0.99 | ±1.81 | ±0.91 | ±1.69 | ±1.30 | |||||
| Net CSF flow volume | μl/beat | −1.00 | −2.00 | 0.152 | −1.00 | −3.00 | 0.232 | 3.00 | 0.00 | 0.080 |
| −0.20 | −2.18 | −0.63 | −2.59 | 1.50 | −0.50 | |||||
| ±6.76 | ±5.39 | ±7.20 | ±5.79 | ±4.57 | ±3.07 | |||||
| ml/min | −0.06 | −0.16 | 0.179 | −0.09 | −0.19 | 0.315 | 0.22 | 0.02 | 0.093 | |
| −0.01 | −0.13 | −0.04 | −0.16 | 0.11 | −0.03 | |||||
| ±0.48 | ±0.39 | ±0.50 | ±0.42 | ±0.34 | ±0.19 | |||||
Abbreviations: MS=multiple sclerosis; RRMS=relapsing-remitting multiple sclerosis; SPMS=secondary progressive multiple sclerosis; CSF=cerebrospinal fluid.
Comparison of parameters in RRMS patients versus control-rr subgroup
Caudocranial flow volume (μl/beat) (p=0.034), (ml\min) (p=0.014), craniocaudal flow volume (ml\min) (p=0.014) and stroke volume (μl/beat) (p=0.046), (ml\min) (p=0.013) were significantly higher in the RRMS patients compared with the control-rr subgroup. There was no statistically significant difference between the two groups in the other parameters compared ( Table II ).
Comparison of parameters in SPMS patients versus control-sp subgroup
No statistically significant difference in any parameter was found between these two groups ( Table II ).
Comparison of parameters in RRMS patients versus SPMS patients
The mean disease duration was 62.31 (±56.46) months in the RRMS patients and 133.50 (±51.49) months in the SPMS patients, thus this parameter was significantly higher in the SPMS patients (p=0.002). The mean EDSS score was 1.91 (±1.00) in the RRMS patients and 5.94 (±1.78) in the SPMS patients and therefore significantly higher in the SPMS patients (p<0.001). No statistically significant difference between the RRMS and SPMS groups was found in any of the other parameters compared (data not shown in Table II ).
Discussion
Reliable flow quantification is reported to be feasible if the diameter of the aqueduct lumen is greater than 1.5 mm 2 ( 14 ) . In our study, the lowest ROI area was 1.7 mm 2 . The mean ROI area was 4.8 mm 2 in the MS patients and 5.1 mm 2 in the control group, and was not found to be significantly different between these two groups.
Cardiac gating can be achieved either prospectively or retrospectively. In this study we used retrospective gating, in which the ECG is recorded as the scanner continuously obtains imaging data. At the end of the scan, software fits the imaging data into the most appropriate portion of the ECG to create the image. In prospective triggering, the scanner uses the QRS complex of the ECG as a signal to begin imaging; there is a necessary “dead zone” at the end of each cardiac cycle for the scanner to await the next QRS complex. The advantage of retrospective gating is that it covers the entire cardiac cycle ( 15 ) .
Nowadays 3.0 T MR imaging of the brain is becoming more widely accepted. 3.0 T MR machines have been used in two other studies ( 8 , 9 ) , whereas we used the 1.5 T MR machine in our study. Almost all the CSF flow parameters (except for CSF production) have proved statistically significantly higher when obtained with the 3.0 T than with the 1.5 T MR equipment ( 16 ) .
Time points per single cardiac cycle can differ due to heart rate. Both 14–16 time points ( 17 ) and 32 time points ( 8 , 9 ) have been used in various studies investigating CSF flow dynamics. There is no publication in the English-language literature that explores alterations in CSF flow parameters due to different time point usage. Additionally, our findings are largely consistent (taking into account differences due to demographic and technical factors) with findings presented in other studies in the literature.
In a study of 16 RRMS patients with CCSVI, Zamboni et al. ( 8 ) found net CSF flow at the cerebral aqueduct to be reduced. In addition, they found a significant relationship between decline in net CSF flow and CCSVI severity. They concluded that CCSVI in MS patients affects CSF dynamics. However, Sunderström et al. ( 9 ) performed a study in 20 RRMS patients and, unlike Zamboni et al. ( 8 ) , did not detect any significant narrowing in neck veins with PC-MRI or contrast-enhanced MR angiography. In the present study, CSF flow parameters, such as net CSF flow volume and stroke volume, evaluated with PC-MRI, were not found to be statistically significantly different between MS patients and the control group.
Craniocaudal CSF flow volume per cardiac beat (μl/beat) was higher in the RRMS patients compared with the control-rr subgroup, but this difference was not statically significant (p=0.062). This finding is consistent with the study of Zamboni et al. ( 8 ) . However, in contrast to our study, Zamboni et al. ( 8 ) found no difference in caudocranial CSF flow volume (μl/beat). Furthermore, even though the net CSF flow volume (μl/beat) was found to be slightly lower in our RRMS patients, this difference was not statistically significant; this finding contrasts with the report of Zamboni et al. ( 8 ) . Sundström et al. ( 9 ) found no difference in the net CFS flow volume (ml/min) in RRMS patients compared with controls, which is consistent with our result. Unlike us, Sundström et al. ( 9 ) found no difference in the stroke volume (μl/beat) in RRMS patients versus controls. Additionally, with regard to the parameters not calculated in the abovementioned studies, we failed to detect differences in some of these parameters, namely mean velocity, peak systolic and peak diastolic velocities and regurgitant fraction, whereas differences did emerge in others, namely the craniocaudal and caudocranial CSF flow volumes (ml/min), and stroke volume (ml/min). Craniocaudal and caudocranial CSF flow volume (ml/min), and stroke volume (ml/min) were found to be higher in the RRMS patients than the control-rr subgroup.
No PC-MRI study investigating CSF dynamics in SPMS patients and controls could be found in the literature. Granted that the small number of SPMS patients in our study precluded any meaningful conclusions, our analysis nevertheless failed to reveal any statistically significant difference in any of the parameters compared. However, it is possible that a PC-MRI study of CSF flow in a larger number of SPMS patients could show a statistically significantly increased CSF regurgitant fraction in the caudocranial direction. In our study, it was found to be higher in SPMS patients (106.7%) than in the control-spms subgroup (98.7%), but this difference was not statistically significant (p=0.058). This finding may be explained by venous hemodynamic impairment in MS patients, which was mentioned in Zamboni’s report ( 8 ) . We think that this hemodynamic impairment causes a mild deficit in CSF reabsorption through the sinuses and, therefore, changes in these parameters. Given the caudocranial direction of net CSF flow volume in SPMS patients, we suggest that CSF reabsorption occurs more in transependymal sites than in the venous sinuses.
When comparing RRMS patients with the control-rr subgroup, statistically significant hyperdynamic properties (increased caudocranial and craniocaudal flow volumes and stroke volume) were observed; these differences did not emerge when comparing the SPMS patients with the control-sp subgroup. This can be explained as follows: the mean age of the healthy control-sp subgroup was higher than that of the control-rr subgroup, albeit not statistically significantly (p=0.095). The literature reports that intracranial CSF volume increases after a person’s twenties ( 18 ) , and that the ratio of cerebral ventricular volume to total brain volume increases with aging ( 19 ) ; a strong correlation between aqueductal CSF flow and ventricular morphology, especially between total ventricular volume and third ventricle width, has also been reported ( 20 ) . Furthermore, various studies have reported an increase in CSF flow dynamics due to aging, albeit not statistically significant ( 21 – 23 ) . Consistent with the literature, we found CSF flow volume (caudocranial and craniocaudal flow volumes and stroke volume) to be higher (albeit not statistically significantly) in the control-sp subgroup, which has an older mean age compared with the control-rr subgroup. This explains the absence of a statistically significant difference between SPMS patients and the control-sp subgroup.
Although craniocaudal (ml/min) and caudocranial CSF flow volume (ml/min), and stroke volume (ml/min) were higher in MS patients, the fact that peak systolic and diastolic velocities were not significantly different between MS patients and controls may be seen as controversial. But peak systolic or peak diastolic velocities (cm/s) are not the only parameters used in calculation of CSF flow volume (ml/min). Heart rate and aqueduct area are also used in the formula to calculate CSF flow volume. The aqueduct area was smaller in the MS patients (p=0.507), whereas peak systolic and peak diastolic velocities, and heart rate were higher in this group, however, these differences were not statistically significant (p=0.411, p=0.086 and p=0.409 respectively). These parameters in MS patients that were increased, but not statistically significantly, are associated with craniocaudal (ml/min) and caudocranial CSF flow volume (ml/min), and stroke volume (ml/min), which, instead, were found to be statistically significantly higher in MS patients than the control group.
We think that the increased craniocaudal and caudocranial CSF flow volume (ml/min) and stroke volume (ml/min) in MS patients can be explained in two ways. First, by the venous hemodynamic impairment in MS patients mentioned in Zamboni’s report ( 8 ) . Although we did not investigate this phenomenon in our study, we suggest that it causes a mild deficit in CSF reabsorption through the sinuses and an increase in the above parameters. Another finding supports this hypothesis: the CSF regurgitant fraction was found to be slightly higher in the MS patients than the control group, however, this difference was not statistically significant. Secondly, although this was not estimated in our study, an atrophy-dependent ventricle volume increase in the MS patients may have caused the increase in the above values, giving rise to an increased mild hyperdynamic situation independent of the venous theory. This idea is supported by Chiang et al. ( 20 ) who found a relationship between ventricular morphology and aqueductal CSF flow in healthy subjects and in patients with communicating hydrocephalus. They concluded that aqueductal CSF flow should not be regarded independently of ventricular morphology. At the same time, Zamboni et. al ( 8 ) showed that the volume of the lateral and third ventricles was increased in MS patients with CCSVI.
Although we had more subjects in our study than others, if more RRMS, SPMS, and primary progressive MS patients could be enrolled in each of these groups, this would allow a more efficient study to be performed. We did not perform Doppler or magnetic resonance venography, and therefore have no information about the jugular and azygos veins. Additionally, the ventricle volumes were not estimated, which weakens our study. The use of 1.5 T MR instead of 3.0 T MR and of 12 time points (a low number) were further weaknesses of our study. There is a clear need for additional studies with more subjects, assessment of the cerebral venous system, cerebral perfusion imaging and estimation of ventricle volume values.
In conclusion, in the present study, the caudocranial and craniocaudal CSF flow volumes and stroke volume were found to be significantly higher in the MS patients. These findings may support the venous theory in MS patients, but may also be explained by atrophy-dependent ventricular dilatation independent of the venous theory in MS patients. Further studies are needed.
References
- 1. Barkovich AJ . Pediatric Neuroimaging . 4th Ed . Philadelphia : Lippincott Williams & Wilkins ; 2005 . [Google Scholar]
- 2. Ge Y , Zohrabian VM , Osa EO , et al. Diminished visibility of cerebral venous vasculature in multiple sclerosis by susceptibility-weighted imaging at 3.0 Tesla . J Magn Reson Imaging . 2009 ; 29 : 1190 – 1194 . doi: 10.1002/jmri.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zamboni P , Menegatti E , Bartolomei I , et al. Intracranial venous haemodynamics in multiple sclerosis . Curr Neurovasc Res . 2007 ; 4 : 252 – 258 . doi: 10.2174/156720207782446298. [DOI] [PubMed] [Google Scholar]
- 4. Zamboni P , Galeotti R , Menegatti E , et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis . J Neurol Neurosurg Psychiatry . 2009 ; 80 : 392 – 399 . doi: 10.1136/jnnp.2008.157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghezzi A , Comi G , Federico A . Chronic cerebro-spinal venous insufficiency (CCSVI) and multiple sclerosis . Neurol Sci . 2011 ; 32 : 17 – 21 . doi: 10.1007/s10072-010-0458-3. [DOI] [PubMed] [Google Scholar]
- 6. Andeweg J . Intracranial venous pressures, hydrocephalus and effects of cerebrospinal fluid shunts . Childs Nerv Syst . 1989 ; 5 : 318 – 323 . doi: 10.1007/BF00274522. [DOI] [PubMed] [Google Scholar]
- 7. Ibrahim I , Rahme R , Hourani R , Ali Y , Melki I , Rizk T . Hydrocephalus following bilateral jugular venous thrombosis in a child: case report and review of the literature . Pediatr Neurosurg . 2008 ; 44 : 68 – 70 . doi: 10.1159/000110667. [DOI] [PubMed] [Google Scholar]
- 8. Zamboni P , Menegatti E , Weinstock-Guttman B , et al. The severity of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis is related to altered cerebrospinal fluid dynamics . Funct Neurol . 2009 ; 24 : 133 – 138 . [PubMed] [Google Scholar]
- 9. Sundström P , Wahlin A , Ambarki K , Birgander R , Eklund A , Malm J . Venous and cerebrospinal fluid flow in multiple sclerosis: a case-control study . Ann Neurol . 2010 ; 68 : 255 – 259 . doi: 10.1002/ana.22132. [DOI] [PubMed] [Google Scholar]
- 10. Poser CM , Paty DW , Scheinberg L , et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols . Ann Neurol . 1983 ; 13 : 227 – 231 . doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 11. Polman CH , Reingold SC , Edan G , et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria” . Ann Neurol . 2005 ; 58 : 840 – 846 . doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 12. Kurtzke JF . Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) . Neurology . 1983 ; 33 : 1444 – 1452 . doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 13. Bradley WG , Jr , Scalzo D , Queralt J , Nitz WN , Atkinson DJ , Wong P . Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging . Radiology . 1996 ; 198 : 523 – 529 . doi: 10.1148/radiology.198.2.8596861. [DOI] [PubMed] [Google Scholar]
- 14. Brinkmann G , Harlandt O , Muhle C , Brossmann J , Heller M . Quantification of fluid flow in magnetic resonance tomography: an experimental study of a flow model and liquid flow measurements in the cerebral aqueduct in volunteers . Rofo . 2000 ; 172 : 1043 – 1051 . doi: 10.1055/s-2000-9217. [Article in German] [DOI] [PubMed] [Google Scholar]
- 15. Fogel MA . Assessment of ventricular function and blood flow . In: Fogel MA , editor. Principles and Practice of Cardiac Magnetic Resonance in Congenital Heart Disease: Form, Function, and Flow . Oxford : Wiley-Blackwell ; 2010 . pp. 51 – 74 . [Google Scholar]
- 16. Flórez N , Moratal D , Forner J , Arana E , Martí-Bonmatí L . Influence of the MR field strength on the PC quantitative analysis of cerebrospinal fluid flow within the aqueduct . 2006 . European Society for Magnetic Resonance in Medicine and Biology. 23rd Annual ESMRMB Meeting. Warsaw . http://riunet.upv.es/bitstream/handle/10251/6029/tesisUPV3059.pdf (accessed June 22, 2012)
- 17. Wentland AL , Wieben O , Korosec FR , Haughton VM . Accuracy and reproducibility of phase-contrast MR imaging measurements for CSF flow . AJNR Am J Neuroradiol . 2010 ; 31 : 1331 – 1336 . doi: 10.3174/ajnr.A2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pfefferbaum A , Mathalon DH , Sullivan EV , Rawles JM , Zipursky RB , Lim KO . A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood . Arch Neurol . 1994 ; 51 : 874 – 887 . doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 19. Akdogan I , Kiroglu Y , Onur S , Karabuluti N . The volume fraction of brain ventricles to total brain volume: a computed tomography stereological study . Folia Morphol (Warsz) . 2010 ; 69 : 193 – 200 . [PubMed] [Google Scholar]
- 20. Chiang WW , Takoudis CG , Lee SH , Weis-McNulty A , Glick R , Alperin N . Relationship between ventricular morphology and aqueductal cerebrospinal fluid flow in healthy and communicating hydrocephalus . Invest Radiol . 2009 ; 44 : 192 – 199 . doi: 10.1097/RLI.0b013e31819a640b. [DOI] [PubMed] [Google Scholar]
- 21. Gideon P , Thomsen C , Ståhlberg F , Henriksen O . Cerebrospinal fluid production and dynamics in normal aging: a MRI phase-mapping study . Acta Neurol Scand . 1994 ; 89 : 362 – 366 . doi: 10.1111/j.1600-0404.1994.tb02647.x. [DOI] [PubMed] [Google Scholar]
- 22. Barkhof F , Kouwenhoven M , Scheltens P , Sprenger M , Algra P , Valk J . Phase-contrast cine MR imaging of normal aqueductal CSF flow. Effect of aging and relation to CSF void on modulus MR . Acta Radiol . 1994 ; 35 : 123 – 130 . [PubMed] [Google Scholar]
- 23. May C , Kaye JA , Atack JR , Schapiro MB , Friedland RP , Rapoport SI . Cerebrospinal fluid production is reduced in healthy aging . Neurology . 1990 ; 40 : 500 – 503 . doi: 10.1212/wnl.40.3_part_1.500. [DOI] [PubMed] [Google Scholar]