Summary
The aim of this study was to assess the prevalence of chronic cerebrospinal venous insufficiency in an unselected cohort of multiple sclerosis (MS) patients. A total of 586 patients with clinically defined MS underwent catheter venography of the internal jugular veins, brachiocephalic veins and azygos vein. The following findings were regarded as pathologic: no outflow, slowed outflow, reversal of flow direction, prestenotic dilation accompanied by impaired outflow, outflow through collaterals, intraluminal structures obstructing the vein, hypoplasia, agenesia or significant narrowing of the vein.
Venous abnormalities were found in 563 patients (96.1%). Lesions in one vein were found in 43.5%, in two veins in 49.5%, and in three veins in 3.1% of patients.
Venous pathologies in the right internal jugular vein were found in 64.0% of patients, in the left internal jugular vein in 81.7%, in the left brachiocephalic vein in 1.0%, and in the azygos vein in 4.9%.
Venous pathologies were found to be highly associated with MS, yet the clinical relevance of this phenomenon remains to be established.
Keywords: azygos vein , brachiocephalic veins , jugular veins , multiple sclerosis , vascular malformations
Introduction
Multiple sclerosis (MS) is a chronic disease of the central nervous system of, as yet, undetermined etiology ( 1 – 3 ) . Recently, a unique vascular pathology comprising stenoses and occlusions in the extracranial veins draining the central nervous system (primarily the internal jugular veins and the azygos vein) was described ( 4 ) . Since this description, this condition, termed chronic cerebrospinal venous insufficiency (CCSVI), has been found to be highly associated with MS ( 5 – 10 ) , a discovery that has shed new light on the potential causes of MS and allowed a reinterpretation of knowledge about this neurologic disease ( 3 , 11 – 14 ) . However, some reports deny the existence of CCSVI ( 15 , 16 ) and, importantly, the actual clinical relevance of this vascular abnormality remains to be established.
Here, we present data on the prevalence of CCSVI in MS patients based on catheter venography findings: the gold standard for the assessment of vascular flow pathologies.
Materials and methods
A total of 586 patients with clinically defined MS were assessed. The patients (416 women and 170 men) were aged 15–70 years, with a median age of 44 years. They had suffered from MS for 0.5 to 40 years (median disease duration: 10 years). Their Extended Disability Status Scale scores ranged from 0.5 to 9.5 with a median score of 5.5. The clinical type of MS could be determined in 441 cases: 156 patients (35.4%) had the relapsing-remitting, 168 (38.1%) the secondary progressive, and 117 (26.5%) the primary progressive course of MS.
All these MS patients underwent catheter venography of the extracranial veins draining the central nervous system: the internal jugular veins, the brachiocephalic veins and the azygos vein. Catheter venography was performed irrespective of the results of noninvasive tests for the assessment of CCSVI: color Doppler sonography and magnetic resonance venography. Catheter venography, undertaken as the first part of endovascular procedures for detected vascular pathologies, was performed under mild sedation and local anesthesia. After introduction of an angiographic catheter into the examined vein, diluted (1:1) iodine-containing contrast, Iodixanolum (Visipaque ® , Amersham Health AS, Norway), was injected. Venographic examination of the veins focused on assessment of outflow. To obtain more information about outflow characteristics, contrast was injected at different levels of the assessed vein; for example, in the case of the internal jugular vein: at the level of the jugular foramen, at the level of the junction with the facial vein, slightly cranially to the valve at the junction with the brachiocephalic vein and also caudally to this valve. The radiograms were also taken from different angles. Usually, each injection consisted of approximately 4–5 ml of diluted contrast (more concentrated contrast can obscure tiny intraluminal structures, such as valve leaflets or membranes). Contrast was injected manually, applying low pressure, since forced injection can produce a false picture of a “reflux”, or even an outflow of contrast toward intracranial sinuses or vertebral veins, which can also be falsely interpreted as a “reflux”. During injection, care was taken to avoid positioning the catheter tip inside or in proximity to a tributary, since this could produce a false picture of outflow via “collaterals” ( 7 , 17 ) . In accordance with the generally accepted venographic signs of impaired venous outflow in other veins (like the iliac or axillary veins), the following venographic flow patterns were regarded as abnormal:
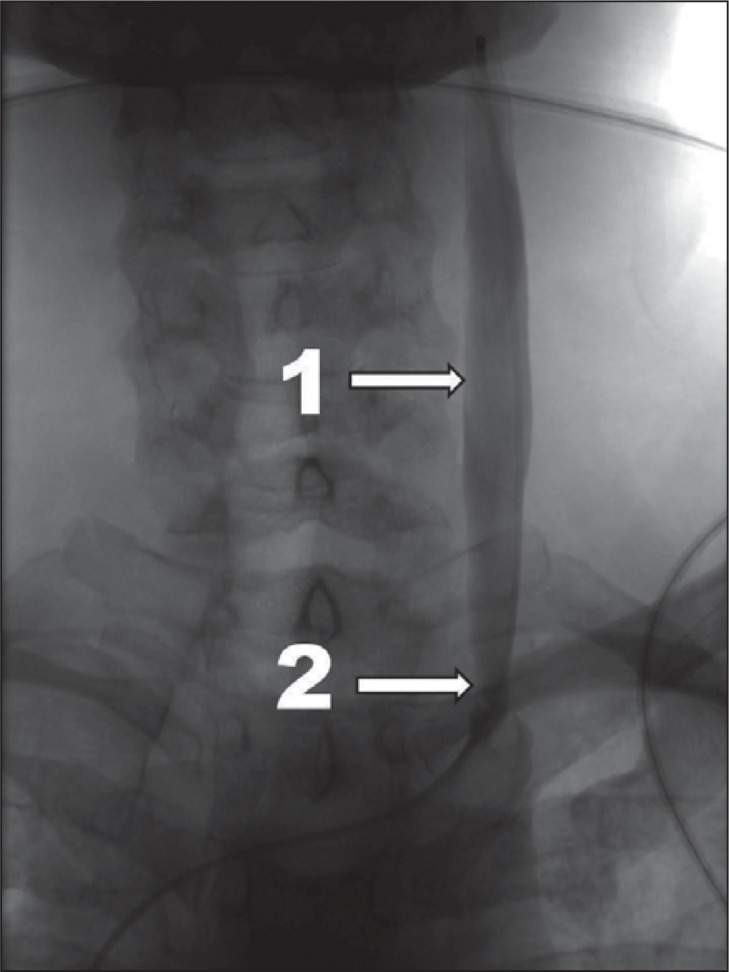
slowed venous outflow, i.e. a retention of injected contrast in the examined vein for longer that one cardiac cycle ( Fig. 1 );
reflux, i.e. a reversal of flow direction;
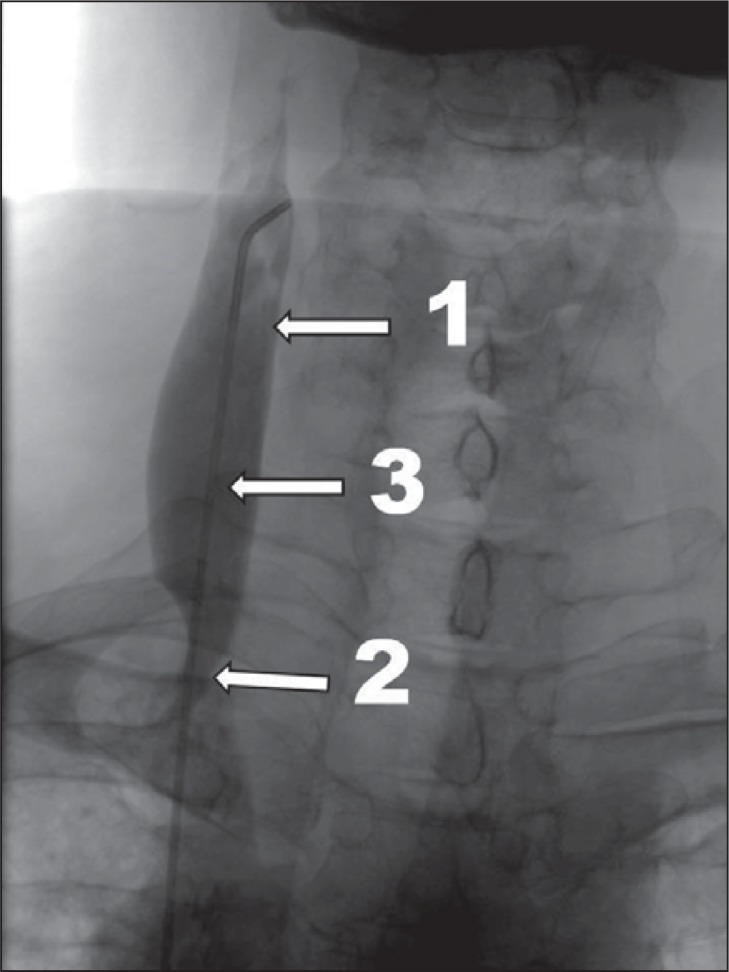
prestenotic dilation of the vein ( Fig. 2 ) associated with slowed outflow or reflux;
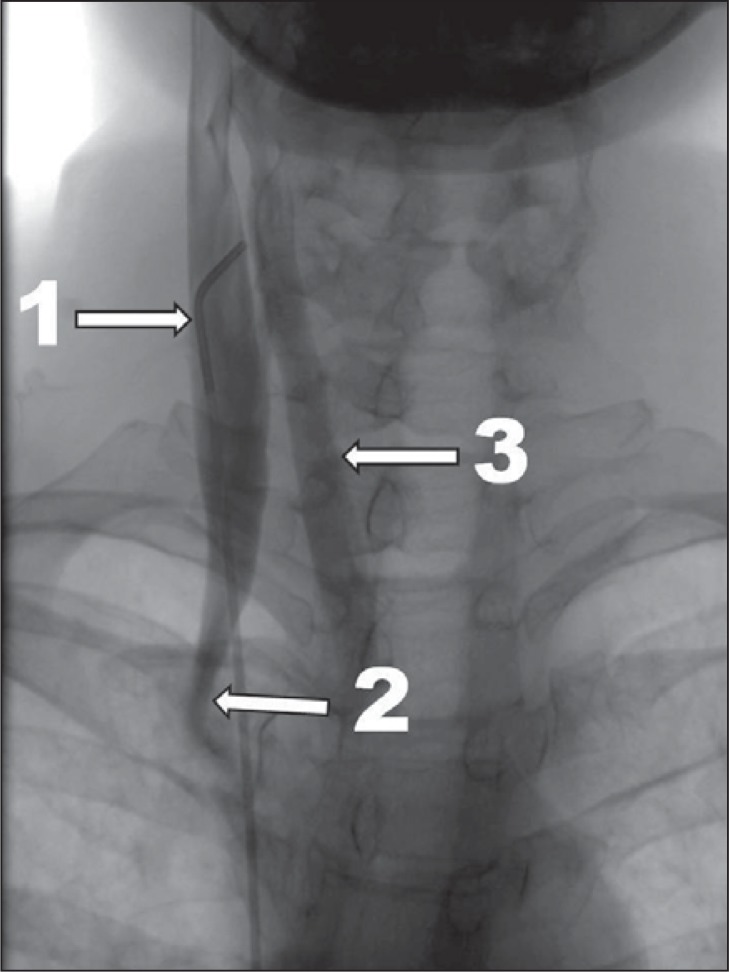
outflow through collaterals ( Fig. 3 );
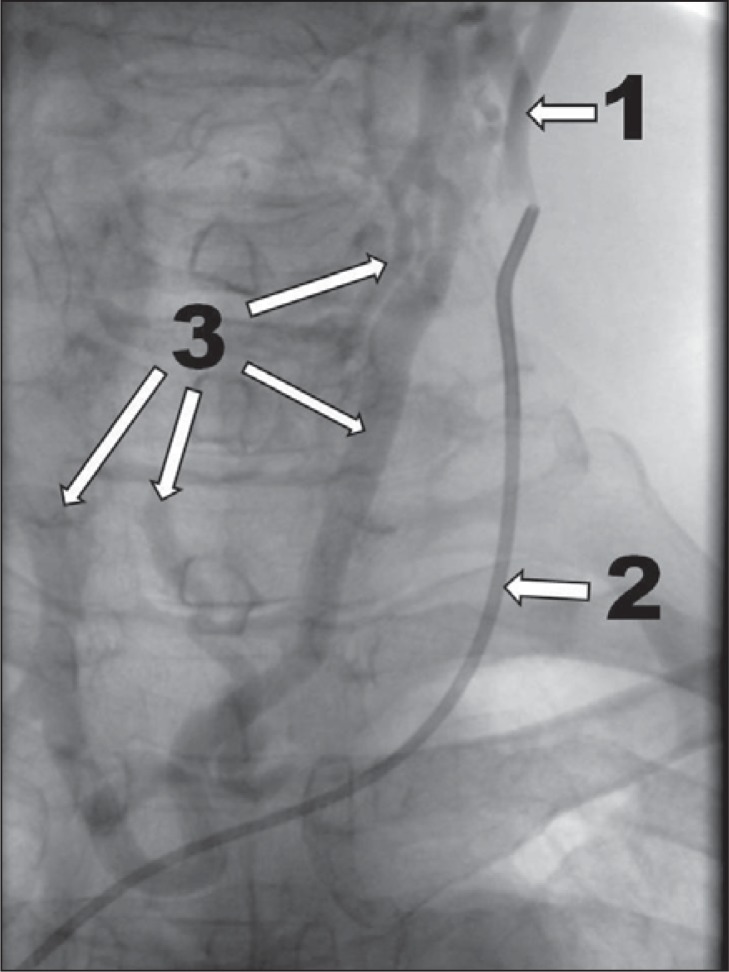
no outflow through the vein ( Fig. 4 );
hypoplasia or narrowing of the vein associated with any of the above-mentioned pathologic flow patterns;
intraluminal structures: webs, septa, membranes;
complete occlusion (for example, post-thrombotic) or agenesia of the vein.
Figure 1 .
Mild CCSVI, venographic grade 1 according to Ludyga et al. ( 7 ): slowed venous outflow, no reflux detected (1 – internal jugular vein; 2 – stenotic valve).
Figure 2 .
More advanced CCSVI, venographic grade 2 according to Ludyga et al. ( 7 ): slowed venous outflow, mild reflux and/or pre-stenotic dilation of the vein (1 – internal jugular vein; 2 – stenotic valve; 3 – prestenotic dilation of the vein).
Figure 3 .
Severe case of CCSVI, venographic grade 3 according to Ludyga et al. ( 7 ): slowed venous outflow with reflux and outflow through collaterals (1 – internal jugular vein; 2 – stenosis at the level of valve; 3 – outflow through collaterals).
Figure 4 .
Very severe case of CCSVI, venographic grade 4 according to Ludyga et al. ( 7 ): no outflow through the vein, huge outflow through collaterals (1 – internal jugular vein; 2 – no outflow through the internal jugular vein; 3 – outflow through collaterals).
Importantly, a narrowing of the vein not accompanied by slowed outflow, reflux or outflow through collaterals was regarded as an anatomic variant and not a pathology. This criterion was also applied to the venous valves – these valves are typically seen at the junction of the internal jugular vein and the brachiocephalic vein and also in the ostium of the azygos vein – which were regarded as pathologic only if they compromised the outflow. The entire study protocol, including imaging diagnostics of extracranial veins (catheter venography, as well as Doppler sonography and MR venography) and also endovascular treatments for detected vascular pathologies, was approved by the Bioethics Committee of the Regional Silesian Board of Physicians in Katowice, Poland (approval No: 7/2010). All patients gave their written consent to undergo these procedures and tests. The study has been registered at ClinicalTrials.gov ; identifier: NCT01264848.
Results
Venous outflow abnormalities of at least one vein draining the central nervous system (left or right internal jugular vein, left or right brachiocephalic vein, or azygos vein) were revealed in 563 cases (96.1% of the patients), whereas in 23 cases (3.9%) no obvious pathology was found. Lesions in one vein were found in 43.5%, in two veins in 49.5% and in three veins in 3.1% of the patients. Interestingly, no outflow pathology was found in the right brachiocephalic vein. Similarly, the prevalence of vascular pathologies was much higher in the left than in the right internal jugular vein. This difference, tested by chi 2 test, was statistically significant (p<0.05). Most of the outflow blockages in the internal jugular veins were caused by malformed valves at the junction with the brachiocephalic veins ( Fig.s 1–3 ). Hypoplastic veins ( Fig. 4 ) or other pathologies were far less common. The majority of the azygos vein lesions (usually stenosis or twisting of the vein) were localized slightly distally to the azygos arch ( Fig. 5 ). Tables 1 and 2 (over) provide details of the distribution of the vascular abnormalities found. We did not find significant differences in terms of distribution of venous lesions between the patients with different clinical courses of MS ( Table 3 , over).
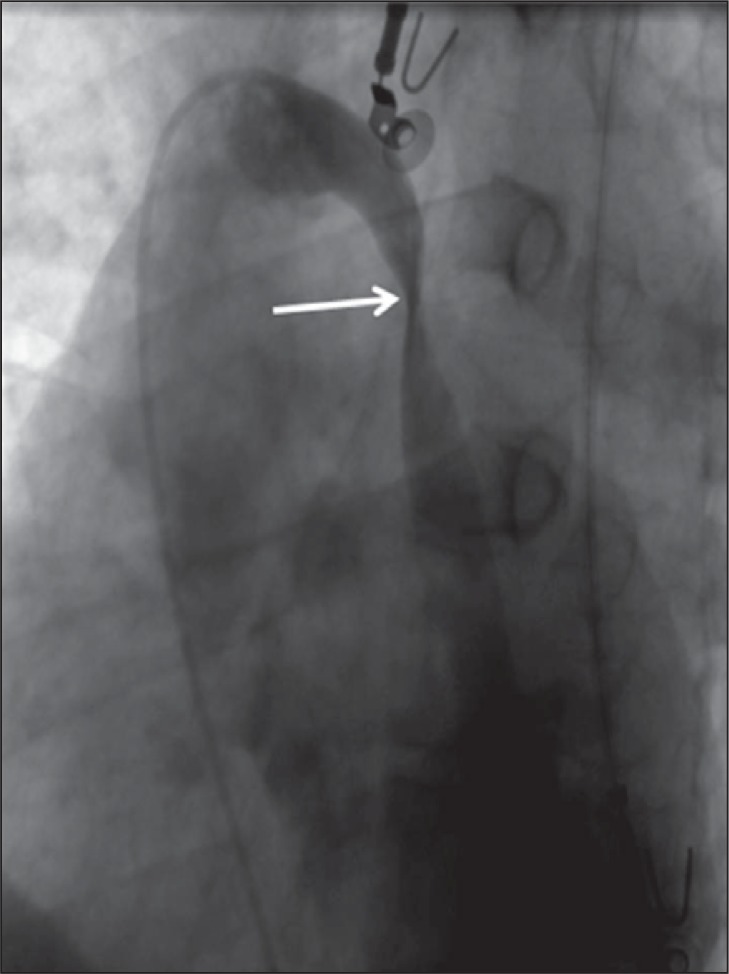
Figure 5 .
Stenosis of the azygos vein caused by twisting (arrow) of the vein.
Table 1 .
Distribution of venous lesions (586 patients)
| Distribution of the lesions | Pattern of the lesions | Number of patients | % |
|---|---|---|---|
| no pathology detected | – | 23 | 3.9% |
|
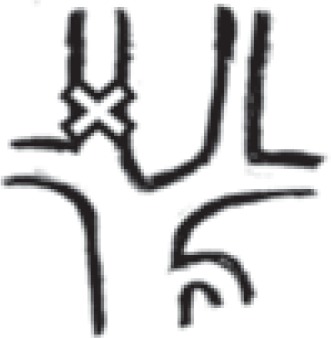
in one internal jugular vein |
|
252 right: 79 left: 173 |
43.0% right: 13.5% left: 29.5% |
|
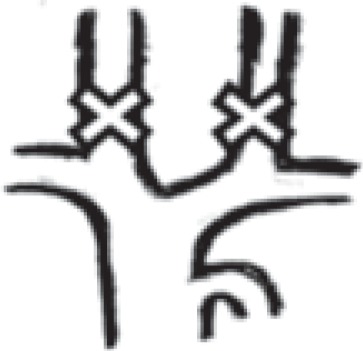
in both internal jugular veins |
|
277 | 47.3% |
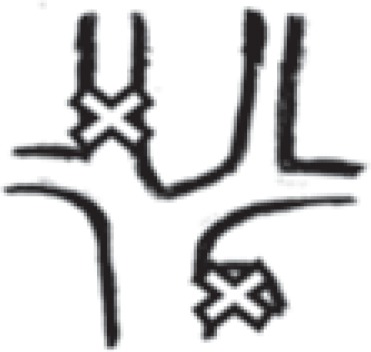
| in one internal jugular vein + azygos vein |
|
12 right: 2 left: 10 |
2.0% right: 0.3% left: 1.7% |
|
in both internal jugular veins + azygos vein |
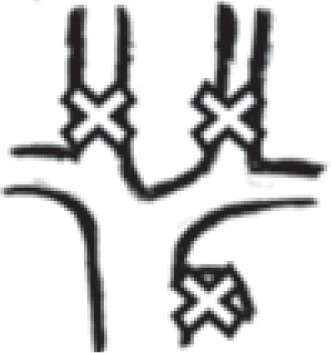
|
13 | 2.2% |
|
in one internal jugular vein + left brachiocephalic vein |
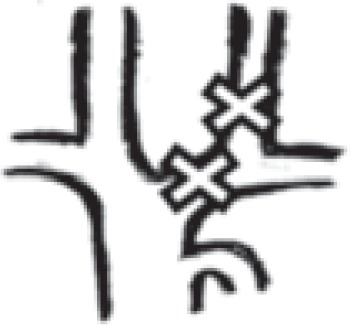
|
1 right: 0 left: 1 |
0.2% right: 0 left: 0.2% |
|
in one internal jugular vein + left brachiocephalic vein + azygos vein |
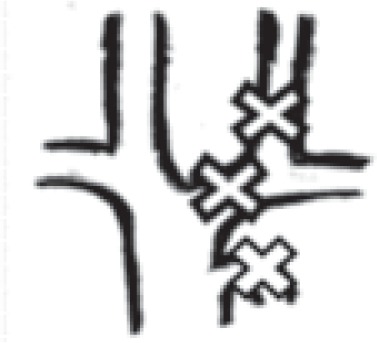
|
1 right: 0 left: 1 |
0.2% right: 0 left: 0.2% |
|
in both internal jugular veins + left brachiocephalic vein |
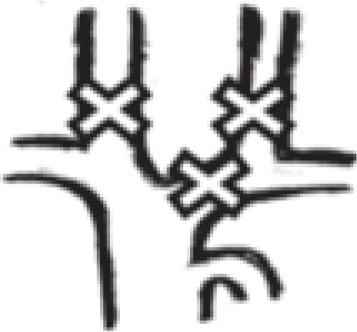
|
4 | 0.7% |
|
only in the azygos vein |
|
3 | 0.5% |
| Total | 586 | 100% |
Table 2 .
Prevalence of pathologies in particular veins
| Vein | Number of patients | % |
|---|---|---|
| Right internal jugular vein | 375 | 64.0% |
| Left internal jugular vein | 479 | 81.7% |
| Right brachiocephalic vein | 0 | 0 |
| Left brachiocephalic vein | 6 | 1.0% |
| Azygos vein | 29 | 4.9% |
Table 3 .
Prevalence of pathologies in particular veins depending on clinical type of MS (441 cases analyzed)
| Distribution of the lesions | Relapsing-remitting MS | Secondary progressive MS | Primary progressive MS |
|---|---|---|---|
| no pathology detected | 7 (4.5%) | 5 (3.0%) | 5 (4.3%) |
|
| |||
| in one internal jugular vein | 70 (44.9%) | 78 (46.4%) | 49 (41.9%) |
| right: 23 (14.7%) | right: 24 (14.3%) | right: 19 (16.2%) | |
| left: 47 (30.1%) | left: 54 (32.1%) | left: 30 (25.6%) | |
|
| |||
| in both internal jugular veins | 73 (46.8%) | 75 (44.6%) | 57 (48.7%) |
|
| |||
| in one internal jugular vein + azygos vein | 3 (1.9%) | 1 (0.6%) | 5 (4.3%) |
| right: 1 (0.6%) | right: 0 | right: 0 | |
| left: 2 (1.3%) | left: 1 (0.6%) | left: 5 (4.3%) | |
|
| |||
| in both internal jugular veins + azygos vein | 2 (1.3%) | 6 (3.4%) | 0 |
|
| |||
| in one internal jugular vein + left brachiocephalic vein | 0 | 1 (0.6%) | 0 |
|
| |||
| in one internal jugular vein + left brachiocephalic vein + azygos vein | 1 (0.6%) | 0 | 0 |
|
| |||
| in both internal jugular veins + left brachiocephalic vein | 0 | 1 (0.6%) | 0 |
|
| |||
| only in the azygos vein | 0 | 1 (0.6%) | 1 (0.9%) |
|
| |||
| Total | 156 | 168 | 117 |
|
| |||
| Total cases with azygos vein involvement | 6 (3.8%) | 8 (4.8%) | 6 (5.1%) |
Discussion
Using catheter venography, we demonstrated a very high prevalence (about 96%) of CCSVI in a group of MS patients who had not been pre-selected using noninvasive tests. Further studies should reveal whether the patients in whom venous pathology was not detected were actually affected by difficult-to-demonstrate vascular problems (such as intracranial stenosis). The alternative possibility is that there is, indeed, a small group of MS patients with normal venous outflow. The latter scenario would mean that MS and CCSVI are not the same, but rather distinct, albeit very closely interconnected, diseases. It should be emphasized that, until the report on MS patients by Zamboni et al. ( 4 ) , no vascular malformations of the azygos vein had been described ( 18 ) , while internal jugular vein pathologies had not been found ( 19 – 21 ) , or been found only very infrequently (3.6% of cases) ( 22 ) . However, the fact that the above papers described non-MS populations seems to indicate that CCSVI is linked especially to this neurologic disease.
Our survey was part of a clinical trial on endovascular treatments for CCSVI, a study entirely planned to assess the safety and efficacy of endovascular procedures performed in order to alleviate venous outflow blockages in the main veins draining the central nervous system. Since endovascular treatments must be preceded by a detailed assessment of the treated veins, primarily by means of catheter venography, this pre-procedural evaluation provided much useful information on the venous system in MS patients. According to the Consensus document of the International Union of Phlebology on the diagnosis and treatment of venous malformations ( 23 ) , CCSVI is recognized as venous truncular lesions obstructing main outflow routes from the central nervous system. The authors of this consensus document recommended diagnostic procedures in suspected CCSVI cases and interventions for proven cases, since CCSVI obstructs outflow from a vital organ, namely the brain. However, for the time being, the existence and, especially, the relevance of CCSVI remain controversial. While many authors have confirmed the discovery of this venous pathology ( 4 – 10 , 24 – 27 ) , others have failed to recognize its existence ( 15 , 16 ) . This discrepancy is very likely associated with the use of Doppler sonography to demonstrate abnormalities of venous outflow. Doppler sonography is a highly operator-dependent test and a pathologic picture can easily be overlooked by an examiner who is not familiar with principles of sonographic diagnostics for CCSVI ( 28 , 29 ) .
The use of catheter venography for the evaluation of the veins seems to be a strength of our study. Unlike Doppler sonography, catheter venography is an established “gold standard” for the diagnosis of vascular diseases. It is widely used in cases of venous outflow pathologies and, importantly, it is far less operator-dependent.
Nevertheless, it must be remembered that an evaluation by means of catheter venography in venous territory differs significantly from a similar evaluation on the arterial side. Consequently, the interventional radiologist must be familiar with angiographic assessment of venous pathologies. Moreover, at present there exist no widely accepted venographic parameters indicating a pathology of the azygos, internal jugular or brachiocephalic veins. Therefore, the use of the criteria presented in the “Materials and methods” section, especially the criterion requiring that a hypoplasia or narrowing of the vein must be associated with another sign of impaired outflow (for example: a reversal of flow direction) in order to give a diagnosis of CCSVI, may constitute a weak point of our study. Other physicians have diagnosed CCSVI more liberally, stating that a narrowing by itself should be regarded as pathologic. For example, Zamboni et al. considered significant stenoses to be any venous lumen reduction greater than 50% ( 25 ) . Consequently, a much higher prevalence of pathologic veins was found by these authors, especially in the case of the azygos vein ( 4 , 26 , 30 ) .
We did not confirm the suggestion by Zamboni et al. ( 4 ) that there exist different distributions of venous lesions in patients with different clinical types of MS ( Table 3 ). However, unlike this author, we did not study the entire azygos and lumbar venous systems and did not compare venous lesion distribution with MRI distribution of plaques in the spinal cord.
Additionally, unlike previous reports, we noticed an asymmetric distribution of the lesions in the internal jugular veins. In our study, venous malformations were more commonly found on the left side. The prevalence of left internal jugular vein malformations was 81.7% vs 64.0% on the right side. Similarly, blockages were found only in the left and not in the right brachiocephalic vein. Currently it is conjectured that CCSVI is a congenital venous malformation. Considering the embryologic development of these veins ( 31 ) , one might expect a higher prevalence of abnormalities on the left side, which is actually what we found in our study. The internal jugular veins that drain the brain in adults develop from precardinal veins in the embryo. These precardinal veins must join the common cardinal veins (in the area where, in an adult, the jugular valve is found). It is easy to imagine that this process is not always perfect, especially on the left side, where part of the left common cardinal vein naturally involutes. Similarly, most of the abnormalities of the azygos vein ( Fig. 5 ) were found distal to the azygos arch, in the area where, embryologically, the proximal part of the left postcardinal vein should join the left supracardinal vein. Probably, in some individuals this process, too, goes awry, resulting in twisting or other malformation of this vein.
The prevalence of CCSVI in our patients was very similar to that reported by Yamout et al. who, using catheter venography, demonstrated a 92% prevalence of CCSVI in their group of 13 MS patients ( 10 ) . Interestingly, they also found that the prevalence of CCSVI in patients with clinically isolated syndrome (CIS), a neurologic syndrome preceding MS, was very low (9%). The authors interpreted this phenomenon as proof of the secondary nature of CCSVI, i.e. that these vascular lesions develop as a result of MS. However, the majority of CCSVI lesions are actually anatomic abnormalities ( 30 ) and it is hard to imagine how inflammatory substances released from the brain in the setting of MS, as claimed by Yamout et al., could evoke such vascular malformations. It is more likely that CIS can progress into clinically overt MS only in the presence of CCSVI (since it is known that only 30–70% of CIS cases progress to MS). This latter scenario seems to be supported by our observation of very weak, statistically insignificant correlations between the severity of CCSVI and the duration of MS ( 32 ) .
Applying our venographic criteria, we found a very high prevalence of CCSVI among MS patients, and can therefore conclude that CCSVI is significantly correlated with MS. However, it should be remembered that, for the time being, the actual prevalence of CCSVI in healthy populations is not known. In the study by Zivadinov et al., who applied Doppler sonography as the diagnostic test, it was found that 23% of healthy individuals presented with sonographic signs of CCSVI, compared with 56% of sonographically positive MS patients ( 33 ) . Although Doppler sonography has some known limitations, on the basis of the findings of the present study it can be predicted that catheter venography would reveal venous blockages in many healthy individuals. However, it seems unlikely that the prevalence would be as high as 96%. Still, the prevalence of CCSVI in healthy populations must continue to be evaluated in ongoing studies, importantly, using objective diagnostic tools and widely accepted criteria. In addition, the clinical significance of these venous anomalies needs to be explained through further research.
References
- 1. Chaudhuri A , Behan PO . Multiple sclerosis: looking beyond autoimmunity . J R Soc Med . 2005 ; 98 : 303 – 306 . doi: 10.1258/jrsm.98.7.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Behan PO . Futility of the autoimmune orthodoxy in multiple sclerosis research . Expert Rev Neurother . 2010 ; 10 : 1023 – 1025 . doi: 10.1586/ern.10.69. [DOI] [PubMed] [Google Scholar]
- 3. Haacke EM . Chronic cerebral spinal venous insufficiency in multiple sclerosis . Expert Rev Neurother . 2011 ; 11 : 5 – 9 . doi: 10.1586/ern.10.174. [DOI] [PubMed] [Google Scholar]
- 4. Zamboni P , Galeotti R , Menegatti E , et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis . J Neurol Neurosurg Psychiatry . 2009 ; 80 : 392 – 399 . doi: 10.1136/jnnp.2008.157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zamboni P , Menegatti E , Galeotti R , et al. The value of cerebral Doppler venous haemodynamics in the assessment of multiple sclerosis . J Neurol Sci . 2009 ; 282 : 21 – 27 . doi: 10.1016/j.jns.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 6. Simka M , Kostecki J , Zaniewski M , Majewski E , Hartel M . Extracranial Doppler sonographic criteria of chronic cerebrospinal venous insufficiency in the patients with multiple sclerosis . Int Angiol . 2010 ; 29 : 109 – 114 . [PubMed] [Google Scholar]
- 7. Ludyga T , Kazibudzki M , Simka M , et al. Endovascular treatment for chronic cerebrospinal venous insufficiency: is the procedure safe? . Phlebology . 2010 ; 25 : 286 – 295 . doi: 10.1258/phleb.2010.010053. [DOI] [PubMed] [Google Scholar]
- 8. Simka M , Kostecki J , Zaniewski M , Majewski E , Szewczyk-Urgacz D . Preliminary report on pathologic flow patterns in the internal jugular and vertebral veins of patients with multiple sclerosis . Przeglad Flebologiczny . 2009 ; 17 : 61 – 64 . [Google Scholar]
- 9. Al-Omari MH , Rousan LA . Internal jugular vein morphology and hemodynamics in patients with multiple sclerosis . Int Angiol . 2010 ; 29 : 115 – 120 . [PubMed] [Google Scholar]
- 10. Yamout B , Herlopian A , Issa Z , et al. Extracranial venous stenosis is an unlikely cause of multiple sclerosis . Mult Scler . 2010 ; 16 : 1341 – 1348 . doi: 10.1177/1352458510385268. [DOI] [PubMed] [Google Scholar]
- 11. Simka M . Blood brain barrier compromise with endothelial inflammation may lead to autoimmune loss of myelin during multiple sclerosis . Curr Neurovasc Res . 2009 ; 6 : 132 – 139 . doi: 10.2174/156720209788185605. [DOI] [PubMed] [Google Scholar]
- 12. Embry AF . Integrating CCSVI and CNS autoimmunity in a disease model for MS . Int Angiol . 2010 ; 29 : 93 – 94 . [PubMed] [Google Scholar]
- 13. Simka M , Zaniewski M . Reinterpreting the magnetic resonance signs of hemodynamic impairment in the brains of multiple sclerosis patients from the perspective of a recent discovery of outflow block in the extracranial veins . J Neurosci Res . 2010 ; 88 : 1841 – 1845 . doi: 10.1002/jnr.22350. [DOI] [PubMed] [Google Scholar]
- 14. Zamboni P , Menegatti E , Weinstock-Guttman B , et al. The severity of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis is related to altered cerebrospinal fluid dynamics . Funct Neurol . 2009 ; 24 : 133 – 138 . [PubMed] [Google Scholar]
- 15. Sundström P , Wåhlin A , Ambarki K , Birgander R , Eklund A , Malm J . Venous and cerebrospinal fluid flow in multiple sclerosis: a case-control study . Ann Neurol . 2010 ; 68 : 255 – 259 . doi: 10.1002/ana.22132. [DOI] [PubMed] [Google Scholar]
- 16. Doepp F , Paul F , Valdueza JM , Schmierer K , Schreiber SJ . No cerebrocervical venous congestion in patients with multiple sclerosis . Ann Neurol . 2010 ; 68 : 173 – 183 . doi: 10.1002/ana.22085. [DOI] [PubMed] [Google Scholar]
- 17. Sclafani S . Chronic cerebrospinal venous insufficiency: a new paradigm and therapy for multiple sclerosis . Endovascular Today . 2010 Jul;: 41 – 46 . [Google Scholar]
- 18. Chasen MH , Charnsangavej C . Venous chest anatomy: clinical implications . Eur J Radiol . 1998 ; 27 : 2 – 14 . doi: 10.1016/s0720-048x(97)00147-2. [DOI] [PubMed] [Google Scholar]
- 19. Mammen T , Keshava SN , Eapen CE , et al. Transjugular liver biopsy: a retrospective analysis of 601 cases . J Vasc Intervent Radiol . 2008 ; 19 : 351 – 358 . doi: 10.1016/j.jvir.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 20. Troianos CA , Kuwik RJ , Pasqual JR , Lim AJ , Odasso DP . Internal jugular vein and carotid artery anatomic relation as determined by ultrasonography . Anesthesiology . 1996 ; 85 : 43 – 48 . doi: 10.1097/00000542-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 21. Lin BS , Kong CW , Tarng DC , Huang TP , Tang GJ . Anatomical variation of the internal jugular vein and its impact on temporary haemodialysis vascular access: an ultrasonographic survey in uraemic patients . Nephrol Dial Transplant . 1998 ; 13 : 134 – 138 . doi: 10.1093/ndt/13.1.134. [DOI] [PubMed] [Google Scholar]
- 22. Denys BG , Uretsky BF , Reddy PS . Ultrasound-assisted cannulation of the internal jugular vein. A prospective comparison to the external landmark-guided technique . Circulation . 1993 ; 87 : 1557 – 1562 . doi: 10.1161/01.cir.87.5.1557. [DOI] [PubMed] [Google Scholar]
- 23. Lee BB , Bergan J , Gloviczki P , et al. Diagnosis and treatment of venous malformations Consensus document of the International Union of Phlebology (IUP)-2009 . Int Angiol . 2009 ; 28 : 434 – 451 . [PubMed] [Google Scholar]
- 24. Zaniewski M , Kostecki J , Ziaja K , et al. Endovascular surgery for the treatment of symptoms of multiple sclerosis. Preliminary report . Chirurgia Polska . 2010 ; 12 : 12 – 17 . [Google Scholar]
- 25. Zamboni P , Menegatti E , Bartolomei I , et al. Intracranial venous haemodynamics in multiple sclerosis . Curr Neurovasc Res . 2007 ; 4 : 252 – 258 . doi: 10.2174/156720207782446298. [DOI] [PubMed] [Google Scholar]
- 26. Zamboni P , Consorti G , Galeotti R , et al. Venous collateral circulation of the extracranial cerebrospinal outflow routes . Curr Neurovasc Res . 2009 ; 6 : 204 – 212 . doi: 10.2174/156720209788970054. [DOI] [PubMed] [Google Scholar]
- 27. Bartolomei I , Salvi F , Galeotti R , et al. Hemodynamic pattern of chronic cerebrospinal venous insufficiency in multiple sclerosis. Correlation with symptoms at onset and clinical course . Int Angiol . 2010 ; 29 : 183 – 188 . [PubMed] [Google Scholar]
- 28. Menegatti E , Genova V , Tessari M , et al. The reproducibility of colour Doppler in chronic cerebrospinal venous insufficiency associated with multiple sclerosis . Int Angiol . 2010 ; 29 : 121 – 126 . [PubMed] [Google Scholar]
- 29. Zamboni P . Regarding “no cerebrocervical venous congestion in patients with multiple sclerosis. Intraluminal jugular septation” . Ann Neurol . 2010 ; 68 : 969 . doi: 10.1002/ana.22152. author reply 970 . [DOI] [PubMed] [Google Scholar]
- 30. Zamboni P , Galeotti R , Menegatti E , et al. A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency . J Vasc Surg . 2009 ; 50 : 1348 – 1358 . doi: 10.1016/j.jvs.2009.07.096. [DOI] [PubMed] [Google Scholar]
- 31. Lee BB , Laredo J , Neville R . Embryological background of truncular venous malformation in the extracranial venous pathways as the cause of chronic cerebrospinal venous insufficiency . Int Angiol . 2010 ; 29 : 95 – 108 . [PubMed] [Google Scholar]
- 32. Simka M , Ludyga T , Kazibudzki M , Latacz P , Âwierad M . Multiple sclerosis: an unlikely cause of chronic cerebrospinal venous insufficiency . JRSM Short Rep . 2011 doi: 10.1258/shorts.2011.010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zivadinov R , Marr K , Cutter G , et al. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS . Neurology . 2011 ; 77 : 138 – 144 . doi: 10.1212/WNL.0b013e318212a901. [DOI] [PubMed] [Google Scholar]