Summary
Chronic cerebrospinal venous insufficiency (CCSVI) is a syndrome characterized by stenoses or obstructions of the internal jugular and/or azygos veins with disturbed flow and formation of collateral venous channels. Ultrasound and venographic studies of the internal jugular and azygos venous systems in patients with multiple sclerosis (MS) have demonstrated a high prevalence of CCSVI (mean 71%, range 0–100%; n=1336) associated with activation of collaterals. By contrast, ultrasound and venographic examinations of normal controls and patients without MS have demonstrated a much lower prevalence (mean 7.1%, range 0–22%; n=505).
Ultrasound in the form of duplex scanning uses a combination of physiological measurements as well as anatomical imaging and has been used for the detection of CCSVI by different centers with variable results. A high prevalence of obstructive lesions, ranging from 62% to 100%, has been found by some teams in patients with MS compared with a low prevalence (0–25%) in controls. However, others have reported absence of these lesions or a lower prevalence (16–52%). This variability could be the result of differences in technique, training, experience or criteria used.
In order to ensure a high reproducibility of duplex scanning with comparable accuracy between centers a detailed protocol with standard methodology and criteria is needed. Also, standardization of the method of reporting of duplex measurements and other findings will facilitate validation of the proposed criteria by different centers. The aim of this document is to produce recommendations for such a protocol and indicate what future research is needed in order to address areas of uncertainty.
Keywords: chronic cerebrospinal venous insufficiency , consensus conference , Doppler ultrasound , multiple sclerosis
Introduction
Background and aims of the document
Chronic cerebrospinal venous insufficiency (CCSVI) is a syndrome characterized by stenoses or obstructions of the internal jugular vein (IJV) and/or azygos (AZ) vein with disturbed flow and formation of collateral venous channels ( 1 , 2 ) . Ultrasound and venographic studies of the internal jugular and azygos venous systems in patients with multiple sclerosis (MS) have demonstrated a high prevalence of CCSVI (mean 71%, range 0–100%; n=1336) associated with activation of collaterals ( 1 – 12 ) . By contrast, ultrasound and venographic examinations of normal controls and patients without MS have demonstrated a much lower prevalence (mean 7.1%, range 0–22%; n=505) ( Table 1 , over).
Table 1 .
Prevalence of CCSVI in patients with multiple sclerosis and in healthy controls
| Author (ref.) | MS Patients | Controls | Investigation | ||
|---|---|---|---|---|---|
|
| |||||
| CCSVI | Total | CCSVI | Total | ||
| Zamboni et al. 2009 ( 1 ) | 65 (100%) | 65 | 0 (0%) | 235 | duplex, venography |
| Zivadinov et al. 2011 ( 4 ) | 162 (56.1%) | 289 | 374 (22.7%) | 163 | duplex |
| Doepp et al. 2011 ( 8 ) | 0 (0%) | 56 | 0 (0%) | 20 | duplex |
| Mayer et al. 2011 ( 9 ) | 0 (0%) | 20 | 1 (5%) | 20 | duplex |
| Yamout et al. 2010 ( 10 ) | 19 (45%) | 42 | – | – | venography |
| Baracchini et al. 2011 ( 11 ) | 8 (16%) | 50 | 1 (2%) | 50 | duplex, venography |
| Al-Omari et al. 2010 ( 5 ) | 21 (84%) | 25 | 0 (0%) | 25 | duplex |
| Simka et al. 2010 ( 6 ) | 64 (91%) | 70 | – | – | duplex |
| Bastianello et al. 2011 ( 7 ) | 610 (86%) | 710 | – | – | duplex |
The origin of venous anomalies is still not completely understood. These lesions are mostly segmental hypoplasia or intraluminal defects, collectively classified as truncular venous malformations ( 13 – 15 ) . Truncular lesions are the result of developmental arrest that occurs during the “later” stages of vascular trunk formation in fetal life. Immature or incomplete development of the main axial veins results in aplasia, hypoplasia or hyperplasia of the vessel or in a defective vessel with obstruction by intraluminal lesions (e.g., vein web, spur, annulus, or septum) or dilatation (e.g., jugular vein ectasia/aneurysm). Such lesions were not detected in radiological studies on healthy subjects ( 16 – 25 ) ; on the contrary, CCSVI-like lesions have been described in association with myelopathies ( 26 , 27 ) .
The increased prevalence of obstruction to the drainage of cerebrospinal veins in patients with MS suggests that venous obstruction may be a contributory factor in the development and progression of this disease. It has also been suggested that relief of such obstruction may produce clinical benefit. In two observational studies involving, overall, 80 MS patients with CCSVI, angioplasty reduced the rate of relapse, improved the Multiple Sclerosis Functional Composite in patients with relapsing-remitting MS ( 28 , 29 ) , and improved physical and mental quality of life (QOL) in relapsing-remitting and primary progressive MS ( 28 ) . In another study of 31 patients, followed up for twelve months, angioplasty reduced chronic fatigue as assessed by the Fatigue Severity Scale and the Fatigue Impact Scale ( 30 ) . Safety was confirmed in more than 500 procedures ( 31 ) . However, the true clinical benefit will be known only when the results of multicenter studies, now in progress, become available. For a list of clinical trials on CCSVI treatment, both completed and ongoing, see Appendix 1 .
Catheter venography is considered the gold standard for determining the anatomical site, type and extent of lesions producing CCSVI. However, venography is invasive and cannot be used as a screening method. By contrast, ultrasound is an ideally suited, non-invasive method of screening and could prove to be a valuable diagnostic test when high sensitivity and specificity are demonstrated. In the presence of high sensitivity and specificity, venography will be needed only when it has already been decided to intervene.
Ultrasound in the form of duplex scanning uses a combination of physiological measurements as well as anatomical imaging and has been used for the detection of CCSVI by different centers with variable results. A high prevalence of obstructive lesions, ranging from 62% to 100%, has been found by some teams in patients with MS compared with a low prevalence of 0–25% in controls ( 1 – 7 ) . However, others have reported absence of such lesions ( 8 , 9 ) or a lower prevalence (16–52%) ( 10 – 12 ) . This variability could be the result of differences in technique, training, experience or criteria used.
In order to ensure a high reproducibility of duplex scanning with comparable accuracy between centers a detailed protocol with standard methodology and criteria is needed. Also, standardization of the method of reporting duplex measurements and other findings will facilitate validation of the proposed criteria. The aims of this document are to produce recommendations for such a protocol and to indicate what future research is needed in order to address areas of uncertainty.
Anatomy of the cerebrospinal venous system
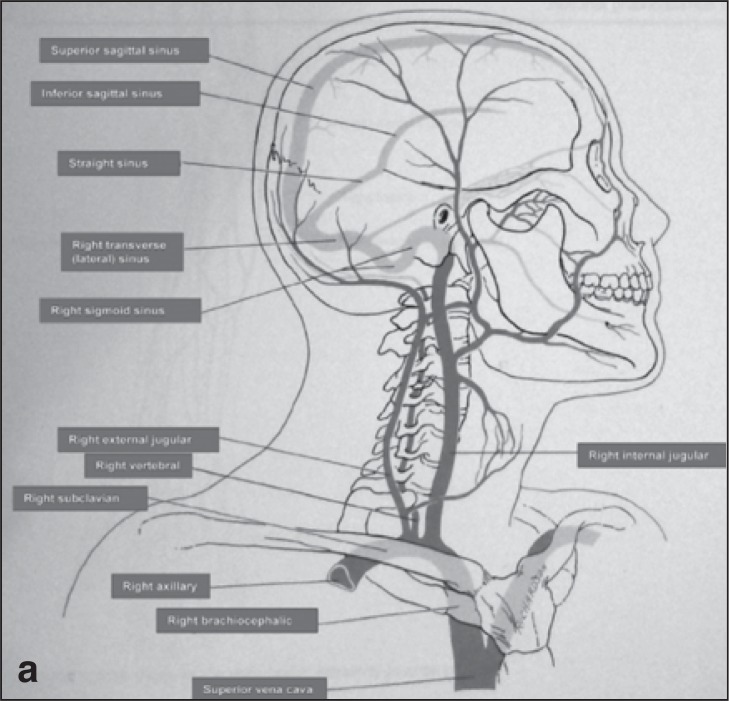
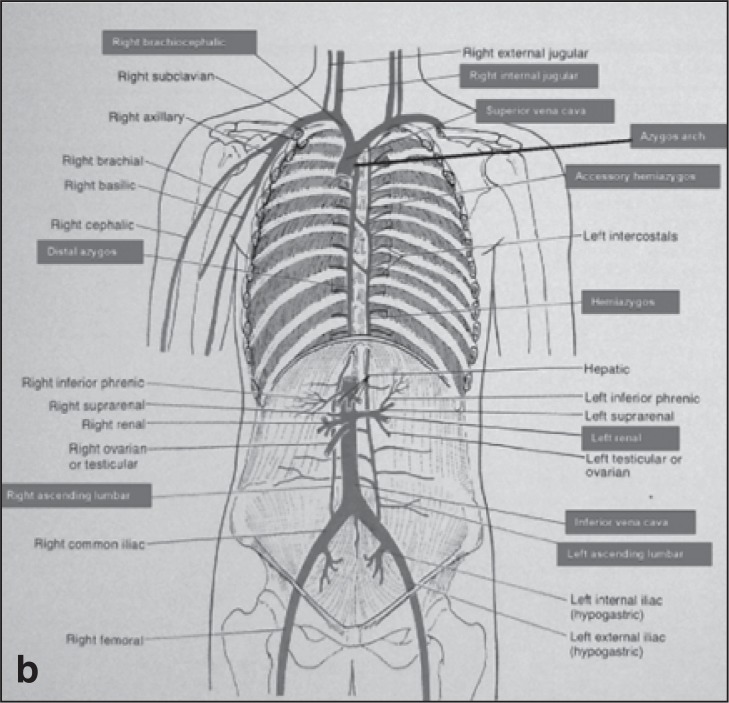
The cerebrospinal venous system is usually asymmetric and has a more variable vessel pattern than the arterial system. The intracranial part is mainly composed of parenchymal veins draining into the dural sinuses. Two main systems are responsible for blood collection, the superficial (cortical) system (blood reaches the dural sinuses by cortical veins and drains blood mainly from the cortex and part of the subcortical white matter), and the deep cerebral venous system, composed of internal cerebral veins, the basal vein of Rosenthal and the great cerebral vein of Galen and their tributaries. They drain the deep “periventricular” white and central gray matter “basal ganglia and thalamus” surrounding the lateral and third ventricles, the brainstem and anterior cerebellum which drains into the straight sinus ( 2 , 32 ) . Blood is collected by the dural sinuses and directed towards the main extracranial venous outflow routes: the IJV and the vertebral system. The IJV drains into the superior vena cava via the brachiocephalic vein ( Fig. 1 a,b ). The vertebral venous system is a valveless system stretching the length of the entire spinal column and it comprises three parts: the internal intraspinal part, the epidural veins, and the extraspinal paravertebral part. The extraspinal part in the neck consists of the vertebral veins (VVs) which accompany the vertebral artery and drain into the innominate vein on the right and into the subclavian vein on the left ( Fig. 1a ). They are reported to be valveless but venographic studies have shown that valves may be present at the junction of the vertebral and subclavian veins. The rest of the vertebral venous system, which is a rich plexus, communicates with the deep thoracic and lumbar veins, intercostal veins, the azygos (AZ) and hemiazygos veins. The lumbar hemiazygos arch is connected with the left renal vein representing a major outflow route for shunting blood into the inferior vena cava. The AZ vein represents the final collector and drains into the superior vena cava with an outlet on the posterior aspect just one cm below the brachiocephalic trunks ( 16 ) ( Fig. 1b ).
Figures 1a and b .
The cerebrospinal veins are a highly complex system rich in anastomoses, extending from the intracranial and intravertebral veins to the neck and the chest, with collaterals also in the retroperitoneal veins.
Ultrasonographic anatomy of the cerebrospinal venous system
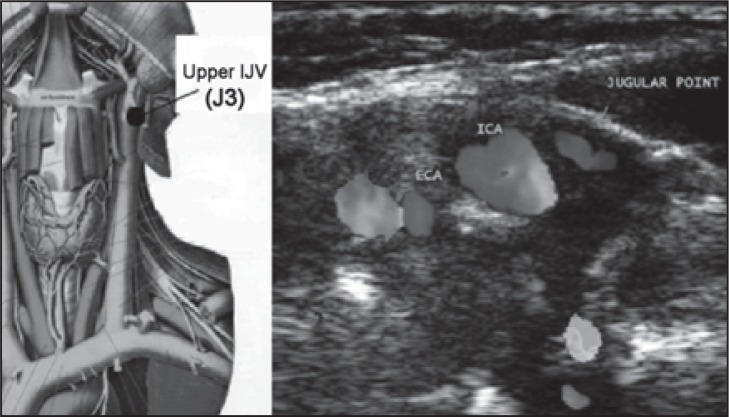
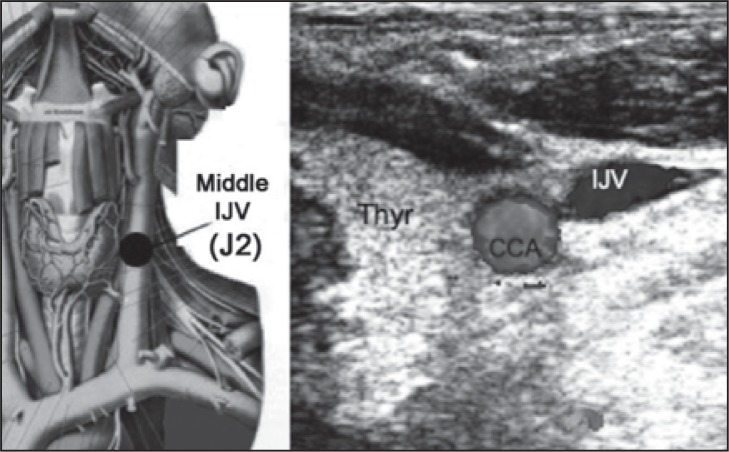
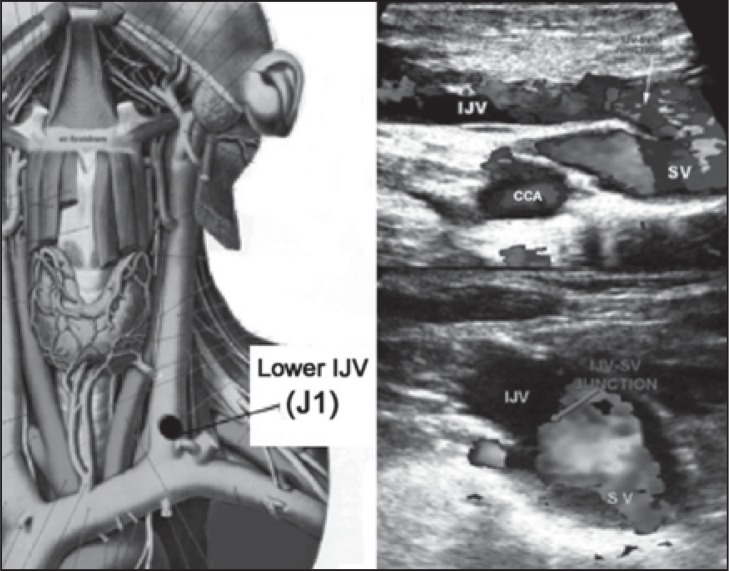
The IJV can be subdivided into three extracranial venous segments: upper (J3), middle (J2), and lower (J1) ( Fig. 2 ). The middle segment is adjacent to the thyroid gland ( Fig. 3 ), and is the segment between the entry of the common facial vein and the beginning of the last 2 cm of the IJV ( Fig. 4 , over). The IJV can be insonated easily at all three levels. The VVs can be easily insonated at all levels, but more easily between the transverse processes of the 5 th and 6 th cervical vertebrae.
Figure 2 .
Left: Upper IJV level (J3) in an anatomical plate modified from the Sobotta Atlas of Anatomy. Right: transverse scan at the upper (J3) level; ECA=external carotid artery; ICA=internal carotid artery. The jugular point corresponds to the position of the internal jugular vein.
Figure 3 .
Left: Middle IJV level (J2) in an anatomical plate modified from the Sobotta Atlas of Anatomy. Right: transverse scan at the middle (J2) level; CCA=common carotid artery; IJV=internal jugular vein; Thyr=thyroid gland.
Figure 4 .
Left: Lower IJV level (J1) in an anatomical plate modified from the Sobotta Atlas of Anatomy. Top right: longitudinal scan at the lower (J1) level; IJV=internal jugular vein; SV=subclavian vein; CCA=common carotid artery. Bottom right: image of the junction in the transverse plane. In both cases, the arrows indicate the junction.
Pathophysiology of the cerebrospinal venous system and CCSVI
Blood leaves the brain as a result of back propulsion of the residual arterial pressure ( vis a tergo ), complemented by a respiratory mechanism ( vis a fronte ) ( 32 , 33 ) . The latter is the thoracic pump which produces a negative intrathoracic pressure during inspiration increasing the aspiration of blood towards the right atrium. In addition to the vis a tergo and vis a fronte , changes in posture and gravity play a main role in ensuring correct cerebral venous return ( 32 – 38 ) .
In the horizontal position the predominant cerebral venous outflow is through the IJVs, whereas in the upright position the VVs become the predominant pathway. This has been demonstrated by angiographic studies and cerebral blood flow measurements using nitrous oxide, labeled erythrocytes and thermodilution techniques and more recently by volume flow measurements using duplex scanning ( 32 ) . In a study by Doepp et al. ( 37 ) a predominant non-jugular drainage pattern in the supine position was found in only 6% of healthy volunteers.
As defined above, CCSVI is characterized by stenoses of the IJVs and/or AZ vein which are shown by B-mode ultrasound to be mostly intraluminal defects ( 1 – 7 , 28 , 29 , 31 , 38 , 39 ) . These stenoses are associated with the development of collateral veins indicating insufficient drainage.
Perfusion of the brain parenchyma has been studied with MRI to assess cerebral blood flow, blood volume and the mean transit time from the arterial to the venous side of the cerebral circulation. In patients with MS, there is evidence that cerebral blood flow is reduced and the mean transit time increased, suggesting abnormalities at the microcirculation level ( 40 , 41 ) . A negative linear relationship between cerebral blood flow measured with MRI perfusion techniques and the Doppler venous hemodynamic insufficiency severity score (VHISS) has been demonstrated ( 41 ) ( Appendix II ). The latter has been used to assess the hemodynamic severity of CCSVI ( 42 , 43 ) . Thus, there appears to be a relationship between the severity of extracranial hemodynamic abnormalities and perfusion within the brain parenchyma ( 41 ) . CCSVI disturbs the normal postural and respiratory mechanisms of cerebral venous return resulting in an abnormal flow ( 1 – 7 ) . Thus, in CCSVI, the main extracranial cerebrospinal veins are obstructed, with venous flow being deviated into collaterals. The main collateral pathways activated are the condylar venous system, pterygoid plexus, and thyroid veins, anterior jugular veins and external jugular veins ( 2 ) .
Five main patterns of distribution of significant (>50%) venous stenoses have been found in large venographic series of patients with MS ( 1 , 31 ) .
Single jugular lesion (30–36%): a significant stenosis in one of the two IJVs with a compensatory enlargement of the contralateral IJV. No data is available concerning VV lesions ( 31 ) .
Double jugular lesion (14–56%): bilateral stenoses of IJVs, with normal AZ venous system.
Double lesion (23%) involving one of the IJVs and the proximal AZ vein.
Triple lesion (3–38%): significant stenosis of both IJVs and the proximal AZ vein.
Multilevel involvement of the AZ and lumbar venous system (18%). Stenosis of IJVs is observed in approximately half of these patients causing additional obstruction ( 1 ) .
A venographic grading system of obstructive lesions has been suggested by one group based on a series of 564 patients. Stage I consists of outflow delay without reflux towards the brain; stage II – outflow delay with mild reflux and/or prestenotic dilatation of the vein; stage III – outflow delay with reflux and outflow through collaterals; stage IV – no outflow through the vein with huge outflow through collaterals ( 31 ) .
Technique of duplex scanning for CCSVI
Equipment and presets
Ideally, a sophisticated duplex scanning system with a linear array broad bandwidth transducer suitable for imaging vessels, such as the carotid artery, should be used. Depending on the system and application, frequency ranges will vary (e.g. 7–5 MHz, 9–3 MHz, 10–5 MHz). When visualization of the lower IJV segment (J1) proves technically challenging, the use of a curvilinear/microconvex probe with a smaller footprint may be useful. For transcranial color Doppler (TCD) a phased array transducer should be used with a lower frequency bandwidth – for example 1.5–2 MHz or 2–3 MHz.
A large number of instrumental settings affect the B-mode, color and Doppler images on ultrasound. However, most manufacturers have chosen default values for these various settings that are pre-programmed for particular clinical applications. It is recommended that the system’s venous presets be activated initially; thereafter these starting values can be altered as required for each patient according to their individual pathology.
Venous velocities are much lower than arterial velocities, so for color Doppler imaging discrepancies are likely to occur. A number of technical considerations are listed below that should help achieve reproducibility.
Color flow
Pulse repetition frequency (PRF)
This is actually preset and it is related to the expected venous velocities. However, it may need to be lowered in order to detect lower flows or increased to avoid aliasing when the actual velocities appear higher than predicted. Newer systems may not have a PRF control but may be controlled by the color “scale”.
Steering angles
To ensure complete color filling of the vessel under examination (if indeed flow is present) one must optimize the color Doppler angle of insonation. This can be done (and generally is done in practice) by using a combination of probe angulation (particularly in transverse plane scanning) and color box steering. The purpose is to avoid an ultrasound beam perpendicular to the vessel which, in turn, would result in no Doppler signal. Therefore one must always steer the color box in a variety of directions in relation to the longitudinal planes of the B-mode image.
In transverse plane scanning the color box must remain at 0 with only probe angulation being used to create the necessary angles.
Color gain
It is universally accepted that arterial flow is conventionally displayed as red color and venous flow as blue. The gain control may need to be adjusted for the single clinical setting. If it is set too low there may be incomplete color filling of the vessel of interest; if it is set too high one will see color outside the vessel and there will be “noise” throughout the image.
Color wall filter
Color wall filter selection is important to optimize detection of low velocity.
Focal zones
Ideally the focal zone must be set to the region of interest. In color mode there normally exists one focal zone (due to frame rate issues) and this may be preset as the image center. The focal zone can be adjusted manually, therefore one should be prepared to alter it when appropriate.
Persistence/frame averaging
Most modern systems have automated frame averaging. For example, when velocities are low, persistence will be high, making pulsatile flow in veins harder to detect. It is important to keep the color box width and depth adjusted to the region of interest as this will result in the best/highest frame rate.
Spectral Doppler
Pulse repetition frequency (PRF)
This will be preset as per manufacturer’s values for a venous setting, but, as with color flow, clinical situations may arise that require the operator to alter these values in order to ensure an optimum Doppler spectral display. If the Doppler PRF setting is too high the slower-moving blood will not be displayed across the spectral display. Therefore one should set the Doppler PRF so that the resultant spectral waveform fills the display without aliasing the scale.
Focal depth
Normally, focal depth is set automatically to follow the sample volume. If it is not, it should be adjusted manually.
Positioning of sample volume and angle of insonation
When examining venous flow the sample volume must be positioned in the center of the vessel under examination. However, to include the full range of velocities, including all areas of reflux in the vein, the gate needs to be open completely to cover the entire lumen.
Ideally, the angle of Doppler beam insonation to the direction of flow should be 60° to keep absolute velocity measurement errors to a minimum. However, because this may be difficult to achieve in the IJV and VVs without applying pressure on the skin when changing the angle of the probe, reducing the angle to 45° is acceptable. The angle must never exceed 60°. Irrespective of the width of the gate, cursor in the middle of the Doppler gate should be positioned in the center of the vessel and parallel to the flow axis.
Patient positioning and technical aspects of ultrasound examination
The CCSVI examination should be performed with the patient in both supine and sitting positions and breathing normally, starting the examination in the supine position. A tilting chair is advisable in order to avoid muscular contractions when changing position. As previously mentioned, each of these positions is associated with a different outflow route ( 32 , 34 , 35 , 44 ) . The patient has to be comfortably positioned on an electro-mechanical chair or a standard examination table. After changing position, an adaptation period of at least 2 minutes should be allowed before any further measurements are made. More than one posture change, from supine to sitting and vice versa, should be avoided, so as not to perturb the distribution of blood volume. A sufficient level of hydration within the twelve hours before the examination must be assured.
We recommend that the subjects breathe normally and where possible through the nose. This allows activation of the thoracic pump without undesirable muscle contraction in the neck. Also, it reduces contraction of the thoracic muscles thereby reducing artifacts, especially when examining the lower segment of the IJV (J1). The pump activation allows the assessment of flow direction. The cross-sectional area (CSA) and the flow direction are measured at the end of the expiratory phase, ensuring that there has been activation of the thoracic pump. The examiner should pay attention to the inclination of the patient’s neck and employ appropriate means of neck support to avoid neck flexion, hyperextension or rotation to the left or right. This will avoid erroneous measurements of the CSA and allow better visualization of the IJV when the patient is in the sitting position.
The operator should use appropriate arm support (especially when the patient is in the sitting position) in order to avoid arm, hand, wrist, back or shoulder strain. A pillow across the patient’s chest, where the operator can rest his/her elbow whilst scanning, will provide good support. The problem of shoulder and arm fatigue is particularly significant when the examination is performed with the patient in the sitting position.
Use a large amount of ultrasonic gel to ensure complete coupling between the transducer and the patient’s skin, thereby avoiding black cones and dark areas on the image. Also, a thick layer of ultrasonic gel avoids excessive pressure on the patient’s neck that may change the shape and dimension of the IJV. Practical maneuvers that may help control the pressure of the transducer against the skin include placing the ring or little finger on the thyroid cartilage in order to ensure better control of applied pressure.
How to perform CCSVI examination of the internal jugular vein (supine and sitting positions)
Investigation of cerebrospinal venous return must be performed with the patient really at rest. In other words, the patient, who may use the deep breathing technique, needs to be calm, not agitated, and with a normal heart rate. The evaluation begins with the patient in the supine position.
B-mode evaluation
With the linear array transducer in the transverse position (with respect to the IJV itself), perform a B-mode evaluation of the IJV from the base of the neck to the angle of the jaw. Although routine use of the Valsalva maneuver is not recommended for CCSVI evaluation, on occasions it can help to visualize the IJV. This is particularly true with the patient in the sitting position (in which the CSA is normally at its smallest), or in situations in which the IJV is completely collapsed as a result of the CCSVI. If a lumen can be opened by the Valsalva maneuver, this means that the lumen is normal. If a lumen cannot be opened by Valsalva this could indicate hypoplasia or agenesis of the IJV.
The Committee recommends performing the Valsalva maneuver at the end of the examination to check for agenesis, especially if no flow was noted in the upright position. Performing Valsalva at the end of the examination avoids redistribution of blood volume, which may affect the entire investigation.
After the evaluation in the transverse plane, the IJV must be completely evaluated in the longitudinal plane.
Valve abnormalities on B-mode
A valve is usually present at the termination of the IJV just before its junction with the subclavian vein. Valves in the lower portion of the IJVs (J1) have been found in 93% of post-mortem studies and in 87% of normal individuals ( 45 ) . Valves were not present in other segments. When a valve is detected it should be evaluated in both the transverse and the longitudinal planes. The cusps of a normal valve should be oriented in the direction of the blood flow and they should move freely with respect to the respiration phases. When the leaflets are open they should be parallel and close to the jugular wall.
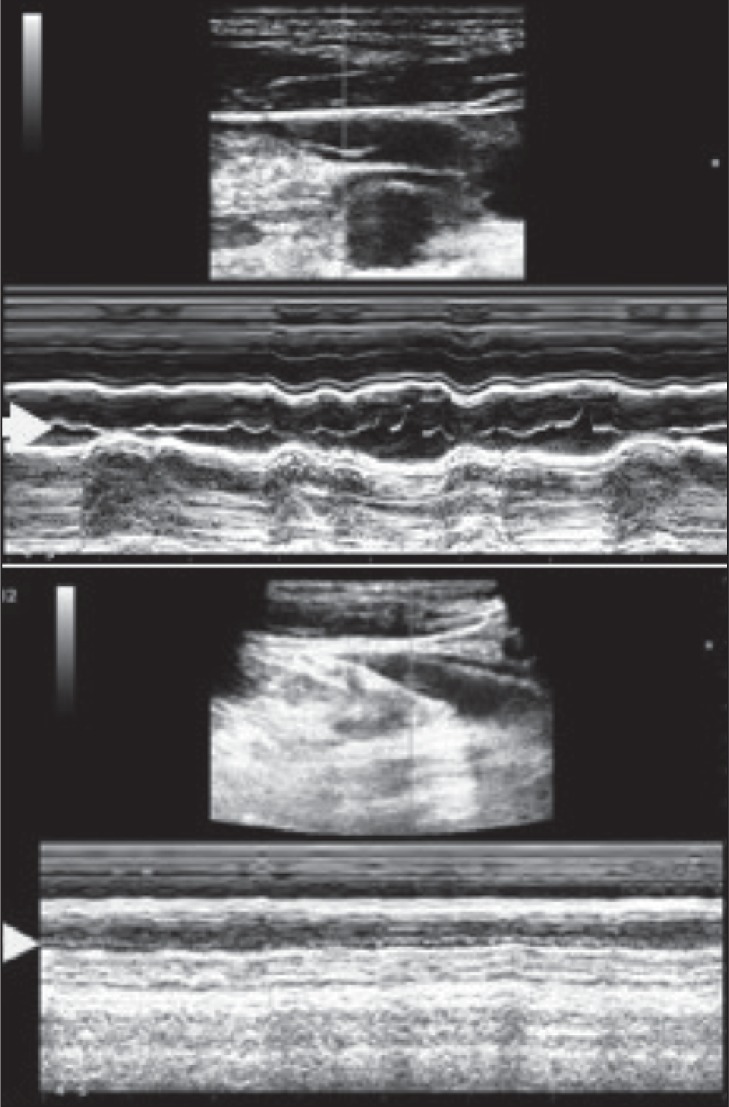
The major anomalies that can be found at the level of the IJV valve are: flap, septum, annulus, immobile leaflets, immobility limited to one of the two leaflets, double channels, anomalous orientation of the valve leaflets (e.g. inverted position of the leaflets, leaflets positioned on the lateral side of the jugular wall) ( 1 – 7 , 30 , 31 , 39 ) . Similarly to cardiac valve evaluation by echo-cardiography and/or in the deep venous system of the lower extremity ( 46 , 47 ) , mobility of the valve cusps can be further documented by using the M-mode function, as shown in figure 5 .
Figure 5 .
Top: M-mode evaluation of the jugular valve showing mobility of the leaflets as indicated by the arrow. Bottom: immobility of valve leaflets demonstrated in M-mode (arrow).
Artifacts on B-mode
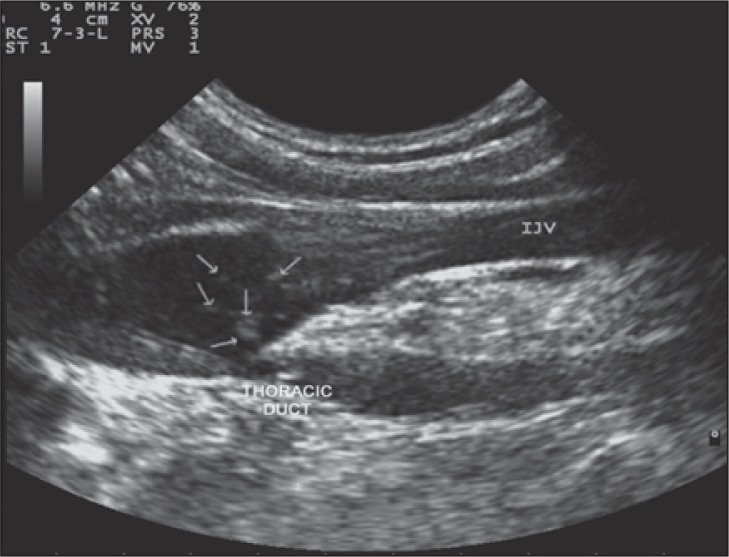
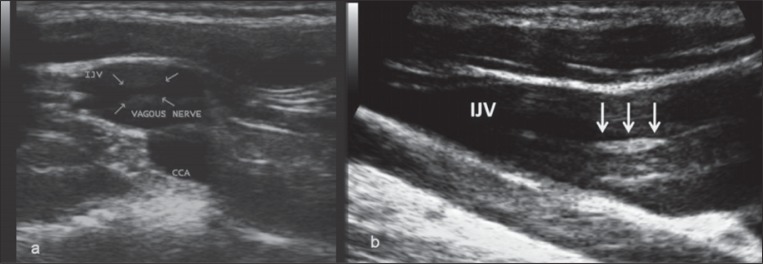
Two major artifacts can be found during the B-mode examination of the IJV. They are believed to be produced by lymph and by the vagus nerve. The thoracic duct, especially after food intake, creates streams of lymph which are seen as hyperechoic filaments within the vessel lumen ( Fig. 6 ). These filaments should not be mistaken for venous valve cusps because they do not show the periodically repeated movements typical of a valve cusp. The vagus nerve is located posterior to the IJV, but on B-mode imaging it may produce an artifact in the middle of the IJV. The vagus nerve may appear as parallel linear echoes within the IJV, which can be misinterpreted as an intraluminal defect ( Fig. 7a ). This artifact may be avoided by angling the probe away from the vagus nerve. Alternatively, this artifact can be provoked by positioning the probe in the longitudinal position, in which the nerve becomes visible on ultrasound because of longitudinal hyperechoic thickening of the neural guaina and fibers. In figure 7a and b the echoes emitted by the transducer reflect a false image of the vagus nerve appearing inside the IJV.
Figure 6 .
B-mode anomaly artifact created by the presence of streams of lymph within the venous blood flow of the IJV.
Figure 7a .
B-mode artifact created by the vagus nerve, in the transverse view at the lower IJV level (J1). The arrows indicate the hyperechoic thickening of the neural guaina and fibers. Figure 7b - Mirror artifact, in the longitudinal aspect of the IJV.
Another artifact, called the mirror artifact, is due to high acoustic reflection of the front wall of the vein. When the sound beam hits a strong interface (such as the anterior wall of the IJV) at an angle other than 90°, it is re-routed. This change in the sound beam course means that it takes longer to return to the machine, which results in the object appearing deeper than it truly is. In the figure, some of the echoes emitted by the transducer reflect a false image of the anterior wall of the vein, which appears as an intraluminal structure ( Fig. 7b ).
Hemodynamic evaluation
Hemodynamic evaluation starts from the lower end of the middle segment of the IJV (J2) which is imaged in transverse view in the color mode. It is suggested that one should follow the IJV upwards to J3, observing the flow direction by means of color mode, asking the patient to breathe quietly through the nose. Any irregularities that may appear (absence of flow, turbulences, reflux) in the color mode ( Fig. 8 ), will require verification by longitudinal imaging ( Fig. 9 , over) in order to study the flow direction and duration of reflux/bidirectional flow by means of Doppler spectral analysis recordings ( Fig. 10 a–c, over) and/or spectral analysis using multi-angle Doppler ( Fig. 11 , over).
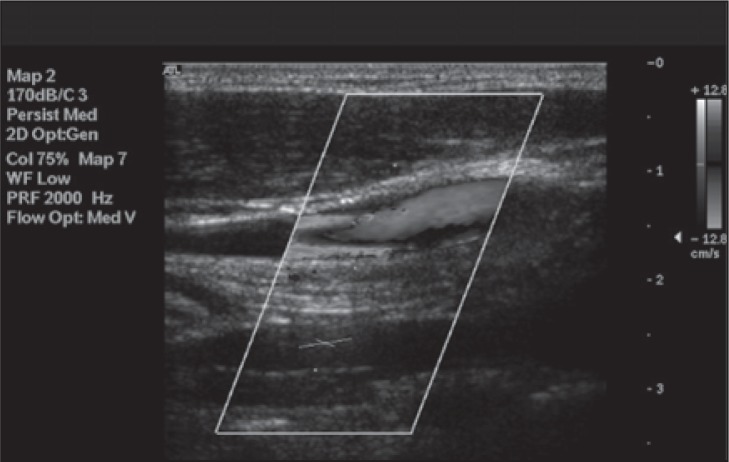
Figure 8 .
Bidirectional flow in the IJV seen in the transverse view needs to be confirmed by longitudinal scans and Doppler waveform recordings ( Fig.s 10–12 ).
Figure 9 .
Bidirectional flow in the IJV seen in longitudinal view. Red color indicates reflux.
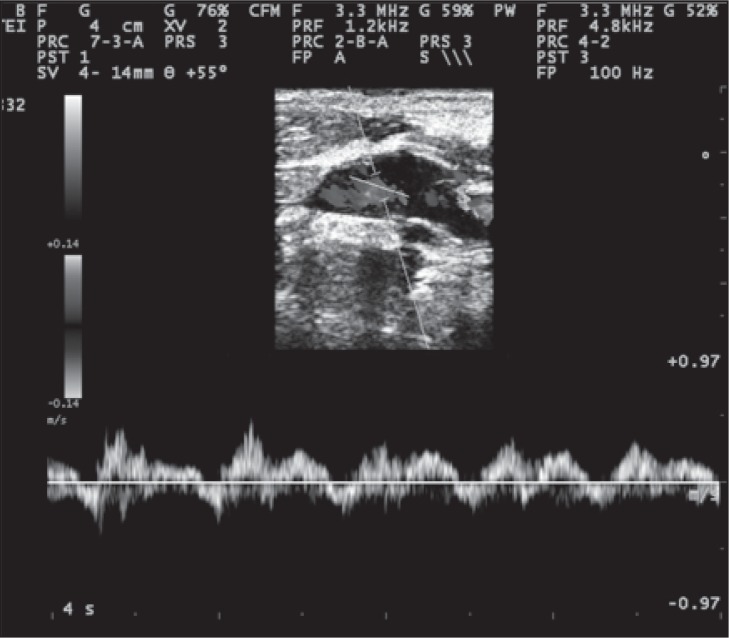
Figure 10 .
Bidirectional Doppler velocities in the IJV recorded with a large sample volume.
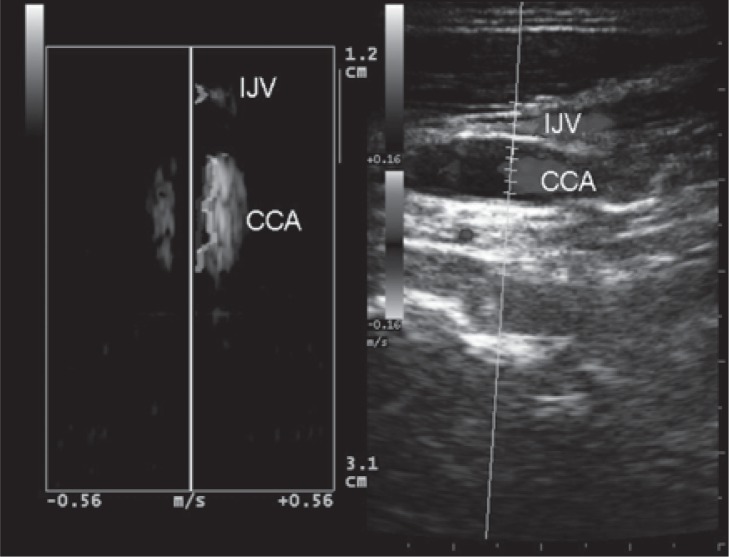
Figure 11 .
A longitudinal image showing a long-lasting reflux. Multi-angle Doppler makes it possible to record the direction of flow in the internal jugular vein (IJV) and in the common carotid artery (CCA) simultaneously.
In the longitudinal plane, careful attention should be paid to the steering of the color flow box, to guarantee the angle of insonation in relation to the vessel ( Fig.s 9 and 10 , over). Although 0.88 sec has been suggested as the cutoff value for reflux through the jugular valve ( 48 ) , this value was originally recorded under Valsalva at J1. By contrast, reflux associated with CCSVI is elicited by activation of the thoracic pump and may last for several seconds ( 1 , 49 ) . Reflux or absence of flow may occur and can be detected in any segment of the IJV. Flow recording of both abnormalities is particularly significant at the end of the expiratory phase; flow evaluation does not require the Valsalva maneuver at all. Recording of flow abnormalities should be made with the Doppler sample gate open wide ( Fig. 10 ). Our suggestion is to insonate the lower part of the IJV (J1) last, after careful evaluation of the J2 and J3 segments.
At J1 level, turbulence and short time reflux (<0.88 sec) should be considered normal because they are the physiological expression of flow close to valve cusps. This reflux is usually confined around the leaflets, and can be increased by respiration. If this reflux can also be seen in the IJV above the origin of the widening (bulb), this has to be considered a pathological reflux ( Fig. 9 ). The optimum cut-off point for the duration of reflux is under review and needs to be established. The value of >0.88 sec for IJV reflux/bidirectional flow has been defined and used in prior studies for Criterion 1. In order to determine the optimum cut-off point for the duration of reflux associated with CCSVI, it is recommended that, in prospective observational studies in which patients undergo venography, recordings be made as shown in figure 10 , together with measurements of duration of reflux. The normal Doppler blood velocity in the IJV (lying or sitting) has been quoted as less than 70 cm/sec ( 8 ) . Since a very high velocity in one IJV ( Fig. 12 ) can point to a stenotic lesion, in such cases it is necessary to conduct a careful assessment for such a lesion, if this was not identified on the initial B-mode evaluation. Although this is not a criterion for CCSVI, it can be a helpful tool. Further research is required, and more data, in order to determine optimum velocity cut-off points. For this reason, it is recommended that in prospective observational studies including normal individuals and patients with MS, velocities be measured in all segments of the IJV (see reporting form in Appendix III ).
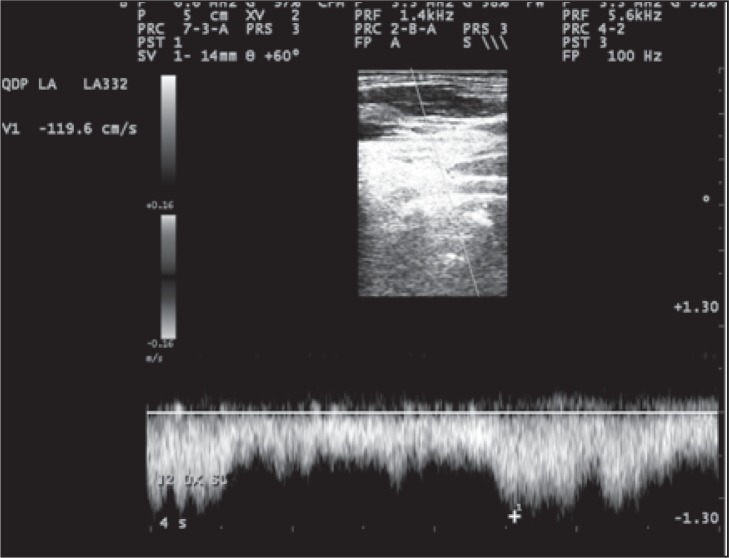
Figure 12 .
Example of increased flow velocity up to 119 cm/sec in the IJV, and absence of reflux. This hemodynamic pattern is not a CCSVI criterion per se, but is highly suggestive of stenosis.
In a large number of cases, no flow can be detected within the IJV, which may suggest the presence of severe obstruction. There are two main possibilities: i) flow is not detected because the IJV is almost completely collapsed and cannot be distended by a Valsalva maneuver (this is compatible with a hypoplastic IJV); ii) flow is not detected (the velocity is too low to be detected even at very low PRF) but the lumen of the IJV is clearly visible. This is compatible with absent flow in a filled IJV.
However, absence of flow in color Doppler mode should always be confirmed by Doppler spectral analysis and absence of thrombosis by compression ( Fig. 13 ). Sometimes, especially when the Doppler sample is placed above the septum/immobile cusps, the spectrum may show very low velocities related to little intraluminal movements and velocity cannot be increased by thoracic pump activation.
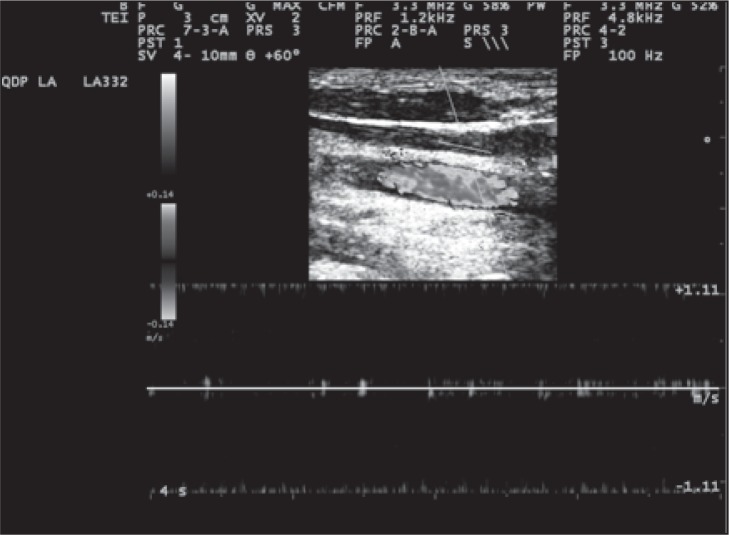
Figure 13 .
Example of absence of detectable flow in longitudinal scan of an IJV in supine position, and PRF is 1.2 kHz.
An IJV blood flow “block” (despite deep inspirations) must be considered a CCSVI criterion if no flow can be detected within an IJV either in the supine or in the sitting position. Alternatively, reflux within a jugular is considered a positive criterion if coupled with absence of flow in the other position, or vice versa.
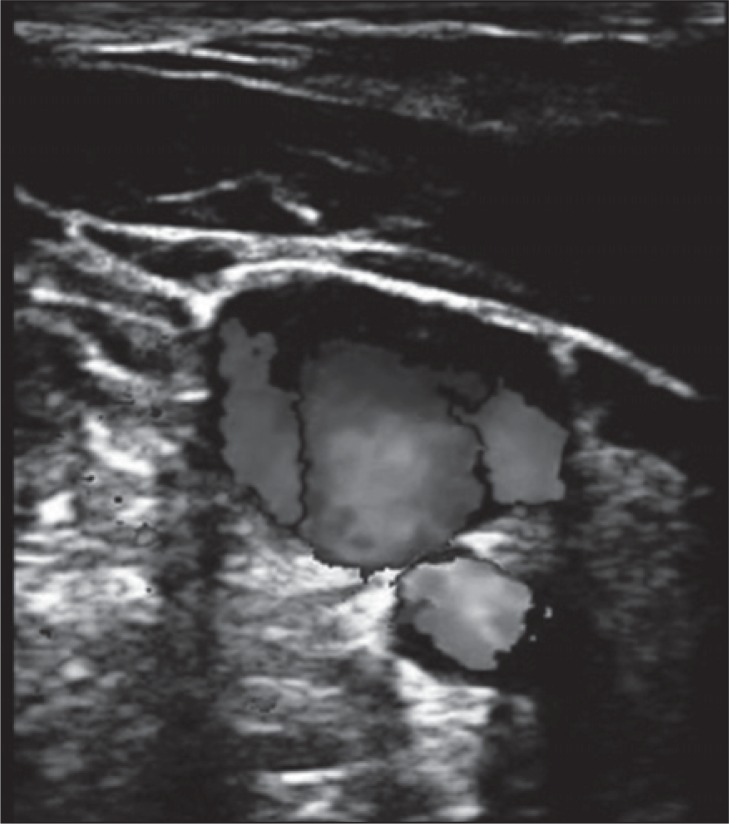
Stenosis in the lower IJV may activate collateral circulation. Collateral circulation may be observed at the levels of the IJV’s junctions with the anterior branch of the retromandibular vein, the common facial vein and the lingual vein, or commonly, at the level of the superior thyroid vein ( Fig. 14 ).
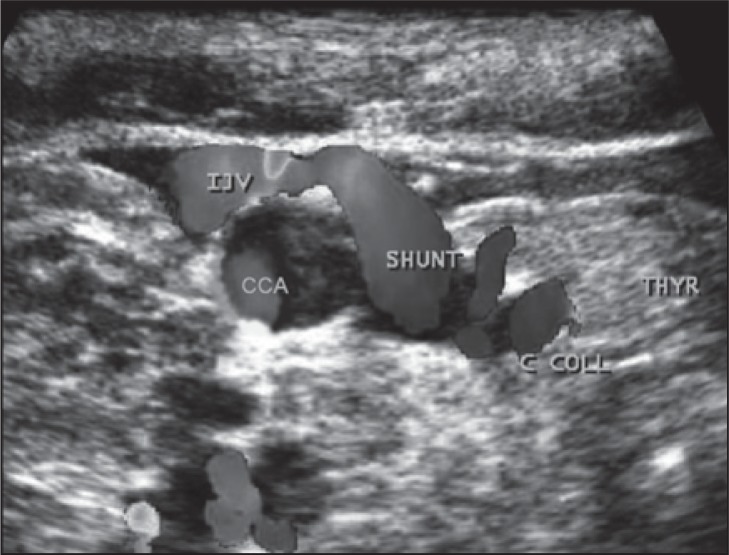
Figure 14 .
Blood from the IJV is shunted into the thyroid vein, a collateral circulation activated by the presence of obstruction at the lower IJV (J1). Note the enlarged veins in the thyroid gland. CCA=common carotid artery; C COLL= collateral circle.
Absence of flow in the upper IJV may also activate collateral circulation; one of the more common examples is that of the shunting flow from the IJV to the superior thyroid vein. The shunt runs through the thyroid gland and re-enters the superior vena cava system through the inferior thyroid vein ( Fig. 14 ).
Measurements of the cross-sectional area (csa)
Measurements of the CSA must be performed in transverse B-mode scans of the middle segment (J2) using the appropriate ellipsoid area measuring tool ( Fig. 15 ). Measurement can be performed with or without activation of the color mode. If the color mode is used, the color gain should be adjusted so that the color does not obscure the vessel wall.
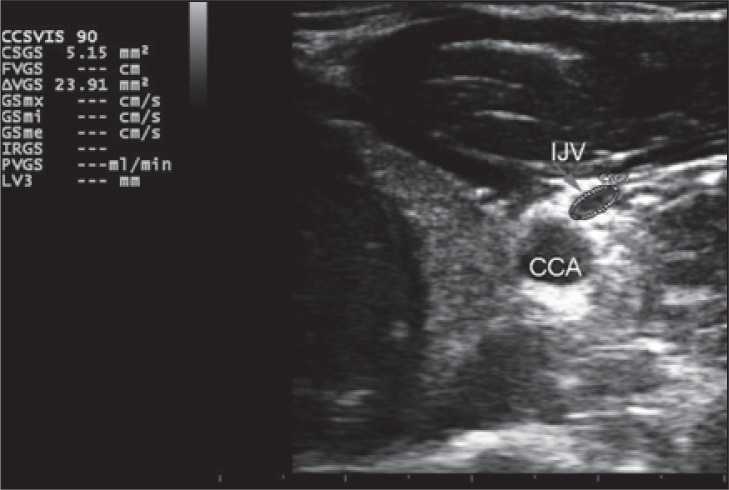
Figure 15 .
Top: Example of cross-sectional area (CSA) measurement of the IJV in supine position. Bottom: Example of CSA measurement of the IJV in sitting position and relative calculation of ΔCSA. In healthy individuals the CSA in the supine position is greater than in the upright position. CSGS=cross sectional jugular area in sitting position; CCA=common carotid artery.
The measurement must be performed at the same point in both supine and sitting positions, initially in the supine position then in the sitting position. The middle section (J2) of the IJV can be identified through the relationship with the thyroid gland or by marking the measurement point on the skin. The transducer must be kept almost perpendicular to the patient’s neck in order to produce a “perfect” transverse section of the IJV (a certain inclination with respect to the 90° angle is still needed in order to get a sufficient color Doppler signal). The perfect circular shape of the common carotid artery can be used as a reference for the correct positioning.
How to perform CCSVI examination of vertebral veins (VVs)
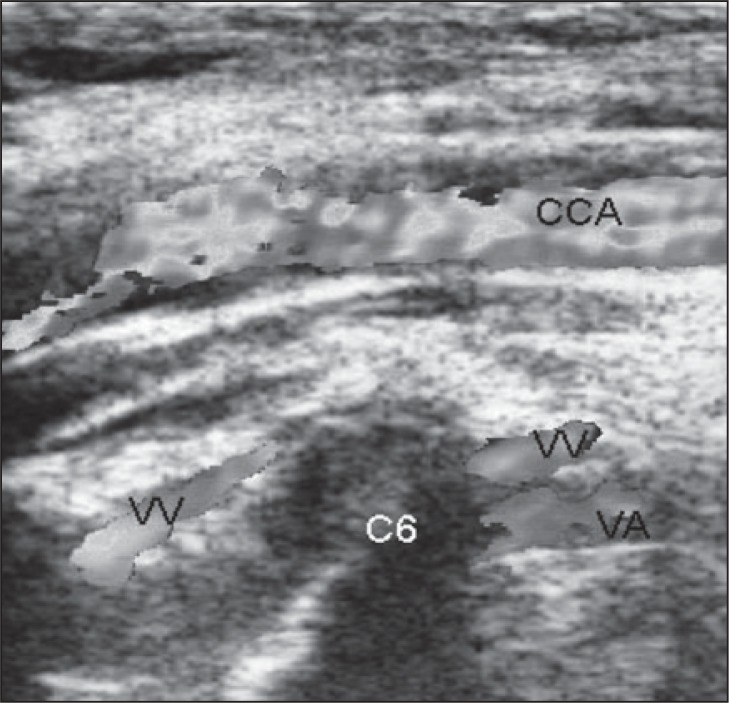
The VVs must be evaluated with the transducer positioned longitudinally. When the patient is in the supine position, the blood flow within the VVs is slower and a lower color Doppler PRF and lower wall filter is needed (with respect to the color Doppler examination of the IJV). Visualization of the VVs is not easy in B-mode and therefore a color Doppler examination should be used from the beginning. The VVs must be examined in visible segments (the easiest segment to examine is between C5 and C6) ( Fig. 16 , over).
Figure 16 .
Example of color Doppler vertebral vein assessment. CCA=common carotid artery; VV=vertebral vein; VA=vertebral artery.
Reflux within VVs is usually activated during the expiratory phase of the respiratory cycle. Reflux within VVs is represented by a complete reversal of blood flow direction lasting more than one second. A Doppler spectral waveform should be recorded. A continuous reversed flow may sometimes be seen (opposite to the direction of physiological flow). This reversed flow, which is also considered abnormal (reflux), may be the result of activation of collaterals connecting extravertebral with intravertebral veins.
Reflux within VVs must be considered a positive CCSVI criterion if it is present in the same VV both in the supine and in the sitting position. Alternatively, a positive criterion can be assigned if reflux in one position is associated with absence of flow in the other position.
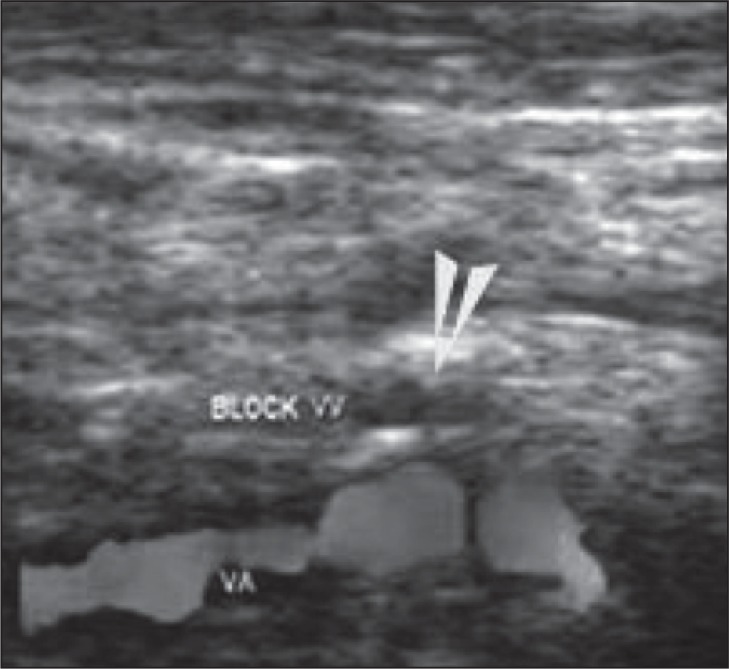
A VV blood flow “block” (despite deep inspirations and low PRF) ( Fig. 17 ) must also be considered a positive CCSVI criterion if no flow can be detected in a VV both in the supine and in the sitting position. VV reflux and/or “block” must be assessed in the same segment at the end of the respiratory phase.
Figure 17 .
Example of a “blocked” vertebral vein (VV), confirmed by Doppler spectrum analysis (indicated by absence of flow at the arrow). VV=vertebral vein; VA=vertebral artery.
CCSVI examination of the intracranial veins
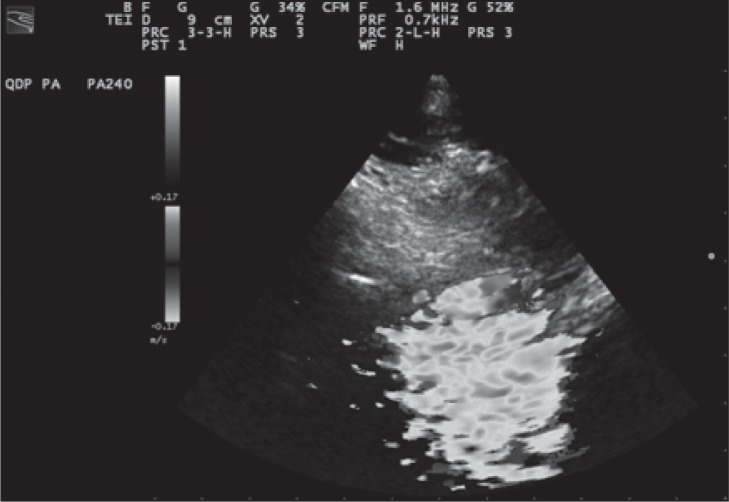
The presence of reflux in the petrosal sinuses detected through the supracondylar window has been proposed as a diagnostic criterion for CCSVI. Because insonation of the petrosal sinuses can be achieved with the Doppler angle close to 90°, detection of reflux in the form of bidirectional flow can be achieved only using a multi-angle Doppler system, such as quality Doppler processing technology (QDP, Esaote Biomedica, Genoa, Italy). QDP enables the operator to identify the blood flow direction in the examined cerebral veins: a proper adjustment of the PRF is necessary in order to visualize the direction clearly, without background Doppler noise ( Fig.18 a , b ).
Figure 18a .
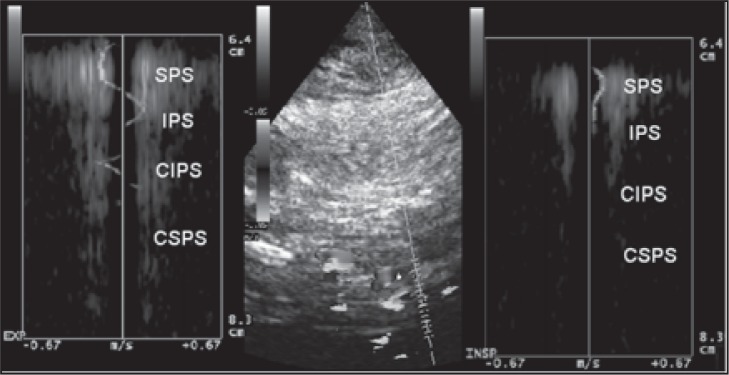
Example of reflux: the blood flow at the level of the superior petrosal sinus (SPS) shows opposite directions between inspiration and expiration; this finding shows the presence of reversed blood flow within the examined vessel between the two phases of respiration. The blood flow direction of the inferior petrosal sinus (IPS) is not detectable during expiration. The blood flow direction of the contralateral inferior petrosal sinus (CIPS) is not detectable during inspiration or expiration.
Figure 18b .
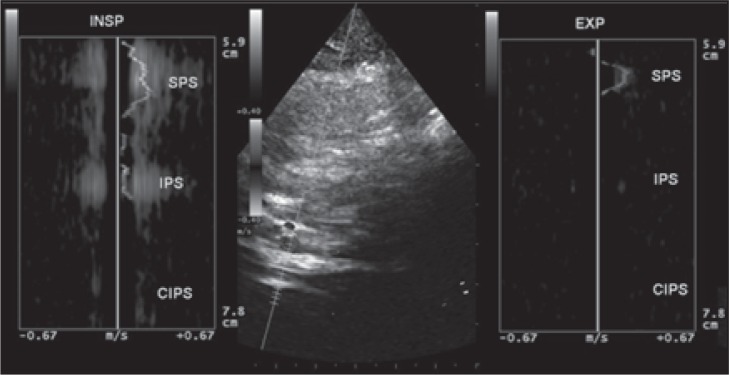
Example of no reflux: the blood flow at the level of the superior petrosal sinus (SPS) shows the same direction between inspiration and expiration; the blood flow direction of the inferior petrosal sinus (IPS) is not detectable during expiration; the blood flow direction of the contralateral inferior petrosal sinus (CIPS) is not detectable during inspiration or expiration.
Because QDP technology is not available on all ultrasound scanning systems, and because more data are needed to define the contribution of intracerebral reflux to the diagnosis of CCSVI, it is not currently recommended as part of the routine procedure. For details see Appendix IV .
ULTRASONOGRAPHIC CRITERIA FOR CCSVI
Introduction
Five criteria for CCSVI were previously described ( 1 – 11 ) and recommended for the diagnosis of CCSVI. The chart below compares the published criteria with the criteria recommended by the Committee in 2011.
| PUBLISHED CRITERIA | 2011 CRITERIA |
|---|---|
| 1. Reflux present in an outflow pathway (IJV and/or VV) with the head at 0° and 90° | 1a. Bidirectional flow in one or both of the IJVs in both positions or bidirectional flow in one position with absence of flow in the other position and/or |
| b. Reversal of flow or bidirectional flow in one or both VVs in both positions. | |
| 2. Reflux in the intracranial veins/deep cerebral veins | The Committee recommends that this criterion be used as an additional criterion (see Appendix V) and defined as bidirectional flow in the intracranial veins and sinuses. |
| 3. High resolution B-mode evidence of proximal IJV stenosis and/or other B-mode anomalies | 3a. Reduction of IJV CSA in supine position to ≤0.3 cm 2 which does not increase with Valsalva maneuver (performed at the end of the examination) and/or |
| b. Intraluminal defects such as flaps, septa or malformed valves combined with hemodynamic changes (block, reflux, increased blood flow velocity). Valve leaflet/s immobility confirmed by M-mode. | |
| 4. Flow not Doppler detectable in the IJVs and/or VVs despite numerous deep inspirations |
4a. Absence of detectable flow in the IJVs and/or VVs, despite numerous deep inspirations, in both sitting and upright positions or |
| b. Absence of detectable flow in IJVs and/or VVs in one position, despite numerous deep inspirations, and bidirectional flow detected in the other position, same side. | |
| 5. Negative ΔCSA in the IJV | 5a. CSA of the IJV is greater in the sitting position than in the lying position or |
| b. appears almost unchanged despite change in position. |
All measurements should be performed on both sides, both in the sitting and in the lying positions. Criteria 1 and 4 are positive only if present in both positions.
The above criteria have been developed empirically, based on the physiology of cerebral venous return, so that the presence of two or more of these would ensure a very high sensitivity.
Table 2 summarizes the prevalence of each of these criteria in patients with MS and in healthy controls based on eight published series. Criterion 3 (detection of stenoses and/or intraluminal defects such as septa and fixed valve leaflets) appeared to show the highest prevalence (67% of MS patients and 18% of healthy controls). Criterion 1 (presence of reflux in the IJVs in both positions and/or presence of reflux in one position and absence of flow in the other) was the second most prevalent criterion (60% of MS patients vs 9% of healthy controls). The prevalence rates of criteria 2, 4 and 5 were much lower, 36%, 26% and 31% respectively. The presence of two or more criteria was found in 73% of the patients with MS and in 8.5% of the controls.
Table 2 .
The prevalence of each of the five criteria in patients with multiple sclerosis and in healthy controls, based on eight published series
| Criteria | References | MS N = 1329 | HC N = 455 | Total MS | Total HC | *OR (95% CI) p |
|---|---|---|---|---|---|---|
| 1. Reflux in IJV and/or VV in supine and upright position OR Reflux in one of the two positions and absence of flow in the other. | Zamboni ( 38 ) | 76/109 | 0/177 | 798/1329 60% | 40/455 9% | 15.6 ( 11 – 22 ) < 0.0001 |
| Al-Omari ( 5 ) | 18/25 | 7/25 | ||||
| Simka ( 6 ) | 31/70 | np | ||||
| Zivadinov ( 4 ) | 130/289 | 33/163 | ||||
| Doepp ( 8 ) | 0/56 | 0/20 | ||||
| Baracchini ( 11 ) | 12/50 | 0/50 | ||||
| Mayer ( 9 ) | 0/20 | 0/20 | ||||
| Bastianello ( 7 ) | 531/710 | np | ||||
|
| ||||||
| 2. Reflux in the intracranial veins | Zamboni ( 38 ) | 55/109 | 0/ | 478/1329 36% | 15/455 3% | 17.1 ( 10 – 29 ) < 0.0001 |
| Al-Omari ( 5 ) | np | np | ||||
| Simka ( 6 ) | np | np | ||||
| Zivadinov ( 4 ) | 104/222 | 15/118 | ||||
| Doepp ( 8 ) | 1/56 | 0/20 | ||||
| Baracchini ( 11 ) | 0/50 | 0/50 | ||||
| Mayer ( 9 ) | 0/20 | 0/20 | ||||
| Bastianello ( 7 ) | 318/710 | np | ||||
|
| ||||||
| 3. High resolution B-mode evidence of proximal IJV stenoses and/or intraluminal defect | Zamboni ( 38 ) | 30/109 | 1/177 | 889/1329 67% | 80/455 17.5% | 9.5 ( 7 – 12 ) < 0.0001 |
| Al-Omari ( 5 ) | 23/25 | 0/25 | ||||
| Simka ( 6 ) | 61/70 | np | ||||
| Zivadinov ( 4 ) | 185/289 | 63/163 | ||||
| Doepp ( 8 ) | 0/56 | 0/20 | ||||
| Baracchini ( 11 ) | 8/50 | 0/50 | ||||
| Mayer ( 9 ) | 13/20 | 16/20 | ||||
| Bastianello ( 7 ) | 569/710 | np | ||||
|
| ||||||
| 4. Flow not Doppler detectable in IJV and/or VV in both supine and upright positions. | Zamboni ( 38 ) | 35/109 | 1/177 | 351/1329 26% | 16/455 3.5% | 9.8 ( 6 – 16 ) < 0.0001 |
| Al-Omari ( 5 ) | 0/25 | 0/25 | ||||
| Simka ( 6 ) | 37/70 | np | ||||
| Zivadinov ( 4 ) | 30/289 | 12/163 | ||||
| Doepp ( 8 ) | 5/56 | 1/20 | ||||
| Baracchini ( 11 ) | 3/50 | 1/50 | ||||
| Mayer ( 9 ) | 0/20 | 1/20 | ||||
| Bastianello ( 7 ) | 241/710 | np | ||||
|
| ||||||
| 5. CSA of the IJV in upright position > CSA supine | Zamboni ( 38 ) | 61/109 | 21/177 | 408/1329 31% | 65/455 14% | 2.7 ( 2 – 4 ) < 0.0001 |
| Al-Omari ( 5 ) | 4/25 | 0/25 | ||||
| Simka ( 6 ) | 28/70 | np | ||||
| Zivadinov ( 4 ) | 33/289 | 11/163 | ||||
| Doepp ( 8 ) | ||||||
| Baracchini ( 11 ) | 8/50 | 3/50 | ||||
| Mayer ( 9 ) | 0/20 | 0/20 | ||||
| Bastianello ( 7 ) | 270/710 | np | ||||
|
| ||||||
| ≥ 2 POSITIVE CRITERIA | Zamboni ( 38 ) | 109/109 | 0/177 | 974/1329 73% | 39/455 8.5% | 29 ( 20 – 41 ) < 0.0001 |
| Al-Omari ( 5 ) | 21/25 | 0/25 | ||||
| Simka ( 6 ) | 63/70 | np | ||||
| Zivadinov ( 4 ) | 162/289 | 37/163 | ||||
| Doepp ( 8 ) | 0/56 | 0/20 | ||||
| Baracchini ( 11 ) | 8/50 | 1/50 | ||||
| Mayer ( 9 ) | 0/20 | 1/20 | ||||
| Bastianello ( 7 ) | 611/710 | np | ||||
Abbreviations: IJV=internal jugular vein; VV=vertebral vein; CSA=cross-sectional area; MS=multiple sclerosis; HC=healthy controls
Two-tailed Fisher’s exact test followed by OR (95% CI) was adopted because all the authors stated that they had used the same ultrasonographic criteria (Zamboni criteria).
Four databases are available, that can be used to analyze the prevalence of all the possible combinations of two or more criteria present in patients with MS, namely those of Bastianello and of Zamboni presented at the Conference, and the publications of Mayer et al. ( 9 ) and Doepp et al. ( 8 ) ( Table 3 ). It can be seen that two criteria were present in 209 (23.3%) of the 895 patients, with 1+3 being the commonest combination followed by 1+5 and 3+4. Three or more criteria were present in 487 (54.3%) of the 897 patients.
Table 3 .
The prevalence of combinations of two or more criteria present in multiple sclerosis patients *
| Criteria and their combination | MS patients n = 895 | % |
|---|---|---|
| 1+2 | 19/895 | 2.1 |
| 1+3 | 81/895 | 9 |
| 1+4 | 13/895 | 1.4 |
| 1+5 | 21/895 | 2.3 |
| 2+3 | 27/895 | 3 |
| 2+4 | 9/895 | 1 |
| 2+5 | 8/895 | 0.9 |
| 3+4 | 20/895 | 2.2 |
| 3+5 | 4/895 | 0.4 |
| 4+5 | 7/895 | 0.7 |
| 1+2+3 | 110/895 | 12.3 |
| 1+2+4 | 9/895 | 1 |
| 1+2+5 | 22/895 | 2.4 |
| 1+3+4 | 42/895 | 4.7 |
| 1+3+5 | 83/895 | 9.3 |
| 1+4+5 | 7/895 | 0.7 |
| 2+3+4 | 7/895 | 0.7 |
| 2+3+5 | 4/895 | 0.4 |
| 2+4+5 | 6/895 | 0.6 |
| 3+4+5 | 11/895 | 1.2 |
| 1+2+3+4 | 33/895 | 3.6 |
| 1+2+3+5 | 44/895 | 4.9 |
| 1+3+4+5 | 61/895 | 6.8 |
| 1+2+4+5 | 8/895 | 0.9 |
| 2+3+4+5 | 6/895 | 0.6 |
| 1+2+3+4+5 | 34/895 | 3.8 |
To date, only two publications have compared ultrasound findings with venographic findings ( Table 1 ). In the presence of two criteria or more, the Ferrara group ( 1 ) found a 100% sensitivity, but the second group, performing venography in seven patients with MS and positive CCSVI criteria ( 11 ) , found no stenotic or obstructive lesions. Small numbers and variability in the way ultrasound examination was performed may explain this discrepancy. Routine venography in 49 patients with MS without prior ultrasound examination performed by the team at the American University of Beirut disclosed stenotic lesions in 7 (24%) of 29 patients with early MS and 12 (92%) of 13 patients with late MS ( 10 ) .
The available data do not make it possible to determine the value, in terms of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), of screening with ultrasound and its overall accuracy compared with the gold standard: venography. In particular, we do not know how many patients with CCSVI lesions are missed in the absence of any ultrasonic criteria, or in the presence of only one. The high prevalence of CCSVI lesions in patients with MS justifies performance of both ultrasound and venography in an adequate series of patients blindly. Ultrasound should be performed by a team that has completed its learning curve and venography should not be withheld in patients with normal ultrasound findings. Patients admitted to such a study should not be preselected on the basis of a previous positive ultrasound test. Such a study will provide information not only on sensitivity, specificity, PPV, NPV and overall accuracy of ultrasound, but also on the contribution of individual criteria and their association with different types of lesion. Pending the availability of such data the Committee suggests that the presence of any two of the revised criteria ( 1 , 3 , 4 , 5 ) described below in detail, be taken to indicate CCSVI.
Criterion 1: Reflux in the IJV and/or VV
A) Bidirectional flow in one or both of the IJVs in both positions or bidirectional flow in one position with absence of flow in the other position (see Criterion 3). These findings suggest IJV stenosis
B) Reversal or bidirectional flow in one or both VVs in both positions. These findings suggest stenosis in the azygos vein, based on reports in which the Doppler parameter was monitored in comparison with catheter venography ( 1 , 2 ) .
Criterion 3: IJV stenosis
A) Severe reduction of the CSA of the IJV in the supine position – less than 0.3 cm 2 – which does not increase with Valsalva maneuver (performed at the end of the examination).
B) Intraluminal defects such as webs, septa or malformed valves combined with hemodynamic changes (increased velocity, absence of flow, reflux/bidirectional flow, etc). M-mode investigation of leaflets may clarify whether they are mobile or not.
Criterion 4: Absence of detectable flow in the IJV and/or VV
Outflow obstruction in the cervical veins indicated by: (a) absence of Doppler signal in the IJV and/or the VV, even after deep inspiration, in both sitting and supine positions, or (b) in one position but with bidirectional flow detected in the other position. These findings are associated with stenosis proximal to the point of assessment.
Criterion 5. Abnormal change in the IJV CSA with change in position (change in hydrostatic pressure)
A CSA of the IJV which (a) is greater in the sitting position than in the lying position, or (b) appears almost unchanged despite change in position.
All measurements should be performed on both sides, and in both positions. Remember that reflux/bidirectional flow and absence of flow become diagnostic criteria only when demonstrated in the same cervical venous segment in both positions. However, absence of flow in one position (e.g. sitting) in the presence of reflux in the other position can be considered a positive criterion, provided they occur in the same vein. The presence of reflux or absence of flow in a single location in the same venous segment in only one position is not in itself a positive criterion. However, it contributes to the score if calculation of the venous hemodynamic insufficiency severity score (VHISS) is contemplated (see Appendix II ).
Criterion 2. Bidirectional flow (or reflux) in the intracranial veins and sinuses
This is potentially an additional criterion (see Appendix V for methodology).
Because not all authors have used the same transcranial approach and because the QDP system is not available on all equipment, the contribution of intracranial reflux is currently under debate. For these reasons, and pending more data, the routine use of this criterion is not currently recommended.
Based on the available evidence ( Table 3 ) omission of criterion 2 in patients with three or more criteria will still result in a positive diagnosis of CCSVI. Omission of criterion 2 in the patients with only 2 positive criteria would result in 63 (7%) of the 895 patients being characterized as having a negative finding ( 1 , 7 ) . Thus, if the QDP system is available, scanning the intracranial veins and using criterion 2 is a logical option when only one of the other criteria is present.
APPENDIX I.
Clinical Trials on CCSVI Treatment
Clinical trials on CCSVI treatment, completed or ongoing, are the following:
- A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency
-
Status: published ( 28 ) .This study evaluated the safety of CCSVI endovascular treatment and its influence on the clinical outcome of the associated MS. Percutaneous transluminal angioplasty (PTA) of venous strictures in patients with CCSVI was shown to be safe, and positively influenced clinical and QOL parameters of the associated MS compared to preoperative treatment. Restenosis rates were elevated in the IJV but promising in the AZ vein, suggesting the need to improve endovascular techniques.
-
- Endovascular treatment for CCSVI
-
Status: published ( 29 ) .This study confirmed the safety and suggests benefits for relapsing-remitting MS patients. Currently it is the only study with a control arm and blinded MRI measure. It has demonstrated a trend towards lower T2 lesion accumulation in treated patients along 6 months.
-
- Multicenter registry for CCSVI testing and treatment
- ClinicalTrials.gov Identifier: NCT01205633
- Status: ongoing
- Patients suspected of having CCSVI should register their participation in magnetic venous resonance (MRV) testing, and if positive, for catheter venoplasty (with a catheter interventionist in their local community). The procedure should but may not be covered by patients’ insurance. Patients must be referred by their treating physician.
- Study to evaluate treating CCSVI in MS patients
- ClinicalTrials.gov identifier: NCT01089686
- Status: ongoing
- The purpose is to evaluate the safety, feasibility and efficacy of PTA in treating extracranial venous obstructive lesions, and its influence on the clinical outcomes of MS patients who have been found to have CCSVI.
- Evaluation of angioplasty in the treatment of CCSVI in MS
- ClinicalTrials.gov identifier: NCT01201707
- Status: recruiting.
- The study is being conducted to determine whether venous angioplasty is an effective treatment for CCSVI. The effectiveness of angioplasty in the treatment of CCSVI is being evaluated by comparison of two groups of patients: one group with CCSVI diagnosed on a venogram and treated with angioplasty, and one group with CCSVI diagnosed on a venogram but not treated.
Endovascular treatment for CCSVI
ClinicalTrials.gov identifier: NCT01264848
Status: recruiting.
This study is designed to evaluate endovascular correction of CCSVI and the influence of these treatments on the symptoms of MS. Prospective open-label cohort study.
- PREMiSe-Prospective Randomized Endovascular therapy in Multiple Sclerosis
- Status: ongoing (Buffalo, NY, USA)
- The aim of this study is to determine whether endovascular intervention via balloon angioplasty to correct the blockages improves MS symptoms or progression.
- BRAVE DREAMS-BRAin VEnous DRainage Exploited Against Multiple Sclerosis
- ClinicalTrials.gov identifier: NCT01371760
-
Status: not yet recruiting (Ferrara, Italy)The aim of this study is to assess, in a double blinded randomized controlled trial (RCT) study design, safety and effectiveness of balloon angioplasty of the main extracranial and extravertebral veins in MS associated with chronic cerebrospinal venous insufficiency (CCSVI).Mean follow-up: 1 year. 10–20 Italian centres; 445 relapsing-remitting (RR) + 234 secondary progressive (SP), overall 679 MS patients will be randomized, with Expanded Disability Status Scale (EDSS score) range 2–5.5; age 18–65 years.
APPENDIX II.
The venous hemodynamic insufficiency severity score (VHISS)
The hemodynamic severity of CCSVI can be scored through the venous hemodynamic insufficiency severity score (VHISS), which was found to have a positive correlation with non-conventional MRI measurements, such as CSF flow dynamics, brain volume, cerebral blood flow, cerebral blood volume, and mean transit time ( 7 , 50 , 51 ) .
The VHISS is an ordinal measure of the overall extent and number of CCSVI criteria with a higher value of VHISS indicating a greater severity of flow pattern anomalies. For each of the five CCSVI criteria a VHISS value can be assigned using the scheme described below. The minimum possible VHISS value is 0 and the maximum 16.
As regards criterion1 (reflux in the IJV and/or VV), there are four venous segments that can potentially exhibit reflux in each position (supine or sitting). One point is assigned for each segment with reflux in any position. Consequently, criterion 1 has a VHISS contribution score that could range from a minimum of 0 to a maximum of eight.
The VHISS contribution score for criterion 2 (IJV stenosis) ranges from 0 to 2, depending on whether B-mode anomalies disturbing outflow are present in none, one or both of the IJVs, respectively. Criterion 2 is assigned a contribution score of 0 should either criterion 1 or criterion 3 be positive for the presence, in either position, of reflux or obstruction in the IJV of interest.
The scoring scheme for the contribution of criterion 3 (absence of flow in the IJV and/or VV) to the VHISS is the same as that for criterion 1, the difference being that only blocks are considered. No points are assigned for segments in which reflux in any position has been detected under criterion 1.
Criterion 4 (change in CSA by changing position) has an overall VHISS contribution score of between 0 and 4, calculated by assigning 0 to 2 points for each IJV. A CSA lying which is greater than CSA sitting by 7mm 2 or more is assigned 0. A CSA lying greater than CSA sitting by less than 7mm 2 is assigned 1. A CSA lying which is less than CSA sitting is assigned 2. (The value of 7 mm 2 corresponds to the 25th percentile of ΔCSA distribution in healthy controls).
Criterion 5 (reflux in intracranial veins) is assigned a VHISS contribution score of 1 if intracranial vein reflux is present in only one position, and of 2 if it was present in both positions. The VHISS contribution score for this criterion is additionally weighted with a factor of 2 if reflux toward the subcortical gray matter can be detected. Consequently, the VHISS contribution score for criterion 2 can range from a minimum of 0 to a maximum of four.
The overall VHISS score is defined as a weighted sum of the scores contributed by each individual CCSVI criterion. The formula for VHISS calculations is:
(The subscripts in this formula indicate the subscores for the five VH criteria).
APPENDIX III.
Screening for Chronic Cerebrospinal Venous Insufficiency (CCSVI)
| Name: | D.O.B.: | Date: | SN: |
| Referring physician: Dr |
| REPORT | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. Supine | |||||||||||
| Right | Left | ||||||||||
| Flow direction | PV cm/sec | Reflux time sec | Area cm² | Valves, septa etc | Flow direction | PV cm/sec | Reflux time sec | Area cm² | Valves, septa etc | ||
| J3 | ----- | J3 | ------ | ||||||||
| J2 | J2 | ||||||||||
| J1 | ----- | J1 | ------ | ||||||||
| VV | ----- | VV | ------ | ||||||||
| Further comments/diagrams | |||||||||||
| B. Sitting | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | ||||||||||
| Flow direction | PV cm/sec | Reflux time sec | Area cm² | Valves, septa etc | Flow direction | PV cm/sec | Reflux time sec | Area cm² | Valves, septa etc | ||
| J3 | ----- | J3 | ------ | ||||||||
| J2 | J2 | ||||||||||
| J1 | ----- | J1 | ------ | ||||||||
| VV | ----- | VV | ----- | ||||||||
| Further comments/diagrams | |||||||||||
| Name: | D.O.B.: | Date: 17-05-11 | SN: |
| Referring physician: |
| EXAMPLE OF A REPORT | |||||||||||
| A. Supine | |||||||||||
| Right | Left | ||||||||||
| Flow direction | PV cm/sec | Reflux time sec | Area cm² | Valves, septa etc | Flow direction | PV cm/sec | Reflux time sec | Area cm² | Valves, septa etc | ||
| J3 | ↓ | 59 | ----- | J3 | ↓ | 56 | ------ | ||||
| J2 | ↓↑ | 57 | 0.90 | 0.36 | J2 | ↓ | 55 | 0.66 | |||
| J1 | ↓↑ | 57 | 1.5 | ----- | FIXED SEPTUM | J1 | ↓↑ | 47 | 1.5 | ------ | MISALIGNED VALVE |
| VV | ↓ | 32 | ----- | VV | ↓ | 20 | ------ | ||||
| Further comments/diagrams | |||||||||||
| B. Sitting | |||||||||||
| Right | Left | ||||||||||
| Flow direction | PV cm/sec | Reflux time sec | Area cm² | Valves, septa etc | Flow direction | PV cm/sec | Reflux time sec | Area cm² | Valves, septa etc | ||
| J3 | ↓ | 32 | ----- | J3 | ↓ | 40 | ------ | ||||
| J2 | ↓ | 153 | 0.26 | J2 | ↓ | 18 | 0.10 | ||||
| J1 | ↓↑ | 32 | ----- | FIXED SEPTUM | J1 | ↓↑ | 170 | ------ | MISALIGNED VALVE | ||
| VV | ↓ | 70 | ----- | VV | ↓ | 35 | ----- | ||||
| Further comments/diagrams | |||||||||||
ADDITIONAL INVESTIGATION: INTRACRANIAL VEINS
| Flow Direction | Reflux> 0.5 sec | |||
|
TRANS
TEMPORAL Rosenthal vein |
↑ | ↓↑ | Y | N |
|
TRANS
CONDYLAR Sup Petr Sin Inf Petr Sin Controlat Inf Petr Sin Controlat Sup Petr Sin Cavernous Sin |
↑ | ↓↑ | Y | N |
Example of Final Report and Conclusions
| CRITERIA | Description |
|
1.
Reflux IJV and\or VV in both positions Reflux in one position and absence of flow in the other |
Yes, No Segments: IJVr, IJVl, VVr,VVl |
|
3.
High resolution B-mode evidence of proximal IJV stenoses and/or other B-mode anomalies |
Yes, No: Segments IJVr, IJVl CSA < 0.3: Segments IJVr, IJVl Flow velocity > 90 cm/sec: Segments IJVr, IJVl Fixed M-mode Evaluation: Segments IJVr, IJVl |
|
4.
Absence of flow in IJV and\or VV in both positions |
Yes, No Segments: IJVr, IJVl, VVr,VVl |
|
5.
CSA sitting > supine in the IJV |
Left, Right |
|
ADDITIONAL CRITERION:
2. Reflux in the intracranial veins |
Yes, No |
| CONCLUSIONS |
CCSVI YES, CCSVI NO N° of fulfilled criteria…./ Affected segments: IJVr, IJVl, suspected AZY |
| Center: | Investigator |
| Signature |
APPENDIX IV.
How to perform CCSVI examination of the intracranial veins
The classical transtemporal and more recently described transoccipital and frontal bone windows allow insonation of intracranial arteries and veins including deep cerebral veins (the Rosenthal vein, the Galen vein, and the internal cerebral vein) and the contralateral transverse sinus ( 50 – 52 ) . In Figure 1 , we show a well established plan of insonation of the midbrain. The midbrain appears as a butterfly-like structure in B-mode. The parenchymal vein most easily detected is the Rosenthal vein because of its anatomical relation with the posterior cerebral artery and midbrain. The blood is expected to flow away from the probe in the direction of the Galen vein-straight sinus.
Figure 1 .
Transtemporal approach at the level of midbrain (e) contralateral skull (g). The ultrasonic beam makes it possible to identify: the middle cerebral artery (a) and the contralateral skull (f), the posterior cerebral artery, pre-peduncular part (b), the Rosenthal vein (c) and the posterior cerebral artery, post-peduncular part (d).
For the veins at the base of the brain (superior and inferior petrosal sinuses) a different transcranial approach by the means of fusion imaging technology with MRI has been demonstrated ( 53 ) . The transducer should be placed at the level of the mandibular condyle, sloping the tail approximately 10° downwards with the insonation depth adjusted to 11 cm. Deep inspiration should elicit venous flow at a depth of about 7 cm. This maneuver helps to identify a cloud composed of color Doppler signals, sometimes within a hyperechoic bright-line representing the sinus wall, visible immediately below the color flow dynamic image ( Fig. 2 ). Due to the lack of clearly visible anatomical B-mode reference markers, the color Doppler must be activated from the beginning of the intracranial examination. At the low PRF setting, a huge color cloud artifact tells the operator that the probe is positioned correctly. After this color cloud has been detected, the operator must increase the PRF and, possibly, activate the power Doppler, in order to have a better color Doppler signal spatial definition ( Fig. 3 ): the PRF must be increased up to the level at which the color cloud artifact disappears and only the spatially well-defined venous color Doppler signal remains on the screen ( 49 , 53 ) .
Figure 2 .
Color cloud artifact seen from the transcranial window at the level of the condyloid process of the mandible (PRF 0.7 kHz). This artifact represents a really powerful “Doppler” anatomical marker in order to find the plane in which one or more of the cerebral veins we want to examine are positioned.
Figure 3 .
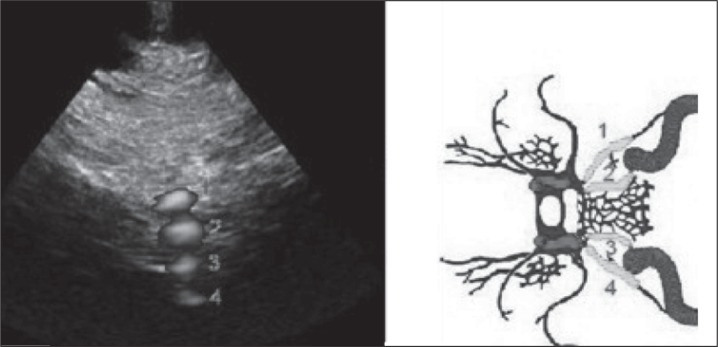
Visualization of the power Doppler signal (instead of color Doppler for increased spatial resolution) of the superior petrosal sinus ( 1 ), inferior petrosal sinus ( 2 ), contralateral inferior petrosal sinus ( 3 ) and (partially) contralateral superior petrosal sinus ( 4 ), from the transcranial window at the level of the condyloid process of the mandible.
This novel approach to the veins of the base of the skull may more constantly insonate venous flow without anatomical reference to structures of the brain parenchyma. It also gives the possibility of examining, according to the depth of insonation, the superior petrosal sinus, the inferior petrosal sinus, and the contralateral inferior petrosal sinus ( Fig. 3 ). Sometimes the cavernous sinus, the intercavernous sinus, and, even more rarely, the vein of Rosenthal and basilar plexus can be insonated.
Because the PW angle of insonation is not favorable from the condylar approach, a multiangle Doppler system composed of more than 250 Doppler samples has been developed (QDP system, Esaote Biomedica, Genoa, Italy). Through a software algorithm it is possible to clearly detect the flow direction within the insonated venous structures.
References
- 1. Zamboni P , Galeotti R , Menegatti E , et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis . J Neurol Neurosurg Psychiatry . 2009 ; 80 : 392 – 399 . doi: 10.1136/jnnp.2008.157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zamboni P , Consorti G , Galeotti R , et al. Venous collateral circulation of the extracranial cerebrospinal outflow routes . Curr Neurovasc Res . 2009 ; 6 : 204 – 212 . doi: 10.2174/156720209788970054. [DOI] [PubMed] [Google Scholar]
- 3. Zivadinov R , Galeotti R , Hojnacki D , et al. Value of MR venography for detection of internal jugular vein anomalies in multiple sclerosis: a pilot longitudinal study . AJNR Am J Neuroradiol . 2011 ; 32 : 938 – 946 . doi: 10.3174/ajnr.A2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zivadinov R , Marr K , Cutter G , et al. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS . Neurology . 2011 ; 77 : 138 – 144 . doi: 10.1212/WNL.0b013e318212a901. [DOI] [PubMed] [Google Scholar]
- 5. Al-Omari MH , Rousan LA . Internal jugular vein morphology and hemodynamics in patients with multiple sclerosis . Int Angiol . 2010 ; 29 : 115 – 120 . [PubMed] [Google Scholar]
- 6. Simka M , Kostecki J , Zaniewski M , Majewski E , Hartel M . Extracranial Doppler sonographic criteria of chronic cerebrospinal venous insuffitiency in the patients with multiple sclerosis . Int Angiol . 2010 ; 29 : 109 – 114 . [PubMed] [Google Scholar]
- 7. Bastianello S , Bergamaschi F , Viselner G , et al. An international multicenter observatory on prevalence of CCSVI in MS . BMC Neurology . 2011 (In press) [Google Scholar]
- 8. Doepp F , Paul F , Valdueza JM , Schmierer K , Schreiber SJ . No cerebrocervical venous congestion in patients with multiple sclerosis . Ann Neurol . 2010 ; 68 : 173 – 183 . doi: 10.1002/ana.22085. [DOI] [PubMed] [Google Scholar]
- 9. Mayer CA , Pfeilschifter W , Lorenz MW , et al. The perfect crime? CCSVI not leaving a trace in MS . J Neurol Neurosurg Psychiatry . 2011 ; 82 : 436 – 440 . doi: 10.1136/jnnp.2010.231613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamout B , Herlopian A , Issa Z , et al. Extracranial venous stenosis is an unlikely cause of multiple sclerosis . Mult Scler . 2010 ; 16 : 1341 – 1348 . doi: 10.1177/1352458510385268. [DOI] [PubMed] [Google Scholar]
- 11. Baracchini C , Perini P , Calabrese M , Causin F , Rinaldi F , Gallo P . No evidence of chronic cerebrospinal venous insufficiency at multiple sclerosis onset . Ann Neurol . 2011 ; 69 : 90 – 99 . doi: 10.1002/ana.22228. [DOI] [PubMed] [Google Scholar]
- 12. Wattjes MP , van Oosten BW , de Graaf WL , et al. No association of abnormal cranial venous drainage with multiple sclerosis: a magnetic resonance venography and flow-quantification study . J Neurol Neurosurg Psychiatry . 2011 ; 82 : 429 – 435 . doi: 10.1136/jnnp.2010.223479. [DOI] [PubMed] [Google Scholar]
- 13. Lee BB , Laredo J , Neville R . Embryological background of truncular venous malformation in the extracranial venous pathways as the cause of chronic cerebrospinal venous insufficiency . Int Angiol . 2010 ; 29 : 95 – 108 . [PubMed] [Google Scholar]
- 14. Lee BB , Bergan J , Gloviczki P , et al. International Union of Phlebology (IUP). Diagnosis and treatment of venous malformations - Consensus document of the International Union of Phlebology (IUP)-2009 . Int Angiol . 2009 ; 28 : 434 – 451 . [PubMed] [Google Scholar]
- 15. Lee BB , Laredo J , Lee TS , Huh S , Neville R . Terminology and classification of congenital vascular malformations . Phlebology . 2007 ; 22 : 249 – 252 . doi: 10.1177/026835550702200604. [DOI] [PubMed] [Google Scholar]
- 16. Uflacker R . Atlas of Vascular Anatomy. An Angiographic Approach . Philadephia : Lippincot, Williams and Wilkins ; 1997 . [Google Scholar]
- 17. Hamoud S , Nitecky S , Engel A , Goldsher D , Hayek T . Hypoplasia of the inferior vena cava with azygous continuation presenting as recurrent leg deep vein thrombosis . Am J Med Sci . 2000 ; 319 : 414 – 416 . doi: 10.1097/00000441-200006000-00013. [DOI] [PubMed] [Google Scholar]
- 18. Gates J , Hartnell GG . Demonstration of inferior vena cava patency by retrograde azygous venography . Cardiovasc Intervent Radiol . 1995 ; 18 : 419 – 421 . doi: 10.1007/BF00338314. [DOI] [PubMed] [Google Scholar]
- 19. Lane EJ , Heitzman ER , Dinn WM . The radiology of the superior intercostal veins . Radiology . 1976 ; 120 : 263 – 267 . doi: 10.1148/120.2.263. [DOI] [PubMed] [Google Scholar]
- 20. Chasen MH , Charnsangavej C . Venous chest anatomy: clinical implications . Eur J Radiol . 1998 ; 27 : 2 – 14 . doi: 10.1016/s0720-048x(97)00147-2. [DOI] [PubMed] [Google Scholar]
- 21. Mammen T , Keshava SN , Eapen CE , et al. Transjugular liver biopsy: a retrospective analysis of 601 cases . J Vasc Interv Radiol . 2008 ; 19 : 351 – 358 . doi: 10.1016/j.jvir.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 22. Dilenge D , Perey B , Geraud G , Nutik S . Angiographic demonstration of the cervical vertebral venous plexus in man . J Can Assoc Radiol . 1975 ; 26 : 77 – 81 . [PubMed] [Google Scholar]
- 23. Zelli GP , Messinetti S , Condorelli S . Original technique of internal jugular phlebography by puncture of the external jugular vein with retrograde emission of the contrast media . Prog Med . 1964 ; 15 : 681 – 688 . [Italian] [PubMed] [Google Scholar]
- 24. Gejrot T , Lauren T . Retrograde venography of the internal jugular veins and transverse sinuses; technique and roentgen anatomy . Acta Otolaryngol . 1964 ; 57 : 556 – 570 . doi: 10.3109/00016486409137117. [DOI] [PubMed] [Google Scholar]
- 25. Gejrot T . Retrograde jugularography in the diagnosis of abnormalities of the superior bulb of the internal jugular vein . Acta Otolaryngol . 1964 ; 57 : 177 – 180 . doi: 10.3109/00016486409136958. [DOI] [PubMed] [Google Scholar]
- 26. Leriche H , Aubin ML , Aboulker J . Cavo-spinal phlebography in myelopathies. Stenoses of internal jugular and azygos veins, venous compressions and thromboses . Acta Radiol Suppl . 1976 ; 347 : 415 – 417 . [French] [PubMed] [Google Scholar]
- 27. Tzuladze II . The selective phlebography of the large tributaries of the vena cava system in the diagnosis of venous circulatory disorders in the spinal complex . Zh Vopr Neirokhir Im N N Burdenko . 1999 ; 2 : 8 – 13 . [Russian] [PubMed] [Google Scholar]
- 28. Zamboni P , Galeotti R , Menegatti E , et al. A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency . J Vasc Surg . 2009 ; 50 : 1348 – 1358 . doi: 10.1016/j.jvs.2009.07.096. [DOI] [PubMed] [Google Scholar]
- 29. Zamboni P , Galeotti R , Weinstock-Guttman B , Kennedy C , Zivadinov R . Venous angioplasty in patients with multiple sclerosis: results of a pilot study . Eur J Vasc Endovasc Surg . 2012 ; 43 : 116 – 122 . doi: 10.1016/j.ejvs.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 30. Malagoni AM , Galeotti R , Menegatti E , et al. Is chronic fatigue the symptom of venous insufficiency associated with multiple sclerosis? A longitudinal pilot study . Int Angiol . 2010 ; 29 : 176 – 182 . [PubMed] [Google Scholar]
- 31. Ludyga T , Kazibudzki M , Simka M , et al. Endovascular treatment for chronic cerebrospinal venous insufficiency: is the procedure safe? . Phlebology . 2010 ; 25 : 286 – 295 . doi: 10.1258/phleb.2010.010053. [DOI] [PubMed] [Google Scholar]
- 32. Schaller B . Physiology of cerebral venous blood flow: from experimental data in animals to normal function in humans . Brain Res Brain Res Rev . 2004 ; 46 : 243 – 260 . doi: 10.1016/j.brainresrev.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 33. Singh AV , Zamboni P . Anomalous venous blood flow and iron deposition in multiple sclerosis . J Cereb Blood Flow Metab . 2009 ; 29 : 1867 – 1878 . doi: 10.1038/jcbfm.2009.180. [DOI] [PubMed] [Google Scholar]
- 34. Menegatti E , Zamboni P . Doppler haemodynamics of cerebral venous return . Curr Neurovasc Res . 2008 ; 5 : 260 – 265 . doi: 10.2174/156720208786413442. [DOI] [PubMed] [Google Scholar]
- 35. Valdueza JM , von Münster T , Hoffman O , Schreiber S , Einhaüpl KM . Postural dependency of the cerebral venous outflow . Lancet . 2000 ; 355 : 200 – 201 . doi: 10.1016/s0140-6736(99)04804-7. [DOI] [PubMed] [Google Scholar]
- 36. Schreiber SJ , Lurtzing F , Gotze R , Doepp F , Klingebiel R , Valdueza JM . Extrajugular pathways of human cerebral venous blood drainage assessed by duplex ultrasound . J Appl Physiol . 2003 ; 94 : 1802 – 1805 . doi: 10.1152/japplphysiol.00782.2002. [DOI] [PubMed] [Google Scholar]
- 37. Doepp F , Schreiber SJ , von Münster T , Rademacher J , Klingebiel R , Valdueza JM . How does the blood leave the brain? A systematic ultrasound analysis of cerebral venous drainage patterns . Neuroradiology . 2004 ; 46 : 565 – 570 . doi: 10.1007/s00234-004-1213-3. [DOI] [PubMed] [Google Scholar]
- 38. Zamboni P , Menegatti E , Galeotti R , et al. The value of cerebral Doppler venous haemodynamics in the assessment of multiple sclerosis . J Neurol Sci . 2009 ; 282 : 21 – 27 . doi: 10.1016/j.jns.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 39. Zamboni P . Regarding “no cerebrocervical venous congestion in patients with multiple sclerosis. Intraluminal jugular septation” . Ann Neurol . 2010 ; 68 : 969 . doi: 10.1002/ana.22152. [DOI] [PubMed] [Google Scholar]
- 40. Law M , Saindane AM , Ge Y , et al. Microvascular abnormality in relapsing-remitting multiple sclerosis: perfusion MR imaging findings in normal-appearing white matter . Radiology . 2004 ; 231 : 645 – 652 . doi: 10.1148/radiol.2313030996. [DOI] [PubMed] [Google Scholar]
- 41. Zamboni P , Menegatti E , Weinstock-Guttman B , et al. Hypoperfusion of brain parenchyma is associated with the severity of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis: a cross-sectional preliminary report . BMC Med . 2011 ; 7 : 9 – 22 . doi: 10.1186/1741-7015-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zamboni P , Menegatti E , Weinstock-Guttman B , et al. The severity of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis is related to altered cerebrospinal fluid dynamics . Funct Neurol . 2009 ; 24 : 133 – 138 . [PubMed] [Google Scholar]
- 43. Zamboni P , Menegatti E , Weinstock-Guttman B , et al. CSF dynamics and brain volume in multiple sclerosis are associated with extracranial venous flow anomalies: a pilot study . Int Angiol . 2010 ; 29 : 140 – 148 . [PubMed] [Google Scholar]
- 44. Gisolf J , van Lieshout JJ , van Heusden K , Pott F , Stok WJ , Karemaker JM . Human cerebral venous outflow pathway depends on posture and central venous pressure . J Physiol . 2004 ; 560 : 317 – 327 . doi: 10.1113/jphysiol.2004.070409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lepori D , Capasso P , Fournier D , Genton CY , Schnyder P . High-resolution ultrasound evaluation of internal jugular venous valves . Eur Radiol . 1999 ; 9 : 1222 – 1226 . doi: 10.1007/s003300050822. [DOI] [PubMed] [Google Scholar]
- 46. Kotani A , Hirano Y , Yasuda C , Ishikawa K . A new ultrasonographic technique for diagnosing deep venous insufficiency – imaging and functional evaluation of venous valves by ultrasonography with improved resolution . Int J Cardiovasc Imaging . 2007 ; 23 : 493 – 500 . doi: 10.1007/s10554-006-9163-y. [DOI] [PubMed] [Google Scholar]
- 47. Feigenbaum H . Role of M-mode technique in today’s echocardiography . J Am Soc Echocardiogr . 2010 ; 23 : 240 – 257 . 335 – 337 . doi: 10.1016/j.echo.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 48. Nedelmann M , Eicke BM , Dieterich M . Functional and morphological criteria of internal jugular valve insufficiency as assessed by ultrasound . J Neuroimaging . 2005 ; 15 : 70 – 75 . doi: 10.1177/1051228404267997. [DOI] [PubMed] [Google Scholar]
- 49. Zamboni P , Galeotti R . The chronic cerebrospinal venous insufficiency syndrome . Phlebology . 2010 ; 25 : 269 – 279 . doi: 10.1258/phleb.2010.009083. [DOI] [PubMed] [Google Scholar]
- 50. Baumgartner RW , Nirkko AC , Müri RM , Gönner F . Transoccipital power-based color-coded Duplex sonography of cerebral sinuses and veins . Stroke . 1997 ; 28 : 1319 – 1323 . doi: 10.1161/01.str.28.7.1319. [DOI] [PubMed] [Google Scholar]
- 51. Stolz E , Kaps M , Kern A , Babacan SS , Dorndorf W . Transcranial color-coded duplex sonography of intracranial veins and sinuses in adults. Reference Data From 130 volunteers . Stroke . 1999 ; 30 : 1070 – 1075 . doi: 10.1161/01.str.30.5.1070. [DOI] [PubMed] [Google Scholar]
- 52. Walter U . Transcranial sonography-assisted stereotaxy and follow-up of deep brain implants in patients with movement disorders . Int Rev Neurobiol . 2010 ; 90 : 274 – 285 . doi: 10.1016/S0074-7742(10)90019-6. [DOI] [PubMed] [Google Scholar]
- 53. Zamboni P , Menegatti E , Viselner G , Bastianello S . Fusion imaging technology of the intracranial veins . Phlebology . 2011 doi: 10.1258/phleb.2011.011069. (In press) [DOI] [PubMed] [Google Scholar]