Abstract
IL-10 production during intracellular bacterial infections is generally thought to be detrimental because of its role in suppressing protective T-helper cell 1 (Th1) responses. Francisella tularensis is a facultative intracellular bacterium that activates both Th1 and Th17 protective immune responses. Herein, we report that IL-10–deficient mice (Il10−/−), despite having increased Th1 and Th17 responses, exhibit increased mortality after pulmonary infection with F. tularensis live vaccine strain. We demonstrate that the increased mortality observed in Il10−/−-infected mice is due to exacerbated IL-17 production that causes increased neutrophil recruitment and associated lung pathology. Thus, although IL-17 is required for protective immunity against pulmonary infection with F. tularensis live vaccine strain, its production is tightly regulated by IL-10 to generate efficient induction of protective immunity without mediating pathology. These data suggest a critical role for IL-10 in maintaining the delicate balance between host immunity and pathology during pulmonary infection with F. tularensis live vaccine strain.
Francisella tularensis, a facultative intracellular bacterium, because of its infectious nature and the severe disease caused by low doses of airborne bacteria, has been classified as a category A select bioterrorism agent.1 Infection in humans is caused by two main subspecies, F. tularensis (type A) and Francisella holarctica (type B).2 An F. tularensis live vaccine strain (LVS) has been developed from the F. tularensis B strain as an experimental vaccine, but is not licensed for use in humans.1 F. tularensis LVS has been used as a representative attenuated model to address the immune requirements for protection against Francisella. By using this model, the importance of IL-12 in driving interferon γ (IFN-γ) and T-helper cell 1 (Th1) responses in immunity to F. tularensis LVS infection is well described.3–5 In contrast, IL-17 is generally thought to play a role in protection against extracellular, but not intracellular, pathogens.6 However, we and others recently identified a protective role for IL-17 in the induction of cellular immunity to F. tularensis LVS pulmonary infection,7–9 by driving the production of IFN-γ through IL-12 induction.7 IL-17 is a proinflammatory cytokine also known to induce chemokines, such as keratinocyte chemoattractant, macrophage inflammatory protein 2 (MIP-2), and granulocyte colony-stimulating factor (G-CSF), to mediate granulopoiesis, neutrophil recruitment, and inflammation.6 Accordingly, the absence of IL-17 during F. tularensis LVS pulmonary infection also results in decreased induction of G-CSF and MIP-2, as well as decreased accumulation of neutrophils and lung inflammation.7 Neutrophil depletion alone does not affect bacterial control after pulmonary infection with F. tularensis LVS,10 suggesting that the role for IL-17 in driving Th1 responses, and not neutrophil recruitment, was the primary immune mechanism mediating protection in this model.7 These data together suggest that both IL-17 and IFN-γ are required for generating protective immunity to pulmonary F. tularensis LVS infection.
IL-10 is an anti-inflammatory cytokine best studied for its inhibitory effects on IL-12 production and down-regulation of Th1 responses.11 Accordingly, IL-10–deficient mice show enhanced protection in models of intracellular bacterial infections, such as Mycobacterium tuberculosis12 and Listeria monocytogenes.13 In addition, in a cutaneous model of F. tularensis LVS infection, IL-10–deficient mice exhibit increased protection, and this was reversed when IL-17 was depleted.14 In contrast to these published studies, in the current study, we report that after pulmonary infection with F. tularensis LVS, mice deficient in IL-10 (Il10−/−) exhibit increased mortality. We clearly demonstrate that the increased mortality in the Il10−/−-infected mice is not associated with loss of protective immunity, because bacterial burden between wild-type and Il10−/− mice is similar, but is caused by exacerbated inflammation and increased lung pathology. We demonstrate that the exacerbated inflammation observed in Il10−/−-infected mice is the result of unrestrained IL-17 production and IL-17–dependent recruitment of neutrophils and resulting lung pathology. These data together suggest that, although IL-17 is required for protective immunity against pulmonary infection with F. tularensis LVS,7,9 IL-17 production is tightly regulated by anti-inflammatory cytokines, such as IL-10. Our studies highlight how inflammatory cytokines, such as IL-17, can be beneficial for host protection, but when produced unrestrained, can mediate host pathology.
Materials and Methods
Animals and Experimental Infection
C57BL/6 (B6) and Il10−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Il17−/− mice15 were crossed to the Il10−/− mice, and Il17−/−/Il-10−/− double-deficient mice were generated in house on the B6 background and were used in accordance with University of Pittsburgh Institutional Animal Care and Use Committee guidelines. F. tularensis LVS (BEI Resources, Manassas, VA) were grown in Mueller-Hinton (MH) broth or agar.5 For pulmonary infections, mice were infected with 1000 colony-forming units (CFUs) of F. tularensis LVS. On day 6 after infection, serial dilutions of the homogenized infected lungs were plated on MH agar plates and lung CFUs were determined. In some experiments, survival was monitored in infected B6- and gene-deficient mice. For depletion of neutrophils, mice were treated with 300 μg of Gr1-depleting antibody (clone IA8; BioXCell, West Lebanon, NH) or isotype control antibody (BioXCell) every 48 hours, as previously described.16 In some experiments, single-cell lung suspensions were prepared, as previously described, and used for flow cytometric analyses.7
Histological and Immunofluorescence Data
Lungs from mice were inflated with 10% neutral-buffered formalin and embedded in paraffin. Lung sections were stained with H&E stain (Colorado Histo-Prep, Fort Collins, CO) and processed routinely for light microscopy. Slides were scored by one of the authors (T.D.O.), who was blinded to the sample groups. Briefly, every field in the entire lung was observed with a light microscope and scored for inflammation, as previously described.17 Scoring was based on the percentage of alveolar tissue with inflammation, according to the following scale: 0, no inflammation; 1, 1% to <25%; 2, 25% to <50%; 3, 50% to <75%; and 4, 75% to 100% inflammation. For immunofluorescence, paraffin was removed from the formalin-fixed lung sections, as previously described,7 and samples were probed with biotinylated rat, anti-mouse GR1 (Rat AL-21; BD Pharmingen). Slow-fade gold antifade with DAPI (Molecular Probes, Grand Island, NY) was used to counterstain tissues and detect nuclei. Images were obtained with a Zeiss Axioplan 2 microscope (Carl Zeiss Microscopy, Jena, Germany) and were recorded with a Zeiss AxioCam digital camera (Carl Zeiss Microscopy).
Determination of Protein Amounts
IL-10, IL-12, and IL-23 protein levels were measured using a Mouse Duoset enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). Other cytokine and chemokine protein levels were determined with a mouse Luminex assay (Linco/Millipore, Billerica, MA). Myeloperoxidase (MPO) chlorination and peroxidase activity were determined in lung homogenates using the MPO activity assay kit (Invitrogen, Grand Island, NY).
Flow Cytometry
Single-cell suspensions were stained with fluorochrome-labeled antibodies specific for CD11c (HL3), Gr1 (RB6-8C5), CD11b (M1/70), CD3 (145-2C11), CD4 (RM4-5), NK1.1 (PK136), γδ T cells (GL3), IFN-γ (XMG1.2), IL-10 (JES5-16E3), and IL-17 (TC11-18H10) or relevant isotype control antibodies. For analysis of intracellular cytokines, cells were stimulated with 50 ng/mL phorbol myristate acetate; 750 ng/mL ionomycin (Sigma Aldrich, St. Louis, MO) in the presence of Golgistop (BD Pharmingen, San Jose, CA) was surface stained, permeabilized with Cytofix-Cytoperm solution (BD Pharmingen), and stained for relevant cytokines. Cells were collected in a Becton Dickinson FACS Aria flow cytometer with FACS Diva software version 6.1.2. Cells were gated based on their forward-by-side scatter characteristics, and the frequency of specific cell types was calculated using FlowJo version 7.6.5 (Tree Star Inc., San Carlos, CA). CD11c+ cells with low autofluorescence were designated as lung dendritic cells (DCs), and CD11c+ cells with high autofluorescence were designated as lung macrophages, as previously described.16,18,19
Generation and Stimulation of BMDCs
Bone marrow dendritic cells (BMDCs) were generated from bone marrow.7 On day 7, nonadherent cells were infected with F. tularensis LVS (multiplicity of infection, 1:100) alone or in combination with 10 μg/mL αIL-10 antibody (clone: JES5-2A5; BioXCell) or isotype control antibody (Rat IgG1; BioXCell) in antibiotic-free Dulbecco’s modified Eagle’s medium for 48 hours. Naïve CD4+ T cells were isolated from OT-II T-cell receptor αβ Tg mice using magnetic CD4+ beads (GK1.5) (Miltenyi Biotec, Auburn, CA) and were cultured with 1 × 106 cells F. tularensis LVS-stimulated or unstimulated BMDCs/mL, with 5 μmol/L ovalbumin323-339 peptide for 6 days, as previously described.7 Culture supernatants were then analyzed by ELISA.
Statistical Analysis
Differences between the means of two experimental groups were analyzed using the two-tailed Student’s t-test; a one-way analysis of variance test was used when more than two groups were analyzed. The log-rank test was used for statistical analyses for the survival studies. Differences were considered significant when P ≤ 0.05.
Results
F. tularensis LVS–Induced IL-10 Production Limits Both Th1 and Th17 Responses
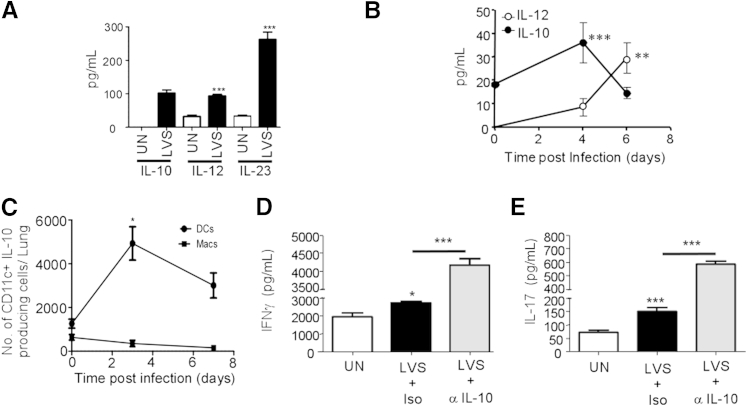
During intracellular bacterial infections, IL-10 expression is detrimental,12,13 because IL-10–deficient mice (Il10−/−) show enhanced protection and decreased bacterial burdens. However, the functional role for IL-10 in the context of F. tularensis LVS infection has not been completely explored. We found that BMDCs from B6 mice produced IL-10 and other polarizing cytokines, such as IL-12 and IL-23, on infection with F. tularensis LVS (Figure 1A). In addition, IL-10 protein was induced early in the lung, whereas IL-12 levels were induced at a later time point after pulmonary F. tularensis LVS infection (Figure 1B). Lung IL-23 levels were lower than detectable levels (data not shown). Lung CD11c+ DCs, but not lung macrophages, were one of the primary sources of IL-10 and accumulated at early time points after pulmonary infection (Figure 1C). Both Th1 and Th17 responses are required for protective immunity against F. tularensis LVS pulmonary infection.5,7 To address the role of F. tularensis LVS-induced IL-10 on Th1 and Th17 responses, we treated F. tularensis LVS-infected B6 BMDCs with IL-10–neutralizing antibody in a DC:T-cell co-culture system and found that IL-10 neutralization resulted in enhanced IFN-γ and IL-17 production by CD4+ T cells (Figure 1, D and E, respectively). These data suggest that IL-10, induced after F. tularensis LVS infection, may limit both Th1 and Th17 responses.
Figure 1.
IL-10 is induced after infection with F. tularensis live vaccine strain (LVS) infection and restrains Th1 and Th17 responses. A: B6 bone marrow dendritic cells (BMDCs) were left uninfected (UN) or infected with F. tularensis LVS (multiplicity of infection, 1:100), and IL-10, IL-12, and IL-23 levels were determined in 48-hour culture supernatants by ELISA. B: B6 mice were infected with 1000 CFUs F. tularensis LVS intratracheally and lung homogenates from 0, 4, and 6 days after infection were assayed for IL-12 and IL-10 levels by ELISA. C: Lung suspensions from uninfected and infected mice were assayed for IL-10 production in CD11c+ DCs and macrophages (Macs) by intracellular staining and flow cytometry, and the number of IL-10-producing cells was calculated. D and E: B6 BMDCS were left untreated (UN), stimulated with F. tularensis LVS, and isotype control antibody (LVS + Iso) or with 10 μg/mL anti–IL-10 antibody (LVS + α-IL-10) for 24 hours, after which OT-II T-cell receptor Tg CD4+ T cells were added to the wells, along with 5 μmol/L ovalbumin323-339 peptide. After 6 days in culture, supernatants were assayed for IFN-γ (D) or IL-17 (E) production. The data points represent the means ± SD of values from three to four samples (A, D, and E) or four to six mice (B and C). ∗P ≤ 0.05, ∗∗P ≤ 0.005, and ∗∗∗P ≤ 0.0005. One experiment is representative of two or more.
IL-17–Dependent Inflammation Mediates Increased Mortality in Il10−/− Mice after F. tularensis LVS Pulmonary Infection
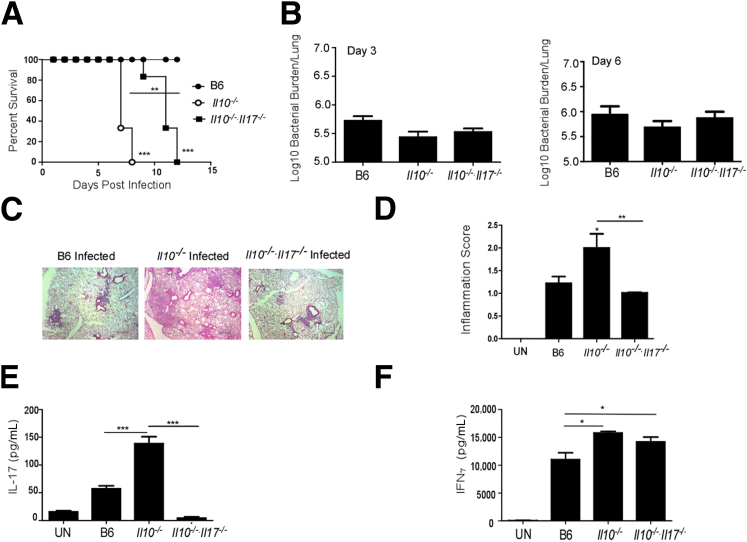
In vitro data suggest that IL-10 may limit both Th1 and Th17 responses, and its presence may be detrimental for protective immunity against F. tularensis LVS infection. Unexpectedly, when Il10−/− mice were infected intratracheally with F. tularensis LVS, they demonstrated decreased survival and increased mortality, when compared with control B6-infected mice (Figure 2A). Interestingly, Il10−/−-infected mice had similar levels of bacterial burden in the lungs at both early and later time points, suggesting that the decreased survival was not associated with increased bacterial burden (Figure 2B). We also found that Il10−/− mice displayed heightened pathology and severe pulmonary inflammation (Figure 2, C and D), suggesting that Il10−/−-infected mice die as a consequence of increased inflammation rather than loss of protective immunity. In vitro data using DC:T-cell co-cultures suggest that IL-10 production restrains both IL-17 and IFN-γ responses in CD4+ T cells (Figure 1, D and E). Therefore, we next addressed whether IL-10 also restrains IL-17 and IFN-γ responses in vivo, and whether dysregulated expression of these proinflammatory cytokines mediates pathology and inflammation during pulmonary tularemia. We found that, when compared with B6 F. tularensis LVS-infected lungs, Il10−/−-infected lungs expressed significantly higher levels of IL-17 (Figure 2E) and IFN-γ protein (Figure 2F). Both γδ T cells and CD4+ T cells are major producers of IL-17 in response to pulmonary infection with F. tularensis LVS.7,9 Accordingly, although numbers of CD4+ and γδ T cells producing IL-17 were increased in B6-infected lungs compared with uninfected lungs, their numbers were further enhanced in Il10−/−-infected lungs (Table 1). These data suggest that, in addition to its known role in restraining Th1 responses,11 IL-10 also has a role in restraining Th17 responses after pulmonary infection with F. tularensis LVS.
Figure 2.
A: Dysregulated IL-17 production in Il10−/− mice mediates increased mortality and lung pathological characteristics after pulmonary F. tularensis LVS infection. B6, Il10−/−, and Il10−/−/Il17−/− mice were infected with 1000 CFUs F. tularensis LVS intratracheally, and the mice that survived were monitored over time. Statistical significance was determined by log-rank test between survival in B6-infected and Il10−/− mice, and between B6-infected and Il10−/−/Il17−/−-infected mice. B: On days 3 and 6 after infection, lung bacterial burden was determined by plating on MH agar plates. C and D: Day 6, formalin-fixed, paraffin-embedded (FFPE) serial lung sections were stained with H&E(C), and sections were scored for percentage of inflammation, as described in Materials and Methods (D). E and F: Lung homogenates from infected B6 and gene-deficient mice were assayed for IL-17 (E) and IFN-γ (F) levels. The data points represent the means ± SD of values from 6 to 10 mice. ∗P ≤ 0.05, ∗∗P ≤ 0.005, and ∗∗∗P ≤ 0.0005. Original magnification, ×10 (C). One experiment is representative of two. UN, uninfected.
Table 1.
IL-17 Production on F. tularensis LVS Infection
| Type of cell | Uninfected lungs | B6-infected lungs | Il10−/−-infected lungs |
|---|---|---|---|
| CD4+ IL-17+ T cells | 1.8 × 104 ± 6 × 103 | 2.4 × 105 ± 9.2 × 104∗ | 4.2 × 105 ± 1.5 × 104† |
| γδ+ IL-17+ T cells | 1.2 × 104 ± 6.2 × 103 | 1.7 × 105 ± 7.4 × 104∗ | 2.9 × 105 ± 6.1 × 104† |
B6 and Il10−/− mice were left uninfected or were infected with 1000 CFUs F. tularensis LVS intratracheally. On day 6, single-cell lung suspensions were assayed for IL-17 production by intracellular staining and flow cytometry.
P ≤ 0.005 between B6-infected and uninfected mice.
P ≤ 0.05 between B6-infected and Il10−/−-infected mice.
The absence of IL-17 during pulmonary tularemia results in decreased inflammation in the infected lung.7 Therefore, we hypothesized that excess IL-17, rather than excess IFN-γ, caused the inflammation, and increased the mortality observed in the Il10−/−-infected mice. To address this, we generated IL-10/IL-17 double-deficient mice (Il10−/−/Il17−/−) and infected them with F. tularensis LVS via the pulmonary route. We found that absence of IL-17 significantly reversed the increased susceptibility seen in Il10−/− mice (Figure 2A). More importantly, we found that the heightened pathological characteristics observed in the lungs of infected Il10−/− mice were also reversed in lungs from Il10−/−/Il17−/−-infected mice (Figure 2, C and D). We have recently shown that IL-17 is protective and is required to drive IL-12–driven Th1-protective cell immunity against F. tularensis LVS.7 This role for IL-17 is likely to overcome the inhibitory effects of IL-10 on IL-12 production, as demonstrated by us in another intracellular bacterial model (namely, Mycobacterium bovis bacillus Calmette-Guerin exposure).20 In support of a role for IL-17 in driving IFN-γ responses specifically to overcome IL-10–mediated inhibition, significantly higher levels of IFN-γ were found in lungs of both Il10−/− and Il10−/−/Il17−/− mice when compared with B6-infected lungs (Figure 2F). In addition, Il10−/−/Il17−/−-infected mice showed a similar bacterial burden to B6- and Il10−/−-infected mice at both early and later time points (Figure 2B). These data together suggest that when IL-10 is absent, IL-17 is not required for generation of Th1 responses or overall protective immunity against pulmonary F. tularensis LVS infection. Instead, excess IL-17 mediates pathology during pulmonary F. tularensis LVS infection.
Dysregulated IL-17 Production Mediates Neutrophil Recruitment and Increased Susceptibility in Il10−/−F. tularensis LVS-Infected Mice
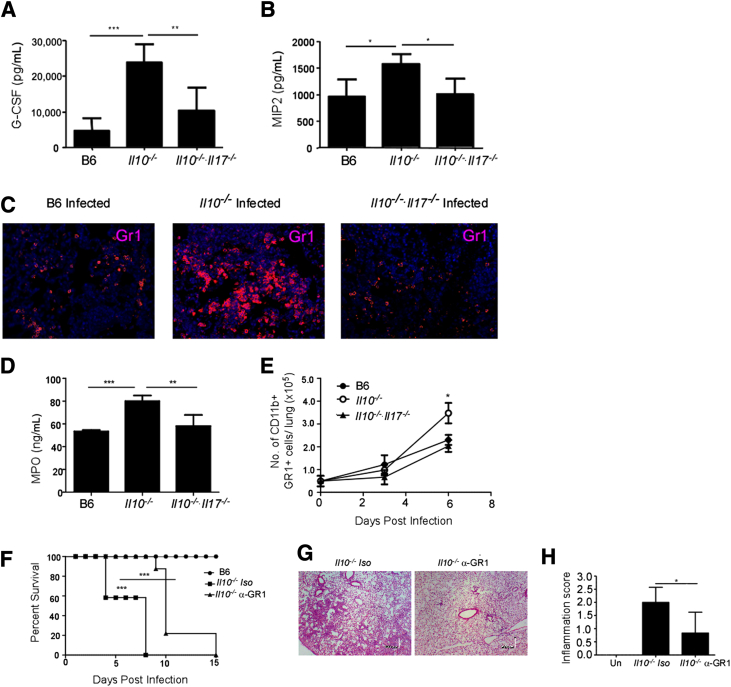
The absence of IL-17 during pulmonary tularemia results in decreased induction of G-CSF, neutrophil recruitment, and resulting inflammation in the infected lung.7 Correlating with the increased IL-17 levels in infected Il10−/− mice, we also found increased levels of G-CSF and the neutrophil-attracting chemokine, MIP-2, in Il10−/−-infected lungs (Figure 3, A and B, respectively). This also coincided with increased accumulation of neutrophils in the severely inflamed lungs of Il10−/−-infected mice (Figure 3C). The activity of MPO, an enzyme that is associated with neutrophil activation and generation of reactive oxygen species, leading to oxidative damage,21 was also notably enhanced in lungs of infected Il10−/− mice (Figure 3D). More importantly, increased numbers of neutrophils also accumulated in day 6–infected lungs of Il10−/− mice (Figure 3E). In contrast, we found that absence of IL-17 in Il10−/− mice reversed the induction of G-CSF and MIP-2 (Figure 3, A and B) and reduced neutrophil accumulation within lung sections (Figure 3C). This coincided with reversal of inflammation in the lung and decreased expression of MPO and decreased neutrophil accumulation in day 6–infected lungs from Il10−/−/Il17−/− mice, compared with Il10−/− mice (Figure 3, D and E). Together, our new data show that exacerbated IL-17 production is pathological during pulmonary infection with F. tularensis LVS.
Figure 3.
A and B: Neutrophils contribute to lung pathological characteristics and increased mortality in Il10−/− mice after F. tularensis LVS infection. B6, Il10−/−, and Il10−/−/Il17−/− mice were infected with 1000 CFUs F. tularensis LVS intratracheally, and on day 6, lung homogenates from infected B6 and gene-deficient mice were assayed for G-CSF (A) and MIP-2 (B) protein levels. C: Serial sections of day 6–infected, formalin-fixed, paraffin-embedded (FFPE) lungs were stained with antibodies specific to GR1. D and E: The level of MPO activity was determined in lung homogenates (D), and neutrophil accumulation (CD11b+ GR1+ cells) in the lungs was enumerated using flow cytometry (E). The data points represent the means ± SD of values from 6 to 10 mice. F: Il10−/− mice were infected with 1000 CFUs F. tularensis LVS intratracheally and treated with Gr1-depleting antibody or isotype control and monitored for survival. G and H: Statistical significance was by log-rank test for the survival studies. Day 6, formalin-fixed, paraffin-embedded (FFPE) serial lung sections were stained with H&E (G), and sections were scored for percentage of inflammation, as described in Materials and Methods (H). The data points represent the means ± SD of values from 6 to 10 mice. ∗P ≤ 0.05, ∗∗P ≤ 0.005, and ∗∗∗P ≤ 0.0005. One experiment is representative of two or more.
To further confirm that the exacerbated inflammation caused because of increased neutrophil recruitment was mediating the increased mortality in Il10−/− mice, we specifically depleted Gr1+ neutrophils using a monoclonal antibody (clone 1A8), which is known to specifically deplete neutrophils without affecting Gr1+ monocyte populations.22 We treated F. tularensis LVS-infected Il10−/− mice with 1A8 antibody or isotype control antibody every 48 hours, and found that Il10−/− mice depleted of neutrophils exhibited decreased susceptibility (Figure 3F) and decreased lung inflammation (Figure 3, G and H). These data together demonstrate that exacerbated neutrophil recruitment and associated inflammation mediate the increased mortality seen in Il10−/− F. tularensis LVS-infected mice.
Discussion
Production of IL-10 is largely believed to be detrimental for immunity against intracellular pathogens, such as M. tuberculosis12 and L. monocytogenes.13 In addition, IL-10 also limits the protective efficacy of M. bovis bacillus Calmette-Guerin vaccination after M. tuberculosis challenge,20,23 suggesting use of anti–IL-10 as a potential adjuvant to improve Th1 responses and immunity against intracellular pathogens. However, in pathological conditions, such as chronic inflammatory bowel disease,24 IL-10 deficiency unleashes an inflammatory response, which earlier was thought to be associated with enhanced CD4+ Th1 cells,25 but was later attributed to inflammatory Th17 cells.26 In contrast, IL-10 deficiency is associated with enhanced Th17 responses during influenza challenge, and a cutaneous model of F. tularensis LVS infection,14 in which it is associated with better protective outcomes.27 In this context, the interesting and unexpected finding reported in this study is that unrestrained IL-17, induced in response to pulmonary F. tularensis LVS infection in Il10−/− mice, mediates neutrophil recruitment and associated pathological characteristics. However, because IL-17 is also required to drive IL-12 production and generate Th1 responses during pulmonary tularemia,7 our studies together propose the new model that IL-17 is required to drive IL-12 and Th1 responses, likely to overcome the inhibitory effects of IL-10 on IL-12 production. In contrast, in the absence of IL-10, dysregulated and exacerbated production of IL-17 production is pathological and mediates inflammation, suggesting a critical role for IL-10 in maintaining the delicate balance between host immunity and pathological characteristics during pulmonary infection with F. tularensis LVS.
Our data show that IL-10 is produced early in the lung after pulmonary F. tularensis LVS infection and that lung DCs are one of the primary cells producing IL-10. In addition, data show that infection of BMDCs with F. tularensis LVS induces IL-10 production and IL-10 neutralization in DC:T-cell co-cultures increased both IL-17 and IFN-γ production in CD4+ T cells. These data are consistent with a previous study that also showed that BMDCs induced IL-10 mRNA and protein in response to F. tularensis LVS infection, in a toll-like receptor-2–dependent manner.28,29 In addition, in vitro studies have demonstrated that F. tularensis LVS, cultured in MH broth, induces more robust IL-10 production in DCs, when compared with host-adapted bacteria cultured in brain heart infusion media.29 However, these differences are only apparent at early time points after infection,30 and our studies, both in vitro and in vivo, were performed over a period of 6 days or more, by which time the bacteria are host adapted. In addition, the observed increased mortality of Il10−/− mice after F. tularensis LVS infection substantiates that in vivo IL-10 is produced and is critical for restraining Th1 and Th17 responses after infection. Accordingly, in this study, increased IFN-γ and IL-17 levels were observed in Il10−/− F. tularensis LVS-infected lungs. A previous study has demonstrated that Il10−/− DCs secrete high levels of IL-12/IL-23 p40 when pulsed overnight with cecal bacterial lysate.31 Thus, it is possible that the increased expression of these Th1- and Th17-polarizing cytokines and other molecules, such as CD86, in Il10−/− DCs31 drives the increased expression of IL-17 and IFN-γ in CD4+ T cells. This finding is consistent with other pulmonary intracellular bacterial infection models, such as pulmonary infection with M. tuberculosis, in which Il10−/−-infected mice demonstrated increased lung IFN-γ and IL-17 levels.12 However, in contrast to our study, M. tuberculosis–infected Il10−/− mice demonstrated better bacterial control and no remarkable differences in lung pathology.12 These data suggest that in some pulmonary infection models, such as F. tularensis LVS, IL-10 plays dual roles. On one hand, IL-10 restrains the inflammatory effects of IL-17 and is beneficial, whereas on the other hand, IL-10 restrains IL-12 and IFN-γ responses and is detrimental to host immunity. Thus, our studies further suggest that the indirect role for IL-17 in driving IL-12 production and Th1 responses is to overcome the inhibitory effects of IL-10 on IL-12 and Th1 responses. This hypothesis is consistent with the finding that, although Il17−/− mice are more susceptible to pulmonary F. tularensis LVS infection and have increased lung bacterial burden and decreased lung IFN-γ levels,7 this requirement for IL-17 in driving Th1 immunity is lost when IL-10 is absent. Interestingly, in a cutaneous model of F. tularensis LVS infection, increased IL-17 that is induced in the IL-10–deficient mice results in improved survival of mice.14 Thus, a recent study,14 along with data shown in this study, demonstrate that, depending on the route of F. tularensis LVS infection, excess IL-17 induced in response to infection can drive either protective or pathological responses.
Recent data suggest that in vitro infection of human monocytes with the virulent strain F. tularensis SCHU S4 induces IL-23 expression,32 and that pulmonary infection with F. tularensis LVS induces lung Th17 cells.33 Exogenous administration of IL-17 delays the time of death from lethal intranasal F. tularensis LVS infection,9 and rescues IFN-γ production in the F. tularensis LVS-infected lung.7 In addition, IL-17 neutralization enhances bacterial burden in previously immunized mice that were challenged with virulent aerosolized F. tularensis SCHU S4.34 More important, peripheral blood mononuclear cells from F. tularensis LVS-immunized human volunteers produce IL-17 in memory CD4+ and CD8+ T cells on stimulation with Francisella antigens,35 suggesting that IL-17 could serve as a good immune correlate for both primary and secondary immunity against tularemia. In this context, it is interesting that prostaglandin E2 is critical for induction of IL-23 and Th17 responses,36,37 whereas it inhibits IL-12 responses through production of IL-10.38 In this context, IL-10 restrains IL-17 production and resulting neutrophil recruitment and inflammation during pulmonary infection with F. tularensis LVS. This is clearly demonstrated in our study because the increased inflammation, induction of G-CSF and MIP-2, and neutrophil accumulation seen in Il10−/−-infected lungs was completely reversed in the lungs of Il10−/−/Il17−/−-infected mice. More important, we also provide evidence that the increased mortality seen in Il10−/−-infected mice is the result of exacerbated neutrophilic inflammation, because Il10−/− mice depleted of neutrophils result in decreased mortality, improved survival, and decreased inflammation. Previous studies showed that depletion of Gr1 cells (using the RB6-8c5 antibody) in wild-type BALB/c mice infected with aerosolized F. tularensis LVS did not affect bacterial burden in the lung.10 This supports our findings that the improved survival observed in Il10−/−-infected mice, which underwent neutrophil depletion, was likely due to decreased inflammatory responses observed, rather than a direct role for neutrophils, in protective immunity. The absence of IL-17 in the Il10−/− mice does not completely rescue susceptibility and improve survival to levels observed in B6-infected mice, suggesting that the increased IFN-γ responses observed in Il10−/−-infected lungs may also be playing a secondary role in this model, and should be further explored.
In summary, our data together suggest that, although IL-17 is required for protective immunity against pulmonary infection with F. tularensis LVS,7,9 its production is tightly regulated by anti-inflammatory cytokines, such as IL-10. However, when IL-17 production is dysregulated, it can cause exacerbated inflammation, associated pathological characteristics, and increased mortality after F. tularensis LVS infection.
Acknowledgments
We thank Stacey Yeh and Daniel Mallon for technical help with some assays.
Footnotes
Supported by NIH grants HL105427 (S.A.K.), T32 AI065380-08 (S.R.S.), and U19 AI91036 (J.R.-M.); Children’s Hospital of Pittsburgh Research Advisory Committee, Children’s Hospital of Pittsburgh, University of Pittsburgh Medical Center Health System grants (L.M. and S.R.S.); and Department of Medicine, University of Rochester, funds (J.R.-M.).
References
- 1.Dennis D.T., Inglesby T.V., Henderson D.A., Bartlett J.G., Ascher M.S., Eitzen E., Fine A.D., Friedlander A.M., Hauer J., Layton M., Lillibridge S.R., McDade J.E., Osterholm M.T., O’Toole T., Parker G., Perl T.M., Russell P.K., Tonat K. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 2.Conlan J.W. Vaccines against Francisella tularensis–past, present and future. Expert Rev Vaccines. 2004;3:307–314. doi: 10.1586/14760584.3.3.307. [DOI] [PubMed] [Google Scholar]
- 3.Anthony L.S., Ghadirian E., Nestel F.P., Kongshavn P.A. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb Pathog. 1989;7:421–428. doi: 10.1016/0882-4010(89)90022-3. [DOI] [PubMed] [Google Scholar]
- 4.Elkins K.L., Cooper A., Colombini S.M., Cowley S.C., Kieffer T.L. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect Immun. 2002;70:1936–1948. doi: 10.1128/IAI.70.4.1936-1948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duckett N.S., Olmos S., Durrant D.M., Metzger D.W. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect Immun. 2005;73:2306–2311. doi: 10.1128/IAI.73.4.2306-2311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolls J.K., Khader S.A. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010;21:443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin Y., Ritchea S., Logar A., Slight S., Messmer M., Rangel-Moreno J., Guglani L., Alcorn J.F., Strawbridge H., Park S.M., Onishi R., Nyugen N., Walter M.J., Pociask D., Randall T.D., Gaffen S.L., Iwakura Y., Kolls J.K., Khader S.A. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowley S.C., Meierovics A.I., Frelinger J.A., Iwakura Y., Elkins K.L. Lung CD4- CD8- double-negative T cells are prominent producers of IL-17A and IFN-gamma during primary respiratory murine infection with Francisella tularensis live vaccine strain. J Immunol. 2010;184:5791–5801. doi: 10.4049/jimmunol.1000362. [DOI] [PubMed] [Google Scholar]
- 9.Markel G., Bar-Haim E., Zahavy E., Cohen H., Cohen O., Shafferman A., Velan B. The involvement of IL-17A in the murine response to sub-lethal inhalational infection with Francisella tularensis. PLoS One. 2010;5:e11176. doi: 10.1371/journal.pone.0011176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conlan J.W., KuoLee R., Shen H., Webb A. Different host defences are required to protect mice from primary systemic vs pulmonary infection with the facultative intracellular bacterial pathogen, Francisella tularensis. LVS Microb Pathog. 2002;32:127–134. doi: 10.1006/mpat.2001.0489. [DOI] [PubMed] [Google Scholar]
- 11.Saraiva M., O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 12.Redford P.S., Boonstra A., Read S., Pitt J., Graham C., Stavropoulos E., Bancroft G.J., O’Garra A. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur J Immunol. 2010;40:2200–2210. doi: 10.1002/eji.201040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai W.J., Kohler G., Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- 14.Metzger D.W., Salmon S.L., Kirimanjeswara G. Differing effects of interleukin-10 on cutaneous and pulmonary Francisella tularensis live vaccine strain infection. Infect Immun. 2013;81:2022–2027. doi: 10.1128/IAI.00024-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakae S., Komiyama Y., Nambu A., Sudo K., Iwase M., Homma I., Sekikawa K., Asano M., Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 16.Kang D.D., Lin Y., Moreno J.R., Randall T.D., Khader S.A. Profiling early lung immune responses in the mouse model of tuberculosis. PLoS One. 2011;6:e16161. doi: 10.1371/journal.pone.0016161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manni M.L., Epperly M.W., Han W., Blackwell T.S., Duncan S.R., Piganelli J.D., Oury T.D. Leukocyte-derived extracellular superoxide dismutase does not contribute to airspace EC-SOD after interstitial pulmonary injury. Am J Physiol Lung Cell Mol Physiol. 2012;302:L160–L166. doi: 10.1152/ajplung.00360.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khader S.A., Partida-Sanchez S., Bell G., Jelley-Gibbs D.M., Swain S., Pearl J.E., Ghilardi N., Desauvage F.J., Lund F.E., Cooper A.M. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006;203:1805–1815. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slight S.R., Rangel-Moreno J., Gopal R., Lin Y., Fallert Junecko B.A., Mehra S., Selman M., Becerril-Villanueva E., Baquera-Heredia J., Pavon L., Kaushal D., Reinhart T.A., Randall T.D., Khader S.A. CXCR5+ T helper cells mediate protective immunity against tuberculosis. J Clin Invest. 2013;123:712–726. doi: 10.1172/JCI65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gopal R., Lin Y., Obermajer N., Slight S., Nuthalapati N., Ahmed M., Kalinski P., Khader S.A. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol. 2012;42:364–373. doi: 10.1002/eji.201141569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinecke J.W., Li W., Francis G.A., Goldstein J.A. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J Clin Invest. 1993;91:2866–2872. doi: 10.1172/JCI116531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daley J.M., Thomay A.A., Connolly M.D., Reichner J.S., Albina J.E. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 23.Pitt J.M., Stavropoulos E., Redford P.S., Beebe A.M., Bancroft G.J., Young D.B., O’Garra A. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J Immunol. 2012;189:4079–4087. doi: 10.4049/jimmunol.1201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berg D.J., Davidson N., Kuhn R., Muller W., Menon S., Holland G., Thompson-Snipes L., Leach M.W., Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson N.J., Leach M.W., Fort M.M., Thompson-Snipes L., Kuhn R., Muller W., Berg D.J., Rennick D.M. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996;184:241–251. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yen D., Cheung J., Scheerens H., Poulet F., McClanahan T., McKenzie B., Kleinschek M.A., Owyang A., Mattson J., Blumenschein W., Murphy E., Sathe M., Cua D.J., Kastelein R.A., Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKinstry K.K., Strutt T.M., Buck A., Curtis J.D., Dibble J.P., Huston G., Tighe M., Hamada H., Sell S., Dutton R.W., Swain S.L. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H., Nookala S., Bina X.R., Bina J.E., Re F. Innate immune response to Francisella tularensis is mediated by TLR2 and caspase-1 activation. J Leukoc Biol. 2006;80:766–773. doi: 10.1189/jlb.0406294. [DOI] [PubMed] [Google Scholar]
- 29.Periasamy S., Singh A., Sahay B., Rahman T., Feustel P.J., Pham G.H., Gosselin E.J., Sellati T.J. Development of tolerogenic dendritic cells and regulatory T cells favors exponential bacterial growth and survival during early respiratory tularemia. J Leukoc Biol. 2011;90:493–507. doi: 10.1189/jlb.0411197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hazlett K.R., Caldon S.D., McArthur D.G., Cirillo K.A., Kirimanjeswara G.S., Magguilli M.L., Malik M., Shah A., Broderick S., Golovliov I., Metzger D.W., Rajan K., Sellati T.J., Loegering D.J. Adaptation of Francisella tularensis to the mammalian environment is governed by cues which can be mimicked in vitro. Infect Immun. 2008;76:4479–4488. doi: 10.1128/IAI.00610-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albright C.A., Sartor R.B., Tonkonogy S.L. Endogenous antigen presenting cell-derived IL-10 inhibits T lymphocyte responses to commensal enteric bacteria. Immunol Lett. 2009;123:77–87. doi: 10.1016/j.imlet.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butchar J.P., Rajaram M.V., Ganesan L.P., Parsa K.V., Clay C.D., Schlesinger L.S., Tridandapani S. Francisella tularensis induces IL-23 production in human monocytes. J Immunol. 2007;178:4445–4454. doi: 10.4049/jimmunol.178.7.4445. [DOI] [PubMed] [Google Scholar]
- 33.Woolard M.D., Hensley L.L., Kawula T.H., Frelinger J.A. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect Immun. 2008;76:2651–2659. doi: 10.1128/IAI.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen H., Harris G., Chen W., Sjostedt A., Ryden P., Conlan W. Molecular immune responses to aerosol challenge with Francisella tularensis in mice inoculated with live vaccine candidates of varying efficacy. PLoS One. 2010;5:e13349. doi: 10.1371/journal.pone.0013349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paranavitana C., Zelazowska E., DaSilva L., Pittman P.R., Nikolich M. Th17 cytokines in recall responses against Francisella tularensis in humans. J Interferon Cytokine Res. 2010;30:471–476. doi: 10.1089/jir.2009.0108. [DOI] [PubMed] [Google Scholar]
- 36.Khayrullina T., Yen J.H., Jing H., Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181:721–735. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boniface K., Bak-Jensen K.S., Li Y., Blumenschein W.M., McGeachy M.J., McClanahan T.K., McKenzie B.S., Kastelein R.A., Cua D.J., de Waal Malefyt R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalinski P., Hilkens C.M., Snijders A., Snijdewint F.G., Kapsenberg M.L. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]