Abstract
Bile acids play a critical role in liver injury and regeneration, but their role in acetaminophen (APAP)–induced liver injury is not known. We tested the effect of bile acid modulation on APAP hepatotoxicity using C57BL/6 mice, which were fed a normal diet, a 2% cholestyramine (CSA)–containing diet for bile acid depletion, or a 0.2% cholic acid (CA)–containing diet for 1 week before treatment with 400 mg/kg APAP. CSA-mediated bile acid depletion resulted in significantly higher liver injury and delayed regeneration after APAP treatment. In contrast, 0.2% CA supplementation in the diet resulted in a moderate delay in progression of liver injury and significantly higher liver regeneration after APAP treatment. Either CSA-mediated bile acid depletion or CA supplementation did not affect hepatic CYP2E1 levels or glutathione depletion after APAP treatment. CSA-fed mice exhibited significantly higher activation of c-Jun N-terminal protein kinases and a significant decrease in intestinal fibroblast growth factor 15 mRNA after APAP treatment. In contrast, mice fed a 0.2% CA diet had significantly lower c-Jun N-terminal protein kinase activation and 12-fold higher fibroblast growth factor 15 mRNA in the intestines. Liver regeneration after APAP treatment was significantly faster in CA diet–fed mice after APAP administration secondary to rapid cyclin D1 induction. Taken together, these data indicate that bile acids play a critical role in both initiation and recovery of APAP-induced liver injury.
Bile acids are versatile biological molecules that regulate energy homeostasis, activate nuclear receptors and cell signaling pathways, and control cell proliferation and inflammatory processes in the liver and gastrointestinal tract.1,2 Bile acids maintain their own homeostasis by activating a complex signaling network involving hepatic and intestinal farnesoid X receptor (FXR), small heterodimer partner, and intestinal fibroblast growth factor (FGF) 15 (FGF19 in human) expression, culminating in inhibition of the primary bile acid–synthesizing enzyme, CYP7A1.3–6 Although bile acids are potent signaling molecules at pathophysiological concentrations, they cause apoptosis, necrosis, and oxidative stress.3,7–10 Bile acids have also been implicated in stimulation of liver regeneration.11–14 Studies in recent years indicate that the bile acid–mediated gut-liver signaling axis may play a critical role in regulation of liver homeostasis.6,15,16
Acetaminophen (APAP) is the most commonly used analgesic and antipyretic agent.17 An overdose of APAP is the major cause of acute liver failure in the United States.18,19 The mechanisms of APAP-induced liver injury and subsequent liver regeneration are the focus of intense investigation.20–22 In an overdose situation, excess APAP is mainly metabolized by CYP2E1 to a reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI). In hepatocytes, conjugation of NAPQI to GSH is the key mechanism for detoxification of NAPQI. Once the GSH is depleted, NAPQI attacks cellular proteins, especially mitochondrial proteins, to form protein adducts. This triggers a cascade of intracellular signaling events involving c-Jun N-terminal protein kinase (JNK) activation and mitochondrial permeability transition, finally culminating in necrotic cell death.20 Liver injury is followed by compensatory liver regeneration, which is a critical determinant of final outcome of liver injury.23 Despite decades of research, how these intracellular events are affected by extracellular signaling is not known.
The current study was designed to explore the role of bile acids in initiation of liver injury and stimulation of liver regeneration after APAP overdose. These studies are highly significant because the data reveal a novel role of bile acids in cellular protection and liver regeneration after APAP overdose, and these studies investigate the effect of resin-mediated bile acid depletion, a commonly used therapy, on APAP toxicity.
Materials and Methods
Animals, Treatments, and Tissue Collection
The C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, MN). Two-month-old male mice (n = 5 to 8 per group per time point) were used in these studies. All animal studies were approved by and performed in accordance with the Institutional Animal Care and Use Committee at University of Kansas Medical Center (Kansas City). Mice were fed a normal diet, a 2% cholestyramine (CSA)–containing diet, or a 0.2% cholic acid (CA)–containing diet for 1 week. After a 1-week feeding of specific diets, mice were injected i.p. with 400 mg/kg APAP (Sigma, St. Louis, MO) dissolved in warm sterile saline solution. Mice were not fasted before APAP treatment. Mice were sacrificed at 0, 1, 4, 8, 12, and 24 hours after APAP treatment, and livers, intestines, and serum were collected and processed as previously described.14
Histological and IHC Data
Paraffin-embedded liver sections (4 μm thick) were used for H&E staining and immunohistochemical (IHC) detection of proliferating cell nuclear antigen (PCNA), as previously described.14 H&E-stained slides were used for necrosis scoring, as previously described,24 and PCNA-stained slides were used for PCNA scoring, as previously described.25
Protein Extraction, Microsome Preparation, and Western Blot Analysis
Protein extracts were prepared from frozen liver tissues using radioimmunoprecipitation assay buffer, containing fresh protease and phosphatase inhibitors, and used for Western blot analysis, as previously described.14 Primary and secondary antibodies were purchased from Cell Signaling Technologies (Danvers, MA).
Microsomes were prepared from murine liver by homogenizing tissue using a Teflon homogenizer in phosphate buffer (pH 7.4) and centrifuged at 9000 × g for 30 minutes at 4°C. The supernatant from this centrifugation was centrifuged at 100,000 × g for 60 minutes at 4°C. The microsomal pellet was resuspended in phosphate buffer with 0.1 mmol/L EDTA (pH 7.4). Microsomes (50 μg) were used for CYP2E1 Western blot analysis.
Real-Time PCR Analysis
Total RNA was extracted from all murine intestines using TRIzol, per the manufacturer’s protocol (Sigma), and reverse transcribed as previously described.14 FGF15 mRNA levels were determined by real-time PCR assays on the Applied Biosystems Prism 7300 Instrument (Grand Island, NY), as previously described.14 18S RNA levels were used for data normalization.
Serum ALT Measurement
Serum alanine aminotransferase (ALT) was measured by kinetic assay method using the Infinity ALT kit (Thermo Scientific, Waltham, MA), according to the manufacturer’s protocol.
Bile Acid Analysis
Serum, liver, and intestinal total bile acids were measured enzymatically using a Total Bile Acid Assay Kit (BQ kits, San Diego, CA), per the manufacturer’s protocol. Briefly, in a 96-well plate, 20 μL of bile acid standard and murine serum samples were added in triplicate. Diaphorase in phosphate buffer (150 μL) was then added and incubated at 37°C for 4 minutes. After adding 30 μL of 3-α-hydroxy steroid dehydrogenase in Tris buffer, absorbance was measured at 540 nm kinetically for 5 minutes. Specific hepatic bile acids were analyzed by Ultra Performance Liquid Chromatography-Mass Spectrometry (Waters, Milford, MA), as previously described.14
Glutathione Analysis
Total GSH was measured in liver homogenates by the 5,5′-dithiobis-(2- nitrobenzoic acid)-glutathione reductase recycling assay (Sigma). Briefly, total glutathione disulfide (GSH) was extracted from liver homogenates in 5% sulfosalicylic acid and oxidized to GSSG by 5,5′-dithiobis-(2- nitrobenzoic acid). After adding NADPH, the rate of GSH formation in the presence of glutathione reductase was measured kinetically at 412 nm.
Statistical Analysis
All data presented as bar graphs are expressed as means ± SEM. An unpaired Student’s t-test was used to evaluate statistically significant differences between groups. P < 0.05 was considered statistically significant.
Results
Increase in APAP-Induced Liver Injury and Delayed Liver Regeneration after Cholestyramine-Mediated Bile Acid Depletion
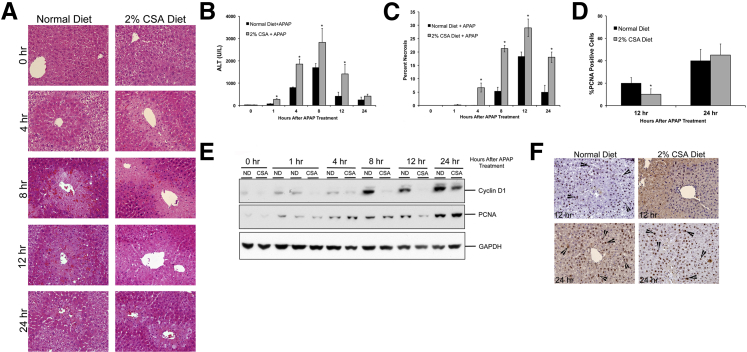
The CSA-fed mice showed a rapid and higher liver injury within 1 hour after APAP treatment compared with control mice, as demonstrated by histopathological analysis of H&E-stained liver sections (Figure 1A), serum ALT levels (Figure 1B), and necrosis scoring (Figure 1C). Centrilobular necrosis was clearly evident at 4 hours after APAP treatment in CSA-fed mice but not until 8 hours after APAP treatment in normal diet–fed mice. Peak liver injury was observed at 8 hours in both groups. Injury decreased significantly in the normal diet–fed mice at 24 hours after APAP treatment. Although serum ALT values declined at 24 hours in CSA-fed mice, necrosis scoring demonstrated that significant liver cell death was still evident in CSA-fed mice at 24 hours after APAP administration. Liver regeneration was studied using PCNA analysis for determining cell proliferation and Western blot analysis of cyclin D1 and PCNA (Figure 1, D–F). PCNA staining (Figure 1, D and F) and cyclin D1 Western blot data (Figure 1E) indicate that the CSA-fed mice experienced a delay in initiation of liver regeneration after APAP overdose and exhibited significantly lower cell proliferation at 12 hours after APAP administration. However, this delay in cell proliferation was overcome by 24 hours after APAP treatment, and both normal diet–fed and CSA-fed mice had similar liver regeneration.
Figure 1.
Increased acetaminophen (APAP)-induced liver injury after bile acid depletion. A: Representative photomicrographs of H&E-stained liver sections obtained over a time course of 0 to 24 hours after APAP treatment of mice fed either a normal diet or a 2% CSA diet. B: Serum ALT activity measured over a time course of 0 to 24 hours after APAP treatment of mice fed either a normal diet or a 2% CSA diet. C: Percentage necrosis after APAP treatment. D: Percentage PCNA-positive cells at 12 and 24 hours in liver sections of APAP-treated mice fed either a normal diet or a 2% CSA diet. E: Western blot analysis of cyclin D1 and PCNA using total liver cell extracts of normal diet– or CSA diet–fed mice treated with APAP. F: Representative photomicrographs of PCNA IHC staining of liver sections of APAP-treated mice fed either a normal diet or a 2% CSA diet at 12 and 24 hours after APAP treatment. Arrowheads indicate cells in the S phase. ∗P < 0.05 (B–D). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Mechanisms of Increased APAP-Induced Injury after Bile Acid Depletion
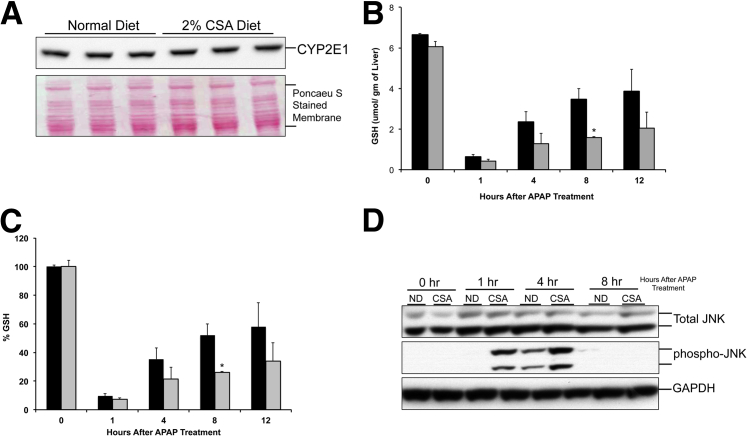
To determine the mechanism behind increased APAP-induced cell injury in CSA-fed mice, we analyzed the levels of APAP-metabolizing enzyme, CYP2E1, antioxidant GSH, and JNK activation, which is known to be a critical step in APAP-induced liver injury. Western blot analysis showed no difference in microsomal CYP2E1 expression between CSA diet–fed mice and normal diet–fed mice (Figure 2A). Analysis of total GSH levels in liver revealed that CSA diet feeding did not have any effect on hepatic GSH levels before APAP treatment. Furthermore, similar GSH depletion (90% for control) was observed at 1 hour after APAP treatment in both normal diet– and CSA-fed mice (Figure 2B). Interestingly, the recovery of hepatic GSH was significantly delayed in the CSA diet–fed mice after APAP administration (Figure 2C). By 12 hours, the normal diet–fed mice had recovered GSH up to 60% of the pre-APAP levels, but the CSA diet–fed mice had recovered only 30% of their pre-APAP levels. Western blot analysis of total and phosphorylated JNK indicated significant activation of JNK in CSA-fed mice within 1 hour after APAP treatment, which further increased and was higher than the normal diet–fed mice at 4 hours after APAP administration (Figure 2D). In contrast, JNK activation was evident in normal diet–fed mice only at 4 hours after APAP treatment.
Figure 2.
Mechanisms of increased acetaminophen (APAP)-induced injury after bile acid depletion. A: Western blot analysis of microsomal CYP2E1 using total liver cell extract of normal diet (ND)–fed and cholestyramine (CSA)-fed murine livers obtained before APAP treatment at 0 hours. B: GSH levels in the livers of ND-fed (black bars) and CSA-fed (gray bars) mice during a time course of 0 to 12 hours after APAP treatment. C: Percentage of GSH depletion in the livers of control (black bars) and CSA-fed (gray bars) mice during a time course of 0 to 12 hours after APAP treatment. D: Western blot analysis of total and phosphorylated JNK using total liver cell extracts of ND-fed and CSA-fed murine livers obtained at various time points after APAP treatment. ∗P < 0.05 (B and C). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Changes in Bile Acid Composition in Livers of CSA-Fed Mice
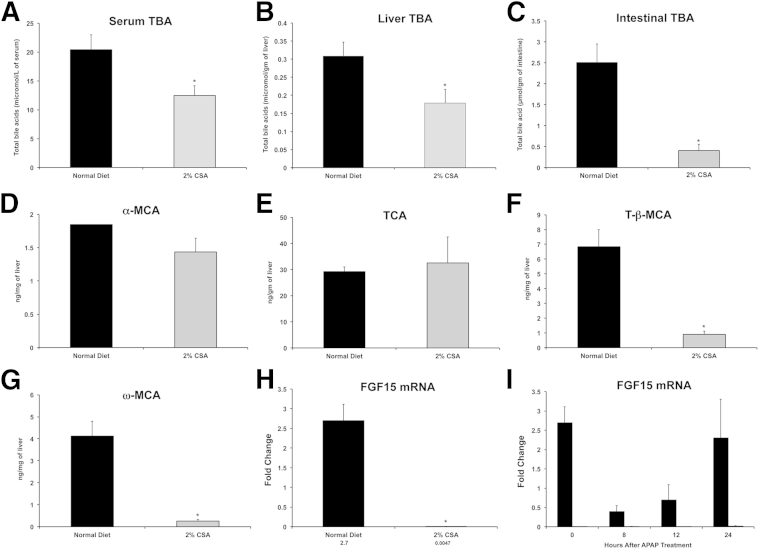
Next, we determined changes in total bile acid levels in serum, liver, and intestine in normal diet–fed and CSA-fed mice. The data indicate a significant decrease in serum, hepatic, and intestinal bile acid levels after CSA treatment (Figure 3, A–C). We further quantified specific bile acid species in the livers of normal diet–fed and CSA-fed mice, which indicated a significant decrease in tauro-β-muricholic acid and ω-muricholic acid in CSA-fed mice (Figure 3, D–G).
Figure 3.
Changes in total bile acids (TBAs) and intestinal FGF15 mRNA after CSA treatment. Total bile acids in serum (A), liver (B), and intestines (C) in normal diet–fed (black bars) or CSA-diet (gray bars) fed mice. UPLC-MS–mediated quantification of specific hepatic bile acids, including α-muricholic acid (MCA; D), tauro-β-muricholic acid (TCA; E), tauro-β-MCA (F), and ω-MCA (G). Serum and liver samples at 0 hours before APAP administration were used for the analysis. H: Real-time PCR analysis of FGF15 mRNA in the intestine of control and CSA-fed mice before APAP treatment at 0 hours. I: Real-time PCR analysis of FGF15 mRNA in the intestine of control and CSA-fed mice at various time points after APAP treatment. ∗P < 0.05 (A–C and F–H).
Significant Inhibition of Intestinal FGF15 Expression after CSA Treatment
Bile acids are known to regulate their own biosynthesis via the liver-gut FXR-FGF15 signaling axis. Bile acids are absorbed in the ileal cells, where they activate FXR, which, in turn, stimulates FGF15 expression and secretion. FGF15 comes to liver via portal circulation and via binding to FGF receptor 4, inhibits hepatic CYP7A1 expression, and down-regulates bile acid synthesis. The effect of CSA-mediated bile acid reduction on intestinal FGF15 expression in normal diet–fed and CSA diet–fed mice was determined using real-time PCR. The data indicate that CSA-fed mice had >500-fold lower intestinal FGF15 than normal diet–fed mice, even before APAP treatment (Figure 3H). After APAP treatment, FGF15 gene expression decreased significantly by 8 hours after APAP treatment but returned to pre-APAP levels by 24 hours after APAP treatment in normal diet–fed mice. In contrast, FGF15 expression remained extremely low to undetectable throughout the time course after APAP treatment in CSA-fed mice (Figure 3I).
Delayed Initiation and Rapid Resolution of APAP-Induced Liver Injury in 0.2% CA Diet–Fed Mice
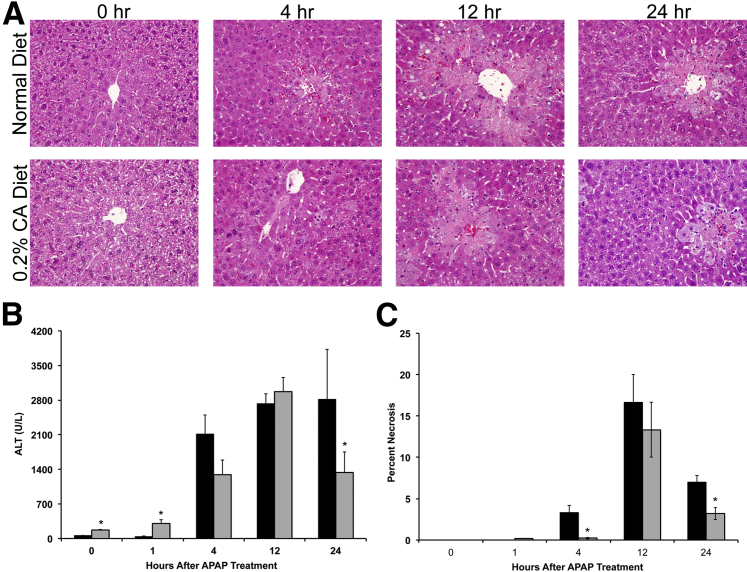
To determine whether bile acid supplementation modulates APAP hepatotoxicity, we fed C57BL/6 mice either a normal diet or a 0.2% CA diet for 1 week before treatment of 400 mg/kg of APAP. Liver injury was determined using serum ALT levels (Figure 4B) and necrosis scoring of H&E-stained paraffin sections (Figure 4, A and C). CA diet–fed mice showed a moderate increase in serum ALT levels before APAP treatment, which continued at 1 hour after APAP treatment. However, necrosis was not evident in histological examinations in CA diet–fed mice before (0 hours) and 1 hour after APAP treatment. After APAP treatment, CA diet–fed mice exhibited a delayed initiation of liver injury, as shown by a lower (but not statistically significant) serum ALT level and necrosis score at 4 hours, which were statistically significant. However, liver injury increased thereafter and was similar in normal diet–fed and CA diet–fed mice at its peak at 12 hours. Interestingly, APAP-induced liver injury decreased much faster and was significantly lower at 24 hours in CA diet–fed mice compared with normal diet–fed mice, as shown by lower serum ALT levels and necrosis scores.
Figure 4.
Cholic acid supplementation delays initiation of injury and stimulates rapid recovery after APAP treatment. A: Representative photomicrographs of H&E-stained liver sections of normal diet– and 0.2% CA diet–fed mice treated with APAP during a time course of 0 to 24 hours. B: Serum ALT levels in normal diet–fed (black bars) and 0.2% CA diet–fed mice (gray bars) treated with APAP. C: Percentage of cells in necrosis in the livers of normal diet–fed (black bars) and 0.2% CA diet–fed mice (gray bars) treated with APAP. ∗P < 0.05 (B and C).
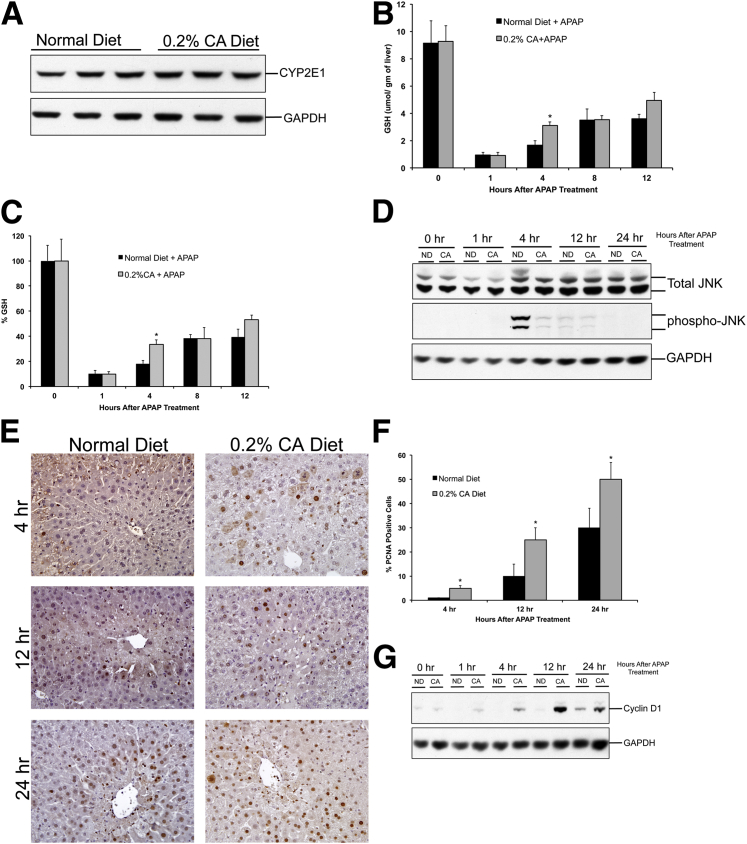
Mechanisms of Delayed Initiation of Liver Injury after CA Supplementation
To determine the mechanisms of delayed initiation of APAP-induced liver injury in CA diet–fed mice, we determined hepatic CYP2E1 levels and GSH depletion after APAP treatment. Western blot analysis using total cell lysates indicated no difference in hepatic CYP2E1 protein expression (Figure 5A). Total GSH-level analysis revealed that cholic acid supplementation did not change hepatic GSH content before APAP treatment. Furthermore, an equal GSH depletion was observed in normal diet–fed and CA diet–fed mice 1 hour after APAP treatment (Figure 5B). GSH recovery was moderately faster in CA diet–fed mice after APAP administration (Figure 5C). We next determined activation of JNK using Western blot analysis, which showed that JNK activation was significantly lower in CA diet–fed mice compared with normal diet–fed mice after APAP treatment (Figure 5D). Substantial JNK phosphorylation was observed in normal diet–fed mice at 4 hours after APAP treatment. In contrast, only moderate JNK phosphorylation was observed in CA diet–fed mice.
Figure 5.
Mechanisms of acetaminophen (APAP)-induced liver injury and regeneration after cholic acid supplementation. Western blot analysis of CYP2E1 using total liver cell extract (A), GSH levels in the livers (B), percentage of GSH depletion (C), Western blot analysis of total and phosphorylated JNK using total liver cell extract (D), representative photomicrographs of PCNA IHC staining in the livers (E), bar graph showing percentage of PCNA-positive cells in livers (F), and Western blot analysis of cyclin D1 using total liver cell extracts (G) of normal diet (ND)–fed and 0.2% CA diet–fed mice after APAP treatment. ∗P < 0.05 (B, C, and F). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Mechanisms of Rapid Recovery from APAP-Induced Liver Injury after CA Supplementation
We further determined the level of liver regeneration after APAP-induced liver injury using PCNA analysis and cyclin D1 protein expression. Our data indicate a rapid and higher increase in liver cell proliferation in CA diet–fed mice after APAP treatment (Figure 5, E and F). There was no ongoing cell proliferation in CA diet–fed mice before APAP administration (data not shown). However, a significant number of hepatocytes were PCNA positive at 4 hours after APAP treatment, which further increased at 12 and 24 hours in CA diet–fed mice. Normal diet–fed mice also showed an increase in liver regeneration, but it was significantly slower and at a lower extent compared with CA diet–fed mice. The increase in cell proliferation was consistent with a rapid and higher increase in cyclin D1 expression in CA diet–fed mice after APAP treatment (Figure 5G).
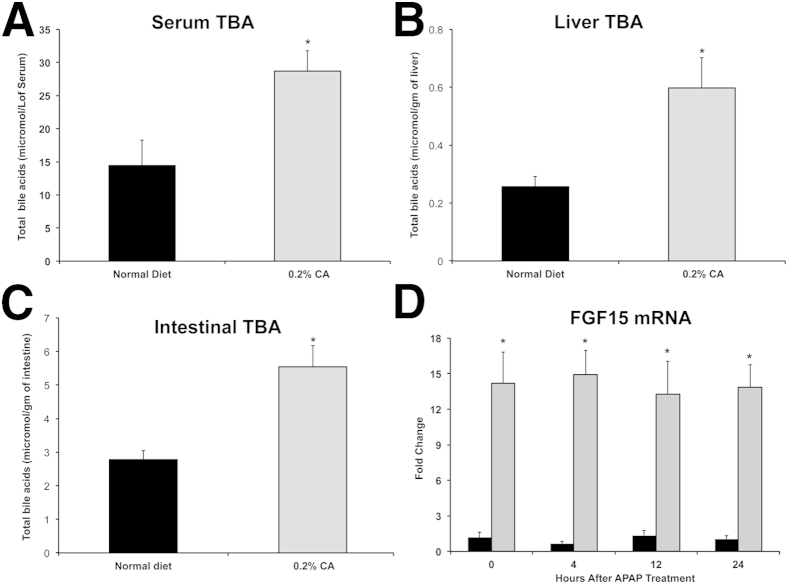
Increase in Total Bile Acids and FGF15 mRNA in CA Diet–Fed Mice
An analysis of total bile acid levels in serum, liver, and intestines of normal diet–fed and CA diet–fed mice revealed that CA supplementation substantially increased total bile acid content in all three compartments (Figure 6, A–C). Further analysis revealed that intestinal FGF15 mRNA was consistently 12- to 14-fold higher in CA diet–fed mice compared with normal diet–fed mice throughout the time course of 0 to 24 hours after APAP treatment (Figure 6D).
Figure 6.
Changes in total bile acids and FGF15 mRNA after cholic acid supplementation. Total bile acids in serum (A), liver (B), and intestines (C) of normal diet–fed and 0.2% cholic acid (CA) diet–fed mice before acetaminophen (APAP) treatment. D: Intestinal FGF15 mRNA expression in normal diet–fed (black bars) and 0.2% CA diet–fed (gray bars) mice before and after APAP administration. ∗P < 0.05 (A–D).
Discussion
Overdose of APAP, the most commonly used analgesic drug, is the main cause of acute liver failure in the Western world.26 Despite decades of research, the mechanisms of APAP-induced liver injury are not completely known.27 Even more complicated and unknown are the mechanisms of APAP overdose on the background of existing disease conditions.27 Furthermore, the contribution of extrahepatic factors to APAP-induced liver injury and subsequent liver regeneration is completely unknown.
Extensive studies in the past decade have demonstrated that bile acids are excellent signaling molecules and that bile acid signaling is involved in several physiological and pathological conditions.1,4 Previous studies have shown that bile acids can signal via multiple receptors, including FXR, TGR5, and epidermal growth factor receptor.3,4,28 At a normal concentration, bile acids seem to be protective against cell stress and injury, but are known to be toxic at higher concentrations.4 Reduction of bile acids using resins, such as cholestyramine, is a common therapy against hypercholesterolemia and in cholestasis.29 Similarly, ursodeoxycholic acid has been used as a treatment for several biliary diseases.30 However, the role of bile acids in APAP-induced liver injury is not known. Our data indicate that depletion of bile acids using CSA results in increased APAP-induced liver injury and slower recovery. In contrast, supplementation of cholic acid in diet resulted in delayed initiation of liver injury and rapid recovery after APAP treatment. Taken together, our data indicate that bile acids may be protective against APAP-induced liver injury.
Our observations are also consistent with previous reports in long-lived Little mice, which were shown to be protected from APAP and bromobenzene hepatotoxicity.31 These mice are also known to have increased bile acids, which have been implicated in several phenotypes observed in these mice, including prolongation of life span and protection from APAP. Furthermore, our data are also consistent with previous studies that activation of FXR, the primary bile acid receptor, provides protection from APAP-induced liver injury.32 However, the mechanisms behind these observations may be different from those underlying our observations.
Our data indicate that neither bile acid depletion by CSA nor CA supplementation interferes with initial bioactivation of APAP to its reactive metabolite, NAPQI. This is evident from rapid and equal depletion of hepatic GSH after APAP treatment in all treatment groups. Previous studies have shown that depletion of GSH is a sensitive indicator of APAP metabolism, especially in overdose situations.21 Recent studies indicate that GSH recovery also plays a critical role in injury development after APAP overdose.33 It is known that GSH recovery, driven by de novo GSH synthesis catalyzed by the enzyme glutamate-cysteine ligase (Gclc), is delayed at higher doses of APAP, which exhibit higher liver injury.33 Consistent with this observation, our results showed that GSH recovery is significantly delayed after APAP administration in mice with CSA-mediated bile acid depletion. Similarly, the GSH recovery was faster in mice fed a cholic acid diet. These data indicate that bile acids may be involved in GSH recovery after APAP overdose. The exact mechanisms behind this effect are not known. It is known that Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is a major regulator of Gclc expression, and studies have shown that bile acids can affect Nrf2 activation.34 This raises the possibility that bile acids may stimulate Gclc expression via Nrf2, which may be involved in the protective effect shown by bile acids.
Hepatic JNK activation is a critical step in the intracellular signaling involved in APAP-induced liver injury.20,35,36 Our data show that bile acid depletion results in rapid activation of JNK and that cholic acid supplementation results in a decreased activation of JNK compared with normal diet control mice. These data indicate that bile acids may suppress JNK activation during APAP-induced liver injury. Whether bile acids directly affect JNK activation or induce this effect via an upstream regulator is not known. Bile acids are known to signal via multiple pathways in the liver, including FXR, TGR5, and epidermal growth factor receptor. It is possible that bile acids may use one of these pathways to suppress JNK signaling after APAP-induced liver injury.
It is known that bile acids play a critical role in the gut-liver signaling axis by stimulating FGF15 (FGF19 in humans) expression in the ileal cells via an FXR-dependent mechanism.6 This gut-liver axis of FGF15 signaling has been implicated in liver regeneration.13,14,37,38 In our studies, CSA-mediated bile acid depletion, which resulted in exacerbation of APAP toxicity, reduced FGF15 expression to undetectable levels. Similarly, 0.2% CA supplementation, which resulted in delayed initiation and rapid recovery from APAP toxicity, resulted in 15-fold up-regulation of FGF15 mRNA. These data suggest a role for FGF15 in the bile acid–mediated protection from APAP hepatotoxicity. However, the exact role of FGF15 remains to be investigated. It is possible that bile acid–stimulated FGF15 has a cytoprotective role during the initiation phase of injury. This notion is supported by previous observations that FGF15-knockout mice exhibited extensive liver injury after partial hepatectomy (PHX).38 It is known that the regenerating lobes after PHX do not normally exhibit necrotic cell death.39 However, recent studies have demonstrated that FGF15-knockout mice have significant necrosis in regenerating lobes after PHX,38 indicating that lack of FGF15 may remove an important cytoprotective mechanism in hepatocytes.
Several studies have demonstrated that compensatory liver regeneration after APAP-induced liver injury is a critical determinant of final outcome of APAP overdose.40–44 Our data clearly demonstrate that bile acid depletion results in delayed liver regeneration and that bile acid supplementation stimulates rapid liver regeneration after APAP overdose. These data are consistent with previous observations that bile acid supplementation stimulates liver regeneration.13 Recent studies have elegantly shown that FGF15 may be a primary mediator of the bile acid–induced stimulation of liver regeneration after PHX.38,45 It remains to be seen whether these same mechanisms apply in stimulating regeneration after APAP overdose.
Taken together, our data indicate that bile acids play a central role in modulating APAP-induced liver injury and regeneration. The observation that cholestyramine-mediated bile acid depletion exacerbates APAP-induced liver injury and delays regeneration is significant because of the routine use of cholestyramine in clinical practice. These data suggest a possible drug-drug interaction between APAP and CSA, and caution should be taken in administering APAP for patients taking CSA. Our data also indicate that bile acids have a cytoprotective and proregenerative role in APAP-induced liver injury, which could potentially be used for treatment of APAP-induced patients with acute liver failure.
Footnotes
Supported by NIH-NIDDK grant R01 DK98414 and American Association for the Study of Liver Diseases/American Liver Foundation Liver Scholar Award (U.A.).
B.B. and P.B. contributed equally to this work.
References
- 1.Hylemon P.B., Zhou H., Pandak W.M., Ren S., Gil G., Dent P. Bile acids as regulatory molecules. J Lipid Res. 2009;50:1509–1520. doi: 10.1194/jlr.R900007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trauner M., Claudel T., Fickert P., Moustafa T., Wagner M. Bile acids as regulators of hepatic lipid and glucose metabolism. Dig Dis. 2010;28:220–224. doi: 10.1159/000282091. [DOI] [PubMed] [Google Scholar]
- 3.Allen K., Jaeschke H., Copple B.L. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178:175–186. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gadaleta R.M., van Mil S.W., Oldenburg B., Siersema P.D., Klomp L.W., van Erpecum K.J. Bile acids and their nuclear receptor FXR: relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta. 2010;1801:683–692. doi: 10.1016/j.bbalip.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y.D., Chen W.D., Moore D.D., Huang W. FXR: a metabolic regulator and cell protector. Cell Res. 2008;18:1087–1095. doi: 10.1038/cr.2008.289. [DOI] [PubMed] [Google Scholar]
- 6.Inagaki T., Choi M., Moschetta A., Peng L., Cummins C.L., McDonald J.G., Luo G., Jones S.A., Goodwin B., Richardson J.A., Gerard R.D., Repa J.J., Mangelsdorf D.J., Kliewer S.A. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Copple B.L., Jaeschke H., Klaassen C.D. Oxidative stress and the pathogenesis of cholestasis. Semin Liver Dis. 2010;30:195–204. doi: 10.1055/s-0030-1253228. [DOI] [PubMed] [Google Scholar]
- 8.Fang Y., Han S.I., Mitchell C., Gupta S., Studer E., Grant S., Hylemon P.B., Dent P. Bile acids induce mitochondrial ROS, which promote activation of receptor tyrosine kinases and signaling pathways in rat hepatocytes. Hepatology. 2004;40:961–971. doi: 10.1002/hep.20385. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S., Natarajan R., Payne S.G., Studer E.J., Spiegel S., Dent P., Hylemon P.B. Deoxycholic acid activates the c-Jun N-terminal kinase pathway via FAS receptor activation in primary hepatocytes: role of acidic sphingomyelinase-mediated ceramide generation in FAS receptor activation. J Biol Chem. 2004;279:5821–5828. doi: 10.1074/jbc.M310979200. [DOI] [PubMed] [Google Scholar]
- 10.Jaeschke H., Gores G.J., Cederbaum A.I., Hinson J.A., Pessayre D., Lemasters J.J. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 11.Chen W.D., Wang Y.D., Meng Z., Zhang L., Huang W. Nuclear bile acid receptor FXR in the hepatic regeneration. Biochim Biophys Acta. 2011;1812:888–892. doi: 10.1016/j.bbadis.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Meng Z., Wang Y., Wang L., Jin W., Liu N., Pan H., Liu L., Wagman L., Forman B.M., Huang W. FXR regulates liver repair after CCl4-induced toxic injury. Mol Endocrinol. 2010;24:886–897. doi: 10.1210/me.2009-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang W., Ma K., Zhang J., Qatanani M., Cuvillier J., Liu J., Dong B., Huang X., Moore D.D. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 14.Borude P., Edwards G., Walesky C., Li F., Ma X., Kong B., Guo G.L., Apte U. Hepatocyte specific deletion of farnesoid X receptor delays, but does not inhibit liver regeneration after partial hepatectomy in mice. Hepatology. 2012;56:2344–2352. doi: 10.1002/hep.25918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiang J.Y. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim I., Ahn S.H., Inagaki T., Choi M., Ito S., Guo G.L., Kliewer S.A., Gonzalez F.J. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Nourjah P., Ahmad S.R., Karwoski C., Willy M. Estimates of acetaminophen (Paracetomal)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15:398–405. doi: 10.1002/pds.1191. [DOI] [PubMed] [Google Scholar]
- 18.Lee W.M. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217–226. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- 19.Lee W.M. Etiologies of acute liver failure. Semin Liver Dis. 2008;28:142–152. doi: 10.1055/s-2008-1073114. [DOI] [PubMed] [Google Scholar]
- 20.Jaeschke H., Bajt M.L. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 21.Jaeschke H., McGill M.R., Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones D.P., Lemasters J.J., Han D., Boelsterli U.A., Kaplowitz N. Mechanisms of pathogenesis in drug hepatotoxicity putting the stress on mitochondria. Mol Interv. 2010;10:98–111. doi: 10.1124/mi.10.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apte U., Mehendale H.M. Hepatic defenses against toxicology: liver regeneration and tissue repair. In: McQueen C.A., editor. Comprehensive Toxicology. ed 2. Elsevier; Philadelphia: 2009. pp. 339–367. [Google Scholar]
- 24.Apte U.M., Banerjee A., McRee R., Wellberg E., Ramaiah S.K. Role of osteopontin in hepatic neutrophil infiltration during alcoholic steatohepatitis. Toxicol Appl Pharmacol. 2005;207:25–38. doi: 10.1016/j.taap.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Walesky C., Edwards G., Borude P., Gunewardena S., O’Neil M., Yoo B., Apte U. Hepatocyte nuclear factor 4 alpha deletion promotes diethylnitrosamine-induced hepatocellular carcinoma in rodents. Hepatology. 2013;57:2480–2490. doi: 10.1002/hep.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee W.M. Acetaminophen-related acute liver failure in the United States. Hepatol Res. 2008;38:S3–S8. doi: 10.1111/j.1872-034X.2008.00419.x. [DOI] [PubMed] [Google Scholar]
- 27.Jaeschke H., McGill M.R., Williams C.D., Ramachandran A. Current issues with acetaminophen hepatotoxicity–a clinically relevant model to test the efficacy of natural products. Life Sci. 2011;88:737–745. doi: 10.1016/j.lfs.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pols T.W., Noriega L.G., Nomura M., Auwerx J., Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol. 2011;54:1263–1272. doi: 10.1016/j.jhep.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Out C., Groen A.K., Brufau G. Bile acid sequestrants: more than simple resins. Curr Opin Lipidol. 2012;23:43–55. doi: 10.1097/MOL.0b013e32834f0ef3. [DOI] [PubMed] [Google Scholar]
- 30.Poupon R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: an overview of their mechanisms of action. Clin Res Hepatol Gastroenterol. 2012;36(Suppl 1):S3–S12. doi: 10.1016/S2210-7401(12)70015-3. [DOI] [PubMed] [Google Scholar]
- 31.Amador-Noguez D., Dean A., Huang W., Setchell K., Moore D., Darlington G. Alterations in xenobiotic metabolism in the long-lived Little mice. Aging Cell. 2007;6:453–470. doi: 10.1111/j.1474-9726.2007.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee F.Y., de Aguiar Vallim T.Q., Chong H.K., Zhang Y., Liu Y., Jones S.A., Osborne T.F., Edwards P.A. Activation of the farnesoid X receptor provides protection against acetaminophen-induced hepatic toxicity. Mol Endocrinol. 2010;24:1626–1636. doi: 10.1210/me.2010-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGill M.R., Lebofsky M., Norris H.R., Slawson M.H., Bajt M.L., Xie Y., Williams C.D., Wilkins D.G., Rollins D.E., Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269:240–249. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu S.C. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han D., Shinohara M., Ybanez M.D., Saberi B., Kaplowitz N. Signal transduction pathways involved in drug-induced liver injury. Handb Exp Pharmacol. 2010;(196):267–310. doi: 10.1007/978-3-642-00663-0_10. [DOI] [PubMed] [Google Scholar]
- 36.Seki E., Brenner D.A., Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L., Wang Y.D., Chen W.D., Wang X., Lou G., Liu N., Lin M., Forman B.M., Huang W. Promotion of liver regeneration/repair by farnesoid X receptor in both liver and intestine in mice. Hepatology. 2012;56:2336–2343. doi: 10.1002/hep.25905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uriarte I., Fernandez-Barrena M.G., Monte M.J., Latasa M.U., Chang H.C., Carotti S., Vespasiani-Gentilucci U., Morini S., Vicente E., Concepcion A.R., Medina J.F., Marin J.J., Berasain C., Prieto J., Avila M.A. Identification of fibroblast growth factor 15 as a novel mediator of liver regeneration and its application in the prevention of post-resection liver failure in mice. Gut. 2013;62:899–910. doi: 10.1136/gutjnl-2012-302945. [DOI] [PubMed] [Google Scholar]
- 39.Michalopoulos G.K. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehendale H.M. Tissue repair: an important determinant of final outcome of toxicant-induced injury. Toxicol Pathol. 2005;33:41–51. doi: 10.1080/01926230590881808. [DOI] [PubMed] [Google Scholar]
- 41.Apte U., Singh S., Zeng G., Cieply B., Virji M.A., Wu T., Monga S.P. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol. 2009;175:1056–1065. doi: 10.2353/ajpath.2009.080976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chanda S., Mangipudy R.S., Warbritton A., Bucci T.J., Mehendale H.M. Stimulated hepatic tissue repair underlies heteroprotection by thioacetamide against acetaminophen-induced lethality. Hepatology. 1995;21:477–486. [PubMed] [Google Scholar]
- 43.Dalhoff K., Laursen H., Bangert K., Poulsen H.E., Anderson M.E., Grunnet N., Tygstrup N. Autoprotection in acetaminophen intoxication in rats: the role of liver regeneration. Pharmacol Toxicol. 2001;88:135–141. doi: 10.1034/j.1600-0773.2001.d01-94.x. [DOI] [PubMed] [Google Scholar]
- 44.Fontana R.J. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92 doi: 10.1016/j.mcna.2008.03.005. 761-794, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L., Wang Y.D., Chen W.D., Wang X., Lou G., Liu N., Lin M., Forman B.M., Huang W. Promotion of liver regeneration/repair by farnesoid X receptor in both liver and intestine in mice. Hepatology. 2012;56:2336–2343. doi: 10.1002/hep.25905. [DOI] [PMC free article] [PubMed] [Google Scholar]