Abstract
Recent evidence indicates that foamy viruses (FVs) are the oldest retroviruses (RVs) that we know and coevolved with their hosts for several hundred million years. This coevolution may have contributed to the non-pathogenicity of FVs, an important factor in development of foamy viral vectors in gene therapy. However, various questions on the molecular evolution of FVs remain still unanswered. The analysis of the spectrum of animal species infected by exogenous FVs or harboring endogenous FV elements in their genome is pivotal. Furthermore, animal studies might reveal important issues, such as the identification of the FV in vivo target cells, which than require a detailed characterization, to resolve the molecular basis of the accuracy with which FVs copy their genome. The issues of the extent of FV viremia and of the nature of the virion genome (RNA vs. DNA) also need to be experimentally addressed.
Keywords: foamy viruses, retroviruses, hepadnaviruses, evolution, genetic conservation, recombination
1. Introduction
Retroviruses have gained a lot of general interest, because of the devastating human AIDS pandemic over the last 35 years, making HIV one of the best-studied viruses of all times. However, lentiviruses, such as HIV, are not the most widespread of Retroviridae. The most prevalent of all retroviruses (RVs) are probably spumaretroviruses (or foamy viruses), a subfamily of RVs that has attracted altogether a group of approximately only 250 researchers from 10–15 labs worldwide over the same period of 35 years.
This life in a scientific niche is also illustrated by the fact that while the PubMed database lists more than 330,000 items for the search criteria “HIV or AIDS”, the hits are only around 1,000 for “foamy virus”. The interest in foamy viruses (FVs) is increasing, since these viruses offer unique opportunities for RV research, due to their distinct replication strategy, distinguishing them from orthoretroviruses [1,2,3], in clinical applications as potential retroviral vectors [4,5,6], due to their non-pathogenicity in any species [3,4], and as a model virus for studying antiretroviral drugs for HIV-1, since FV integrase has been crystallized [7,8,9]. However, the particular molecular characteristics of a virus may be a reflection of its evolutionary success or failure. Additionally, in terms of evolutionary success and genome stability, no RV compares to FVs, as suggested by a number of recent studies.
2. FV Sequence Conservation
Viruses with an RNA genome or an RNA (pre-) genome phase in their replication cycle are likely to accumulate genetic changes over time [10,11]. Individuals infected chronically by such viruses generally present (if untreated for their infection) a dynamic swarm of genetically slightly different viral variants at any given moment [10,11,12]. In virology, this has been termed the quasispecies concept [10,11,12]. At the basis of this concept is the error-prone replication by the RNA polymerase or reverse transcriptase (RT) together with a high in vivo replication rate [10,11,12]. The errors due to these types of mutations can be in the range of 1:103 to 1:105 point mutations per replication cycle [10,11].
This scenario may be further complicated by recombination events exchanging larger pieces of genetic information mainly between related viruses by replicase during genome copying [10,11]. Recombination events by template switching are well known for several virus families, such as picornaviruses, and have been investigated extensively (for a review, see [13]). Furthermore, in the case of viruses with segmented genomes, such as orthomyxoviruses, the exchange of whole segments may alter the features of the progeny substantially [10,11,12,13]. It is evident that genetic recombination or gene exchange (reassortment) in vivo requires the infection of one cell within one host by at least two viruses.
Numerous examples of high genetic variability of the retroviral, hepadnaviral and positive (+) strand RNA virus families have been investigated in detail (HIV, hepatitis B virus (HBV) and hepatitis C virus (HCV) are only the most prominent ones causing chronic infections in humans [14,15,16,17,18,19,20,21,22,23,24]), while only a handful of examples for recombination were described for the non-segmented minus (−) strand RNA viruses [25].
However, FVs appear to be an exception to these general rules of RNA virus and retrovirus genetic variability, because their genome is highly stable [26,27,28,29,30]. The in vivo variation of FV genomes has been estimated to be around 1.7 × 10−8 substitutions per site per year, which was quite close to that of mitochondrial DNA of 1.16 × 10−8; these values are unprecedented among viruses having an RNA phase in their replication cycle [28].
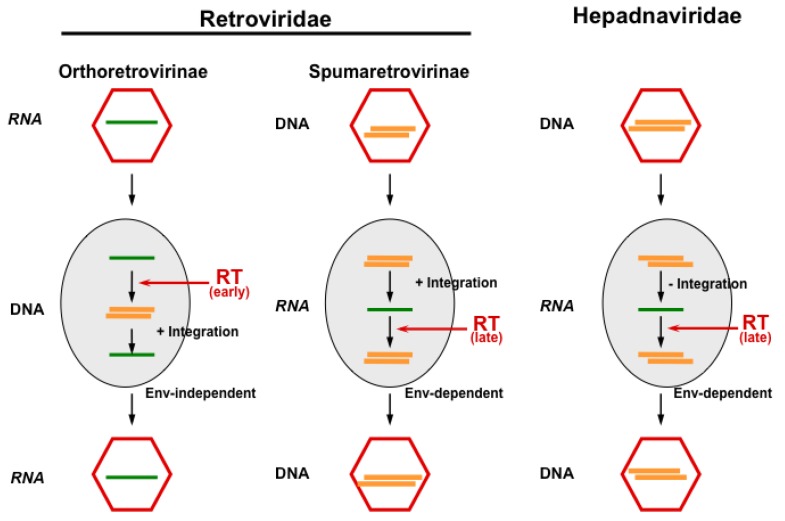
Even hepadnaviruses, which are related to FVs in terms of their replication pathway (Figure 1), show a one thousand-fold higher in vivo point mutation rate upon chronic human infections [16], despite the fact that they have a much more compact genetic order with largely overlapping reading frames that puts constraints on the variability of hepadnaviruses [31]. Indeed, the common origin of retroviruses with hepadnaviruses has been suggested long ago [32,33], and FVs might represent this evolutionary link, from which both viral families evolved [34,35,36]. However, fossil viral records are not available to address this question. Thus, for the time being, the relation of FV genomes to orthoretroviruses and hepadnaviruses is more or less a functional one. Therefore, due to the accuracy of genome copying and sequence stability, an FV sequence can be used to determine the animal subspecies of its origin, provided the exclusion of trans-species infection [37]. This feature has practical consequences, for instance, by designing breeding strategies for non-human primates (NHPs). For obvious reasons, it is often impossible to obtain blood samples from wild or quasi-wild animals, particularly the great apes. However, methods have been adapted from experiences in AIDS-research to amplify FV sequences from fecal samples obtained without disturbing the animals [38]. Due to the enormous genome conservation of FVs, such analyses revealed a phylogenetic co-distribution of the viruses with their hosts [37].
Figure 1.
The principal replication strategies of viruses making use of reverse transcription. While orthoretroviruses are RNA viruses, which replicate through a DNA intermediate and require integration for reproduction, hepadnaviruses are DNA viruses replicating through an RNA intermediate without the integration of their genome. FVs appear to functionally bridge these pathways, since they reverse transcribe (at least to a significant extent) late in replication (like hepadnaviruses) and must integrate their genome into the host cell genome (like orthoretroviruses). Furthermore, cellular exit of FV particles depends on their cognate glycoprotein as in hepadnaviruses, while budding of orthoretroviral particles is Env-independent (Figure adapted from [3]). RT, reverse transcriptase.
Investigations of whether the exceptionally high processivity, observed for the FV RT enzyme [39], reflected by the accuracy of in vitro genome copying and, thus, possibly an intrinsic feature of the FV RT, revealed a surprising observation: analysis of bacterially-expressed protein indicated that the fidelity of the FV RT enzyme approximated the one of HIV RT in a similar assay [40]. The overall mutation rates were 1.7 × 10−4 for prototype FV (PFV) and 7.5 × 10−5 for HIV, respectively [40]. Two thirds of PFV RT errors were due to deletions or small insertions [40]. Apparently, many more deletions or insertions were discovered for the FV enzyme than for the HIV RT. These in vitro findings resembled, in some way, deletions observed in the long terminal repeats (LTRs) upon FV amplification in cell culture [41,42].
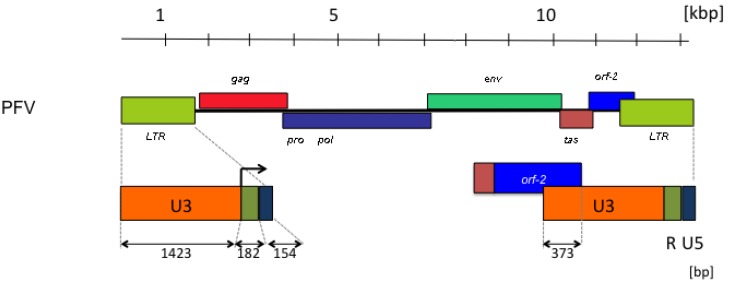
The length of the LTRs is a striking feature of FV genomes (Figure 2). The U3 regions contribute to these by approximately 85%. In particular, the LTRs of FVs from primates have extraordinarily long U3 regions of more than 1,400 bps [41]. Approximately one third of the U3 regions of primate FVs are protein-encoding bet sequences (Figure 2). The possible functions of the sequence remainder, aside from relatively short DNA motifs required for the regulation of gene expression [43,44], are inadequately defined and need further characterization. Because the rest of the genome has protein-encoding and cis-acting RNA or DNA functions, or both [2,45,46], the deletions in the U3 LTR region may be tolerated by virus replication in cell culture [41,42]. Such deletions were actually found upon obtaining proviral FV molecular clones from higher primates [47], and the detection of the undeleted LTRs [41,48] delayed their initial identification [49,50].
Figure 2.
Prototype foamy virus (PFV) genome organization with particular reference to the length and structure of the long terminal repeat (LTR) with the canonical U3, R and U5 regions. The retroviral genes (gag, pro-pol, env) are abbreviated as usual. The FV accessory tas and orf-2 reading frames are shown, as well as the spliced reading frame, giving rise to the Bet protein. The start of transcription in the LTR is indicated by an arrow in the enlargement at the bottom. The numbers refer to lengths in kilobase pairs (kbp) or base pairs (bp).
Another deletion that also occurs in vivo [41,48] is located in the coding region of the tas gene and contains the DNA copy of an almost full-length viral RNA, except approximately 200 bps, that includes the splice, which is normally needed to encode the Bet protein [51,52]. Since Tas is an essential viral accessory protein that exerts its function in trans [49,53], the so-called ∆Tas genome behaves like a defective-interfering particle [2,3].
The low fidelity detected by the in vitro study of the FV RT prompted the analysis of viral replication in cell culture [54], similar to investigations correcting the initially-reported very low in vitro fidelity of HIV RT [55,56]. The determination of the FV RT error rate on a lacZ-gene fragment that also was used in vitro [40,54] revealed that the frequency of deletions now dropped to insignificant values, and that of miss-incorporations, due to point mutations, was now found to be around 4 × 10−4 per site and replication cycle [54]. The vast majority of the point mutations were G –> A transitions, suggesting a role of APOBEC3 proteins in shaping the FV genome sequences (see below) [57].
The FV accessory Bet protein has been identified to counteract cellular APOBEC3 proteins [58,59]. However, the prototype FV (PFV) isolate Bet is inefficient compared, for instance, to the Bet protein of feline FV (FFV) proviral plasmids in this respect ([60], M. Löchelt, personal communication). Probably this inefficiency, partially due to the adaptation of the PFV isolate to cell culture conditions, is responsible for some misleading findings on Bet function [61]. Furthermore, it remained unclear whether this inefficiency to counteract APOBEC3 or the rather low cellular bet gene expression levels obtained with the particular expression plasmid [54,62] or both reasons led only to an around 50% reduction of G –> A mutations identified in the target cells transduced by FV vectors [54]. In contrast, normally, the bet gene is highly expressed in naturally FV-infected cells [63,64,65].
It should be noted that even if one ignores all G –> A mutations, by assuming that Bet will prevent these in the in vivo situation, such an “idealized” FV RT error rate of around 1.1 × 10−5 point mutations per replication cycle would still remain [54]. This is significantly more than observed for FV in vivo replication and indicates that more constraints act on the virus that do not allow for in vivo genome variability. This difference may be due to the potential presence of a factor that negatively influences the accuracy of FV genome copying in the HEK 293T cells, used to produce the vector particles, or the cells lack a factor that positively regulates the FV RT fidelity, which is present in vivo. However, the amount of virus produced by transfection of HEK 293T cells (usually on the order of 106 virions/mL of supernatant) is probably much higher than in vivo values, although these await a more detailed characterization [66,67].
The “idealized” FV mutation rate is even higher than that of primate T-cell leukemia viruses (PTLVs), which influences infected cells to multiply and, thus, amplify the viral genome by proviral expansion, thus avoiding an RNA phase [68,69]. With respect to sequence conservation, it is worth mentioning that PTLVs also show weak evidence of human adaptation and human viruses in a given area resembling their simian virus counterparts in the same area more than human isolates from a different geographic region [70]. It has been suggested that PTLVs are not in need of a genetic adaptation to the human host, since they appear to be readily adapted [70]. The multiple trans-species transmissions of simian PTLVs to humans represent a comparable situation to FVs [70]. However, PTLVs are transmitted among humans, while FVs are apparently not [3,70].
Compared to the persistent infections observed with HIV, HBV and HCV, FVs give rise to a rather low in vivo replication rate in blood and most tissues [48,66,71]. This low in vivo replication might be in part responsible for the observed low FV mutation rate; however, it should be noted that the mutation rate in cell culture was calculated per replication cycle and the in vivo evolutionary mutation rate per year. However, the low FV viremia and the different scales of mutation rate measurements probably do not represent the whole story, because in the SFV system, active viral replication has been found in cells of the oral mucosa [66,67], which is consistent with the relative ease of isolating virus from NHPs by throat swaps [72]. Thus, the constraints put on the virus in vivo cannot currently be reproduced by cell culture experiments, and much more work is needed to understand the FV replication in greater detail. In particular, the identification of the exact target cells should be followed by a detailed characterization of the process of foamy viral reverse transcription.
3. FV Recombination
As noted above, one requisite for virus recombination to occur in the offspring is the infection of one host cell by at least two distinct, but genetically-related, viruses; although, a so-called illegitimate recombination event between genetically unrelated sequences can occur, but seems to be very rare compared to a homologous recombination [10,11]. However, if it takes place, it can have really bizarre and unpredictable results. In RV replication, recombination is an obligatory event: during reverse transcription, the RT enzyme has to switch RNA templates in order to synthesize the double-stranded cDNA genome. From HIV-infection, circulating recombinant forms (CRFs) are known that significantly contribute to virus resistance and diversity [23,73]. It has been estimated that HIV RT crosses its template at a minimum of 2.8-times per replication cycle [74].
Given the distinct replication pathways and probably different in vivo target cells between orthoretroviruses and spumaretroviruses, a recombination event between these viruses belonging to distinct retroviral subfamilies appears very unlikely. Furthermore, such a nota bene illegitimate non-homologous recombination event has not been reported from the supposedly thousands of NHPs dually-infected with lentiviruses and FVs in NHP centers.
However, in the unlikely event such a new virus develops, such a new kind of “FV” may acquire the pathogenic importance the current FVs lack. A dual infection of FV-HIV in a human case has already been reported [75]. In addition, according to Murphy’s Law (“Whatever can go wrong will go wrong”), it is advisable to be prepared for a worst-case scenario. Thus, research and surveillance may aid in the early detection of recombination leading to novel viruses.
The first evidence for homologous recombination in FVs in vivo came from the report by Liu et al. in which SFVs from chimpanzees were analyzed by single-genome amplification [37]. It was found that the original simian FV from chimpanzee (SFVcpz), which apes were infected with, recombined with SFVs of lower primates that the chimpanzees hunt for prey [37]. Furthermore, it was shown very recently by Galvin et al. [76] that SFVmcy-2 (better known as the laboratory strain Macaca mulatta SFV (SFVmac-2)) represents a recombinant form of SFVmcy-1 (SFVmac-1) and an African green monkey SFV (SFVagm (SFV-3))-like virus in the receptor-binding domain of env [76]. Furthermore, a cell culture model of FV recombination by template switching has been developed that indicated an almost 100% probability of the FV RT to switch to a closely-related template, provided both templates were expressed in the producer cells at equivalent levels [54]. However, so far, no clear picture has emerged from the considerable co-infection by different SFVs among NHPs in the wild [37,48] or in captivity [77], because in some monkeys, a recombinant virus appeared, and in others, the apparently peaceful coexistence of two strains took place [30,37,78]. In the event of persistently infected chimpanzees by lower primate FVs, the situation resembles somehow the reported zoonotic infections of humans by NHP FVs, except that there is no evidence for human-to-human transmission of FVs [79]. Given their overall common strategy for genome amplification [2,80], it is very unlikely, however, not completely formally excluded yet, that different FVs in vivo make use of different target cells (in particular, when heterologous hosts become infected).
4. Retroviral Genome Diversity
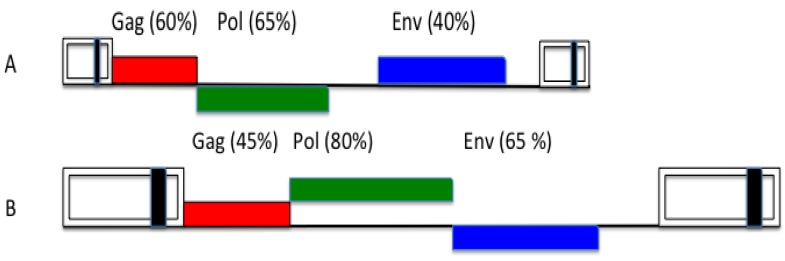
The overall orthoretroviral genome diversity indicates that env genes are more variable than gag. The variability of lentiviral envs is well-known (Figure 3). FVs also appear to be an exception to this rule, because they show the opposite feature [81], since their env sequences are much more highly conserved than the respective gag genes (Figure 3). One hypothetical argument is that, probably, all FVs make use of the same cellular receptor superfamily [82,83] and must conserve recognizing epitopes. However, this argument is valid for HIV, as well. Another point concerns the FV replication strategy, in particular, the Env topology with leader peptide (LP), surface (SU) and transmembrane (TM) proteins that may exert a more rigid Env configuration and, consequently, sequence conservation of the FV tripartite than of the normal orthoretroviral bipartite protein [84]. However, this cannot be the whole story: e.g., why do we find conserved motifs in all FV LPs that are probably responsible for directly contacting cognate FV capsids [85,86], while the latter appear to show such a high variability?
Figure 3.
Overall nucleotide genome conservation of primate lentiviral (A) and primate foamy viral (B) genomes. The values are approximate and show a reciprocal feature of lentiviral and foamy viral gag and env genes. For clarity, accessory reading frames have been omitted (adapted from [81]).
A different aspect deals with the amino acid distribution in Gag of FVs. More or less all the basic residues in foamy viral capsid proteins are represented by arginines [87]. Lysine-poor viral proteins are very rare in virology [87]. The FV codon usage indicates that several of the arginine-specifying bases in contemporary gag genes were probably lysine-specifying in previous gag genes [87]. The analysis of PFV mutants, in which some arginine codons were mutated “back” to lysine codons, excluded a role of anti-retroviral restriction factors in lysine exclusion (see below). However, they showed a strong interferon (IFN) sensitivity as a possible explanation for lysine-poor Gag in FVs [87]. Thus, host IFN selection appears to be a potential long-term driving force shaping current FV Gags (see below).
Since a functional explanation has not been defined yet, the distribution of variable and conserved motifs in FV genomes requires a closer look. Furthermore, it may be that some characteristic features of PFV and other Gags of primate FV origin, such as the nuclear pathway [88,89] or the intra-cellular retrotransposition [90,91], may turn out to be particular to PFV and close relatives, uncommon in other FVs and not actually representative of the FV-specific replication pathway [80,92].
5. Innate Host Defense and Viral Counter-Defense
Innate host defense and viral counter-defense can determine the outcome of many viral infections, resulting in the survival of hosts and viral genomes (positive selection). Central to the innate host defense is the interferon (IFN) system. Due to the profound effect of IFN on viral replication, there has been mutual evolutionary modulation by viruses on the host’s IFN system and by the host’s IFN system on viral genomes [93,94,95]. Thus, a constant arms race between viruses and hosts emerged [93,94], and retroviruses were no exception to the rule [93,94].
Early studies have indicated the sensitivity of FV replication to IFNs [96,97]. However, in these studies, FVs were regarded as very weak, if at all, inducers of an IFN response [96]. The vulnerability, in particular, to type II IFN, was later investigated from a very different angle, when it was shown that the addition of γ-antibodies greatly enhanced the chances to isolate FVs in cell culture from peripheral blood lymphocytes (PBLs) ex vivo [98] based upon that only 10–20 cells in 106 PBLs appear to contain FV DNA on average [48,66,99]. In summary, these studies indicated the extreme vulnerability of FV replication to IFN, but left the question of IFN induction by FVs completely open. This was addressed in a more recent study by Rua et al. [48], who showed that as with other retroviruses [100,101], the toll-like receptor 7 (TLR7) is the main molecule in plasmacytoid dendritic cells (pDCs), the cells in the body that preferentially secret type I IFN [93], to sense FVs and to induce a strong IFN response [102]. TLR7 recognizes single-stranded RNA. Thus, although reverse transcription has commenced in the virus-producing cell far enough to make the virion DNA “infectious” [80,87,103,104], there is sufficient virion RNA present to trigger the IFN response. This strong host IFN response might indicate that natural infections by FVs were, in ancient times, not as harmless as they are apparently now. This scenario can be possibly mimicked by infection of specific IFN knockout mice. On the other hand, the vigorous IFN response may be a reason for the inert infection by FVs.
The induction of IFNs may have resulted in various adaptations that allowed the virus to replicate even in their presence. The mode of action of IFNs to establish the antiretroviral state includes the hyper-induction of cellular proteins (restriction factors) by which the battle of host vs. virus begins. The restriction factors acting on retroviruses have been described in a number of excellent reviews [95,105,106,107]. Therefore, their mechanisms of action against orthoretroviruses will be mentioned here only when it is relevant to FV replication.
However, with FVs, it appears that the host-virus battle is supposedly over and may have occurred in evolutionary history. If FV infections were to be completely non-pathogenic, there would be no need for the host to adapt to these viruses nor for the virus to counteract the host response. In this situation the only “interest” of the virus would be to multiply its genome and propagate without affecting the host. This may be an additional reason for the initial difficulty in assigning Bet an APOBEC3-counteracting function [61] and for the finding of a bet-minus FV in humans [108]. However, we will leave this question open for the time being and will briefly look at the already described antiretroviral restriction factors:
(A) TRIM5α
The oligomeric tripartite motif (TRIM) proteins of primates include a large family of antiviral effectors that are made of conserved building blocks, namely an N-terminal RING finger domain, one or two B-box motifs, a coiled-coil motif (these three are collectively called the RBCC-domain) and the C-terminal B30.2/SPRY domain, which confers retrovirus capsid-binding [109,110].
TRIM5α proteins inhibit a very early stage of orthoretroviral replication before reverse transcription occurs by interfering with capsid breakdown and uncoating of the viral RNA, which is a requisite for reverse transcription to take place. To do this, they physically bind to capsids and might induce their proteasomal degradation [111,112,113,114,115]. However, the last point is dubious, since inhibition of the proteasome does not inhibit the anti-viral activity of TRIM5α [116,117]. Although regions and motifs in both proteins have been mapped [116,118], molecular details of how exactly TRIM5α can inhibit such diverse retroviruses are largely unknown. Very recently the crystallographic structure of an FV N-terminal Gag fragment has been resolved [119]. This analysis revealed not only clues about how FV Gag may interact with autologous Env, but also, how TRIM5α might bind to these unique retroviral capsids [119].
TRIM5α proteins act in a species-specific manner [116]. For instance, the rhesus macaque (mac) TRIM5α is active against HIV-1, and vice versa, human TRIM5α prevents an infection by SIVmac [116,118]. Since the effect of TRIM5α on viral replication is profound (up to two orders of magnitude are regularly observed in experimental settings [116,120]), the presence of TRIM5α has been discussed as a strong barrier against viral trans-species transmissions [116,118,121]. However, all TRIM5α proteins are inactive against the autologous virus, e.g., HIV-1 will replicate regardless of the presence or absence of human TRIM5α, because the retroviral capsid genes evolved to be insensitive to “their” TRIM5α proteins, arguing for a very long virus-host adaption.
FVs are also vulnerable to these proteins [120,122]. As with orthoretroviruses, the restriction is dictated by the B30.2 domain and exhibits some species-specificity [120], which suggests a more or less development by chance rather than having specific evolutionary implications. Moreover, concerning their protein and nucleic acid composition, FVs capsids are very much different from their orthoretroviral cousins [2,3]. Thus, the mechanism of TRIM5α action must be very much determined from a common structural motif, provided it is similar in both retroviral subfamilies. Since details on this mechanism have not been investigated for any of the spumaretroviruses, one cannot tell which evolutionary signature in contemporary foamy viral gag genes was imprinted by TRIM5α selection, except that such a signature must exist.
(B) APOBEC3
If the retroviral genomes were to be reverse transcribed (note that there is a distinctive difference between orthoretroviruses and spumaretroviruses with respect to the time-point of reverse transcription in the viral replication cycle, which, in the case of the latter, appears to take place to a very large extent in virus-producing cells [2,3]), intermediate RNA-DNA hybrids will be generated (Figure 1). The single-stranded DNA in these hybrids is the target of apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3 (APOBEC3) proteins, which deaminate cytosines to uridines [106,123,124]. Furthermore, there is a deamination-independent APOBEC3 effect on the reverse transcription of lentiviruses [106,125,126,127]. It is worth mentioning here that there is a marked difference in how lentiviruses and spumaretroviruses react to prevent APOBEC3 packaging. Both virus genera generate a viral protein to antagonize APOBEC3. While in most lentiviruses, the Vif protein prevents APOBEC3 (mainly APOBEC3G and 3F) protein packaging by delivering them to the cellular proteasome for degradation [106,128], the foamy viral accessory Bet protein prevents encapsidation by blocking APOBEC3 dimerization and targeting them to form insoluble complexes incapable of incorporation into nascent FV capsids [129,130]. FV Bet proteins are not initiating APOBEC3 protein destruction [57,58,59].
Domains of physical interaction on the side of effector molecules and targets have been mapped [57,130]. Recent results from the feline FV system indicate that ORF-2 encodes the APOBEC3-interacting sequences [130]. On the other hand, the tas-exon of bet does not contribute directly in distracting APOBEC3; however, it may participate in the high bet expression level.
The ORF-2 sequences evolutionarily shaped the genomic APOBEC3 locus and vice versa [57]. ORF-2/bet sequences belong to the more variable genomic regions of FVs and impose a high value of non-synonymous vs. synonymous mutations. This implies that, similar to the situation in the lentivirus genus [123,131], there is some species specificity in ORF-2/Bet to antagonize APOBEC3 proteins [57,129,130].
For lentiviruses, it has been proposed that the virus (HIV-1) benefits from a partially active APOBEC3 by exploiting this particular cellular mechanism of innate immunity to enhance viral diversity [132,133,134]. The strict conservation of their genomes illustrates that this is not an option for FVs. Moreover, the development of a viral protein to counteract APOBEC instead of evolving the gag genes, as a way not to package APOBECs, shows that either the latter is not so easily possible or that homologous-insensitive TRIM5α (see above) constitutes the older antiviral host activity. However, sequence evidence argues against the latter possibility [135]. Clearly, the clarification of this response needs further experimental approaches, in particular, the species specificity of FVs to antagonize APOBEC3 proteins requires further analysis.
(C) Tetherin
From the viewpoint of virus replication, tetherin (CD317) is the last of the IFN-inducible factors that restrict FV replication, because it acts on the cell-free virion [136,137]. Tetherin is a type II trans-membrane protein with an N-terminal trans-membrane domain, followed by an extra-cellular domain that adopts a coiled-coil structure and a C-terminal glycosyl-phosphatidylinositol (GPI) anchor [107,138]. Due to its membrane-sticking features, tetherin inhibits the generation of free enveloped virions (not only form orthoretroviruses) [139,140].
Tetherin forms homodimers [107,138,141,142]. Dimerization is a requisite to act on lentiviruses; thereby, the Vpu or the Nef proteins have evolved to counteract and degrade tetherin [107,138,141,142]. In HIV-2, where a vpu ORF does not exist, the Env protein fulfils this function [107,138,141,142]. However, dimerization appears to be not essential for FV restriction, whereas the membrane insertion on both sides is [143]. FVs have no particular ORF to counteract tetherin. This feature might result in the low viremia and cell-associated nature of FV infections. Similar to FIV, which also does not contain a tetherin-antagonizing protein [144], this obviously does not prevent infections by FVs.
(D) SamHD1
Weather the Sterile motif domain- and HD domain-containing protein 1 (SamHD1), which restricts HIV replication in myeloid cells by alteration of the nucleotide pool required for reverse transcription and which is counteracted by HIV-2 (and related viruses), Vpx protein [145] is also able to restrict FV replication and was investigated with a negative result recently [146]. Since SanHD1 acts on the nucleotide pool required for reverse transcription, it appeared a priori unlikely that it is able to restrict FVs, if added to target cells. Due to the feature of late reverse transcription, the FV genome already consists of DNA (Figure 1).
6. On the Origin of PFV and Further Human FV Infections
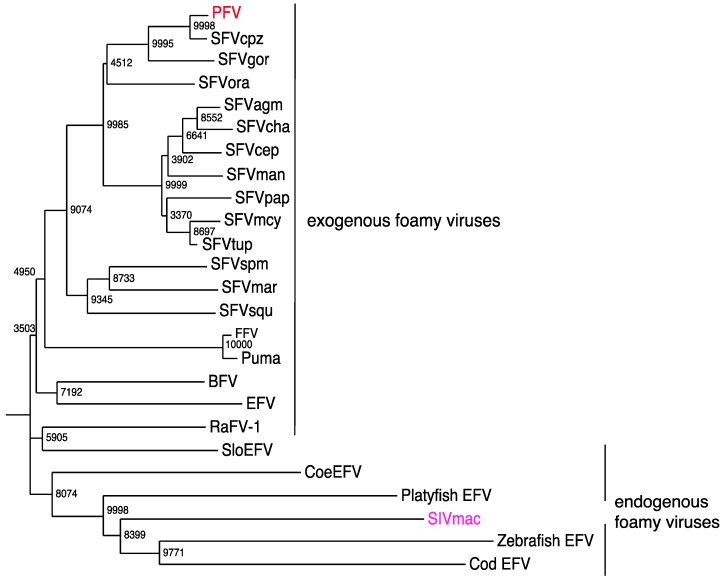
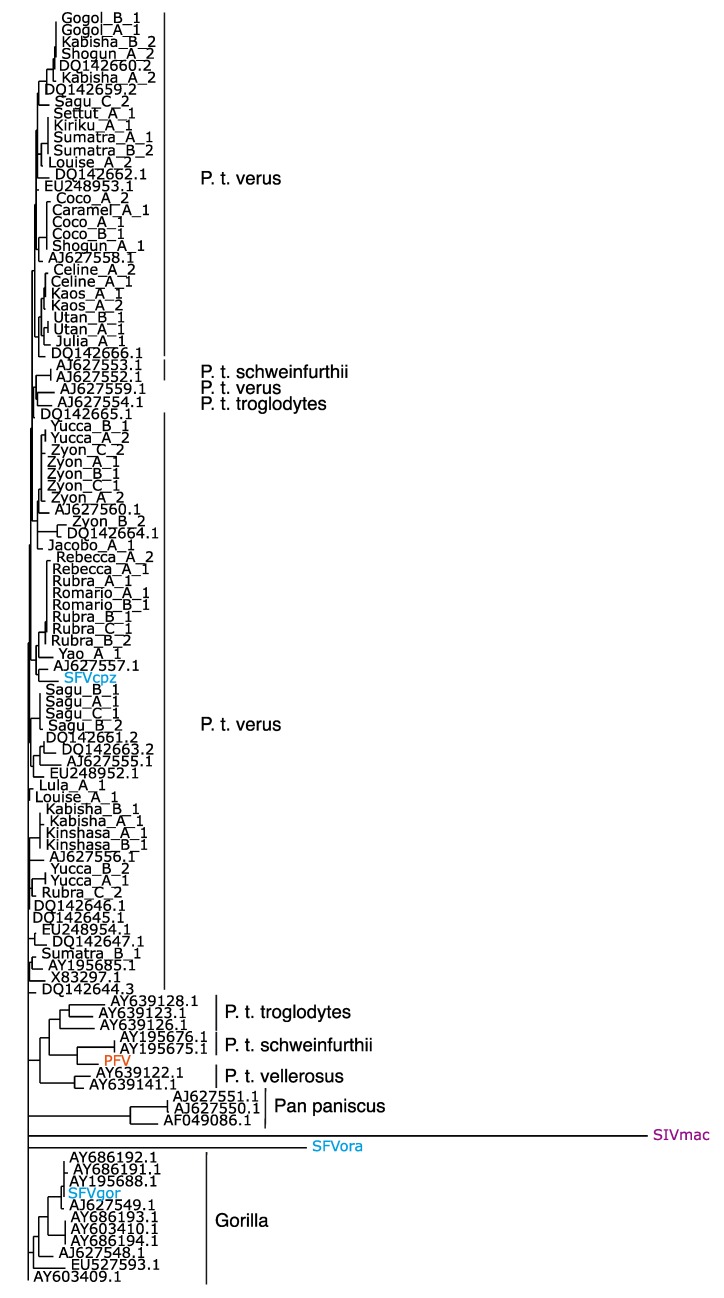
Early reports presented serological evidence of a human FV [147,148]. However, these studies could not be confirmed upon further investigations [149,150]. Furthermore, when an FV from chimpanzees was molecularly cloned and sequenced, it was suggested that the PFV isolate, which was obtained in 1970 from a Kenyan patient [151], might actually represent an ape virus [152] (Figure 4).
Figure 4.
Phylogeny of current endogenous and exogenous FV pol amino acid sequences (from the active center-specifying integrase sequences and comprising 142 codons). A neighbor-joining tree was calculated by using the Maximum Composite Likelihood method with a bootstrap test of 10,000 replicates. BFV, bovine FV (NP_044929.1); EFV equine FV (NP_054716.1); PFV, prototypic FV (in red) (Y07725.1); SFVcpz, SFV from chimpanzee (CCP47057); SFVgor, SFV from gorilla (AY195688.1); SFVora, SFV from orangutan (CAD67562); SFVagm, African green monkey (Cercopithecus aethiops) FV (YP_001956722.2); SFVcha, SFV from Chlorocebus aethiops (CAM34599); SFVcep, SFV from Cercopithecus pygerythrus (AAV92627); SFVman, SFV from mandrill (ADO65890.1); SFVpap, SFV from Papio (CAM34655); SFVmcy (previously SFVmac) from Macaca mulatta, SFV from Macaca cyclopis (CAA41394.1); SFVtup, SFV from Tupaia [155] (AGN49359); SFVspm, SFV from spider monkey (ABV59399.1); SFVmar, SFV from marmosets (ADE06000.1); SFVsqu, SFV from squirrel monkeys (ADE05995.1); FFV, feline FV (NP_056914); Puma, FV from Puma concolor (AGC11913); RaFV-1, FV from the bat Rhinolophus affinis (AFK85015); SloEFV, endogenous FV from sloths (Katzourakis et al., 2009); CoeEFV, endogenous FV in the coelacanth (Latimeria) genome (JX006241.1); platyfish EFV, endogenous FV from platyfish (M. Schartl, personal communication); zebrafish EFV, zebrafish (M. Schartl, personal communication); and Cod EFV, codfish (M. Schartl, personal communication) genomes; integrase encoding sequences of the macaque simian immunodeficiency virus (SIVmac in magenta) (AAC57420.1) served as the outlier; the endogenous FV sequence from aye-aye (PSFVaye) [156] was not incorporated, because integrase sequences are not available.
However, a more detailed evaluation, including DNA sequences, which became only available recently [48,50], reveals that the SFVs from chimpanzees present a wider sequence spectrum than the SFVs from gorillas (Figure 5). This finding represents only a very indirect support for the phylogeographic origin of FV sequences, because the actual geographic origin of the gorilla whose SFV sequence was reported first [48,50] is dubious. All other SFVgor sequences [48] were derived from animals stemming from a more limited area compared to the FVs derived from chimpanzees. These data are best compatible with the assumption of a strain-specific transmission pattern according to mother-to-child transmission and social groups [30]. In addition, they reflect behavioral differences between chimpanzees and gorillas, e.g., a more aggressive behavior of the former. Surely, more sequencing data from great ape FVs should reveal a better evolutionary relationship of FVs. However, PFV to SFV being almost identical in P.t. schweinfurthii points to these chimpanzee subspecies as a potential transmitter of this virus.
Figure 5.
A 425-nucleotide fragment from the conserved region of the integrase domain of pol was used to establish the phylogenetic tree demonstrating the phylogenetic relations of SFVcpz, PFV and SFVgor. The position of the proviral PFV (red) sequence and other proviral plasmids (blue) are indicated. SIVmac (magenta) served as an outlier. Pan paniscus is Bonobo.
Aside from PFV, there are many examples of human infections by NHP FVs among personnel in primate centers, zoos or other facilities housing NHPs or due to NHP biological materials [79]. Furthermore, African bushmeat hunters appear to be at high risk of SFV infections [48]. Another risk to acquire an FV infection exists for humans living close to Asian temple sites or other places, where humans often encounter free roaming monkeys [153]. In most cases, when humans acquired an FV infection, it was due to wounds in recipients and mostly from donor bites or deep scratches [48,79]. The infected humans remained unaware of their infection until—sometimes after decades [48,79]—researchers investigated their blood and were able to isolate virus in addition to finding serologic and nucleic acid evidence of infection [3,48]. However, retroviral infections sometimes go for long periods of time unnoticed and, in addition, pathological consequences may be rather subtle [154]; therefore, a careful follow up of the human cases is advisable. However, up to now, not a single case of human-to-human transmission has been reported. It appears as if mainly social behavior changes associated with human evolution were linked to a loss in FV susceptibility.
One point deserving further discussion is that FV transmitted from an NHP to a human did not yet evolve into a human FV, in sharp contrast to the development of HIV from simian immunodeficiency virus (SIV) transmitted to humans [175]. Even after decades of infection, the FV remained stable and maintained the genetic signature of the original infectious agent, i.e., the viral mutation rate in the new human host was not different from that in the natural ape or monkey host [48,79]. This is one of the clearest arguments for the non-pathogenic nature of FV infections. On the other hand, lessons from PTLV teach that the lack of human adaptation does not automatically preclude viral pathogenesis [70].
7. Exogenous FVs in the Animal Kingdom
The overall distribution of FV sequences indicates that these viruses are extremely widespread in the animal kingdom (Figure 4). FVs have not only been frequently found in NHPs of Old and New World origins, with a prevalence depending on the individual age and mounting up to 100% in grown up animals in the wild [30,37,77]. FVs are also present in prosimians, felines, bovines and equines [3,155,157,158]. Isolated reports on FVs from sheep [159], bison [160] and sea lions [161] suggest their presence in Bovidae and sea mammals. A notable exception is rodents, for which a specific FV has not been reported yet. Recently, Blasse et al. reported even the age-dependent infection of chimpanzees by more than one FV [30]. However, as to the extent of cross-neutralization of the superinfecting virus by immune response against the originally infecting FV, this has not been analyzed yet.
The various bat species are a major reservoir of viral infections, which may be eventually transmitted to humans as zoonoses; rhabdo-, corona-, filo-, orthomyxo-, paramyxo- and herpes-viruses are only some examples [162,163,164,165]. It came, therefore, as no major surprise that the detection of exogenous FV sequences upon screening of fecal samples from Asian bat species was reported recently by Wu et al. [166]. The finding of FVs in a such a diverse and old order illustrates the very old age of these viruses and asks for a more systematic screening and molecular characterization of FVs isolated from animals (not necessarily only mammals; see below) close to evolutionary branch points or otherwise interesting to science.
8. Endogenous FVs
If retroviruses get access to gametocytes, the result can be an endogenous form of the virus, which than mutates “neutrally” and is passaged in the germline along with the host cell genome and no longer transmitted as an exogenous virus [167,168]. In the vast majority of cases, “lethal” mutations accumulate over time in replication-required viral reading frames [167]. Approximately 8% of the human genome is made up by endogenous retroviruses, and a further 30%–40% of sequences are the result of reverse transcription [167].
Based on some sequence homology, the endogenous retrovirus-like (ERV) family L has been proposed to represent distantly related human and animal endogenous FVs [169]. However, the stretch of homology is rather short and does not hold up for the rest of the viral genome [170]. Thus, it was almost two decades before researchers again looked for endogenous FVs. They came up with some interesting findings from several pretty much different species. In any case, they demonstrated the very old age of FVs.
Firstly, Katzourakis et al. [171] reported their presence in the two- and three-toed sloths from South America (SloEFV) (Figure 4). Both species were separated approximately 21 million years ago (MYA). Since these endogenous elements were absent in other Xenarthrans species (e.g., ant bears and armadillos), which separated from the sloths 55 MYA, the authors conclude the establishment of SloEFV during this period (55 MYA–21 MYA) and the previous presence of exogenous FVs [171].
Secondly, Han and Worobey published [156] an endogenous FV sequence (PSFVaye), which was derived from the Madagascar aye-aye lemur (Daubentonia madagascarensis), a primitive prosimian. This suggests that exogenous FVs were present before the split of Strepsirrhini (mainly galagos, loris and lemurs) and Haplorhini (mainly the simians of the Old and New World) around 85 MYA, because the aye-aye is phylogenetically basal to the current lemurs [156].
Thirdly, it was reported again by Han and Worobey [172] that, surprisingly, the Coelacanth (Latimeria) genome harbors endogenous FV-like elements (CoeEFV) (Figure 4). Latimeria is a “living fossil”, believed to be a side branch of those now extinct lobe-finned fish, from which all current land vertebrates developed. If FVs were indeed to be present in these species, the FV-host coevolution represents an age of over 400 MYA, by far oldest known virus-host relationship. For instance, they would be approximately 30-times older than the oldest endogenous lentivirus we know [173].
Fourthly, Schartl et al. recently reported the discovery of fish endogenous FVs from the platyfish (Xiphophorus) and the cod species [174]. Furthermore, FV-like sequences were previously described in the zebra fish genome [175]. These discoveries do not necessarily extend the age of FVs; rather, the finding in another vertebrate order, in addition to mammals, supports the notion of the remarkable evolutionary success of this retroviral subfamily. Not all fish species harbor endogenous FVs [174], and the finding of almost intact copies of some FV genes [174], that is, without the accumulation of “lethal” mutations, might indicate the recent evolutionary invasion from exogenous FV.
9. Conclusions
1. Exogenous FVs might be present in species outside the class of placental Mammalia. Therefore, it will be scientifically extremely interesting to see FV isolates/sequences from Marsupialia, monotremes, birds, reptiles and amphibians. Even their presence as exogenous viruses in some fish species is not excluded, and the very much cell-associated nature of the agents might have yet prevented their detection.
2. The very long coevolution with animals may be the real reason for the benign nature of FV infections. For reasons of scientific consistency, it might be theoretically distracting and, for reasons of vector application, probably, medically beneficial that a genuine human FV does apparently not exist.
Acknowledgements
The authors would like to thank Manfred Schartl and Fabian Leendertz for providing the fish and simian FV sequences and the reviewers for their suggestions. We are indebted to Kristin Kaufmann for editorial assistance and the DFG for continuous support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Linial M.L. Foamy viruses are unconventional retroviruses. J. Virol. 1999;73:1747–1755. doi: 10.1128/jvi.73.3.1747-1755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rethwilm A. Molecular biology of foamy viruses. Med. Microbiol. Immunol. 2010;199:197–207. doi: 10.1007/s00430-010-0158-x. [DOI] [PubMed] [Google Scholar]
- 3.Rethwilm A., Lindemann D. Foamy Viruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. 6th ed. Volume 6. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013. pp. 1613–1632. [Google Scholar]
- 4.Lindemann D., Rethwilm A. Foamy virus biology and its application for vector development. Viruses. 2011;3:561–585. doi: 10.3390/v3050561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rethwilm A. Foamy virus vectors: An awaited alternative to gammaretro- and lentiviral vectors. Curr. Gene Ther. 2007;7:261–271. doi: 10.2174/156652307781369092. [DOI] [PubMed] [Google Scholar]
- 6.Trobridge G.D. Foamy virus vectors for gene transfer. Expert Opin. Biol. Ther. 2009;9:1427–1436. doi: 10.1517/14712590903246388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hare S., Gupta S.S., Valkov E., Engelman A., Cherepanov P. Retroviral intasome assembly and inhibition of DNA strand transfer. Nature. 2010;464:232–236. doi: 10.1038/nature08784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maertens G.N., Hare S., Cherepanov P. The mechanism of retroviral integration from X-ray structures of its key intermediates. Nature. 2010;468:326–329. doi: 10.1038/nature09517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valkov E., Gupta S.S., Hare S., Helander A., Roversi P., McClure M., Cherepanov P. Functional and structural characterization of the integrase from the prototype foamy virus. Nucleic Acids Res. 2009;37:243–255. doi: 10.1093/nar/gkn938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domingo E., Holland J.J. The Origin and Evolution of Viruses. In: Mahe B.W.J., ter Meulen V., editors. Virology. Volume 1. Edward Arnold; London, UK: 2005. pp. 11–23. [Google Scholar]
- 11.Domingo E. Virus Evolution. In: Knipe D.M., Howley P.M., editors. Fields Virology. 5th ed. Volume 5. Lippincott Williams; Philadelphia, PA, USA: 2007. pp. 389–421. [Google Scholar]
- 12.Domingo E., Sheldon J., Perales C. Viral quasispecies evolution. Microbiol. Mol. Biol. Rev.: MMBR. 2012;76:159–216. doi: 10.1128/MMBR.05023-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon-Loriere E., Holmes E.C. Why do RNA viruses recombine? Nat. Rev. Microbiol. 2011;9:617–626. doi: 10.1038/nrmicro2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buonaguro L., Tornesello M.L., Buonaguro F.M. Human immunodeficiency virus type 1 subtype distribution in the worldwide epidemic: Pathogenetic and therapeutic implications. J. Virol. 2007;81:10209–10219. doi: 10.1128/JVI.00872-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korber B.T., Letvin N.L., Haynes B.F. T-cell vaccine strategies for human immunodeficiency virus, the virus with a thousand faces. J. Virol. 2009;83:8300–8314. doi: 10.1128/JVI.00114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osiowy C., Giles E., Tanaka Y., Mizokami M., Minuk G.Y. Molecular evolution of hepatitis B virus over 25 years. J. Virol. 2006;80:10307–10314. doi: 10.1128/JVI.00996-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmonds P., Mellor J., Sakuldamrongpanich T., Nuchaprayoon C., Tanprasert S., Holmes E.C., Smith D.B. Evolutionary analysis of variants of hepatitis C virus found in South-East Asia: Comparison with classifications based upon sequence similarity. J. Gen. Virol. 1996;77:3013–3024. doi: 10.1099/0022-1317-77-12-3013. [DOI] [PubMed] [Google Scholar]
- 18.Andernach I.E., Jutavijittum P., Samountry B., Yousukh A., Thammavong T., Hubschen J.M., Muller C.P. A high variability of mixed infections and recent recombinations of hepatitis B virus in Laos. PLoS One. 2012;7:e30245. doi: 10.1371/journal.pone.0030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemelaar J. The origin and diversity of the HIV-1 pandemic. Trends Mol. Med. 2012;18:182–192. doi: 10.1016/j.molmed.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Hemelaar J., Gouws E., Ghys P.D., Osmanov S. Global trends in molecular epidemiology of HIV-1 during 2000–2007. AIDS. 2011;25:679–689. doi: 10.1097/QAD.0b013e328342ff93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simmonds P. Genetic diversity and evolution of hepatitis C virus--15 years on. J. Gen. Virol. 2004;85:3173–3188. doi: 10.1099/vir.0.80401-0. [DOI] [PubMed] [Google Scholar]
- 22.Wang G.P., Sherrill-Mix S.A., Chang K.M., Quince C., Bushman F.D. Hepatitis C virus transmission bottlenecks analyzed by deep sequencing. J. Virol. 2010;84:6218–6228. doi: 10.1128/JVI.02271-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M., Foley B., Schultz A.K., Macke J.P., Bulla I., Stanke M., Morgenstern B., Korber B., Leitner T. The role of recombination in the emergence of a complex and dynamic HIV epidemic. Retrovirology. 2010;7:25. doi: 10.1186/1742-4690-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi W., Carr M.J., Dunford L., Zhu C., Hall W.W., Higgins D.G. Identification of novel inter-genotypic recombinants of human hepatitis B viruses by large-scale phylogenetic analysis. Virology. 2012;427:51–59. doi: 10.1016/j.virol.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 25.Han G.Z., Worobey M. Homologous recombination in negative sense RNA viruses. Viruses. 2011;3:1358–1373. doi: 10.3390/v3081358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schweizer M., Neumann-Haefelin D. Phylogenetic analysis of primate foamy viruses by comparison of pol sequences. Virology. 1995;207:577–582. doi: 10.1006/viro.1995.1120. [DOI] [PubMed] [Google Scholar]
- 27.Schweizer M., Schleer H., Pietrek M., Liegibel J., Falcone V., Neumann-Haefelin D. Genetic stability of foamy viruses: Long-term study in an African green monkey population. J. Virol. 1999;73:9256–9265. doi: 10.1128/jvi.73.11.9256-9265.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Switzer W.M., Salemi M., Shanmugam V., Gao F., Cong M.E., Kuiken C., Bhullar V., Beer B.E., Vallet D., Gautier-Hion A., et al. Ancient co-speciation of simian foamy viruses and primates. Nature. 2005;434:376–380. doi: 10.1038/nature03341. [DOI] [PubMed] [Google Scholar]
- 29.Verschoor E.J., Langenhuijzen S., Bontjer I., Fagrouch Z., Niphuis H., Warren K.S., Eulenberger K., Heeney J.L. The phylogeography of orangutan foamy viruses supports the theory of ancient repopulation of Sumatra. J. Virol. 2004;78:12712–12716. doi: 10.1128/JVI.78.22.12712-12716.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blasse A., Calvignac-Spencer S., Merkel K., Goffe A.S., Boesch C., Mundry R., Leendertz F.H. Mother-offspring transmission and age-dependent accumulation of simian foamy virus in wild chimpanzees. J. Virol. 2013;87:5193–5204. doi: 10.1128/JVI.02743-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mason W.S., Burrell C.J., Casey J., Gerlich W.H., Howard C.R., Kann M., Lanford R., Newbold J., Schaefer S., Taylor J.M., et al. Hepadnaviruses. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. Virus Taxonomy. Elsevier Academic Press; London, UK: 2005. pp. 373–384. [Google Scholar]
- 32.Miller R.H., Robinson W.S. Common evolutionary origin of hepatitis B virus and retroviruses. Proc. Natl. Acad. Sci. USA. 1986;83:2531–2535. doi: 10.1073/pnas.83.8.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zlotnick A., Stahl S.J., Wingfield P.T., Conway J.F., Cheng N., Steven A.C. Shared motifs of the capsid proteins of hepadnaviruses and retroviruses suggest a common evolutionary origin. FEBS Lett. 1998;431:301–304. doi: 10.1016/S0014-5793(98)00755-8. [DOI] [PubMed] [Google Scholar]
- 34.Enssle J., Jordan I., Mauer B., Rethwilm A. Foamy virus reverse transcriptase is expressed independently from the Gag protein. Proc. Natl. Acad. Sci. USA. 1996;93:4137–4141. doi: 10.1073/pnas.93.9.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lecellier C.H., Saib A. Foamy viruses: Between retroviruses and pararetroviruses. Virology. 2000;271:1–8. doi: 10.1006/viro.2000.0216. [DOI] [PubMed] [Google Scholar]
- 36.Rethwilm A. Unexpected replication pathways of foamy viruses. J. Acquir Immune Defic. Syndr. Hum. Retrovirol. 1996;13(Suppl. 1):S248–S253. doi: 10.1097/00042560-199600001-00037. [DOI] [PubMed] [Google Scholar]
- 37.Liu W., Worobey M., Li Y., Keele B.F., Bibollet-Ruche F., Guo Y., Goepfert P.A., Santiago M.L., Ndjango J.B., Neel C., et al. Molecular ecology and natural history of simian foamy virus infection in wild-living chimpanzees. PLoS Pathog. 2008;4:e1000097. doi: 10.1371/journal.ppat.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharp P.M., Hahn B.H. Origins of HIV and the AIDS pandemic. Cold Spring Harb. Perspect. Med. 2011;1:a006841. doi: 10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rinke C.S., Boyer P.L., Sullivan M.D., Hughes S.H., Linial M.L. Mutation of the catalytic domain of the foamy virus reverse transcriptase leads to loss of processivity and infectivity. J. Virol. 2002;76:7560–7570. doi: 10.1128/JVI.76.15.7560-7570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyer P.L., Stenbak C.R., Hoberman D., Linial M.L., Hughes S.H. In vitro fidelity of the prototype primate foamy virus (PFV) RT compared to HIV-1 RT. Virology. 2007;367:253–264. doi: 10.1016/j.virol.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt M., Herchenröder O., Heeney J., Rethwilm A. Long terminal repeat U3 length polymorphism of human foamy virus. Virology. 1997;230:167–178. doi: 10.1006/viro.1997.8463. [DOI] [PubMed] [Google Scholar]
- 42.De Celis J., Tobaly-Tapiero J., Hampe A., Emanoil-Ravier R. Structure and function of the long terminal repeat of the chimpanzee foamy virus isolates (SFV-6) Arch. Virol. 1994;138:345–355. doi: 10.1007/BF01379137. [DOI] [PubMed] [Google Scholar]
- 43.Kang Y., Blair W.S., Cullen B.R. Identification and functional characterization of a high-affinity Bel-1 DNA binding site located in the human foamy virus internal promoter. J. Virol. 1998;72:504–511. doi: 10.1128/jvi.72.1.504-511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He F., Blair W.S., Fukushima J., Cullen B.R. The human foamy virus Bel-1 transcription factor is a sequence-specific DNA binding protein. J. Virol. 1996;70:3902–3908. doi: 10.1128/jvi.70.6.3902-3908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hartl M.J., Bodem J., Jochheim F., Rethwilm A., Rosch P., Wöhrl B.M. Regulation of foamy virus protease activity by viral RNA - a novel and unique mechanism among retroviruses. J. Virol. 2011;85:4462–4469. doi: 10.1128/JVI.02211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spannaus R., Hartl M.J., Wohrl B.M., Rethwilm A., Bodem J. The prototype foamy virus protease is active independently of the integrase domain. Retrovirology. 2012;9:41. doi: 10.1186/1742-4690-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rethwilm A., Darai G., Rösen A., Maurer B., Flügel R.M. Molecular cloning of the genome of human spumaretrovirus. Gene. 1987;59:19–28. doi: 10.1016/0378-1119(87)90262-9. [DOI] [PubMed] [Google Scholar]
- 48.Rua R., Betsem E., Calattini S., Saib A., Gessain A. Genetic characterization of simian foamy viruses infecting humans. J. Virol. 2012;86:13350–13359. doi: 10.1128/JVI.01715-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Löchelt M., Zentgraf H., Flügel R.M. Construction of an infectious DNA clone of the full-length human spumaretrovirus genome and mutagenesis of the bel 1 gene. Virology. 1991;184:43–54. doi: 10.1016/0042-6822(91)90820-2. [DOI] [PubMed] [Google Scholar]
- 50.Schulze A., Lemey P., Schubert J., McClure M.O., Rethwilm A., Bodem J. Complete nucleotide sequence and evolutionary analysis of a gorilla foamy virus. J. Gen. Virol. 2011;92:582–586. doi: 10.1099/vir.0.027011-0. [DOI] [PubMed] [Google Scholar]
- 51.Saib A., Koken M.H., van der Spek P., Peries J., de The H. Involvement of a spliced and defective human foamy virus in the establishment of chronic infection. J. Virol. 1995;69:5261–5268. doi: 10.1128/jvi.69.9.5261-5268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saib A., Peries J., de The H. A defective human foamy provirus generated by pregenome splicing. EMBO J. 1993;12:4439–4444. doi: 10.1002/j.1460-2075.1993.tb06129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rethwilm A., Erlwein O., Baunach G., Maurer B., ter Meulen V. The transcriptional transactivator of human foamy virus maps to the bel 1 genomic region. Proc. Natl. Acad. Sci. USA. 1991;88:941–945. doi: 10.1073/pnas.88.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gärtner K., Wiktorowicz T., Park J., Mergia A., Rethwilm A., Scheller C. Accuracy estimation of foamy virus genome copying. Retrovirology. 2009;6:32. doi: 10.1186/1742-4690-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mansky L.M., Temin H.M. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J. Virol. 1995;69:5087–5094. doi: 10.1128/jvi.69.8.5087-5094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roberts J.D., Bebenek K., Kunkel T.A. The accuracy of reverse transcriptase from HIV-1. Science. 1988;242:1171–1173. doi: 10.1126/science.2460925. [DOI] [PubMed] [Google Scholar]
- 57.Perkovic M., Schmidt S., Marino D., Russell R.A., Stauch B., Hofmann H., Kopietz F., Kloke B.P., Zielonka J., Strover H., et al. Species-specific inhibition of APOBEC3C by the prototype foamy virus protein bet. J. Biol. Chem. 2009;284:5819–5826. doi: 10.1074/jbc.M808853200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Löchelt M., Romen F., Bastone P., Muckenfuss H., Kirchner N., Kim Y.B., Truyen U., Rosler U., Battenberg M., Saib A., et al. The antiretroviral activity of APOBEC3 is inhibited by the foamy virus accessory Bet protein. Proc. Natl. Acad. Sci. USA. 2005;102:7982–7987. doi: 10.1073/pnas.0501445102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russell R.A., Wiegand H.L., Moore M.D., Schäfer A., McClure M.O., Cullen B.R. Foamy virus Bet proteins function as novel inhibitors of the APOBEC3 family of innate antiretroviral defense factors. J. Virol. 2005;79:8724–8731. doi: 10.1128/JVI.79.14.8724-8731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zemba M., Alke A., Bodem J., Winkler I.G., Flower R.L., Pfrepper K., Delius H., Flügel R.M., Löchelt M. Construction of infectious feline foamy virus genomes: Cat antisera do not cross-neutralize feline foamy virus chimera with serotype-specific Env sequences. Virology. 2000;266:150–156. doi: 10.1006/viro.1999.0037. [DOI] [PubMed] [Google Scholar]
- 61.Delebecque F., Suspene R., Calattini S., Casartelli N., Saib A., Froment A., Wain-Hobson S., Gessain A., Vartanian J.P., Schwartz O. Restriction of foamy viruses by APOBEC cytidine deaminases. J. Virol. 2006;80:605–614. doi: 10.1128/JVI.80.2.605-614.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bock M., Heinkelein M., Lindemann D., Rethwilm A. Cells expressing the human foamy virus (HFV) accessory Bet protein are resistant to productive HFV superinfection. Virology. 1998;250:194–204. doi: 10.1006/viro.1998.9362. [DOI] [PubMed] [Google Scholar]
- 63.Baunach G., Maurer B., Hahn H., Kranz M., Rethwilm A. Functional analysis of human foamy virus accessory reading frames. J. Virol. 1993;67:5411–5418. doi: 10.1128/jvi.67.9.5411-5418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Löchelt M. Foamy virus transactivation and gene expression. Curr. Top. Microbiol. Immunol. 2003;277:27–61. doi: 10.1007/978-3-642-55701-9_2. [DOI] [PubMed] [Google Scholar]
- 65.Muranyi W., Flügel R.M. Analysis of splicing patterns of human spumaretrovirus by polymerase chain reaction reveals complex RNA structures. J. Virol. 1991;65:727–735. doi: 10.1128/jvi.65.2.727-735.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Falcone V., Leupold J., Clotten J., Urbanyi E., Herchenröder O., Spatz W., Volk B., Bohm N., Toniolo A., Neumann-Haefelin D., et al. Sites of simian foamy virus persistence in naturally infected African green monkeys: Latent provirus is ubiquitous, whereas viral replication is restricted to the oral mucosa. Virology. 1999;257:7–14. doi: 10.1006/viro.1999.9634. [DOI] [PubMed] [Google Scholar]
- 67.Murray S.M., Picker L.J., Axthelm M.K., Hudkins K., Alpers C.E., Linial M.L. Replication in a superficial epithelial cell niche explains the lack of pathogenicity of primate foamy virus infections. J. Virol. 2008;82:5981–5985. doi: 10.1128/JVI.00367-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wattel E., Cavrois M., Gessain A., Wain-Hobson S. Clonal expansion of infected cells: A way of life for HTLV-I. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996;13(Suppl. 1):S92–S99. doi: 10.1097/00042560-199600001-00016. [DOI] [PubMed] [Google Scholar]
- 69.Mortreux F., Leclercq I., Gabet A.S., Leroy A., Westhof E., Gessain A., Wain-Hobson S., Wattel E. Somatic mutation in human T-cell leukemia virus type 1 provirus and flanking cellular sequences during clonal expansion in vivo. J. Natl. Cancer Inst. 2001;93:367–377. doi: 10.1093/jnci/93.5.367. [DOI] [PubMed] [Google Scholar]
- 70.Gessain A., Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 2012;3:388–410. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan A.S., Kumar D. Simian foamy virus infection by whole-blood transfer in rhesus macaques: Potential for transfusion transmission in humans. Transfusion. 2006;46:1352–1359. doi: 10.1111/j.1537-2995.2006.00862.x. [DOI] [PubMed] [Google Scholar]
- 72.Neumann-Haefelin D., Rethwilm A., Bauer G., Gudat F., zur Hausen H. Characterization of a foamy virus isolated from Cercopithecus aethiops lymphoblastoid cells. Med. Microbiol. Immunol. (Berl.) 1983;172:75–86. doi: 10.1007/BF02124508. [DOI] [PubMed] [Google Scholar]
- 73.Nora T., Charpentier C., Tenaillon O., Hoede C., Clavel F., Hance A.J. Contribution of recombination to the evolution of human immunodeficiency viruses expressing resistance to antiretroviral treatment. J. Virol. 2007;81:7620–7628. doi: 10.1128/JVI.00083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhuang J., Jetzt A.E., Sun G., Yu H., Klarmann G., Ron Y., Preston B.D., Dougherty J.P. Human immunodeficiency virus type 1 recombination: Rate, fidelity, and putative hot spots. J. Virol. 2002;76:11273–11282. doi: 10.1128/JVI.76.22.11273-11282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Switzer W.M., Garcia A.D., Yang C., Wright A., Kalish M.L., Folks T.M., Heneine W. Coinfection with HIV-1 and simian foamy virus in West Central Africans. J. Infect. Dis. 2008;197:1389–1393. doi: 10.1086/587493. [DOI] [PubMed] [Google Scholar]
- 76.Galvin T.A., Ahmed I.A., Shahabuddin M., Bryan T., Khan A.S. Identification of Recombination in the Envelope Gene of Simian Foamy Virus Serotype 2 Isolated from Macaca cyclopis. J. Virol. 2013;87:8792–8797. doi: 10.1128/JVI.03555-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hooks J.J., Gibbs C.J., Jr. The foamy viruses. Bacteriol. Rev. 1975;39:169–185. doi: 10.1128/br.39.3.169-185.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leendertz F.H., Zirkel F., Couacy-Hymann E., Ellerbrok H., Morozov V.A., Pauli G., Hedemann C., Formenty P., Jensen S.A., Boesch C., et al. Interspecies transmission of simian foamy virus in a natural predator-prey system. J. Virol. 2008;82:7741–7744. doi: 10.1128/JVI.00549-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boneva R.S., Switzer W.M., Spira T.J., Bhullar V.B., Shanmugam V., Cong M.E., Lam L., Heneine W., Folks T.M., Chapman L.E. Clinical and virological characterization of persistent human infection with simian foamy viruses. AIDS Res. Hum. Retrovir. 2007;23:1330–1337. doi: 10.1089/aid.2007.0104. [DOI] [PubMed] [Google Scholar]
- 80.Roy J., Rudolph W., Juretzek T., Gartner K., Bock M., Herchenröder O., Lindemann D., Heinkelein M., Rethwilm A. Feline foamy virus genome and replication strategy. J. Virol. 2003;77:11324–11331. doi: 10.1128/JVI.77.21.11324-11331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rethwilm A. Foamy Viruses. In: Mahe B.W.J., ter Meulen V., editors. Virology. Volume 2. Edward Arnold; London, UK: 2005. pp. 1304–1321. [Google Scholar]
- 82.Plochmann K., Horn A., Gschmack E., Armbruster N., Krieg J., Wiktorowicz T., Weber C., Stirnnagel K., Lindemann D., Rethwilm A., et al. Heparan sulfate is an attachment factor for foamy virus entry. J. Virol. 2012;86:10028–10035. doi: 10.1128/JVI.00051-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nasimuzzaman M., Persons D.A. Cell Membrane-associated heparan sulfate is a receptor for prototype foamy virus in human, monkey, and rodent cells. Mol. Ther. 2012;20:1158–1166. doi: 10.1038/mt.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hunter E. Viral Entry and Receptors. In: Coffin J.M., Hughes S.H., Varmus H.E., editors. Retroviruses. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York, NY 11724, USA: 1997. [PubMed] [Google Scholar]
- 85.Lindemann D., Pietschmann T., Picard-Maureau M., Berg A., Heinkelein M., Thurow J., Knaus P., Zentgraf H., Rethwilm A. A particle-associated glycoprotein signal peptide essential for virus maturation and infectivity. J. Virol. 2001;75:5762–5771. doi: 10.1128/JVI.75.13.5762-5771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilk T., Geiselhart V., Frech M., Fuller S.D., Flügel R.M., Löchelt M. Specific interaction of a novel foamy virus Env leader protein with the N-terminal Gag domain. J. Virol. 2001;75:7995–8007. doi: 10.1128/JVI.75.17.7995-8007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matthes D., Wiktorowicz T., Zahn J., Bodem J., Stanke N., Lindemann D., Rethwilm A. Basic residues in the foamy virus gag protein. J. Virol. 2011;85:3986–3995. doi: 10.1128/JVI.01906-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schliephake A.W., Rethwilm A. Nuclear localization of foamy virus Gag precursor protein. J. Virol. 1994;68:4946–4954. doi: 10.1128/jvi.68.8.4946-4954.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Müllers E., Stirnnagel K., Kaulfuss S., Lindemann D. Prototype foamy virus gag nuclear localization: A novel pathway among retroviruses. J. Virol. 2011;85:9276–9285. doi: 10.1128/JVI.00663-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heinkelein M., Pietschmann T., Jarmy G., Dressler M., Imrich H., Thurow J., Lindemann D., Bock M., Moebes A., Roy J., et al. Efficient intracellular retrotransposition of an exogenous primate retrovirus genome. EMBO J. 2000;19:3436–3445. doi: 10.1093/emboj/19.13.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heinkelein M., Rammling M., Juretzek T., Lindemann D., Rethwilm A. Retrotransposition and cell-to-cell transfer of foamy viruses. J. Virol. 2003;77:11855–11858. doi: 10.1128/JVI.77.21.11855-11858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bodem J., Zemba M., Flügel R.M. Nuclear localization of the functional Bel 1 transactivator but not of the gag proteins of the feline foamy virus. Virology. 1998;251:22–27. doi: 10.1006/viro.1998.9369. [DOI] [PubMed] [Google Scholar]
- 93.Pitha P.M. Innate antiviral response: Role in HIV-1 infection. Viruses. 2011;3:1179–1203. doi: 10.3390/v3071179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meyerson N.R., Sawyer S.L. Two-stepping through time: Mammals and viruses. Trends Microbiol. 2011;19:286–294. doi: 10.1016/j.tim.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blanco-Melo D., Venkatesh S., Bieniasz P.D. Intrinsic cellular defenses against human immunodeficiency viruses. Immunity. 2012;37:399–411. doi: 10.1016/j.immuni.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rhodes-Feuillette A., Lasneret J., Paulien S., Ogunkolade W., Peries J., Canivet M. Effects of human recombinant alpha and gamma and of highly purified natural beta interferons on simian Spumavirinae prototype (simian foamy virus 1) multiplication in human cells. Res. Virol. 1990;141:31–43. doi: 10.1016/0923-2516(90)90054-M. [DOI] [PubMed] [Google Scholar]
- 97.Rhodes-Feuillette A., Saal F., Lasneret J., Santillana-Hayat M., Peries J. Studies on in vitro interferon induction capacity and interferon sensivity of simian foamy viruses. Arch. Virol. 1987;97:77–84. doi: 10.1007/BF01310735. [DOI] [PubMed] [Google Scholar]
- 98.Falcone V., Schweizer M., Toniolo A., Neumann-Haefelin D., Meyerhans A. Gamma interferon is a major suppressive factor produced by activated human peripheral blood lymphocytes that is able to inhibit foamy virus-induced cytopathic effects. J. Virol. 1999;73:1724–1728. doi: 10.1128/jvi.73.2.1724-1728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Von Laer D., Neumann-Haefelin D., Heeney J.L., Schweizer M. Lymphocytes are the major reservoir for foamy viruses in peripheral blood. Virology. 1996;221:240–244. doi: 10.1006/viro.1996.0371. [DOI] [PubMed] [Google Scholar]
- 100.Liberatore R.A., Bieniasz P.D. Sensing retroviruses. Immunity. 2011;35:8–10. doi: 10.1016/j.immuni.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kane M., Case L.K., Wang C., Yurkovetskiy L., Dikiy S., Golovkina T.V. Innate immune sensing of retroviral infection via Toll-like receptor 7 occurs upon viral entry. Immunity. 2011;35:135–145. doi: 10.1016/j.immuni.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rua R., Lepelley A., Gessain A., Schwartz O. Innate sensing of foamy viruses by human hematopoietic cells. J. Virol. 2012;86:909–918. doi: 10.1128/JVI.06235-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moebes A., Enssle J., Bieniasz P.D., Heinkelein M., Lindemann D., Bock M., McClure M.O., Rethwilm A. Human foamy virus reverse transcription that occurs late in the viral replication cycle. J. Virol. 1997;71:7305–7311. doi: 10.1128/jvi.71.10.7305-7311.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu S.F., Sullivan M.D., Linial M.L. Evidence that the human foamy virus genome is DNA. J. Virol. 1999;73:1565–1572. doi: 10.1128/jvi.73.2.1565-1572.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wolf D., Goff S.P. Host restriction factors blocking retroviral replication. Annu. Rev. Genet. 2008;42:143–163. doi: 10.1146/annurev.genet.42.110807.091704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Malim M.H., Bieniasz P.D. HIV Restriction Factors and Mechanisms of Evasion. Cold Spring Harb. Perspect. Med. 2012;2:a006940. doi: 10.1101/cshperspect.a006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng Y.H., Jeang K.T., Tokunaga K. Host restriction factors in retroviral infection: Promises in virus-host interaction. Retrovirology. 2012;9:112. doi: 10.1186/1742-4690-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Callahan M.E., Switzer W.M., Matthews A.L., Roberts B.D., Heneine W., Folks T.M., Sandstrom P.A. Persistent zoonotic infection of a human with simian foamy virus in the absence of an intact orf-2 accessory gene. J. Virol. 1999;73:9619–9624. doi: 10.1128/jvi.73.11.9619-9624.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nisole S., Stoye J.P., Saib A. TRIM family proteins: Retroviral restriction and antiviral defence. Nat. Rev. Microbiol. 2005;3:799–808. doi: 10.1038/nrmicro1248. [DOI] [PubMed] [Google Scholar]
- 110.Towers G.J. The control of viral infection by tripartite motif proteins and cyclophilin A. Retrovirology. 2007;4:40. doi: 10.1186/1742-4690-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ganser-Pornillos B.K., Chandrasekaran V., Pornillos O., Sodroski J.G., Sundquist W.I., Yeager M. Hexagonal assembly of a restricting TRIM5alpha protein. Proc. Natl. Acad. Sci. USA. 2011;108:534–539. doi: 10.1073/pnas.1013426108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Black L.R., Aiken C. TRIM5alpha disrupts the structure of assembled HIV-1 capsid complexes in vitro. J. Virol. 2010;84:6564–6569. doi: 10.1128/JVI.00210-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lukic Z., Hausmann S., Sebastian S., Rucci J., Sastri J., Robia S.L., Luban J., Campbell E.M. TRIM5alpha associates with proteasomal subunits in cells while in complex with HIV-1 virions. Retrovirology. 2011;8:93. doi: 10.1186/1742-4690-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rold C.J., Aiken C. Proteasomal degradation of TRIM5alpha during retrovirus restriction. PLoS Pathog. 2008;4:e1000074. doi: 10.1371/journal.ppat.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stremlau M., Perron M., Lee M., Li Y., Song B., Javanbakht H., Diaz-Griffero F., Anderson D.J., Sundquist W.I., Sodroski J. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc. Natl. Acad. Sci. USA. 2006;103:5514–5519. doi: 10.1073/pnas.0509996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Perez-Caballero D., Hatziioannou T., Yang A., Cowan S., Bieniasz P.D. Human tripartite motif 5alpha domains responsible for retrovirus restriction activity and specificity. J. Virol. 2005;79:8969–8978. doi: 10.1128/JVI.79.14.8969-8978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu X., Anderson J.L., Campbell E.M., Joseph A.M., Hope T.J. Proteasome inhibitors uncouple rhesus TRIM5alpha restriction of HIV-1 reverse transcription and infection. Proc. Natl. Acad. Sci. USA. 2006;103:7465–7470. doi: 10.1073/pnas.0510483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yap M.W., Nisole S., Stoye J.P. A single amino acid change in the SPRY domain of human Trim5alpha leads to HIV-1 restriction. Curr. Biol.: CB. 2005;15:73–78. doi: 10.1016/j.cub.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 119.Goldstone D.C., Flower T.G., Ball N.J., Sanz-Ramos M., Yap M.W., Ogrodowicz R.W., Stanke N., Reh J., Lindemann D., Stoye J.P., et al. A unique spumavirus Gag N-terminal domain with functional properties of orthoretroviral matrix and capsid. PLoS Pathog. 2013;9:e1003376. doi: 10.1371/journal.ppat.1003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yap M.W., Lindemann D., Stanke N., Reh J., Westphal D., Hanenberg H., Ohkura S., Stoye J.P. Restriction of foamy viruses by primate Trim5alpha. J. Virol. 2008;82:5429–5439. doi: 10.1128/JVI.02462-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hatziioannou T., Princiotta M., Piatak M., Jr., Yuan F., Zhang F., Lifson J.D., Bieniasz P.D. Generation of simian-tropic HIV-1 by restriction factor evasion. Science. 2006;314:95. doi: 10.1126/science.1130994. [DOI] [PubMed] [Google Scholar]
- 122.Pacheco B., Finzi A., McGee-Estrada K., Sodroski J. Species-specific inhibition of foamy viruses from South American monkeys by New World Monkey TRIM5{alpha} proteins. J. Virol. 2010;84:4095–4099. doi: 10.1128/JVI.02631-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cullen B.R. Role and mechanism of action of the APOBEC3 family of antiretroviral resistance factors. J. Virol. 2006;80:1067–1076. doi: 10.1128/JVI.80.3.1067-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Malim M.H. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:675–687. doi: 10.1098/rstb.2008.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bishop K.N., Holmes R.K., Malim M.H. Antiviral potency of APOBEC proteins does not correlate with cytidine deamination. J. Virol. 2006;80:8450–8458. doi: 10.1128/JVI.00839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Holmes R.K., Malim M.H., Bishop K.N. APOBEC-mediated viral restriction: Not simply editing? Trends Biochem. Sci. 2007;32:118–128. doi: 10.1016/j.tibs.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 127.Newman E.N., Holmes R.K., Craig H.M., Klein K.C., Lingappa J.R., Malim M.H., Sheehy A.M. Antiviral function of APOBEC3G can be dissociated from cytidine deaminase activity. Curr. Biol.: CB. 2005;15:166–170. doi: 10.1016/j.cub.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 128.Yu X., Yu Y., Liu B., Luo K., Kong W., Mao P., Yu X.F. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- 129.Jaguva Vasudevan A.A., Perkovic M., Bulliard Y., Cichutek K., Trono D., Haussinger D., Munk C. Prototype Foamy Virus Bet Impairs the Dimerization and Cytosolic Solubility of Human APOBEC3G. J. Virol. 2013 doi: 10.1128/JVI.03385-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Slavkovic Lukic D., Hotz-Wagenblatt A., Lei J., Räthe A.M., Mühle M., Denner J., Münk C., Löchelt M. Identification of the feline foamy virus Bet domain essential for APOBEC3 counteraction. Retrovirology. 2013;10:76. doi: 10.1186/1742-4690-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chiu Y.L., Greene W.C. The APOBEC3 cytidine deaminases: An innate defensive network opposing exogenous retroviruses and endogenous retroelements. Annu. Rev. Immunol. 2008;26:317–353. doi: 10.1146/annurev.immunol.26.021607.090350. [DOI] [PubMed] [Google Scholar]
- 132.Kim E.Y., Bhattacharya T., Kunstman K., Swantek P., Koning F.A., Malim M.H., Wolinsky S.M. Human APOBEC3G-mediated editing can promote HIV-1 sequence diversification and accelerate adaptation to selective pressure. J. Virol. 2010;84:10402–10405. doi: 10.1128/JVI.01223-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sadler H.A., Stenglein M.D., Harris R.S., Mansky L.M. APOBEC3G contributes to HIV-1 variation through sublethal mutagenesis. J. Virol. 2010;84:7396–7404. doi: 10.1128/JVI.00056-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wood N., Bhattacharya T., Keele B.F., Giorgi E., Liu M., Gaschen B., Daniels M., Ferrari G., Haynes B.F., McMichael A., et al. HIV evolution in early infection: Selection pressures, patterns of insertion and deletion, and the impact of APOBEC. PLoS Pathog. 2009;5:e1000414. doi: 10.1371/journal.ppat.1000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Perez-Caballero D., Soll S.J., Bieniasz P.D. Evidence for restriction of ancient primate gammaretroviruses by APOBEC3 but not TRIM5alpha proteins. PLoS Pathog. 2008;4:e1000181. doi: 10.1371/journal.ppat.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Neil S.J., Zang T., Bieniasz P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 137.Van Damme N., Goff D., Katsura C., Jorgenson R.L., Mitchell R., Johnson M.C., Stephens E.B., Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hammonds J., Wang J.J., Spearman P. Restriction of retroviral replication by Tetherin/BST-2. Mol. Biol. Int. 2012;2012:424768. doi: 10.1155/2012/424768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Neil S.J., Sandrin V., Sundquist W.I., Bieniasz P.D. An interferon-alpha-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe. 2007;2:193–203. doi: 10.1016/j.chom.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jouvenet N., Neil S.J., Zhadina M., Zang T., Kratovac Z., Lee Y., McNatt M., Hatziioannou T., Bieniasz P.D. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 2009;83:1837–1844. doi: 10.1128/JVI.02211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Harris R.S., Hultquist J.F., Evans D.T. The restriction factors of human immunodeficiency virus. J. Biol. Chem. 2012;287:40875–40883. doi: 10.1074/jbc.R112.416925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Perez-Caballero D., Zang T., Ebrahimi A., McNatt M.W., Gregory D.A., Johnson M.C., Bieniasz P.D. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell. 2009;139:499–511. doi: 10.1016/j.cell.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu F., Tan J., Liu R., Xu D., Li Y., Geng Y., Liang C., Qiao W. Tetherin inhibits prototypic foamy virus release. Virol. J. 2011;8:198–207. doi: 10.1186/1743-422X-8-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dietrich I., McMonagle E.L., Petit S.J., Vijayakrishnan S., Logan N., Chan C.N., Towers G.J., Hosie M.J., Willett B.J. Feline tetherin efficiently restricts release of feline immunodeficiency virus but not spreading of infection. J. Virol. 2011;85:5840–5852. doi: 10.1128/JVI.00071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hrecka K., Hao C., Gierszewska M., Swanson S.K., Kesik-Brodacka M., Srivastava S., Florens L., Washburn M.P., Skowronski J. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gramberg T., Kahle T., Bloch N., Wittmann S., Mullers E., Daddacha W., Hofmann H., Kim B., Lindemann D., Landau N.R. Restriction of diverse retroviruses by SAMHD1. Retrovirology. 2013;10:26. doi: 10.1186/1742-4690-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Loh P.C., Matsuura F., Mizumoto C. Seroepidemiology of human syncytial virus: Antibody prevalence in the Pacific. Intervirology. 1980;13:87–90. doi: 10.1159/000149112. [DOI] [PubMed] [Google Scholar]
- 148.Mahnke C., Kashaiya P., Rössler J., Bannert H., Levin A., Blattner W.A., Dietrich M., Luande J., Löchelt M., Friedman-Kien A.E., et al. Human spumavirus antibodies in sera from African patients. Arch. Virol. 1992;123:243–253. doi: 10.1007/BF01317261. [DOI] [PubMed] [Google Scholar]
- 149.Schweizer M., Turek R., Hahn H., Schliephake A., Netzer K.O., Eder G., Reinhardt M., Rethwilm A., Neumann-Haefelin D. Markers of foamy virus infections in monkeys, apes, and accidentally infected humans: Appropriate testing fails to confirm suspected foamy virus prevalence in humans. AIDS Res. Hum. Retrovir. 1995;11:161–170. doi: 10.1089/aid.1995.11.161. [DOI] [PubMed] [Google Scholar]
- 150.Ali M., Taylor G.P., Pitman R.J., Parker D., Rethwilm A., Cheingsong-Popov R., Weber J.N., Bieniasz P.D., Bradley J., McClure M.O. No evidence of antibody to human foamy virus in widespread human populations. AIDS Res. Hum. Retrovir. 1996;12:1473–1483. doi: 10.1089/aid.1996.12.1473. [DOI] [PubMed] [Google Scholar]
- 151.Achong B.G., Mansell P.W., Epstein M.A., Clifford P. An unusual virus in cultures from a human nasopharyngeal carcinoma. J. Natl. Cancer Inst. 1971;46:299–307. [PubMed] [Google Scholar]
- 152.Herchenröder O., Renne R., Loncar D., Cobb E.K., Murthy K.K., Schneider J., Mergia A., Luciw P.A. Isolation, cloning, and sequencing of simian foamy viruses from chimpanzees (SFVcpz): High homology to human foamy virus (HFV) Virology. 1994;201:187–199. doi: 10.1006/viro.1994.1285. [DOI] [PubMed] [Google Scholar]
- 153.Jones-Engel L., May C.C., Engel G.A., Steinkraus K.A., Schillaci M.A., Fuentes A., Rompis A., Chalise M.K., Aggimarangsee N., Feeroz M.M., et al. Diverse contexts of zoonotic transmission of simian foamy viruses in Asia. Emerg. Infect. Dis. 2008;14:1200–1208. doi: 10.3201/eid1408.071430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Keele B.F., Jones J.H., Terio K.A., Estes J.D., Rudicell R.S., Wilson M.L., Li Y., Learn G.H., Beasley T.M., Schumacher-Stankey J., et al. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature. 2009;460:515–519. doi: 10.1038/nature08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huang F., Yu W. Foamy virus in the tree shrew Tupaia belangeri is highly related to simian foamy virus in Macaca Mulatta. AIDS Res. Hum. Retrovir. 2013;29:1177–1178. doi: 10.1089/aid.2013.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Han G.Z., Worobey M. An endogenous foamy virus in the aye-aye (Daubentonia madagascariensis) J. Virol. 2012;86:7696–7698. doi: 10.1128/JVI.00650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Saib A. Non-primate foamy viruses. Curr. Top. Microbiol. Immunol. 2003;277:197–211. doi: 10.1007/978-3-642-55701-9_9. [DOI] [PubMed] [Google Scholar]
- 158.Meiering C.D., Linial M.L. Historical perspective of foamy virus epidemiology and infection. Clin. Microbiol. Rev. 2001;14:165–176. doi: 10.1128/CMR.14.1.165-176.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Flanagan M. Isolation of a spumavirus from a sheep. Aust. Vet. J. 1992;69:112–113. doi: 10.1111/j.1751-0813.1992.tb07464.x. [DOI] [PubMed] [Google Scholar]
- 160.Amborski G.F., Storz J., Keney D., Lo J., McChesney A.E. Isolation of a retrovirus from the American bison and its relation to bovine retroviruses. J. Wildl. Dis. 1987;23:7–11. doi: 10.7589/0090-3558-23.1.7. [DOI] [PubMed] [Google Scholar]
- 161.Kennedy-Stoskopf S., Stoskopf M.K., Eckhaus M.A., Strandberg J.D. Isolation of a retrovirus and a herpesvirus from a captive California sea lion. J. Wildl. Dis. 1986;22:156–164. doi: 10.7589/0090-3558-22.2.156. [DOI] [PubMed] [Google Scholar]
- 162.Luis A.D., Hayman D.T., O’Shea T.J., Cryan P.M., Gilbert A.T., Pulliam J.R., Mills J.N., Timonin M.E., Willis C.K., Cunningham A.A., et al. A comparison of bats and rodents as reservoirs of zoonotic viruses: Are bats special? Proc. Biol. Sci. R. Soc. 2013;280:20122753. doi: 10.1098/rspb.2012.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Calisher C.H., Childs J.E., Field H.E., Holmes K.V., Schountz T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tong S., Li Y., Rivailler P., Conrardy C., Castillo D.A., Chen L.M., Recuenco S., Ellison J.A., Davis C.T., York I.A., et al. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. USA. 2012;109:4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Drexler J.F., Seelen A., Corman V.M., Fumie Tateno A., Cottontail V., Melim Zerbinati R., Gloza-Rausch F., Klose S.M., Adu-Sarkodie Y., Oppong S.K., et al. Bats worldwide carry hepatitis E virus-related viruses that form a putative novel genus within the family Hepeviridae. J. Virol. 2012;86:9134–9147. doi: 10.1128/JVI.00800-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu Z., Ren X., Yang L., Hu Y., Yang J., He G., Zhang J., Dong J., Sun L., Du J., et al. Virome analysis for identification of novel mammalian viruses in bat species from Chinese provinces. J. Virol. 2012;86:10999–11012. doi: 10.1128/JVI.01394-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Katzourakis A., Gifford R.J. Endogenous viral elements in animal genomes. PLoS Genet. 2010;6:e1001191. doi: 10.1371/journal.pgen.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Holmes E.C. The evolution of endogenous viral elements. Cell Host Microbe. 2011;10:368–377. doi: 10.1016/j.chom.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cordonnier A., Casella J.F., Heidmann T. Isolation of novel human endogenous retrovirus-like elements with foamy virus-related pol sequence. J. Virol. 1995;69:5890–5897. doi: 10.1128/jvi.69.9.5890-5897.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Benit L., Lallemand J.B., Casella J.F., Philippe H., Heidmann T. ERV-L elements: A family of endogenous retrovirus-like elements active throughout the evolution of mammals. J. Virol. 1999;73:3301–3308. doi: 10.1128/jvi.73.4.3301-3308.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Katzourakis A., Gifford R.J., Tristem M., Gilbert M.T., Pybus O.G. Macroevolution of complex retroviruses. Science. 2009;325:1512. doi: 10.1126/science.1174149. [DOI] [PubMed] [Google Scholar]
- 172.Han G.Z., Worobey M. An endogenous foamy-like viral element in the coelacanth genome. PLoS Pathog. 2012;8:e1002790. doi: 10.1371/journal.ppat.1002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gifford R.J., Katzourakis A., Tristem M., Pybus O.G., Winters M., Shafer R.W. A transitional endogenous lentivirus from the genome of a basal primate and implications for lentivirus evolution. Proc. Natl. Acad. Sci. USA. 2008;105:20362–20367. doi: 10.1073/pnas.0807873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schartl M., Walter R.B., Shen Y., Garcia T., Catchen J., Amores A., Braasch I., Chalopin D., Volff J.N., Lesch K.P., et al. The genome of the platyfish, Xiphophorus maculatus, provides insights into evolutionary adaptation and several complex traits. Nat. Genet. 2013;45:567–572. doi: 10.1038/ng.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Llorens C., Munoz-Pomer A., Bernad L., Botella H., Moya A. Network dynamics of eukaryotic LTR retroelements beyond phylogenetic trees. Biol. Direct. 2009;4:41. doi: 10.1186/1745-6150-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]