Abstract
Background:
Newborns and young infants suffer high rates of infections in South Asia and sub-Saharan Africa. Timely access to appropriate antibiotic therapy is essential for reducing mortality. In an effort to develop community case management guidelines for young infants, 0–59 days old, with clinically diagnosed severe infections, or with fast breathing, 4 trials of simplified antibiotic therapy delivered in primary care clinics (Pakistan, Democratic Republic of Congo, Kenya and Nigeria) or at home (Bangladesh and Nigeria) are being conducted.
Methods:
This article describes the scientific rationale for these trials, which share major elements of trial design. All the trials are in settings of high neonatal mortality, where hospitalization is not feasible or frequently refused. All use procaine penicillin and gentamicin intramuscular injections for 7 days as reference therapy and compare this to various experimental arms utilizing comparatively simpler combination regimens with fewer injections and oral amoxicillin.
Conclusion:
The results of these trials will inform World Health Organization policy regarding community case management of young infants with clinical severe infections or with fast breathing.
Keywords: newborn, young infant, sepsis, pneumonia, meningitis, clinically severe infections, fast breathing, community management, community case management, antibiotic, gentamicin, amoxicillin, procaine penicillin, South Asia, sub-Saharan Africa
Infections in the neonatal period are responsible for an estimated 12% of under-5 child mortality.1 Most newborns with severe infections (sepsis, pneumonia, meningitis) in South Asia and sub-Saharan Africa die without access to hospitalization and appropriate therapy,2–6 prompting the need for alternative strategies for home or first-level facility-based management in countries with a high burden of neonatal deaths.3–5,7 This article describes the scientific rationale for the study design of 3 randomized controlled equivalence trials evaluating simplified antibiotic regimens for management of clinically diagnosed severe infections in newborns and young infants in community settings in Bangladesh, Pakistan and a multicountry study in Africa (Democratic Republic of Congo, Kenya and Nigeria). Additionally, the scientific reasoning underlying a trial of simplified antibiotic therapy for management of young infants who present with fast breathing alone is also described. Although the individual study designs are adapted to site and country needs and are detailed in the site-specific methods articles,8–11 here we focus on the overarching scientific approach to case definitions, selection of trial sites, participant inclusion/exclusion criteria, choice of antibiotics and regimens and outcomes. The common design elements were made possible by extensive collaboration between the study sponsors12 and investigators and will enhance the interpretation and application of the studies’ results, in part by enabling future pooled analyses for outcomes of subgroups of interest (eg, treatment failure in newborns with early-onset infections, newborns with multiple clinical signs of illness vs. single signs, etc.).
STUDY AGE GROUP
Newborns have increased vulnerability to infection-related morbidity and mortality because of their immature immune systems and underdeveloped skin barrier.13 However, a decision to include infants beyond the newborn period, that is, in the second month of life, was made for 2 reasons. First, the newborn immune response matures on a gradient with age14 with increased susceptibility to infection persisting into the second month of life, and second, the current World Health Organization (WHO)/United Nations International Children’s Emergency Fund Integrated Management of Childhood Illness addresses children aged 0–59 days as a separate group (“young infants”) from children 2–59 months old.15 Therefore, any new case management strategy for use in developing countries needs to be applicable to infants who are 0–59 days old.
DIAGNOSTIC ALGORITHM TO STANDARDIZE DEFINITION OF CLINICALLY DIAGNOSED SEVERE INFECTIONS IN NEWBORNS AND YOUNG INFANTS ACROSS THE TRIAL SITES
Infections in the newborn and young infant present with nonspecific signs and symptoms and can be difficult to diagnose even by experienced health professionals.13 A high index of suspicion and prompt initiation of antibiotic therapy are required to save lives because of the often rapidly fulminant course of invasive bacterial infections in young infants. This practice is followed in industrialized countries where clinically ill-appearing newborns and young infants are treated with parenteral antibiotics on the basis of a diagnosis of “presumed sepsis,” although blood cultures eventually are found to yield no growth in the vast majority.8,16–18
In an effort to promote the early detection and appropriate referral of sick young infants by frontline health workers in resource-limited settings, WHO, United States Agency for International Development and the Saving Newborn Lives Initiative of Save the Children (funded by the Bill and Melinda Gates Foundation) sponsored a multicountry study to evaluate several clinical signs for their utility in making referral decisions compared with the judgment of an experienced clinician.19 Participating countries included Bangladesh, Bolivia, Ghana, India, Pakistan and South Africa. The Young Infant Clinical Signs Study resulted in the development of a simple clinical algorithm based on 7 signs and symptoms for predicting severe illness in the young infant with high sensitivity and moderate specificity for use in first-level health facilities.19 The 7 clinical signs and symptoms are history of difficulty feeding, history of convulsions, movement only when stimulated, respiratory rate of 60 breaths per minute or more, severe chest indrawing, temperature of 37.5°C or more or less than 35.5°C. The presence of any one triggers a decision to refer the infant. The availability of the validated Young Infant Clinical Signs of Severe Illness algorithm presented an evidence-based starting point for the development of a clinical definition of severe infection in the young infant for use in the proposed trials. However, certain limitations of this algorithm influence specificity for the diagnosis of infection, considerations that are important in equivalence trial settings where a nonspecific definition of infection could dilute out important differences in therapeutic options among a smaller group of infants who truly had severe bacterial infections included within a larger group of mildly ill infants who would recover without antibiotic therapy. In order to increase specificity of diagnosis of clinically diagnosed severe infection, the following modifications have been made: the fast respiratory rate sign has been dropped from our case definition as accumulating evidence indicates that it substantively reduces specificity of the case definition (see discussion below), the upper temperature cutoff (for fever) has been increased to 38°C (instead of 37.5°C) and history of difficulty feeding requires confirmation by observation of infant feeding (African sites) or determination of poor suck through digital examination if feeding cannot be observed (Asian sites). Presence of convulsions is considered to indicate critical illness, and infants with convulsions are excluded. Table 1 presents the case definition used for diagnosis of clinical severe infections in these trials.
TABLE 1.
Comparison of WHO Integrated Management of Neonatal Child Illness Algorithm and Case Definition of Clinically Diagnosed Infection Used in the Young Infant Simplified Antibiotic Therapy Trials

Fast breathing (respiratory rate >60 per minutes) is frequently present as an isolated sign in young infants who do not otherwise appear ill.19 Fast breathing did not predict mortality in young infants in studies from Bangladesh and India.20–22 However, fast breathing even as a single sign is part of the WHO Integrated Management of Childhood Illness clinical algorithm and indicates very severe disease and need for hospital referral and parenteral antibiotics.23 This has created a management dilemma, whether to treat young infants with fast breathing alone as a mild illness or as a severe illness. In some settings from South Asia, neonates and young infants with fast breathing have been successfully treated by oral antibiotics.6,24,25 There are no studies from Africa, and none have compared oral antibiotics with injectable therapy for management of infants with fast breathing as the only sign of illness. Thus, there is a need to inform policy by providing evidence whether young infants with fast breathing alone can be treated with oral antibiotics. Such a study is being undertaken in the multicenter African sites.
CHOICE OF ANTIBIOTICS, REGIMENS, DOSAGE, DURATION AND DELIVERY STRATEGIES
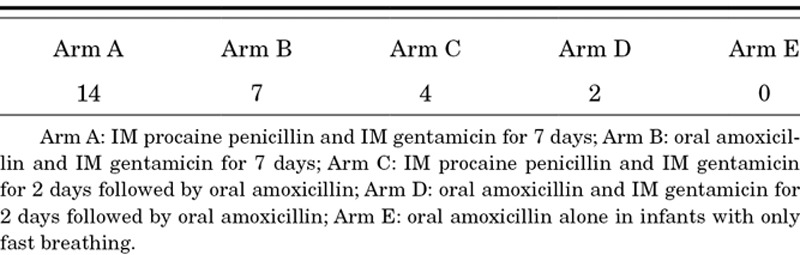
Ideally, the selection of an appropriate antimicrobial agent is based on its ability to kill bacteria that are responsible for the infection in the majority of cases, based on efficacy and safety data, and pharmacokinetic information from relevant pediatric age groups.26 For the simplified antibiotic therapy trials, additional considerations include ease of delivery in community settings and cost—both factors with significant implications for future scale-up. The experimental arms, therefore, comprise combinations of injectable and oral antibiotics or switch therapy from injectable to oral antibiotics (Tables 2 and 3).
TABLE 2.
Antibiotics Regimens Evaluated in Trials of Simplified Antibiotic Therapy for Management of Newborns and Young Infants With Clinically Diagnosed Severe Infections (CSIs) or Fast Breathing
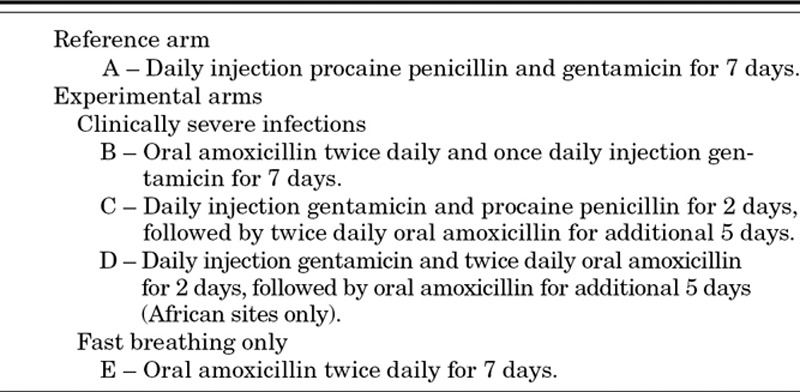
TABLE 3.
Number of of Intramuscular (IM) Injections in Each Therapeutic Arm
Penicillin and gentamicin are used globally for treating sepsis and presumed sepsis in the neonatal age group and the second month of life.15,27,28 Extended-interval (24-hourly) gentamicin regimens using doses ranging from 4 to 5 mg/kg/day have been shown to be as effective as traditional dosing regimens for treating neonatal sepsis.29–31 The combination of penicillin/amoxicillin and gentamicin targets common neonatal pathogens such as Escherichia coli, other enteric gram-negative rods, streptococci and pneumococci.
Benzyl penicillin and ampicillin need to be given 4 times a day, which is impractical in the outpatient setting. Based on recommendations from the 2007 London consultation on community-based strategies for management of severe infections in young infants born in high mortality settings,12 the pharmacologic profile of antibiotics32,33 and experience of their use,3–5 we decided to use a combination of procaine penicillin and gentamicin delivered by intramuscular (IM) injection as the reference arm to treat clinically diagnosed severe infection because both can be given in once daily dosing and together can successfully treat vast majority of bacterial infections in young infants.
For the simplified experimental regimens, amoxicillin was selected as the oral agent. Oral antibiotics have successfully been used in community-based management of neonatal pneumonia, resulting in significant reduction in pneumonia and overall neonatal mortality.6,24 There is extensive experience with use of oral amoxicillin in newborns and young infants, and safety of this drug is well-established in this age group.27,34 In full-term neonates, the bioavailability of oral amoxicillin is high at approximately 80%.35 Similar serum amoxicillin concentrations were achieved in neonates in the first 4 days of life with intravenous and oral routes, with the exception of the first 30 minutes after dosing when levels were lower with oral administration.36,37
All the simplified experimental arms in these studies employ oral amoxicillin, yet in varying ways (Table 2). Arm D (injection gentamicin for 2 days and oral amoxicillin for 7 days) is being undertaken only in the African sites because of reservations by the Technical Advisory Group about achieving an adequate sample size in Pakistan and Bangladesh within the funding period. The study comparing reference therapy with oral amoxicillin alone in infants who have only fast breathing is also being conducted in African young infants.
Amoxicillin has been recommended for use in doses ranging from 50 to 150 mg/kg/day. Higher doses are recommended to achieve minimum inhibitory concentrations that can overcome pneumococcal resistance. High-dose oral amoxicillin (80–90 mg/kg/day) is the therapy of choice for young children with otitis media and pneumonia due to suspected drug-resistant pneumococcal infection.38–40 The recommended intravenous dose of amoxicillin for neonates in the first week of life is 100 mg/kg divided every 12 hours, whereas 150 mg/kg/day divided every 8 hours is recommended for older neonates.27 In the African and South Asian trials, amoxicillin is given in doses ranging from 80 to 100 mg/kg/day based on 1 of 6 weight bands in which the infant falls.
The duration of antibiotic therapy is based on achievement of cure for an infection. The WHO recommendation of 10 or more days duration of therapy for antibiotic treatment is not based on strong evidence. Studies in Bangladesh and India have used 10 days of antibiotic therapy,4,5 whereas in Pakistan, 7 days of procaine penicillin and gentamicin once daily was found to be effective in the management of possible serious bacterial infection in young infants when families refused referral care.3 Very severe pneumonia in older children has been treated with 7 days of injectable antibiotics with good results,41 whereas severe pneumonia has been treated with 5 days of oral antibiotics.42 Switch therapy of injectable to oral antibiotics has been demonstrated to be efficacious for treatment of serious infections in neonates18 and in older children.43 For young infants with clinical severe infection included in this study, IM procaine penicillin will be used in a dose of 50,000 units/kg once daily IM and IM gentamicin in a 4–7.5 mg/kg/day once daily dose IM (depending on weight band of the young infant). Duration of all regimens in this study will be 7 days.
The trials are designed to be open-label because of the difficulty in ethically justifying use of placebo injections in the population under study and because acceptability of simplified regimens with lower number of injections is an important secondary outcome for the trials.
SELECTION CRITERIA FOR TRIAL PARTICIPANTS AND TRIAL SITES
The current standard of care for young infants with clinical signs suggestive of severe infection is hospitalization and parenteral antibiotic therapy for 10 or more days.23 As discussed elsewhere in this supplement44 and previously reported,4,45 this standard of care is rarely practiced in developing countries with high neonatal mortality rates. For ethical reasons, in the design of these trials, we felt compelled to only include sites where neonatal mortality rates and hospitalization refusal rates were known to be high,8–10 and those young infants whose families refused referral advice for hospitalization for their sick young infants. Documented refusal of hospital admission thus forms an important inclusion criterion for enrolment of study subjects. The Bangladesh and Pakistan investigators have reported their experience with family acceptance for hospitalization.22,45 Other site characteristics are reported in the site-specific articles in this supplement. The African trial sites in Democratic Republic of Congo, Kenya and Nigeria were chosen to be broadly representative of central, eastern and western sub-Saharan African countries with neonatal mortality rates exceeding 40 per 1000 live births and remote locations making access to hospital facilities difficult for many families.2
Despite the effect on study generalizability, we are excluding young infants with clinical signs of critical illness (unconscious, convulsions, apnea, unable to feed, unable to cry, cyanosed, bulging fontanelle, persistent vomiting), infants with weight less than 1500 g and infants with surgical comorbidities because we cannot ethically justify random allocation of such infants to therapeutic options with oral antibiotics.
SELECTION OF PRIMARY STUDY OUTCOME
Treatment failure on or before 7 days of therapy has been chosen as the primary study outcome in these trials and defined as a composite of death, clinical deterioration, serious adverse event, hospitalization or persistence of clinical signs beyond specified days. Death, although a hard outcome, has not by itself been selected as a primary outcome because we expect the number of deaths to be low (around 2%) based on prior experience3 as rescue therapy is going to be provided and it will be unethical to withhold such therapy. Therefore, using the comparatively rare outcome of death will inflate the sample size to unfeasible levels. Because of the subjective nature of clinical signs of deterioration, their definitions and determination have been standardized across sites and are described further in the site-specific articles.8–10
CONCLUSION
This article describes the scientific rationale for the study designs of 3 trials of simplified antibiotic therapy for the management of clinically diagnosed severe infections and a trial of management of fast breathing in newborns and young infants currently being conducted in Bangladesh, Pakistan, Democratic Republic of Congo, Kenya and Nigeria. Although designed as independent studies, extensive collaboration between study sponsors and among study investigators has resulted in harmonization of study protocols wherever possible, which will allow subsequent pooled analyses by providing sufficient power to address important policy questions regarding outcomes in subgroups such as newborns with early-onset sepsis, and infants with multiple clinical signs (indicating more severe illness) versus single signs. These trials will inform the development of policies and guidelines regarding community case management of newborns and young infants with clinically diagnosed severe infections as well as for infants with fast breathing alone.
Footnotes
Accepted for publication June 5, 2013.
The views and opinions expressed in this article are those of the author(s) and not necessarily the views and opinions of the United States Agency for International Development or World Health Organization.
This journal article was supported with funds provided by Save the Children’s Saving Newborn Lives Program through a grant from the Bill and Melinda Gates Foundation. Its contents are solely the responsibility of the authors and do not necessarily reflect the views of Save the Children or the Bill and Melinda Gates Foundation.
The Pakistan study was funded by the Saving Newborn Lives program of Save the Children Federation, Inc, through a grant from the Bill and Melinda Gates Foundation and the World Health Organization. S.S.T. received training support from the Fogarty International Center, National Institute of Health, grant D43TW007585.
The Bangladesh study was funded by the United States Agency for International Development, through a cooperative agreement (GHS-A-00-09-00004-00) with the Johns Hopkins Bloomberg School of Public Health, World Health Organization and by the Saving Newborn Lives program of Save the Children Federation, Inc, through a grant from the Bill and Melinda Gates Foundation. The authors have no other funding or conflicts of interest to disclose.
The African study was funded by the World Health Organization through a grant from the Bill and Melinda Gates Foundation.
Address for correspondence: Anita K. M. Zaidi, MB BS, SM, Professor and Chair, Department of Paediatrics and Child Health, Aga Khan University, Stadium Road, PO Box 3500, Karachi, Pakistan. E-mail: anita.zaidi@aku.edu.
Copyright © 2013 by Maharaj Kishan Bhan. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License, where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially.
REFERENCES
- 1.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. The Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.Lawn JE, Cousens S, Darmstadt GL, et al. Why are 4 million newborn babies dying every year? Lancet. 2004;364:2020. doi: 10.1016/S0140-6736(04)17511-9. [DOI] [PubMed] [Google Scholar]
- 3.Zaidi AK, Tikmani SS, Warraich HJ, et al. Community-based treatment of serious bacterial infections in newborns and young infants: a randomized controlled trial assessing three antibiotic regimens. Pediatr Infect Dis J. 2012;31:667–672. doi: 10.1097/INF.0b013e318256f86c. [DOI] [PubMed] [Google Scholar]
- 4.Bang AT, Bang RA, Baitule SB, et al. Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet. 1999;354:1955–1961. doi: 10.1016/S0140-6736(99)03046-9. [DOI] [PubMed] [Google Scholar]
- 5.Baqui AH, El-Arifeen S, Darmstadt GL, et al. Projahnmo Study Group. Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: a cluster-randomised controlled trial. Lancet. 2008;371:1936–1944. doi: 10.1016/S0140-6736(08)60835-1. [DOI] [PubMed] [Google Scholar]
- 6.Zaidi AK, Ganatra HA, Syed S, et al. Effect of case management on neonatal mortality due to sepsis and pneumonia. BMC Public Health. 2011;11(suppl 3):S13. doi: 10.1186/1471-2458-11-S3-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darmstadt GL, Black RE, Santosham M. Research priorities and postpartum care strategies for the prevention and optimal management of neonatal infections in less developed countries. Pediatr Infect Dis J. 2000;19:739–750. doi: 10.1097/00006454-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Baqui AH, Samir Kumar Saha SK, Nawshad Uddin Ahmed ASM, et al. Safety and efficacy of simplified antibiotic regimens for outpatient treatment of serious infection in neonates and young infants 0–59 days of age in Bangladesh. Pediatr Infect Dis J. 2013;32(suppl):S12–S18. doi: 10.1097/INF.0b013e31829ff790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaidi AKM, Tikmani SS, Sultana S, et al. Simplified antibiotic regimens for the management of clinically diagnosed severe infections in newborns and young infants in first-level facilities in Karachi, Pakistan: study design for an outpatient randomized controlled equivalence trial. Pediatr Infect Dis J. 2013;32(suppl):S19–S25. doi: 10.1097/INF.0b013e31829ff7aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.AFRINEST (AFRIcan NEonatal Sepsis Trial) Group. Simplified regimens for management of neonates and young infants with severe infection when hospital admission is not possible: Study protocol for a randomized, open-label equivalence trial. Pediatr Infect Dis J. 2013;32(suppl):S26–S32. doi: 10.1097/INF.0b013e31829ff7d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AFRINEST (AFRIcan NEonatal Sepsis Trial) Group. Treatment of fast breathing in neonates and young infants with oral amoxicillin compared with penicillin–gentamicin combination: study protocol for a randomized, open-label equivalence trial. Pediatr Infect Dis J. 2013;32(suppl):S33–S39. doi: 10.1097/INF.0b013e31829ff7eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khanal S, Sharma J, Gc VS, et al. Community health workers can identify and manage possible infections in neonates and young infants: MINI–a model from Nepal. J Health Popul Nutr. 2011;29:255–264. doi: 10.3329/jhpn.v29i3.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edmond K, Zaidi A. New approaches to preventing, diagnosing, and treating neonatal sepsis. PLoS Med. 2010;7:e1000213. doi: 10.1371/journal.pmed.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuenca AG, Wynn JL, Moldawer LL, et al. Role of innate immunity in neonatal infection. Am J Perinatol. 2013;30:105–112. doi: 10.1055/s-0032-1333412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UNICEF WHO. IMCI Chart Booklet—Standard. Available at: http://www.who.int/maternal_child_adolescent/documents/IMCI_chartbooklet/en/index.html. 2008.
- 16.Neal PR, Kleiman MB, Reynolds JK, et al. Volume of blood submitted for culture from neonates. J Clin Microbiol. 1986;24:353–356. doi: 10.1128/jcm.24.3.353-356.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurlat I, Stoll BJ, McGowan JE., Jr Time to positivity for detection of bacteremia in neonates. J Clin Microbiol. 1989;27:1068–1071. doi: 10.1128/jcm.27.5.1068-1071.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manzoni P, Esposito S, Gallo E, et al. Switch therapy in full-term neonates with presumed or proven bacterial infection. J Chemother. 2009;21:68–73. doi: 10.1179/joc.2009.21.1.68. [DOI] [PubMed] [Google Scholar]
- 19.Group YICSS. Clinical signs that predict severe illness in children under age 2 months: a multicentre study. Lancet. 2008;12:8. doi: 10.1016/S0140-6736(08)60106-3. [DOI] [PubMed] [Google Scholar]
- 20.Bang AT, Reddy HM, Deshmukh MD, et al. Neonatal and infant mortality in the ten years (1993 to 2003) of the Gadchiroli field trial: effect of home-based neonatal care. J Perinatol. 2005;25(suppl 1):S92–S107. doi: 10.1038/sj.jp.7211277. [DOI] [PubMed] [Google Scholar]
- 21.Bang AT, Bang RA, Reddy MH, et al. Simple clinical criteria to identify sepsis or pneumonia in neonates in the community needing treatment or referral. Pediatr Infect Dis J. 2005;24:335–341. doi: 10.1097/01.inf.0000157094.43609.17. [DOI] [PubMed] [Google Scholar]
- 22.Baqui AH, Arifeen SE, Williams EK, et al. Effectiveness of home-based management of newborn infections by community health workers in rural Bangladesh. Pediatr Infect Dis J. 2009;28:304–310. doi: 10.1097/INF.0b013e31819069e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Handbook IMCI: Integrated Management of Childhood Illness. Geneva: Switzerland: World Health Organization; 2005. [Google Scholar]
- 24.Sazawal S, Black RE Pneumonia Case Management Trials Group. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infect Dis. 2003;3:547–556. doi: 10.1016/s1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- 25.Bhutta ZA, Zaidi AK, Thaver D, et al. Management of newborn infections in primary care settings: a review of the evidence and implications for policy? Pediatr Infect Dis J. 2009;28(suppl 1):S22–S30. doi: 10.1097/INF.0b013e31819588ac. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Rahman SMKG. The pharmacokinetic-pharmacodynamic interface: Determinants of anti-infective drug action and efficacy in pediatrics. In: Feigin RD, Demmler-Harrison GJ, Kaplan SL, editors. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. Philadelphia, PA: Saunders-Elsevier; 2009. pp. 3156–3177. [Google Scholar]
- 27.Fanos V, Dall’Agnola A. Antibiotics in neonatal infections: a review. Drugs. 1999;58:405–427. doi: 10.2165/00003495-199958030-00003. [DOI] [PubMed] [Google Scholar]
- 28.Saez-Llorens XMCG. Clinical pharmacology of antibacterial agents. In: Remington JSKJ, editor. Infectious Disease of the Fetus, Newborn and Infants. Philadelphia, PA: Saunders; 2001. pp. 1419–1466. [Google Scholar]
- 29.Nestaas E, Bangstad HJ, Sandvik L, et al. Aminoglycoside extended interval dosing in neonates is safe and effective: a meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2005;90:F294–F300. doi: 10.1136/adc.2004.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao S, Ahmed M, Hagan R. One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database Syst Rev. 2006;1:CD005091. doi: 10.1002/14651858.CD005091.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Darmstadt GL, Hossain MM, Jana AK, et al. Determination of extended-interval gentamicin dosing for neonatal patients in developing countries. Pediatr Infect Dis J. 2007;26:501–507. doi: 10.1097/INF.0b013e318059c25b. [DOI] [PubMed] [Google Scholar]
- 32.Darmstadt GL, Batra M, Zaidi AK. Oral antibiotics in the management of serious neonatal bacterial infections in developing country communities. Pediatr Infect Dis J. 2009;28(suppl 1):S31–S36. doi: 10.1097/INF.0b013e3181958794. [DOI] [PubMed] [Google Scholar]
- 33.Darmstadt GL, Batra M, Zaidi AK. Parenteral antibiotics for the treatment of serious neonatal bacterial infections in developing country settings. Pediatr Infect Dis J. 2009;28(suppl 1):S37–S42. doi: 10.1097/INF.0b013e31819588c3. [DOI] [PubMed] [Google Scholar]
- 34.Gras-Le Guen C, Boscher C, Godon N, et al. Therapeutic amoxicillin levels achieved with oral administration in term neonates. Eur J Clin Pharmacol. 2007;63:657–662. doi: 10.1007/s00228-007-0307-3. [DOI] [PubMed] [Google Scholar]
- 35.Cohen MD, Raeburn JA, Devine J, et al. Pharmacology of some oral penicillins in the newborn infant. Arch Dis Child. 1975;50:230–234. doi: 10.1136/adc.50.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Autret E, Laugier J, Marimbu J, et al. Comparison of plasma levels of amoxicillin administered by oral and intravenous routes in neonatal bacterial colonization. Arch Fr Pediatr. 1988;45:679–682. [PubMed] [Google Scholar]
- 37.Giustardi A, Coppola G. Comparison of plasma concentrations of amoxicillin administered by oral and venous routes in neonatal bacterial colonizations. Pediatr Med Chir. 1992;14:447–449. [PubMed] [Google Scholar]
- 38.Dowell SF, Butler JC, Giebink GS, et al. Acute otitis media: management and surveillance in an era of pneumococcal resistance-a report from the Drug-resistant Streptococcus pneumoniae Therapeutic Working Group. Pediatr Infect Dis J. 1999;18:1. [PubMed] [Google Scholar]
- 39.Pediatrics AAo. Report of the Committee on Infectious Diseases. Grove Village: 2001. [Google Scholar]
- 40.Hale KA, Isaacs D. Antibiotics in childhood pneumonia. Paediatr Respir Rev. 2006;7:145–151. doi: 10.1016/j.prrv.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Asghar R, Banajeh S, Egas J, et al. Chloramphenicol versus ampicillin plus gentamicin for community acquired very severe pneumonia among children aged 2–59 months in low resource settings: multicentre randomised controlled trial (SPEAR study). BMJ. 2008;336:80–84. doi: 10.1136/bmj.39421.435949.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hazir T, Fox LM, Nisar YB, et al. New Outpatient Short-Course Home Oral Therapy for Severe Pneumonia Study Group. Ambulatory short-course high-dose oral amoxicillin for treatment of severe pneumonia in children: a randomised equivalency trial. Lancet. 2008;371:49–56. doi: 10.1016/S0140-6736(08)60071-9. [DOI] [PubMed] [Google Scholar]
- 43.Vouloumanou EK, Rafailidis PI, Kazantzi MS, et al. Early switch to oral versus intravenous antimicrobial treatment for hospitalized patients with acute pyelonephritis: a systematic review of randomized controlled trials. Curr Med Res Opin. 2008;24:3423–3434. doi: 10.1185/03007990802550679. [DOI] [PubMed] [Google Scholar]
- 44.Qazi SA, Wall S, Brandes N, et al. An innovative mutlipartner research programme to effectively address detection, assessment and treatment of neonatal infections in low resource settings. Pediatr Infect Dis J. 2013;32(suppl):S3–S6. doi: 10.1097/INF.0b013e31829ff5e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owais A, Sultana S, Stein AD, et al. Why do families of sick newborns accept hospital care? a community-based cohort study in Karachi, Pakistan. J Perinatol. 2011;31:586–592. doi: 10.1038/jp.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]


