Supplemental Digital Content is Available in the Text.
Key Words: antiretroviral therapy, prevention, elimination, HIV transmission, Vietnam, people who inject drugs
Abstract
Background:
Few studies have assessed the effects of antiretroviral therapy (ART) to prevent HIV transmission in Asian HIV epidemics. Vietnam has a concentrated HIV epidemic with the highest prevalence among people who inject drugs. We investigated the impact of expanded HIV testing and counseling (HTC) and early ART, combined with other prevention interventions on HIV transmission.
Methods:
A deterministic mathematical model was developed using HIV prevalence trends in Can Tho province, Vietnam. Scenarios included offering periodic HTC and immediate ART with and without targeting subpopulations and examining combined strategies with methadone maintenance therapy and condom use.
Results:
From 2011 to 2050, maintaining current interventions will incur an estimated 18,115 new HIV infections and will cost US $22.1 million (reference scenario). Annual HTC and immediate treatment, if offered to all adults, will reduce new HIV infections by 14,513 (80%) and will cost US $76.9 million. Annual HTC and immediate treatment offered only to people who inject drugs will reduce new infections by 13,578 (75%) and will cost only US $23.6 million. Annual HTC and immediate treatment for key populations, combined with scale-up of methadone maintenance therapy and condom use, will reduce new infections by 14,723 (81%) with similar costs (US $22.7 million). This combination prevention scenario will reduce the incidence to less than 1 per 100,000 in 14 years and will result in a relative cost saving after 19 years.
Conclusions:
Targeted periodic HTC and immediate ART combined with other interventions is cost-effective and could lead to potential elimination of HIV in Can Tho.
INTRODUCTION
Antiretroviral therapy (ART) prevents the transmission of HIV by suppressing viral load, one of the most important determinants of the risk of HIV transmission.1 The concept of HIV treatment as prevention (TasP) of HIV transmission has been proven by the use of antiretrovirals to prevent mother-to-child transmission2 and by observational studies assessing sexual transmission among serodiscordant couples.3-4 A randomized control trial comparing early ART with deferred ART showed that early ART reduces sexual HIV transmission by 96% in serodiscordant couples.5 Community-based studies from Taiwan,6 San Francisco,7 Vancouver,8 and KwaZulu-Natal, South Africa9 have shown that the number of newly reported HIV cases or the hazard of HIV acquisition fell as ART coverage was expanded and as community viral load declined. HIV incidence in a cohort of people who inject drugs (PWID) was positively associated with the mean viral load in HIV-positive PWID, providing additional evidence that ART prevents needle-borne HIV transmission.8,10
Mathematical models have also been developed to estimate the impact of TasP on generalized epidemics.11,12 Modeling studies consistently show a significant impact of ART on incidence in generalized settings.11,12 For example, modeling suggested that annual HIV testing and counseling (HTC) followed by immediate ART for people diagnosed with HIV could greatly lead to the elimination of HIV in South Africa.12 Although models have also been developed to estimate the preventive effect of ART in PWID,13–16 they have only examined the impact of ART provided to those with CD4 count below 350 cells per cubic millimeter.
Although a number of studies on TasP have been started or are being planned,17 relatively few are focused on the HIV epidemic in Asia. Asia has the second largest number of people living with HIV (PLHIV) after Africa, and most Asian countries have low or concentrated HIV epidemics.18 Opportunities exist to significantly reduce new infections through improved access to earlier testing and treatment in some Asian countries with relatively low HIV prevalence. In Vietnam, the HIV epidemic is concentrated in certain key populations. In 2011, HIV prevalence was highest in PWID at 13.4%, whereas the estimated prevalence in the general population aged 15–49 years was only 0.45%.19 In 2011, there were an estimated 249,000 people living with HIV.19 ART has been rapidly scaled-up since 2005, and 60,924 people were receiving ART at the end of 2011.19
Although emphasizing the priority to further scale-up ART among those with CD4 count less than 350 cells per cubic millimeter, World Health Organization advises countries to consider expanding ART for certain subpopulations in which the greatest health benefits and prevention impact can be expected.20,21 Can Tho is one of the 2 provinces chosen by the Vietnam Ministry of Health to demonstrate proactive expansion and simplification of HIV treatment including improved access to earlier HIV testing and treatment.22,23 We use Can Tho provincial data to analyze the potential benefits and strategies of expanding access to HTC and ART in combination with other prevention interventions.
METHODS
Model Structure
A standard compartmental model with susceptible people, PLHIV not on ART, and PLHIV on ART24,25 was developed for each of 7 subpopulations. Although generalized epidemics are often modeled assuming 1 population with similar characteristics, concentrated epidemics require the establishment of subpopulations with different HIV prevalence, different roles in HIV transmission dynamics, and different interventions. To address this issue, a network structure that links subpopulations as seen in Vietnam was used to construct the model (Fig. 1) The model includes the following: PWID, men who have sex with men (MSM), female sex workers (FSWs), male clients of female sex workers (MCF), and low-risk women (LRW). MSM and FSWs are further divided into 2 groups: those who inject drugs and those who do not. It is assumed that a certain proportion of men in all male groups visit FSW or have regular female partners (see Appendix, Supplemental Digital Content, http://links.lww.com/QAI/A431). We also assumed that although LRW can be infected by their male sexual partners, they do not infect other adults. The low-risk men, including low-risk MSM, were excluded from the model, with the assumption that their contribution to the epidemic and studied outcomes are negligible. However, if a person in key populations ceases to be engaged in the risk behavior, but is infected with HIV, he or she was retained in the model and counted in the original risk groups. The equations and further details for the model are given in the Appendix (see Supplemental Digital Content, http://links.lww.com/QAI/A431) and are implemented in Microsoft Excel. The model is fitted using maximum likelihood, allowing for overdispersion of the data where necessary, and checking that the fit is statistically acceptable for each subpopulation separately and collectively.
FIGURE 1.
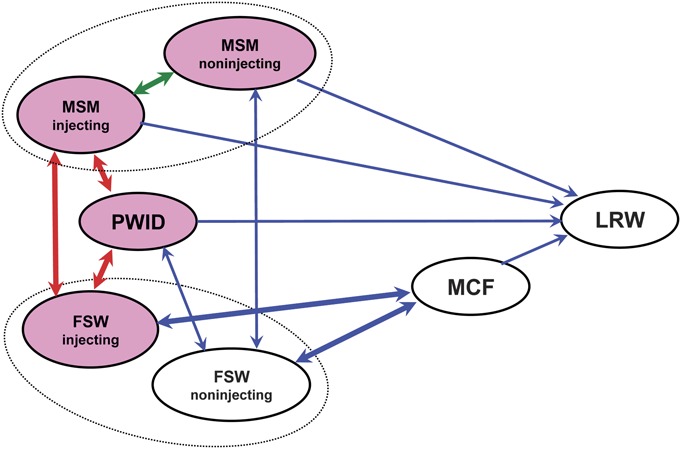
Schematic diagram showing the links between the different subpopulations in the model. Red arrow shows HIV transmission via needle sharing, green arrow shows sexual transmission among MSM, and blue arrow shows heterosexual transmission. The groups coloured pink indicate that HIV transmission happens within the group.
Data Sources
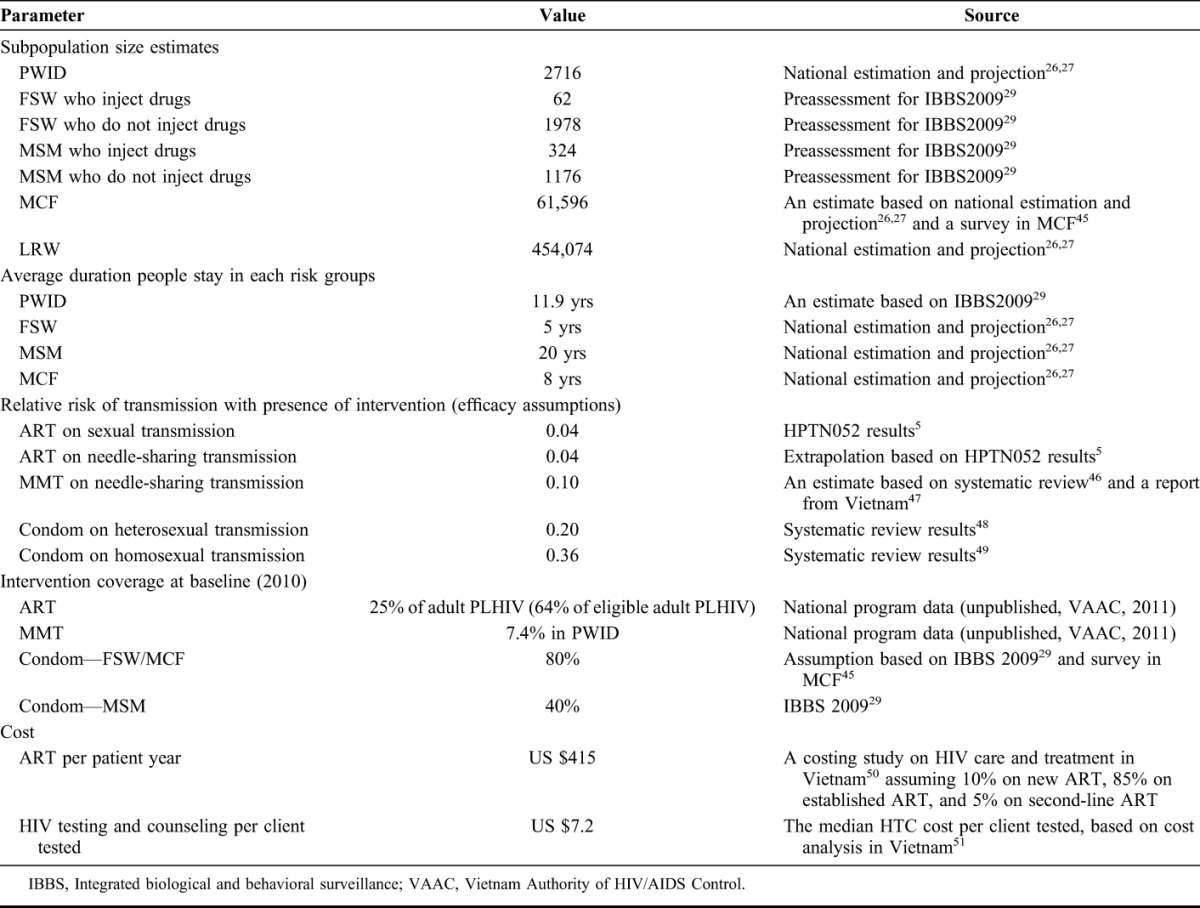
The trend data for HIV prevalence in each subpopulation in Can Tho are drawn from data set used by the Vietnam’s national working group on estimation and projection,26,27 The data set is derived from annual National Sentinel Surveillance from 1994 to 2010 (unpublished data, Vietnam Authority of HIV/AIDS Control, 2011) and Integrated Biological and Behavioral Surveillance (IBBS) in 200628 and 2009.29 For FSWs and MSM, sentinel surveillance does not distinguish between those who do and do not inject drugs, but estimates from IBBS 200929 were used to calibrate the prevalence in those 2 groups. To estimate the subpopulation sizes (Table 1), the data were triangulated using numbers used for the national estimation and projection,26,27 data from various Ministries, mapping exercises conducted by various projects, and HIV case reports. The unit cost of ART per patient-year and that of HTC were obtained from the costing studies in Vietnam (Table 1). Further details of the data sources are available in the Appendix (see Supplemental Digital Content, http://links.lww.com/QAI/A431).
TABLE 1.
Key Input Parameters and Assumptions Used in the Model
Interventions and Scenarios
This study assessed the effects of “periodic testing and immediate treatment (PTIT),” for which we assume people voluntarily receive HTC at regular intervals and, if diagnosed with HIV, start ART immediately irrespective of CD4 count. We define “universal PTIT” as PTIT provided to all adults, and “targeted PTIT” as PTIT offered to 1 or more selected subpopulations. It was assumed that PTIT is introduced in 2011 and expanded to the defined coverage by 2015 and then maintained until 2050. We also define standard ART as the ART provided when people have CD4 count less than 350 cells per cubic millimeter. The following 4 scenario sets, 13 in total, were assessed.
Baseline Scenarios
(1) No intervention counter factual scenario, assuming that neither ART nor other prevention intervention had ever been made available; (2) reference scenario, in which ART and other prevention intervention coverage (Table 1) are maintained at the level in 2010 up to 2050.
Universal PTIT Scenarios
All adults receive PTIT with periodic HTC offered at varying frequency including every 2 years, every year, or every 6 months. Other prevention interventions were maintained at the 2010 level.
Targeted PTIT Scenarios
PTIT is provided to each of the 5 subpopulations separately with annual HTC. Other prevention interventions are maintained at the 2010 level.
Potential Policy Scenarios
(1) Standard ART scale-up: ART expanded to 90% of eligible adults (CD4 ≤ 350 cells/mm3) by 2020; coverage of other prevention interventions is maintained at the 2010 level. (2) Combination prevention scale-up: methadone maintenance therapy (MMT) expanded to 50% of PWID; consistent condom use expanded to 85% of FSW and 60% of MSM; ART to 90% of eligible adults (CD4 ≤ 350 cells/mm3), by 2020. (3) Combination prevention scale-up including the interventions in the previous scenario 2 in combination with PTIT with annual HTC offered to PWID, FSW, and MSM.
For the universal and targeted PTIT scenarios, it was assumed 100% of people offered services will accept HTC at regular intervals; for the potential policy scenarios that involve PTIT, it was assumed 70% of PWID and MSM and 80% of FSW would be reached and accept annual HTC. For both PTIT and standard ART, it was assumed 95% of those tested HIV positive will start ART immediately based on respective eligibility criteria. All the scenarios assume that current level of clean syringe accessibility be maintained over the studied period. The assumptions for intervention efficacy are shown in Table 1.
Outcome Measures and Uncertainty Analysis
The following measurements were determined for each scenario: cumulative new HIV infections and deaths, cumulative costs of HTC and ART, and cost per disability-adjusted life years (DALYs) averted, in the period from 2011 to 2050. Costs are discounted at 3% per year against the baseline year at 2010. To estimate DALYs, we ignored the relatively modest disability component (about 1.5%), as described previously, 30 because for HIV prevention and life-prolonging ART, DALYs are almost entirely captured by the change in mortality.
Uncertainty concerning ART efficacy to reduce the probability of needle-borne HIV transmission was studied by changing the efficacy step-wise from 96% to 70%. This analysis was performed for the following key scenarios: (1) reference, (2) Universal PTIT with annual HTC, (3) Targeted PTIT focusing on PWID, and (4) Combination prevention scale-up with PTIT for key populations.
RESULTS
Modeling of the Epidemic
The fitted values of the HIV prevalence in 2010 were as follows: 46% in PWID, 55% in FSWs who inject drugs, 3.8% in FSWs who do not inject drugs, 27% in MSM who inject drugs, 3.6% in MSM who do not inject drugs, 1.0% in MCF, and 0.35% in LRW (for Figure of best fit to HIV trend data, see Figure S3, Supplemental Digital Content, http://links.lww.com/QAI/A431).
Baseline Scenarios
If neither ART nor other interventions had ever been made available, there would have been an estimated 30,329 new infections and 17,301 AIDS deaths from 2011 to 2050 in Can Tho province. With the reference scenario, in which ART and other prevention interventions are maintained at the coverage in 2010, there are a projected 18,115 new HIV infections and 7624 AIDS deaths, with a cumulative cost of US $22.1 million.
Universal PTIT Scenarios
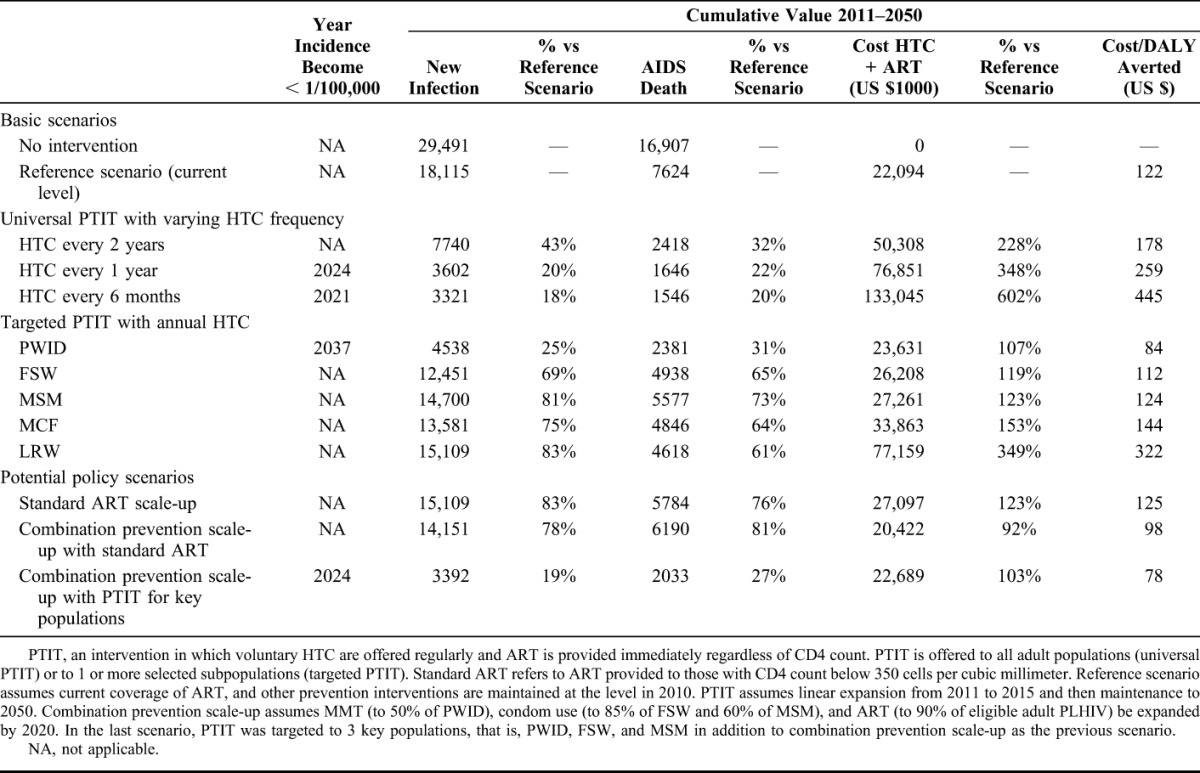
When compared with the reference scenario, universal PTIT with testing once every 2 years, 1 year, and 6 months will avert 57%, 80%, and 82% of new infections; and 68%, 78%, and 80% of deaths, respectively (Table 2). The cumulative HTC and ART costs with testing once every 2 years, 1 year, and 6 months will be 2.3-fold, 3.5-fold, and 6-0 fold as high as those in the reference scenario (Table 2).
TABLE 2.
Effects of Various Scenarios on Cumulative New HIV Infection, AIDS Death, Cost, and Cost-Effectiveness From 2011 to 2050
Targeted PTIT Scenarios
The impact of PTIT differs greatly depending on which subpopulation is targeted. When compared with the reference scenario, the largest reduction in the cumulative new infections (75% reduction) and deaths (69% reduction) is achieved when the target group is PWID. If the target group is FSWs, new infections and deaths are reduced by 31% and 35%, respectively. If the target group is MSM, MCF, and LRW, new infections are reduced by 19%, 25%, and 17%, although there will still be modest reductions in deaths of 27%, 36%, and 39%, respectively. Relative to the costs associated with the reference scenario, only a small additional cost is required to target key populations: 107%, 119% and 123% for PWID, FSW and MSM, respectively (Table 2). In contrast, when MCF or LRW are targeted, the costs are much greater at 359% and 349%, respectively.
Potential Policy Scenarios
Increasing standard ART coverage to 90% of currently eligible adults (CD4 ≤ 350 cells/mm3) reduces cumulative new infections by 17% and of deaths by 24% compared with the reference scenario, although costs increase by 23% (Table 2). Adding the expansion of combination prevention including MMT, condom use and standard ART decreases cumulative new infections and deaths by 22% and 19%, respectively, and would cost 8% less than the reference. Relative to the reference scenario, adding PTIT targeting for PWID, FSW, and MSM to the combination prevention scale-up results in a decrease in cumulative new infections and deaths by 81% and 73%, respectively, and only a 3% increase in costs. This scenario will reduce HIV incidence from 61 per 100,000 person-years in 2010 to 6.2 per 100,000 person-years (90% reduction) in 10 years, and to less than 1 per 100,000 person-years in 2024. Although the annual costs of the PTIT-containing combination prevention is higher than that of the reference scenario until 2029, it will be cost saving after 2029 (Fig. 2C).
FIGURE 2.
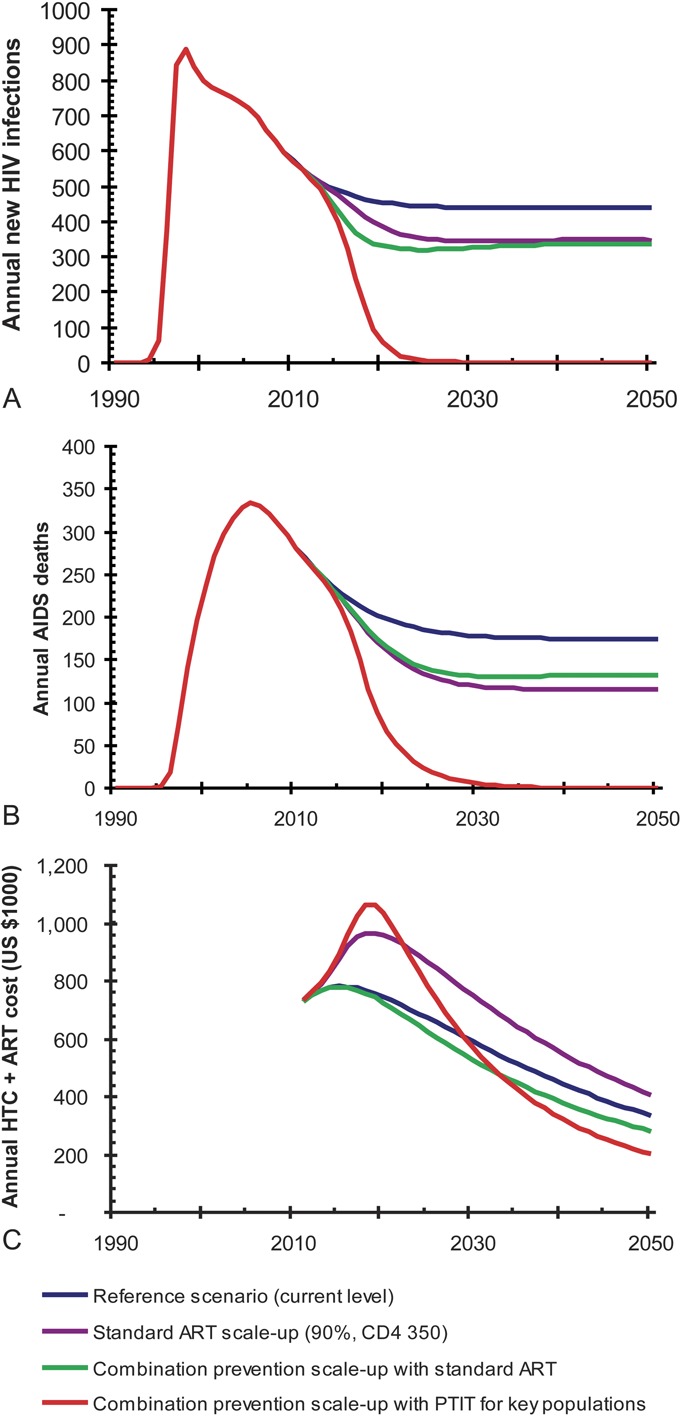
Annual new HIV infection (A), AIDS death (B), and ART and HTC cost (C) with 3 potential policy scenarios. Reference scenario assumes current coverage of ART, and other prevention interventions are maintained at the level in 2010. Combination prevention scale-up assumes expansion of MMT, condom use, and ART. In the last scenario, PTIT focusing on 3 key populations, that is, PWID, FSW, and MSM, was added to combination prevention scale-up.
Cost-Effectiveness Analysis
Figure 3 maps the scenarios against cumulative cost and DALYs averted. The reference scenario costs US $ 122 per DALY saved. There were 2 nondominated scenarios as follows: combination prevention scale-up (US $98/DALY averted) and combination prevention with PTIT for key populations (US $78/DALY averted). Incremental cost effectiveness ratio of the latter against the former is US $28 per DALY. Targeted PTIT focusing on PWID also had similar cost-effectiveness profile as the latter, and was close to “efficiency frontier” (Fig. 3). Other scenarios studied were dominated.
FIGURE 3.
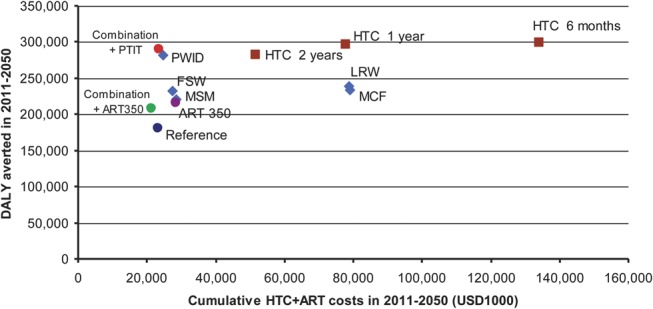
Cumulative DALY saved and cost of HTC and ART from 2011 to 2050 of 13 analyzed scenarios. Brown box refers to universal PTIT scenarios with varying HTC interval (2 year, 1 year, and 6 months). Blue diamond refers to targeted PTIT scenarios with annual HTC focusing on single subpopulations (PWID, FSW, MSM, MCF, and LRW). SA, standard ART scale-up to 90% of those with CD4 below 350 cells per cubic millimeter. Combination + ART 350, combination prevention scale-up with standard ART. Combination + PTIT, combination prevention scale-up with PTIT for PWID, FSW, and MSM.
Uncertainty Analysis
Figure S5 (see Supplemental Digital Content, http://links.lww.com/QAI/A431) shows the effects of varying assumed ART efficacy on needle-borne transmission on cumulative new infections and costs from 2011 to 2050 for the 4 key scenarios. The impact of PTIT to reduce new infection is attenuated in efficacy-dependent manner, although there is still substantial reduction of cumulative new infections by PTIT; for example, cumulative new infections will be reduced by 54% with “combination prevention scale-up with PTIT” scenario even when the efficacy is assumed at 70%. The cumulative costs increase by only 6%–18% depending on the scenarios, when the efficacy is changed from 96% to 70%. (see Figure S5, Supplemental Digital Content, http://links.lww.com/QAI/A431). When the efficacy is 90% or greater, the “combination prevention scale-up with PTIT” scenario reduces HIV incidence to below 10 in 100,000.
DISCUSSION
Our model suggests that active HIV case finding and earlier access to ART for key populations combined with other interventions could lead to highly effective and cost-effective reductions in HIV transmission in this concentrated epidemic setting. The best strategy consisted of delivering services to key populations including early HIV diagnosis, immediate ART, MMT, condom use alongside the current approach to providing of HTC and ART for those at lower risk. It is important to note that all of the scenarios assume that the current high level of access to clean syringes is maintained. Projections using this strategy suggest that it would dramatically reduce HIV incidence to 1/100,000 in 14 years and achieve annual cost savings after around 20 years.
Our model suggests that providing periodic testing and immediate ART for key populations, especially for PWID, is the least expensive and most effective intervention for reducing the new infections and deaths in the entire population. In contrast, offering PTIT universally to all adults or targeting PTIT for populations with low HIV incidence, such as MCF or LRW, is far less cost-effective given its smaller epidemiological impact and much larger costs. Since MCF and LRW are large populations, more HIV tests are required to offer HTC periodically (see Figure S6, Supplemental Digital Content, http://links.lww.com/QAI/A431). These findings suggest that different testing and treatment strategies could be considered depending on the population, such as periodic HTC and earlier ART for higher risk key populations, and client-initiated and provider-initiated HTC at health facilities for MCF and LRW. Because in Vietnam, LRW are primarily infected by their male sexual partners who have risk behaviors,31,32 offering HTC to partners of PLHIV could potentially facilitate reaching LRW who are living with HIV earlier although avoiding the need to test the entire LRW population.
Although offering annual testing and immediate ART requires a substantial initial investment, projections suggest that focusing on key populations will ensure a substantial return on this medium-term investment. Additionally, our analysis shows that the cumulative person-years of ART required for 2011–2050 will be similar regardless of whether PTIT is introduced or the current ART program is maintained. The earlier ART intervention will reduce new infections to a much greater extent than the current approach, which balances the initial increase in the number of people starting ART with a subsequent decrease in people in need for ART in later years. These findings are in line with modeling results regarding the projected impact of ART in generalized epidemics.12,30
Our analysis suggested that PTIT would have greater impact and is cost-saving when implemented as part of a combination prevention strategy.33 As part of combination prevention efforts, Vietnam has effectively implemented large-scale harm reduction programs.34 At the end of 2011, 6900 people were receiving MMT at 41 clinics,19 and in 2011, existing needle-syringe programs (NSPs) distributed approximately 30 million needles and syringes or 140 syringes per PWID per year.19 Importantly, a combination prevention strategy could harness programmatic synergy between those behavioural interventions and PTIT. For example, peer educators working for NSP and condom promotion would likely be the most effective agents who can directly reach key populations and facilitate their early uptake of HIV diagnosis and treatment. In 2011, in Vietnam, only 29% of PWID, 44% of FSW, and 30% of MSM reported that they had received HTC in the past 12 months and knew their test results.19 Improving demand and earlier and more frequent access to HIV testing is critical but unlikely to be feasible without capitalizing on the already effective community-based NSP and condom promotion program. Likewise, opioid substitution therapy also improves ART uptake, adherence, and outcomes35,36 and the Government’s plan to expand MMT to 80,000 PWID by 201519 could serve as an effective platform for delivering periodic HTC and earlier ART in PWID.
Our model did not examine scenarios that varied NSP coverage because the available data and anecdotal information suggests that PWID in Vietnam already have a high level of access to clean needles not only through NSP but also from other sources (eg, pharmacies). For example, the majority of PWID (85%–98%, depending on provinces) reported using clean needles and syringes at the last injection,29 although the same survey found a large variation in access to NSP in the last 6 months by provinces ranging from 0.7% to 84%.29 Although the scientific evidence is clear that access to clean needles is an effective steps in reducing the needle-borne HIV transmission, for the purposes of modeling, the already high level of access to clean needles would mean that changes in current background NSP coverage would have little impact in Viet Nam.
The precise efficacy of ART in reducing probability of parenteral HIV transmission is not yet established. Although per-contact risk of HIV transmission associated with needle-sharing is higher than that associated with sexual intercourse,37–39 it would be plausible to assume that the transmission risk is proportional to viral load, and thus the risk reduction attributable to ART is likely similar irrespective of transmission route. With this assumption, we extrapolated the extent of risk reduction by ART on sexual transmission to that on needle-borne transmission, although we also performed uncertainty analysis by varying the ART efficacy assumptions. As expected, the results showed that the impact of ART on new HIV infection is attenuated proportionally to the assumed efficacy; however, ART still has substantial impact in reducing new infection even if the efficacy is reduced to 70%, suggesting that ART could still be effective intervention to reduce new infection. There are also uncertainties in program parameters, for example, uptake of HIV testing and ART, retention and adherence to ART, and behavioral risk reduction, but our goal was to examine a well-performing program and we aim to study the individual effects of these parameters on program performance in future studies. There have also been limited data on the role of acute HIV infection on the onward transmission; however, the recent debate suggests that acute infection will not compromise predicted impact of test-and-treat strategies.40
A recent review reported that social and structural factors have placed significant barriers to optimal ART use among PWID and called for removal of those in order for TasP strategies to be maximally effective.41 In Vietnam, punitive policies and associated stigma and discrimination against people who use drugs have been recognized as critical barriers for their timely access to health and HIV services.42,43 However, in late 2012, the Government responded to the United Nations’ statement calling for the closure of compulsory drug detention centres44 and made the initial step by expressing its vision to shift toward a community-based voluntary drug treatment approach. For the modeled strategy to be successful, it is essential that the society addresses the structural barriers so that key populations can safely access HIV services. The interventions need to be implemented in a way that respects human rights, and successful design and implementation would depend on the extent of the support of local communities of key populations and PLHIV.
There are limitations in our analysis. First, there are a number of uncertainties in our modeling assumptions. We plan to further analyze the effects of program parameters in future studies. Second, we did not include the cost of outreach for key populations, which is an essential intervention to facilitate their HTC uptake. Third, we did not include the cost of other prevention interventions, that is, MMT, NSP, and condom promotion, and thus a direct comparison of the complete cost associated with combination prevention and PTIT is not possible.
Our analysis represents the only scientific efforts we are aware of to estimate potential impact of active case finding and immediate HIV treatment in a concentrated epidemic in Asia. The analysis suggests that upfront investment in offering access to periodic HTC and immediate treatment to key populations is likely to save a substantial number of lives and costs in the near future. Targeted and frequent HTC and earlier ART significantly decreases HIV infections and deaths, and when combined with other interventions, it could make a substantial contribution to the earlier achievement of HIV control and elimination.
ACKNOWLEDGMENTS
The authors are grateful to Dr Lai Kim Anh, Director of Can Tho Provincial AIDS Center, for her support for the present study. The authors thank Dr Rod Bennett for the technical guidance in economic analysis; Dr Bruce Struminger, Dr. Michelle McConnell, Dr Nguyen Thi Thuy Van, and Ms. Nguyen Thi Minh Thu for their technical comments; and Ms Nguyen Thi Vinh for her secretarial support. Open access was made possible with support by US CDC.
Footnotes
M.K., R.G., and B.W. contributed to conception of the study. B.W., M.K., R.G., D.D.B., A.B.S., F.M., and Y.R.L. contributed to design of the study. Data synthesis was undertaken by M.K., H.V.T., P.N., D.J., and K.S. Mathematical modeling was done by B.W. and A.B.S. The authors M.K., R.G., H.V.T., P.N., A.B.S., and Y.R.L. contributed in the literature review. M.K., B.W., and R.G. drafted the report. M.K., R.G., D.D.B., H.V.T., P.N., A.B.S., D.J., K.S., F.M., Y.R.L., and B.W. contributed to review and editing of the publication.
Supported by United Nations in Vietnam. Modeling work by B.W. was supported by United Nations in Vietnam through One United Nations fund to World Health Organization.
The opinions and statements in this article are those of the authors and do not represent the official policy, endorsement, or views of World Health Organization and US CDC
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929 [DOI] [PubMed] [Google Scholar]
- 2.CDC. Achievements in public health. Reduction in perinatal transmission of HIV infection–United States, 1985–2005. MMWR Morb Mortal Wkly Rep. 2006;55:592–597 [PubMed] [Google Scholar]
- 3.Attia S, Egger M, Muller M, et al. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404 [DOI] [PubMed] [Google Scholar]
- 4.Anglemyer A, Rutherford GW, Baggaley RC, et al. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. Cochrane Database Syst Rev. 2011;10:CD009153 [DOI] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang CT, Hsu HM, Twu SJ, et al. Decreased HIV transmission after a policy of providing free access to highly active antiretroviral therapy in Taiwan. J Infect Dis. 2004;190:879–885 [DOI] [PubMed] [Google Scholar]
- 7.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanser F, Barnighausen T, Grapsa E, et al. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood E, Kerr T, Marshall BD, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton JW, Johnson LF, Salomon JA, et al. HIV Treatment as Prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med. 2012;9:e1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granich RM, Gilks CF, Dye C, et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57 [DOI] [PubMed] [Google Scholar]
- 13.Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. PLoS Med. 2011;8:e1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degenhardt L, Mathers B, Vickerman P, et al. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet. 2010;376:285–301 [DOI] [PubMed] [Google Scholar]
- 15.Strathdee SA, Hallett TB, Bobrova N, et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet. 2010;376:268–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Gilmour S, Zhang H, et al. The epidemiological impact and cost-effectiveness of HIV testing, antiretroviral treatment and harm reduction programs. AIDS. 2012;26:2069–2078 [DOI] [PubMed] [Google Scholar]
- 17.Granich R, Gupta S, Suthar AB, et al. Antiretroviral therapy in prevention of HIV and TB: update on current research efforts. Curr HIV Res. 2011;9:446–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UNAIDS. UNAIDS Report on the Global AIDS Epidemic 2010. Geneva, Switzerland: UNAIDS; 2010 [Google Scholar]
- 19.Viet Nam AIDS Response Progress Report 2012. Hanoi, Vietnam: National Committee for AIDS, Drugs and prostitution prevention and control; 2012. http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/ce_VN_Narrative_Report.pdf (Accessed on 2 March 2013). [Google Scholar]
- 20.WHO. The Strategic Use of Antiretrovirals to Help End the HIV Epidemic. Geneva, Switzerland: World Health Organization; 2012 [Google Scholar]
- 21.WHO. Programmatic Update: Antiretroviral Treatment as Prevention (TasP) of HIV and TB. Geneva, Switzerland: World Health Organization; 2012 [Google Scholar]
- 22.Hirnschall G, Schwartlander B. Treatment 2.0: catalysing the next phase of scale-up. Lancet. 2011; 378:209–11 [DOI] [PubMed] [Google Scholar]
- 23.Bui DD, Mesquita F, Do TN, et al. Treatment 2.0 Pilot in Viet Nam—early progress and challenges. World J AIDS. 2012;2:64–70 [Google Scholar]
- 24.Williams BG, Granich R, De Cock KM, et al. Antiretroviral therapy for tuberculosis control in nine African countries. Proc Natl Acad Sci U S A. 2010;107: 19485–19489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams BG, Granich R, Dye C. Role of acute infection in HIV transmission. Lancet. 2011;378:1913; author reply 1914–1915 [DOI] [PubMed] [Google Scholar]
- 26.Viet Nam HIV/AIDS Estimates and Projections 2007–2012. Hanoi, Viet Nam: Viet Nam Authority of HIV/AIDS Control, Ministry of Health; 2009 [Google Scholar]
- 27.Viet Nam HIV/AIDS Estimates and Projections 2011–2015. Hanoi, Viet Nam: Viet Nam Authority of HIV/AIDS Control, Ministry of Health; 2012 [Google Scholar]
- 28.Results from the HIV/STI Integrated Biolgoical and Behavioral Surveillance (IBBS) in Viet Nam 2005–2006. Hanoi, Viet Nam: Ministry of Health - Viet Nam; 2006 [Google Scholar]
- 29.Results from the HIV/STI Integrated Biological and Behavioral Surveillance (IBBS) in Viet Nam – Round II 2009. Ministry of Health – Viet Nam. Hanoi, Viet Nam. 2011 [Google Scholar]
- 30.Granich R, Kahn JG, Bennett R, et al. Expanding ART for treatment and prevention of HIV in South Africa: estimated cost and cost-effectiveness 2011–2050. PLoS One. 2012;7:e30216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Travis L, Tran TD, Tran HM. Triangulation Exercise on Intimate Partner Transmission of HIV in Viet Nam. Hanoi, Viet Nam: UNAIDS and UN Women; 2012 [Google Scholar]
- 32.Baseline Surveys in Female Sexual Partners of Male IDUs in Dien Bien and Ho Chi Minh City. Hanoi, Viet Nam: Partners in Health Research; 2012 [Google Scholar]
- 33.Merson MH, O'Malley J, Serwadda D, et al. The history and challenge of HIV prevention. Lancet. 2008;372:475–488 [DOI] [PubMed] [Google Scholar]
- 34.WHO. Good Practice in Asia: Targeted HIV Prevention for IDU and Sex Workers. Viet nam's First Large-Scale National Arm Reduction Initiative WHO/WPRO and Partners. Hanoi, Vietnam: World Health Organization; 2010. http://www.who.int/hiv/pub/idu/wpro_vietnam/en/ Accessed on 2 March 2013 [Google Scholar]
- 35.Malta M, Magnanini MM, Strathdee SA, et al. Adherence to antiretroviral therapy among HIV-infected drug users: a meta-analysis. AIDS Behav. 2010;14:731–747 [DOI] [PubMed] [Google Scholar]
- 36.Wood E, Kerr T, Tyndall MW, et al. A review of barriers and facilitators of HIV treatment among injection drug users. AIDS. 2008;22:1247–1256 [DOI] [PubMed] [Google Scholar]
- 37.Baggaley RF, Boily MC, White RG, et al. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS. 2006;20:805–812 [DOI] [PubMed] [Google Scholar]
- 38.Hudgens MG, Longini IM, Jr, Halloran ME, et al. Estimating the transmission probability of human immunodeficiency virus in injecting drug users in Thailand. Appl Stat. 2001;50:1–14 [Google Scholar]
- 39.Royce RA, Sena A, Cates W, Jr, et al. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078 [DOI] [PubMed] [Google Scholar]
- 40.Cohen MS, Dye C, Fraser C, et al. HIV treatment as prevention: debate and commentary—will early infection compromise treatment-as-prevention strategies? PLoS Med. 2012;9:e1001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milloy MJ, Montaner J, Wood E. Barriers to HIV treatment among people who use injection drugs: implications for 'treatment as prevention'. Curr Opin HIV AIDS. 2012;7:332–338 [DOI] [PubMed] [Google Scholar]
- 42.Maher L, Coupland H, Musson R. Scaling up HIV treatment, care and support for injecting drug users in Vietnam. Int J Drug Policy. 2007;18:296–305 [DOI] [PubMed] [Google Scholar]
- 43.WHO. Assessment of Compulsory Treatment of People Who Use Drugs in Cambodia, China, Malaysia and Viet Nam: An Application of Selected Human Rights Principles. Manila, Philippines: World Health Organization Western Pacific Regional Office; 2009 [Google Scholar]
- 44.UN Joint Statement. Compulsory drug detention and rehabilitation centres. 2012. Available at: http://www.unodc.org/documents/eastasiaandpacific//2012/03/drug-detention-centre/JC2310_Joint_Statement6March12FINAL_En.pdf (Accessed on 2 March 2013).
- 45.Behavioral Survey of Male Clients of Female Sex Workers Across Seven Provinces in Viet Nam. Hanoi, Viet Nam: Population Service International Viet Nam; 2009 [Google Scholar]
- 46.Gowing L, Farrell MF, Bornemann R, et al. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2011:CD004145 [DOI] [PubMed] [Google Scholar]
- 47.Evaluation of the Pilot Model for the Substitution Treatment of Opiate Dependence Using Methadone in Hai Phong and Ho Chi Minh City (Phase 1). Hanoi, Viet Nam: Viet Nam Ministry of Health; 2009 [Google Scholar]
- 48.Weller S, Davis K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev. 2002:CD003255 [DOI] [PubMed] [Google Scholar]
- 49.WHO. Prevention and Treatment of HIV and Other Sexually Transmitted Infections Among Men Who Have Sex With Men and Transgender People: Recommendations for a Public Health Approach 2011-Annexes. Geneva, Switzerland: World Health Organization;2011 [PubMed] [Google Scholar]
- 50.Duong AT, Kato M, Bales S, et al. Costing analysis of national HIV treatment and care programme in Vietnam. JAIDS 2013 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 51.Cost analyses of selected USAID-supported HIV interventions managed by FHI 360 in Vietnam: outreach, HIV testing and counseling (HTC), and anti-retroviral treatment (ART) 2009–2010. FHI 360/Urban Care. Hanoi, Vietnam. 2012


