Abstract
Preeclampsia is a major pregnancy complication with potential short- and long-term consequences for both mother and fetus. Understanding its pathogenesis and causative biomarkers is likely to yield insights for prediction and treatment. Herein, we provide evidence that transthyretin, a transporter of thyroxine and retinol, is aggregated in preeclampsia and is present at reduced levels in sera of preeclamptic women, as detected by proteomic screen. We demonstrate that transthyretin aggregates form deposits in preeclampsia placental tissue and cause apoptosis. By using in vitro approaches and a humanized mouse model, we provide evidence for a causal link between dysregulated transthyretin and preeclampsia. Native transthyretin inhibits all preeclampsia-like features in the humanized mouse model, including new-onset proteinuria, increased blood pressure, glomerular endotheliosis, and production of anti-angiogenic factors. Our findings suggest that a focus on transthyretin structure and function is a novel strategy to understand and combat preeclampsia.
Preeclampsia occurs in 5% to 8% of pregnancies worldwide and is a major cause of fetal and maternal morbidity and mortality.1–3 It is a heterogeneous disease with varied presentations from mild self-limited hypertension and proteinuria to severe forms with significant end-organ dysfunction and HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets).3 Although the cause of preeclampsia and its appropriate treatment remain elusive, this syndrome has been proposed to reflect at least two stages of complications during pregnancy. These begin with preclinical manifestations at the maternal-fetal interface, followed by systemic clinical symptoms.1,2 Hypertension, proteinuria, and edema, with a variable degree of fetal growth restriction, are the cardinal features of preeclampsia.3 Because the placenta is the nutritional and immunological gateway to normal fetal development and pregnancy outcome, placenta-related events are believed to be central to the pathogenesis of this disease. Evidence exists for the release of disease-initiating molecules into maternal circulation that triggers the clinical symptoms.1,4 Placental and systemic anomalies reflected by circulating placental debris, inflammation, impaired remodeling of spiral arteries, placental hypoxia/ischemia, excess production of anti-angiogenic factors [soluble fms-like tyrosine kinase-1 (sFlt-1)], and soluble endoglin (sEng), and angiotensin receptor autoantibodies have all emerged as contributors to the pathophysiological characteristics of preeclampsia.2,4–14
Preeclampsia has remained enigmatic because of lack of well-defined etiology and animal models. Although normal mice do not develop preeclampsia spontaneously, mouse models have been judged to be particularly useful to uterine diseases and pregnancy complications because many similarities in female reproduction and placentation have been identified between the two species.15 Moreover, their tractable genetics provide an effective way to probe mechanisms more deeply than many other species.15–17 We recently showed that sera from preeclamptic women could function as a source of novel causative factors that induced hypertension, proteinuria, and kidney pathological characteristics, as well as intrauterine growth restriction (IUGR), in IL-10−/− mice in a pregnancy-specific manner.18 IL-10 functions as a potent vascular and anti-inflammatory cytokine and has been shown to be present at significantly reduced levels in preeclampsia placental tissue.19,20 Preeclampsia serum (PES) was found to disrupt endovascular cross talk between trophoblasts and endothelial cells and to induce placental hypoxia and excess production of sFlt-1 and sEng,18 soluble factors known to precipitate maternal symptoms.21,22 These results from our serum-based humanized mouse model suggest that the pathophysiological characteristics of preeclampsia are more complex than previously thought and are likely to involve interactions and dysregulation of multiple factors. By using serum proteomic screening by surface-enhanced laser-desorption ionization-time-of-flight (SELDI-TOF), our results suggest that PES contains a reduced abundance of transthyretin, a plasma transport protein for the thyroid hormone, thyroxine, and retinol-binding protein.23 More important, transthyretin has been widely studied for its role in amyloid diseases associated with protein misfolding and aggregation, resulting in deposits of toxic, fibrillar aggregates in specific organs.24–26 Dysregulated or reduced transthyretin has also been implicated in Alzheimer disease, and overexpression of a wild-type human transthyretin transgene has been shown to ameliorate the disease in the transgenic murine model of human Alzheimer disease.27,28 Transthyretin in its native form assumes a homotetrameric quaternary configuration (approximately 14 kDa per monomer). Post-translational modifications of the monomer result in detection of several isoforms.29 Circulating transthyretin is also a validated marker of malnutrition and has a putative role in oocyte maturation and inflammation.30–32 Although the presence of transthyretin during implantation in mice and in the placenta and trophoblasts in humans has been reported,33,34 its functional role in normal pregnancy or adverse pregnancy outcomes has not been recognized. We hypothesize that transthyretin in preeclampsia is structurally and functionally dysregulated and contributes to the onset of this serious pregnancy complication. Herein, we present complementary in vitro and in vivo approaches, which show that endogenously altered transthyretin is a preeclampsia-causing agent and that native transthyretin has the ability to block the onset of preeclampsia-like features.
Materials and Methods
Human Subjects
Preeclampsia was defined by new-onset systolic and diastolic blood pressures of >140 and 90 mm Hg, respectively, and proteinuria (>300 mg of protein in a 24-hour urine collection or a random urine protein/creatinine ratio of >0.3) after 20 weeks’ gestation. Severe preeclampsia was further defined by systolic and diastolic blood pressures of >160 and 110 mm Hg, respectively. For the purposes of this study, we followed the exclusion and inclusion criteria as described elsewhere.18 Gestational age-matched healthy, normotensive, pregnant women (the normal group) were included as controls. Blood was obtained from pregnant women during 32 to 36 weeks of pregnancy with informed consent, under the approved protocols by the Institutional Review Boards of Women and Infants Hospital of Rhode Island (Providence), Linkoping University Hospital (Linkoping, Sweden), and University of Jena (Jena, Germany). Serum was separated and frozen as aliquots at −80°C until further use. Patient characteristics included for the study are provided in Supplemental Table S1.
Proteomics and Two-Dimensional Gel Electrophoresis
Two different groups of gestational age-matched serum samples were analyzed by SELDI-TOF. In the first group, normal pregnancy serum (NPS) (n = 16) or PES (n = 53) samples were analyzed by SELDI-TOF using the anionic exchange Q10 chip and analyzed with a ProteinChip Reader (series 4000; Bio-Rad, Munich, Germany). The bioinformatical analysis was performed using Ciphergen Express Client version 3.0 software. Arrays were exposed to 2200 nJ laser energy to detect proteins in the range of 2 to 20 kDa and to 3500 nJ for proteins in the range of 20 to 200 kDa at a pressure of <150 μPa. Calibration was performed externally through the use of a protein molecular weight standard kit (Bio-Rad, Waltham, MA). A similar SELDI-TOF analysis was performed using an additional second group of serum samples from normal pregnancy (n = 7) and preeclampsia (n = 8) from an independent cohort of subjects to validate the proteomics findings from the first group.
To identify the protein(s) that is altered in PES, we performed two-dimensional gel electrophoresis using both NPS and PES samples. Briefly, 50 μL of serum was precipitated in 50 μL of 20% trifluoroacetic acid and 50% acetonitrile for 30 minutes at −20°C. Protein pellets were washed twice in ice-cold 80% acetone and rehydrated for 48 hours in 125 μL of 2% immobilized pH gradient buffer (0.5% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane-sulfonate, 8 urea, and 0.002% bromophenol blue). Concentrated protein solution was applied on a 7-cm nonlinear Immobiline DryStrip pH 3-11 (GE Healthcare, Boston, MA) through incubation for 14 hours under mineral oil using a Dry Strip reswelling tray. Isoelectric focusing was performed using a Multiphor II Flatbed Electrophoresis System (Amersham, Piscataway, NJ). Subsequently, the 2% immobilized pH gradient strips were transferred into 10 mL of an equilibration solution consisting of 23% glycerol, 4.6 mol/L urea, 30 mmol/L Tris(hydroxymethyl)aminomethane (Tris), 0.03 mmol/L SDS with 65 mmol/L dithiothreitol (DTT) for 15 minutes, followed by incubation in a 10-mL equilibration solution (135 mmol/L iodoacetamide and Serva Blue G-250). The second dimension was performed using a NuPAGE 4% to 12% Bis-Tris Zomm Gel (Invitrogen, Carlsbad, CA) in a Novex Mini- Cell (Invitrogen). Spots in the appropriate molecular mass range were cut out from the gel, destained, dried, and trypsin digested. The peptide fingerprints obtained from trypsin-digested spots were analyzed using the ProFound database (The Rockefeller University, http://prowl.rockefeller.edu/prowl-cgi/profound.exe, last accessed August 20, 2011).
ELISA Data
Serum levels of human transthyretin were measured by using a transthyretin-specific enzyme-linked immunosorbent assay (ELISA) kit (ALPCO kit, Salem, NH), according to the manufacturer’s protocol. Briefly, the serum samples were diluted 1:10,000 or 1:20,000 in wash buffer and incubated on a precoated plate for 1 hour at room temperature, washed, and incubated with peroxidase-labeled antibody for 1 hour. Color was developed using TMB substrate for 10 minutes, stop solution was added, and the plate was read at 450 nm. The serum levels of mouse sFlt-1 and sEng were measured by ELISA (R&D Systems, Minneapolis, MN), as per the manufacturer’s protocol.
Immunodepletion of Transthyretin
Approximately 10 μL of protein A agarose beads was washed in coimmunoprecipitation buffer and incubated with either 5 μL anti-human transthyretin antibody (whole antiserum; Sigma-Aldrich, St. Louis, MO) or isotype IgG (Dako, Carpentaria, CA). After blocking with 3% milk and being washed, the beads were incubated with serum (1:50 dilution). The supernatants after centrifugations were used for SELDI-TOF analysis or for in vivo experiments. Immunodepletion of transthyretin was confirmed by using Western blot analysis.
Immunoprecipitation of Transthyretin
Transthyretin antibody-bound γ bind Plus Sepharose beads were incubated with 100 μL serum overnight and centrifuged, and the immunoprecipitates and supernatant were used for experiments. Western blot analysis using a reducing gel confirmed the depletion of transthyretin (TTR). For protein-protein interaction studies, individual serum samples were spiked with equal amounts of purified sEng protein before immunoprecipitation.
In Vivo Studies
All animal protocols were approved by the Lifespan Institutional Animal Care and Use Committee. IL-10−/− mice with a C57BL/6 background were housed and mated in a specific pathogen-free facility under the care of the Central Research Department of Rhode Island Hospital. All mating experiments were repeated at least three times, with at least four to seven mice per treatment. The day of vaginal plug appearance was designated gestational day (gd) 0. Animals received i.p. injections of severe preeclampsia serum (100 μL) per mouse or an equivalent volume of normal pregnancy serum as control on gd 10, as described.18 For gain-of-function studies, PES was incubated with 20 or 100 μg transthyretin protein purified from human serum (AbD Serotec, Oxford, UK) at 37°C for 15 minutes before injection on gd 10. Similarly, immunoprecipitate or supernatant obtained from serum immunodepleted using transthyretin or isotype antibody (Dako, Glostrup, Denmark), as described later, was injected on gd 10.
Assessment of Proteinuria, Blood Pressure, Fetal Weight, Renal Pathological Characteristics, and ELISA
On gd 16, the animals were transferred to metabolic cages for 24-hour urine collection for assessing proteinuria. Total urinary albumin was measured using Albumin (mouse) ELISA kit (ALPCO Diagnostics, Germany). To normalize the albumin, urinary creatinine was measured using Metra Creatinine Kit (Quidel Corporation, San Diego, CA), according to the manufacturer’s protocol. Proteinuria was represented as the ratio of urinary albumin/creatinine and was expressed as μg/mg. On gd17, blood pressure was recoding by the tail-cuff method, as described earlier.14,18 The animals were then euthanized, and the uteroplacental units were surgically removed, the placenta was separated from the fetus and imaged, and fetal weights were recoded. Kidney tissue was harvested from gd17 mice, fixed in 10% buffered formalin, and stained with H&E and PAS for histopathological examination, as previously described.14,18 Morphological changes were recorded using SPOT Advanced software (Diagnostic Instruments Inc., Sterling Heights, MI) at ×100 magnification (Nikon Eclipse 80i microscope, Tokyo, Japan).
In Vitro Three-Dimensional Tube Formation Assay
A three-dimensional tube formation assay has been used to mimic in vitro the endovascular interaction between trophoblasts and endothelial cells. Briefly, the first-trimester extravillous trophoblast cell line, HTR8,35 and 2.5 × 104 human umbilical cord endothelial cells were co-cultured on Matrigel in the presence of NPS or PES, as described.18,36 For gain- or loss-of-function studies, PES used in these experiments was either incubated with a different concentration of transthyretin (AbD Serotec) or immunodepleted using transthyretin (Dako), or isotype antibody was used for endovascular activity.
Transthyretin Aggregation Studies
Phosphate-buffered serum from normal pregnancy or preeclampsia, 0.4 mg/mL human transthyretin, or 0.4 mg/mL bovine serum albumin (BSA) were diluted 1:1 with a buffer (100 mmol/L KCl and 1 mmol/L EDTA) to achieve the desired pH (sodium citrate for pH <3.2, sodium acetate for pH 3.5 to 5.3, and sodium phosphate buffer for pH >5.3).37 The solutions were incubated at 37°C in Eppendorf tubes. At measurement, the tubes were vortex mixed for 5 seconds, and turbidity was measured at 330 nm in triplicate using a nanodrop. The percentage of aggregation was calculated, with the 100% value corresponding to the maximum signal of native TTR protein or PES. All measurements were made in triplicate in at least two independent experiments.
Western Blot Analysis
Serum samples or immunoprecipitates were separated on 12% native PAGE (aggregated TTR) or precast 4% to 15% SDS-PAGE gel (TTR monomers) under reducing conditions and electroblotted on a polyvinylidene difluoride membrane. The membranes were subsequently blocked with 5% milk in PBS with Tween 20 for 1 hour at room temperature. Blots were then probed with rabbit anti-human TTR antibody (1:500; Dako, Glostrup, Denmark) in 1% PBS with Tween 20 for 1 hour at room temperature. The bands were visualized using horseradish peroxidase–conjugated secondary antibodies (α Diagnostics, San Antonio, TX), followed by enhanced chemiluminescence (Pierce, Rockford, IL). The protein bands were recorded using a Konica SRX 101A developer (Tokyo, Japan). For protein-protein interaction studies, transthyretin immunoprecipitates from NPS or PES were probed under reducing conditions with biotinylated goat anti-human sEng or biotinylated mouse anti-human retinol binding protein (RBP)-4 (1:2000).
Far Western Analysis
Recombinant retinol binding protein-4, 3.75 μg (Fitzgerald Industries, Acton, MA), 0.75 μg endoglin (R&D Systems), 1.5 μg sFlt-1 (Fitzgerald Industries), and 6 μg vitronectin (Promega, Madison, WI) were separated on a 4% to 15% precast SDS gel (Bio-Rad) and electroblotted on a polyvinylidene difluoride membrane. The proteins were denatured and renatured on the membrane using a modified AC buffer [100 mmol/L NaCl, 20 mmol/L Tris (pH 7.6), 0.5 mmol/L EDTA, 10% glycerol, 0.1% Tween-20, 2% BSA, and 1 mmol/L DTT] by gradually reducing the guanidine-HCl concentration, as per the protocol described.38 The membranes were subsequently blocked with 5% BSA in PBS with Tween 20 for 1 hour at room temperature and incubated with 10 μg TTR protein (AbD Serotec) in a modified protein binding buffer [100 mmol/L NaCl, 20 mmol/L Tris (pH 7.6), 0.5 mmol/L EDTA, 10% glycerol, 0.1% Tween-20, 2% BSA, and 1 mmol/L DTT] overnight at 4°C. Blots were then probed with 1:500 TTR antibody (Dako) in 1% PBS with Tween 20 containing BSA for 1 hour at room temperature. The bands were visualized using horseradish peroxidase–conjugated secondary antibodies (α Diagnostics), followed by enhanced chemiluminescence (Pierce). The protein bands were imaged (Gel Document System, GDS 8000; Bio-Rad Laboratories, Hercules, CA).
IHC and Thioflavin S Staining
Antigen retrieval of deparaffinized term human placental sections was performed using a citric acid–based unmasking solution (Vector, Burlingame, CA) and microwave oven technique. Sections from normal and preeclampsia specimens were blocked for 1 hour with 20% goat serum and incubated with primary transthyretin polyclonal rabbit antibody (1:400; Dako) in a humidified chamber for 1 hour in room air, followed by biotinylated secondary anti-rabbit antibody (VectaStain Elite Kit, Burlingame, CA) for 45 minutes. Immunolabeling was performed by a standard avidin-biotin technique, as per the manufacturer’s protocol. Labeling was developed with 0.05% 3,3′-diaminobenzidine (Sigma), and slides were counterstained with hematoxylin (Fisher Scientific, Kalamazoo, MI). Human transthyretin in the mouse placenta was probed using transthyretin polyclonal rabbit antibody (1:200, overnight at 4°C; Dako) and detected using Cy-3–conjugated anti-rabbit IgG (1:100; Invitrogen). Sections were counterstained with DAPI. Thioflavin S staining was performed as described.39 Briefly, deparaffinized sections were sequentially treated with 0.25% potassium permanganate solution for 5 minutes, bleaching solution (1% potassium metabisulfite and 1% oxalic acid) for 5 minutes, rinsed with water, and stained with 0.02% thioflavin S (Sigma) for 3 to 5 minutes. Finally, the sections were rinsed twice with 80% alcohol and water, dehydrated, and mounted with coverslips.
TUNEL Staining
TUNEL staining for apoptotic nuclei was performed using the ApopTag Peroxidase in Situ Apoptosis Detection Kit (Millipore, Billerica, MA), according to the manufacturer’s instructions. Labeling reactions were performed for 60 minutes at 37°C in a humidified chamber. Color development was accomplished using 3,3′-diaminobenzidine for 5 minutes. Sections were counterstained with methyl green.
Statistical Analysis
SELDI-TOF raw data were analyzed using the software Ciphergen express client version 3.0 in two separate parts, in accordance with laser energy. First, peaks were automatically detected and manually adjusted, followed by linear normalization to compensate for differences in total protein concentration. Spectra showing normalization factors <0.5 or >3, which shows low quality of the spectra, were excluded from analysis. Subsequently, P values were calculated by using a two-sided t-test (n = 2 groups), a paired t-test (n = 2 paired groups), or single-factor/one-way analysis of variance (n = ≥3 groups) for normally distributed data, and a U-test (n = 2 groups), a Wilcoxon t-test (n = 2 paired groups), or a Kruskal-Wallis H test (n = ≥3 groups) for not normally distributed data. For normally distributed animal and ELISA data, two-tailed Student’s t-tests were used in our analysis. P < 0.05 was considered significant.
Results
Proteomic Analysis Reveals Reduced Levels of Transthyretin in Preeclampsia Serum
We recently showed that PES, but not NPS, induces a full spectrum of preeclampsia-like features in pregnant IL-10−/− mice.18 To identify the potential causative factor(s) in PES, we undertook a comparative proteomic analysis of PES and NPS. PES and control gestational age-matched NPS were analyzed by SELDI-TOF (2 to 200 kDa). A protein of approximately 14 kDa molecular mass was found to be consistently present at reduced levels in PES (Figure 1A). Because the number of NPS samples in initial analysis was comparatively fewer than PES, this observation was confirmed using an independent cohort of 15 serum samples (Supplemental Table S2), where all four isoforms of transthyretin were recorded at reduced intensities in PES. Two-dimensional gel electrophoresis and peptide mass fragmentation of the trypsin-digested spots identified an approximately 14 kDa protein cluster that was determined to be transthyretin by comparison with the ExPASy database (Figure 1B) (P02766; Swiss Institute of Bioinformatics, http://www.expasy.org, last accessed August 20, 2011). The identity of the protein was further confirmed by the loss of transthyretin signal after its immunodepletion from NPS (Figure 1C) and by direct comparison of the molecular mass with commercial human transthyretin (Figure 1D). Each peak in the group was assigned to be either native protein or cysteinylated, cysteinglycinylated, and glutathionylated isoforms (Supplemental Figure S1). The identities of these isoforms were based on their molecular mass, as described in the literature.40 As evident from the data, the relative abundance of native transthyretin and all of its isoforms was reduced in PES compared with NPS (Supplemental Figure S1). In contrast, the level of albumin was unchanged between PES and NPS samples.
Figure 1.
Surface enhanced laser desorption ionization-time-of-flight (SELDI-TOF) and biochemical analyses of transthyretin in preeclampsia serum. A: Preeclampsia serum (PES) (n = 53) and normal pregnancy serum (NPS) (n = 16) were analyzed by SELDI-TOF in the molecular mass range of 2 to 200 kDa. In the entire molecular weight (MW) range, the time of flight scatterplot shows most significant changes (reduction) in the median intensities of a 14-kDa protein group in PES when compared with NPS samples. B: Isoelectric point (pI) and molecular weight of a protein spot (approximately 14 kDa) separated by two-dimensional gel electrophoresis from a representative NPS sample are shown. The mass fragmentation pattern of the corresponding trypsin-digested spot and molecular mass (m/z) of the peptide fragments are also shown. The sequence coverage obtained by comparison with the ExPASy database (P02766; Swiss Institute of Bioinformatics, http://www.expasy.org, last accessed August 20, 2011) identified the protein spot (approximately 14 kDa) to be transthyretin. C: Normalized Protein Chip array profiles are shown of a representative NPS sample immunodepleted with a transthyretin-neutralizing antibody or an isotype-matched antibody. Successful immunodepletion of transthyretin (pink highlighted area) confirmed that the protein with a molecular mass of approximately 14 kDa was transthyretin. D: Comparison of transthyretin protein peaks (molecular mass of approximately 14,000 Da) in NPS, PES, and commercial human transthyretin (cTTR) is shown.
Evidence for the Presence of Transthyretin Protein Aggregates in Preeclampsia
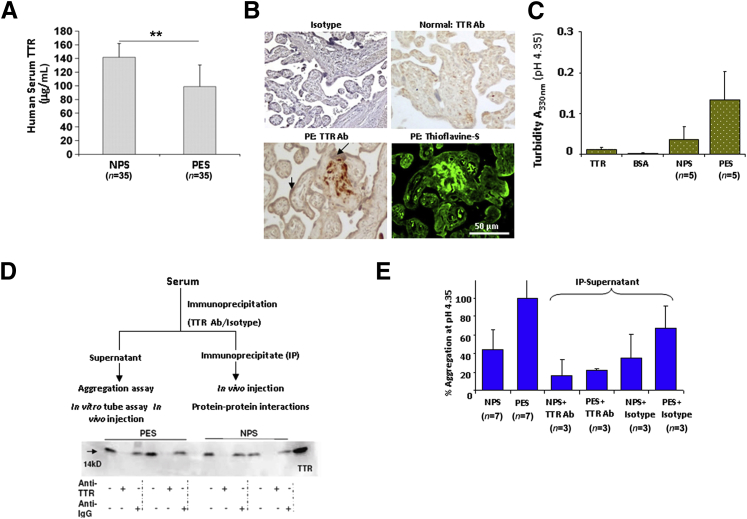
We next examined whether the SELDI-TOF data could be confirmed by ELISA that recognizes all forms of transthyretin. As shown in Figure 2A, the SELDI-TOF data were confirmed by ELISA and a significant reduction (P < 0.001) was observed in transthyretin levels between NPS (n = 35) and PES (n = 35). However, our initial Western blot analysis under reducing conditions provided contrasting data in that when an equal amount of total serum protein was probed for transthyretin between NPS and PES, it resulted in a similar band intensity profile. Because the protein chips used in SELDI-TOF are derivatized to interact and sequester proteins according to their surface interaction potential,41 it is possible that structurally altered transthyretin in PES showed reduced binding efficiency. Similarly, antibodies used in the ELISA kit may show poor affinity for altered transthyretin. In amyloid diseases, aggregation of transthyretin leads to toxic tissue deposits.42–45 This prompted us to probe possible aggregation properties of transthyretin in preeclampsia. We first examined human placental tissue from normal pregnancy and preeclampsia deliveries for transthyretin immunostaining and deposits. When probed with a transthyretin-specific antibody, preeclampsia placental tissue, in general, showed intense transthyretin staining that was particularly apparent in the extravillous trophoblast domain regions, as assessed by perinatal pathologists (Figure 2B and Supplemental Figure S2A). Furthermore, staining with thioflavin S, a specific fluorescent dye that detects extracellular deposits,39 displayed an intense signal that colocalizes with transthyretin-immunoreactive regions from an adjacent placental section from preeclampsia, suggestive of the amyloidal nature of transthyretin deposits (Figure 2B). Thioflavin S staining from additional placental tissue sections is shown in Supplemental Figure S2B. These findings support our contention that aggregated transthyretin forms extracellular amyloid deposits in the placenta, which may cause toxic effects and disrupt placental function.
Figure 2.
Transthyretin in preeclampsia is dysregulated and forms aggregates. A: Analysis of serum transthyretin in normal pregnancy serum (NPS) and preeclampsia serum (PES) by ELISA. Transthyretin is present at significantly reduced levels in PES (P < 0.001). B: Immunohistochemical (IHC) analysis using transthyretin-specific antibody (Ab) shows strong transthyretin staining in the extravillous domain of human placental section from preeclampsia, not normal pregnancy, in addition to staining in the trophoblast layer (arrows) lining the placental villi. Adjacent section of the same preeclampsia placenta shows intense fluorescence for amyloid-specific thioflavin S staining that overlaps with extracellular transthyretin-positive deposits. Additional placental tissue sections are shown in Supplemental Figure S2. C: Comparative analysis of aggregation of purified transthyretin, NPS, PES, and albumin indicated by turbidity measurements (330 nm) is shown as average values of multiple serum samples analyzed. All of the serum samples used were normalized to 0.4 mg/mL transthyretin, as determined by ELISA. PES showed a higher propensity to aggregate, as reflected by higher turbidity. On an average, eight different samples of NPS and PES were analyzed. D: Experimental flow chart for transthyretin IP and depletion is presented. SDS-PAGE immunoblotting shows the presence or absence of transthyretin monomer in the immunoprecipitate or supernatant of PES or NPS, respectively. E: Turbidity (330 nm) of supernatants obtained from transthyretin-depleted NPS or PES samples was measured and expressed as percentage aggregation. Antibody-mediated transthyretin depletion abolished aggregation associated with PES. ∗∗P < 0.01.
Sera from Preeclampsia Patients Exhibit Increased Propensity for Transthyretin-Specific Protein Aggregation
In solution, transthyretin and its mutant variants are known to undergo pH-dependent monomerization and aggregation.37,42,43 Transthyretin aggregation in NPS and PES was monitored by measuring solution turbidity at 330 nm, a measurement previously considered valid for quantification of transthyretin misfolding and aggregation.37,42 Consistent with previous reports,42 purified (native) transthyretin exhibited weak aggregated characteristics (turbidity) between pH 3.5 and 4.5, with peak aggregation at 4.35, whereas albumin exhibited no turbidity over this pH range (Supplemental Figure S2C). Under identical conditions, we then assessed NPS and PES for intrinsic and transthyretin-mediated aggregation. As observed in Figure 2C, PES exhibited higher aggregation propensity than NPS. To assess transthyretin-mediated aggregation propensity of PES, we performed experiments by immunodepleting transthyretin, as shown in the scheme (Figure 2D). The depletion of transthyretin in serum samples was confirmed by immunoblotting for the protein. Transthyretin antibody, not isotype control IgG, depleted the protein (Figure 2D). In protein aggregation studies at pH 4.35 (Figure 2E), depletion of transthyretin in PES significantly abolished aggregation, confirming its specific contribution in this process. Isotype control antibody had a minimal effect on the aggregation profile of NPS or PES.
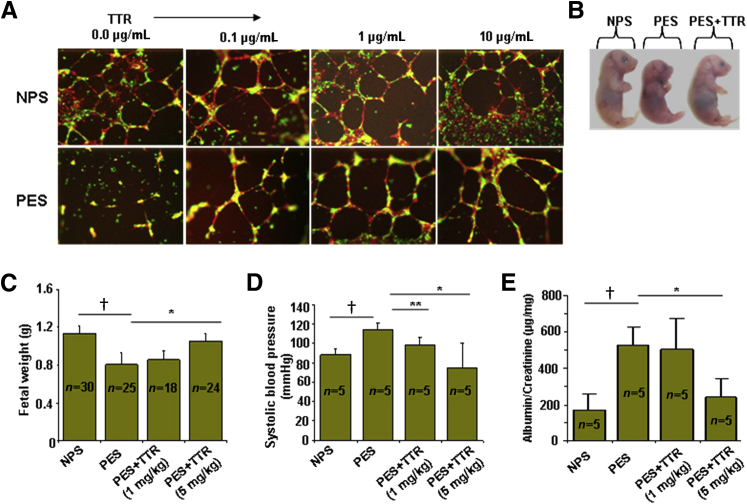
Exogenous Transthyretin Rescues PES-Induced Disruption of in Vitro Three-Dimensional Tube Formation and in Vivo Disease Features
We have previously shown that PES disrupts endovascular cross talk between endothelial cells and trophoblasts,18,36 a process required for spiral artery remodeling. We next evaluated the effect of native transthyretin on PES-mediated disruption of three-dimensional tube formation between endothelial cells and trophoblasts. Replenishment of PES with exogenous transthyretin restored capillary tube formation in a dose-dependent manner, whereas PES disrupted this interaction (Figure 3A). We next examined whether exogenous human transthyretin could inhibit the PES-induced onset of preeclampsia pathological characteristics in IL-10−/− mice. Co-administration of a single dose of 5 mg/kg transthyretin and PES on gd10 prevented the PES-induced IUGR (Figure 3, B and C), reversed PES-induced hypertension (Figure 3D), and restored proteinuria (Figure 3E) to normal values. These observations clearly suggest that exogenous (normal) transthyretin has the potential to counter the effects of altered transthyretin present in PES.
Figure 3.
Exogenous transthyretin inhibits preeclampsia-associated features in vitro and in vivo. A: Serum-induced and transthyretin-modified endovascular interaction between endothelial cells (human umbilical cord endothelial cells, cell tracker red) and first-trimester trophoblasts (HTR8, cell tracker green) was analyzed by three-dimensional tube formation on Matrigel.18 Native transthyretin rescued tube formation disrupted by preeclampsia serum (PES). B: Reversal of PES-induced intrauterine growth restriction (IUGR) by exogenous transthyretin in IL-10−/− mice. A representative image of gd 17 fetus is shown. A total of five animals were used in each condition. C: The average fetal weight from IL-10−/− mice receiving different treatments was evaluated. D: Systolic blood pressure in pregnant mice on gd 17 was evaluated in response to different treatments. E: Proteinuria in pregnant mice was assessed on gd 17 in response to various treatments. The results are expressed as a ratio of albumin/creatinine excretion (μg/mg). All values represent means ± SD of at least 5 to 30 animals per group, depending on the experiment. ∗P < 0.01, ∗∗P < 0.05 between PES and transthyretin treatment groups; †P < 0.05 between NPS and PES groups.
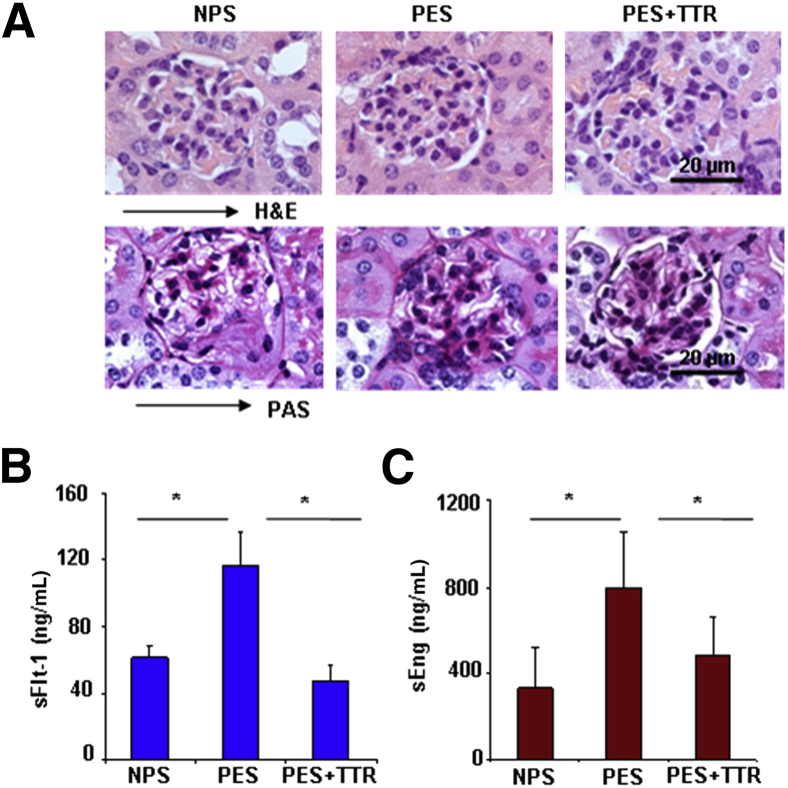
We have demonstrated that PES causes renal pathological characteristics and induces production of anti-angiogenic factors, sFlt-1 and sEng.18 Next, we evaluated whether exogenous transthyretin inhibited production of sFlt-1 and sEng and protected against glomerular endotheliosis in response to PES. As shown in Figure 4A, human transthyretin prevented the PES-induced glomerular endotheliosis in pregnant IL-10−/− mice, as suggested by H&E and PAS staining. We assessed mouse serum levels of sFlt-1 and sEng in response to treatment with NPS, PES, or PES spiked with transthyretin. As shown in Figure 4, B and C, exogenous human transthyretin significantly inhibited the PES-induced increase of circulating sFlt-1 and sEng to levels comparable to the NPS-treated group, suggesting that dysregulated transthyretin affects overall preeclampsia pathological characteristics and that native protein can blunt all these contributing factors.
Figure 4.
Exogenous transthyretin rescues glomerular integrity and inhibits production of sFlt-1 and sEng in preeclamptic IL-10−/− mice. A: Histopathological analysis of H&E- or periodic acid schiff (PAS)-stained renal tissues from NPS−, PES−, or PES+ transthyretin-treated (5 mg/kg) pregnant IL-10−/− mice are shown. Original magnification, ×100. The H&E stain shows the normalization of PES-induced capillary occlusion, enlarged glomeruli, and swollen endothelial cells by transthyretin treatment that is comparable to NPS-treated control mice. PAS-based staining indicates that transthyretin treatment reverses PES-induced inflammation of capillary endothelial cells (endotheliosis). These pathological changes are absent in the NPS-treated mice. A representative image from staining of at least three animals per group is shown. B: Serum levels of mouse sFlt-1 from pregnant IL-10−/− mice obtained on gd 17 from different treatment groups are shown. Treatment with 5 mg/kg transthyretin reverses the PES-induced excess production of sFlt-1 in IL-10−/− mice. C: Serum levels of mouse sEng from pregnant IL-10−/− mice obtained on gd 17 from different treatment groups are shown. Treatment with transthyretin reverses the preeclampsia serum (PES)-induced excess production of sEng to levels comparable to normal pregnancy serum (NPS) treatment control group. All values are expressed as means ± SD obtained from at least five animals per treatment group. ∗P < 0.05 represents the significance between different treatment groups.
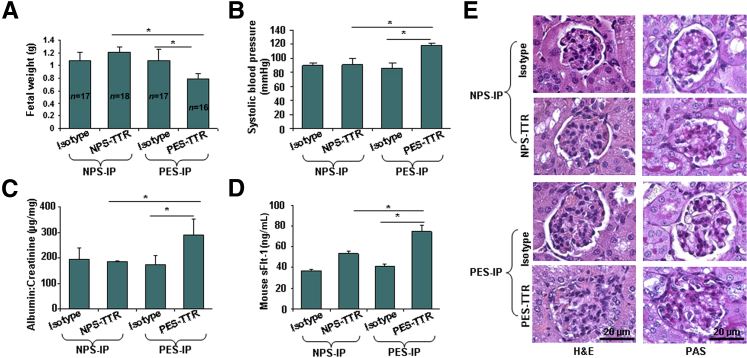
Evidence that Transthyretin Immunoprecipitated from PES Induces Disease Features in Pregnant IL-10−/− Mice
To obtain direct evidence that dysregulated transthyretin from PES contributes to preeclampsia-like features in IL-10−/− mice, we evaluated the effect of transthyretin immunoprecipitated from NPS (NPS-transthyretin) or PES (PES-transthyretin), as shown in the flow chart (Figure 2D). The presence of approximately equal amounts of transthyretin in immunoprecipitates was confirmed by using Western blot analysis (Figure 2D). The administration of PES-transthyretin on gd 10 resulted in IUGR, as indicated by reduced fetal weight (Figure 5A), elevated hypertension (Figure 5B) and proteinuria (Figure 5C), and induced production of sFlt-1 (Figure 5D). Furthermore, as shown in Figure 5E, administration of PES-transthyretin resulted in signature features of glomerular endotheliosis, including inflammation of endothelium and swelling, as indicated by H&E and PAS staining. In contrast, NPS-transthyretin did not cause significant changes in all of the parameters monitored. These findings provide direct evidence that dysregulated transthyretin contributes to preeclampsia-like features in pregnant IL-10−/− mice.
Figure 5.
Immunoprecipitated (IP) transthyretin from preeclampsia serum (PES) induces disease features in IL-10−/− mice. Pregnant mice were injected (i.p.) on gd 10 with transthyretin immunoprecipitate obtained from 100 μL normal pregnancy serum (NPS) or PES each, as described in Materials and Methods. A: The average weight of fetal units derived from pregnant mice on gd 17 receiving different treatments is shown. B: The systolic blood pressure of pregnant mice on gd 17 was evaluated in response to different treatments. C: Proteinuria (albumin/creatinine ratio, in μg/mg) is shown in response to various treatments. PES-transthyretin caused significant proteinuria compared with NPS-transthyretin. Isotype-matched IgG-mediated immunoprecipitation from PES resulted in values similar to NPS, confirming the validity of transthyretin-specific antibody. D: Transthyretin immunoprecipitate from PES induced excess production of mouse sFlt-1 in pregnant IL-10−/− mice. E: Histological analysis using H&E or periodic acid schiff (PAS) staining of renal tissue from pregnant IL-10−/− mice in response to transthyretin immunoprecipitates from NPS and PES is shown. A representative image from staining of at least three animals per group is presented. All values represent means ± SD of at least 3 to 18 animals per group. ∗P < 0.05 between treatment groups indicated.
Does PES transthyretin form toxic deposits in the mouse placenta? We evaluated the placenta from NPS− and PES-transthyretin–treated IL-10−/− mice for human transthyretin. As shown in Supplemental Figure S3, immunoprecipitated transthyretin from both NPS and PES reached the placenta (Supplemental Figure S3, C and D), whereas saline control (Supplemental Figure S3A) or isotype-matched IgG-mediated immunoprecipitates from PES showed no evidence of human transthyretin (Supplemental Figure S3B). More important, transthyretin antibody-mediated immunoprecipitates from PES formed heavy deposits (Supplemental Figure S3D) and caused apoptosis (Supplemental Figure S3F) in the placental region, as assessed by TUNEL staining. In contrast, although NPS-transthyretin reached the placenta (Supplemental Figure S3C), it did not cause cell death (Supplemental Figure S3E). These results suggest that PES-transthyretin elicits toxic effects in the mouse placenta.
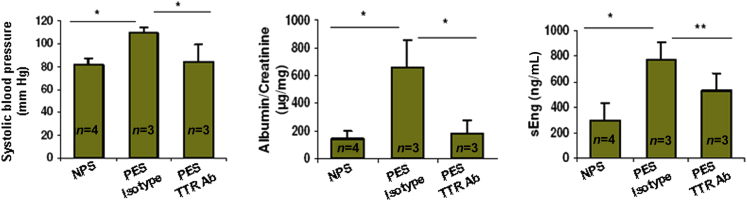
We also screened flow through from transthyretin-immunodepleted serum samples for their ability to cause preeclampsia-like features in IL-10−/− mice. The basic premise was that depletion of dysregulated transthyretin from PES should result in normal pregnancy, with no increased blood pressure, new-onset proteinuria, and production of factors, such as sEng. As shown in Figure 6, transthyretin-depleted PES failed to induce blood pressure increase, proteinuria, and production of sEng. PES treated with isotype-matched IgG still maintained pathological characteristics compared with NPS.
Figure 6.
Transthyretin-immunodepleted preeclampsia serum (PES) fails to induce preeclampsia-like features. Transthyretin-depleted PES (100 μL) was injected on gd10 in IL-10−/− mice, and its effects on fetal size, blood pressure, proteinuria, and serum levels of sEng were compared with injection of an equal volume of normal pregnancy serum (NPS) or isotype-matched IgG-depleted PES. Immunodepletion of transthyretin in PES fails to cause significant changes in blood pressure, proteinuria, or sEng levels, suggesting that dysregulated transthyretin in PES is responsible for induction of preeclampsia pathological characteristics in IL-10−/− mice. All values represent means ± SD of at least three to four animals per group. ∗P < 0.05 between treatment groups tested.
Transthyretin Functions as a Binding Partner for Anti-Angiogenic Protein sEng
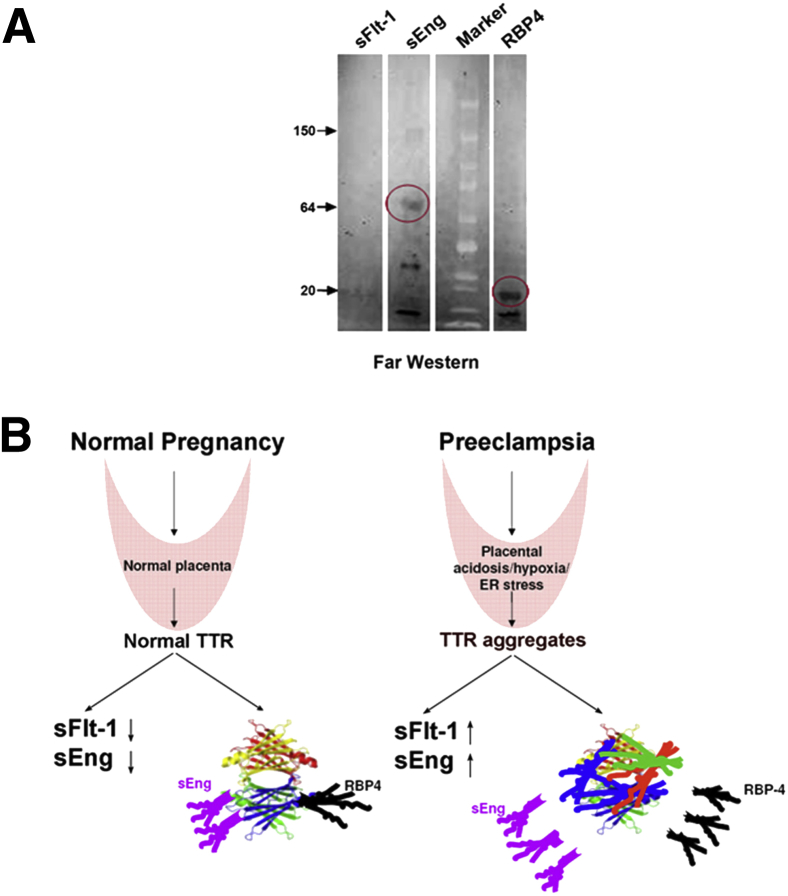
Because transthyretin functions as a binding partner for thyroxine, RBP-4, and β-amyloid protein,23,28 we hypothesized that native transthyretin could function as a scavenger protein for preeclampsia-associated factors. Initially, we searched the SELDI-TOF mass spectrometry profile for additional proteins that disappeared with transthyretin depletion in NPS. Along with several proteins, the analysis suggested that a protein of approximately 66.5 kDa could be a part of the complex (Supplemental Figure S4 and Supplemental Table S3). Because sEng is 67 kDa molecular weight and its levels in preeclampsia are altered,7 we pursued the hypothesis that transthyretin interacts with this protein. We confirmed this interaction using the Far Western technique, which depends on binding of a prey protein to bait proteins.38 Native transthyretin was used as the bait protein and purified sFlt-1, sEng, and RBP-4 as prey proteins. RBP-4 was included as a positive control. As shown in Figure 7A, transthyretin binds to sEng and RBP4. We did not observe significant binding of transthyretin to sFlt-1 under these experimental conditions.
Figure 7.
Transthyretin binds to soluble endoglin and its possible mode of action. A: Far Western blot showing the binding of transthyretin with sEng and RBP-4 (red circle) and lack of binding to sFlt-1. Purified sFlt-1, sEng, and RBP-4 were used as prey proteins, and purified human transthyretin was used as probing bait protein. A representative immunoblot from multiple independent experiments is shown. B: Proposed model showing the role of upstream factors, such as hypoxia, acidosis, and stress, in triggering aggregation of transthyretin that induces production of anti-angiogenic factors and impairs the binding and scavenging of preeclampsia-causing soluble factors.
Discussion
In this study, we report novel findings associated with dysregulated transthyretin in preeclampsia. By using PES or transthyretin immunoprecipitated from PES, we demonstrate that administration of aberrant transthyretin to pregnant IL-10−/− mice induces a full spectrum of preeclampsia-like features. The relevance of using IL-10−/− mice lies in the fact that IL-10 is a potent anti-inflammatory and vascular cytokine.20,46,47 IL-10 is a potent inducer of heme oxygenase-1, which has been shown to be critical for placentation and fetal development.48,49 IL-10 and heme oxygenase-1 exert protective effects against oxidative stress and induce negative regulation of sFlt-1 and sEng.50–52 Thus, the absence of IL-10 may predispose to inflammatory activities and poor vascularization at the maternal-fetal interface. In this regard, it is tempting to speculate that aggregated and toxic transthyretin may cause preeclampsia-like pathological characteristics in IL-10−/− mice, even at low doses.
The extraordinary observation of aberrant transthyretin in preeclampsia is further supported by immunodepletion of transthyretin from PES, which ameliorates most of the disease features. Measurement of serum transthyretin from normal pregnancy and preeclampsia by SELDI-TOF suggested that this protein was significantly reduced in PES compared with NPS. On the other hand, we observed transthyretin deposits in preeclampsia placental tissue and in the mouse placenta from animals administered with human transthyretin from PES. This prompted us to focus on the aggregation properties of endogenous transthyretin, a feature widely associated with unscheduled misfolding and aggregation in amyloid diseases and possibly in Alzheimer disease.24–26,44 By using several complementary approaches, we provide evidence for aggregation of transthyretin protein in PES and in placental tissue from preeclampsia pregnancies. NPS and PES differed in their susceptibility to pH-dependent aggregation that was abolished after immunodepletion of transthyretin. Thus, we propose that preeclampsia is a disease of protein misfolding and aggregation. A similar scenario has been proposed for human serine protease inhibitor A1,53 although it is not yet clear how this protease inhibitor is involved in preeclampsia. Serine protease inhibitor A1 has been shown to undergo fragmentation, misfolding, and aggregation in response to oxidative stress.54
Transthyretin is protective in a murine model of Alzheimer disease because of its ability to sequester β-amyloid peptides in a chaperone-like manner.27,28 Our gain-of-function studies support a similar protective role of exogenous transthyretin in preeclampsia. The rescue of maternal symptoms in mice was associated with reduction in sFlt-1 and sEng. The role of native transthyretin in inhibiting production of anti-angiogenic factors is noteworthy because these factors are thought to be key regulators of local and systemic manifestations of preeclampsia.21,22 On the other hand, immunoprecipitated transthyretin from PES induces production of these factors in pregnant IL-10−/− mice, implying a direct role of dysregulated protein in programming the events that lead to the onset of preeclampsia pathological characteristics. We provide evidence that dysregulated transthyretin reaches the mouse placenta, forms deposits, and induces placental apoptosis.
Our findings raise questions about the mechanisms and microenvironment that lead to transthyretin misfolding and aggregation in preeclampsia. Several factors, such as mutations in transthyretin, local change in pH at membranes, and oxidative stress, are likely to contribute to destabilization of transthyretin, causing tetramer dissociation, a rate-limiting step in transthyretin amyloidogenesis.25,42 However, both natural transthyretin and mutant variants form aggregates and amyloid fibrils.24,25,45 How may this happen at the maternal-fetal interface during pregnancy, even without mutations? Hypoxia is thought to be associated with the induction of endoplasmic reticulum (ER) stress and, possibly, preeclampsia pathological characteristics.55,56 The ER is a hub for proper folding and export of peptides, guided by ER-specific chaperones. ER stress can dysregulate the function of chaperones, resulting in export of misfolded proteins into circulation.56 Such a scenario is manifest in the placenta of IUGR.57 These conditions can also overlap in preeclampsia, leading to poor placentation. In this context, our published studies have shown that maternal hypoxia causes preeclampsia-like features in pregnant mice.14 Interestingly, hypoxia has been shown to control TTR expression and uptake.58 Thus, it is plausible that chronic hypoxia and ER stress destabilize transthyretin into a misfolded conformation and aggregation. More important, failure to adapt to a challenging intrauterine milieu may trigger conformational changes in proteins, causing aggregation and loss of function that lead to development of preeclampsia.
Our SELDI-TOF analysis and Far Western data on protein-protein interactions reveal novel transthyretin binding partners, including sEng (Supplemental Figure S4 and Supplemental Table S3). Because normal transthyretin binds to sEng, we propose that aggregated transthyretin fails to trap factors, such as sEng, which allows such proteins to contribute to the pathogenesis of this syndrome. Autophagy is known to dispose unwanted proteins and maintain homeostasis,59 but it has been shown to be impaired in preeclampsia.60 Its impairment is caused by endoglin.60 Interestingly, dysregulated transthyretin present in PES induces production of sFlt-1 and sEng. It is, thus, possible that free soluble endoglin inhibits autophagy, and this effect could be imparted by aggregated transthyretin. When homotetrameric transthyretin transforms into misfolded and self-aggregated structures, the binding and scavenging potential of this protein is lost while acquiring the potential to induce production of sFlt-1, sEng, and other unknown factors (Figure 7B). Thus, preeclampsia pathological characteristic–associated aggregation of transthyretin is most likely an upstream event of production of anti-angiogenic factors.
Overall, our studies provide compelling evidence for the concept that preeclampsia is, in part, a syndrome of protein misfolding and aggregation. These observations offer a new framework for understanding the complex nature of preeclampsia pathogenesis. Intriguingly, exogenous transthyretin can reverse the features of the syndrome in a humanized mouse model of preeclampsia. These studies reveal innovative frontiers for identifying potential translational targets for therapy.
Acknowledgments
We thank the members of the Sharma laboratory for critical discussion, Phil Gruppuso for insightful comments, Sheryl-Vi Rico for transthyretin measurement by ELISA, Paula Weston for histological data, Christian Melle and Nico Escher for their support in SELDI-TOF analyses, and Justine S. Fitzgerald and Andreas Brückmann for providing patient serum samples for supplemental experiments.
Footnotes
Supported by NIH grant P20RR018728 (J.P.), National Institute of Environmental Health Sciences grant P42ES013660 (S.S.), and Rhode Island Research Alliance Collaborative Research Award 2009-28 (S.S.).
Disclosures: A patent application (US patent 13/147,993) for compositions, formulations, and methods for treating preeclampsia-type disorders of pregnancy was filed in 2010.
Supplemental Data
Surface enhanced laser desorption ionization-time-of-flight (SELDI-TOF) proteomic analysis of preeclampsia serum (PES) shows reduction in different isoforms of transthyretin. Differential protein profile of transthyretin (TTR) isoforms in normal pregnancy serum (NPS) and PES. Dot plot shows consistent reduction of the intestines to native TTR, cysteinglycinylated TTR, and glutathionylated TTR in multiple PES (n = 53) compared with NPS (n = 16) samples. Unlike transthyretin and its modified isoforms, albumin levels were not significantly different between the NPS and PES group.
Transthyretin in preeclampsia is dysregulated. A: Staining by IHC shows marked staining for transthyretin in the syncytiotrophoblast layer lining in the placental villi in preeclampsia compared with normal placenta. More important, intense transthyretin staining is prominent in the extravillous domain region in the preeclampsia placental sections. For simplicity, only one representative placental tissue section from normal pregnancy is included because multiple tissue sections did not provide for extracellular-type transthyretin staining. B: Thioflavin S staining of random preeclampsia placental sections shows aggregated attainment in the extravillous domain. Original magnification, ×20 (A and B). Noncellular thioflavin S staining was detected in preeclampsia placental sections but not in gestational age-matched normal pregnancy placental sections (white arrows). C: pH dependence of aggregation of native transthyretin (black bars) and albumin (white bars) incubated for 3 days at 37°C. Turbidity data were normalized to the highest value of transthyretin aggregation at pH 4.35.
Dysregulated transthyretin from PES forms toxic aggregates and induces apoptosis in the placenta from IL-10−/− mice. Administration of immunoprecipitated PES-transthyretin results in protein deposits and apoptosis in the placenta of pregnant IL-10−/− mice. A–D: Representative immunofluorescence staining of human transthyretin in mouse placental sections from gd17 gestation in response to treatments: saline (A), isotype IgG immunoprecipitate from PES (B), transthyretin immunoprecipitate from NPS (C), and transthyretin immunoprecipitate from PES (D). The placental tissue is marked for the mesometrial (M), deciduas basalis (DB), and labyrinthine (L) regions. PES-transthyretin shows excessive tissue deposits (D). E and F: A representative image of TUNEL staining of the labyrinthine area of mouse placental sections in response to treatment with transthyretin immunoprecipitate from NPS (E) and transthyretin immunoprecipitate from PES (F). A significant TUNEL-positive signal (arrows) is seen in placental sections obtained from PES-transthyretin immunoprecipitate-treated mice. Original magnifications: ×10 (A–D); ×20 (E and F).
Transthyretin-interacting proteins, as revealed by SELDI-TOF analysis. SELDI-TOF profile shows the absence of different molecular mass proteins (6000 to 70,000 Da) after immunodepletion of NPS with transthyretin antibody (Ab) but not with isotype IgG antibody.
References
- 1.Roberts J.M., Hubel C.A. Is oxidative stress the link in the two-stage model of pre-eclampsia? Lancet. 1999;354:788–789. doi: 10.1016/S0140-6736(99)80002-6. [DOI] [PubMed] [Google Scholar]
- 2.Redman C.W., Sargent I.L. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 3.Dekker G., Sibai B. Primary, secondary and tertiary prevention of pre-eclampsia. Lancet. 2001;357:209–215. doi: 10.1016/S0140-6736(00)03599-6. [DOI] [PubMed] [Google Scholar]
- 4.Steegers E.A., Dadelszen P., Duvekot J.J., Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 5.Parikh S.M., Karumanchi S.A. Putting pressure on pre-eclampsia. Nat Med. 2008;14:810–812. doi: 10.1038/nm0808-810. [DOI] [PubMed] [Google Scholar]
- 6.Meekins J.W., Pijnenborg R., Hanssens M., McFadyen I.R., van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 7.Levine R.J., Lam C., Qian C., Yu K.F., Maynard S.E., Sachs B.P., Sibai B.M., Epstein F.H., Romero R., Thadani R., Karumanchi S.A., CPEP Study Group Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 8.Zhou C.C., Zhang Y., Irani R.A., Zhang H., Mi T., Popek E.J., Hicks M.J., Ramin S.M., Kellems R.E., Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redman C.W., Sargent I.L. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;29:S73–S77. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y., Damsky C.H., Fisher S.J. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype: one cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hupperts B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;5:970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- 12.Hung T.H., Skepper J.N., Charnock-Jones D.S., Burton G.J. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90:1274–1281. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- 13.Sharma S., Norris W.E., Kalkunte S. Beyond the threshold: an etiological bridge between hypoxia and immunity in preeclampsia. J Reprod Immunol. 2010;85:112–116. doi: 10.1016/j.jri.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai Z., Kalkunte S., Sharma S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. 2011;57:505–514. doi: 10.1161/HYPERTENSIONAHA.110.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim H.J., Wang H. Uterine disorders and pregnancy complications: insights from mouse models. J Clin Invest. 2010;120:1004–1015. doi: 10.1172/JCI41210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson L. Cancer: clinical trials unite mice and humans. Nature. 2012;483:546–548. doi: 10.1038/483546a. [DOI] [PubMed] [Google Scholar]
- 17.Gitler A.D., Lehmann R. Modeling human diseases. Science. 2012;337:269. doi: 10.1126/science.1227179. [DOI] [PubMed] [Google Scholar]
- 18.Kalkunte S., Boij S.R., Norris W., Friedman J., Lai Z., Kurtis J., Lim K.H., Padbury J.F., Matthiesen L., Sharma S. Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay. Am J Pathol. 2010;177:2387–2398. doi: 10.2353/ajpath.2010.100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennessy A., Pilmore H.L., Simmons L.A., Painter D.M. A deficiency of placental IL-10 in preeclampsia. J Immunol. 1999;163:3491–3495. [PubMed] [Google Scholar]
- 20.Kalkunte S., Nevers T., Norris W.E., Sharma S. Vascular IL-10: a protective role in preeclampsia. J Reprod Immunol. 2011;88:65–69. doi: 10.1016/j.jri.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatesha S., Toporsian M., Lam C., Hanai J., Yamamoto T., Kim Y.M., Bdolah Y., Lim K.H., Yuan H.T., Libermann T.A., Stillman I.E., Roberts D., D’Amore P.A., Epstein F.H., Sellke F.W., Romero R., Sukhatme V.P., Latarte M., Karumanchi S.A. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 22.Maynard S.E., Min J.Y., Merchan J., Lim K.H., Li J., Mondal S., Libermann T.A., Morgan J.P., Sellke F.W., Stillman I.E., Epstein F.H., Sukhatme V.P., Karumanchi S.A. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raz A., Goodman D.S. The interaction of thyroxine with human plasma prealbumin and with the prealbumin-retinol-binding protein complex. J Biol Chem. 1969;244:3230–3237. [PubMed] [Google Scholar]
- 24.Dobson C.M. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 25.Hammarström P., Wiseman R.L., Powers E.T., Kelly J.W. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science. 2003;299:713–716. doi: 10.1126/science.1079589. [DOI] [PubMed] [Google Scholar]
- 26.Goldsteins G., Persson H., Anderson K., Olofsson A., Dacklin I., Edvinsson A., Saraiva M.J., Lundgren E. Exposure of cryptic epitopes on transthyretin only in amyloid and in amyloidogenic mutants. Proc Natl Acad Sci U S A. 1999;96:3108–3113. doi: 10.1073/pnas.96.6.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buxbaum J.N., Ye Z., Reixach N., Friske L., Levy C., Das P., Golde T., Masliah E., Roberts A.R., Bartfai T. Transthyretin protects Alzheimer’s mice from the behavioral and biochemical effects of Abeta toxicity. Proc Natl Acad Sci U S A. 2008;105:2681–2686. doi: 10.1073/pnas.0712197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein T.D., Anders N.J., DeCarli C., Chan S.L., Mattson M.P., Johnson J.A. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPSW mice resulting in tau phosphorylation and loss of hippocampal neurons: support for the amyloid hypothesis. J Neurosci. 2004;24:7707–7717. doi: 10.1523/JNEUROSCI.2211-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altland K., Winter P., Saraiva M.J., Suhr O. Sulfite and base for the treatment of familial amyloidotic polyneuropathy: two additive approaches to stabilize the conformation of human amyloidogenic transthyretin. Neurogenetics. 2004;5:61–67. doi: 10.1007/s10048-003-0160-1. [DOI] [PubMed] [Google Scholar]
- 30.Henze A., Rohn S., Gericke B., Raila J., Schweigert F.J. Structural modifications of serum transthyretin in rats during protein-energy malnutrition. Rapid Commun Mass Spectrom. 2008;22:3270–3274. doi: 10.1002/rcm.3728. [DOI] [PubMed] [Google Scholar]
- 31.Schweigert F.J., Gericke B., Wolfram W., Kaisers U., Dudenhausen J.W. Peptide and protein profiles in serum and follicular fluid of women undergoing IVF. Hum Reprod. 2006;21:2960–2968. doi: 10.1093/humrep/del257. [DOI] [PubMed] [Google Scholar]
- 32.Gericke B., Raila J., Deja M., Rohn S., Donaubauer B., Nagl B., Haebel S., Schweigert F.J., Kaisers U. Alteration of transthyretin microheterogeneity in serum of multiple trauma patients. Biomark Insights. 2007;2:299–306. [PMC free article] [PubMed] [Google Scholar]
- 33.Burnum K.E., Tranguch S., Mi D., Daikoku T., Dey S.K., Caprioli R.M. Imaging mass spectrometry reveals unique protein profiles during embryo implantation. Endocrinology. 2008;149:3274–3278. doi: 10.1210/en.2008-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKinnon B., Li H., Richard K., Mortimer R. Synthesis of thyroid hormone binding proteins transthyretin and albumin by human trophoblast. J Clin Endocrinol Metab. 2005;90:6714–6720. doi: 10.1210/jc.2005-0696. [DOI] [PubMed] [Google Scholar]
- 35.Graham C.H., Connelly I., MacDougall J.R., Kerbel R.S., Stetler-Stevenson W.G., Lala P.K. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 36.Kalkunte S., Lai Z., Tewari N., Chichester C., Romero R., Padbury J., Sharma S. In vitro and in vivo evidence for lack of endovascular remodeling by third trimester trophoblasts. Placenta. 2008;29:871–878. doi: 10.1016/j.placenta.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reixach N., Foss T.R., Santelli E., Pascual J., Kelly J.W., Buxbaum J.N. Human-murine transthyretin heterotetramers are kinetically stable and non-amyloidogenic: a lesson in the generation of transgenic models of diseases involving oligomeric proteins. J Biol Chem. 2008;283:2098–2107. doi: 10.1074/jbc.M708028200. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y., Li Q., Chen X.Z. Detecting protein-protein interactions by Far western blotting. Nat Protoc. 2007;2:3278–3284. doi: 10.1038/nprot.2007.459. [DOI] [PubMed] [Google Scholar]
- 39.Guntern R., Bouras C., Hof P.R., Vallet P.G. An improved thioflavine S method for staining neurofibrillary tangles and senile plaques in Alzheimer’s disease. Experientia. 1992;48:8–10. doi: 10.1007/BF01923594. [DOI] [PubMed] [Google Scholar]
- 40.Schweigert F.J., Wirth K., Raila J. Characterization of the microheterogeneity of transthyretin in plasma and urine using SELDI-TOF-MS immunoassay. Proteome Sci. 2004;2:5. doi: 10.1186/1477-5956-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vorderwülbecke S., Cleverley S., Weinberger S.R., Wiesner A. Protein quantification by the SELDI-TOF-MS–based ProteinChip System. Nat Methods. 2005;2:393–395. [Google Scholar]
- 42.Kelly J.W. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr Opin Struct Biol. 1998;8:101–106. doi: 10.1016/s0959-440x(98)80016-x. [DOI] [PubMed] [Google Scholar]
- 43.Lai Z., Colón W., Kelly J.W. The acid-mediated denaturation pathway of transthyretin yields a conformational intermediate that can self-assemble into amyloid. Biochemistry. 1996;35:6470–6482. doi: 10.1021/bi952501g. [DOI] [PubMed] [Google Scholar]
- 44.Koo E.H., Lansbury P.T., Jr., Kelly J.W. Amyloid diseases: abnormal protein aggregation in neurodegeneration. Proc Natl Acad Sci U S A. 1999;96:9989–9990. doi: 10.1073/pnas.96.18.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karlsson A., Olofsson A., Eneqvist T., Sauer-Eriksson A.E. Cys114-linked dimers of transthyretin are compatible with amyloid formation. Biochemistry. 2005;44:13063–13070. doi: 10.1021/bi050795s. [DOI] [PubMed] [Google Scholar]
- 46.Thaxton J.E., Sharma S. Interleukin-10: a multifaceted agent of pregnancy. Am J Reprod Immunol. 2010;63:482–491. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Didion S.P., Kinzenbaw D.A., Schrader L.I., Chu Y., Faraci F.M. Endogenous IL-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension. 2009;54:619–624. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee T.S., Chau L.Y. Heme oxygenase-1 mediates anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 49.Zenclussen A.C., Lim E., Knoeller S., Knackstedt M., Hertwig K., Hagen E., Klapp B.F., Arck P.C. Heme oxygenases in pregnancy II: HO-2 is downregulated in human pathologic pregnancies. Am J Reprod Immunol. 2003;50:66–76. doi: 10.1034/j.1600-0897.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 50.Huet O., Laemmel E., Fu Y., Dupic L., Aprico A., Andrews K.L., Moore S.L., Harrois A., Meikle P.L., Vicaut E., Chin-Dusting J.P., Duranteau J. Interleukin 10 antioxidant effect decreases leukocytes/endothelial interaction induced by tumor necrosis factor α. Shock. 2013;39:83–88. doi: 10.1097/SHK.0b013e318278ae36. [DOI] [PubMed] [Google Scholar]
- 51.Soares M.P., Bach F.H. Heme oxygenase 1: from biology to therapeutic potential. Trends Immunol. 2009;15:50–58. doi: 10.1016/j.molmed.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Cudmore M., Ahmed S., Al-Ani B., Fujisawa T., Coxall H., Chudasama K., Devey L.R., Wigmore S.J., Abbas A., Hewett P.W., Ahmed A. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 53.Buhimschi I.A., Zhao Z., Funai E.F., Harris N., Bernstein I.M., Saade G.R., Buhimschi C.S. Proteomic profiling of urine identifies specific fragments of SERPINA1 and albumin as biomarkers of preeclampsia. Am J Obstet Gynecol. 2008;199:551.e1–551.e16. doi: 10.1016/j.ajog.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matheson N.R., Wong P.S., Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979;88:402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- 55.Feldman D.E., Chauhan V., Koong A.C. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol Cancer Res. 2005;3:597–605. doi: 10.1158/1541-7786.MCR-05-0221. [DOI] [PubMed] [Google Scholar]
- 56.Redman C.W. The endoplasmic reticulum stress of placental impoverishment. Am J Pathol. 2008;173:311–314. doi: 10.2353/ajpath.2008.080412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yung H.W., Calabrese S., Hynx D., Hemmings B.A., Cetin I., Charnock-Jones D.S., Burton G.J. Evidence of placental translation, inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol. 2008;173:451–462. doi: 10.2353/ajpath.2008.071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel J., Landers K., Li H., Mortimer R.H., Richard K. Oxygen concentration regulates expression and uptake of transthyretin, a thyroxine binding protein, in JEG-3 choriocarcinoma cells. Placenta. 2011;32:128–133. doi: 10.1016/j.placenta.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 59.Klionsky D.J. Good riddance to bad rubbish. Nature. 2006;441:819–820. doi: 10.1038/441819a. [DOI] [PubMed] [Google Scholar]
- 60.Nakashima A., Yamanaka-Tatemastu M., Fujita N., Koizumi K., Shima T., Yoshida T., Nikaido T., Okamoto A., Yoshimori T., Siato S. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation and preeclampsia. Autophagy. 2013;9:1–14. doi: 10.4161/auto.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Surface enhanced laser desorption ionization-time-of-flight (SELDI-TOF) proteomic analysis of preeclampsia serum (PES) shows reduction in different isoforms of transthyretin. Differential protein profile of transthyretin (TTR) isoforms in normal pregnancy serum (NPS) and PES. Dot plot shows consistent reduction of the intestines to native TTR, cysteinglycinylated TTR, and glutathionylated TTR in multiple PES (n = 53) compared with NPS (n = 16) samples. Unlike transthyretin and its modified isoforms, albumin levels were not significantly different between the NPS and PES group.
Transthyretin in preeclampsia is dysregulated. A: Staining by IHC shows marked staining for transthyretin in the syncytiotrophoblast layer lining in the placental villi in preeclampsia compared with normal placenta. More important, intense transthyretin staining is prominent in the extravillous domain region in the preeclampsia placental sections. For simplicity, only one representative placental tissue section from normal pregnancy is included because multiple tissue sections did not provide for extracellular-type transthyretin staining. B: Thioflavin S staining of random preeclampsia placental sections shows aggregated attainment in the extravillous domain. Original magnification, ×20 (A and B). Noncellular thioflavin S staining was detected in preeclampsia placental sections but not in gestational age-matched normal pregnancy placental sections (white arrows). C: pH dependence of aggregation of native transthyretin (black bars) and albumin (white bars) incubated for 3 days at 37°C. Turbidity data were normalized to the highest value of transthyretin aggregation at pH 4.35.
Dysregulated transthyretin from PES forms toxic aggregates and induces apoptosis in the placenta from IL-10−/− mice. Administration of immunoprecipitated PES-transthyretin results in protein deposits and apoptosis in the placenta of pregnant IL-10−/− mice. A–D: Representative immunofluorescence staining of human transthyretin in mouse placental sections from gd17 gestation in response to treatments: saline (A), isotype IgG immunoprecipitate from PES (B), transthyretin immunoprecipitate from NPS (C), and transthyretin immunoprecipitate from PES (D). The placental tissue is marked for the mesometrial (M), deciduas basalis (DB), and labyrinthine (L) regions. PES-transthyretin shows excessive tissue deposits (D). E and F: A representative image of TUNEL staining of the labyrinthine area of mouse placental sections in response to treatment with transthyretin immunoprecipitate from NPS (E) and transthyretin immunoprecipitate from PES (F). A significant TUNEL-positive signal (arrows) is seen in placental sections obtained from PES-transthyretin immunoprecipitate-treated mice. Original magnifications: ×10 (A–D); ×20 (E and F).
Transthyretin-interacting proteins, as revealed by SELDI-TOF analysis. SELDI-TOF profile shows the absence of different molecular mass proteins (6000 to 70,000 Da) after immunodepletion of NPS with transthyretin antibody (Ab) but not with isotype IgG antibody.