Abstract
While nephrologists often observe reduced hematocrit associated with inhibitors of angiotensin-converting enzyme (ACE), the basis for this effect is not well understood. We now report that two strains of ACE knockout mice have a normocytic anemia associated with elevated plasma erythropoietin levels. 51Cr labeling of red cells showed that the knockout mice have a normal total blood volume but a reduced red cell mass. ACE knockout mice, which lack tissue ACE, are anemic despite having normal renal function. These mice have increased plasma levels of the peptide acetyl-SDKP, a possible stem cell suppressor. However, they also show low plasma levels of angiotensin II. Infusion of angiotensin II for 2 weeks increased hematocrit to near normal levels. These data suggest that angiotensin II facilitates erythropoiesis, a conclusion with implications for the management of chronically ill patients on inhibitors of the renin-angiotensin system.
Introduction
The renin-angiotensin system plays a critical role in blood pressure regulation and fluid hemodynamics. Pharmacologic inhibitors of this system are routinely used to treat hypertension and congestive heart failure. One of the most controversial effects of the renin-angiotensin system has been the interplay of this system with erythrocyte production. A variety of clinical reports have noted an association between activation of the renin-angiotensin system and increased erythropoiesis (1–3). These studies have come from analyses of patients with a variety of chronic diseases including chronic obstructive pulmonary disease, heart failure, and renal transplantation. Other investigators have suggested a link between angiotensin-converting enzyme (ACE) inhibitors and worsened anemia, particularly in patients with chronic renal failure (4–6). While research has focused on the interplay of the renin-angiotensin system and erythropoietin, no mechanistic explanation for these observations has been generally accepted.
Central to the renin-angiotensin system is ACE, a peptidase that converts angiotensin I to angiotensin II (7). In mammals, most ACE is bound to tissues such as endothelium, but enzymatic cleavage results in a circulating form within plasma. In vitro, ACE is capable of cleaving many small peptides besides angiotensin I. However, in vivo, with the exception of bradykinin, the significance of nonangiotensin peptides as ACE substrates is not well understood. ACE is a protein with two independent catalytic domains. While both catalytic sites hydrolyze angiotensin I with roughly equal efficiency, the amino- and carboxy-terminal catalytic domains differ in their rate constants for other peptides.
Using targeted homologous recombination in embryonic stem (ES) cells, our laboratory created two lines of mice with modifications of the ACE gene (8, 9). These animals are termed ACE.1 and ACE.2. Mice homozygous for the ACE.1 allele (ACE.1 knockout mice) are null for all ACE production. They have a marked reduction of blood pressure, and a renal lesion characterized by hypoplasia of the renal medulla and papilla. In contrast to this null phenotype, animals homozygous for the ACE.2 allele (ACE.2 knockout mice) have a partial restoration of ACE activity. These animals express a truncated ACE protein containing only the amino-terminal catalytic domain. Since this shortened ACE protein lacks the carboxy-terminal domain that normally anchors ACE to cell membranes, the ACE.2 protein is exported from cells into blood and other extracellular fluids. Thus, while the plasma of ACE.2 mice converts angiotensin I to angiotensin II with about 34% of the activity of wild-type mouse plasma, tissues such as the lung and kidney completely lack ACE protein or activity. The systolic blood pressure of ACE.2 knockout mice averaged 75 mmHg, as low as that of the ACE.1 knockout animals.
Here, we investigate an unexpected finding concerning the phenotypes of both the ACE.1 and ACE.2 mice. These animals are anemic. ACE.2 knockout mice are a particularly useful model to study the role of the renin-angiotensin system in erythropoiesis, since these mice have normal renal development and glomerular function. Our data suggest that the anemia associated with interruption of the renin-angiotensin system results from a lack of angiotensin II production, supporting the concept that angiotensin II facilitates erythropoiesis. These conclusions have implications for the clinical management of chronically ill patients on inhibitors of the renin-angiotensin system.
Methods
Generation of ACE.1 and ACE.2 mice.
ACE knockout mouse strains were created using targeted homologous recombination in ES cells as previously described (8, 9). Both strains were propagated by the breeding of heterozygotes, with periodic backcrossing to C57BL/6 mice. The ACE.1 mice used in this paper were at the 6th and 7th generations of backcross, while the ACE.2 mice were at the 3rd through 5th generations of backcross. Genotyping of pups from both strains was by PCR analysis as previously described.
Hemoglobin and hematocrit.
Blood samples were collected via the tail vein, and hemoglobin concentration was determined using a Hemocue β-hemoglobin photometer from Auratech Inc. (Greensboro, North Carolina, USA). Additional blood was collected in microcapillary tubes. These were centrifuged for 4 minutes at 12,000 g and placed into a manual microcapillary reader for the determination of hematocrit.
Red cell indices and serum chemistries.
ACE.1 and ACE.2 mice were bled either by the tail vein or by cardiac puncture. For the determination of mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC), and reticulocyte counts, blood was collected into tubes containing potassium-EDTA to prevent clotting. For other serum chemistries, blood was placed in 1.5-ml Eppendorf tubes, permitted to clot, and then centrifuged for the collection of serum. All analyses were performed either at the clinical laboratories of Emory University Hospital or at the veterinary division of Antech Diagnostics (Farmingdale, New York, USA).
Creatinine and creatinine clearance.
Mice were placed in metabolic cages for 24 hours without food but with water ad libitum. Total urine volume was measured. Urine creatinine concentration was determined using a Beckman LX20 (Beckman Coulter Inc., Fullerton, California, USA). After the collection period, plasma creatinine concentration was determined in venous blood obtained from the tail. The creatinine clearance was calculated using the standard equation: creatinine clearance = (urine creatinine / plasma creatinine) × urine volume.
Plasma erythropoietin, angiotensin I, and angiotensin II levels.
Mice were exsanguinated by cardiac puncture, and blood was collected on ice in tubes containing 1.6 mg/ml potassium-EDTA, 100 μM amastatin , 100 μM bestatin, and 4 μg/ml lisinopril (10). Plasma was frozen immediately after blood collection and stored frozen until assays were performed. Erythropoietin was measured using a radioimmunoassay kit (bioMérieux SA, Marcy-l’Etoile, France). Standard curves were obtained using human erythropoietin and recombinant murine erythropoietin (Boehringer Mannheim Corp., Meylan, France). The standard curve with murine erythropoietin was strictly parallel to the standard curve with human erythropoietin. Angiotensin I and angiotensin II were also measured by radioimmunoassay as previously described (11). The assay background was determined by measuring peptide levels in angiotensinogen knockout mice, animals genetically modified to lack angiotensin I and angiotensin II. Background values were subtracted from the ACE.1 and ACE.2 measurements to obtain the final data.
Acetyl-SDKP.
Acetyl-SDKP was measured in plasma by a competitive enzyme immunoassay as previously described (12). Polyclonal antibodies were obtained after immunization of acetyl-SDKP bound to Electrophorus electricus acetylcholinesterase. The lower limit of this assay was estimated as 0.2 nM.
Blood pressure determination.
Systolic blood pressure analysis was determined using a Visitech Systems (Apex, North Carolina, USA) automated tail cuff system (13). All animals were trained for 5 days prior to data collection. They were then subjected to three sets of data acquisition each day for the next 4 days. The first set of five data points was discarded whereas the next two sets of ten measurements were kept and averaged. Data sets with a standard deviation greater than 9 mmHg were not considered. The measured systolic blood pressure for an animal was the average blood pressure obtained over 4 days and consisted of eight sets of ten measurements.
Angiotensin II infusion into ACE.2 mice.
The base-line blood pressure was measured for a cohort of ten ACE.2 knockout mice and six littermate wild-type mice. The mice were then bled from the tail vein for determination of hematocrit and plasma hemoglobin concentration. The mice were weighed, and Alzet model 1002 osmotic minipumps (ALZA Corp., Palo Alto, California, USA) were implanted subcutaneously into the dorsum. Six ACE.2 knockout mice and six wild-type mice received minipumps delivering a final dose of 0.3 mg/kg/d angiotensin II diluted in sterile 0.9% saline. Four ACE.2 knockout mice received pumps loaded only with saline. All mice received the infusion during 14 days. The mice were retrained for blood pressure measurement during the last part of the infusion. Final systolic blood pressure was measured during the last 4 days of the infusion. On day 14, each animal was bled from the tail vein for the determination of hematocrit and plasma hemoglobin concentration.
Red cell mass.
The measurement of red cell mass and blood volume in mice using 51Cr-labeled erythrocytes has been previously described (14, 15). Briefly, whole blood was harvested from donor ACE.2 heterozygous mice and incubated with 100 μCi Na51CrO4 for 30 minutes at room temperature. During the incubation, the tubes were gently inverted every 5 minutes to ensure adequate mixing. The erythrocytes were then pelleted at 2500 g for 10 minutes and washed twice in PBS. Finally, they were resuspended to a hematocrit of approximately 50%.
ACE.2 mice were anesthetized with ketamine, xylazine, and butorphanol, and the carotid arteries were catheterized with Micro-Renathane tubing (Braintree Scientific Inc., Braintree, Massachusetts, USA) tapered in sesame oil heated to 200°C. The mice were infused with 100 μl of labeled erythrocytes, followed by a flush of 300 μl heparin/PBS (2000 U/ml). After 10 minutes, they were exsanguinated by cardiocentesis and their blood divided into 100 μl aliquots. One aliquot was used to determine the postinfusion hematocrit. Two more were directly counted in a γ counter, while the final two were centrifuged and washed before being counted. Data were adjusted for the infusion volume of 400 μl and then normalized for the weight of the animals.
Results
Anemia.
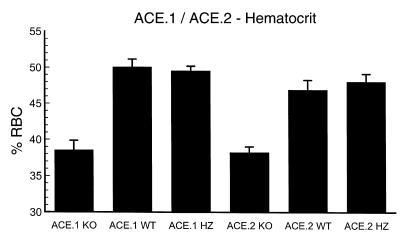
Evaluation of ACE.1 and ACE.2 mice demonstrated a persistent anemia (Figure 1). Both wild-type mice and mice heterozygous for the ACE.1 or ACE.2 mutation had hematocrits that averaged 47–50%. In contrast, the average hematocrits of ACE.1 and ACE.2 knockout mice were 39% and 38% respectively. This reduction was both consistently observed and highly significant for both knockout genotypes as compared with control mice (P < 0.0001). Hemoglobin levels were also reduced in both knockout genotypes (Table 1). There were no significant differences in MCV, MCH, and MCHC, indicating a normocytic, normochromic anemia. None of the genotypes showed a gross abnormality of platelets or white cell number.
Figure 1.
Hematocrit. Tail vein blood was collected into microcapillary tubes and the hematocrit was determined for ACE.1 and ACE.2 knockout (KO), wild-type (WT), and heterozygous (HZ) mice. The number of mice in each group was as follows: ACE.1 KO, 12; ACE.1 WT, 13; ACE.1 HZ, 11; ACE.2 KO, 24; ACE.2 WT, 15; ACE.2 HZ, 14. The reduction in hematocrit between knockout and wild-type mice was highly significant, with P < 0.0001 for both knockout genotypes as compared with control mice. No significant difference was observed between wild-type and heterozygous mice. Data are presented as mean ± SE. RBC, red blood cell.
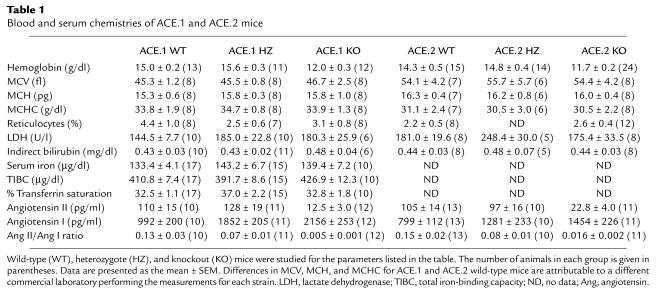
Table 1.
Blood and serum chemistries of ACE.1 and ACE.2 mice
ACE.1 mice were used to study iron metabolism by evaluating serum iron, total iron binding capacity, and serum iron saturation. As presented in Table 1, the values for the ACE.1 knockout mice were no different from those measured in littermate wild-type or heterozygous animals. Stainable bone marrow iron was readily apparent in both ACE.1 and ACE.2 knockout mice (data not shown). These data argue against iron deficiency as the cause of anemia. At necropsy, the histologic appearance of the bone marrow in knockout animals was not different from the bone marrow of control mice. Specifically, the cellularity of bone marrow from knockout mice was greater than 95% with a myeloid-to-erythroid ratio of 4:1. Megakaryocytes were relatively numerous and diffusely spaced. Normal maturation was observed in myeloid, erythroid, and megakaryocytic lineages.
We also investigated hemolysis as a factor contributing to the anemia. This was evaluated in ACE.1 and ACE.2 mice by studying serum indirect bilirubin, lactate dehydrogenase, and reticulocyte levels (Table 1). As with the evaluation of iron, we found no significant differences in these indices between knockout mice and both littermate wild-type and heterozygous animals. While serum haptoglobin is useful in assessing hemolysis in humans, this measurement is not easily applied to mice, since even wild-type mice have undetectable serum haptoglobin levels. Thus, our data suggest that hemolysis does not play a major role in the anemia observed in these animals.
Erythropoietin.
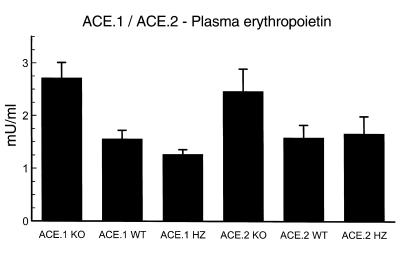
In the evaluation of human anemia due to inhibition of the renin-angiotensin system, erythropoietin levels have been controversial; some groups have reported lowered levels, while others find no significant change (4). To measure plasma erythropoietin in ACE.1 and ACE.2 mice, animals were exsanguinated by cardiac puncture and erythropoietin was measured by radioimmunoassay (Figure 2). These data show that ACE.1 and ACE.2 knockout mice have significantly higher plasma erythropoietin levels than wild-type or heterozygous littermate controls. For example, ACE.1 knockout mice averaged 2.72 ± 0.29 mU/ml, while wild-type mice averaged 1.56 ± 0.16 mU/ml (P < 0.01). A similar comparison of ACE.2 knockout mice (2.46 ± 0.42 mU/ml) with wild-type (1.59 ± 0.24 mU/ml) was also significant (P < 0.05). While the ACE.1 knockout animals tended to have higher serum erythropoietin levels than the ACE.2 knockout animals, this difference did not achieve statistical significance. Thus, despite a relatively elevated plasma erythropoietin, ACE.1 and ACE.2 knockout mice are anemic.
Figure 2.
Plasma erythropoietin. Anesthetized mice were bled by cardiac puncture and plasma was immediately frozen. Erythropoietin levels were determined by radioimmunoassay. The number of mice in each group was as follows: ACE.1 KO, 10; ACE.1 WT, 9; ACE.1 HZ, 12; ACE.2 KO, 8; ACE.2 WT, 13; ACE.2 HZ, 9. The P value comparing ACE.1 knockout mice with wild-type mice is less than 0.01. A similar comparison of ACE.2 knockout mice with wild-type gave P < 0.05. Thus, the anemia present in ACE.1 and ACE.2 knockout mice was associated with elevated plasma levels of erythropoietin. Data are presented as mean ± SE.
Renal function.
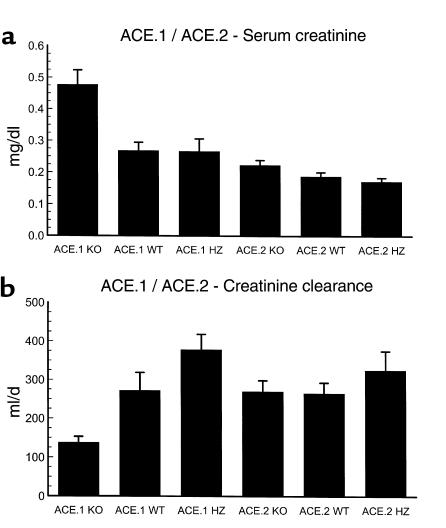
We considered renal failure as a possible explanation for anemia in these mice. In order to compare the renal function of the two strains of mice, we evaluated the serum creatinine and creatinine clearance of a large cohort of animals (Figure 3, a and b). These data show a discrepancy between the renal function of the ACE.1 and ACE.2 knockout mice. ACE.1 knockout mice have chemical evidence of renal failure as indicated by an elevation of serum creatinine and a significant reduction of renal creatinine clearance. For instance, the creatinine clearance of ACE.1 knockout mice was 138 ± 15 ml/d, while littermate wild-type mice had a creatinine clearance of 272 ± 46 ml/d (P < 0.05). In contrast, ACE.2 knockout mice had no evidence of renal failure, in that their serum creatinine and creatinine clearance values were not significantly different from wild-type littermate controls. The difference in renal function between ACE.1 and ACE.2 knockout mice is consistent with the known renal histology of the two strains of mice (9). ACE.1 knockout mice have a renal lesion typified by medullary underdevelopment. In contrast, the kidneys of most ACE.2 knockout mice show normal renal medullary development. As anticipated, heterozygous mice of both ACE strains have renal function indistinguishable from that of wild-type mice. Thus, while renal failure may be a potential explanation for anemia in the ACE.1 knockout mice, it seems very unlikely that renal failure is the explanation for the anemia observed in ACE.2 knockout animals.
Figure 3.
Renal function. (a) Serum creatinine was measured from venous blood obtained from the tail. The number of mice in each group was as follows: ACE.1 KO, 11; ACE.1 WT, 12; ACE.1 HZ, 14; ACE.2 KO, 30; ACE.2 WT, 50; ACE.2 HZ, 50. (b) The creatinine clearance was measured as described in Methods. The number of mice in each group was as follows: ACE.1 KO, 5; ACE.1 WT, 6; ACE.1 HZ, 6; ACE.2 KO, 30; ACE.2 WT, 21; ACE.2 HZ, 19. All data are presented as mean ± SE. ACE.1 mice have a significant elevation of serum creatinine as compared with wild-type and heterozygous mice (P < 0.01). They also have a significant reduction of creatinine clearance as compared with these same control animals (P < 0.05). In contrast, ACE.2 knockout mice have no evidence of renal failure in that their serum creatinine and creatinine clearance values were not significantly different from wild-type littermate controls. While the creatinine clearance of ACE.1 and ACE.2 heterozygous mice is somewhat elevated above the levels of wild-type mice, these differences do not reach statistical significance. The difference in serum creatinine between ACE.1 wild-type mice and ACE.2 wild-type mice is probably due to the use of different reference laboratories to obtain these data.
Plasma peptide levels.
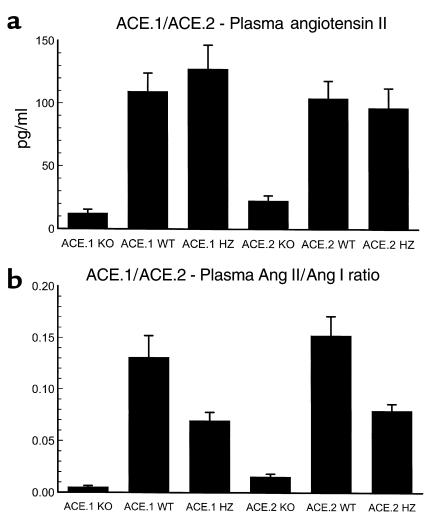
An important difference between ACE.1 and ACE.2 knockout mice is the effect of the mutated ACE gene. ACE.1 knockout mice are null for all ACE activity. In contrast, ACE.2 knockout mice lack tissue-bound ACE but do have some restoration of ACE activity in plasma. Despite this difference, both the blood pressure and the degree of anemia present in these two strains of knockout mice are essentially indistinguishable. To investigate this in detail, we measured the plasma levels of the peptides angiotensin I and angiotensin II by radioimmunoassay (Figure 4, a and b, and Table 1). As an important control for this study, we determined angiotensin I and angiotensin II levels in the plasma of angiotensinogen knockout mice. These animals genetically lack angiotensin I and angiotensin II, allowing us to determine the cross-reactivity of our assay for extraneous peptides. This value was subtracted from the ACE.1 and ACE.2 measurements to obtain the final data. As one would anticipate, both ACE.1 and ACE.2 knockout mice have elevated levels of angiotensin I and much-decreased angiotensin II. In contrast, heterozygous mice have plasma angiotensin II levels that are not significantly different from those of wild-type mice. They achieve this, and a normal blood pressure, through the upregulation of angiotensin I levels (Figure 4b, Table 1). There is a small difference in the plasma levels of angiotensin II between ACE.1 and ACE.2 knockout mice (12.5 ± 2.9 vs. 22.8 ± 4.0 pg/ml, P < 0.05). While this difference is significant, knockout mice of both strains possess only a small fraction of the plasma angiotensin II present in wild-type mice. Interestingly, while angiotensin II levels in ACE.1 knockout mice are reduced by about 90%, they are not zero, probably because of non–ACE-dependent degradation of the elevated levels of angiotensin I present in these animals.
Figure 4.
Plasma angiotensin peptide levels. Anesthetized mice were bled by cardiac puncture and plasma was immediately frozen. Plasma angiotensin I and angiotensin II peptide levels were determined by radioimmunoassay. The number of mice in each group was as follows: ACE.1 KO, 12; ACE.1 WT, 10; ACE.1 HZ, 11; ACE.2 KO, 11; ACE.2 WT, 13; ACE.2 HZ, 10. (a) Both ACE.1 and ACE.2 knockout mice have a marked reduction of plasma angiotensin II, with P < 0.0001 compared with either wild-type or heterozygous mice. (b) Both ACE.1 and ACE.2 knockout mice have a marked reduction of the angiotensin II / angiotensin I ratio. This is due to a reduction of plasma angiotensin II and an elevation of plasma angiotensin I levels (see Table 1). While ACE.1 and ACE.2 heterozygous mice have normal levels of plasma angiotensin II (a), the elevation of angiotensin I present in these mice resulted in a significant reduction of the angiotensin II / angiotensin I ratio as compared with wild-type mice (P < 0.01). Data are presented as mean ± SE.
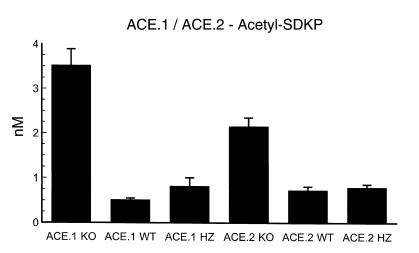
Acetyl-SDKP is a peptide that is normally degraded by the amino-terminal catalytic region of ACE. While not extensively studied, this peptide has been implicated as a bone marrow stem cell suppressor (16–18). Using a radioimmunoassay, we studied plasma acetyl-SDKP levels in the ACE.1 and ACE.2 mice (Figure 5). Both the ACE.1 and ACE.2 knockout mice showed a very significant elevation of plasma acetyl-SDKP as compared with wild-type or heterozygous mice (P < 0.0001). In addition, there was a significant difference between the plasma levels of the ACE.1 knockout mice and those of the ACE.2 knockout mice (3.5 ± 0.4 nM vs. 2.2 ± 0.2 nM, P < 0.01). One might anticipate such a difference in acetyl-SDKP peptide levels between the ACE.1 and ACE.2 knockout mice, since previous analysis has shown that the plasma activity of ACE.2 knockout mice, as measured with the amino-terminal–specific substrate acetyl-SDKP, is almost 90% that of plasma from wild-type mice (9). Thus, ACE.1 and ACE.2 knockout mice have an equivalent degree of anemia, despite the disparity of plasma acetyl-SDKP levels. While one may hypothesize that acetyl-SDKP is not the primary cause of anemia in these mice, formal studies, such as investigations of the kinetics of SDKP inhibition, need to be performed to fully evaluate this question.
Figure 5.
Acetyl-SDKP. Venous blood was obtained from the tail and plasma was immediately frozen. Acetyl-SDKP peptide levels were measured by radioimmunoassay. The number of mice in each group was as follows: ACE.1 KO, 7; ACE.1 WT, 6; ACE.1 HZ, 6; ACE.2 KO, 18; ACE.2 WT, 9; ACE.2 HZ, 10. Both ACE.1 and ACE.2 knockout mice have an elevation of plasma acetyl-SDKP as compared with wild-type or heterozygous mice. The highest levels of peptide were present in the ACE.1 knockout mice, animals completely null for ACE activity. Data are presented as the means ± SE.
Red cell mass.
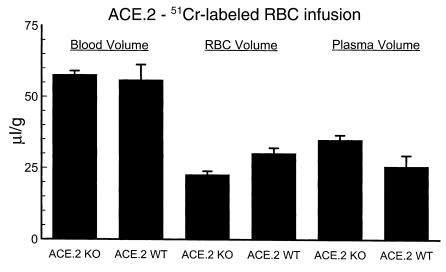
A critical question concerning the anemia present in ACE knockout mice is the effect of vasodilation in these animals. Angiotensin II is a potent vasoconstrictor, and the marked reduction of this peptide in the ACE knockout mice should promote vasodilation. Hypothetically, this might lead to volume expansion and a dilutional anemia. We used 51Cr labeling of red blood cells to determine blood volume, red blood cell volume, and plasma volume in ACE.2 knockout mice (Figure 6). ACE.2 knockout mice have a total blood volume comparable with that of wild-type mice (58 μl/g vs. 56 μl/g). This value is similar to the total blood volumes of several strains of mice previously reported in the literature (14, 19). However, ACE.2 knockout mice have a 25% reduction of red cell mass as compared with wild-type mice (22.7 μl/g ± 1.1 μl/g vs. 30.3 μl/g ± 1.7 μl/g, P < 0.01). These data show that the anemia observed in ACE.2 knockout mice is real and not due to excessive volume expansion. Thus, our data indicate that ACE.2 knockout mice have roughly an equivalent reduction of both hematocrit (Figure 1) and red cell mass.
Figure 6.
Red cell mass. Whole blood was obtained by cardiac puncture from donor ACE.2 heterozygous mice. After labeling with 51Cr, an aliquot was infused via a carotid artery catheter. After allowing for equilibration, blood was obtained by cardiac puncture and used to determine total blood volume and total red cell volume. An implied plasma volume was then calculated. All data were normalized for the weight of the animal. The number of mice in each group was as follows: ACE.2 KO, 7; ACE.2 WT, 7. While the blood volume of ACE.2 knockout mice is equivalent to that of wild-type mice, the data show that the ACE.2 knockout mice have roughly a 25% reduction of red cell mass (P < 0.01). Data are presented as mean ± SE.
Response of anemia to angiotensin II.
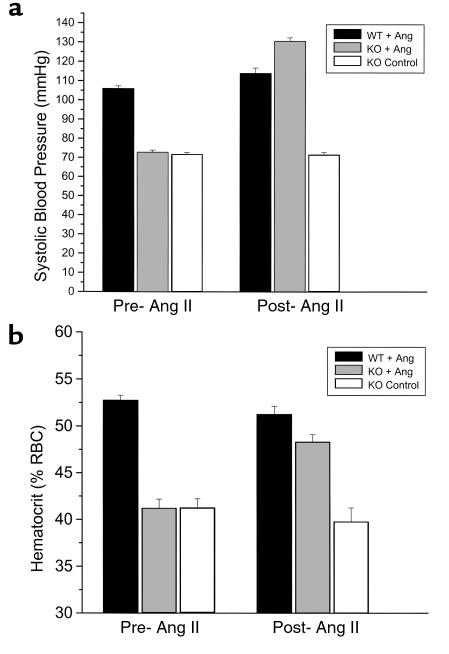
In evaluating the anemia in the ACE.2 knockout mice, we questioned whether the anemia was due to the lack of angiotensin II production or to an effect of some other peptide such as acetyl-SDKP accumulation. This can be experimentally investigated by infusing angiotensin II into ACE.2 knockout mice and examining the effect on hematocrit. If the anemia is the result of a nonangiotensin peptide such as acetyl-SDKP, then angiotensin II peptide infusion should have a minimal effect. An important preliminary study was to determine the dose of angiotensin II sufficient to raise the blood pressure of ACE.2 knockout animals to roughly wild-type levels. ACE.2 knockout mice are far more sensitive to exogenous angiotensin II than wild-type mice. As an example, the constant infusion of 1.1 mg/kg/d, a dose of angiotensin II often used to raise the blood pressure of rodents by roughly 40 mmHg, caused extreme hypertension and death in ACE.2 knockout mice. Finally, we determined that the infusion by osmotic minipump of 0.3 mg/kg/d of angiotensin II for 2 weeks was well tolerated and raised the systolic blood pressure of ACE.2 knockout mice from 73 mmHg to 131 mmHg (Figure 7A). This small dose of angiotensin II had a minimal effect on ACE.2 wild-type mice, raising systolic blood pressure from 106 mmHg to 114 mmHg.
Figure 7.
Angiotensin II infusion increases hematocrit. A cohort of wild-type and ACE.2 knockout mice were evaluated for systolic blood pressure and hematocrit. Animals were then implanted with osmotic minipumps delivering either angiotensin II (+ Ang) or vehicle (Control). After 2 weeks, blood pressure and hematocrit were reassessed. (a) The systolic blood pressure of the mice before and after angiotensin II infusion. The number of mice in each group was as follows: ACE.2 WT, 6; ACE.2 KO + Ang, 6; ACE.2 KO control, 4. Infusion of small amounts of angiotensin II raised the blood pressure of ACE.2 knockout mice to levels comparable with those of wild-type mice. (b) The hematocrits of the mice described in a were studied before and after angiotensin II infusion. ACE.2 knockout mice treated with angiotensin II showed a significant increase of hematocrit (P < 0.001) to levels near those of wild-type mice.
A cohort of ACE.2 knockout mice was treated with either 0.3 mg/kg/d angiotensin II or saline for 2 weeks (Figure 7). Systolic blood pressure and hematocrit were determined immediately before and at the end of the infusion period. A group of wild-type ACE.2 mice were treated in a similar fashion. This study showed a marked increase of hematocrit in ACE.2 knockout mice receiving angiotensin II from the preinfusion level of 41.2 ± 1.0% to the postinfusion value of 48.3 ± 0.8% (P < 0.001). Thus, treatment of ACE.2 knockout mice with a low dose of angiotensin II raised the blood pressure and corrected the hematocrit to near wild-type levels. This study suggests that the anemia in ACE.2 knockout mice is directly related to the lack of angiotensin II generation in these animals.
Discussion
While the role of the renin-angiotensin system in blood pressure control is widely appreciated, there is no theoretical underpinning to explain why mice deficient in ACE present with anemia. This anemia parallels a variety of clinical studies suggesting that the renin-angiotensin system plays a role in human erythrocyte production. For instance, an early study of the ACE inhibitor enalapril in both hypertensive patients and normal volunteers found a small reduction of hematocrit levels (20). Many more studies have examined the effect of ACE inhibitors and AT1 receptor antagonists in renal dialysis and renal transplantation patients. In particular, a substantial number of clinical studies have commented that ACE inhibitors are often effective in reducing the erythrocytosis observed after renal transplantation (21–25). These studies have disagreed as to the role of ACE inhibitors on erythropoietin production and effectiveness (4). Some groups suggest that ACE inhibitors reduce erythropoietin levels or induce resistance to erythropoietin (6, 22, 23, 26). Other studies find no causative link between erythropoietin and the anemia induced by ACE inhibitors or AT1 receptor antagonists (3, 24, 27, 28).
ACE.2 knockout mice are an excellent model system for studying the role of the renin-angiotensin system in erythropoiesis (9). These mice have stable renal function similar to that of wild-type mice. The mice do not have a chronic disease, and they do not take medications with secondary actions. Yet they consistently present with anemia characterized by a reduction of red cell mass of approximately 20%. These mice have adequate iron stores and no evidence of hemolysis. Serum erythropoietin levels are elevated as compared with control mice. Whether this is an appropriate level of elevation given the degree of anemia is difficult to evaluate. What we have observed is that ACE.2 knockout mice respond to pharmacologic administration of recombinant erythropoietin with a brisk elevation of hematocrit (data not shown). Thus, the anemia in ACE.2 mice does not appear to be associated with a gross reduction of erythropoietin levels or responsiveness.
Strikingly, ACE.2 knockout mice respond to a small dose of angiotensin II by increasing hematocrit to near normal levels. This implies that, in response to angiotensin II, there is an increase of red blood cell mass and a decrease of plasma volume to near normal levels. Thus, our data suggest that the anemia in these animals results from a lack of angiotensin II, and not from the effects of a nonangiotensin peptide such as the accumulation of acetyl-SDKP. Mrug et al. studied the effect of angiotensin II on the proliferation of erythroid progenitors and reported that angiotensin II enhanced erythropoietin-stimulated erythroid proliferation in vitro (29). This group also showed that burst-forming units–erythroid (BFU-E) colonies possess angiotensin II AT1 receptors, and that the effect of angiotensin II was blocked by the AT1 receptor antagonist losartan. Erythropoietin, the main stimulator of erythrocyte formation, binds a surface receptor and signals to the nucleus through the Jak-STAT pathway (30). This signaling pathway is important, as evidenced by the embryonic lethality of Jak2 kinase knockout mice due to gross defects in erythrocyte generation (31, 32). There is now abundant evidence that angiotensin II, acting through the AT1 receptor, also stimulates Jak2 kinase activation and STAT nuclear translocation (33, 34). Whether the lack of angiotensin II–mediated Jak-STAT signaling contributes to the anemia observed in ACE knockout mice is not known.
ACE inhibitors and AT1 receptor antagonists are widely used in clinical medicine. While the hematocrit-lowering effects of these agents in hypertensive patients is typically insignificant, this may not be true in patients with chronic diseases associated with anemia. In such patients, clinicians need to be aware that angiotensin II appears to play a role in erythropoiesis.
Acknowledgments
This work was supported by grants from the NIH, Warner-Lambert, Novartis Pharmaceuticals, Bristol-Myers Squibb Co., and Institut National de la Santé et de la Recherche Médicale (INSERM). Oliver Smithies (University of North Carolina) provided the angiotensinogen mice. The authors would like to thank the following individuals at Emory University: Ginny Secor, William E. Mitch, Jeff Sands, Janice Lea, and Jeannine Holden. The authors would also like to thank Pierre Meneton (INSERM, Paris, France) and Marie-Hélène Schlageter and J. Larghero (Hôpital St. Louis, Paris, France).
Footnotes
Justin Cole and Dilek Ertoy contributed equally to this work.
References
- 1.Vlahakos DV, et al. Association between activation of the renin-angiotensin system and secondary erythrocytosis in patients with chronic obstructive pulmonary disease. Am J Med. 1999;106:158–164. doi: 10.1016/s0002-9343(98)00390-8. [DOI] [PubMed] [Google Scholar]
- 2.Jensen JD, Eiskjaer H, Bagger JP, Pedersen EB. Elevated serum level of erythropoietin in congestive heart failure related to renal function. J Intern Med. 1993;233:125–130. doi: 10.1111/j.1365-2796.1993.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 3.Julian BA, et al. Erythropoiesis after withdrawal of enalapril in post-transplant erythrocytosis. Kidney Int. 1994;46:1397–1403. doi: 10.1038/ki.1994.411. [DOI] [PubMed] [Google Scholar]
- 4.Macdougall IC. The role of ACE inhibitors and angiotensin II receptor blockers in the response to epoetin. Nephrol Dial Transplant. 1999;14:1836–1841. doi: 10.1093/ndt/14.8.1836. [DOI] [PubMed] [Google Scholar]
- 5.Cruz DN, Perazella MA, Abu-Alfa AK, Mahnensmith RL. Angiotensin-converting enzyme inhibitor therapy in chronic hemodialysis patients: any evidence of erythropoietin resistance? Am J Kidney Dis. 1996;28:535–540. doi: 10.1016/s0272-6386(96)90464-3. [DOI] [PubMed] [Google Scholar]
- 6.Kamper A-L, Nielsen OJ. Effect of enalapril on haemoglobin and serum erythropoietin in patients with chronic nephropathy. Scand J Clin Lab Invest. 1990;50:611–618. doi: 10.3109/00365519009089178. [DOI] [PubMed] [Google Scholar]
- 7.Corvol P, Williams TA, Soubrier F. Peptidyl dipeptidase A: angiotensin I-converting enzyme. Methods Enzymol. 1995;248:283–305. doi: 10.1016/0076-6879(95)48020-x. [DOI] [PubMed] [Google Scholar]
- 8.Esther CR, Jr, et al. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- 9.Esther CR, Jr, et al. The critical role of the tissue angiotensin-converting enzyme as revealed by gene targeting in mice. J Clin Invest. 1997;99:2375–2385. doi: 10.1172/JCI119419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azizi M, et al. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J Clin Invest. 1996;97:839–844. doi: 10.1172/JCI118484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lachurie ML, Azizi M, Guyenne TT, Alhenc-Gelas F, Ménard J. Angiotensin-converting enzyme gene polymorphism has no influence on the circulating renin-angiotensin-aldosterone system or blood pressure in normotensive subjects. Circulation. 1995;91:2933–2942. doi: 10.1161/01.cir.91.12.2933. [DOI] [PubMed] [Google Scholar]
- 12.Pradelles P, et al. Negative regulator of pluripotent hematopoietic stem cell proliferation in human white blood and plasma as analyzed by enzyme immunoassay. Biochem Biophys Res Commun. 1990;170:986–993. doi: 10.1016/0006-291x(90)90489-a. [DOI] [PubMed] [Google Scholar]
- 13.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 14.Sluiter W, Oomens LWM, Brand A, Van Furth R. Determination of blood volume in the mouse with 51chromium-labeled erythrocytes. J Immunol Methods. 1984;73:221–225. doi: 10.1016/0022-1759(84)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Seferynska I, Brookins J, Rice JC, Fisher JW. Erythropoietin production in exhypoxic polycythemic mice. Am J Physiol. 1989;256:C925–C929. doi: 10.1152/ajpcell.1989.256.4.C925. [DOI] [PubMed] [Google Scholar]
- 16.Lenfant M, et al. Inhibitor of hematopoietic pluripotent stem cell proliferation: purification and determination of its structure. Proc Natl Acad Sci USA. 1989;86:779–782. doi: 10.1073/pnas.86.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnet D, et al. Direct and reversible inhibitory effect of the tetrapeptide acetyl-N-Ser-Asp-Lys-Pro (Serapenide) on the growth of human CD34 plus subpopulations in response to growth factors. Blood. 1993;82:3307–3314. [PubMed] [Google Scholar]
- 18.Robinson S, Lenfant M, Wdziecazk-Bakala J, Melville J, Riches A. The mechanism of action of the tetrapeptide acetyl-N-Ser-Asp-Lys-Pro (AcSDKP) in the control of haematopoietic stem cell proliferation. Cell Prolif. 1992;25:623–632. doi: 10.1111/j.1365-2184.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- 19.Bernstein, S.E. 1966. Physiological characteristics. In Biology of the laboratory mouse. E.L. Green, editor. McGraw-Hill. New York, New York, USA. 337–350.
- 20.Griffing GT, Melby JC. Enalapril (MK-421) and the white cell count and haematocrit. Lancet. 1982;1:1361. doi: 10.1016/s0140-6736(82)92430-8. [DOI] [PubMed] [Google Scholar]
- 21.Gaston RS, Julian BA, Diethelm AG, Curtis JJ. Effects of enalapril on erythrocytosis after renal transplantation. Ann Intern Med. 1991;115:954–955. doi: 10.7326/0003-4819-115-12-954. [DOI] [PubMed] [Google Scholar]
- 22.Conlon PJ, Farrell J, Donohoe J, Walshe JJ. The beneficial effect of enalapril on erythrocytosis after renal transplantation. Transplantation. 1993;56:217–219. [PubMed] [Google Scholar]
- 23.Gossmann J, et al. Mechanism of angiotensin converting enzyme inhibitor-related anemia in renal transplant recipients. Kidney Int. 1996;50:973–978. doi: 10.1038/ki.1996.398. [DOI] [PubMed] [Google Scholar]
- 24.Perazella M, et al. Enalapril treatment of posttransplant erythrocytosis: efficacy independent of circulating erythropoietin levels. Am J Kidney Dis. 1995;26:495–500. doi: 10.1016/0272-6386(95)90496-4. [DOI] [PubMed] [Google Scholar]
- 25.Suh B, Suh J, Kwun K. Effect of captopril in the treatment of erythrocytosis after renal transplantation. Transplant Proc. 1996;28:1557–1558. [PubMed] [Google Scholar]
- 26.Albitar S, Genin R, Fen-Chong M, Serveaux M-O, Bourgeon B. High dose enalapril impairs the response to erythropoietin treatment in haemodialysis patients. Nephrol Dial Transplant. 1998;13:1206–1210. doi: 10.1093/ndt/13.5.1206. [DOI] [PubMed] [Google Scholar]
- 27.Charytan C, Goldfarb-Rumyantzev A, Wang YF, Schwenk MH, Spinowitz BS. Effect of angiotensin-converting enzyme inhibitors on response to erythropoietin therapy in chronic dialysis patients. Am J Nephrol. 1998;18:498–503. doi: 10.1159/000013394. [DOI] [PubMed] [Google Scholar]
- 28.Chew CG, Weise MD, Disney AP. The effect of angiotensin II receptor antagonist on the exogenous erythropoietin requirement of haemodialysis patients. Nephrol Dial Transplant. 1999;14:2047–2049. [PubMed] [Google Scholar]
- 29.Mrug M, Stopka T, Julian BA, Prchal JF, Prchal JT. Angiotensin II stimulates proliferation of normal early erythroid progenitors. J Clin Invest. 1997;100:3210–3214. doi: 10.1172/JCI119769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remy I, Wilson IA, Michnick SW. Erythropoietin receptor activation by a ligand-induced conformation change. Science. 1999;283:990–993. doi: 10.1126/science.283.5404.990. [DOI] [PubMed] [Google Scholar]
- 31.Parganas E, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 32.Neubauer H, et al. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 33.Marrero MB, et al. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature. 1995;375:247–250. doi: 10.1038/375247a0. [DOI] [PubMed] [Google Scholar]
- 34.Bhat GJ, Thekkumkara TJ, Thomas WG, Conrad KM, Baker KM. Angiotensin II stimulates sis-inducing factor-like DNA binding activity. J Biol Chem. 1994;269:31443–31449. [PubMed] [Google Scholar]