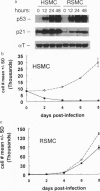
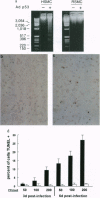
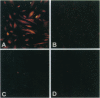
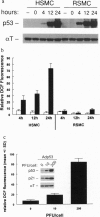
Abstract
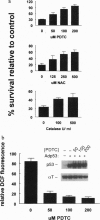
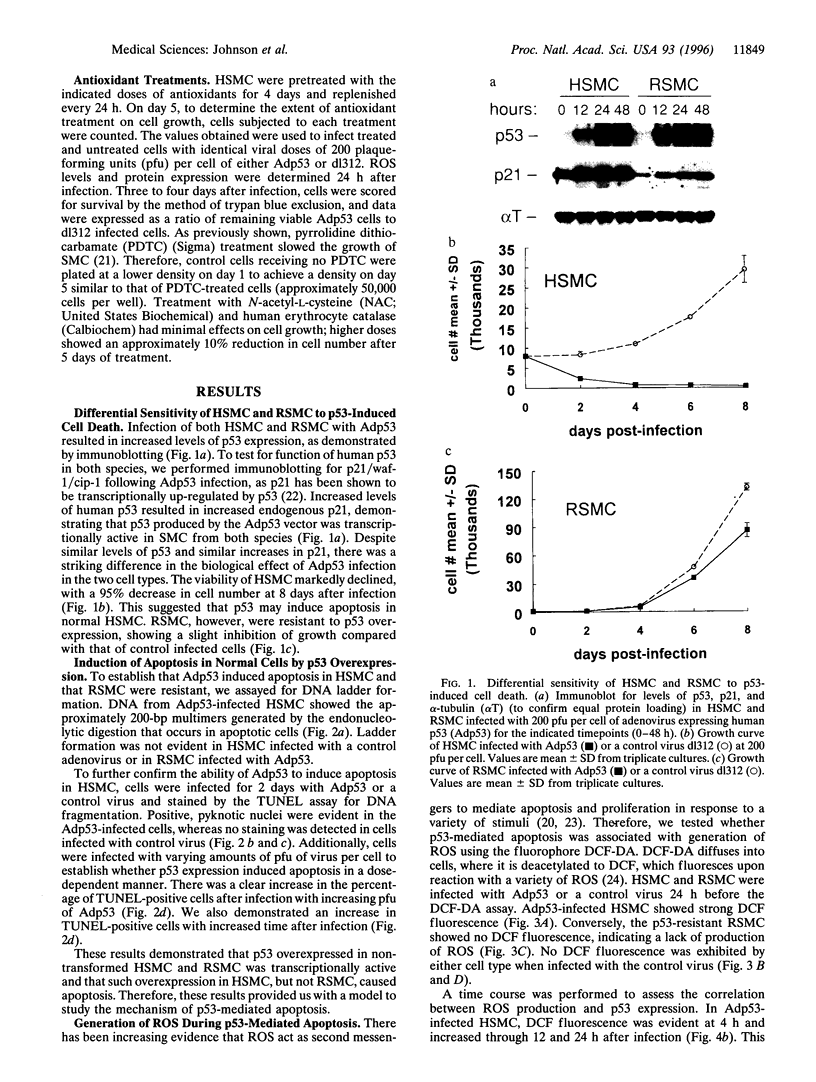
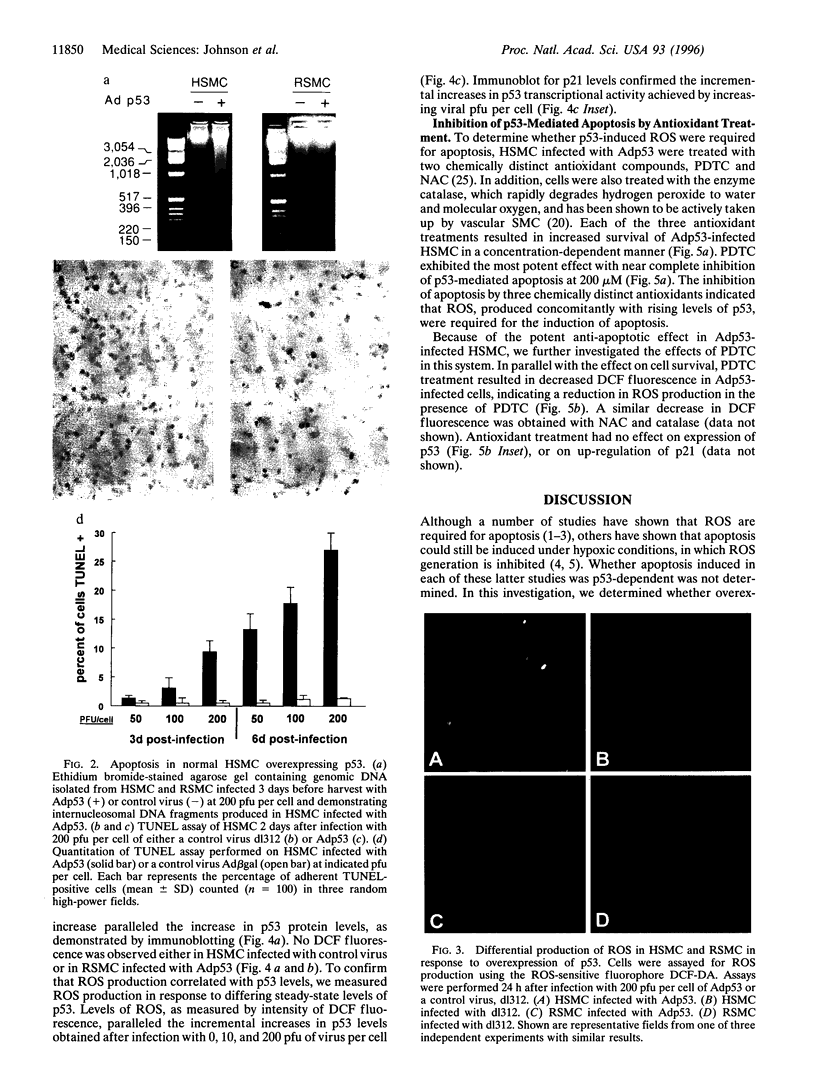
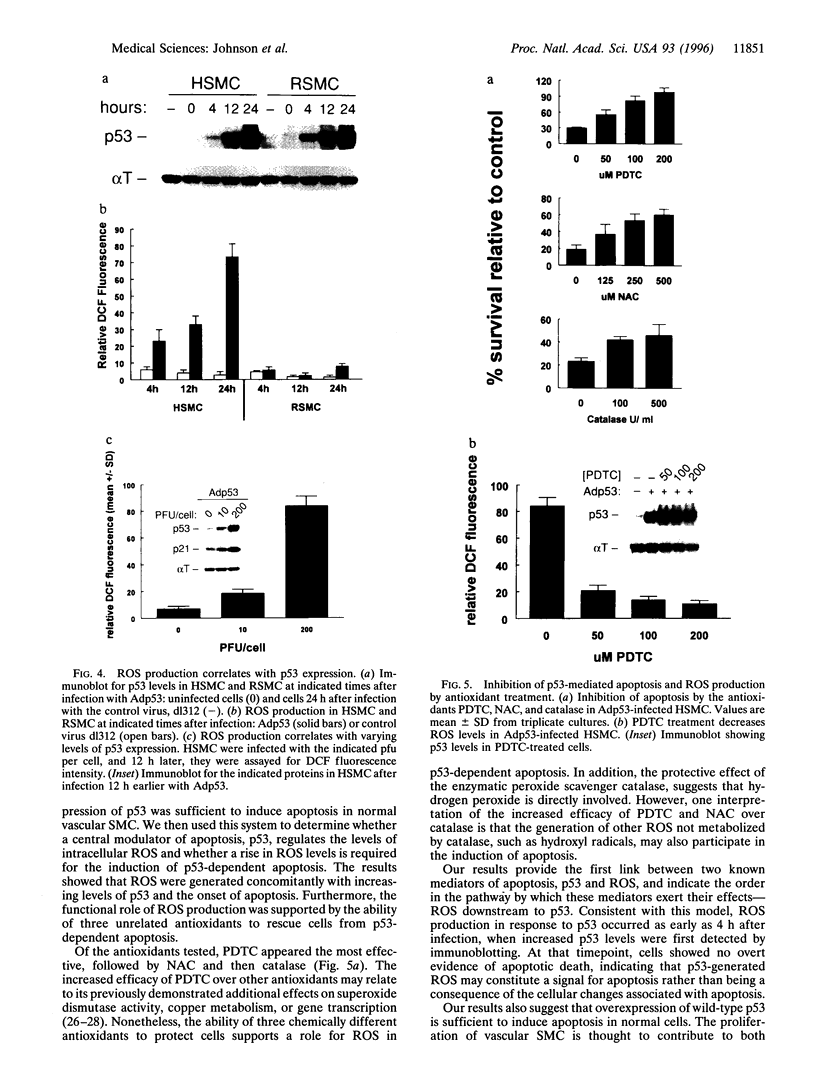
Reactive oxygen species (ROS) have been implicated as potential modulators of apoptosis. Conversely, experiments under hypoxic conditions have suggested that apoptosis could occur in the absence of ROS. We sought to determine whether a central modulator of apoptosis, p53, regulates the levels of intracellular ROS and whether a rise in ROS levels is required for the induction of p53-dependent apoptosis. We transiently overexpressed wild-type p53, using adenoviral gene transfer, and identified cell types that were sensitive or resistant to p53-mediated apoptosis. Cells sensitive to p53-mediated apoptosis produced ROS concomitantly with p53 overexpression, whereas cells resistant to p53 failed to produce ROS. In sensitive cells, both ROS production and apoptosis were inhibited by antioxidant treatment. These results suggest that p53 acts to regulate the intracellular redox state and induces apoptosis by a pathway that is dependent on ROS production.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aragonés J., López-Rodríguez C., Corbí A., del Arco P. G., López-Cabrera M., de Landázuri M. O., Redondo J. M. Dithiocarbamates trigger differentiation and induction of CD11c gene through AP-1 in the myeloid lineage. J Biol Chem. 1996 May 3;271(18):10924–10931. doi: 10.1074/jbc.271.18.10924. [DOI] [PubMed] [Google Scholar]
- Bellas R. E., Lee J. S., Sonenshein G. E. Expression of a constitutive NF-kappa B-like activity is essential for proliferation of cultured bovine vascular smooth muscle cells. J Clin Invest. 1995 Nov;96(5):2521–2527. doi: 10.1172/JCI118313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busciglio J., Yankner B. A. Apoptosis and increased generation of reactive oxygen species in Down's syndrome neurons in vitro. Nature. 1995 Dec 21;378(6559):776–779. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- Chang M. W., Barr E., Lu M. M., Barton K., Leiden J. M. Adenovirus-mediated over-expression of the cyclin/cyclin-dependent kinase inhibitor, p21 inhibits vascular smooth muscle cell proliferation and neointima formation in the rat carotid artery model of balloon angioplasty. J Clin Invest. 1995 Nov;96(5):2260–2268. doi: 10.1172/JCI118281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. W., Barr E., Seltzer J., Jiang Y. Q., Nabel G. J., Nabel E. G., Parmacek M. S., Leiden J. M. Cytostatic gene therapy for vascular proliferative disorders with a constitutively active form of the retinoblastoma gene product. Science. 1995 Jan 27;267(5197):518–522. doi: 10.1126/science.7824950. [DOI] [PubMed] [Google Scholar]
- Chang M. W., Ohno T., Gordon D., Lu M. M., Nabel G. J., Nabel E. G., Leiden J. M. Adenovirus-mediated transfer of the herpes simplex virus thymidine kinase gene inhibits vascular smooth muscle cell proliferation and neointima formation following balloon angioplasty of the rat carotid artery. Mol Med. 1995 Jan;1(2):172–181. [PMC free article] [PubMed] [Google Scholar]
- Geng Y. J., Libby P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1 beta-converting enzyme. Am J Pathol. 1995 Aug;147(2):251–266. [PMC free article] [PubMed] [Google Scholar]
- Guzman R. J., Hirschowitz E. A., Brody S. L., Crystal R. G., Epstein S. E., Finkel T. In vivo suppression of injury-induced vascular smooth muscle cell accumulation using adenovirus-mediated transfer of the herpes simplex virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10732–10736. doi: 10.1073/pnas.91.22.10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haecker G., Vaux D. L. Viral, worm and radical implications for apoptosis. Trends Biochem Sci. 1994 Mar;19(3):99–100. doi: 10.1016/0968-0004(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Han D. K., Haudenschild C. C., Hong M. K., Tinkle B. T., Leon M. B., Liau G. Evidence for apoptosis in human atherogenesis and in a rat vascular injury model. Am J Pathol. 1995 Aug;147(2):267–277. [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Isner J. M., Kearney M., Bortman S., Passeri J. Apoptosis in human atherosclerosis and restenosis. Circulation. 1995 Jun 1;91(11):2703–2711. doi: 10.1161/01.cir.91.11.2703. [DOI] [PubMed] [Google Scholar]
- Jacobson M. D., Raff M. C. Programmed cell death and Bcl-2 protection in very low oxygen. Nature. 1995 Apr 27;374(6525):814–816. doi: 10.1038/374814a0. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Kane D. J., Sarafian T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S., Ord T., Bredesen D. E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993 Nov 19;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- Katayose D., Gudas J., Nguyen H., Srivastava S., Cowan K. H., Seth P. Cytotoxic effects of adenovirus-mediated wild-type p53 protein expression in normal and tumor mammary epithelial cells. Clin Cancer Res. 1995 Aug;1(8):889–897. [PubMed] [Google Scholar]
- Lowe S. W., Bodis S., McClatchey A., Remington L., Ruley H. E., Fisher D. E., Housman D. E., Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994 Nov 4;266(5186):807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- Nobel C. I., Kimland M., Lind B., Orrenius S., Slater A. F. Dithiocarbamates induce apoptosis in thymocytes by raising the intracellular level of redox-active copper. J Biol Chem. 1995 Nov 3;270(44):26202–26208. doi: 10.1074/jbc.270.44.26202. [DOI] [PubMed] [Google Scholar]
- Ohba M., Shibanuma M., Kuroki T., Nose K. Production of hydrogen peroxide by transforming growth factor-beta 1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J Cell Biol. 1994 Aug;126(4):1079–1088. doi: 10.1083/jcb.126.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T., Gordon D., San H., Pompili V. J., Imperiale M. J., Nabel G. J., Nabel E. G. Gene therapy for vascular smooth muscle cell proliferation after arterial injury. Science. 1994 Aug 5;265(5173):781–784. doi: 10.1126/science.8047883. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Schreck R., Baeuerle P. A. A role for oxygen radicals as second messengers. Trends Cell Biol. 1991 Aug;1(2-3):39–42. doi: 10.1016/0962-8924(91)90072-h. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Bennett M. R. Death by any other name. Am J Pathol. 1995 Aug;147(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Eguchi Y., Kosaka H., Kamiike W., Matsuda H., Tsujimoto Y. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature. 1995 Apr 27;374(6525):811–813. doi: 10.1038/374811a0. [DOI] [PubMed] [Google Scholar]
- Speir E., Modali R., Huang E. S., Leon M. B., Shawl F., Finkel T., Epstein S. E. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994 Jul 15;265(5170):391–394. doi: 10.1126/science.8023160. [DOI] [PubMed] [Google Scholar]
- Sundaresan M., Yu Z. X., Ferrans V. J., Irani K., Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995 Oct 13;270(5234):296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- Symonds H., Krall L., Remington L., Saenz-Robles M., Lowe S., Jacks T., Van Dyke T. p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell. 1994 Aug 26;78(4):703–711. doi: 10.1016/0092-8674(94)90534-7. [DOI] [PubMed] [Google Scholar]
- Wiedau-Pazos M., Goto J. J., Rabizadeh S., Gralla E. B., Roe J. A., Lee M. K., Valentine J. S., Bredesen D. E. Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science. 1996 Jan 26;271(5248):515–518. doi: 10.1126/science.271.5248.515. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]