Abstract
Despite consented efforts in prevention, mycotoxins remain a problem of human health concern in several parts of the world including developed countries. Within the same range of toxins concentrations in the blood some people develop a disease while others do not. Could this inequality in front of mycotoxins effects be explained by environment factors and/or genetic predisposition? Among recent advances in environmental health research Correlation between chronic diseases and mycotoxins in humans deserves attention through several questions: Are genetic factors involved in disease causation of mycotoxins? How much are these factors currently taken into account for mycotoxins risk assessment and how much should we involve them? Answers are still to come. Genetic and environment factors deserve therefore more attention when dealing with regulatory limits, since among the general population, those who are at risk and will develop specific diseases are likely those bearing genetic predispositions. We have addressed these questions for the specific case of ochratoxin A in humans by investigating in Tunisia, county of Jelma, in four rural families forming a household of 21 persons all exposed to ochratoxin A in diet. Our results confirm that ochratoxin A induces chronic tubular nephropathy in humans and mainly point at those having the HLA haplotype A3, B27/35, DR7 to be more sensitive to the disease for quantitatively similar or lower exposure. Persons with such haplotype were found to bear chronic interstitial nephropathy with tubular karyomegalic cells while others were apparently healthy. Godin et al. (1996) in France have also found in sibling (a sister and her brother from urban area) that have similar HLA haplotype B35-patern, OTA-related renal tubulopathy with mild proteinuria including β2-microglobulinuria. Several mechanisms are discussed that could be put ahead to explain how the HLA haplotype could lead to tubular cells lyses and renal failure. In the mean time it is urgent to search for mass screening biomarkers for mycotoxins in humans and related genetic factors to set-up more appropriate regulation.
Keywords: Mycotoxins in humans, environment and genetic factors, ochratoxin A, HLA haplotype A3, B27/35
Introduction
The most frequent toxinogenic fungi in the world are Aspergillus, Penicillium and Fusarium species. They produce Aflatoxin B1 transformed into Aflatoxin M1 found in milk, Ochratoxins and Zearalenone, Fumonisin B1, T-2 toxin, HT-2 toxin and Deoxynivalenol (vomitoxin) that are increasingly of human health concern [1–2]. Some of their metabolites are still toxic and may be involved in human diseases, they are not destroyed by normal industrial processing, [2–7]. Their toxic effects, liver, kidney and hematopoetic toxicity, immune toxicity, reproduction toxicity, foetal toxicity, teratogenicity, and mainly carcinogenicity are mostly known in experimental models, the extrapolation to humans being sometime impossible due to lack of appropriate tools and biomarkers [2, 8–11]. The inaccuracy of extrapolation of experimental data to human may be explained by several reasons: (i) discrepancy between human and animal exposure, (ii) lack of precise health risks associated with specific proposed limits, (iii) possibility of synergism or antagonism with other mycotoxins present in the same diets [2, 4, 8, 12], (iv) coexistence of other pathologies such as viral hepatitis, immune or hormonal deficiencies or organs dysfunction. In contrast to experimental animals, humans are placed in more complex environment in which their genetic imprinting may be influenced, modulated and/or modified by many factors including mycotoxins.
Even when a specific biomarker of a given mycotoxin is identified in humans, it remains difficult to establish the relationship with a given illness because of above quoted reasons and because of the genotoxic and epigenetic effects of mycotoxins. These can be strongly influenced by the metabolome as the expression of individual genetic polymorphism. Actually for example, in anyone continuously exposed to aflatoxin B1 hepatocarcinoma would be expected. However, according to environment factors and to his individual genetic polymorphism, some other cancers or diseases may be found. Possible beneficial influence of diet may mask or counterbalance the expected pathology as previously observed in experimental models.
People may develop diverse degree of the same disease for similar exposure to mycotoxins. It is clear that individuals do not react the same way for similar exposure to harmful mycotoxins. If one compares OTA blood levels from certain Balkan Endemic Nephropathy (BEN) areas and some regions in Western European countries, it appears that while some people have BEN there is apparently no disease in Western Europe for similar blood concentrations [2–3, 13]. It is presently believed that this discrepancy is explained by diets and eating habits. Is that enough?
How these facts influence the risk assessment in humans and how much should we take them into account? The toxic effects of mycotoxins are currently explained by several mechanisms, (i) direct cytotoxicity of the parent compound and derived metabolites, (ii) DNA damages leading to mutagenesis, (iii) indirect DNA bases modifications such as oxidation and/or methylation leading to epigenetic promoter pathways This applies to aflatoxin B1, ochratoxin A, Zearalenone and partly to fumonisin B1 since no direct genotoxic effects have been demonstrated for the latest, [2, 13–15].
In these genotoxic and epigenetic promoter pathways, several enzymes are involved such as repair enzymes. Modification occurring in the encoding genes may modulate and/or reduce their activity. Furthermore it is accepted that mutations occurring in key genes such as p53 gene mutation, frequent in human tumours, or in the genes encoding the proteins Bax or Bcl2 or in oncogenes [16, 17], will strongly influence cell survival versus cell death thus promoting casually cell transformation and tumour genesis.
All these pathways cannot exclude either the participation of endogenous effectors such as hormones (some mycotoxins being moreover hormonal disrupters such as zearalenone) or that of xenobiotics present in our environment, (air, water, and diet) possibly acting in concert with mycotoxins [2, 18]. Several mechanisms of action have been demonstrated in experimental models and in humans that do not take into account genetic pre disposition of people either before exposure, and/or after exposure to environment factors. This applies actually to the rationales for the establishment of limits and regulations for mycotoxins [19]. For all the above reasons we have to address the question: how much should we involve genetic and environmental factors in the risk assessment of mycotoxins in humans?
Taking as example the case of human intake of ochratoxin A in which it is obvious that not all heavily exposed persons are ill, the implication of genetic pre disposition has been investigated in Tunisia (specially the county of Jelma) that is known to be a high spot of OTA exposure in Northern Africa. Ochratoxin A (OTA) (Fig. 1) is produced by several species of fungal genera [1] that is widespread in human food, animal feed and detected in blood and tissues in Tunisia as well as in the Balkans. This mycotoxin has several adverse effects the most prominent being nephrotoxicity and carcinogenicity [2–3, 7, 9].
Figure 1.
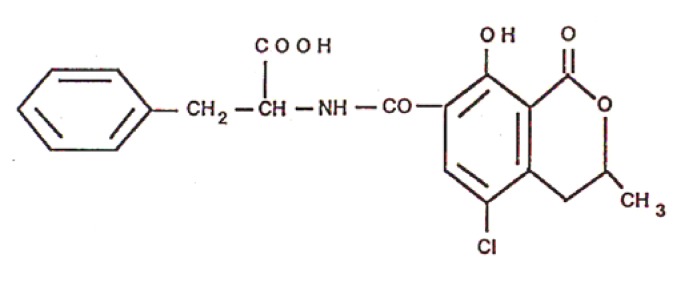
Structure of ochratoxin A
Exposure to OTA has been linked with BEN, a chronic kidney disease associated with urothelial tumours, [2, 4, 20]. In Tunisia, a Chronic Interstitial Nephropathy (CIN) of unknown aetiology bearing striking similarities with BEN in its pathogenic characteristics was under investigation for more than one decade for implication of OTA [2, 7, 21]. Taking into account the high prevalence of ochratoxin A in blood of Tunisian people and the above quoted disease as compared to other kidney diseases and, considering that healthy populations are equally exposed, links were established that tend to demonstrate the causative role of OTA in ill persons [5, 7, 21].
Whether the causative role of OTA in inducting this human nephropathy is correlated to some specific genetic imprinting of ill people is an obsessing question for risk assessment. To enlighten this point, investigations were designed on a rural household, consisting of 21 people for kidney impairment, OTA assays in food, blood and urine in addition to HLA (Human Leucocyte histocompatibility locus A) fingerprinting.
Materials and Methods
The household
People presenting the clinical signs of CIN related to OTA similarly to BEN that are lombalgia, polyuria, and absences of oedema, hypertension and hematuria. Echography and radiography also reveal small size-kidneys with asymmetry and irregular renal contours [3, 7, 21]. They live in a rural environment in the western centre of Tunisia (Jelma). This area belongs to a region that was found to be one of most affected Tunisian areas of high OTA contamination, according to previous investigations and even considered as an area of endemic OTA-related nephropathy [2, 5, 7]. Indeed, high concentrations of OTA in blood were found. This region is warm and humid.
Diagnosis of Chronic Interstitial Nephropathy of Unknown Aetiology
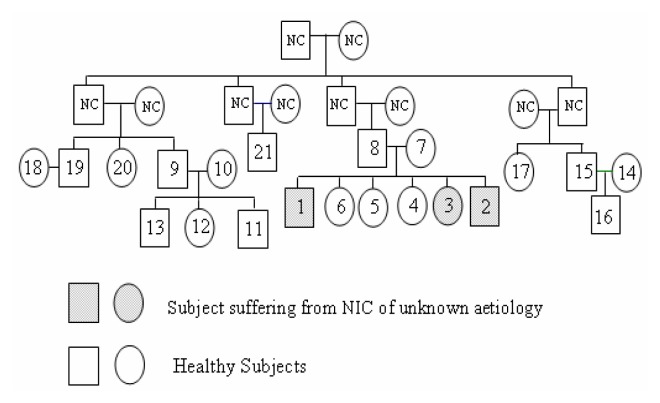
The prospected community is made up of 21 members (11 women and 10 men, aged from 32 to 65 years) of four families (see genealogical tree, Fig. 2). For each subject, blood and urine samples were collected for OTA assays and for the evaluation of the renal function. For this nephropathy, several etiological agents have been incriminated seriously considered and disqualified. The cases that remained without any possible aetiology are categorised as CIN of unknown aetiology presumably related to OTA since only this causal agent remained correlated to the disease [5, 7].
Figure 2.
Genealogical tree of people included in the study.
Bio Analysis
β2-Microglobulinuria Assay on Urines
β2-microglobulin was quantified in urines using an immunoenzymatic competitive test in microplate coated with monoclonal antibody, in the presence of a tracer consisting in a β2-microglobulin conjugated with alkaline phosphatase. After washing to remove excess tracer bound enzymatic activity is measured by the colour developed by a chromogenic substrate in a plate reader. The concentration of β2-microglobulin is then obtained by a standard curve built using the material provided with the ELISA kit by Immunodiagnostik, Benscheim, Germany.
Analysis of Ochratoxin A in Blood, Urine and Food Samples
Blood samples were collected in heparinized tubes. After centrifugation, (850g/10min at 4°C) the plasma samples (2ml) were frozen at −80°C until analysis. The plasma and urine samples were acidified with acetic acid 1M (pH 4.5) then cleaned on C18 Sep-Pak cartridge (10 × 20mm), Waters (Supelco, Sigma-Aldrich, France). After washing with acetic acid 2% (v/v), OTA was eluted with HCl 1% in methanol (v/v). Samples were then separated by HPLC system and quantified by fluorescence detection (Varian Pro Star) with an excitation wavelength of 340 nm and an emission wavelength of 465nm at ambient temperature according to known quantities of standard OTA as previously described, [2, 5, 7, 21]. Detection and quantification limits were confirmed to be 0.1ng/ml and 0.5ng/ml [7, 9].
For each family, three types of daily-consumed basic food (cereals, cereal made-foods and beans) were collected for OTA assays. OTA-positive samples were confirmed by hydrolysis of OTA by 100μl of carboxypeptidase (Bovine pancreas, Sigma) (100 IU/ml) in the buffer Tris-HCl 0.04 M (pH 7.6) and NaCl 1M during 2h at 37°C and analysis by HPLC using the conditions described above. Ochratoxin alpha (OTα) appears instead of OTA [5, 7, 21].
Histology
Light microscopy observations were performed with magnification of 400 on sections of kidney biopsies stained using trichrome de Maçon dye to confirm karyomagaly in renal tubules [7, 22] that are BEN-like lesions.
HLA Antigens Determination
On blood samples, HLA-A and HLA-B antigens have been typed by a standard microlymphocytotoxicity technique using a panel of standard sera (One Lambda, CA).
Results
The 21 subjects were selected from the rural population of Tunisia in the region of Jelma, known for high OTA exposure.
Presence of Ochratoxin A in Food, Blood and Urine
OTA assays in blood showed that 19 persons are contaminated among the 21 prospected in the range of 8ng/ml to 1468ng/ml, (Table 1). High OTA concentrations were found in blood of the three cases (n°1, n°2 and n°3), respectively 505.83ng/ml, 102.63 ng/ml and 1023ng/ml. Higher OTA blood concentrations were found in the blood of other members of the family I (n° 7, 1332ng/ml) and from family II, (n° 9 and n°11, respectively 1348 and 1334ng/ml), (Table 1). The ill persons are three siblings, two brothers and their sister which kidney biopsies showed karyomegaly in renal tubules, Fig 2. They have altered renal function, high uremia and creatinemia, without hypertension, β2-microglobulinuria was respectively 10400μg/l, 440μg/l and 360μg/l. All these signs are characteristic of BEN, (Table 2). Some others have more OTA in food and blood although they do not show any sign of BEN, (Table 2).
Table 1.
Ochratoxin A contaminations, in food, blood and urine of the 21 exposed people, and β2-microglobulinuria.
| Case N° | OTA in Food μg/kg | OTA in Blood, ng/ml (OTA in Urine, ng/ml) | β2-Microglobulinuria μg/l | CIN of Unknown Etiology | |
|---|---|---|---|---|---|
| Family I | 1 | 0.88 ± 0.45 | 505.83 (10.18) | 10400 | + |
| 2 | 102.36 (94.4) | 440 | + | ||
| 3 | 1023 (limit) | 360 | + | ||
| 4 | 378 (limit) | 230 | − | ||
| 5 | 20.4 (limit) | 260 | − | ||
| 6 | 0 | 220 | − | ||
| 7 | 1332 (limit) | 300 | − | ||
| 8 | 15 (limit) | 850 | − | ||
|
| |||||
| Family II | 9 | 5.29 ±7.19 | 1348 (limit) | 300 | − |
| 10 | 702(limit) | 260 | − | ||
| 11 | 1334 (41.5) | 1300 | − | ||
| 12 | 8 (limit) | 240 | − | ||
| 13 | 15 (limit) | 230 | − | ||
|
| |||||
| Family III | 14 | 0.94 ± 0.45 | 967 (limit) | 225 | − |
| 15 | 218 (limit) | 700 | − | ||
| 16 | 210 (1000) | 250 | − | ||
| 17 | 33 (limit) | 210 | − | ||
|
| |||||
| 18 | 0.31 ± 0.21 | 0 | 260 | − | |
| 19 | 9 (limit) | 280 | − | ||
|
|
|||||
| 20 | NC | 268 (limit) | 310 | − | |
| 21 | 8 (limit) | 300 | − | ||
Table 2.
HLA haplotype of patients CIN BEN-like and daily intake of OTA for the subjects calculated from the plasma clearance, blood concentration and bioavailability of OTA in human according to the following equation, in which K= daily intake, Cpl= OTA plasma clearance ml/min, Cp=OTA plasma concentration and A= bioavailability.
| Case N° | OTA in Food μg/kg | OTA in Blood/ng/ml | OTA Daily intake ng/kg bw/Day | CIN BEN-Like | HLA Determinant | |
|---|---|---|---|---|---|---|
| Family I | 1 | 0.88 ± 0.45 | 505.8 | 658 | + | A3/28; B 27/35; DR 7/52 |
| 2 | 102.4 | 133 | + | A3/28; B 27/35; DR 7/11 | ||
| 3 | 1023 | 1330 | + | A3/40; B 27/35; DR 7/52 | ||
| 4 | 378 | 491 | − | |||
| 5 | 20.4 | 27 | − | |||
| 6 | 0 | 0 | − | |||
| 7 | 1332 | 1732 | − | |||
| 8 | 15 | 20 | − | |||
|
| ||||||
| Family II | 9 | 5.29 ±7.19 | 1348 | 1752 | − | |
| 10 | 702 | 912.6 | − | |||
| 11 | 1334 | 1734 | − | |||
| 12 | 8 | 10.4 | − | |||
| 13 | 15 | 20 | − | |||
|
|
||||||
| Family III | 14 | 0.94 ± 0.45 | 967 | 1257 | − | |
| 15 | 218 | 283 | − | |||
| 16 | 210 | 273 | − | |||
| 17 | 33 | 42.9 | − | |||
|
| ||||||
| 18 | 0.31 ± 0.21 | 0 | 0 | − | ||
| 19 | 9 | 12 | − | |||
| 20 | ND | 268 | 348 | − | ||
| 21 | 8 | 10 | − | |||
Genetic Imprinting of HLA
The 21 poeple were tested for their HLA haplotype because this is known to be implicated in renal tubulopathy and karyomegaly in human by several groups working on the subject [23–25]. The three cases showing BEN-like nephropathy with high OTA concentrations in blood and urines belong respectively to the haplotype, A 3/28; B 27/35; DR 7/52; A 3/28; B 27/35; DR 7/11 - A 3/40; B 27/35; DR 7/52. They share the following elements of phenotype A3, B27/35 and DR7.
Discussion
In these investigations, it is obvious that all people from rural areas enrolled are exposed to ochratoxin A. The two individuals considered to be negative have shown in their blood and urine OTA values that fall in detection and quantification limits and were not confirmed further as previously described. They need to be followed-up to know whether their low exposure remained low or not. The urine OTA-concentrations of other apparently healthy people fall also in this range. However their case needs to be discussed according to the knowledge we have concerning the elimination of OTA in urine. OTA is actually eliminated by glomerular filtration, tubular re-absorption and tubular secretion, [2]. Those who have limited amount of OTA in urine have likely no alteration at any of these processes. Those who have appreciable OTA concentrations in urine without symptoms of tubulopathy have likely asymptomatic glomerular disease that leads to urinary elimination of proteins that bind more than 90% of available OTA in blood [2]. They are under investigation for that reason. All asymptomatic subjects also need to be seriously surveyed. Only three of OTA-positive people are ill, showing BEN-like nephropathy although others have similar OTA concentrations in blood and urine. Could this be explained by environment factors only or genetic factors only or both? It is to be emphasised that for ethical reasons no biopsy has been performed on those who were apparently healthy.
The ochratoxin A concentrations found in either food or biological fluids during the present investigations in Tunisia are even higher than those observed in BEN areas in the Balkans. These should then normally elicit kidney tubules damages, provided that people are exposed for long enough. There is no doubt that most individuals enrolled in the present investigations have been exposed for many days or weeks according to the half-life time of OTA in human body (more than 30 days) and to the concentrations found that cannot likely be ingested in a single dose. The two people who do not have OTA in blood could have ingested also substances bearing the capability of preventing OTA tissue distribution, metabolism and of enhancing its elimination such as OTA-binding peptides, Aspartame, antioxidant such as tea, inhibitors of prostaglandin synthetase, etc., [2].
The renal lesions in ill people are clearly established, since β2-microglobulinuria is high and karyomegaly is histologically demonstrated. This has been also found in north of France in sibling (a sister and her brother from urban area) that bear similar HLA hoplotype B35-patern, [25] and also mild proteinuria including β2-microglobulinuria. This implicates a particular genetic imprinting and evidence for genetic defects, [24, 25]. The two individuals (n°8 and 15) who have elevated β2-microglobulinuria seem to be very atypical cases for a reason we now ignore. Their HLA haplotype is under verification.
Ochratoxin A is nephrotoxic to all animal species tested so far including pigs and induces karyomegaly in proximal tubules in rats, [11, 22]. These data have to be put in parallel with data in humans, [5, 7, 20, 21, 25] and karyomegaly has to be considered as a marker of pathology in both humans and animals exposed to OTA. Two mechanisms could be put ahead to explain how the HLA haplotype could lead to tubular cells lyses and renal failure. First, Hanada et al. 2004, propose that cytotoxic T lymphocytes (CTLs) could detect and destroy cells displaying modified class I molecules of the major histocompatibility complex, [26]. OTA is known to be mutagenic and may induce modification in HLA complex favouring renal tubule cell lyses and/or karyomegaly in tubular regenerating cells, [2, 3, 15, 20–22]. Second, Morozov et al. 1991, propose a possible mechanism for the release of beta 2-microglobulin from HLA complex to make it available to exert toxic effects on the kidney as a potential pathogenesis of Balkan endemic nephropathy, [27]. Both mechanisms may be co-acting on the renal tubules of people having a given HLA haplotype and high beta 2-microglobulinuria. One may then hypothesize that genetic pre-disposition are involved in this disease causation.
Other genetic polymorphisms or pre-dispositions that also can influence human genotoxic risk are those of glutathione s-transferase and cytochromes P450, suggesting involvement of oxidative stress, [2, 10, 11, 28]. Recent findings have demonstrated that oxidative damages contribute to the cytotoxicity and carcinogeneticy of mycotoxins such as ochratoxin A, aflatoxin B1 (AfB1), fumonisin B1 and Zearalenone [2, 10–12, 16]. The case of aflatoxins is comparable to that of ochratoxin A in several aspects. Aflatoxins occur in peanut butter, cereals and their metabolism requires allelic polymorphic enzymes such as glutathione-S-transferases encoded by glutathione-S-transferase gene (GSTM1) and glutathione-S-transferase gene (GSTT1) and microsomal epoxide hydrolase encoded by epoxide hydrolase gene (EPHX). The rate at which aflatoxins become activated or detoxified may depend on polymorphisms in the encoding genes. GSTM1 homozygous deletion was indeed found to modify the association between peanut butter consumption and hepato-cellular carcinoma (HCC) [6, 10]. Possible roles of GSTT1 and EPHX polymorphisms in this relationship are well documented [2, 6, 10, 11, 17, 28]. Whether humans vary in their ability to detoxify the active intermediate metabolite of AfB1, AfB1-exo-8, 9-epoxide, is not certain but may explain why all exposed individuals do not develop HCC [6]. The situation is similar for ochratoxin A. If it becomes obvious that in an exposed population to mycotoxins, those who will develop specific diseases are those bearing some genetic pre-disposition or defaults, it would be valuable and indeed it becomes urgent, to take these factors into account for prevention, risk assessment and management. In the mean time, while waiting for more appropriate regulatory limits, the common sense suggests that prevention involves first, reduction of mycotoxins levels in foodstuffs and further implication of diet components such as vitamins, antioxidants and substances known to prevent carcinogenesis.
Currently, environmental factors are those mostly evaluated to determine human exposure. This procedure appears largely insufficient to definitely establish the aetiology (links) of a given intoxication and/or disease with probable toxic agents. The present findings suggest that biomarkers might be more useful tool for epidemiological research in mycotoxins exposure in humans in relation to diseases provided that genetic factors are also taken into account in addition to environmental factors. The whole would then represent a valid instrument for group evaluation. To this objective it is necessary to demonstrate a stronger association with diseases that perhaps the future development of genetic and molecular epidemiology will make possible.
Since there is a real lack of biomarkers for mycotoxins, a review of main methodological questions regarding biomarkers will be needed. This should focus on protocols finalized for the study of multiple panels of cytotoxicity and genotoxicity biomarkers. These should take into account the influence of environment toxicants at low doses, biomarkers associated to risk and genetic polymorphism. In this context it should be suggested to evaluate by genotyping and phenotyping the proportion of the population that bears similar haplotype since they are those who seem really to be at risk for diseases related to ochratoxin A according to life-style. The opportunity is given in northern African to follow up, in cohort study, ochratoxin A-contaminated populations and those who are already in the danger list.
References
- 1.Van der Merwe K. J., Steyn P. S., Fourie L., Scott D. B., Theron J. J. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature. 1965;205:1112–1113. doi: 10.1038/2051112a0. [DOI] [PubMed] [Google Scholar]
- 2.Creppy E. E. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol. Lett. 2002;127:19–28. doi: 10.1016/s0378-4274(01)00479-9. [DOI] [PubMed] [Google Scholar]
- 3.Krogh P. Role of ochratoxin in disease causation. Food Chem. Toxicol. 1992;30:213–224. doi: 10.1016/0278-6915(92)90036-k. [DOI] [PubMed] [Google Scholar]
- 4.Kuiper-Goodman T., Scott P. M. Risk assessment of the mycotoxin ochratoxin A. Biomed. Environ. Sci. 1989;2:179–248. [PubMed] [Google Scholar]
- 5.Abid S., Hassen W., Achour A., Skhiri H., Maaroufi K., Ellouz F., Creppy E., Bacha H. Ochratoxin A and human chronic nephropathy in Tunisia: is the situation endemic? Hum. Exp. Tox. 2003;22:77–84. doi: 10.1191/0960327103ht328oa. [DOI] [PubMed] [Google Scholar]
- 6.McGlynn K. A., Hunter K., LeVoyer T., Roush J., Wise P., Michielli R. A., Shen F. M., Evans A. A., London W. T., Buetow K. H. Susceptibility to aflatoxin B1-related primary hepatocellular carcinoma in mice and humans. Cancer Res. 2003;63:4594–4601. [PubMed] [Google Scholar]
- 7.Hassen W., Abid S., Achour A., Maaroufi K., Creppy E., Bacha H. Ochratoxin A and human nephropathy in Tunisia: a ten year survey. Ann. Toxicol. Anal. 2003;15:21–29. doi: 10.1191/0960327103ht328oa. [DOI] [PubMed] [Google Scholar]
- 8.Ueno Y. The toxicology of mycotoxins. Crit. Rev. Toxicol. 1985;14:99–132. doi: 10.3109/10408448509089851. [DOI] [PubMed] [Google Scholar]
- 9.Creppy E. E., Kane A., Dirheimer G., Lafarge-Frayssinet C., Mousset S., Frayssinet C. Genotoxicity of ochratoxin A in mice: DNA single-strand break evaluation in spleen, liver and Kidney. Toxicol. Lett. 1985;28:29–35. doi: 10.1016/0378-4274(85)90006-2. [DOI] [PubMed] [Google Scholar]
- 10.Tiemersma E. W., Omer R. E., Bunschoten A., van’t Veer P., Kok F. J., Idris M. O., Kadaru A. M., Fedail S. S., Kampman E. Role of genetic polymorphism of glutathione-S-transferase T1 and microsomal epoxide hydrolase in aflatoxin-associated hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2001;10:785–791. [PubMed] [Google Scholar]
- 11.Simarro-Doorten A. Y., Bull S., van der Doelen M. A. M., Fink-Gremmels J. Metabolism-mediated cytotoxicity of ochratoxin A. Toxicology in Vitro. 2004;18:271–277. doi: 10.1016/j.tiv.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Creppy E. E., Chiarappa P., Baudrimont I., Borraci P., Moukha S., Carratu M. R. Synergistic effects of fumonisin B1 and ochratoxin A: are in vitro cytotoxicity data predictive of in vivo acute toxicity? Toxicology. 2004;201:115–123. doi: 10.1016/j.tox.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 13.FAO/WHO Expert Committee on Food Additives. Evaluation of certain mycotoxins in food. Fifty-sixth report of the Joint World Health Organ. Tech. Rep. Ser. 2002;906:1–62. [PubMed] [Google Scholar]
- 14.Mobio T.A., Anane R., Baudrimont I., Carratu M.R., Shier T.W., Dano S.D., Ueno Y., Creppy E.E. Epigenetic properties of fumonisin B1: cell cycle arrest and DNA base modification in C6 glioma cells. Toxicol Appl Pharmacol. 2000;164:91–96. doi: 10.1006/taap.2000.8893. [DOI] [PubMed] [Google Scholar]
- 15.Horvath A., Upham B. L., Ganev V., Trosko J. E. Determination of the epigenetic effects of ochratoxin in a human kidney and a rat liver epithelial cell line. Toxicon. 2002;40:273–82. doi: 10.1016/s0041-0101(01)00219-7. [DOI] [PubMed] [Google Scholar]
- 16.Assaf H., Azouri H., Pallardy M. Ochratoxin A induces apoptosis in human lymphocytes through down regulation of Bcl-xL. Toxicol Sci. 2004;79:335–344. doi: 10.1093/toxsci/kfh123. [DOI] [PubMed] [Google Scholar]
- 17.Lühe A., Hildebrand H., Bach U., Dingermann T., Ahr H-J. A new approach to studying ochratoxin A(OTA)-induced nephrotoxicity: expression profiling in vivo and in vitro employing cDNA microarrays. Toxico. Sci. 2003;73:315–328. doi: 10.1093/toxsci/kfg073. [DOI] [PubMed] [Google Scholar]
- 18.Ouanes Z., Abid S., Ayed I., Anane R., Mobio T., Creppy E. E., Bacha H. Induction of micronuclei by zearalenone in Vero monkey kidney cells and in bone marrow cells of mice: protective effect of vitamin E. Mut Res. 2003;538:63–70. doi: 10.1016/s1383-5718(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 19.Stoloff L., Van Egmond H. P., Park D. L. Rationales for the establishment of limits and regulations for mycotoxins in mammals. Food Addit Contam. 1999;36:175–181. doi: 10.1080/02652039109373971. [DOI] [PubMed] [Google Scholar]
- 20.Pfohl-Leszkowicz A., Grosse Y., Castegnaro M., Nicolov I. G., Chernozemsky I. N., Bartsch H., Betbeder A. M., Creppy E. E., Dirheimer G. Ochratoxin A-related DNA adducts in urinary tract tumours of Bulgarian subjects. IARC Sci Publ. 1993;124:141–148. [PubMed] [Google Scholar]
- 21.Hassen W., Abid S., Achour A., Creppy E., Bacha H. Ochratoxin A and β2-microglobulinuria in healthy individuals and in chronic interstitial nephropathy patients in the centre of Tunisia: a hot spot of Ochratoxin A exposure. Toxicology. 2004;199:185–93. doi: 10.1016/j.tox.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 22.Baudrimont I., Betbeder A-M., Creppy E. E. Protective effect of aspartame on the karyomegaly induced by ochratoxin a in rat kidney. Toxicol Lett. 1996;88:21–22. doi: 10.1007/s002040100229. [DOI] [PubMed] [Google Scholar]
- 23.Mihatsch M. J., Gudat F., Zollinger H. U., Heierli C., Tholen H., Reutter F. W. Systemic karyomegaly associated with chronic interstitial nephritis. A new disease entity? Clin Nephrol. 1979;12:54–62. [PubMed] [Google Scholar]
- 24.Spoendlin M., Moch H., Brunner F., Brunner W., Burger H. R., Kiss D., Wegmann W., Dalquen P., Oberholzer M., Thiel G. Karyomegalic interstitial nephritis: further support for a distinct entity and evidence for a genetic defect. Am J Kidney Dis. 1995;25:242–252. doi: 10.1016/0272-6386(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 25.Godin M., Francois A., Le Roy F., Morin J. P., Creppy E. E., Hemet J., Fillastre J. P. Karyomegalic interstitial nephritis. Am J Kidney Dis. 1996;27(1):166. doi: 10.1016/s0272-6386(96)90047-5. [DOI] [PubMed] [Google Scholar]
- 26.Hanada K., Yewdell J. W., Yang J. C. Immune recognition of a human renal cancer antigen through post-translational protein splicing. Nature. 2004;427:252–256. doi: 10.1038/nature02240. [DOI] [PubMed] [Google Scholar]
- 27.Morozov V. N., Morozova T., Bray P., Hranisavljevic J., Vucelic D. Survey of small molecule and ion binding to beta 2-microglobulin--possible relation to BEN. Kidney Int Suppl. 1991;34:S85–88. [PubMed] [Google Scholar]
- 28.Andonova I. E., Sarueva R. B., Horvath A. D., Simeonov V. A., Dimitrov P. S., Petropoulos E. A., Ganev V. S. Balkan endemic nephropathy and genetic variants of glutathione S-transferases. J. Nephrol. 2004;17:390–398. [PubMed] [Google Scholar]
- 29.Gautier J. P., Holzhaeuser D., Markovic J., Germaud E., Schilter B., Ad Turesky R. J. Oxidative damage and stress response from ochratoxin A exposure in rats. Free Radical Biology and Medicine. 2001;30:1089–1098. doi: 10.1016/s0891-5849(01)00507-x. [DOI] [PubMed] [Google Scholar]