Abstract
Cannabinoid receptor 2 (CB2) is highly expressed in immune cells and stimulation decreases inflammatory responses. We tested the idea that selective CB2 activation in human monocytes suppresses their ability to engage the brain endothelium and migrate across the blood-brain barrier (BBB), preventing consequent injury. Intravital videomicroscopy was used to quantify adhesion of leukocytes to cortical vessels in lipopolysaccharide-induced neuroinflammation, after injection of ex vivo CB2–activated leukocytes into mice; CB2 agonists markedly decreased adhesion of ex vivo labeled cells in vivo. In an in vitro BBB model, CB2 activation in monocytes largely attenuated adhesion to and migration across monolayers of primary human brain microvascular endothelial cells and diminished BBB damage. CB2 stimulation in monocytes down-regulated active forms of integrins, lymphocyte function-associated antigen 1 (LFA-1), and very late antigen 4 (VLA-4). Cells treated with CB2 agonists exhibited increased phosphorylation levels of inhibitory sites of the actin-binding proteins cofilin and VASP, which are upstream regulators of conformational integrin changes. Up-regulated by relevant stimuli, Rac1 and RhoA were suppressed by CB2 agonists in monocytes. CB2 stimulation decreased formation of lamellipodia, which play a key role in monocyte migration. These results indicate that selective CB2 activation in leukocytes decreases key steps in monocyte–BBB engagement, thus suppressing inflammatory leukocyte responses and preventing neuroinflammation.
CME Accreditation Statement: This activity (“ASIP 2013 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2013 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Two subtypes of receptors mediate most cannabinoid actions: the CB1 receptor, located in the central nervous system and in peripheral tissues,1,2 and the CB2 receptor, found in the periphery and mainly in immune cells expressing high levels of CB2.3,4 The level of their expression is dependent on the activation state of the cell and the type of stimulus.5 Cannabinoids modulate immune functions and therefore have therapeutic potential for the treatment of inflammatory diseases.4,6,7 Recruitment of inflammatory cells and their adhesion and transendothelial migration are triggered by local production of cytokines, chemokines, and adhesion molecules.6 Our research group recently showed that CB2 activation reduces expression of adhesion molecules in brain endothelium, decreasing leukocyte engagement and recruitment.8 Cannabinoids can inhibit chemokine-induced chemotaxis of various immune cells.7,9,10 However, the mechanisms underlying the effects of cannabinoids in human monocytes, such as adhesion and migration, are largely unknown.
The monocyte-macrophage system exists in at least two distinct phenotypes of differentiation: proinflammatory (M1) and anti-inflammatory (M2).11 Extensive data suggest that microglial activity is not always adequate to support repair of the central nervous system and that recruitment of myeloid cells (M2) from the blood stream contributes to recovery after spinal cord injury.12 Microglia restricted amyloid-β plaques in a mouse model of Alzheimer’s disease13,14 and reversed paralysis in an experimental autoimmune encephalomyelitis model for multiple sclerosis.15 However, because the CNS is an immune privileged site, it is assumed to exclude excessive leukocyte trafficking,16,17 especially the proinflammatory (M1) type, which is prevalent during infection.18,19
Circulating leukocytes are recruited to sites of injury, infection, and inflammation by well-coordinated sequential steps (including tethering, rolling, arrest, firm adhesion, and migration across the endothelium20,21) mediated by selectin and integrin proteins.21 Many integrins are expressed in an inactive state.22 Integrin ligand binding is rapidly and transiently activated by cytoplasmic signals initiated by stimulation of diverse cell surface receptors, such as cytokines (inside-out signaling), or by ligand binding to integrin or integrin cross-linking (outside-in signaling).21,23 Development of antibodies that detect expression of active integrin conformation has led to better understanding of the interactions between leukocytes and the endothelium.24 Leukocyte integrins, like lymphocyte function-associated antigen-1 (LFA-1) and very late antigen 4 (VLA-4), recognize their respective endothelial adhesion molecules (ICAM-1 and VCAM-1) and stabilize adhesion under constant shear stress forces. Using HUTS21 and MEM-148 (which detect the active conformation of VLA-4 and LFA-1, respectively24–26) and relevant stimuli, we analyzed the effects of CB2 activation on the processes regulating integrin conformational changes during monocyte adhesion and migration.
In the present study, we tested CB2 agonists in a lipopolysaccharide (LPS)-stimulated encephalitis model to evaluate leukocyte–endothelial cell interactions in brain microvessels.8 Selective ex vivo CB2 activation in leukocytes resulted in a significant diminution in leukocyte–endothelial cell engagement. Conversely, there was a 50% increase in leukocyte adhesion when leukocytes from mice lacking the CB2 receptor (CB2ko) were transferred to wild-type (WT) CB2-expressing animals. Next, we showed that CB2 activation in monocytes significantly reduced adhesion and migration in a human in vitro blood-brain barrier (BBB) model, preserving barrier function. CB2 agonists reduced integrin activation and lamellipodia formation in primary monocytes via inhibition of small GTPases (RhoA and Rac1) and effects on cytoskeletal proteins. These results demonstrate anti-inflammatory effects of selective CB2 agonists in monocytes and suggest treatment opportunities for chronic inflammatory disorders within and outside of the central nervous system.
Materials and Methods
Reagents and Cells
Recombinant hTNF-α and hMCP1/CCL2 were from R&D Systems (Minneapolis, MN). Four selective CB2 agonists were used: JWH133 [(6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran], GP1a [(N-piperidin-1-yl)-1-(2,4-dichlorophenyl)-1,4-dihydro-6-methylindeno[1,2-c]pyrazole-3-carboxamide], AM1241 [(2-Iodo-5-nitrophenyl) (1-([1-methyl-2-piperidin]methyl)-1H-indol-3-yl)methanone], and O-1966 (1-[4-(1,1-dimethyl-heptyl)-2,6-dimethoxy-phenyl]-3-methyl-cyclohexanol). JWH133 and GP1a were from Tocris Bioscience (Bristol, UK), AM1241 from Cayman Chemical (Ann Arbor, MI), and O-1966 from Organix (Woburn, MA). Phorbol 12-myristate 13-acetate (PMA) and the selective CB2 antagonist SR144528 [5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)methyl]-N-[(1S,2S,4R)-1,3,3-trimethylbicyclo[2.2.1]hept-2-yl]-1H-pyrazole-3-carboxamide] were from Cayman Chemical. LPS from Escherichia coli 0111:B4 was from Sigma-Aldrich (St. Louis, MO).
Primary brain microvascular endothelial cells (BMVECs), isolated from vessels from brain resection tissue (showing no abnormalities) of patients undergoing surgery for treatment of intractable epilepsy, were supplied by Michael J. Bernas and Dr. Marlys H. Witte (University of Arizona, Tucson, AZ) and were maintained as described previously.27 Primary human monocytes, supplied by the Human Immunology Core facility of the University of Pennsylvania (Philadelphia, PA), were maintained as described previously27 and were used within 24 hours of isolation. The human myeloid leukemia cell line U937 (ATCC, Manassas, VA) was maintained as described previously.27
In all experiments, primary monocytes or U937 monocytic cells were pretreated with CB2 agonists (O-1966, JWH133, or GP1a at 10 μmol/L or AM1241 at 2 μmol/L) for 30 minutes, except as otherwise stated. CB2 agonists and antagonists were dissolved in ethanol and then diluted into growth medium. In all experiments, for nontreated (NT) cells only diluent was added, without any CB2 agonist or antagonist. Treatment with agonists did not cause any toxic effects on cell viability as determined by a LIVE/DEAD cell viability assay (Life Technologies, Carlsbad, CA) (data not shown).
Animals
Intravital videomicroscopy (IVM) was performed on 8-week-old male C57BL/6 or CB2ko mice (strain B6.129P2-Cnr2tm1Dgen/J) purchased from the Jackson Laboratory (Bar Harbor, MI). In vivo experiments were conducted in accordance with guidelines approved by the Temple University Institutional Animal Care and Use Committee.
Ex Vivo Treatment and Labeling of Leukocytes and IVM
IVM for in vivo leukocyte adhesion was performed on animals subjected to craniotomy and cranial window implantation as described previously.8
Leukocytes were isolated from donor mice using 1× RBC lysis buffer (eBioscience, San Diego, CA), according to the manufacturer’s instructions. For ex vivo treatment, 2 × 106 cells were treated with CB2 agonist, washed with PBS, stained with 1 μmol/L calcein-AM (Life Technologies), washed, and resuspended in PBS for injection. Before IVM, animals were treated with 6 mg/kg LPS for 4 hours, anesthetized, immobilized, and injected intraorbitally with 100 μL calcein-AM–labeled cells.
Leukocytes were visualized by fluorescent light [495 nm excitation for calcein-AM and 601 nm for rhodamine 6G (Sigma-Aldrich)] in cerebral vessels through the cranial window using a SteREO Discovery V20 epifluorescence microscope (Carl Zeiss Microscopy, Jena, Germany) equipped with an AxioCam MR digital camera and analyzed using AxioVision 4.8 imaging software. A 30-second video (time-series image set between 16 and 20 frames per second) was captured using a digital high-speed recorder. Adherent leukocytes were defined as the number of leukocytes firmly attached to the endothelium that did not change location during the observation period, scored as the number of cells per square millimeter of the vascular surface area, calculated from the diameter and length of the vessel segment under observation.
Monocyte Adhesion Assays
Freshly isolated human monocytes were treated with or without CB2 agonist. Treatments were removed from the monocytes before calcein-AM labeling; adhesion assays were performed as described previously.27 Fluorescence of adherent monocytes was measured using a Synergy 2 plate reader (BioTek Instruments, Winooski, VT) and expressed as mean fold adhesion, calculated as the number of adherent monocytes for each experimental condition divided by the basal adhesion of the untreated control.
Transendothelial Migration Assays
Transendothelial migration assays were performed as described previously,27 with monocytes treated and labeled similarly to the adhesion assay. The number of migrated monocytes was determined using ImageJ software version 1.43u (NIH, Bethesda, MD) and expressed as fold difference in migration from triplicate determinations, calculated as the number of migrated monocytes for each experimental condition divided by the number of migrated monocytes in the untreated, no-chemoattractant control.
TEER
Monocytes were treated with or without CB2 agonist. Cells were rinsed, and the medium was replaced before addition of the monocytes (1 × 105 cells per well) to the electrical cell-substrate impedance sensing (ECIS) array. Transendothelial electrical resistance (TEER) was measured using a 1600R ECIS system (Applied BioPhysics, Troy, NY) as described previously27 and was expressed as the mean percent change from baseline TEER from at least two independent experiments (four to six replicates each).
Conformational Changes of VLA-4 and LFA-1 Integrins
We used the VLA-4–specific ligand LDV (l-leucyl-l-aspartyl-l-valyl-l-prolyl-l-alanyl-l-alanyl-l-lysine; Tocris Bioscience) as a specific stimulator of active VLA-4 conformation. VLA-4–activated conformation, detected by HUTS21, was measured by fluorescence-activated cell sorting as described previously.24,27
Primary monocytes (0.5 × 106/mL) in RPMI 1640–1% fetal bovine serum were treated with or without CB2 agonists and then stimulated with 100 ng/mL PMA for 1 hour. Cells were placed on ice and fixed with 4% formaldehyde. LFA-1 conformation, detected by MEM-148 antibody, was measured as described previously.25 Data were acquired with a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA) and analyzed with FlowJo software version 8.7 (Tree Star, Ashland, OR).
RhoA and Rac1 GTPase Activity Assay
RhoA and Rac1 GTPase activity was measured in cell lysates prepared from primary monocytes or U937 cells (untreated or pretreated with CB2 agonists) by stimulation with 100 ng/mL PMA or 100 ng/mL CCL2, known activators of GTPases28 or with 12 nmol/L LDV peptide. For a positive control, cells were stimulated with 1 μg/mL CN04 (Cytoskeleton, Denver, CO). To inhibit RhoA or Rac1 GTPase activity, cells were pretreated with specific inhibitors [1 μg/mL CT04 (Cytoskeleton) or 75 μmol/L NSC23766 (EMD Millipore, Billerica, MA), respectively]. To measure RhoA and Rac1 GTPase activity, G-LISA RhoA and G-LISA Rac1 activation assay kits (Cytoskeleton) were used according to the manufacturer’s instructions.
Western Blotting
For signaling studies, 5 × 106 U937 cells were suspended in 1 mL of RPMI 1640–1% fetal bovine serum, treated with or without CB2 agonist and stimulated with 12 nmol/L LDV, 100 ng/mL CCL2, or 100 ng/mL PMA for 1 hour at 37°C. Then cells were placed on ice and lysed in radioimmunoprecipitation assay buffer. Isolated proteins were separated on SDS-PAGE as described previously.27 Anti–p-cofilin and anti–p-VASP (Cell Signaling Technology, Danvers, MS) and anti–β-tubulin protein loading control (Santa Cruz Biotechnology, Santa Cruz, CA) were used to detect target proteins. Bound primary antibodies were exposed to fluorescent IRDye 680 or IRDye 800 infrared secondary antibodies (LI-COR Biosciences, Lincoln, NE) and were detected using the LI-COR Odyssey imaging system.
Visualization of Lamellipodia
For immunofluorescence studies, cells were treated and stimulated as described above for signaling experiments and then were fixed, permeabilized, and stained as described previously.27 Images of twenty cells per treatment were taken at ×63 magnification (1024 × 1024 pixel area) using a Leica SP5 confocal laser scanning microscope (Leica Microsystems, Wetzlar, Germany) and processed with Adobe Photoshop CS3 software (Adobe Systems, San Jose, CA). The area (in pixels) of each lamellipodium per cell was measured using ImageJ software (NIH), and the average lamellipodium area per cell in each captured image was used for statistical analysis.
Statistical Analysis
Multiple group comparisons were performed by one-way analysis of variance with Dunnett’s post hoc tests (adhesion, migration, fluorescence-activated cell sorting, and G-LISA assay). Statistical analyses were performed using GraphPad Prism software version 5 (GraphPad Software, La Jolla, CA). Differences were considered significant at P < 0.05. Data are expressed as means ± SEM from experiments performed multiple times.
Results
CB2 Activation Decreases Leukocyte Adhesion in Surface Cortical Vessels
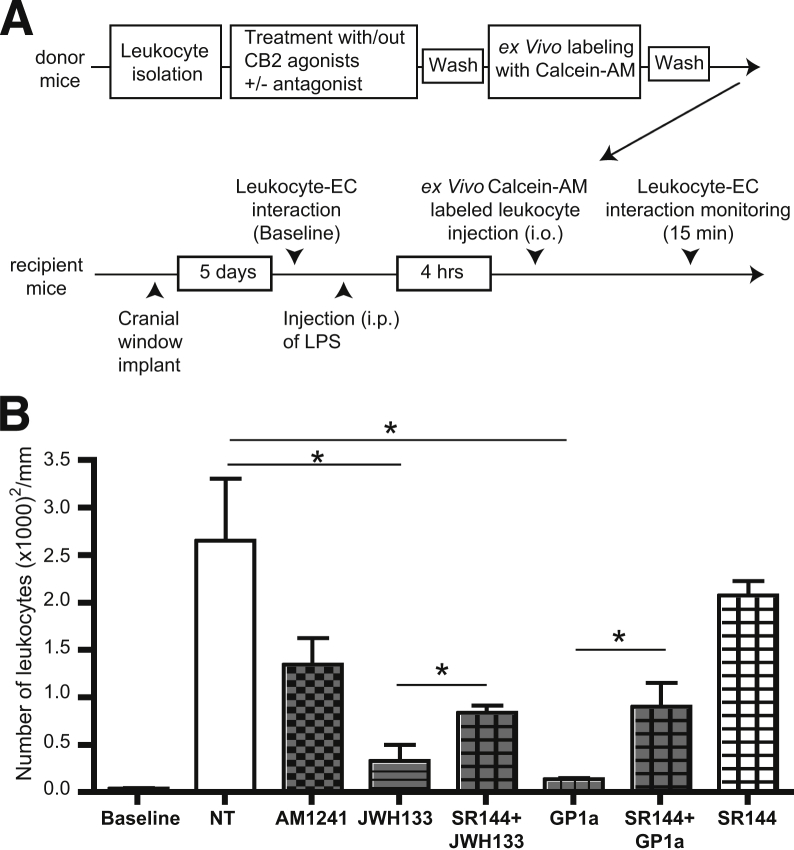
Previous studies have demonstrated increased expression of CB2 in human brain tissues affected by amyotrophic lateral sclerosis, Alzheimer’s disease, and multiple sclerosis.7,29 CB2 is highly expressed on leukocytes, and expression is up-regulated by proinflammatory stimuli.30 CB2 activation reduced inflammation in preclinical disease models.7,29,31–35 We hypothesized that CB2 on leukocytes could serve as a target for the anti-inflammatory effects of CB2 agonists via decreasing leukocyte engagement of endothelium and BBB injury. To investigate this idea, we tested whether CB2 activation reduces leukocyte adhesion in vivo in an animal model of systemic inflammatory response. We used IVM for surveillance of cerebral vascular changes and leukocyte–endothelial cell interactions.8 To evaluate the effects of CB2 activation on leukocytes only (firm leukocyte adhesion), leukocytes isolated from WT mice were treated ex vivo with selective CB2 agonists, stained with calcein-AM, washed, and injected into LPS-treated mice (Figure 1A). Compared with control mice, animals treated with LPS showed a 20-fold increase in firm adhesion of ex vivo labeled cells. The CB2 agonists GP1a, JWH133, and AM1241 decreased leukocyte adhesion by 96%, 91%, and 50%, respectively (Figure 1B and Supplemental Figure S1). Autologous leukocytes (rhodamine-labeled) showed a uniform 20-fold increase in firm adhesion (Supplemental Figure S2), which is indicative of the expected inflammatory response. The ratio of injected versus autologous leukocytes adherent to brain microvessels in LPS-treated mice ranged from 1:8 to 1:10. Pretreatment of leukocytes with the CB2 antagonist and the inverse agonist SR144528 partially reversed the effects of the selective CB2 agonists (Figure 1B). These results indicate major anti-inflammatory effects of selective CB2 activation in leukocytes in vivo.
Figure 1.
Selective CB2 activation in leukocytes reduced their adhesion in LPS-induced encephalitis. A: Experimental design. B: Quantitation of leukocyte firm adhesion (not rolling) during 30 seconds of observation (ex vivo treated, calcein-AM–labeled leukocytes). Leukocytes were treated ex vivo with or without CB2 agonist (2 μmol/L AM1241 or 10 μmol/L JWH133 or GP1a) and with or without a CB2 antagonist [10 μmol/L SR144528 (SR144)]. Representative images from a video of leukocytes labeled with calcein-AM are shown in Supplemental Figure S1. Data are expressed as means ± SEM. n = 4 to 6 animals per treatment. ∗P < 0.05. EC, endothelial cell; i.o., intraorbital.
Next, to confirm involvement of the CB2 receptor, we performed adoptive transfer of WT and CB2ko leukocytes to WT and CB2ko recipient mice. Leukocytes were isolated from WT or CB2ko donor mice, labeled with calcein-AM, washed, and injected into recipient mice (Figure 2A). CB2ko leukocytes transferred into WT recipient mice exhibited an approximately 100% increase in adhesion, compared with WT, CB2-expressing leukocytes; in similar experiments with CB2ko recipients, there was a 40% increase in adhesion of CB2ko leukocytes, compared with WT leukocytes (Figure 2B and Supplemental Figure S3). WT leukocytes exhibited increased adhesion in CB2ko animals versus WT animals treated with LPS. Leukocyte adhesion observed in the same animals injected with autologous leukocytes paralleled results in adoptive transfers, with a 40% increase in adhesion in CB2ko animals, compared with WT animals (Supplemental Figure S4).
Figure 2.
Absence of CB2 increases endothelial–leukocyte adhesion in LPS-associated encephalitis. A: Experimental design. B: Quantitation of leukocytes (calcein-AM–labeled) under firm adhesion (not rolling) during 30 seconds of observation. Representative images from a video of leukocytes labeled with calcein-AM are shown in Supplemental Figure S3. Data are expressed as means ± SEM. n = 4 animals per group. ∗P < 0.05.
CB2 Stimulation in Monocytes Diminishes Their Adhesion to Brain Endothelium, Diminishes Transendothelial Migration, and Attenuates BBB Injury
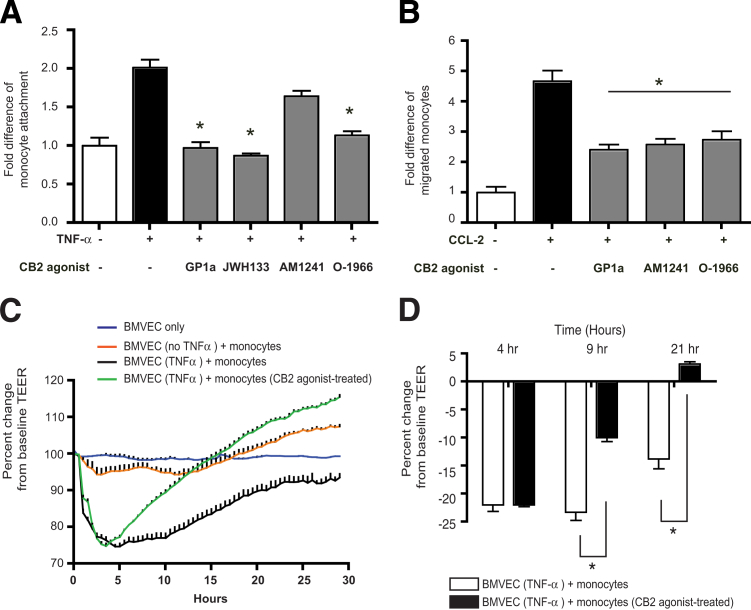
Next, we determined whether CB2 stimulation decreases monocyte adhesion to brain endothelium. TNF-α induced a twofold increase in the number of monocytes attaching to BMVECs, compared with baseline, and the addition of CB2 agonist pretreatment reduced the adhesion of monocytes to BMVECs by approximately 100% (GP1a, JWH133, and AM1241), compared with TNF-α alone (untreated control) (Figure 3A). Using an in vitro BBB model, we tested whether CB2 activation in primary human monocytes prevents their passage across BMVEC monolayers toward CCL2. Application of CCL2 to the lower chamber of BBB constructs increased monocyte migration 4.9-fold. CB2 agonist pretreatment of monocytes then attenuated this increased monocyte migration across endothelial monolayers by approximately 60% (Figure 3B).
Figure 3.
CB2 activation reduces monocyte adhesion to and migration across BMVEC monolayers and improves barrier function. Calcein-AM–labeled primary human monocytes pretreated with CB2 agonists (10 μmol/L O-1966, JWH133 or GP1a or 2 μmol/L AM1241) were washed with PBS and added to BMVECs. A: Fold difference of adherent cells calculated based on a standard curve derived from fluorescence intensity of known numbers of labeled cells. B: Fold difference in transmigrated monocytes. C: TEER was measured in TNF-α–pretreated BMVECs during engagement by monocytes. Results are from a representative experiment with the selective CB2 agonist GP1a (10 μmol/L). D: Percent change in TEER of BMVECs at 4, 9, and 21 hours after addition of monocytes with or without pretreatment with CB2 agonist. Data are expressed as means ± SEM from at least three independent experiments. ∗P < 0.05 versus untreated control (at least three replicates).
Brain endothelial engagement by leukocytes and subsequent migration across the BBB leads to barrier damage. We hypothesized that because CB2 activation in monocytes reduced their ability to attach and migrate through BMVEC monolayers (Figure 3, A and B), it would also prevent monocyte-mediated disruption of the BBB. Integrity of the barrier was evaluated by measuring TEER (Figure 3C). Monocyte addition to BMVECs resulted in a 22% decrease in TEER during the first 4 hours (Figure 3D). CB2 agonist–treated monocytes produced a 10% decrease at 9 hours but then an increase to above baseline by 21 hours, whereas the TEER of BMVEC monolayers with untreated monocytes remained reduced by approximately 14% even at 21 hours (Figure 3D).
Integrin Activation Is Affected in CB2-Activated Monocytes
Monocyte adhesion to activated endothelium is mediated by integrins, such as VLA-4 and LFA-1, whose active conformation is stimulated by inside–out or outside–in activation. Inside–out activation of LFA-1 is induced by chemokine activation of G-protein-coupled receptors and can be mediated by PMA.25 MEM-148 antibody bound weakly on resting monocytes (Figure 4A), but was up-regulated on monocytes activated by PMA (twofold). CB2 activation in PMA-stimulated monocytes decreased expression of activated LFA-1 by 50% to 60% (Figure 4B). Because RhoA and Rac1 control leukocyte cytoskeleton and adhesion and migration, we tested their specific inhibitors in parallel with CB2. Application of the Rac1 inhibitor NSC23766 diminished the amount of activated LFA-1 integrin by 30%, whereas the RhoA inhibitor CT04 did not produce a significant effect. Surprisingly, activation of CB2 with AM1241 or inhibition of both RhoA and Rac1 in monocytes up-regulated LFA-1 activation by 40% to 50%.
Figure 4.
CB2 activation prevents conformational activation of lymphocyte function-associated antigen-1 (LFA-1, integrin β2) in monocytes. Monocytes were treated with GP1a or AM1241 (CB2 agonists) and CT04 or NSC23766 (RhoA and Rac1 GTPase inhibitors, respectively), and the active forms of LFA-1 were detected using the conformation-specific antibody MEM-148. Expression of total LFA-1 (CD18) was not affected by CB2 agonists. A: Stimulation of primary monocytes with PMA changes integrin conformation from a closed (not active) to an open (active) form. Total amount of integrin β2 (CD18) is shown on the x axis. B: Quantitation of integrin β2 conformational activation as percentage of cells with the activated form of the integrin (right upper quadrant in panel A). Data are expressed as means ± SEM from three independent experiments. ∗P < 0.05 versus untreated control.
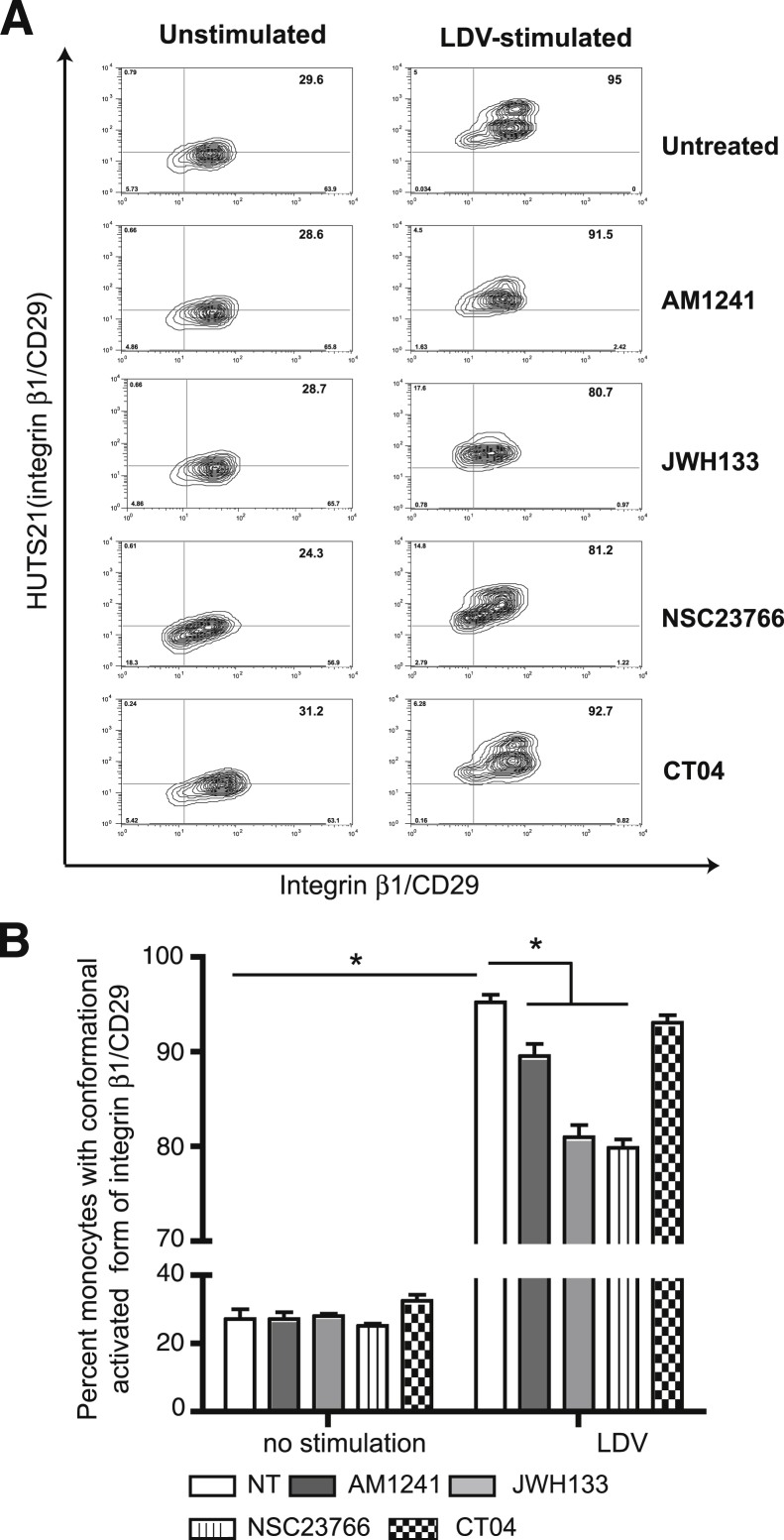
Exposure of monocytes to LDV, which is known to alter VLA-4 (CD29) conformation,24 increased active integrin β1 expression threefold (Figure 5). CB2 agonists decreased active integrin β1 expression by 10% to 17% (Figure 5). Rac1 inhibitor–treated monocytes reduced integrin activation, whereas RhoA inhibition did not produce a significant effect on outside–in activation of VLA-4.
Figure 5.
CB2 activation prevents conformational activation of very late antigen 4 (VLA-4, integrin β1) in monocytes. Monocytes were treated with AM1241 or JWH133 (CB2 agonists) and CT04 or NSC23766 (RhoA and Rac1 GTPase inhibitors, respectively), and the active forms of VLA-4 were detected using the conformation-specific antibody, HUTS21. Expression of total VLA-4 (CD29) was not affected by CB2 agonists. A: Stimulation of primary monocytes with LDV changes integrin conformation from a closed (not active) to an open (active) form. Total amount of integrin β1 (CD29) is shown on the x axis. B: Quantitation of integrin β1 conformational activation as percentage of cells with the activated form of the integrin (right upper quadrant in panel A). Data are expressed as means ± SEM from three independent experiments. ∗P < 0.05 versus untreated control.
Rac1 and RhoA GTPase Activation Is Reduced in CB2-Activated Monocytes
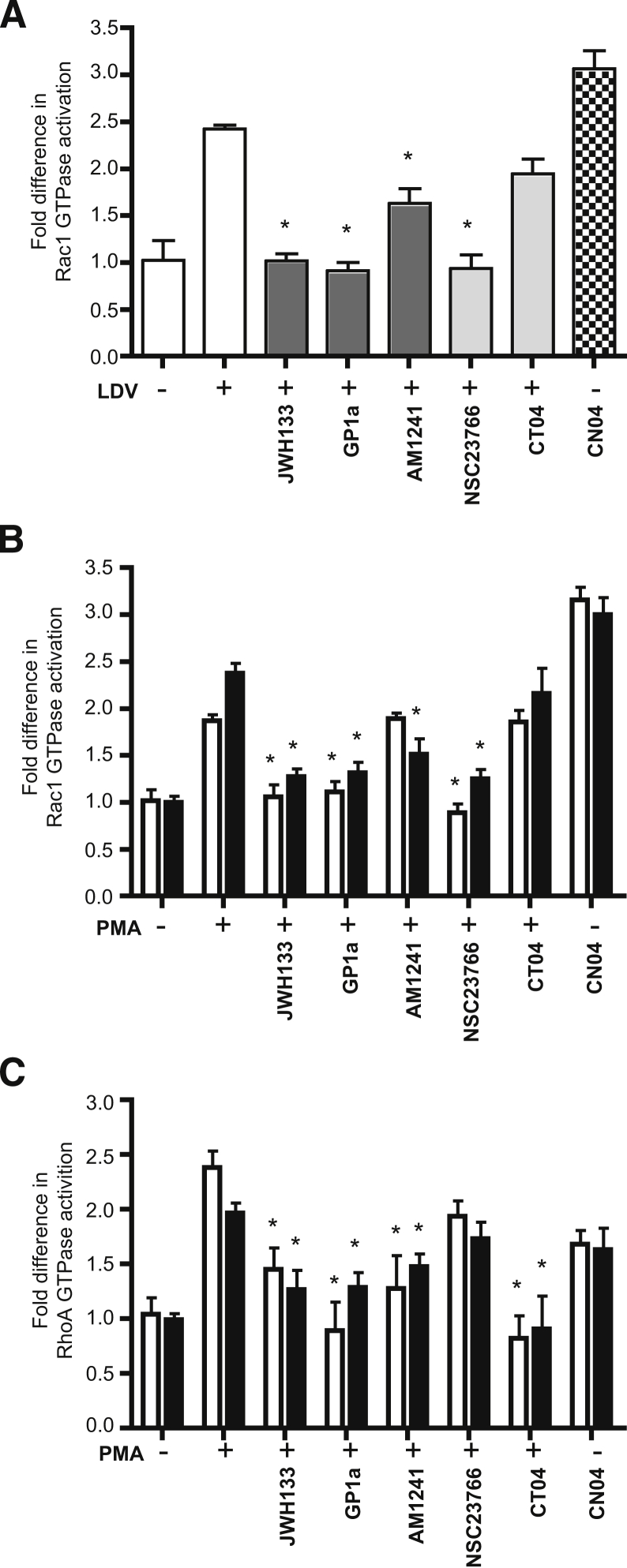
Because RhoA and Rac1 play critical roles in monocyte migration, we explored the possibility that CB2 agonists would suppress their activity in monocytes activated with relevant stimuli. Monocyte activation by LDV peptide increased Rac1 activity by 2.4-fold; three CB2 agonists significantly decreased Rac1 activity (JWH133 and GP1a by 100%, and AM1241 by 50%, respectively) (Figure 6A). No RhoA activation was observed with LDV stimulation (data not shown). Next, we examined whether CB2 agonists would decrease Rac1 and RhoA activation in primary monocytes and U937 cells stimulated by PMA. A significant increase in GTPase activation was detected in monocytes stimulated with PMA (Figure 6, B and C) and a substantial reduction was observed in the presence of CB2 agonists. PMA stimulation of U937 cells and monocytes activated RhoA and Rac1 2.0- to 2.5-fold (Figure 6, B and C); CB2 stimulation diminished their activation in a manner similar to that of primary monocytes. Rac1 or RhoA inhibitor treatment resulted in total inhibition of the respective GTPase. These results suggest that CB2 stimulation inhibits RhoA and Rac1.
Figure 6.
CB2 stimulation suppresses activation of Rac1 and RhoA small GTPases in primary monocytes. Cells were serum-starved for 24 hours (RPMI 1640–1% fetal bovine serum medium supplemented with penicillin–streptomycin) and then were pretreated with CB2 agonist (10 μmol/L JWH133 or GP1a or 2 μmol/L AM1241) before stimulation. A: Rac1 activity under LDV stimulation was measured in primary monocytes. B and C: Rac1 and RhoA activity under PMA stimulation were measured in primary monocytes (white bars) and U937 cells (black bars). The level of RhoA or Rac1 activity in nonstimulated, nontreated cells was assigned a value of 1 to calculate the relative ratio of activation. Data are expressed as means ± SEM from three independent experiments. ∗P < 0.05 versus untreated control.
CB2 Stimulation Prevents Dephosphorylation of Inhibitory Sites of VASP and Cofilin
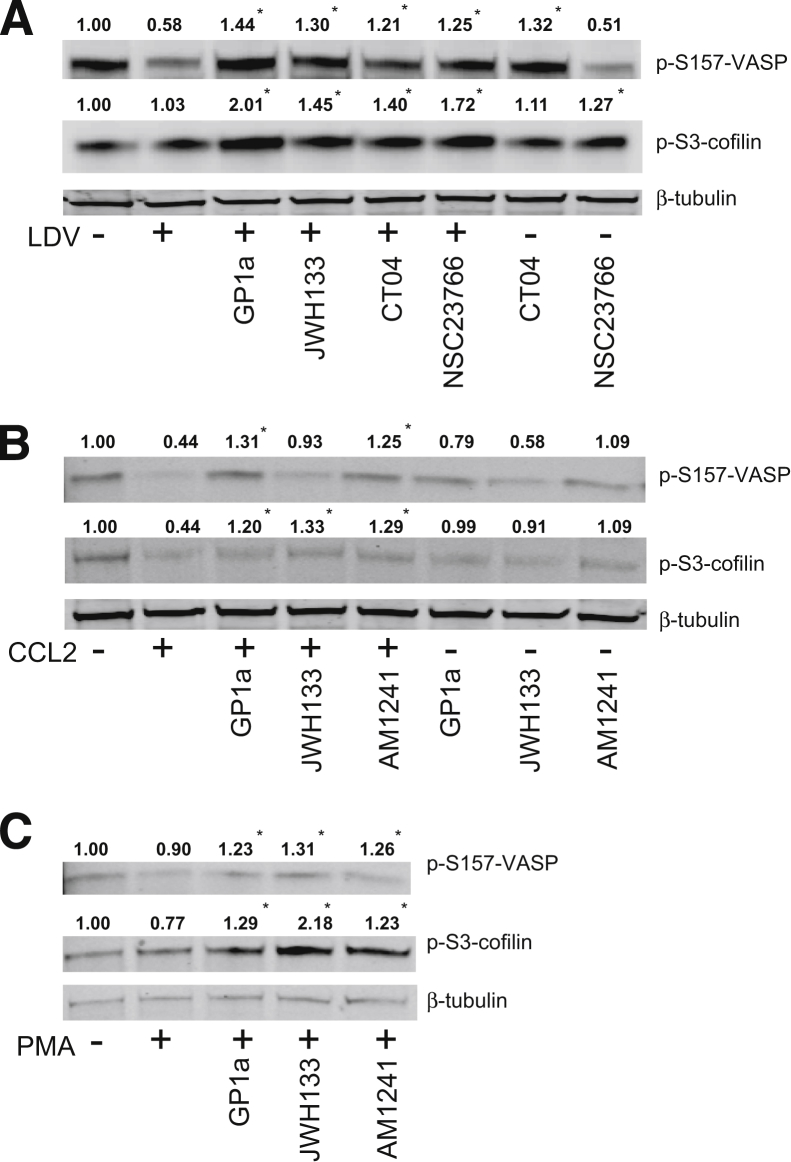
To control and coordinate cell movement and actin filament rearrangements, efficient disassembly of pre-existing actin filaments is necessary. We have previously demonstrated that monocytic cells with down-regulation of Rac1 increase phosphorylation of the inhibitory sites of the actin-binding proteins cofilin and vasodilator-stimulated phosphoprotein (VASP), which regulate conformational changes of integrins.27 Because CB2 agonists decreased RhoA and Rac1 activation, we evaluated changes in the phosphorylated inhibitory sites of cofilin and VASP after stimulation with LDV, PMA, or CCL2 in the presence of CB2 agonists (Figure 7). Because U937 cells exhibit a response in GTPase activation similar to that of primary monocytes, we used them for Western blotting. LDV stimulation reduced inhibitory VASP phosphorylation by 42%, which was increased by 80% to 90% by CB2 agonists. Similar effects on p-VASP were observed by inhibition of RhoA or Rac1 in LDV-stimulated monocytes (Figure 7A). PMA stimulation decreased p-cofilin by 23%, which was up-regulated by 50 ± 4% after treatment with the CB2 agonists GP1a and AM1241 and by 200% after JWH133 treatment (Figure 7C). To mimic the signaling events that occur in the cell during migration, we stimulated monocytes with the monocyte chemoattractant protein CCL2 (Figure 7B), which produced p-cofilin changes similar to that of monocytes stimulated with LDV or PMA during CB2 activation.
Figure 7.
CB2 activation increases levels of p-S3-cofilin and p-S157-VASP. U937 cells were treated with CB2 agonist (10 μmol/L JWH133 or GP1a or 2 μmol/L AM1241) before stimulation with LDV (A), CCL2, (B) or PMA (C). Densitometry of phosphorylated bands was normalized to β-tubulin. The level of phosphorylation in non–cross-linked cells was assigned a value of 1 to calculate the relative ratio. Data are expressed as means ± SEM from three independent experiments. ∗P < 0.05 versus untreated control.
Next, we tested whether LDV, CCL2, or PMA stimulation results in a decrease in p-VASP (Figure 7). All three stimuli decreased p-VASP, and treatment with CB2 agonists increased phosphorylation of S157-VASP by 30% to 50% in LDV-, CCL2-, or PMA-stimulated monocytes (Figure 7). Application of a RhoA-specific inhibitor had similar effects on S157-VASP phosphorylation in LDV-stimulated or nonstimulated cells, whereas the addition of a Rac1 inhibitor affected phosphorylation levels of S157-VASP only in LDV-stimulated monocytes (Figure 7A). Taken together, these results suggest that CB2 activation affects the actin-binding proteins VASP and cofilin via RhoA and Rac1 inhibition.
Lamellipodia Formation Is Diminished in CB2-Activated Monocytes
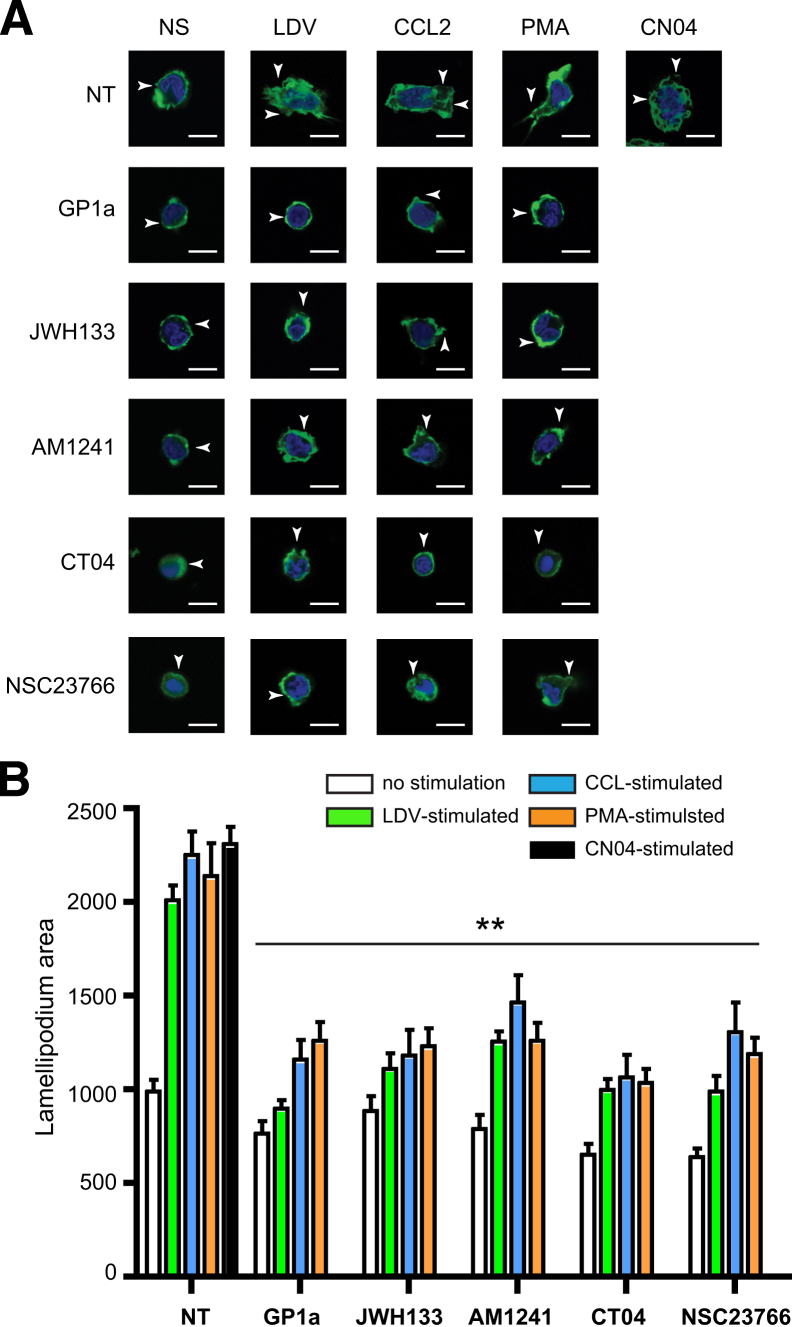
Formation of lamellipodia is necessary for leukocyte migration associated with recruitment of active Rac1 or RhoA into the leading or lagging edge of the cell, respectively.36,37 To evaluate the possibility that CB2 activation affects lamellipodia formation, monocytes were visualized and lamellipodia area was measured (Figure 8). LDV and CN04 stimulation resulted in a 2.0- to 2.3-fold increase in lamellipodia area, and CCL2 or PMA stimulation led to a respective 125 ± 6% or 110 ± 10% increase in lamellipodia formation. After treatment of monocytes with CB2 agonist, lamellipodia area was reduced by 70% to 100% (Figure 8B). After inhibition of either Rac1 or RhoA, lamellipodia area was similar to that of resting monocytes. These data indicate that CB2 activation diminishes lamellipodia formation in monocytes via inhibition of small GTPases.
Figure 8.
Effects of CB2 activation on lamellipodia formation. A: Images of primary monocytes stained with Acti-stain 488 fluorescent phalloidin (Cytoskeleton). Protruding lamellipodia are indicated by arrowheads. B: Average lamellipodium area (pixels) per cell (20 cells per treatment) was calculated. Primary monocytes were left untreated or were treated with CB2 agonist (10 μmol/L JWH133 or GP1a or 2 μmol/L AM1241), CT04 RhoA inhibitor (1 μg/mL), or NSC23766 Rac1 inhibitor (75 μmol/L), with or without stimulation. CN04 GTPase activator (1 μg/mL) was used as a positive control. Data are expressed as means ± SEM from two independent experiments. ∗∗P < 0.01 versus untreated control. Scale bar = 5 μm.
Discussion
Leukocyte–endothelium interaction is at the heart of many inflammatory responses. Infiltration of monocytes across the BBB plays an important role in various neuroinflammatory conditions, including multiple sclerosis, ischemia–reperfusion injury, and experimental autoimmune encephalomyelitis. Application of CB2 agonists has been shown to reduce leukocyte adhesion.31–33,38,39 However, these studies did not address how much of the effects of CB2 activation apply to immune cells or endothelium (or both), nor did they address the underlying mechanisms. In the present study, we used IVM to test the effects of CB2 agonists on leukocyte adhesion to vascular walls in an LPS-induced encephalitis model, in which leukocytes isolated from mice were treated with CB2, labeled with calcein-AM ex vivo, and injected into LPS-treated animals. CB2 activation in leukocytes significantly inhibited leukocyte adhesion to endothelium in cortical vessels (Figure 1), whereas autologous cells did not show any change in adhesion (Supplemental Figure S2). CB2ko animals showed higher levels of inflammation, compared with WT mice (Supplemental Figure S4). Adoptive transfer of CB2ko leukocytes resulted in an increased number of adherent leukocytes in both WT and CB2ko mice (Figure 2).
Enhanced adhesion of monocytes to TNF-α–stimulated BMVECs and their migration across BMVEC monolayers were attenuated in CB2 agonist-treated monocytes. Inability of CB2-activated monocytes to attach and migrate through the endothelial layer resulted in attenuation of BBB disruption, as demonstrated by TEER analysis (Figure 3). Cannabinoids are able to inhibit chemokine-induced chemotaxis of a variety of cell types, including neutrophils, lymphocytes, macrophages, monocytes, and microglia.7 However, the mechanism or mechanisms leading to such inhibition have not been identified.
Small GTPases (Rho, Rac, and Cdc42) play an important role in transendothelial leukocyte migration, oxidative stress, and inflammation by linking surface receptors and the actin cytoskeleton.40,41 In leukocytes, Rac1 and Cdc42 regulate cell polarity, lamellipodia formation, and direct migration, whereas RhoA controls leukocyte tail retraction during transmigration.42 We therefore measured RhoA and Rac1 activation after stimulation with LDV peptide or PMA in monocytes and in U937 cells, and observed a decrease in active GTPases after CB2 stimulation. The increase in activation of RhoA and Rac1 during migration was attenuated by CB2 agonists.43
Because we observed diminished adhesion of monocytes treated with CB2 agonists, we explored the idea that CB2 stimulation results in conformational changes of active integrin and/or total integrin expression. LDV stimulation led to a threefold increase in conformationally active VLA-4 in primary monocytes. CB2 activation and Rac1 inhibition resulted in diminution of active integrin β1 (Figure 5, A and B), but expression of total VLA-4 was not changed after treatment, indicating the importance of VLA-4 conformational status. Memory T cells constitutively expressing activation/ligand-induced epitopes on β1 integrins recognized by HUTS21 exhibit significantly higher rates of attachment and accumulation on VCAM-1 expressing cells, compared with other T-cell subsets without active epitope expression.44 The unbending of integrin leading to conformational activation presumably exposes the VCAM-1 binding site,45 which facilitates both tethering and rolling.46 Chigaev et al24 reported that the rate of HUTS21 binding is related to VLA-4 activation state. We have previously reported that, on treatment with specific GSK3β or Rac-1 inhibitors, expression of active VLA-4 is decreased by 40% to 60% in LDV-stimulated monocytes.27 Ferreira et al47 reported a link between conformational VLA-4 activation and Rac1, which was correlated with decreased THP-1 monocytic cell migration.
Inside–out activation of LFA-1 was induced by PMA in primary monocytes; Rac1 inhibition and CB2 activation decreased LFA-1 conformational change by 30% to 50%, whereas RhoA inhibition had no effect (Figure 4A). Pretreatment of monocytes with both CB2 agonists and RhoA or Rac1 inhibitors resulted in unexpected LFA-1 activation. Type I leukocyte adhesion deficiency is caused by mutations in the β2-subunit of LFA-1, preventing LFA-1 surface expression,48 which is triggered by Rho signaling.49 Semmrich et al22 reported that expression of constitutively active LFA-1 in mice resulted in impaired migration of lymphocytes.
Whether a cell is circulating within the blood or migrating through tissues, the leukocyte cytoskeleton undergoes significant changes. Actin-binding proteins, such as cofilin and VASP, regulate rearrangements for these different cell states.50 VASP is involved in filopodia formation, and p-S157-VASP level is associated with impaired binding to focal adhesion proteins, such as vinculin, paxillin, and talin.51 During inside–out activation of integrins, dephosphorylation of VASP allows its binding to talin, which in turn allows integrin activation.52 Our present findings demonstrate that CB2 activation in monocytes results in increased p-S157-VASP levels. Several studies have demonstrated that the inability to dephosphorylate S157-VASP inhibits neutrophil and monocyte migration, as well as associated integrin regulation.27,53 Cofilin causes disassembly of actin filaments to monomers, so that they can be recycled. Cofilin activity is inhibited by the phosphorylation of S3; it is known that cofilin phosphorylation drastically changes in response to extracellular stimuli and during cell migration and morphogenesis.54 We therefore tested the effects of CB2 activation on p-S3-cofilin levels in U937 cells and found increased levels of p-S3-cofilin in CB2 agonist-treated cells (Figure 7). Specific RhoA and Rac1 inhibitors showed similar results.
Actin cytoskeletal reorganization plays a central role in cell migration. During migration, cells display F-actin–rich lamellipodia formation toward the direction of movement. Cofilin plays an essential role in lamellipodia formation by stimulating actin filament disassembly near the pointed ends, thereby supplying actin monomers for polymerization.55 Stimulation of Rho GTPases by CCL2, PMA, or LDV resulted in increased lamellipodia formation in monocytes, whereas CB2 activation in monocytes led to significant inhibition in lamellipodia formation. Application of specific RhoA and Rac1 inhibitors yielded similar results, further indicating their involvement in this process.
Taken together, our observations suggest that selective CB2 activation in leukocytes affects key steps in leukocyte engagement of brain endothelium (active integrin expression, lamellipodia formation, adhesion and migration via GTPase inhibition, and cytoskeleton regulation) and could thus be an attractive target for therapeutic interference in neuroinflammatory disorders.
Footnotes
Supported in part by NIH grants R01 AA015913 and R01 MH65151 (Y.P.) and by the Intramural Research Program of the NIH-NIAAA (P.P.).
See related Commentary on page 1375.
Contributor Information
Slava Rom, Email: srom@temple.edu.
Yuri Persidsky, Email: yuri.persidsky@tuhs.temple.edu.
Supplemental Data
Ex vivo CB2 activation of leukocytes does not affect adhesion of autologous leukocyte adhesion in LPS-induced encephalitis. Representative images from a 30-second video of leukocytes labeled with calcein-AM. n = 4 to 6 mice per group. Scale bar = 100 μm.
Ex vivo CB2 activation of leukocytes does not affect autologous leukocyte adhesion in LPS-induced encephalitis. A: Representative images from a 30-second video of leukocytes labeled with rhodamine-6G. B: Quantitation of leukocyte firm adhesion (rhodamine 6G–labeled autologous leukocytes) during 30 seconds of observation. Data are expressed as means ± SEM. n = 4 to 6 mice per group. Scale bar = 100 μm. NS, nonsignificant.
Absence of CB2 increases adhesion of autologous leukocytes in LPS-associated encephalitis. At 5 days after cranial window implantation, C57BL/6 mice were injected with LPS and 4 hours later with ex vivo calcein-AM–labeled leukocytes. Representative images from 30-second videos of leukocytes labeled with calcein-AM and visualized by IVM for adhesion in pial venules 30 minutes after injection. n = 4 mice per group.
Absence of CB2 increases adhesion of autologous leukocytes in LPS-associated encephalitis. Five days after cranial window implantation, C57BL/6 mice were injected with LPS and 4 hours later with ex vivo calcein-AM–labeled leukocytes. A: Representative images from 30-second videos of leukocytes labeled with rhodamine-6G (autologous cells) and visualized by IVM for adhesion in pial venules 30 minutes after injection. B: Quantitation of leukocyte firm adhesion (rhodamine 6G–labeled autologous cells) during 30 seconds of observation. Data are expressed as means ± SEM. n = 4 mice per group. ∗P < 0.05.
References
- 1.Anday J.K., Mercier R.W. Gene ancestry of the cannabinoid receptor family. Pharmacol Res. 2005;52:463–466. doi: 10.1016/j.phrs.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Marsicano G., Goodenough S., Monory K., Hermann H., Eder M., Cannich A., Azad S.C., Cascio M.G., Gutiérrez S.O., van der Stelt M., López-Rodriguez M.L., Casanova E., Schütz G., Zieglgänsberger W., Di Marzo V., Behl C., Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 3.Galiègue S., Mary S., Marchand J., Dussossoy D., Carrière D., Carayon P., Bouaboula M., Shire D., Le Fur G., Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 4.Rom S., Persidsky Y. Cannabinoid receptor 2: potential role in immunomodulation and neuroinflammation. J Neuroimmune Pharmacol. 2013;8:608–620. doi: 10.1007/s11481-013-9445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S.F., Newton C., Widen R., Friedman H., Klein T.W. Differential expression of cannabinoid CB(2) receptor mRNA in mouse immune cell subpopulations and following B cell stimulation. Eur J Pharmacol. 2001;423:235–241. doi: 10.1016/s0014-2999(01)01122-0. [DOI] [PubMed] [Google Scholar]
- 6.Montecucco F., Burger F., Mach F., Steffens S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am J Physiol Heart Circ Physiol. 2008;294:H1145–H1155. doi: 10.1152/ajpheart.01328.2007. [DOI] [PubMed] [Google Scholar]
- 7.Pacher P., Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res. 2011;50:193–211. doi: 10.1016/j.plipres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramirez S.H., Hasko J., Skuba A., Fan S., Dykstra H., McCormick R., Reichenbach N., Krizbai I., Mahadevan A., Zhang M., Tuma R., Son Y.J., Persidsky Y. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J Neurosci. 2012;32:4004–4016. doi: 10.1523/JNEUROSCI.4628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller A.M., Stella N. CB2 receptor-mediated migration of immune cells: it can go either way. Br J Pharmacol. 2008;153:299–308. doi: 10.1038/sj.bjp.0707523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacher P., Gao B. Endocannabinoids and liver disease. III. Endocannabinoid effects on immune cells: implications for inflammatory liver diseases. Am J Physiol Gastrointest Liver Physiol. 2008;294:G850–G854. doi: 10.1152/ajpgi.00523.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 12.Shechter R., London A., Varol C., Raposo C., Cusimano M., Yovel G., Rolls A., Mack M., Pluchino S., Martino G., Jung S., Schwartz M. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butovsky O., Kunis G., Koronyo-Hamaoui M., Schwartz M. Selective ablation of bone marrow-derived dendritic cells increases amyloid plaques in a mouse Alzheimer’s disease model. Eur J Neurosci. 2007;26:413–416. doi: 10.1111/j.1460-9568.2007.05652.x. [DOI] [PubMed] [Google Scholar]
- 14.London A., Cohen M., Schwartz M. Microglia and monocyte-derived macrophages: functionally distinct populations that act in concert in CNS plasticity and repair. Front Cell Neurosci. 2013;7:34. doi: 10.3389/fncel.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber M.S., Prod’homme T., Youssef S., Dunn S.E., Rundle C.D., Lee L., Patarroyo J.C., Stüve O., Sobel R.A., Steinman L., Zamvil S.S. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 16.Ransohoff R., Tani M. Do chemokines mediate leukocyte recruitment in post-traumatic CNS inflammation? Trends Neurosci. 1998;21:154–159. doi: 10.1016/s0166-2236(97)01198-3. [DOI] [PubMed] [Google Scholar]
- 17.Ransohoff R.M., Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 18.Mehta A., Brewington R., Chatterji M., Zoubine M., Kinasewitz G.T., Peer G.T., Chang A.C., Taylor F.B., Jr., Shnyra A. Infection-induced modulation of M1 and M2 phenotypes in circulating monocytes: role in immune monitoring and early prognosis of sepsis. Shock. 2004;22:423–430. doi: 10.1097/01.shk.0000142184.49976.0c. [DOI] [PubMed] [Google Scholar]
- 19.Chan G., Bivins-Smith E.R., Smith M.S., Smith P.M., Yurochko A.D. Transcriptome analysis reveals human cytomegalovirus reprograms monocyte differentiation toward an M1 macrophage. J Immunol. 2008;181:698–711. doi: 10.4049/jimmunol.181.1.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 21.Alon R., Shulman Z. Chemokine triggered integrin activation and actin remodeling events guiding lymphocyte migration across vascular barriers. Exp Cell Res. 2011;317:632–641. doi: 10.1016/j.yexcr.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Semmrich M., Smith A., Feterowski C., Beer S., Engelhardt B., Busch D.H., Bartsch B., Laschinger M., Hogg N., Pfeffer K., Holzmann B. Importance of integrin LFA-1 deactivation for the generation of immune responses. J Exp Med. 2005;201:1987–1998. doi: 10.1084/jem.20041850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans R., Lellouch A.C., Svensson L., McDowall A., Hogg N. The integrin LFA-1 signals through ZAP-70 to regulate expression of high-affinity LFA-1 on T lymphocytes. Blood. 2011;117:3331–3342. doi: 10.1182/blood-2010-06-289140. [DOI] [PubMed] [Google Scholar]
- 24.Chigaev A., Waller A., Amit O., Halip L., Bologa C.G., Sklar L.A. Real-time analysis of conformation-sensitive antibody binding provides new insights into integrin conformational regulation. J Biol Chem. 2009;284:14337–14346. doi: 10.1074/jbc.M901178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drbal K., Angelisová P., Cerny J., Hilgert I., Horejsi V. A novel anti-CD18 mAb recognizes an activation-related epitope and induces a high-affinity conformation in leukocyte integrins. Immunobiology. 2001;203:687–698. doi: 10.1016/S0171-2985(01)80017-6. [DOI] [PubMed] [Google Scholar]
- 26.Drbal K., Angelisova P., Hilgert I., Cerný J., Novák P., Horejsí V. A proteolytically truncated form of free CD18, the common chain of leukocyte integrins, as a novel marker of activated myeloid cells. Blood. 2001;98:1561–1566. doi: 10.1182/blood.v98.5.1561. [DOI] [PubMed] [Google Scholar]
- 27.Rom S., Fan S., Reichenbach N., Dykstra H., Ramirez S.H., Persidsky Y. Glycogen synthase kinase 3beta inhibition prevents monocyte migration across brain endothelial cells via Rac1-GTPase suppression and down-regulation of active integrin conformation. Am J Pathol. 2012;181:1414–1425. doi: 10.1016/j.ajpath.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamatovic S.M., Keep R.F., Kunkel S.L., Andjelkovic A.V. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci. 2003;116:4615–4628. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- 29.Benito C., Tolón R.M., Pazos M.R., Núñez E., Castillo A.I., Romero J. Cannabinoid CB2 receptors in human brain inflammation. Br J Pharmacol. 2008;153:277–285. doi: 10.1038/sj.bjp.0707505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han K.H., Lim S., Ryu J., Lee C.W., Kim Y., Kang J.H., Kang S.S., Ahn Y.K., Park C.S., Kim J.J. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc Res. 2009;84:378–386. doi: 10.1093/cvr/cvp240. [DOI] [PubMed] [Google Scholar]
- 31.Bátkai S., Osei-Hyiaman D., Pan H., El-Assal O., Rajesh M., Mukhopadhyay P., Hong F., Harvey-White J., Jafri A., Hasko G., Huffman J.W., Gao B., Kunos G., Pacher P. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 2007;21:1788–1800. doi: 10.1096/fj.06-7451com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajesh M., Mukhopadhyay P., Bátkai S., Haskó G., Liaudet L., Huffman J.W., Csiszar A., Ungvari Z., Mackie K., Chatterjee S., Pacher P. CB2-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am J Physiol Heart Circ Physiol. 2007;293:H2210–H2218. doi: 10.1152/ajpheart.00688.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang M., Adler M.W., Abood M.E., Ganea D., Jallo J., Tuma R.F. CB2 receptor activation attenuates microcirculatory dysfunction during cerebral ischemic/reperfusion injury. Microvasc Res. 2009;78:86–94. doi: 10.1016/j.mvr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacher P., Steffens S. The emerging role of the endocannabinoid system in cardiovascular disease. Semin Immunopathol. 2009;31:63–77. doi: 10.1007/s00281-009-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukhopadhyay P., Rajesh M., Pan H., Patel V., Mukhopadhyay B., Bátkai S., Gao B., Haskó G., Pacher P. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic Biol Med. 2010;48:457–467. doi: 10.1016/j.freeradbiomed.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiosses W.B., Shattil S.J., Pampori N., Schwartz M.A. Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nat Cell Biol. 2001;3:316–320. doi: 10.1038/35060120. [DOI] [PubMed] [Google Scholar]
- 37.Worthylake R.A., Burridge K. RhoA and ROCK promote migration by limiting membrane protrusions. J Biol Chem. 2003;278:13578–13584. doi: 10.1074/jbc.M211584200. [DOI] [PubMed] [Google Scholar]
- 38.Shi C., Pamer E.G. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horváth B., Magid L., Mukhopadhyay P., Bátkai S., Rajesh M., Park O., Tanchian G., Gao R.Y., Goodfellow C.E., Glass M., Mechoulam R., Pacher P. A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury. Br J Pharmacol. 2012;165:2462–2478. doi: 10.1111/j.1476-5381.2011.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alevriadou B.R. CAMs and Rho small GTPases: gatekeepers for leukocyte transendothelial migration. Focus on “VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration.”. Am J Physiol Cell Physiol. 2003;285:C250–C252. doi: 10.1152/ajpcell.00189.2003. [DOI] [PubMed] [Google Scholar]
- 41.Rolfe B.E., Worth N.F., World C.J., Campbell J.H., Campbell G.R. Rho and vascular disease. Atherosclerosis. 2005;183:1–16. doi: 10.1016/j.atherosclerosis.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 42.van Buul J.D., Hordijk P.L. Signaling in leukocyte transendothelial migration. Arterioscler Thromb Vasc Biol. 2004;24:824–833. doi: 10.1161/01.ATV.0000122854.76267.5c. [DOI] [PubMed] [Google Scholar]
- 43.Kurihara R., Tohyama Y., Matsusaka S., Naruse H., Kinoshita E., Tsujioka T., Katsumata Y., Yamamura H. Effects of peripheral cannabinoid receptor ligands on motility and polarization in neutrophil-like HL60 cells and human neutrophils. J Biol Chem. 2006;281:12908–12918. doi: 10.1074/jbc.M510871200. [DOI] [PubMed] [Google Scholar]
- 44.Lim Y.C., Wakelin M.W., Henault L., Goetz D.J., Yednock T., Cabañas C., Sánchez-Madrid F., Lichtman A.H., Luscinskas F.W. Alpha4beta1-integrin activation is necessary for high-efficiency T-cell subset interactions with VCAM-1 under flow. Microcirculation. 2000;7:201–214. [PubMed] [Google Scholar]
- 45.Barthel S.R., Johansson M.W., McNamee D.M., Mosher D.F. Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma. J Leukoc Biol. 2008;83:1–12. doi: 10.1189/jlb.0607344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salas A., Shimaoka M., Kogan A.N., Harwood C., von Andrian U.H., Springer T.A. Rolling adhesion through an extended conformation of integrin alphaLbeta2 and relation to alpha I and beta I-like domain interaction. Immunity. 2004;20:393–406. doi: 10.1016/s1074-7613(04)00082-2. [DOI] [PubMed] [Google Scholar]
- 47.Ferreira A.M., Isaacs H., Hayflick J.S., Rogers K.A., Sandig M. The p110delta isoform of PI3K differentially regulates beta1 and beta2 integrin-mediated monocyte adhesion and spreading and modulates diapedesis. Microcirculation. 2006;13:439–456. doi: 10.1080/10739680600776062. [DOI] [PubMed] [Google Scholar]
- 48.Woska J.R., Jr., Shih D., Taqueti V.R., Hogg N., Kelly T.A., Kishimoto T.K. A small-molecule antagonist of LFA-1 blocks a conformational change important for LFA-1 function. J Leukoc Biol. 2001;70:329–334. [PubMed] [Google Scholar]
- 49.Bolomini-Vittori M., Montresor A., Giagulli C., Staunton D., Rossi B., Martinello M., Constantin G., Laudanna C. Regulation of conformer-specific activation of the integrin LFA-1 by a chemokine-triggered Rho signaling module. Nat Immunol. 2009;10:185–194. doi: 10.1038/ni.1691. [DOI] [PubMed] [Google Scholar]
- 50.Burkhardt J.K., Carrizosa E., Shaffer M.H. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 51.Worth D.C., Hodivala-Dilke K., Robinson S.D., King S.J., Morton P.E., Gertler F.B., Humphries M.J., Parsons M. Alpha v beta3 integrin spatially regulates VASP and RIAM to control adhesion dynamics and migration. J Cell Biol. 2010;189:369–383. doi: 10.1083/jcb.200912014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Askari J.A., Buckley P.A., Mould A.P., Humphries M.J. Linking integrin conformation to function. J Cell Sci. 2009;122:165–170. doi: 10.1242/jcs.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eckert R.E., Jones S.L. Regulation of VASP serine 157 phosphorylation in human neutrophils after stimulation by a chemoattractant. J Leukoc Biol. 2007;82:1311–1321. doi: 10.1189/jlb.0206107. [DOI] [PubMed] [Google Scholar]
- 54.Bernstein B.W., Bamburg J.R. ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 2010;20:187–195. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mizuno K. Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell Signal. 2013;25:457–469. doi: 10.1016/j.cellsig.2012.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ex vivo CB2 activation of leukocytes does not affect adhesion of autologous leukocyte adhesion in LPS-induced encephalitis. Representative images from a 30-second video of leukocytes labeled with calcein-AM. n = 4 to 6 mice per group. Scale bar = 100 μm.
Ex vivo CB2 activation of leukocytes does not affect autologous leukocyte adhesion in LPS-induced encephalitis. A: Representative images from a 30-second video of leukocytes labeled with rhodamine-6G. B: Quantitation of leukocyte firm adhesion (rhodamine 6G–labeled autologous leukocytes) during 30 seconds of observation. Data are expressed as means ± SEM. n = 4 to 6 mice per group. Scale bar = 100 μm. NS, nonsignificant.
Absence of CB2 increases adhesion of autologous leukocytes in LPS-associated encephalitis. At 5 days after cranial window implantation, C57BL/6 mice were injected with LPS and 4 hours later with ex vivo calcein-AM–labeled leukocytes. Representative images from 30-second videos of leukocytes labeled with calcein-AM and visualized by IVM for adhesion in pial venules 30 minutes after injection. n = 4 mice per group.
Absence of CB2 increases adhesion of autologous leukocytes in LPS-associated encephalitis. Five days after cranial window implantation, C57BL/6 mice were injected with LPS and 4 hours later with ex vivo calcein-AM–labeled leukocytes. A: Representative images from 30-second videos of leukocytes labeled with rhodamine-6G (autologous cells) and visualized by IVM for adhesion in pial venules 30 minutes after injection. B: Quantitation of leukocyte firm adhesion (rhodamine 6G–labeled autologous cells) during 30 seconds of observation. Data are expressed as means ± SEM. n = 4 mice per group. ∗P < 0.05.