Background: The biological effects of transthyretin proteins on vasculature remain unknown.
Results: V30M transthyretin tetramer modulates endothelial global gene expression, down-regulating pro-angiogenic genes, inducing apoptosis and inhibiting migration.
Conclusion: Transthyretin proteins regulate angiogenesis by conferring different molecular identities to endothelial cells.
Significance: This work has critical implications in the prevention of early hepatic artery thrombosis in familial amyloidotic polyneuropathy patients after liver transplantation.
Keywords: Angiogenesis, Endothelial Cell, Gene Expression, Microarray, Vascular Biology, Familial Amyloidotic Polyneuropathy, Transthyretin
Abstract
Familial amyloidotic polyneuropathy (FAP) has a high prevalence in Portugal, and the most common form of hereditary amyloidosis is caused by an amyloidogenic variant of transthyretin (TTR) with a substitution of methionine for valine at position 30 (V30M). Until now, the available efficient therapy is liver transplantation, when performed in an early phase of the onset of the disease symptoms. However, transplanted FAP patients have a significantly higher incidence of early hepatic artery thrombosis compared with non-FAP transplanted patients. Because FAP was described as an independent risk factor for early hepatic artery thrombosis, more studies to understand the underlying mechanisms involved in this outcome are of the utmost importance. Knowing that the liver is the major site for TTR production, we investigated the biological effects of TTR proteins in the vasculature and on angiogenesis. In this study, we identified genes differentially expressed in endothelial cells exposed to the WT or V30M tetramer. We found that endothelial cells may acquire different molecular identities when exposed to these proteins, and consequently TTR could regulate angiogenesis. Moreover, we show that V30M decreases endothelial survival by inducing apoptosis, and it inhibits migration. These findings provide new knowledge that may have critical implications in the prevention of early hepatic artery thrombosis in FAP patients after liver transplantation.
Introduction
Familial amyloidotic polyneuropathy (FAP)3 is an inherited autosomal dominant disease that affects primarily the peripheral and autonomic nervous systems. It was first described by Corino de Andrade, in 1952, in the Northern area of Portugal (1), and then many foci of FAP cases were reported worldwide (2–6). FAP is caused by the extracellular deposition of amyloid fibrils composed of transthyretin (TTR) protein. TTR is a homotetramic plasma protein of approximately 55 kDa produced mainly in the liver and choroid plexus of the brain. The known physiological functions of TTR are the transport of thyroid hormone T4 and retinol, through binding to the retinol-binding protein. To date, approximately 112 mutations in the primary structure of TTR have been discovered of which 98 are amyloidogenic. Among the amyloidogenic variants the substitution of Val for Met at position 30 (V30M) is the most common mutation (7, 8). Several studies suggest that amyloidogenic mutations destabilize the native structure of TTR, thereby inducing conformational changes that lead to dissociation of the tetramers into partially unfolded species which can subsequently self-assemble into amyloid fibrils (9). The proposed mechanism under certain physiological conditions, including temperature, pH, ionic strength, and protein concentration, states that mutant TTR molecules can dissociate into non-native monomers with a distinct compact structure, which in turn can partially unfold forming high molecular mass soluble aggregates (10, 11). Quintas et al. have also demonstrated that there is a clear correlation between the stability of the non-native monomer formed upon tetramer dissociation, aggregate formation, and the amyloidogenic potential of different TTR variants (12). Growing evidence has pointed toward a model mechanism of amyloidosis cytotoxicity where amyloidogenic mutations in TTR destabilize the native structure into dimers and monomers that rapidly form oligomers and protofibrillar species which are the cytotoxic intermediates (9, 13, 14).
Although several approaches are being developed to induce the clearance of extracellular deposition of TTR (for review, see Ref. 15), so far orthotopic liver transplantation (OLT) has been the treatment of choice for FAP because this organ is the main source of TTR production. Because the liver of FAP patients is usually fully functional, although it produces mutant TTR, in 1995 a new transplant technique, sequential transplantation, or domino liver transplantation, was developed in Portugal, by a team led by Prof. Linhares Furtado (16). In the domino transplantation FAP patients receive an organ, while the organ of the FAP patient is reused for transplantation into another patient with chronic liver disease. A recent prospective monitoring these transplanted FAP patients has shown prolongation of life, and among the different TTR mutations, the most favorable results were obtained for patients with the V30M TTR mutation (17).
Curry Cabral Hospital is presently the most important liver transplantation center in Portugal. Since the first OLT performed in this center in 1992, there have been marked improvements in surgical technique, anesthesia management, immunosuppressive regimen, and medical care of these patients. However, early postoperative thrombotic complications, particularly hepatic artery thrombosis (HAT), remain critical causes of in-hospital morbidity, potentially culminating in acute graft loss (18, 19). A retrospective analysis of 223 OLTs showed that the incidence of early HAT in FAP patients was 7.7-fold higher compared with non-FAP patients. The cause for this higher incidence of HAT could not be justified based on the technical factors analyzed; preoperative details, surgical features, and postoperative variables were similar for patients with or without HAT (20). FAP is therefore an independent risk for early HAT, and it is therefore vital to clarify what are the mechanisms involved in this outcome.
Because the liver is the major site of TTR production, we may hypothesize that the hepatic microenvironment and liver endothelial cells (ECs) respond differentially to the WT or V30M TTR proteins. To date there is no knowledge on the effects of these proteins in EC biology.
In the present study, we used DNA microarray technology to investigate the endothelial global gene expression patterns in response to tetrameric WT and V30M TTR. We report that genes involved in IFN and TNF pathways are significantly modulated by V30M TTR compared with the WT and may have a role in angiogenesis regulation. Accordingly, V30M induces the down-regulation of several pro-angiogenic genes such as VEGFR1, VEGFR2, FGF2, TGFB2, and ANGPT2. Moreover, our results suggest that V30M decreases EC survival by inducing apoptosis and it inhibits migration. These new insights into the regulation of endothelial function by these proteins may have critical implications in the prevention of early hepatic artery thrombosis in FAP patients after liver transplantation.
EXPERIMENTAL PROCEDURES
Cell Culture
Primary human umbilical vein endothelial cells (HUVECs) were kindly provided by Dr. Shahin Rafii (Cornell University Medical College, New York). ECs, passage 6–8, were cultured as described (21).
Transthyretin Expression and Purification
Recombinant WT and V30M TTR were produced in an Escherichia coli expression system and purified as described previously (22), with minor alterations. WT TTR expression plasmid (pmmHA) was provided by Jeffery Kelly, and V30M TTR expression plasmid (pETM-13) was a courtesy of EMBL-GS (Munich, Germany). E. coli BL21+ transformed with the appropriate plasmid was grown overnight in 15-ml starter cultures (LB broth with 50 μg/ml ampicillin, for WT-TTR, or 50 μg/ml kanamycin, for V30M-TTR) at 37 °C and 200 rpm. One-liter cultures (LB broth with antibiotic) were inoculated with 10 ml of starter cultures and incubated at 37 °C and 200 rpm until A600 nm = 0.4–0.6. Protein expression was then induced by adding 0.1 mm isopropyl 1-thio-β-d-galactopyranoside. The purification procedure was applied both for WT and V30M TTR. After 3 h of protein expression cells were centrifuged in a Centrifuge 5810R (Eppendorf) at 9000 rpm for 3 min, at 4 °C, which was followed by resuspension in TBS, pH 7.6, sonication in a Soniprep 150 (MSE), and centrifugation at 14,000 × g for 5 min, at 4 °C. Because TTR is a soluble protein, cell extracts were then precipitated with (NH4)2SO4 (40% saturation) followed by centrifugation at 12,000 × g for 15 min, at 4 °C. The supernatant was then brought to 90% (NH4)2SO4 saturation, and precipitated proteins were harvested by centrifugation in the same conditions as the previous one. The pellets were resuspended in ionic exchange buffer A (25 mm Tris-HCl, 1 mm EDTA, pH 8) and dialyzed against the same buffer overnight at 4 °C in dialysis tubing cellulose membrane (average flat width, 76 mm) from Sigma-Aldrich. Dialyzed proteins were separated by ionic exchange chromatography with a Resource Q (GE Healthcare) after equilibration with 95% buffer A and 5% buffer B (25 mm Tris-HCl, 1 mm EDTA, 1 m NaCl, pH 8). TTR variants were collected during a 5–35% buffer B gradient and loaded onto a HiLoad 16/600 Superdex 75 pg (GE Healthcare) equilibrated in PBS. Finally, a tetrameric form of both TTRvariants was isolated by gel filtration chromatography as described previously (12), with an analytical Superdex 200 10/300 GL previously equilibrated in Hanks' balanced salt solution.
Transthyretin Quantification and Analysis of the Species in Solution
Protein concentrations were obtained spectrophotometrically at 280 nm using an extinction coefficient of 7.76 × 104 m−1 cm−1 based on a molecular mass of 55 kDa for TTR (A280 nm (1%) = 14.1 mg−1 ml cm−1) (11, 12). To assure that just the presence of the WT and V30M TTR tetramer were in the solution, 100 μl of the final purified TTR was injected on an analytical tricorn Superdex 75 (GE Healthcare). Only the samples with just the TTR tetramer were applied to cells.
RNA Isolation, Target Synthesis, and Hybridization to Affymetrix GeneChips
Total RNA was extracted from HUVECs and cultured for 3 h in the presence of the purified WT and V30M TTR proteins at 4 μm, using RNeasy Micro Kit (Qiagen). Three different RNA isolates were used as replicates, and total RNA was extracted using the RNeasy Mini Kit (Qiagen). Concentration and purity were determined by spectrophotometry, and integrity was confirmed using an Agilent 2100 Bioanalyzer with a RNA 6000 Nano Assay (Agilent Technologies, Palo Alto, CA). RNA was processed for use on Affymetrix GeneChip Human Gene 1.1 ST Array Strips (NuGEN Technologies, San Carlos, CA) Ovation PicoSL WTA System V2, and Encore Biotin Module, according to the manufacturer's protocol. Briefly, 17 ng of total RNA was used in a reverse transcription reaction to obtain first-strand cDNA. After generation and purification of DNA-RNA heteroduplex double-strand cDNA, this was subjected to SPIA amplification. Size distribution of purified SPIA cDNA was assessed using an Agilent 2100 Bioanalyzer with a RNA 6000 Nano Assay, followed by fragmentation and end-labeling of 2.5 μg of SPIA cDNA (Encore Biotin Module, NuGEN) and confirmation using a RNA 6000 Nano Assay. The end-labeled, fragmented cDNA was used in a 130-μl hybridization mixture containing added hybridization controls (GeneAtlas Hybridization, Wash, and Stain Kit for WT Array Strips; Affymetrix) and hybridized on array strips for 20 h at 48 °C. Standard posthybridization wash and double-stain protocols (GeneAtlas Hybridization, Wash, and Stain Kit for WT Array Strips) were used on an Affymetrix GeneAtlas system, followed by scanning of the array strips. Microarray data were deposited in the Gene Expression Omnibus (GEO) repository at the NCBI under accession number GSE44856.
GeneChip Data Analysis
Scanned arrays were analyzed first with Affymetrix Expression Console software for quality control. Subsequent analysis was carried out with Chipster 2.3.0 (23). Robust multi-array average was used to compute expression values for the six arrays in this study and based on custom cdf file included in hugene11stv1hsentrezg.db as available from Brainarray database (24). Next 33% of all genes with the lowest S.D. from the gene mean were filtered out, leaving 13,224 genes of the original 19,738 genes for further analysis. These were subjected to an empirical Bayes two-group test (25) with Benjamini-Hochberg multiple testing correction and a p value cut-off of 0.05, resulting in a list of 436 differentially expressed genes. Ingenuity Pathway Analysis (IPA) (Ingenuity Systems) was used to determine the biological pathways in which the genes that were significantly over- or underexpressed were involved. Our list of significant changed genes, with respective -fold change and p values was uploaded within the IPA database and a core analysis was carried out. Canonical pathways analysis identified the pathways from the IPA library that were most significantly associated with the data set. Genes from the data set that met the p value cut-off of 5% and were associated with a canonical pathway in the Ingenuity Pathways Knowledge Base were considered for the analysis.
Apoptosis Analysis
HUVECs were plated at equal densities, and after 24, 48, 72, 84, or 96 h of incubation with WT and V30M TTR proteins at different concentrations (1, 2, 4, 8, or 10 μm), cells were stained with Annexin V-FITC (Boehringer Mannheim) and propidium iodide (PI) (Interchim). The percentage of apoptotic cells (Annexin V-positive, PI-negative and -positive) was determined by flow cytometry (FACSCalibur; BD Biosciences) and Flowjo 6.4.7 software. Results are shown as the percentage of early and late apoptotic cells (Annexin V-positive/PI-negative and Annexin V-positive/PI-positive, respectively).
Wound Healing Assay
HUVECs were plated to confluence and wounds created in the monolayer by scraping the plate with a pipette tip, after the addition of WT and V30M TTR proteins at 4 μm. Wounded monolayers were photographed immediately after wounding and 7 h later, and the area of the wound was measured. Several regions (n = 3) for each treatment done in triplicate were recorded, and the means ± S.D. of each treatment were calculated after normalizing for wounded area at t = 0 and expressed as percentage of wound healing.
Quantitative RT-PCR (qRT-PCR)
Total RNA from HUVECs cultured in the presence of WT and V30M TTR proteins at 4 μm for 5 or 8 h was isolated using the RNeasy Micro Kit (Qiagen). For each sample, 1 μg of RNA was reverse transcribed into cDNA (Superscript II Kit; Invitrogen). The mRNA levels of the following targets: IFITM1 forward, 5′-TCATCCTGTCACTGGTATTCGGCTC-3′ and reverse, 5′-GTGGGTATAAACTGCTGTATCTAGGG-3′; BAK1 forward, 5′-GGGTCTATGTTCCCCAGGAT-3′ and reverse, 5′-GCAGGGGTAGAGTTGAGCA-3′; TNF forward, 5′-GAGCTGTGGGGAGAACAAAAGGA-3′ and reverse, 5′-TTGGCCCTTGAAGAGGACCTG-3′; TNFAIP3(A20) forward, 5′-CTTGTGGCGCTGAAAACGAA-3′ and reverse, 5′-CCACTGTCCTTCAGGGTCAC-3′; FOS forward, 5′-CTCAATGACCCTGAGCCCA-3′ and reverse, 5′-TTGCTAATGTTCTTGACCGGC-3′; VEGFR1 forward, 5′-CCCTCGCCGGAAGTTGTAT-3′ and reverse, 5′-GTCAAATAGCGAGCAGATTTCTCA-3′; VEGFR2 forward, 5′-ATTCCTCCCCCGCATCA-3′ and reverse, 5′-GCTCGTTGGCGCACTCTT-3′ FGF2 forward, 5′-AAGAGCGACCCTCACATCAAGCTA-3′ and reverse, 5′-TAGCCAGGTAACGGTTAGCACACA-3′; TGFB2 forward, 5′-GCTTTGGATGCGGCCTATTGCTTT-3′ and reverse, 5′-CTCCAGCACAGAAGTTGGCATTGT-3′; ANGPT2 forward, 5′-AGGACACACCACGAATGGCATCTA-3′ and reverse, 5′-TGAATAATTGTCCACCCGCCTCCT-3′; TUB forward, 5′-CATTGGCAATAGCACAGCCATCCA-3′ and reverse, 5′-ACGAGGTCGTTCATGTTGCTCTCA-3′; and 18S forward, 5′-GCCCTATCAACTTTCGATGGTAGT-3′ and reverse, 5′-CCGGAATCGAACCCTGATT-3′ were measured by qRT-PCR performed according to the manufacturer's protocol using Power SYBR® Green (Invitrogen) and an Applied Biosystems 7500 Fast Real-time PCR. The housekeeping gene used to normalize was 18S. The real-time PCR program consisted of an initial denaturation step at 95 °C for 10 min followed by 40 cycles at 95 °C for 15 s and at 60 °C for 1 min. The relative quantification was performed according to the comparative method (2-ΔΔCt; Applied Biosystems User Bulletin 2P/N 4303859), with WT condition as internal calibrator and 18S as normalizer. The ΔCt value for each sample is representative of three different experiments.
RESULTS
Differential Response of ECs to V30M TTR Compared with WT TTR: Human Microarray Study
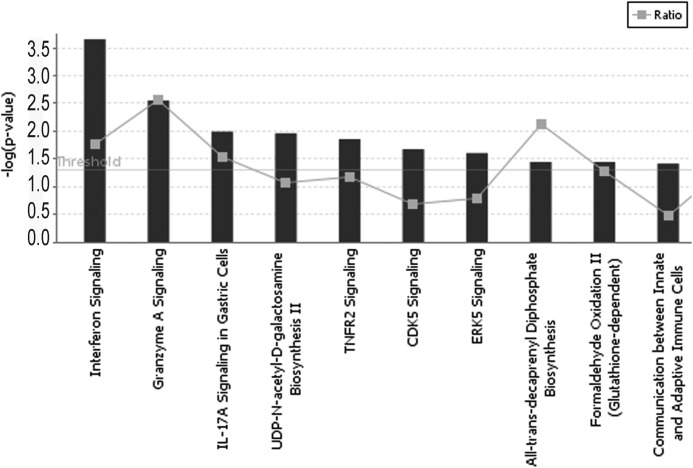
To characterize the EC response to WT and V30M TTR, we employed DNA microarray analysis, which is a powerful tool for analyzing global changes in gene expression. HUVECs were incubated with WT and V30M TTR tetramers at the final concentration of 4 μm for 3 h. We chose this concentration because it is the one that resembles the plasma levels of TTR ∼25 mg/dl (17, 26). Changes in mRNA expression are given as -fold changes comparing V30M TTR versus WT TTR-treated cells. All changes are significant with Benjamini-Hochberg-adjusted p values <0.05. In our study, 213 genes with a ratio of 1.42 or above and 223 genes with a ratio of −1.42 or below were considered up-regulated or down-regulated by V30M, respectively. A list of the top 10 up- or down-regulated genes is presented in Table 1. Of notice are the most down-regulated genes of the data set, c-fos and fosB, with a -fold change of −3.76 and −3.67, respectively, both belonging to the fos gene family. To further investigate the biological relevance of the data obtained from the gene expression microarrays, we used IPA software. The genes considered to have been differentially regulated were uploaded into IPA along with the gene identifiers and corresponding -fold change values. IPA constructs networks that optimize for both interconnectivity and number of focus genes under the constraint of maximal network size. In the graphical representation of a network, genes or gene products are represented as nodes, and the biological relationship between two nodes is represented as an edge (line). The intensity of the node color indicates the degree of up (red) or down (green) regulation of a given gene. Nodes are displayed using various shapes that represent the functional class of the gene product. Edges are displayed with various labels that describe the nature of the relationship between the nodes. By this analysis, we explored the molecular interactions and biological processes in which the genes significantly modified by V30M TTR could be involved. Canonical pathways analysis identified molecular pathways that were most significant to the data set. The top 10 canonical signaling pathways are illustrated in Fig. 1. IFN and TNF are two of the most relevant canonical pathways. In the IFN signaling pathway, the transcript encoding for the T cell protein-tyrosine phosphatase, described as being involved in silencing of VEGFR2 signaling and inhibiting biological responses to VEGF (27), is up-regulated in ECs exposed to V30M TTR when compared with the WT TTR. In contrast, the IFN-induced transmembrane protein 1 (IFITM1), described as regulator of EC sprouting in vitro and angiogenesis in vivo (28), and the pro-apoptotic Bak1 protein are down-regulated in ECs exposed to V30M TTR. In TNF signaling pathway, the transcripts encoding for the cytokine TNF and for the zing finger protein A20 are up-regulated in V30M exposed ECs. TNF is described as a potent inducer of EC apoptosis (29, 30) whereas A20 has been well established as a dual inhibitor of nuclear factor-κB (NF-κB) activation and apoptosis in the TNF receptor 1 signaling pathway. Moreover, in this signaling pathway, the transcript encoding for the proto-oncogene c-fos is down-regulated in ECs exposed to V30M. c-fos dimerizes with members of Jun family to form AP-1, a transcription factor involved in the regulation of VEGF, one of the most important angiogenic factors (31).
TABLE 1.
Top 10 most up-regulated and down-regulated genes induced by V30M TTR compared with WT TTR
A DNA microarray analysis was used to characterize the endothelial cell response to WT and V30M tetrameric TTR proteins. HUVECs were incubated with WT and V30M TTR tetramers at the final concentration of 4 μm for a period of 3 h. Changes in mRNA expression are given as -fold changes comparing V30M TTR-treated cells versus WT TTR-treated cells. All changes are significant with Benjamini-Hochberg-adjusted p values <0.05.
| Gene symbol | Gene description | -Fold change |
|---|---|---|
| Up-regulated | ||
| SNORD59B | Small nucleolar RNA, C/D box 59B | 2.73 |
| POM121L9P | POM121 membrane glycoprotein-like 9, pseudogene | 2.35 |
| SNORD49A | Small nucleolar RNA, C/D box 49A | 2.18 |
| SNORD113-4 | Small nucleolar RNA, C/D box 113-4 | 2.06 |
| SNORD114-3 | Small nucleolar RNA, C/D box 114-3 | 2.06 |
| SNORD14C | Small nucleolar RNA, C/D box 14C | 2.05 |
| HSD17B7 | Hydroxysteroid (17β) dehydrogenase 7 | 2.02 |
| CLK4 | CDC-like kinase 4 | 2.01 |
| MIR186 | MicroRNA 186 | 2.00 |
| SNORD114-2 | Small nucleolar RNA, C/D box 114-2 | 1.95 |
| Down-regulated | ||
| ANAPC1 | Anaphase-promoting complex subunit 1 | −2.01 |
| BAK1 | BCL2-antagonist/killer 1 | −2.04 |
| FAM103A1 | Family with sequence similarity 103, member A1 | −2.05 |
| OR10G9 | Olfactory receptor, family 10, subfamily G, member 9 | −2,07 |
| LOC100286979 | Similar to anaphase-promoting complex subunit 1 | −2.09 |
| EGR1 | Early growth response 1 | −2.16 |
| HIST1H2BK | Histone cluster 1, H2bk | −2.20 |
| CDC20 | Cell division cycle 20 homolog (Saccharomyces cerevisiae) | −2.20 |
| FOSB | FBJ murine osteosarcoma viral oncogene homolog B | −3.67 |
| FOS | FBJ murine osteosarcoma viral oncogene homolog | −3.76 |
FIGURE 1.
IPA software was used to analyze the differentially regulated genes. IPA showing canonical pathways was significantly modulated by V30M TTR compared with WT. Only the 10 pathways with the most significant changes are shown. The p value for each pathway is indicated by the bar and is expressed as negative log of Benjamini-Hochberg-adjusted p value; threshold is at 1.25 = −log (p = 0.05). The gray squares represent the ratio of the number of genes in a given pathway that meet the cut-off criteria divided by the total number of genes that make up that pathway.
qRT-PCR of Microarray Data
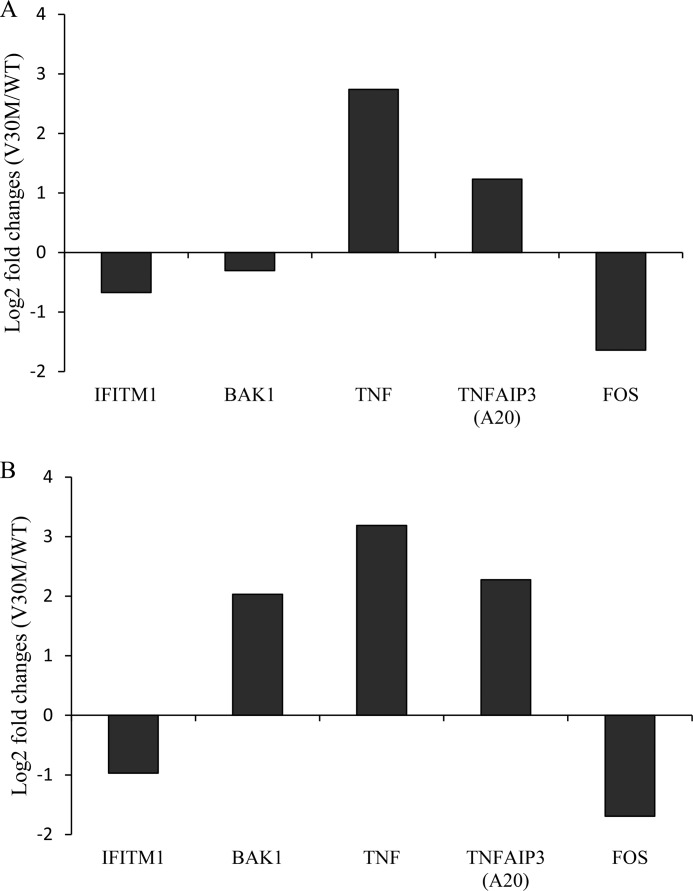
Although microarrays are a powerful tool to study global transcriptional responses, it is important to confirm microarray data. With this objective, HUVECs were cultured in the presence of TTR tetrameric proteins, at 4 μm, for 5 or 8 h, and total RNA was extracted. Quantitative RT-PCR validated decreased IFITM1 and c-fos expression and increased A20 and TNF expression by the V30M TTR tetramer. We found that BAK1 expression, one of the top 10 down-regulated genes in the arrays analysis at 3 h, is marginally down-regulated at 5 h but significantly increased at 8 h (Fig. 2) in ECs exposed to V30M TTR compared with cells exposed to the WT TTR tetramer.
FIGURE 2.
Validation of selected microarray targets by qRT-PCR. HUVECs were incubated for 5 h (A) or 8 h (B) with WT and V30M TTR tetrameric proteins. Relative expression of selected targets from the IFN pathway (IFITM1 and BAK1) and from TNFR2 pathway (TNF, TNFAIP3, c-fos) were quantified by qRT-PCR. Data represent the -fold change in gene expression of each target in V30M TTR-treated cells relative to the internal calibrator (WT TTR-treated cells) in triplicate measurements. Chart values are representative of three independent experiments.
V30M TTR Tetramers Down-regulate Pro-angiogenic Gene Expression in HUVECs
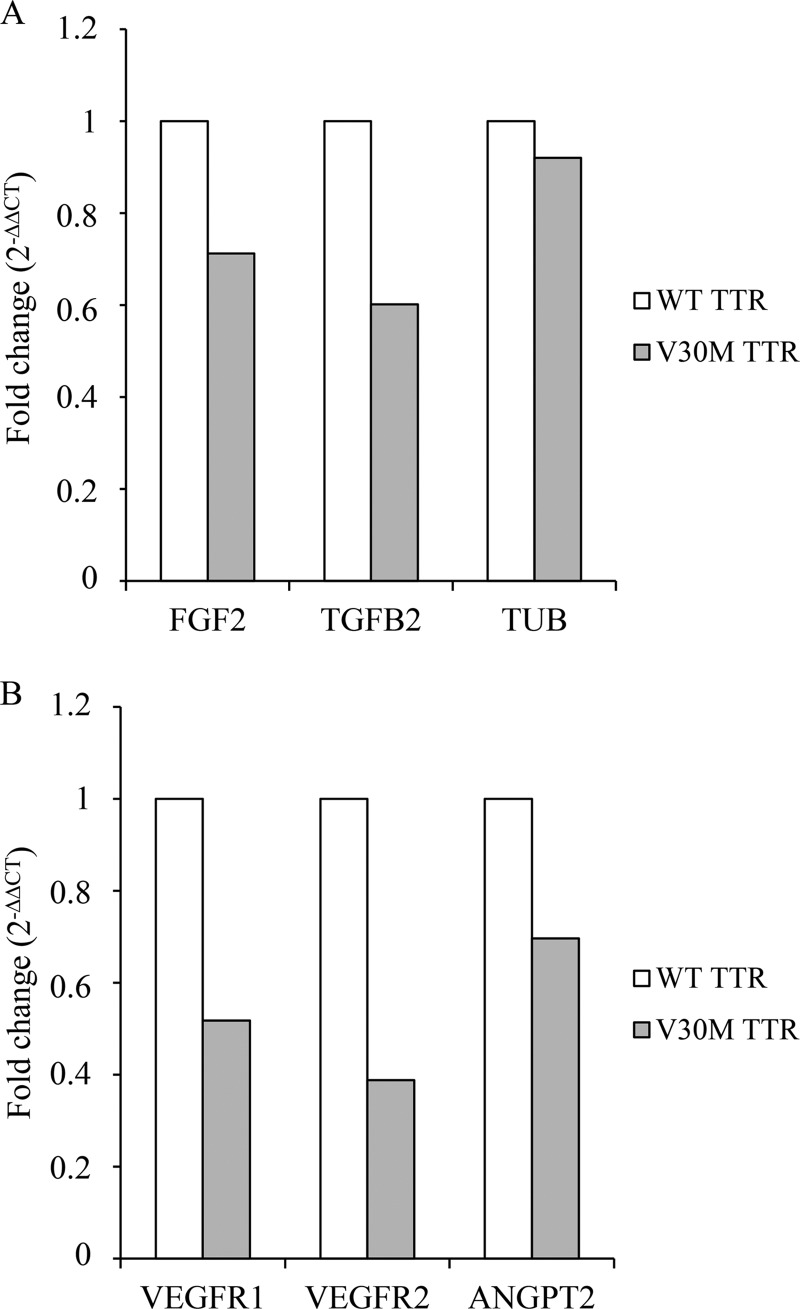
Because our experimental data suggest that angiogenesis may be modulated by V30M TTR, we investigated the levels of expression of other proteins known to be important in angiogenesis such as VEGFR1, VEGFR2, FGF2, TGFB2, and ANGPT2. According to our microarray data the expression of these targets does not change at 3 h by the V30M TTR tetramer. For that reason, the expression of these selected pro-angiogenic molecules was assessed later. Therefore, HUVECs were cultured in the presence of the WT and V30M TTR tetramer, at 4 μm, for 5 or 8 h followed by RNA extraction and quantitative RT-PCR analysis. Our results suggest that V30M induces a down-regulation of all selected genes (Fig. 3). Moreover, the expression of tubulin, here used as a putative unchanged target by the WT and V30M TTR tetramer, remains identical (Fig. 3A).
FIGURE 3.
V30M TTR tetramers modulate the expression of several pro-angiogenic targets in HUVECs. Cells were incubated for 5 h (A) or 8 h (B) with WT and V30M TTR tetrameric proteins. Relative expression of selected pro-angiogenic genes (VEGFR1, VEGFR2, ANGPT2, FGF2, and TGFB2) and cytoskeleton-related protein tubulin (TUB) was quantified by qRT-PCR. Data represent the -fold change in gene expression of each target in V30M TTR-treated cells relative to the internal calibrator (WT TTR-treated cells) in triplicate measurements. Chart values are representative of three independent experiments.
V30M TTR Decreases EC Survival by Inducing Apoptosis
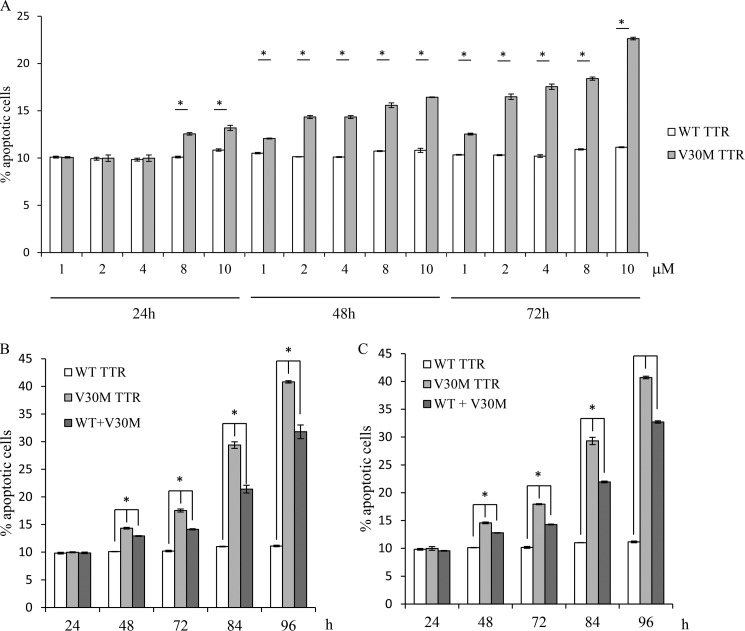
According to our findings, the V30M TTR tetramer regulates genes involved in one of the most important pro-angiogenic pathway, VEGF/VEGFR2, described as being fundamental in endothelial survival. For that reason, we assessed the capacity of V30M to induce apoptosis. HUVECs were incubated with WT and V30M TTR tetrameric proteins, at different concentrations, for 24, 48, and 72 h, and then ECs were labeled with Annexin V-FITC and PI. As shown in Fig. 4 A, there is an increase of the percentage of apoptosis in a concentration-dependent manner when HUVECs were cultured with the V30M compared with HUVECs cultured with the WT TTR tetramer. The values obtained for the WT TTR tetramer do not change with the different concentrations or over time (Fig. 4A) and are similar to those observed when HUVECs are cultured in normal medium in the absence of any type of TTR protein (data not shown).
FIGURE 4.
V30M TTR decreases endothelial cell survival by inducing apoptosis. A, HUVECs were cultured in the presence of WT and V30M TTR tetrameric proteins at 1, 2, 4, 8, or 10 μm for 24, 48, and 72 h. After the mentioned incubation periods cells were stained with Annexin V-FITC and PI. B, HUVECs were cultured (i) in the presence of 4 μm WT TTR; (ii) in the presence of 4 μm V30M TTR; (iii) in the presence of 2 μm WT and 2 μm V30M TTR for 24, 48, 72, 84, and 96 h. After the mentioned incubation periods cells were stained with Annexin V-FITC and PI. C, HUVECs were cultured (i) in the presence of 4 μm WT TTR; (ii) in the presence of 4 μm V30M TTR; (iii) in the presence of 2 μm WT and 2 μm V30M TTR for 24, 48, 72, 84, and 96 h. Every 24 h, the medium was removed, and fresh medium was added with the respective tetramers. After the incubation periods cells were stained with Annexin V-FITC and PI. A–C, values are given as the percentage of apoptotic cells (Annexin V-positive, PI-negative and -positive) in culture. Data are shown as mean ± S.D. of triplicate cultures and are representative of three independent experiments. A, *, p < 0.0005; B and C, *, p < 0.00025.
Because 4 μm is the dose that resembles the plasma levels of TTR ∼25 mg/dl (17, 26), we investigated whether the percentage of EC apoptosis that was obtained with this dose (Fig. 4A, 4 μm) could be augmented if the time of incubation increased. HUVECs were incubated with WT or V30M TTR tetrameric proteins at 4 μm for 24, 48, 72, 84, and 96 h, and as observed (Fig. 4B) V30M TTR tetramer significantly increases the percentage of apoptosis over time in contrast to the WT. Moreover, an additional experimental condition was added (Fig. 4B) where HUVECs were cultured in the presence of both WT and V30M TTR tetrameric proteins (at 2 μm, each). The same procedure was performed as described above, and according to our results the percentage of apoptosis after 48 h is significantly superior when ECs are cultured simultaneously in the presence of both types of proteins (WT and V30M) compared with the values obtained when ECs are cultured exclusively in the presence of WT, but significantly inferior compared with those obtained when ECs are cultured exclusively in the presence of V30M. Furthermore, we investigated whether the percentage of EC apoptosis that was obtained in this particular condition could be changed by the addition of fresh tetramer every 24 h over 96 h. Accordingly, HUVECs were incubated with WT or V30M TTR tetrameric proteins at 4 μm or both proteins (at 2 μm) for 24, 48, 72, 84, and 96 h. Every 24 h, the medium was removed, and fresh medium was added with the respective tetramers. ECs were labeled with Annexin V-FITC and PI and the percentage of apoptosis analyzed at indicated times (Fig. 4C). As observed in Fig. 4C, the results were similar to those obtained when tetramers were added uniquely at the beginning of the experiment (Fig. 4B).
V30M TTR Tetramer Inhibits EC Migration
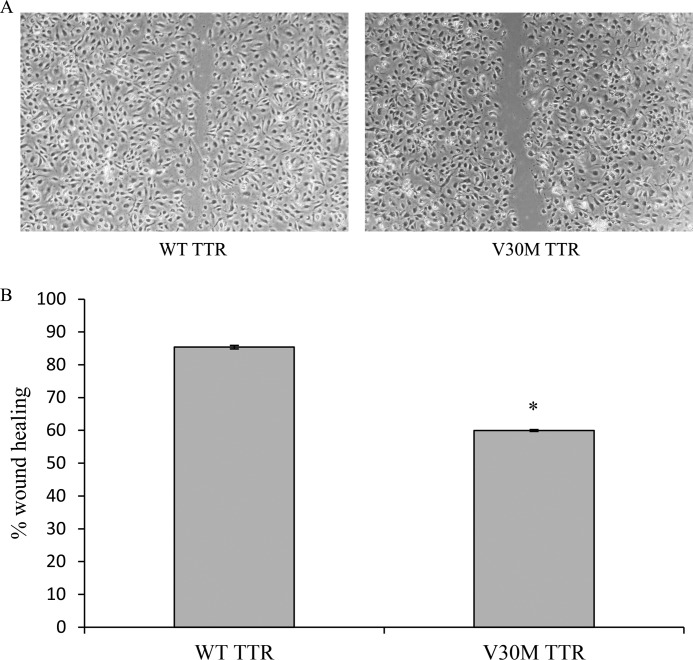
We also investigated the effect of the V30M TTR tetramer in EC migration because our results suggest that TTR may regulate angiogenesis, and we know that in this process cell migration is essential. Confluent HUVEC monolayers were exposed to either protein, at 4 μm, and an in vitro wound healing assay was performed. The EC migratory response to wounding was measured. As represented in Fig. 5, ECs in the presence of the WT achieved almost 85% wound closure after 7 h, whereas V30M TTR inhibited closure by approximately 1.4-fold (60% wound closure). At 7 h after wounding, neither type of TTR protein induces apoptosis (data not shown), suggesting that the result obtained is independent of a putative effect of V30M in EC survival.
FIGURE 5.
V30M TTR tetramer inhibits endothelial cell migration. A, HUVECs were cultured until confluent, and the monolayer was scraped to initiate wounding immediately after the addition of TTR tetrameric proteins at 4 μm. Monolayers were followed over time and the wound area was measured at t = 0 h and t = 7 h. Magnification, ×10. B, quantitation of results from several regions (n = 3) for each treatment done in triplicate was recorded, and the means ± S.D. of each treatment were calculated after normalizing for wounded area at t = 0 and expressed as percentage of wound healing. *, p < 0.0002.
DISCUSSION
Orthotopic liver transplant for FAP has become the usual treatment of choice for this fatal disease. However, transplanted FAP patients have a significantly higher incidence of early hepatic artery thrombosis compared with non-FAP transplanted patients. Because FAP was described as an independent risk factor for early HAT, more studies aiming to clarify this phenomenon are needed to develop specific prevention strategies for this population. Knowing that the livers of FAP patients are anatomically and functionally normal except for the production of the mutant TTR, we hypothesized that ECs may acquire different molecular identities when exposed to WT or mutant TTR proteins, and consequently TTR could regulate angiogenesis. To the best of our knowledge, the biological effects of TTR proteins in the vasculature and on angiogenesis were unknown.
To address this issue, we investigated global gene expression of HUVECs cultured in the presence of the WT and V30M tetrameric TTR proteins, at 4 μm, a concentration similar to the one found physiologically. The first key finding of this work is the identification of differentially expressed genes in ECs exposed to the WT or V30M TTR tetramer. By microarray analysis, we found that the expression of a total of 436 genes differed significantly between the ECs exposed to the WT and V30M tetramer. To identify mechanisms behind experimental observations of changes in gene expression, our data sets were analyzed through the use of IPA canonical pathways. The IFN and TNF signaling pathways were identified as canonical pathways that were most significant to the data set. Interestingly, all of the mediators observed to be up- or down-regulated in these particular pathways, namely T cell protein-tyrosine phosphatase, IFITM1, BAK1, TNF, A20, and c-fos, are involved in endothelial cell survival and angiogenesis regulation, the second key finding of this work. To validate our microarray data set, qRT-PCR was performed. Consistently, the V30M TTR tetramer induces a down-regulation of the transcript levels of IFITM1 and c-fos and an up-regulation of TNF and A20, in HUVECs, at 5 or 8 h of exposure. IFITM1 and c-fos are both involved in angiogenesis regulation. IFITM1 was found to be rapidly induced as EC sprout in vitro and form luminized vessels. Using a vascular bed xenotransplant model, a role for the human IFITM1 was identified during in vivo angiogenesis (28). IFITM1 was described as a molecule that may regulate the transition of ECs from a quiescent to an angiogenic state (28). Because we found that V30M tetrameric protein induces a decrease in IFTM1 expression, this could be related to an anti-angiogenic response that could be in agreement with our results that suggest that V30M inhibits EC migration. The protein c-fos heterodimerizes with Jun proteins to form the transcription factor AP-1. According to our microarray analysis, Jun expression is not significantly changed by V30M, a finding that was confirmed by qRT-PCR (data not shown). Taken together, our results suggest that the expression of AP-1 may be consequently decreased in HUVECs exposed to V30M. One of the genes regulated by AP-1 is one of the most important angiogenic factors, VEGF (31). Moreover, it was also described that, in ECs, AP-1 is also involved in the transcription of interleukin 8 (IL-8), which acts in an autocrine fashion to suppress apoptosis and facilitate cell proliferation and migration (32). In agreement with our results that suggest that V30M induces AP-1 down-regulation, our observations shown that V30M induces apoptosis and inhibits EC migration, the third key finding of our work.
The proteins TNF and A20 are mainly involved in the regulation of EC survival. TNF, up-regulated by V30M TTR tetramer, is a potent inducer of EC apoptosis, consistent with the effect of V30M in inducing EC apoptosis. Concerning A20, it is difficult to predict its biological effect in ECs exposed to V30M, where we found that its expression levels are up-regulated, because this protein has a dual role. Although A20 inhibits apoptosis mediated by the TNF signaling, it paradoxically inhibits NF-κB. In most cell types, it has been well established that activation of NF-κB antagonizes apoptotic cell death triggered by TNF signaling. Moreover, our results suggest that BAK1, one of the 10 top down-regulated genes by V30M TTR in our array analysis (performed at 3 h), is marginally down-regulated at 5 h. This suggests that even though the levels of BAK1 significantly decrease at 3 h of V30M TTR exposure, this slight effect is seen later, at 5 h, and interestingly, at 8 h, the levels of expression of BAK1 transcripts are significantly increased by V30M TTR compared with the WT tetramer. BAK1 is a pro-apoptotic gene, and its up-regulation by V30M TTR is consistent with our results showing that V30M induces HUVECs to undergo apoptosis. According to our findings for BAK1, we hypothesized that other molecular targets playing a role in angiogenesis and that do not appear as significantly altered in our microarray data set (performed at 3 h) could be differentially expressed by V30M TTR tetramer later on. Therefore, we selected several genes such as VEGFR1, VEGFR2, FGF2, TGFB2, and ANGPT2. The receptors 1 and 2 of VEGF (VEGFR1 and VEGFR2) are the main mediators of VEGF-induced endothelial proliferation, survival, migration, tubular morphogenesis, and sprouting in adults (33, 34). Moreover, it has been long recognized that FGF2, TGFB2, and ANGP2 are important in angiogenesis inducing migration and proliferation of ECs, stimulating tube formation and sprouting of new blood vessels (35). Here, we found that HUVECs present a down-regulation of the expression of all of these angiogenic players after 5 or 8 h of exposure to V30M compared with the WT TTR tetramer. Therefore, our results consistently raise the possibility that TTR proteins differentially regulate endothelial gene expression, conferring different molecular identities to ECs. Accordingly, V30M TTR tetramer decreases the expression levels of several genes involved in endothelial proliferation, survival, and migration and could negatively modulate angiogenesis compared with the WT TTR tetramer. Our results provide the important insight that TTR protein plays a key regulatory role in angiogenesis whereby the mutant V30M or WT tetramer differentially activates ECs, inducing a specific pattern of gene expression. According to the cellular pathways and molecular targets that are expressed, distinct vascular responses will be dictated by the V30M or the WT TTR tetramer.
To the best of our knowledge, the evidence that FAP patients could develop diseases associated with angiogenesis deficiency has not been previously noted. We can hypothesize that the normal life span of the FAP patient minimizes the chance of the development of angiogenesis-related diseases. However, FAP was recently identified as an independent risk factor for early thrombotic complications and specifically for HAT following OLT often culminating in acute graft loss (20). The cause of this phenomenon is not known. In light of presented data, we can hypothesize that the sudden change in serum levels of TTR V30M to WT can be challenging to FAP donor ECs. Knowing that FAP is an autosomal dominant disease, it is expectable that in the majority of FAP patients, their ECs could be exposed simultaneously to the WT and V30M proteins, but even so, our results suggest that ECs are biologically affected by V30M because an induction of apoptosis is observed in the presence of both tetrameric proteins. However, according to our results suggesting that the percentage of apoptosis is significantly different among ECs exposed to both types of TTR tetrameric proteins and ECs exposed exclusively to V30M tetrameric protein, we expect that ECs from homozygotic or heterozygotic FAP patients for V30M may respond differentially. Nevertheless, homozygotic or heterozygotic FAP patients for V30M were exposed to TTR V30M through life, which induced a low angiogenic potential. Few hours after OLT, the microenvironment is altered, and the vasculature should respond to a WT protein (produced by the new transplanted liver), which induces different biological pathways and gene expression favoring angiogenesis and EC proliferation. Also, as reported by our surgeons, HAT occurs mainly in the donor side of vascular anastomosis (data not shown). This fact is in accordance with our description. However, HAT is not a concern of FAP patients only, occurring also in non-FAP receptors. This means that local factors must be taken into account. Nonetheless, an existing vascular anastomosis is a huge stress factor for angiogenesis. It may partially explain why the thrombosis events occur in the anastomosis area, not at distant sites. This study highlighted the severe problem of high incidence HAT in FAP recipients during OLT. We consider that with this work new knowledge has been introduced in this area, but the problem remains. Further investigation is needed to clarify this matter, mainly which interventional treatments are required.
Acknowledgment
We thank Mike Saure, from The Scripps Research Institute, the technical support with the procedure of production and purification of transthyretin proteins.
This work was supported by Fundação para a Ciencia e Tecnologia Grant PIC/IC/83062/2007.
The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE44856).
- FAP
- familial amyloidotic polyneuropathy
- HAT
- hepatic artery thrombosis
- HUVEC
- human umbilical vein endothelial cell
- IPA
- Ingenuity Pathway Analysis
- OLT
- orthotopic liver transplantation
- PI
- propidium iodide
- qRT-PCR
- quantitative RT-PCR
- TTR
- transthyretin.
REFERENCES
- 1. Andrade C. (1952) A peculiar form of peripheral neuropathy: familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain 75, 408–427 [DOI] [PubMed] [Google Scholar]
- 2. Benson M. D., Wallace M. R., Tejada E., Baumann H., Page B. (1987) Hereditary amyloidosis: description of a new American kindred with late onset cardiomyopathy. Appalachian amyloid. Arthritis Rheum. 30, 195–200 [DOI] [PubMed] [Google Scholar]
- 3. Dwulet F. E., Benson M. D. (1984) Primary structure of an amyloid prealbumin and its plasma precursor in a heredofamilial polyneuropathy of Swedish origin. Proc. Natl. Acad. Sci. U.S.A. 81, 694–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakazato M., Kangawa K., Minamino N., Tawara S., Matsuo H., Araki S. (1984) Identification of a prealbumin variant in the serum of a Japanese patient with familial amyloidotic polyneuropathy. Biochem. Biophys. Res. Commun. 122, 712–718 [DOI] [PubMed] [Google Scholar]
- 5. Palácios S. A., Bittencourt P. L., Cançado E. L., Farias A. Q., Massarollo P. C., Mies S., Kalil J., Goldberg A. C. (1999) Familial amyloidotic polyneuropathy type 1 in Brazil is associated with the transthyretin V30M variant. Amyloid 6, 289–291 [DOI] [PubMed] [Google Scholar]
- 6. Suhr O. B., Svendsen I. H., Andersson R., Danielsson A., Holmgren G., Ranløv P. J. (2003) Hereditary transthyretin amyloidosis from a Scandinavian perspective. J. Intern. Med. 254, 225–235 [DOI] [PubMed] [Google Scholar]
- 7. Saraiva M. J., Birken S., Costa P. P., Goodman D. S. (1984) Amyloid fibril protein in familial amyloidotic polyneuropathy, Portuguese type: definition of molecular abnormality in transthyretin (prealbumin). J. Clin. Invest. 74, 104–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benson M. D., Kincaid J. C. (2007) The molecular biology and clinical features of amyloid neuropathy. Muscle Nerve 36, 411–423 [DOI] [PubMed] [Google Scholar]
- 9. Hou X., Aguilar M. I., Small D. H. (2007) Transthyretin and familial amyloidotic polyneuropathy. FEBS J. 274, 1637–1650 [DOI] [PubMed] [Google Scholar]
- 10. Quintas A., Saraiva M. J., Brito R. M. (1997) The amyloidogenic potential of transthyretin variants correlates with their tendency to aggregate in solution. FEBS Lett. 418, 297–300 [DOI] [PubMed] [Google Scholar]
- 11. Quintas A., Saraiva M. J., Brito R. M. (1999) The tetrameric protein transthyretin dissociates to a non-native monomer in solution: a novel model for amyloidogenesis. J. Biol. Chem. 274, 32943–32949 [DOI] [PubMed] [Google Scholar]
- 12. Quintas A., Vaz D. C., Cardoso I., Saraiva M. J., Brito R. M. (2001) Tetramer dissociation and monomer partial unfolding precedes protofibril formation in amyloidogenic transthyretin variants. J. Biol. Chem. 276, 27207–27213 [DOI] [PubMed] [Google Scholar]
- 13. Sousa M. M., Cardoso I., Fernandes R., Guimarães A., Saraiva M. J. (2001) Deposition of transthyretin in early stages of familial amyloidotic polyneuropathy: evidence for toxicity of nonfibrillar aggregates. Am. J. Pathol. 159, 1993–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reixach N., Deechongkit S., Jiang X., Kelly J. W., Buxbaum J. N. (2004) Tissue damage in the amyloidoses: transthyretin monomers and nonnative oligomers are the major cytotoxic species in tissue culture. Proc. Natl. Acad. Sci. U.S.A. 101, 2817–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Almeida M. R., Saraiva M. J. (2012) Clearance of extracellular misfolded proteins in systemic amyloidosis: experience with transthyretin. FEBS Lett. 586, 2891–2896 [DOI] [PubMed] [Google Scholar]
- 16. Furtado A., Tomé L., Oliveira F. J., Furtado E., Viana J., Perdigoto R. (1997) Sequential liver transplantation. Transplant. Proc. 29, 467–468 [DOI] [PubMed] [Google Scholar]
- 17. Benson M. D. (2013) Liver transplantation and transthyretin amyloidosis. Muscle Nerve 47, 157–162 [DOI] [PubMed] [Google Scholar]
- 18. Stange B. J., Glanemann M., Nuessler N. C., Settmacher U., Steinmüller T., Neuhaus P. (2003) Hepatic artery thrombosis after adult liver transplantation. Liver Transpl. 9, 612–620 [DOI] [PubMed] [Google Scholar]
- 19. Mueller A. R., Platz K. P., Kremer B. (2004) Early postoperative complications following liver transplantation. Best Pract. Res. Clin. Gastroenterol. 18, 881–900 [DOI] [PubMed] [Google Scholar]
- 20. Bispo M., Marcelino P., Freire A., Martins A., Mourão L., Barroso E. (2009) High incidence of thrombotic complications early after liver transplantation for familial amyloidotic polyneuropathy. Transpl. Int. 22, 165–171 [DOI] [PubMed] [Google Scholar]
- 21. Sofia Vala I., Martins L. R., Imaizumi N., Nunes R. J., Rino J., Kuonen F., Carvalho L. M., Rüegg C., Grillo I. M., Barata J. T., Mareel M., Santos S. C. (2010) Low doses of ionizing radiation promote tumor growth and metastasis by enhancing angiogenesis. PloS ONE 5, e11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Foss T. R., Kelker M. S., Wiseman R. L., Wilson I. A., Kelly J. W. (2005) Kinetic stabilization of the native state by protein engineering: implications for inhibition of transthyretin amyloidogenesis. J. Mol. Biol. 347, 841–854 [DOI] [PubMed] [Google Scholar]
- 23. Kallio M. A., Tuimala J., Hupponen T., Klemelä P., Gentile M., Scheinin I., Koski M., Käki J., Korpelainen E. (2011) Chipster: user-friendly analysis software for microarray and other high-throughput data. BMC Genomics 12, 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sandberg R., Larsson O. (2007) Improved precision and accuracy for microarrays using updated probe set definitions. BMC Bioinformatics 8, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smyth G. K. (2004) Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, article 3 [DOI] [PubMed] [Google Scholar]
- 26. Buxbaum J. N., Reixach N. (2009) Transthyretin: the servant of many masters. Cell Mol. Life Sci. 66, 3095–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ivaska J., Mattila E. (May 13, 2008) Method for inhibiting or stimulating angiogenesis in an individual. U. S. Patent 20,100,160,228
- 28. Popson S. A., Hughes C. C. (2010) A role for IFITM proteins in angiogenesis. FASEB J. 24, 750–75119887652 [Google Scholar]
- 29. Robaye B., Mosselmans R., Fiers W., Dumont J. E., Galand P. (1991) Tumor necrosis factor induces apoptosis (programmed cell death) in normal endothelial cells in vitro. Am. J. Pathol. 138, 447–453 [PMC free article] [PubMed] [Google Scholar]
- 30. Polunovsky V. A., Wendt C. H., Ingbar D. H., Peterson M. S., Bitterman P. B. (1994) Induction of endothelial cell apoptosis by TNFα: modulation by inhibitors of protein synthesis. Exp. Cell Res. 214, 584–594 [DOI] [PubMed] [Google Scholar]
- 31. Tischer E., Mitchell R., Hartman T., Silva M., Gospodarowicz D., Fiddes J. C., Abraham J. A. (1991) The human gene for vascular endothelial growth factor: multiple protein forms are encoded through alternative exon splicing. J. Biol. Chem. 266, 11947–11954 [PubMed] [Google Scholar]
- 32. Abdel-Malak N. A., Srikant C. B., Kristof A. S., Magder S. A., Di Battista J. A., Hussain S. N. (2008) Angiopoietin-1 promotes endothelial cell proliferation and migration through AP-1-dependent autocrine production of interleukin-8. Blood 111, 4145–4154 [DOI] [PubMed] [Google Scholar]
- 33. Orecchia A., Lacal P. M., Schietroma C., Morea V., Zambruno G., Failla C. M. (2003) Vascular endothelial growth factor receptor-1 is deposited in the extracellular matrix by endothelial cells and is a ligand for the α5β1 integrin. J. Cell Sci. 116, 3479–3489 [DOI] [PubMed] [Google Scholar]
- 34. Ferrara N., Gerber H. P., LeCouter J. (2003) The biology of VEGF and its receptors. Nat. Med. 9, 669–676 [DOI] [PubMed] [Google Scholar]
- 35. Distler J. H., Hirth A., Kurowska-Stolarska M., Gay R. E., Gay S., Distler O. (2003) Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q. J. Nucl. Med. 47, 149–161 [PubMed] [Google Scholar]