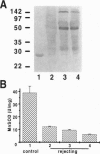
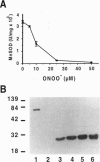
Abstract
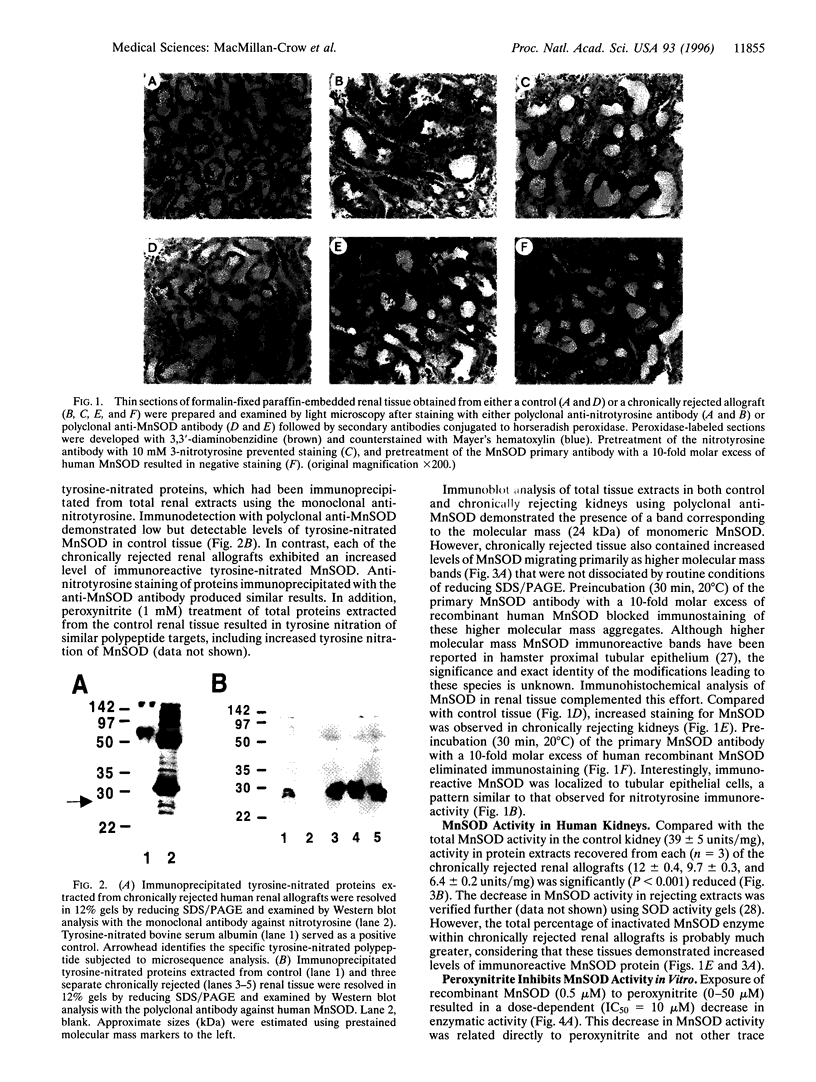
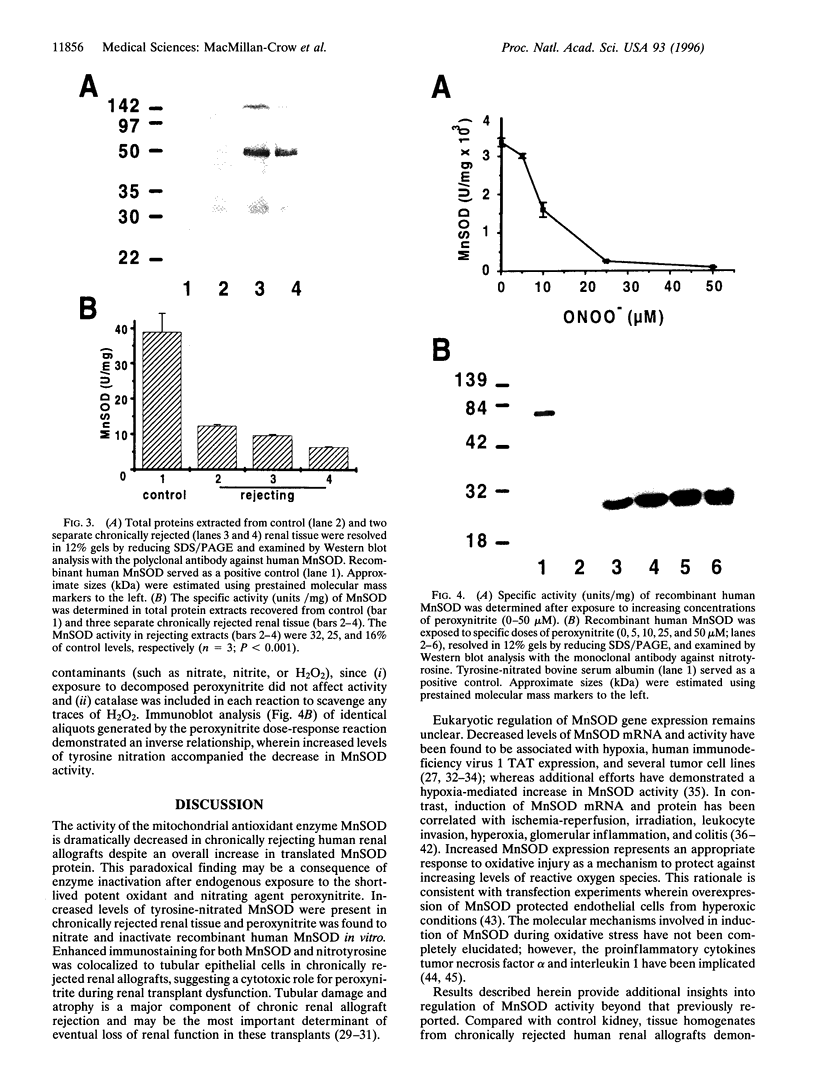
Inflammatory processes in chronic rejection remain a serious clinical problem in organ transplantation. Activated cellular infiltrate produces high levels of both superoxide and nitric oxide. These reactive oxygen species interact to form peroxynitrite, a potent oxidant that can modify proteins to form 3-nitrotyrosine. We identified enhanced immunostaining for nitrotyrosine localized to tubular epithelium of chronically rejected human renal allografts. Western blot analysis of rejected tissue demonstrated that tyrosine nitration was restricted to a few specific polypeptides. Immunoprecipitation and amino acid sequencing techniques identified manganese superoxide dismutase, the major antioxidant enzyme in mitochondria, as one of the targets of tyrosine nitration. Total manganese superoxide dismutase protein was increased in rejected kidney, particularly in the tubular epithelium; however, enzymatic activity was significantly decreased. Exposure of recombinant human manganese superoxide dismutase to peroxynitrite resulted in a dose-dependent (IC50 = 10 microM) decrease in enzymatic activity and concomitant increase in tyrosine nitration. Collectively, these observations suggest a role for peroxynitrite during development and progression of chronic rejection in human renal allografts. In addition, inactivation of manganese superoxide dismutase by peroxynitrite may represent a general mechanism that progressively increases the production of peroxynitrite, leading to irreversible oxidative injury to mitochondria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almond P. S., Matas A., Gillingham K., Dunn D. L., Payne W. D., Gores P., Gruessner R., Najarian J. S. Risk factors for chronic rejection in renal allograft recipients. Transplantation. 1993 Apr;55(4):752–757. doi: 10.1097/00007890-199304000-00013. [DOI] [PubMed] [Google Scholar]
- Alvarez B., Rubbo H., Kirk M., Barnes S., Freeman B. A., Radi R. Peroxynitrite-dependent tryptophan nitration. Chem Res Toxicol. 1996 Mar;9(2):390–396. doi: 10.1021/tx950133b. [DOI] [PubMed] [Google Scholar]
- Asahi M., Fujii J., Suzuki K., Seo H. G., Kuzuya T., Hori M., Tada M., Fujii S., Taniguchi N. Inactivation of glutathione peroxidase by nitric oxide. Implication for cytotoxicity. J Biol Chem. 1995 Sep 8;270(36):21035–21039. doi: 10.1074/jbc.270.36.21035. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S., Carson M., Smith C. D., Koppenol W. H. ALS, SOD and peroxynitrite. Nature. 1993 Aug 12;364(6438):584–584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Crow J. P. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem Soc Trans. 1993 May;21(2):330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- Beckmann J. S., Ye Y. Z., Anderson P. G., Chen J., Accavitti M. A., Tarpey M. M., White C. R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994 Feb;375(2):81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- Bohle A., Grund K. E., Mackensen S., Tolon M. Correlations between renal interstitium and level of serum creatinine. Morphometric investigations of biopsies in perimembranous glomerulonephritis. Virchows Arch A Pathol Anat Histol. 1977 Feb 18;373(1):15–22. doi: 10.1007/BF00432465. [DOI] [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973 Jul;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassina A., Radi R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys. 1996 Apr 15;328(2):309–316. doi: 10.1006/abbi.1996.0178. [DOI] [PubMed] [Google Scholar]
- Castro L., Rodriguez M., Radi R. Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J Biol Chem. 1994 Nov 25;269(47):29409–29415. [PubMed] [Google Scholar]
- Cecka J. M. Early rejection: determining the fate of renal transplants. Transplant Proc. 1991 Feb;23(1 Pt 2):1263–1264. [PubMed] [Google Scholar]
- Chang L. Y., Kang B. H., Slot J. W., Vincent R., Crapo J. D. Immunocytochemical localization of the sites of superoxide dismutase induction by hyperoxia in rat lungs. Lab Invest. 1995 Jul;73(1):29–39. [PubMed] [Google Scholar]
- Crow J. P., Beckman J. S. Reactions between nitric oxide, superoxide, and peroxynitrite: footprints of peroxynitrite in vivo. Adv Pharmacol. 1995;34:17–43. doi: 10.1016/s1054-3589(08)61079-0. [DOI] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Estévez A. G., Radi R., Barbeito L., Shin J. T., Thompson J. A., Beckman J. S. Peroxynitrite-induced cytotoxicity in PC12 cells: evidence for an apoptotic mechanism differentially modulated by neurotrophic factors. J Neurochem. 1995 Oct;65(4):1543–1550. doi: 10.1046/j.1471-4159.1995.65041543.x. [DOI] [PubMed] [Google Scholar]
- Fellström B., Klareskog L., Larsson E., Tufveson G., Wahlberg J., Rönnstrand L., Heldin C. H., Terracio L., Rubin K. Tissue distribution of macrophages, class II transplantation antigens, and receptors for platelet-derived growth factor in normal and rejected human kidneys. Transplant Proc. 1987 Oct;19(5):3625–3627. [PubMed] [Google Scholar]
- Flores S. C., Marecki J. C., Harper K. P., Bose S. K., Nelson S. K., McCord J. M. Tat protein of human immunodeficiency virus type 1 represses expression of manganese superoxide dismutase in HeLa cells. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7632–7636. doi: 10.1073/pnas.90.16.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Lehninger A. L. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J Cell Biol. 1982 Nov;95(2 Pt 1):527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinner W., Tisher C. C., Nick H. S. Regulation of manganese superoxide dismutase in glomerular epithelial cells: mechanisms for interleukin 1 induction. Kidney Int. 1995 Aug;48(2):354–362. doi: 10.1038/ki.1995.303. [DOI] [PubMed] [Google Scholar]
- HUME D. M., MERRILL J. P., MILLER B. F., THORN G. W. Experiences with renal homotransplantation in the human: report of nine cases. J Clin Invest. 1955 Feb;34(2):327–382. doi: 10.1172/JCI103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huie R. E., Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18(4):195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Beckman J. S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992 Nov 1;298(2):446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Chen J., Tsai M., Martin J. C., Smith C. D., Beckman J. S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992 Nov 1;298(2):431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., al-Mehdi A. B., Fisher A. B. Reactive species in ischemic rat lung injury: contribution of peroxynitrite. Am J Physiol. 1995 Aug;269(2 Pt 1):L158–L164. doi: 10.1152/ajplung.1995.269.2.L158. [DOI] [PubMed] [Google Scholar]
- Jacoby D. B., Choi A. M. Influenza virus induces expression of antioxidant genes in human epithelial cells. Free Radic Biol Med. 1994 Jun;16(6):821–824. doi: 10.1016/0891-5849(94)90198-8. [DOI] [PubMed] [Google Scholar]
- Kaur H., Halliwell B. Evidence for nitric oxide-mediated oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett. 1994 Aug 15;350(1):9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- Kerby J. D., Verran D. J., Luo K. L., Ding Q., Tagouri Y., Herrera G. A., Diethelm A. G., Thompson J. A. Immunolocalization of FGF-1 and receptors in glomerular lesions associated with chronic human renal allograft rejection. Transplantation. 1996 Jul 27;62(2):190–200. doi: 10.1097/00007890-199607270-00008. [DOI] [PubMed] [Google Scholar]
- Kiyama S., Yoshioka T., Burr I. M., Kon V., Fogo A., Ichikawa I. Strategic locus for the activation of the superoxide dismutase gene in the nephron. Kidney Int. 1995 Feb;47(2):536–546. doi: 10.1038/ki.1995.67. [DOI] [PubMed] [Google Scholar]
- Kooy N. W., Royall J. A., Ye Y. Z., Kelly D. R., Beckman J. S. Evidence for in vivo peroxynitrite production in human acute lung injury. Am J Respir Crit Care Med. 1995 Apr;151(4):1250–1254. doi: 10.1164/ajrccm/151.4.1250. [DOI] [PubMed] [Google Scholar]
- Land W., Schneeberger H., Schleibner S., Illner W. D., Abendroth D., Rutili G., Arfors K. E., Messmer K. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation. 1994 Jan;57(2):211–217. doi: 10.1097/00007890-199401001-00010. [DOI] [PubMed] [Google Scholar]
- Li Y., Huang T. T., Carlson E. J., Melov S., Ursell P. C., Olson J. L., Noble L. J., Yoshimura M. P., Berger C., Chan P. H. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995 Dec;11(4):376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- Lin K. T., Xue J. Y., Nomen M., Spur B., Wong P. Y. Peroxynitrite-induced apoptosis in HL-60 cells. J Biol Chem. 1995 Jul 14;270(28):16487–16490. doi: 10.1074/jbc.270.28.16487. [DOI] [PubMed] [Google Scholar]
- Lindau-Shepard B., Shaffer J. B., Del Vecchio P. J. Overexpression of manganous superoxide dismutase (MnSOD) in pulmonary endothelial cells confers resistance to hyperoxia. J Cell Physiol. 1994 Nov;161(2):237–242. doi: 10.1002/jcp.1041610207. [DOI] [PubMed] [Google Scholar]
- MURRAY J. E., MERRILL J. P., HARRISON J. H. Kidney transplantation between seven pairs of identical twins. Ann Surg. 1958 Sep;148(3):343–359. doi: 10.1097/00000658-195809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Moreno J. J., Pryor W. A. Inactivation of alpha 1-proteinase inhibitor by peroxynitrite. Chem Res Toxicol. 1992 May-Jun;5(3):425–431. doi: 10.1021/tx00027a017. [DOI] [PubMed] [Google Scholar]
- Nadeau K. C., Azuma H., Tilney N. L. Sequential cytokine dynamics in chronic rejection of rat renal allografts: roles for cytokines RANTES and MCP-1. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8729–8733. doi: 10.1073/pnas.92.19.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley L. W., St Clair D. K., Autor A. P., Oberley T. D. Increase in manganese superoxide dismutase activity in the mouse heart after X-irradiation. Arch Biochem Biophys. 1987 Apr;254(1):69–80. doi: 10.1016/0003-9861(87)90082-8. [DOI] [PubMed] [Google Scholar]
- Oberley T. D., Schultz J. L., Li N., Oberley L. W. Antioxidant enzyme levels as a function of growth state in cell culture. Free Radic Biol Med. 1995 Jul;19(1):53–65. doi: 10.1016/0891-5849(95)00012-m. [DOI] [PubMed] [Google Scholar]
- Packer M. A., Murphy M. P. Peroxynitrite causes calcium efflux from mitochondria which is prevented by Cyclosporin A. FEBS Lett. 1994 May 30;345(2-3):237–240. doi: 10.1016/0014-5793(94)00461-7. [DOI] [PubMed] [Google Scholar]
- Prütz W. A., Mönig H., Butler J., Land E. J. Reactions of nitrogen dioxide in aqueous model systems: oxidation of tyrosine units in peptides and proteins. Arch Biochem Biophys. 1985 Nov 15;243(1):125–134. doi: 10.1016/0003-9861(85)90780-5. [DOI] [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991 Mar 5;266(7):4244–4250. [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991 Aug 1;288(2):481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- Radi R., Rodriguez M., Castro L., Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994 Jan;308(1):89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- Ratner L. E., Hadley G. A., Hanto D. W., Mohanakumar T. Immunology of renal allograft rejection. Arch Pathol Lab Med. 1991 Mar;115(3):283–287. [PubMed] [Google Scholar]
- Risdon R. A., Sloper J. C., De Wardener H. E. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968 Aug 17;2(7564):363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- Rubbo H., Denicola A., Radi R. Peroxynitrite inactivates thiol-containing enzymes of Trypanosoma cruzi energetic metabolism and inhibits cell respiration. Arch Biochem Biophys. 1994 Jan;308(1):96–102. doi: 10.1006/abbi.1994.1014. [DOI] [PubMed] [Google Scholar]
- Russell W. J., Jackson R. M. MnSOD protein content changes in hypoxic/hypoperfused lung tissue. Am J Respir Cell Mol Biol. 1993 Dec;9(6):610–616. doi: 10.1165/ajrcmb/9.6.610. [DOI] [PubMed] [Google Scholar]
- Schainuck L. I., Striker G. E., Cutler R. E., Benditt E. P. Structural-functional correlations in renal disease. II. The correlations. Hum Pathol. 1970 Dec;1(4):631–641. doi: 10.1016/s0046-8177(70)80061-2. [DOI] [PubMed] [Google Scholar]
- Schulz J. B., Matthews R. T., Muqit M. M., Browne S. E., Beal M. F. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole protects against MPTP-induced neurotoxicity in mice. J Neurochem. 1995 Feb;64(2):936–939. doi: 10.1046/j.1471-4159.1995.64020936.x. [DOI] [PubMed] [Google Scholar]
- Seo H. G., Takata I., Nakamura M., Tatsumi H., Suzuki K., Fujii J., Taniguchi N. Induction of nitric oxide synthase and concomitant suppression of superoxide dismutases in experimental colitis in rats. Arch Biochem Biophys. 1995 Dec 1;324(1):41–47. doi: 10.1006/abbi.1995.9932. [DOI] [PubMed] [Google Scholar]
- Shuker D. E., Prevost V., Friesen M. D., Lin D., Ohshima H., Bartsch H. Urinary markers for measuring exposure to endogenous and exogenous alkylating agents and precursors. Environ Health Perspect. 1993 Mar;99:33–37. doi: 10.1289/ehp.939933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom K., Crapo J. D. Adaptation to oxygen by preexposure to hypoxia: enhanced activity of manganese superoxide dismutase. Bull Eur Physiopathol Respir. 1981;17 (Suppl):111–116. [PubMed] [Google Scholar]
- Solez K., Axelsen R. A., Benediktsson H., Burdick J. F., Cohen A. H., Colvin R. B., Croker B. P., Droz D., Dunnill M. S., Halloran P. F. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int. 1993 Aug;44(2):411–422. doi: 10.1038/ki.1993.259. [DOI] [PubMed] [Google Scholar]
- Stadler J., Curran R. D., Ochoa J. B., Harbrecht B. G., Hoffman R. A., Simmons R. L., Billiar T. R. Effect of endogenous nitric oxide on mitochondrial respiration of rat hepatocytes in vitro and in vivo. Arch Surg. 1991 Feb;126(2):186–191. doi: 10.1001/archsurg.1991.01410260074010. [DOI] [PubMed] [Google Scholar]
- Szabó C., Zingarelli B., O'Connor M., Salzman A. L. DNA strand breakage, activation of poly (ADP-ribose) synthetase, and cellular energy depletion are involved in the cytotoxicity of macrophages and smooth muscle cells exposed to peroxynitrite. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):1753–1758. doi: 10.1073/pnas.93.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill C. L., Stevenot S. A., Campbell-Thompson M., Nick H. S., Valentine J. F. Induction and immunolocalization of manganese superoxide dismutase in acute acetic acid-induced colitis in the rat. Gastroenterology. 1995 Sep;109(3):800–811. doi: 10.1016/0016-5085(95)90387-9. [DOI] [PubMed] [Google Scholar]
- Torres J., Darley-Usmar V., Wilson M. T. Inhibition of cytochrome c oxidase in turnover by nitric oxide: mechanism and implications for control of respiration. Biochem J. 1995 Nov 15;312(Pt 1):169–173. doi: 10.1042/bj3120169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U. G., Werber M. M., Beck Y., Hartman J. R., Frolow F., Sussman J. L. Characterization of crystals of genetically engineered human manganese superoxide dismutase. J Mol Biol. 1989 Apr 20;206(4):787–788. doi: 10.1016/0022-2836(89)90586-x. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]
- Wharton M., Granger D. L., Durack D. T. Mitochondrial iron loss from leukemia cells injured by macrophages. A possible mechanism for electron transport chain defects. J Immunol. 1988 Aug 15;141(4):1311–1317. [PubMed] [Google Scholar]
- Wong G. H. Protective roles of cytokines against radiation: induction of mitochondrial MnSOD. Biochim Biophys Acta. 1995 May 24;1271(1):205–209. doi: 10.1016/0925-4439(95)00029-4. [DOI] [PubMed] [Google Scholar]