Abstract
Background
As patients experience transitions in care, there is a need to share information between care providers in an accurate and timely manner. With the push towards electronic medical records and other electronic tools (eTools) (and away from paper-based health records) for health information exchange, there remains uncertainty around the impact of eTools as a form of communication.
Objective
To examine the impact of eTools for health information exchange in the context of care coordination for individuals with chronic disease in the community.
Data Sources
A literature search was performed on April 26, 2012, using OVID MEDLINE, OVID MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, EBSCO Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Wiley Cochrane Library, and the Centre for Reviews and Dissemination database, for studies published until April 26, 2012 (no start date limit was applied).
Review Methods
A systematic literature search was conducted, and meta-analysis conducted where appropriate. Outcomes of interest fell into 4 categories: health services utilization, disease-specific clinical outcomes, process-of-care indicators, and measures of efficiency. The quality of the evidence was assessed individually for each outcome. Expert panels were assembled for stakeholder engagement and contextualization.
Results
Eleven articles were identified (4 randomized controlled trials and 7 observational studies). There was moderate quality evidence of a reduction in hospitalizations, hospital length of stay, and emergency department visits following the implementation of an electronically generated laboratory report with recommendations based on clinical guidelines. The evidence showed no difference in disease-specific outcomes; there was no evidence of a positive impact on process-of-care indicators or measures of efficiency.
Limitations
A limited body of research specifically examined eTools for health information exchange in the population and setting of interest. This evidence included a combination of study designs and was further limited by heterogeneity in individual technologies and settings in which they were implemented.
Conclusions
There is evidence that the right eTools in the right environment and context can significantly impact health services utilization. However, the findings from this evidence-based analysis raise doubts about the ability of eTools with care-coordination capabilities to independently improve the quality of outpatient care. While eTools may be able to support and sustain processes, inefficiencies embedded in the health care system may require more than automation alone to resolve.
Plain Language Summary
Patients with chronic diseases often work with many different health care providers. To ensure smooth transitions from one setting to the next, health care providers must share information and coordinate care effectively. Electronic medical records (eTools) are being used more and more to coordinate patient care, but it is not yet known whether they are more effective than paper-based health records. In this analysis, we reviewed the evidence for the use of eTools to exchange information and coordinate care for people with chronic diseases in the community. There was some evidence that eTools reduced the number of hospital and emergency department visits, as well as patients' length of stay in the hospital, but there was no evidence that eTools improved the overall quality of patient care.
Background
In July 2011, the Evidence Development and Standards (EDS) branch of Health Quality Ontario (HQO) began developing an evidentiary framework for avoidable hospitalizations. The focus was on adults with at least 1 of the following high-burden chronic conditions: chronic obstructive pulmonary disease (COPD), coronary artery disease (CAD), atrial fibrillation, heart failure, stroke, diabetes, and chronic wounds. This project emerged from a request by the Ministry of Health and Long-Term Care for an evidentiary platform on strategies to reduce avoidable hospitalizations.
After an initial review of research on chronic disease management and hospitalization rates, consultation with experts, and presentation to the Ontario Health Technology Advisory Committee (OHTAC), the review was refocused on optimizing chronic disease management in the outpatient (community) setting to reflect the reality that much of chronic disease management occurs in the community. Inadequate or ineffective care in the outpatient setting is an important factor in adverse outcomes (including hospitalizations) for these populations. While this did not substantially alter the scope or topics for the review, it did focus the reviews on outpatient care. HQO identified the following topics for analysis: discharge planning, in-home care, continuity of care, advanced access scheduling, screening for depression/anxiety, self-management support interventions, specialized nursing practice, and electronic tools for health information exchange. Evidence-based analyses were prepared for each of these topics. In addition, this synthesis incorporates previous EDS work, including Aging in the Community (2008) and a review of recent (within the previous 5 years) EDS health technology assessments, to identify technologies that can improve chronic disease management.
HQO partnered with the Programs for Assessment of Technology in Health (PATH) Research Institute and the Toronto Health Economics and Technology Assessment (THETA) Collaborative to evaluate the cost-effectiveness of the selected interventions in Ontario populations with at least 1 of the identified chronic conditions. The economic models used administrative data to identify disease cohorts, incorporate the effect of each intervention, and estimate costs and savings where costing data were available and estimates of effect were significant. For more information on the economic analysis, please contact either Murray Krahn at murray.krahn@theta.utoronto.ca or Ron Goeree at goereer@mcmaster.ca.
HQO also partnered with the Centre for Health Economics and Policy Analysis (CHEPA) to conduct a series of reviews of the qualitative literature on “patient centredness” and “vulnerability” as these concepts relate to the included chronic conditions and interventions under review. For more information on the qualitative reviews, please contact Mita Giacomini at giacomin@mcmaster.ca.
The Optimizing Chronic Disease Management in the Outpatient (Community) Setting mega-analysis series is made up of the following reports, which can be publicly accessed at http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/ohtas-reports-and-ohtac-recommendations.
Optimizing Chronic Disease Management in the Outpatient (Community) Setting: An Evidentiary Framework
Discharge Planning in Chronic Conditions: An Evidence-Based Analysis
In-Home Care for Optimizing Chronic Disease Management in the Community: An Evidence-Based Analysis
Continuity of Care: An Evidence-Based Analysis
Advanced (Open) Access Scheduling for Patients With Chronic Diseases: An Evidence-Based Analysis
Screening and Management of Depression for Adults With Chronic Diseases: An Evidence-Based Analysis
Self-Management Support Interventions for Persons With Chronic Diseases: An Evidence-Based Analysis
Specialized Nursing Practice for Chronic Disease Management in the Primary Care Setting: An Evidence-Based Analysis
Electronic Tools for Health Information Exchange: An Evidence-Based Analysis
Health Technologies for the Improvement of Chronic Disease Management: A Review of the Medical Advisory Secretariat Evidence-Based Analyses Between 2006 and 2011
Optimizing Chronic Disease Management Mega-Analysis: Economic Evaluation
How Diet Modification Challenges Are Magnified in Vulnerable or Marginalized People With Diabetes and Heart Disease: A Systematic Review and Qualitative Meta-Synthesis
Chronic Disease Patients' Experiences With Accessing Health Care in Rural and Remote Areas: A Systematic Review and Qualitative Meta-Synthesis
Patient Experiences of Depression and Anxiety With Chronic Disease: A Systematic Review and Qualitative Meta-Synthesis
Experiences of Patient-Centredness With Specialized Community-Based Care: A Systematic Review and Qualitative Meta-Synthesis
Objective of Analysis
The objective of this analysis was to examine the impact of electronic tools (eTools) for health information exchange in the context of care coordination for individuals with chronic disease in the community. Of particular interest was the use of eTools by community-based primary care physicians (PCPs) to share information in an accurate and timely manner with laboratories, pharmacies, and other health care providers as patients transition between PCPs and acute care or other specialists. This evidence-based analysis is a part of the mega-analysis Optimizing Chronic Disease Management in the Community.
Clinical Need and Target Population
Continuity of Care
Continuity of care can be categorized into 3 domains: relational, management, and informational. Informational continuity of care (the focus of this analysis) is the continuous flow of information between multiple care providers across different parts of the health care system.
Overall sustained continuity of care has been associated with fewer hospitalizations and emergency department (ED) visits, as well as improved patient satisfaction and receipt of preventive services. (1) As patients experience transitions in care (such as between primary care, specialists, and hospitalists) they are at increased risk for adverse events as a result of errors in information transmission. (2) As such, formal efforts towards informational continuity of care have become a key component of care coordination. (3)
Care Coordination
Care coordination involves the exchange of information about a patient's care history, current health status, and/or care plan. (4) It accompanies breaks in continuity of care and is carried out to facilitate the appropriate delivery of health care services by various health care providers. (4) Even the best continuity of care efforts cannot entirely eliminate the need for care coordination during patient transitions; for example, there will always be a need for care coordination between PCPs and specialists.
As a patient navigates the health care system, complex networks of providers require careful care coordination to ensure information continuity (Figure 1). To be well informed, PCPs must coordinate with specialists, EDs, hospital-based physicians, and sources of diagnostic data (e.g., laboratory and imaging results), as well as communicating with nurses and other allied health care professionals. Failures in care coordination can contribute to serious adverse events. (4)
Figure 1: Example of Complex Flow of Information Involved in Care Coordination.
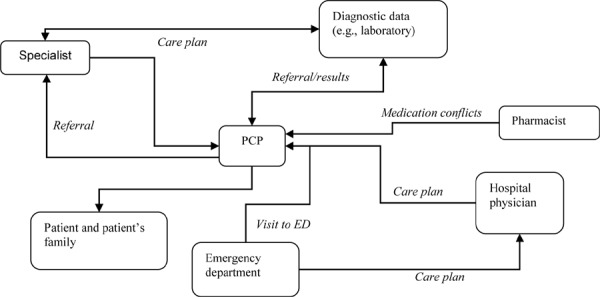
Abbreviations: ED, emergency department; PCP, primary care physician.
Tools for Care Coordination
Care coordination may take many different forms. Informal methods include “hallway handoffs” (i.e., person-to-person communication), e-mail, phone calls, and even sticky notes on patient charts. (5) More formal techniques involve standardized levels of information and include structured person-to-person handoffs, discharge summaries with medication history, and organized shared care. (5)
Care coordination is increasingly being conducted using computer-based programs to facilitate information transfer and shared care. (6) There are a number of perceived potential benefits to this approach, including improved provider communication and coordination (as a result of standardized documentation), and speed of availability. (4;5) However, some health care providers are hesitant to adopt computer-assisted management; reasons for concern include security and privacy issues, depersonalization of care, and the up-front costs of incorporating an electronic system. (7)
Care Coordination and Chronic Disease
Individuals with a chronic disease often have multiple concurrent chronic conditions and complications that require regular visits with a number of different specialists in addition to their PCP. As well, these patients may have intermittent interactions with the ED and other acute care settings. (2;3) As such, they may be at increased risk for severe adverse events if information does not flow between health care settings in a timely and accurate manner. (2;3;8) Given the potential patient safety risks associated with poor care coordination, many institutions and health care systems are exploring means of improving care coordination. (6)
Technology
Electronic Tools for Health Information Exchange
Currently, the use of eTools ranges from a single point of information exchange between 2 health care providers to real-time complete sharing of patient electronic medical records (EMRs) between everyone involved in a patient’s care. Given the current rate of evolution of computer-assisted communication in health care, the terminology used to describe eTools is almost as varied as the tools themselves. Table 1 describes common terminology and potential applications for a number of eTools used in modern health care systems.
Table 1: Description and Potential Applications for Various eTools.
| eTool | Description | Application |
|---|---|---|
| Alerts and reminders | A system that uses patient-level data and clinical guidelines to prompt physicians with alerts and reminders for patient check-ups and treatments | Usually part of a CPOE or EMR system |
| CDSS | A system that uses patient-level data and clinical guidelines to prompt physicians with treatment and prevention opportunities for their patients | May be part of a comprehensive EMR system or implemented as a stand-alone system |
| CPOE | A system to share physician orders with multiple care providers, including nurses, pharmacists, and other allied health care professionals | May be part of a comprehensive EMR system or implemented as a stand-alone system |
| Disease registry | A system that maintains lists of patients with a particular diagnosis or who require routine health maintenance manoeuvres | Used to track patients who need regular follow-up and to conduct population health status and service utilization monitoring |
| EHR | Linked health records to identify a patient’s interaction with multiple points of contact in the health care system | Used to monitor and manage the population health to identify trends in prevalence rates and risk assessments |
| EMR | A comprehensive health record at the level of the patient within a single health care system | Typically applied at the level of a single institution or network; may or may not be accessible to health care professionals outside of that institution (e.g., PCPs sharing EMRs with hospital physicians) |
| e-Prescribing | A system to add, adjust, edit, monitor, and share prescribing orders | May be part of a comprehensive EMR system or implemented as a stand-alone system |
| Health information system or health information tool | Generic term to describe electronic systems that manage, store, and/or retrieve health data | May be used to describe any combination of eTools used in health information management |
| PACS | A system to manage, store, and retrieve results of certain health tests, such as an MRI or CT scan | May be part of a comprehensive EMR system or implemented as a stand-alone system |
| Patient portal | Extensions of existing EMR systems that allow patients to view and interact with at least part of the EMR under the responsibility of physicians and hospitals | Used to facilitate patient interactions with their physicians and other health care professionals; may be used to assist with self-management programs that are guided and monitored by health care providers |
| PHR | Patient-accessible health record; may or may not include a mechanism to facilitate monitoring by, and communication with, health care providers | May be used to assist with patient self-management, specifically with chronic disease (e.g., monitoring blood glucose levels in patients with diabetes). Usually used to give patients access to their own health records |
| Risk assessment tool | A system that uses patient-level data and validated risk assessment tools to identify patients at risk (e.g., for diabetes, cardiovascular disease, or rehospitalization) | May be implemented at the level of the individual patient, physician practice, or population level |
Abbreviations: CDSS, clinical decision support system; CPOE, computerized physician (or provider) order entry; CT, computed tomography; EHR, electronic health record; EMR, electronic medical record; eTool, electronic tool; MRI, magnetic resonance imaging; PACS, picture archiving communication system; PCP, primary care physician; PHR, personal (or patient) health record.
Dissemination of eTools for Health Information Exchange
The adoption of EMRs has been steadily on the rise. One study commissioned by Canada Health Infoway examined automation in general practice across 10 countries (8 European nations, Australia, and New Zealand). (9) The authors found that nearly all physicians in these countries had computers (90 to 100%) and that in Denmark and Norway, more than 75% of physician offices conducted business in a “paper-light” manner. (9) Overall, the most common application was medication prescribing and monitoring, whether or not it was a mandated component of government regulations. (9)
Denmark is considered a successful example of the adoption of information and communication technology in PCP offices; it had more than 80% dissemination of EMRs among its PCPs by 2009. (10) EMRs were equipped, at a minimum, with the ability to record patient appointments, generate medication prescriptions, send orders and requests to laboratories, include clinical notes, and receive results from other physicians (including discharge summaries). (10) Additionally, as many as 60% of all physicians had EMRs in 2009, facilitating communication with specialists and hospitals for referrals and shared-care functionalities. (10) Where success in EMR uptake has been observed, it has largely been attributed to a central body as the national health system integrator; in the case of Denmark, this is the government agency MedCom. (10) Similar trends have been observed in the United Kingdom, where there has been substantial uptake in computer use in primary care since the late 1980s, specifically to assist with the management of diabetes care. (11) In 1988, 20% of family practices had computers; that number rose to 70% by 1992 and 92% by 1997. (11)
In contrast, North America has been significantly slower to reach the same degree of uptake. The United States Centers for Disease Control and Prevention determined via survey that as of 2010, 48.3% of physicians reported using at least partial EMR/electronic health record (EHR) systems in their practice. (12) This was an increase of 6.3% from 1 year earlier, but part of a growth trend since 2003, when only 17.3% of physicians reported using EMRs/EHRs. (12)
Ontario Context
Ontario’s primary health teams are generally supportive of computer-assisted communication. (5) There is consensus that eTools can facilitate the sharing of information, providing greater ease, speed, and accuracy. (5) However, some health care providers maintain a preference for face-to-face communication. (5) This may be attributed to lack of time to sit and read email, lack of familiarity with technology, and/or concerns that it would be time-consuming to learn. (5)
The Ontario government agency e-Health Ontario is mandated to “play a leading role in harnessing [information technology] and innovation to improve patient care, safety and access…” (13) Among its numerous initiatives is the creation of a funding program to encourage community physicians to adopt EMRs and the launch of a comprehensive e-prescribing system at 2 pilot sites. (14)
OntarioMD, an eHealth Ontario partner agency, operates the “new EMR adopter” funding program. This program grants physicians as much as $30,000 (Cdn) in subsidies over the first 3 years of EMR implementation in a previously paper-based practice. (15) The program has a predefined list of standards that must be met for an EMR system to be eligible. As of February 2012, more than 7,000 community-based physicians (including both general practitioners [GPs] and specialists) had been funded via government programs. (16)
Evidence-Based Analysis
Research Questions
What is the impact of eTools for health information exchange on patient outcomes and health services utilization when used to improve the care coordination of adults with chronic disease?
What specifications of eTools contribute to their effectiveness?
Research Methods
Literature Search
Search Strategy
A literature search was performed on April 26, 2012, using OVID MEDLINE, OVID MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, EBSCO Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Wiley Cochrane Library, and the Centre for Reviews and Dissemination database, for studies published before April 26, 2012 (no start date limit was applied). Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search.
Inclusion Criteria*
English language, full-reports
published before April 26, 2012
tools and systems for electronic health information exchange that facilitate provider-provider communication in the outpatient community setting (including but not limited to referrals, prescribing, computerized physician order entries, and intra-team communication)
covering 1 or more of the chronic conditions of interest (chronic obstructive pulmonary disease, coronary artery disease, congestive heart failure, atrial fibrillation, diabetes, stroke, chronic wounds) or otherwise identified with general terms for chronic conditions or multiple chronic conditions/multi-morbidity
Exclusion Criteria*
eTools to facilitate communication between patient and health care provider
patient health records and patient self-monitoring devices
database risk-assessment tools
eTools to facilitate improved management or care of patients within a single physician’s practice (e.g., clinical decision-support and patient data management systems)
studies where no outcomes of interest could be extracted, or where there was substantial confounding in the exposure of interest
letters, comments, editorials, surveys, and other publications based primarily on expert opinion
Outcomes of Interest
Primary Outcomes
-
health services utilization
– hospitalizations
– readmissions
– length of stay
– ED use
– mortality
– health-related quality of life
– patient satisfaction
disease-specific clinical outcomes (e.g., hemoglobin A1c [HbA1c], blood pressure, total cholesterol)
Secondary Outcomes
-
process-of-care indicators
– achievement of a clinical outcome (e.g., HbA1c < 7%)
– rate of clinical tests/examinations conducted or recorded (e.g., rate of conducting eye examinations among patients with diabetes)
-
measures of efficiency
– record keeping (e.g., accuracy of information)
– informational continuity (e.g., time to receive discharge summary)
– time
– subjective impact on efficiency (e.g., self-identified provider workload)
Statistical Analysis
Where appropriate, a meta-analysis was performed using Review Manager Version 5. (17) A fixed-effect model was used, unless significant heterogeneity was observed (P ≤ 0.10); then, a random-effects model was used to address significant heterogeneity. A P value of < 0.05 was considered statistically significant.
Where meta-analysis was not appropriate and where sufficient data were provided, effect estimates were calculated and presented descriptively. Some studies presented adjusted effect estimates; these were extracted directly, but they limited the potential for meta-analysis.
Patient-level data were prioritized over population-level data (e.g., number of ED visits per patient versus proportion of the population who had an ED visit), as they were considered to more accurately represent the impact on health services utilization.
Quality of Evidence
The quality of the body of evidence for each outcome was examined according to the GRADE Working Group criteria. (18) The overall quality was determined to be very low, low, moderate, or high using a step-wise structural methodology.
Study design was the first consideration; the starting assumption was that randomized controlled trials (RCTs) are high quality, whereas, observational studies are low quality. Five additional factors—risk of bias, inconsistency, indirectness, imprecision and publication bias—were then taken into account. Limitations in these areas resulted in downgrading the quality of evidence. Finally, 3 main factors that may raise the quality of evidence were considered: large magnitude of effect, dose response gradient, and accounting for all residual confounding factors. (18) For more detailed information, please refer to the latest series of GRADE articles. (18)
As stated by the GRADE Working Group, the final quality score can be interpreted using the following definitions:
| High | Very confident that the true effect lies close to that of the estimate of the effect |
| Moderate | Moderately confident in the effect estimate—the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| Low | Confidence in the effect estimate is limited—the true effect may be substantially different from the estimate of the effect |
| Very Low | Very little confidence in the effect estimate – the true effect is likely to be substantially different from the estimate of effect |
Results of Evidence-Based Analysis
The database search yielded 2,723 citations published before April 26, 2012 (with duplicates removed). Articles were excluded based on information in the title and abstract. The full texts of potentially relevant articles were obtained for further assessment. Figure 2 shows the breakdown of when and for what reason citations were excluded in the analysis.
Figure 2: Citation Flow Chart.
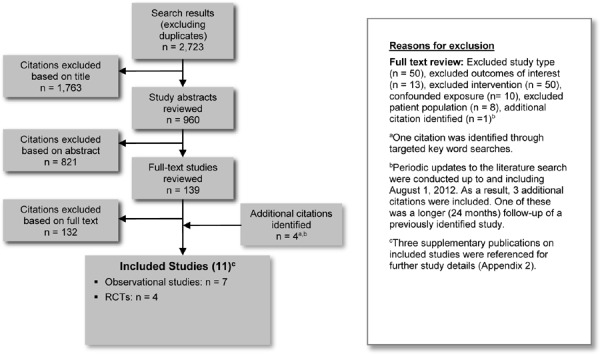
Seven studies (3 RCTs and 4 observational studies) met the inclusion criteria. The reference lists of the included studies were hand searched to identify any additional potentially relevant studies, and 4 additional citations (1 RCT and 3 observational studies) were included, for a total of 11 citations.
For each included study, the study design was identified and is summarized in Table 2, which is a modified version of a hierarchy of study design by Goodman. (19)
Table 2: Body of Evidence Examined According to Study Design.
| Study Design | Number of Eligible Studies |
|---|---|
| RCT Studiesa | |
| Systematic review of RCTs | |
| Large RCT | 4 |
| Small RCT | |
| Observational Studiesb | |
| Systematic review of non-RCTs with contemporaneous controls | |
| Non-RCT with contemporaneous controls | 2 |
| Systematic review of non-RCTs with historical controls | |
| Non-RCT with historical controls | 1 |
| Database, registry, or cross-sectional study | |
| Case series | |
| Retrospective review, modelling | 4 |
| Studies presented at an international conference | |
| Expert opinion | |
| Total | 11 |
Abbreviation: RCT, randomized controlled trial.
Includes 2 cluster RCTs.
Includes 3 studies that are self-identified as controlled trials, but methodology is that of observational studies.
Summary of Other Evidence
Ten systematic reviews based on original research were identified but not included in the analysis. (20-29) No systematic review was found to be representative of the population, setting, and interventions of interest. Most were narrative reviews that applied no meta-analyses or regression analyses.
The reviews identified components of data management systems that may contribute to the improved care of patients with chronic disease. All acknowledged that there are limitations in the current body of literature, mostly because of significant heterogeneity among interventions and varying degrees of integration of eTools in established organizational structures. None of the reviews identified eTool components that could be clearly attributed to the optimization of chronic disease management in the community, but additional systematic reviews have noted the potential impact of health information exchange in a general primary care population. (30;31)
Characteristics of Included Studies
Eleven studies were included in the evidence-based analysis (Table 3). The studies were from 4 different countries (Australia 1, Netherlands 1, United Kingdom 1, United States 8) and included 4 different populations of interest (coronary artery disease 1, diabetes 7, heart failure 1, multiple chronic conditions 2). Study sample sizes ranged from 235 to 27,207 patients; 1 study reported number of patient encounters (125,700).
Table 3: Description of Included Studies.
| Author, Year | Country, Sites | Study Design | Length of Study | Patient Population | Mean Age, yearsa (Intervention/Control) | Female, % (Intervention/Control) | Sample Size, nb (Intervention/Control) | Loss to Follow-up (Intervention/Control) | List of All Outcomes Reported |
|---|---|---|---|---|---|---|---|---|---|
| Branger et al, 1999 (32) | Netherlands(Apeldoorn region) | Case-control | 1 year | Patients with diabetes | 58/62 | 53/53 | 215/60 | None | Number of tests recorded per patient for 11 clinical tests; number of patient contacts with GP and consultant; number of letters between GP and consultants |
| Cebul et al, 2011 (38) | United States (Ohio) | Case-control | 1 year | Adults (18-75 years) with diabetes | 58/53 | 52/57 | 24,547/2,660 | NA | 4 measures of care, 5 clinical outcomes, and composite outcomes for each; trends by type of clinical practice and insurance |
| Crosson et al, 2012 (39) | United States (New Jersey, Pennsylvania) | Case-control | 3 years | Patients with diabetes | 59/61 | 53/51 | 306/492 | 21 practices withdrew, closed, or otherwise excluded after study recruitment | 5 process-of-care measures, 3 treatment measures, 3 outcome measures, and composite outcomes for each |
| Graumlich et al, 2009 (34) | United States (Illinois) | Cluster RCT | 6 months | Patients (18-98 years) with the probability of repeat admission ≥ 0.40c | Age presented categorically: 27% were 55-64 years/30% were 18-44 years | 57/53 | 316/315 | 29 (10 deaths)/32 (10 deaths) | Readmissions, ED visits, adverse events, type of adverse event, time to readmission, time to ED visit, time to receive discharge summary |
| Henderson et al, 2010 (36) | Australia (multiple regions) | Non-RCT | 16 months | All patients in GP practiced | NR; logistic regression model adjusted for differences in baseline characteristics | NR; logistic regression model adjusted for differences in baseline characteristics | 106,900/18,800 patient encounters | NA | Consultation length; multivariate analyses for 33 other quality indicators, most of which are rate of conducting clinical tests |
| Herrin et al, 2012 (40) | United States (Texas) | Case-control | 5 years | Patients with diabetes and ≥ 40 years of age | Age presented categorically: 34% were 51-60 years/38% were 51-60 years | 50/50 | 6,376/7,675 patients 10,171/35,033 patient years |
NA; patient years are accounted | 11 process-of-care measures, 6 clinical outcome thresholds, and composite of these outcomes |
| Khan et al, 2010 (35) | United States (Vermont, New York) | Cluster RCT | 32 months(average) | Adult patients with diabetes | 62/63 | 52/50 | 3,856/3,512 | NR | Hospital admission, readmission, length of stay, ED admission, money in patient charges; stratified by gender and age |
| Lester et al, 2005 (33) | United States (Massachusetts) | RCT | 12 months | Adult patients (>30 years of age) with CAD or CAD risk equivalent | 64/62 | 57/60 | 118/117 | All randomized patients received allocated intervention; only 81 patientsin the intervention group and 82 in the control group had LDL-C measures taken | Proportion with change in statin prescription, time to change in prescription, repeat LDL-C, reason for deferred action after referral |
| Montori et al, 2002 (37) | United States (Minnesota—Mayo clinic) | Cluster RCT | 24 months | Adult (≥18 years of age) patients with diabetes (type I or II) | 69/72 | 56/60 | 399/208 | NR | 12 performance measures of compliance with clinical tests, 8 metabolic outcomes, 3 health care use outcomes |
| Walsh et al, 2012 (41) | United States(multiple regions) | Case-control | 24 months | Patients with heart failuree | 70 (median) | 28 | 4,220/2,950 | NR | Physician practice characteristics, conformity with 7 quality measures |
| Wells et al, 1996 (42) | United Kingdom (Bedfordshire) | Case series | 23 months | Patients with diabetes | NR | NR | 2,049 (after)/ 1,190 (before) | NR | Compliance with 9 performance measures |
Abbreviations: CAD, coronary artery disease; ED, emergency department; GP, general practitioner; LDL-C, low-density lipoprotein cholesterol; NA, not applicable; NR, not reported; RCT, randomized controlled trial.
Unless otherwise specified.
Number of patients unless otherwise specified.
Based on age, health status, number of physician visits, CAD, and diabetes, among other factors.
Results stratified and 3 groups of interest were identifiable: 1) diabetes; 2) left ventricular failure, ischemic heart disease, diabetes, or cerebrovascular disease; and 3) atrial fibrillation.
Based on myocardial infarction history and left ventricular systolic dysfunction.
The eTools applied in each study were unique, as were the conditions under which they were applied (Table 4). Some were used to coordinate care between hospital-based and outpatient/community-based health care providers; (32-35) some were applied in a community setting to help coordinate care between PCPs and other health care professionals (e.g., nurses and pharmacists); (36;37) the rest were applied in multiple care coordination efforts and/or did not specify their points of care coordination communication. (38-42)
Table 4: Description of Individual Technologies Applied.
| Author, Year | Care Coordination Communication Sites |
Intervention | Control | Description and Context of Intervention Technology |
|---|---|---|---|---|
| Branger et al, 1999 (32) | PCPs (GPs) ↕ Hospital outpatient clinic diabetes specialists |
GPs with the highest number of referred patients through the EDI system to the specialists in the outpatient clinic (20 GPs; 215 patients) | GPs not in the intervention group (12 GPs; 60 patients) | EDI system that fully replaced paper records and has the capability for communication with other electronic information systems; an EDI system has been in place in the study region since 1989, with increasing levels of detail and sophistication since its inception |
| Cebul et al, 2011 (38) | PCPs ↕ Various sources, including fellow health care team members |
Practices using EHRs (3 care organizations; 33 practices; 516 providers; 24,547 patients) | Practices using paper-based records (4 care organizations; 13 practices; 53 providers; 2,660 patients) | Details of individual EHR systems were not specified |
| Crosson et al, 2012 (39) | PCPs ↕ Various sources, including fellow health care team members |
Practices using EHRs for the duration of the study (16 practices; 306 patients at end of study) | Practices not using EHRs (therefore paper records) for the duration of the study (26 practices; 492 patients at end of study) | Details of individual EHR systems were not specified; at the time of this study there were local incentive programs designed to encourage the adoption of EHRs by smaller practices, but it is not clear whether the funders had required components to be eligible for the financial incentive programs |
| Graumlich et al, 2009 (34) | Hospital internists ↓ Outpatient physicians and dispensing pharmacists in the community |
Use of computer software to automatically generate personalized discharge summaries (35 physicians; 316 patients) | Usual care, handwritten discharge summaries (35 physicians; 315 patients) | A CPOE with automatically generated discharge documents, including prescriptions with details for dispensing pharmacist; included decision support software |
| Henderson et al, 2010 (36) | GPs, PCPs ↕ Various health care providers, including laboratories, pharmacies, and specialists |
GPs who were clinical computer users defined as using their computers for prescribing or ordering tests or medical records; this may or may not include the Internet or email (1,069 GPs) | GPs using computers for administrative functions only; this may or may not include the Internet or email capability; this group also included any physicians who did not use a computer at all (188 GPs) | Details of individual computer programs used were not specified; at the time of this study over 97% of Australian GPs had a computer available at their practice |
| Herrin et al, 2012 (40) | GPs, PCPs ↕ Various sources, including fellow health care team members |
Practices using EHRs at some point during the study period (6,376 unique patients throughout study duration of 5 years; 10,017 patient years) | Practices and patients never exposed to EHRs (7,675 unique patients throughout study duration of 5 years; 35,033 patient years) | The local health authority implemented a network of EHRs rolled out to various primary care practices over the study period; these EHRs included CDSSs, order entry, and alerts/reminders, in addition to patient data management and shared care capabilities |
| Khan et al, 2010 (35) | Laboratories ↓ PCPs |
Vermont Diabetes Information System (3,856 patients) | Usual care (3,512 patients) | The Vermont Diabetes Information System compiles lab results, maintains a registry and produces a report for primary care providers and patients; this report includes guideline-based recommendations, and alert letters are issued on an as-needed basis; a regional network of hospital-based laboratories has been in place since 1996, and at the time of the study it included 13 of the 14 regional hospitals |
| Lester et al, 2005 (33) | Hospital specialists ↓ PCPs and patients |
Automated identification of patients and emailed outreach to PCPs of patients at high risk; email included best practice decision support, as well as electronic physician order entry and integration into existing EHR (118 patients) | Usual care with EHR system (117 patients) | A total of 14 physicians were invited to participate; each physician had patients in both the intervention and control groups; to be eligible, physicians must have already demonstrated competence with an EHR system |
| Montori et al, 2002 (37) | Primary care (physicians, nurses, clinical assistants, and diabetes educators) ↕ Various sources, including fellow health care team members |
DEMS (16 PCPs; 6,336 patients at end of study) | Before introduction of DEMS (6,646 patients at start of study) | DEMS includes laboratory, medication, examination, and clinical notes in a manner for sharing among different health care providers; it also includes reminders based on clinical guidelines |
| Walsh et al, 2012 (41) | Not specified | Practices using an EHR alone or in combination with paper records (78 practices; 4,220 patients) | Practices using only paper records (61 practices; 2,950 patients) | Details of individual EHR systems were not specified; EHR use was self-identified in the IMPROVE-HF survey |
| Wells et al, 1996 (42) | GPs ↕ Various sources, including local hospital, diabetes specialist centre, and fellow health care team members |
Shared care as facilitated by the introduction of a computerized system to support diabetes management | Baseline (1,190 patients at start of study) | Information regarding a patient in response to computer-generated prompts or otherwise of clinical importance was transcribed into a central database at the diabetes information centre, which was opened in 1990 to facilitate a shared care structure between the community and hospital physicians |
Abbreviations: CDSS, clinical decision support system; CPOE, computerized physician (or provider) order entry; DEMS, diabetes electronic management system; EDI, electronic data interchange; EHR, electronic health record; GP, general practitioner; PCP, primary care physician.
The quality of evidence was evaluated individually for each outcome. When evaluating the quality of evidence, further study details were sought from additional articles published on the same study if possible (Appendix 2). Details of the quality of evidence evaluation are available in Appendix 3.
Analysis
The included studies reported on 5 of the 8 primary outcomes of interest (Table 5). No studies reported mortality, health-related quality of life, or patient satisfaction. Studies also reported a number of process-of-care indicators and measures of efficiency.
Table 5: Studies and Outcomes by Chronic Disease Group.
| Author, Year | Primary Outcomes of Interest | Process of Care Indicator |
Measures of Efficiency | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Health Services Utilization | Disease-Specific Clinical Outcomes | |||||||||||
| Hospitalizations | Length of Stay | ED Visits | Readmissions | HbA1c | BP | Cholesterol | Triglycerides | Othera | Achievement of Clinical Guidelines | |||
| Diabetes | ||||||||||||
| Branger et al, 1999 (32) | ✓ | ✓ | ✓ | |||||||||
| Cebul et al, 2011 (38) | ✓ | ✓ | ||||||||||
| Crosson et al, 2012 (39) | ✓ | ✓ | ||||||||||
| Herrin et al, 2012 (40) | ✓ | ✓ | ||||||||||
| Khan et al, 2010 (35) | ✓ | ✓ | ✓ | |||||||||
| Montori et al, 2002 (37) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Wells et al, 1996 (42) | ✓ | |||||||||||
| CAD | ||||||||||||
| Lester et al, 2005 (33) | ✓ | ✓ | ✓ | |||||||||
| Heart Failure | ||||||||||||
| Walsh et al, 2012 (41) | ✓ | |||||||||||
| Multiple Chronic Conditions | ||||||||||||
| Graumlich et al, 2009 (34) | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Henderson, et al 2010 (36) | ✓ | |||||||||||
Abbreviations: BP, blood pressure; CAD, coronary artery disease; ED, emergency department; HbA1c, hemoglobin A1c; PCP, primary care physician.
Includes PCP visits and adverse events.
Health Services Utilization
Five health services utilization outcomes were reported in the included studies: hospitalizations, length of stay, ED visits, readmissions, and primary care visits.
Hospitalizations
One study identified a statistically significant decrease in hospital admissions (relative reduction 15%) in the intervention group (Table 6) (GRADE quality of evidence: moderate).
Table 6: Impact of eTools on Hospitalizations.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Admissions Per Patient, n (Intervention/Control) | Effect Estimate(95% Cl) |
|---|---|---|---|---|---|
| Khan et al, 2010 (35) | RCT | 32 months (average) | 3,856/3,512 | 0.17/0.20 | Mean difference −0.03 (−0.05 to −0.01) |
Abbreviations: CI, confidence interval; eTool, electronic tool; RCT, randomized controlled trial.
Montori et al also commented that their research did not identify a statistically significant difference between study groups with respect to number of hospitalizations, but they did not provide data to support this statement. (37)
Length of Stay
One study identified a statistically significant decrease in hospital length of stay (relative reduction 10%) in the intervention group (Table 7) (GRADE quality of evidence: moderate).
Table 7: Impact of eTools on Length of Stay.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Days Per Patient, n (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Khan et al, 2010 (35) | RCT | 32 months (average) | 3,856/3,512 | 0.99/1.1 | Mean difference −0.11 (−0.19 to −0.03) |
Abbreviations: CI, confidence interval; eTool, electronic tool; RCT, randomized controlled trial.
ED Visits
One study identified a statistically significant decrease in number of ED visits (relative reduction 25%) in the intervention group (Table 8) (GRADE quality of evidence: moderate).
Table 8: Impact of eTools on Number of ED Visits.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Visits Per Patient, n (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Khan et al, 2010 (35) | RCT | 32 months (average) | 3,856/3,512 | 0.27/0.36 | Mean difference −0.09 (−0.14 to −0.04) |
Abbreviations: CI, confidence interval; ED, emergency department; eTool, electronic tool; RCT, randomized controlled trial.
aAdjusted with cluster correction.
Patient-level data were prioritized for this review; however, Graumlich et al conducted a smaller RCT that found no statistically significant difference between study groups in proportion of patients with an ED visit (risk difference adjusted for cluster correction −0.052% [95% confidence interval (CI) −0.115 to 0.011]). (34)
Montori et al also commented that their research did not identify a statistically significant difference between study groups with respect to number of ED visits, but they did not provide data to support this statement. (37)
Readmissions
One study identified no statistically significant difference between study groups in patient readmission rates (Table 9) (GRADE quality of evidence: high).
Table 9: Impact of eTools on Readmissions.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Readmissions, n (%) (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Graumlich et al, 2009 (34) | RCT | 6 months | 316/315 | 117 (37.0)/119 (37.8) | aDiffa −0.005 (−0.074 to 0.065) |
Abbreviations: aDiff, adjusted risk difference; CI, confidence interval; ED, emergency department; eTool, electronic tool; RCT, randomized controlled trial.
Adjusted for previous hospitalizations, ED visits, heart failure, and physician function.
Other Health Services Utilization: Primary Care Visits
Montori et al commented that their research did not identify a statistically significant difference between study groups with respect to number of primary care visits, but they did not provide data to support this statement. (37)
Disease-Specific Clinical Outcomes
Eight disease-specific outcomes were reported in the included studies: HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, low-density lipoprotein cholesterol (LDL-C), triglycerides, proportion of patients who experienced an adverse event, and achievement of clinical guidelines.
HbA1c
One RCT and 1 observational study reported on HbA1c levels. Neither study identified a statistically significant difference between study groups in HbA1c levels (Table 10) (GRADE quality of evidence: low to very low).
Table 10: Impact of eTools on HbA1c.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | HbA1c, % (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 | NR | Mean difference 0.01 [−0.3 to 0.4) |
| Branger et al, 1999 (32) | Observational | 6 months | 215/60 | −0.21/−0.12 | Mean difference −0.09 [−0.69 to 0.51) |
Abbreviations: CI, confidence interval; eTool, electronic tool; HbA1c, hemoglobin A1c; NR, not reported; RCT, randomized controlled trial.
Blood Pressure
One study identified no statistically significant difference between study groups in mean difference in systolic or diastolic blood pressure (Table 11) (GRADE quality of evidence: low).
Table 11: Impact of eTools on Blood Pressure.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | BP, mm Hg (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Systolic Blood Pressure | |||||
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 | NR | Mean difference −0.8 (−5.0 to 3.4) |
| Diastolic Blood Pressure | |||||
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 | NR | Mean difference −0.6 (−2.4 to 1.1) |
Abbreviations: BP, blood pressure; CI, confidence interval; eTool, electronic tool; NR, not reported; RCT, randomized controlled trial.
Lipids
One RCT identified no statistically significant difference between study groups with respect to mean difference in total cholesterol (Table 12) (GRADE quality of evidence: low). Two RCTs identified no statistically significant difference between study groups with respect to mean difference in LDL-C (due to different patient populations, estimates could not be pooled) (GRADE quality of evidence: low). One study identified no statistically significant difference between study groups with respect to mean difference in triglycerides (GRADE quality of evidence: low).
Table 12: Impact of eTools on Lipids.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Lipids (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Total Cholesterol, mmol/L | |||||
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 | NR | Mean difference −0.1 (−3.5 to 1.8) |
| LDL-C, mg/dL | |||||
| Lester et al, 2005 (33) | RCT | 1 month | 81/82 | 106.8/111.5 | Mean difference −4.7 (−13.4 to 4.0) |
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 | NR | Mean difference −0.1 (−3.0 to 2.8) |
| Triglycerides, mg/dL | |||||
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 | NR | Mean difference 0.1 (−1.7 to 3.5) |
Abbreviations: CI, confidence interval; eTool, electronic tool; NR, not reported; LDL-C, low-density lipoprotein cholesterol; RCT, randomized controlled trial.
Lester et al also examined differences in LDL-C levels at the first measures after the introduction of eTools and found no statistically significant difference in LDL-C between patient groups (intervention 111.7 mg/dL, control 118.1mg/dL, P = 0.2). (33)
Adverse Events
One study found no statistically significant difference between study groups with respect to the proportion of patients with an adverse event within 1 month after hospital discharge (Table 13) (GRADE quality of evidence: high).
Table 13: Impact of eTools on Adverse Events.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Adverse Events, n (%)(Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Graumlich et al, 2009 (34) | RCT | 1 month | 316/315 | 117 (37.0)/119 (37.8) | aDiff a 0.003 (−0.037 to 0.043) |
Abbreviations: aDiff, adjusted risk difference; CI, confidence interval; eTool, electronic tool; RCT, randomized controlled trial.
Adjusted with cluster correction.
Other Disease-Specific Clinical Outcome: Achievement of Clinical Guidelines
The proportion of patients who met a pre-defined threshold of various clinical outcomes was examined in several observational studies (Table 14). An observed increase in the proportion of patients who achieved the clinical threshold was considered an indication of good clinical practice (GRADE quality of evidence: very low).
Table 14: Impact of eTools on Achievement of Clinical Guidelines.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results, % (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| HbA1c Managed and Below Guideline Threshold | |||||
| Cebul et al, 2011 (38) | Observational | 1 year | 24,547/2,660 |
HbA1c < 8% 70.5/48.0 |
aDiffa 10.9 (−1.7 to 23.6) |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
HbA1c ≤ 8% 78.9/80.7 |
aORb 0.9 (0.8–1.0) |
| BP Managed and Below Guideline Threshold | |||||
| Cebul et al, 2011 (38) | Observational | 1 year | 24,547/2,660 |
BP < 140/80 mm Hg 55.8/38.9 |
aDiffa 11.1 (−1.0 to 23.2) |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
SBP < 130 mm Hg 52.2/46.1 |
aORb 1.2 (1.1–1.3) |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
DBP < 80 mm Hg 63.6/53.0 |
aORb 1.3 (1.2–1.3) |
| LDL-C Managed and Below Guideline Thresholdc | |||||
| Cebul et al, 2011 (38) | Observational | 1 year | 24,547/2,660 | 87.0/66.1 | aDiffa 18.1 (11.8–24.4) |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
71.3/65.5 | aORb 0.7 (0.6−0.8) |
| Triglycerides < 150 mg/dL | |||||
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
54.8/52.0 | aORb 0.9 (0.8–1.0) |
| BMI < 30 kg/m2 | |||||
| Cebul et al, 2011 (38) | Observational | 1 year | 24,547/2,660 | 32.8/34.1 | aDiffa −2.9 (−8.0 to −2.1) |
| Behavioural Intervention: Nonsmoker | |||||
| Cebul et al, 2011 (38) | Observational | 1 year | 24,547/2,660 | 82.1/52.3 | aDiffa 17.0 (5.3–28.6) |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
86.9/82.5 | aORb 1.1 (1.0–1.2) |
| Composite | |||||
| Cebul et al, 2011 (38) | Observational | 1 year | 24,547/2,660 |
Composited 43.7/15.7 |
aDiffa 15.2 (4.5–25.9) |
| Crosson et al, 2012 (39) | Observational | 3 years | 306/492 |
All targets mete NR |
aORf 1.42 (1.12–2.51) |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
Optimal careg 20.2/11.0 |
aORb 1.5 (1.3–1.6) |
Abbreviations: aDiff, adjusted risk difference; aOR, adjusted odds ratio; BP, blood pressure; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; eTool, electronic tool; HbA1c, hemoglobin A1c; LDL-C, low-density lipoprotein cholesterol; NR, not reported; SBP, systolic blood pressure.
Adjusted for insurance type, age, sex, race/ethnic group, language preference, estimated household income, and education level.
Adjusted for age, sex, insulin usage, and year of study.
Cebul et al outcome is LDL-C < 100 mg/dL or use of a statin; Lester et al outcome calculated using reported proportion of patients with LDL-C > 130 mg/dL.
Composite of HbA1c < 8%, blood pressure < 140/80 mm Hg, LDL-C < 100 mg/dL or use of statin, BMI < 30 kg/m2, or nonsmoker.
Criteria: HbA1c < 7%, LDL-C ≤ 100 mg/dL, or BP ≤ 130/85 mm Hg.
Adjusted for clustering effect.
Achieving HbA1c ≤ 8%, LDL-C <100 mg/dL, blood pressure < 130/80 mm Hg, nonsmoker, and Aspirin use.
Crosson et al also examined a composite outcome of achievement of 2 of 3 targets met and found a statistically significant improvement in the intervention group compared to control group (odds ratio [OR] 1.54, 95% CI 1.06–2.25). (39) They also examined the composite outcome of achievement of all criteria related to appropriate treatment (HbA1c ≤ 8% or > 8% and on an antihyperglycemic agent; LDL-C ≤ 100 mg/dL or > 100 mg/dL and on a lipid-lowering agent; and blood pressure ≤ 130/85 mm Hg or > 130/85 mm Hg and on an antihypertensive agent). They observed no statistically significant difference in the intervention group compared with the control group (OR 1.42, 95% CI 0.81–2.41). (39)
Process-of-Care Indicators
Some studies reported the rate at which clinically important tests or examinations were conducted (or recorded). An observed increase in the rate at which these tests were conducted was considered an indication of good clinical practice.
Blood Pressure Measures Conducted
Three studies examined the number of blood pressure measures conducted upon the implementation of eTools (Table 15) (GRADE quality of evidence: very low).
Table 15: Impact of eTools on Blood Pressure Measures Conducted.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Branger et al, 1999 (32) | Observational | 1 year | 215/60 | 417(1.9)/81(1.4) measures (per patient) |
Mean difference 0.50 (0.28−0.72) |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
100%/99.9% of patients |
aORa 36.5 (6.0–105.9) |
| Wells et al, 1996 (42) | Observational | 23 months | 2,049/1,190 | 92%/74% of patients |
OR 4.12 (3.35–5.07) |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; eTool, electronic tool; OR, odds ratio.
Adjusted for age, sex, insulin usage, and year of study.
Lipid Tests Conducted
Three studies found no difference between study groups with respect to total cholesterol and triglyceride measurements (Table 16) (GRADE quality of evidence: low to very low).
Table 16: Impact of eTools on Lipid Tests Conducted.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Total Cholesterol | |||||
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 | 84%/79% of patients |
aORb 1.4 (0.8–2.3) |
| Branger et al, 1999 (32) | Observational | 1 year | 215/60 | 149(0.7)/25(0.4) measures (per patient) |
Mean difference 0.30 (0.03−0.57) |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
93.7/87.4 of patients |
aORa 0.9 (0.8–1.0) |
| Triglycerides | |||||
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 | 82%/75% of patients |
aORb 5.0 (0.9–2.4) |
| Branger et al, 1999 (32) | Observational | 1 year | 215/60 | 52 (0.2)/7 (0.1) measures (per patient) |
Mean difference 0.10 (0.02−0.18) |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
94.9%/89.7% of patients |
aORa 0.8 (0.7−0.9) |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; eTool, electronic tool; RCT, randomized controlled trial.
Adjusted for age, sex, insulin usage, and year of study.
Adjusted with logistic regression; no further details available.
Montori et al also examined high-density lipoprotein cholesterol and found no statistically significant difference between groups in the proportion of patients receiving the test. (37)
HbA1c Tests Conducted
One RCT found no statistically significant difference between study groups with respect to HbA1c measurements (Table 17) (GRADE quality of evidence: low). Five observational studies found a trend towards increased proportion of patients who received HbA1c tests in the intervention group compared to the control group (GRADE quality of evidence: very low).
Table 17: Impact of eTools on HbA1c Tests Conducted.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 | 99%/94% of patients | aORa4.5 (1.0–19.5) |
| Branger et al, 1999 (32) | Observational | 1 year | 215/60 | 177 (0.8)/9 (0.2) measures (per patient) | Mean differenceb 0.60 (0.21−0.99) |
| Cebul et al, 2011 (38) | Observational | 1 year | 24,547/2,660 | 94.6%/85.6% of patients | aDiffb 7.2 (0.4–14.0) |
| Henderson et al, 2010 (36) | Observational | 16 months | 3,432/688 encounters | 25.1/17.6 per 100 encounters | aRCc 3.10 (NR) P = 0.24 |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years | 97.6%/92.7% of patients | aORd 0.6 (0.5−0.6) |
| Wells et al, 1996 (42) | Observational | 23 months | 2,049/1,190 | 93%/73% of patients | OR 4.89 (3.95–6.04) |
Abbreviations: aDiff, adjusted risk difference; aOR, adjusted odds ratio; aRC, adjusted regression correlation; CI, confidence interval; eTool, electronic tool; FRACGP, Fellowship of the Royal Australian College of General Practitioners; GP, general practitioner; HbA1c, hemoglobin A1c; NR, not reported; OR, odds ratio; RCT, randomized controlled trial.
Adjusted with logistic regression, further details not provided.
Adjusted for insurance type, age, sex, race/ethnic group, language preference, estimated household income, and education level.
Adjusted for GP age, GP sex, FRACGP status, work in deputizing services in preceding month, bulk billing for all patients, practice accreditation status, presence of a practice nurse.
Adjusted for age, sex, insulin usage, and year of study.
Blood Glucose/Fructosamine Tests Conducted
One observational study found no significant difference in the number of blood glucose tests conducted between study groups; it did find an increase in the intervention group in number of fructosamine tests conducted per patient (Table 18) (GRADE quality of evidence: very low).
Table 18: Impact of eTools on Blood Glucose and Fructosamine Tests Conducted.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Blood Glucose | |||||
| Branger et al, 1999 (32) | Observational | 1 year | 215/60 | 400 (1.9)/105 (1.8) measures (per patient) | Mean difference 0.10 (−0.04 to 0.24) |
| Fructosamine | |||||
| Branger et al, 1999 (32) | Observational | 1 year | 215/60 | 47 (0.2)/0 (0.0) measures (per patient) | Mean difference 0.20 (0.05−0.35) |
Abbreviations: CI, confidence interval; eTools, electronic tools.
Eye Examinations Conducted
One RCT found a statistically significant increase in number of eye examinations conducted in the intervention group (Table 19) (GRADE quality of evidence: low). Five observational studies and found a statistically significant increase in the intervention groups (GRADE quality of evidence: very low).
Table 19: Impact of eTools on Eye Examinations Conducted.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 |
Retina examination 69%36% of patients |
aORa2.4 (1.5–3.9) |
| Branger et al, 1999 (32) | Observational | 1 year | 215/60 |
Ophthalmologist assessment 64 (0.3)/18 (0.3) assessments (per patient) |
Mean difference 0.0 (0.0−0.0) |
| Cebul et al, 2011 (38) | Observational | 1 year | 24,547/2,660 |
Eye examinations 62.6%/30.8% of patients |
aDiffb 25.0 (18.7–31.2) |
| Henderson et al, 2010 (36) | Observational | 16 months | 3,432/688 encounters |
Referral to ophthalmologist or allied health professional 7.1/3.6 per 100 encounters |
aRCc 2.94 (NR) P = 0.002 |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
Eye examinations 41.8%/20.0% of patients |
aORd 1.5 (1.4–1.7) |
| Wells et al, 1996 (42) | Observational | 23 months | 2,049/1,190 |
Fundoscopy 90%/78% of patients |
OR2.54 (2.08–3.10) |
Abbreviations: aDiff, adjusted risk difference; aOR, adjusted odds ratio; aRC, adjusted regression correlation; CI, confidence interval; eTool, electronic tool; FRACGP, Fellowship of the Royal Australian College of General Practitioners; GP, general practitioner; NR, not reported; OR, odds ratio; RCT, randomized controlled trial.
Adjusted with logistic regression, further details not provided.
Adjusted for insurance type, age, sex, race/ethnic group, language preference, estimated household income, and education level.
Adjusted for GP age, GP sex, FRACGP status, work in deputizing services in preceding month, bulk billing for all patients, practice accreditation status, presence of a practice nurse.
Adjusted for age, sex, insulin usage, and year of study.
In addition, Wells et al examined visual acuity and found a statistically significant OR of 2.79 (95% CI 2.39 to 3.26) for the number of visual acuity examinations conducted in the intervention groups versus the control groups. (42)
Foot Examinations Conducted
One RCT found a statistically significant increase in number of foot examinations conducted in the intervention group (Table 20) (GRADE quality of evidence: low). Two observational studies found a statistically significant increase in the intervention group (GRADE quality of evidence: very low).
Table 20: Impact of eTools on Foot Examinations Conducted.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 | 88%/66% of patients | aORa2.3 (1.2–4.4) |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years | 56.6%/10.8% of patients | aORb 2.8 (2.6–3.0) |
| Wells et al, 1996 (42) | Observational | 23 months | 2,049/1,190 | 96%/89% of patients | OR2.97 (2.23–3.95)P ≤ 0.01 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; eTool, electronic tool; OR, odds ratio; RCT, randomized controlled trial.
Adjusted with logistic regression, further details not provided.
Adjusted for baseline performance and cohort.
A pooled estimate also demonstrated a significant increase in number of foot examinations in the intervention group (Figure 3).
Figure 3: Pooled Effect Estimate of Foot Examinations Conducted in Observational Studies.
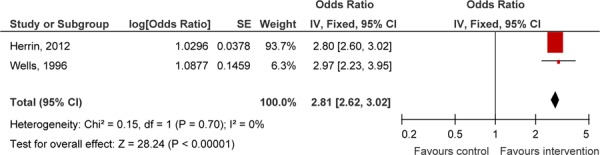
Abbreviations: CI, confidence interval; IV, instrumental variable; RCT, randomized controlled trial; SE, standard error.
Urine Protein Tests Conducted for Kidney Management
One RCT found a statistically significant increase in number of urine protein tests conducted in the intervention group (Table 21) (GRADE quality of evidence: low). Three observational studies found no statistically significant increase in the intervention group (GRADE quality of evidence: very low).
Table 21: Impact of eTools on Urine Protein Tests Conducted for Kidney Management.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 |
Microalbuminuria 55%/27% of patients |
aORa3.2 (1.9–5.2) |
| Branger et al, 1999 (32) | Observational | 1 year | 215/60 |
Proteinuria level 20 (0.1)/29 (0.5) measures (per patient) |
Mean difference −0.40 (−0.95 to 0.15) |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
Microalbumin 71.5%/54.8% of patients |
aORb 1.2 (1.1–1.3) |
| Wells, et al, 1996 (42) | Observational | 23 months | 2,049/1,190 |
Urine protein 84%/57% of patients |
OR 3.96 (3.4–4.7) |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; eTool, electronic tool; OR, odds ratio; RCT, randomized controlled trial.
Adjusted with logistic regression; further details not provided.
Adjusted for age, sex, insulin usage, and year of study.
Other Tests for Kidney Management Conducted
One observational study found no statistically significant difference between study groups in number of creatinine tests conducted (Table 22) (GRADE quality of evidence: very low). One observational study examined a composite kidney management outcome and demonstrated a statistically significant increase in appropriate kidney management in the intervention group (GRADE quality of evidence: very low). One observational study found that the number of patients who received urinalysis testing was significantly lower in the intervention group (GRADE quality of evidence: very low).
Table 22: Impact of eTools on Other Tests Conducted for Kidney Management.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Branger et al, 1999 (32) | Observational | 1 year | 215/60 |
Creatinine levels 106 (0.5)/21 (0.4)measures (per patient) |
Mean difference 0.10 (−0.04 to 0.24) |
| Cebul et al, 2011 (38) | Observational | 1 year | 24,547/2,660 |
Kidney management(microalbumin or ACE inhibitor or ARB) 93.4%/78.2% of patients |
aDiffa 13.3 (8.4–18.3) |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
Urinalysis 47.6%/50.6% of patients |
aORb 0.8 (0.7−0.8) |
Abbreviations: ACE, angiotensin-converting enzyme; aDiff, adjusted risk difference; aOR, adjusted odds ratio; ARB, angiotensin receptor blocker; CI, confidence interval; eTool, electronic tool.
Adjusted for insurance type, age, sex, race/ethnic group, language preference, estimated household income, and education level.
Adjusted for age, sex, insulin usage, and year of study.
Weight Measures Conducted
One study found a statistically significant increase in the number of weight measures in the intervention group (Table 23) (GRADE quality of evidence: very low).
Table 23: Impact of eTools on Weight Measures Conducted.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Branger et al, 1999 (32) | Observational | 1 year | 215/60 | 448 (2.1)/27 (0.5) measures (per patient) | Mean difference 1.6 (0.62–2.58) |
Abbreviations: CI, confidence interval; eTools, electronic tools.
Height Measures Conducted
One study found a statistically significant increase in the proportion of patients with a height measure recorded in the intervention group (Table 24) (GRADE quality of evidence: very low).
Table 24: Impact of eTools on Height Measures Conducted.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Wells et al, 1996 (42) | Observational | 23 months (41) | 2,049/1,190 | 90%/80% of patients | OR 2.25 (1.84–2.75) |
Abbreviations: CI, confidence interval; eTool, electronic tool; OR, odds ratio.
Vaccinations and Immunizations Administered
One RCT found a statistically significant increase in immunizations in the intervention group (Table 25) (GRADE quality of evidence: low). Two observational studies found an increase in vaccinations in the intervention groups (Table 25) (GRADE quality of evidence: very low).
Table 25: Impact of eTools on Immunizations Administered.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Montori et al, 2002 (36;37) | RCT | 24 months | 399/208 |
Immunization 80/64 |
aORa 1.7 (1.1–2.7) |
| Cebul et al, 2011 (38) | Observational | 1 year | 24,547/2,660 |
Pneumococcal vaccination 83.0/15.0 |
aDiffb 57.1 (43.6–70.5) |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
Influenza vaccination 61.6/50.5 |
aORc 1.1 (1.0 -1.1) |
Abbreviations: aDiff, adjusted risk difference; aOR, adjusted odds ratio; CI, confidence interval; eTool, electronic tool; RCT, randomized controlled trial.
Adjusted with logistic regression; further details not provided.
Adjusted for insurance type, age, sex, race/ethnic group, language preference, estimated household income, and education level.
Adjusted for age, sex, insulin usage, and year of study.
Appropriately Managed Medications
Two observational studies found no difference between study groups with respect to number of angiotensin-converting enzyme (ACE) inhibitors_prescriptions per patient encounter or in proportion of patients with prescriptions (Table 26) (GRADE quality of evidence: very low).
Table 26: Impact of eTools on Appropriately Prescribed ACE Inhibitors.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Henderson et al, 2010 (36) | Observational | 16 months | 5,838/1,075 encounters | 5.9/4.5 per 100 encounters | aRCa 0.16 (NR) P = 0.86 |
| Walsh et al, 2012 (41) | Observational | 24 months | 4,220/2,950 | ACE inhibitor/ARB improvement in use of therapy from baseline 7.3%/8.6% | aORb 0.83 (0.63–1.09) |
Abbreviations: ACE inhibitor, angiotensin-converting enzyme inhibitor; aOR, adjusted odds ratio; ARB, angiotensin receptor blocker; aRC, adjusted regression correlation; CI, confidence interval; eTool, electronic tool; FRACGP, Fellowship of the Royal Australian College of General Practitioners; GP, general practitioner; NR, not reported.
Adjusted for GP age, GP sex, FRACGP status, work in deputizing services in preceding month, bulk billing for all patients, practice accreditation status, presence of a practice nurse.
Adjusted for patient and practice characteristics.
Two observational studies found no difference between study groups in anticoagulation prescriptions for atrial fibrillation (Table 27) (GRADE quality of evidence: very low).
Table 27: Impact of eTools on Appropriately Prescribed Anticoagulation for Atrial Fibrillation.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Henderson et al, 2010 (36) | Observational | 16 months | 906/145 encounters | Warfarin 35.4/40.0 per 100 encounters | aRCa −5.23 (NR) P = 0.14 |
| Walsh et al, 2012 (41) | Observational | 24 months | 4,220/2,950 | Anticoagulation for atrial fibrillation improvement in use of therapy from baseline 6.4%/8.6% | aORb 0.65 (0.40–1.05) |
Abbreviations: aOR, adjusted odds ratio; aRC, adjusted regression correlation; CI, confidence interval; eTool, electronic tool; FRACGP, Fellowship of the Royal Australian College of General Practitioners; GP, general practitioner.
Adjusted for GP age, GP sex, FRACGP status, work in deputizing services in preceding month, bulk billing for all patients, practice accreditation status, presence of a practice nurse.
Adjusted for patient and practice characteristics.
Two observational studies examined appropriately prescribed Aspirin. One study found no significant difference between study groups in the prescribing of Aspirin or clopidogrel, while the other found a statistically significant increase in the proportion of patients who received Aspirin in the intervention group (Table 28) (GRADE quality of evidence: very low).
Table 28: Impact of eTools on Appropriately Prescribed Aspirin.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Henderson et al, 2010 (36) | Observational | 16 months | 5,838/1,075 encounters |
Aspirin or clopidogrel 8.7/9.6 per 100 encounters |
aRCa −1.93 (NR) P = 0.14 |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
Aspirin 82.2%51.4% of patients |
aORb 4.8 (4.4–5.3) |
Abbreviations: aOR, adjusted odds ratio; aRC, adjusted regression correlation; CI, confidence interval; eTool, electronic tool; FRACGP, Fellowship of the Royal Australian College of General Practitioners; GP, general practitioner; NR, not reported.
Adjusted for GP age, GP sex, FRACGP status, work in deputizing services in preceding month, bulk billing for all patients, practice accreditation status, presence of a practice nurse.
Adjusted for age, sex, insulin usage and year of study.
A number of other outcomes related to appropriately prescribed medications were examined; no statistically significant results were observed, with the exception of the proportion of patients prescribed beta-blockers (Table 29) (GRADE quality of evidence: very low).
Table 29: Impact of eTools on Other Outcomes of Appropriately Managed Medications.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results, % (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Walsh et al, 2012 (41) | Observational | 24 months | 4,220/2,950 |
Aldosterone antagonist 17.4/20.7 |
aORa 0.86 (0.49–1.50) |
| Walsh et al, 2012 (41) | Observational | 24 months | 4,220/2,950 |
ICD/CRT-D 19.1/18.0 |
aORa 1.06 (0.78–1.44) |
| Walsh et al, 2012 (41) | Observational | 24 months | 4,220/2,950 |
Beta-blocker 6.9/5.3 |
aORa 1.43 (1.05–1.93) |
| Walsh et al, 2012 (41) | Observational | 24 months | 4,220/2,950 |
CRT-P/CRT-D 33.6/31.1 |
aORa 1.33 (0.73–2.43) |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; CRT-D, cardio-resynchronization therapy with defibrillator; CRT-P, cardio-resynchronization therapy with pacemaker; eTool, electronic tool; ICD, implantable cardioverter defibrillator.
Adjusted for patient and practice characteristics.
Finally, 1 RCT found a statistically significant increase in the number of changes in statin prescriptions in the intervention group at 1 month, but not at 1 year (Table 30) (GRADE quality of evidence: low at 1 month and moderate at 1 year; difference is due to wide confidence intervals at 1 month).
Table 30: Impact of eTools on Appropriate Changes Made to Statin Prescriptions.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results, % (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Lester et al, 2005 (33) | RCT | 1 month | 118/117 |
At 1 month 15.3/2.0 |
OR 10.35 (2.34–45.71) |
| Lester et al, 2005 (33) | RCT | 1 year | 118/117 |
At 1 year 24.6/17.1 |
OR 1.58 (0.83–2.99) |
Abbreviations: CI, confidence interval; eTool, electronic tool; OR, odds ratio; RCT, randomized controlled trial.
Behavioural Management Interventions
Two studies found a statistically significant increase in the proportion of patients receiving diet advice in the intervention groups (Table 31) (GRADE quality of evidence: low to very low).
Table 31: Impact of eTools on Behavioural Management Interventions.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results, % of patients (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Diet Advice | |||||
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 | 70/60 | aORa 1.9 (1.2–3.0) |
| Wells et al, 1996 (42) | Observational | 23 months | 2,049/1,190 |
Saw dietitian 91/81 |
OR 2.36 (1.92–2.91) |
| Smoking | |||||
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 |
Tobacco advice 94/87 |
aORa 2.0 (0.9–4.3) |
| Herrin et al, 2012 (40) | Observational | 5 years | 10,017/35,033 patient years |
Smoking assessment 98.6/94.3 |
aORb 2.6 (2.2–3.1) |
| Other | |||||
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 |
Exercise advice 80/52 |
aORa 2.7 (1.6–4.5) |
| Montori et al, 2002 (37) | RCT | 24 months | 399/208 |
Self-management support 61/38 |
aORa 2.6 (1.7–3.8) |
| Walsh et al, 2012 (41) | Observational | 24 months | 4,220/2,950 |
Heart failure education improvement in use of therapy from baseline 24.7/26.6 |
aORc 0.95 (0.67–1.35) |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; eTool, electronic tool; NR, not reported; OR, odds ratio; RCT, randomized controlled rial.
Adjusted with logistic regression; further details not provided.
Adjusted for age, sex, insulin usage, and year of study.
Adjusted for patient and practice characteristics.
One RCT found no significant change in the proportion of patients receiving tobacco advice, but 1 observational study found a statistically significant increase in the proportion of patients receiving a smoking assessment in the intervention group (GRADE quality of evidence: low to very low).
One RCT found a statistically significant increase in the proportion of patients receiving exercise and self-management advice in the intervention group (GRADE quality of evidence: low). One observational study found a statistically significant improvement in heart failure education in the intervention group (GRADE quality of evidence: very low).
Composite Outcomes
Two observational studies examined a composite outcome of conducting or recording certain examinations and tests as good clinical practice measures. One study found a statistically significant increase in the proportion of patients who had an HbA1c measurement, kidney management, eye examination, or pneumococcal vaccination in the intervention group (Table 32). The other study did not find a statistically significant difference between study groups for meeting 3 of the following criteria: HbA1c assessed within previous 6 months, urine microalbumin assessed within the previous 12 months, smoking status assessed within the previous 6 months, LDL-C assessed within the previous 12 months, or blood pressure recorded at the previous 3 visits (GRADE quality of evidence: very low).
Table 32: Impact of eTools on Composite Outcomes of Tests Conducted.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Cebul et al, 2011 (38) | Observational | 1 year | 24,547/2,660 |
Compositea 50.9/6.6% of patients |
aDiffb 35.1 (28.3–41.9) P < 0.001 |
| Crosson et al, 2012 (39) | Observational | 3 years | 306/492 | 3 of 5 criteriac met NR | aORd 1.60 (0.93–2.74) P = 0.09 |
Abbreviations: aDiff, adjusted risk difference; aOR, adjusted odds ratio; CI, confidence interval; eTool, electronic tool; HbA1c, hemoglobin A1c; LDL-C, low density lipoprotein cholesterol; NR, not reported.
Composite of measurement of HbA1c, kidney management, eye examination, and pneumococcal vaccination.
Adjusted for insurance type, age, sex, race/ethnic group, language preference, estimated household income, and education level.
Criteria: HbA1c assessed within last 6 months, urine microalbumin assessed within last 12 months, smoking status assessed within last 6 months, LDL-C assessed within last 12 months, blood pressure recorded at each of 3 previous visits.
Adjusted for clustering effect.
Measures of Efficiency
Various measures of efficiency in the context of the utilization of electronic tools for health information exchange as a means of chronic disease management in the community were identified in the included studies. Specifically, 2 categories of efficiency examined: time and communication.
Time
One RCT found no statistically significant difference between study groups in time to receipt of discharge summary when comparing electronic discharge summaries and handwritten structure summaries (Table 33) (GRADE quality of evidence: high).
Table 33: Impact of eTools on Time.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Time to Receive Discharge Summary | |||||
| Graumlich et al, 2009 (34) | RCT | 6 months | 316/315 | Proportion of physicians to receive discharge summaries within 1–7 days 56.0%/57.1 | aDiffa −1.1% (−9.2%-6.9%) |
| Time to Receive Clinical Intervention | |||||
| Lester et al, 2005 (33) | RCT | 1 year | 118/117 |
Time to first measure of LDL-C 99 days/121 days |
Mean difference −22.0 (−82.9 to 38.9) |
| Lester et al, 2005 (33) | RCT | 1 year | 118/117 |
Time to change in statin prescription (median) 0 months/7.1 months |
Mean difference −7.1 (−12.0 to −2.2) |
| Time Spent With Patients | |||||
| Montori et al, 2002 (37) | Before/after evaluation for this outcome; RCT | 2 years | 399/208 |
Time spent with patients (provider) Start of implementation: median 5 min (range 0–30 min) 2 years after implementation: median 9.5 min (range 0–34) Time spent with patients (nurse) Start of implementation: median 15 min (range 4–45 min) 2 years after implementation: median 18 min (range 10–55) |
Mean difference 4.5 (1.83–7.17) Mean difference 3.00 (0.67–5.33) |
Abbreviations: aDiff, adjusted risk difference; CI, confidence interval; eTool, electronic tool; LDL-C, low-density lipoprotein cholesterol; RCT, randomized controlled trial.
Adjusted with cluster correction.
One RCT found a statistically significant shorter time to change in a statin medication among patients whose care providers received an electronic outreach summary report, but found no difference between study groups in time to first measurement of LDL-C (Table 33) (GRADE quality of evidence: moderate).
One observational evaluation found a statistically significant increase in the length of time PCPs and nurses spent with their patients 2 years after implementation of the electronic diabetes management system (Table 33) (GRADE quality of evidence: very low).
Additionally, the RCT by Lester et al found that it took physicians less than 60 seconds to complete the emailed report. (33)
Communication
One observational study identified a statistically significant increase in the number of letters sent from consultants to GPs in the intervention group, but not from GPs to consultants or in the number of patient contacts with either GP or consultant (Table 34) (GRADE quality of evidence: very low).
Table 34: Impact of eTools on Frequency of Communication.
| Author, Year | Study Design | Length of Follow-up | Sample Size, n (Intervention/Control) | Results (Intervention/Control) | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
| Branger et al, 1999 (32) | Observational | 1 year | 215/60 | Number of letters sent from GPs to consultants 151 (0.7)/14 (0.2) total (per patient) P ≥ 0.05 | Not estimable |
| Number of letters sent from consultants to GPs 339 (1.6)/24 (0.4) total (per patient) P = 0.00 | |||||
| Number of patient contacts with GPs and consultants 14 with GP, 4 with consultant/14 with GP, 4 with consultant P ≥ 0.05 |
Abbreviations: CI, confidence interval; eTool, electronic tool; GP, general practitioner.
Potential Trends in Analysis Results
The second research question was aimed at identifying any potential factors that contribute to the observed outcomes of interest, and 96 different outcomes were extracted. Given that most of the included studies did not report outcomes in a consistent manner, a simple accounting summary was constructed to explore any potential trends. If a trend existed, we would expect to see mostly positive outcomes in 1 component while mostly nonsignificant outcomes in another with the same categorical exploration.
Three different potential trends were examined: 1) impact of eTools by specific disease population; 2) impact of eTools by targeted care coordination aspect; and 3) impact of eTools by technology.
Overall, no outstanding trends were identified, indicating that there was no single disease group, care coordination aspect, or technology that contributed more significantly to the observed impacts of eTools. This observed trend of no difference held when a subgroup analysis was conducted, limiting the analysis to an examination of only process-of-care outcomes (Figure 4).
Figure 4: Subgroup Analysis: Process-of-Care Outcomes By Disease, Care Coordination Aspect, and Technology.
Abbreviations: EHR, electronic health record; EMR, electronic medical record; PCP, primary care physician.
Summary of Results
Eleven articles were identified from a systematic literature search that examined the application of eTools for health information exchange to assist with the management of patients with chronic disease in the community setting. There was a substantial amount of technological, clinical, and methodological diversity among the included studies.
Three categories of outcomes of interest were examined: 1) the primary outcomes of interest, which included both health services utilization and disease-specific clinical outcomes; 2) process-of-care indicators; and 3) measures of efficiency.
Primary Outcomes (Health Services Utilization and Disease-Specific Clinical Outcomes)
In summary, 1 RCT demonstrated a reduction in hospitalizations, length of stay, and ED visits (Table 35). In this study, the intervention was an electronic laboratory report generated and forwarded to PCPs with recommendations linked to guidelines. (35) Among the other studies examining various eTools, there was evidence of no difference in readmissions and various disease-specific outcomes between study groups.
Table 35: Summary of Health Services Utilization and Disease-Specific Clinical Outcomes.
| Outcome | Number of Studies | Statistical Method | Effect Estimate (95% CI) | GRADEa |
|---|---|---|---|---|
| Hospitalizations | 1 (RCT) | Mean difference | −0.03 (−0.05 to −0.01) | Moderate |
| Length of stay, days | 1 (RCT) | Mean difference | −0.11 (−0.19 to −0.03) | Moderate |
| ED visits | 1 (RCT) | Mean difference | −0.09 (−0.14 to −0.04) | Moderate |
| Readmissions | 1 (RCT) | Risk difference | −0.005 (−0.074 to 0.065) | High |
| Disease-Specific Outcomes | ||||
| HbA1c%, | 1 (RCT) | Mean difference | 0.01 (−0.3 to 0.4) | Low |
| 1 (Observational) | Mean difference | −0.09 (−0.69 to 0.51) | Very low | |
| SBP, mm Hg | 1 (RCT) | Mean difference | −0.8 (−5.0 to 3.4) | Low |
| DBP, mm Hg | 1 (RCT) | Mean difference | −0.6 (−2.4 to 1.1) | Low |
| Total cholesterol, mmol/L | 1 (RCT) | Mean difference | −0.1 (−3.5 to 1.8) | Low |
| LDL-C, mg/dL | 2 (RCT) | Mean difference | −4.7 (−13.4 to 4.0) | Low |
| Mean difference | −0.1 (−3.0 to 2.8) | Low | ||
| Triglycerides, mg/dL | 1 (RCT) | Mean difference | 0.1 (−1.7 to 3.5) | Low |
| Adverse events | 1 (RCT) | Risk difference | 0.003 (−0.037 to 0.043) | High |
| Achievement of Clinical Outcomes | ||||
| HbA1c < 8% | 2 (Observational) | Risk difference | 10.9 (−1.7 to 23.6) | Very low |
| HbA1c ≤ 8% | Odds ratio | 0.9 (0.8–1.0) | ||
| BP < 140/80 mm Hg | 1 (Observational) | Risk difference | 11.1 (−1.0 to 23.2) | Very low |
| SBP < 130 mm Hg | 1 (Observational) | Odds ratio | 1.2 (1.1–1.3) | |
| DBP < 80 mm Hg | 1 (Observational) | Odds ratio | 1.3 (1.2–1.3) | |
| LDL-C < 100 mg/dL or statin | 2 (Observational) | Risk difference | 18.1 (11.8–24.4) | Very low |
| LDL-C < 100 mg/dL | Odds ratio | 0.7 (0.6−0.8) | ||
| Triglycerides < 150 mg/dL | 1 (Observational) | Odds ratio | 0.9 (0.8–1.0) | Very low |
| BMI < 30 kg/m2 | 1 (Observational) | Risk difference | −2.9 (−8.0 to −2.1) | Very low |
| Nonsmoker | 2 (Observational) | Risk difference | 17.0 (5.3–28.6) | Very low |
| Odds ratio | 1.1 (1.0–1.2) | |||
| Composite of targets metb | 1 (Observational) | Risk difference | 15.2 (4.5–25.9) | Very low |
| Composite—3 of 3 targets metc | 1 (Observational) | Odds ratio | 1.42 (1.12–2.51) | |
| Composite—optimal care a | 1 (Observational) | Odds ratio | 1.5 (1.3–1.6) |
Abbreviations: BMI, body mass index; BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; ED, emergency department; HbA1c, hemoglobin A1c; LDL-C, low density lipoprotein cholesterol; RCT, randomized controlled trial; SBP, systolic blood pressure.
Details of individual GRADE assessments are available in Appendix 3.
Composite of HbA1c < 8%, blood pressure <140/80 mm Hg, LDL-C <100 mg/dL or use of statin, BMI < 30 kg/m2, or nonsmoker.
Criteria: HbA1c < 7%, LDL-C ≤ 100 mg/dl, or blood pressure ≤ 130/85 mm Hg.
Achieving HbA1c ≤ 8%, LDL-C <100 mg/dL, blood pressure < 130/80 mm Hg, nonsmoker, and Aspirin use.
Process-of-Care Indicators
All process of care measures reported were related to the frequency of which certain tests or examinations were conducted (or recorded). Results for this grouping of outcomes were inconclusive. Additionally, there was no observed trend of an impact based on the disease-specific grouping of patients, the care coordination aspect targeted, or the technology applied (Table 36).
Table 36: Summary of Process-of-Care Indicators.
| Outcome | Number of Studies | Statistical Method | Effect Estimate (95% CI) | GRADEa |
|---|---|---|---|---|
| Rate of Conducting (or Recording) Clinical Tests | ||||
| BP measures | 3 (Observational) | Mean difference | 0.50 (0.28−0.72) | Very low |
| Odds ratio | 36.5 (6.0–105.9) | |||
| Odds ratio | 4.12 (3.35–5.07) | |||
| Total cholesterol | 1 (RCT) | Odds ratio | 1.4 (0.8–2.3) | Low |
| 2 (Observational) | Mean difference | 0.30 (0.03−0.57) | Very low | |
| Odds ratio | 0.9 (0.8–1.0) | |||
| Triglycerides | 1 (RCT) | Odds ratio | 5.0 (0.9–2.4) | Low |
| 2 (Observational) | Mean difference | 0.10 (0.02−0.18) | Very low | |
| Odds ratio | 0.8 (0.7−0.9) | |||
| HbA1c | 1 (RCT) | Odds ratio | 4.5 (1.0–19.5) | Low |
| 5 (Observational) | Mean difference | 0.6 (0.21−0.99) | Very low | |
| Risk difference | 7.2 (0.4–14.0) | |||
| Regression correlation | 3.10 (NR), P = 0.24 | |||
| Odds ratio | 0.6 (0.5−0.6) | |||
| Odds ratio | 4.89 (3.95–6.04) | |||
| Blood glucose | 1 (Observational) | Mean difference | 0.10 (−0.04 to 0.24) | Very low |
| Fructosamine | 1 (Observational) | Mean difference | 0.20 (0.05−0.35) | Very low |
| Eye examinations | 1 (RCT) | Odds ratio | 2.4 (1.5–3.9) | Low |
| 5 (Observational) | Mean difference | 0.0 (0.0−0.0) | Very low | |
| Risk difference | 25.0 (18.7–31.2) | |||
| Regression correlation | 2.94 (NR), P = 0.002 | |||
| Odds ratio | 1.5 (1.4–1.7) | |||
| Odds ratio | 2.54 (2.08–3.10) | |||
| Foot examinations | 1 (RCT) | Odds ratio | 2.3 (1.2–4.4) | Low |
| 2 (Observational) | Odds ratio | 2.81 (2.62–3.02)b | Very low | |
| Kidney management: urine protein | 1 (RCT) | Odds ratio | 3.2 (1.9–5.2) | Low |
| 3 (Observational) | Mean difference | −0.40 (−0.95 to 0.15) | Very low | |
| Odds ratio | 1.2 (1.1–1.3) | |||
| Odds ratio | 3.96 (3.4–4.7) | |||
| Kidney management: creatinine | 1 (Observational) | Mean difference | 0.10 (−0.04 to 0.24) | Very low |
| Kidney management: composite outcome | 1 (Observational) | Risk difference | 13.3 (8.4–18.3) | Very low |
| Kidney management: urinalysis | 1 (Observational) | Odds ratio | 0.8 (0.7−0.8) | Very low |
| Weight | 1 (Observational) | Mean difference | 1.6 (0.62–2.58) | Very low |
| Height | 1 (Observational) | Odds ratio | 2.25 (1.84–2.75) | Very low |
| Vaccinations and immunizations | 1 (RCT) | Odds ratio | 1.7 (1.1–2.7) | Low |
| 2 (Observational) | Risk difference | 57.1 (43.6–70.5) | Very low | |
| Odds ratio | 1.1 (1.0–1.1) | |||
| Medications: ACE inhibitors | 2 (Observational) | Regression correlation | 0.16 (NR), P = 0.86 | Very low |
| Odds ratio | 0.83 (0.63–1.09) | |||
| Medications: anticoagulation | 2 (Observational) | Regression correlation | −5.23 (NR), P = 0.14 | Very low |
| Odds ratio | 0.65 (0.40–1.05) | |||
| Medications: Aspirin (or clopidogrel) | 2 (Observational) | Regression correlation | −1.93 (NR), P = 0.14 | Very low |
| Odds ratio | 4.8 (4.4–5.3) | |||
| Medications: aldosterone antagonist | 1 (Observational) | Odds ratio | 0.86 (0.49–1.50) | Very low |
| Medications: ICD/CRT-D | 1 (Observational) | Odds ratio | 1.06 (0.78–1.44) | Very low |
| Medications: beta-blocker | 1 (Observational) | Odds ratio | 1.43 (1.05–1.93) | Very low |
| Medications: CRT-P/CRT-D | 1 (Observational) | Odds ratio | 1.33 (0.73–2.43) | Very low |
| Medications: changes in statins (1 month) | 1 (RCT) | Odds ratio | 10.35 (2.34–45.71) | Low |
| Medications: changes in statins (1 year) | 1 (RCT) | Odds ratio | 1.58 (0.83–2.99) | Moderate |
| Behavioural interventions: diet advice | 1 (RCT) | Odds ratio | 1.9 (1.2–3.0) | Low |
| 1 (Observational) | Odds ratio | 2.36 (1.92–2.91) | Very low | |
| Behavioural interventions: smoking assessment | 1 (RCT) | Odds ratio | 2.0 (0.9–4.3) | Low |
| 1 (Observational) | Odds ratio | 2.6 (2.2–3.1) | Very low | |
| Behavioural interventions: exercise advice | 1 (RCT) | Odds ratio | 2.7 (1.6–4.5) | Low |
| Behavioural interventions: self-management support | 1 (RCT) | Odds ratio | 2.6 (1.7–3.8) | Low |
| Behavioural interventions: HF education | 1 (Observational) | Odds ratio | 0.95 (0.67–1.35) | Very low |
| Composite outcomes | 1 (Observational) | Risk difference | 35.1 (28.3–41.9) | Very low |
| 1 (Observational) | Odds ratio | 1.60 (0.93–2.74) | ||
Abbreviations: ACE inhibitor, angiotensin-converting enzyme inhibitor; BP, blood pressure; CI, confidence interval; CRT-D, cardio-resynchronization therapy with defibrillator; CRT-P, cardio-resynchronization therapy with pacemaker; HbA1c, hemoglobin A1C; ICD, implantable cardioverter defibrillator; NR, not reported; RCT, randomized clinical trial.
Details of individual GRADE assessments are available in Appendix 3.
Pool effect estimate.
Measures of Efficiency
There was evidence that an electronic discharge summary was received in as timely a manner as paper-based discharge summaries; overall, the evidence did not demonstrate improved efficiency (Table 37).
Table 37: Summary of Measures of Efficiency.
| Outcome | Number of Studies | Statistical Method | Effect Estimate (95% CI) | GRADEa |
|---|---|---|---|---|
| Impact on Time | ||||
| Proportion of PCPs receiving discharge summary within 1–7 days | 1 (RCT) | Risk difference | 1.1 (−9.2 to 6.9) | High |
| Time to first measure of LDL-C, days | 1 (RCT) | Mean difference | −22.0 (−82.9 to 38.9) | Moderate |
| Time to change in statin prescription | 1 (RCT) | Mean difference | −7.1 (−12.0 to−2.2) | Moderate |
| Time spent by providers with patients | 1 (Observational) | Mean difference | 4.5 (1.83–7.17) | Very low |
| Time spent by nurses with patients | 1 (Observational) | Mean difference | 3.00 (0.67–5.33) | Very low |
| Impact on Communication | ||||
| Number of letters from GP to consultant | 1 (RCT) | NR | Not significant | Very low |
| Number of letters from consultant to GP | 1 (RCT) | NR | Significant increase | Very low |
| Number of patient contacts with GP | 1 (RCT) | NR | Not significant | Very low |
| Number of patient contacts with consultant | 1 (RCT) | NR | Not significant | Very low |
Abbreviations: CI, confidence interval; GP, general practitioner; LDL-C, low-density lipoprotein cholesterol; NR, not reported; PCP, primary care physician; RCT, randomized clinical trial.
Details of individual GRADE assessments are available in Appendix 3.
Conclusions
The findings from this evidence-based analysis call into question the ability of eTools to independently improve the quality of outpatient care coordination. Although automation is intended to facilitate consistency in application and measurement, eTools may not be able to overcome underlying process inefficiencies. That said, based on the findings from this report, there does not appear to be evidence of patient harm with the implementation of eTools in various contexts and settings. (Note: All conclusions are from the perspective of implementation of eTools versus comparator groups.)
Health Services Utilization
When an automated laboratory results report with clinical alerts mapped to guidelines was shared with primary care, there was evidence of a reduction in the following:
hospitalizations (relative reduction 15%), based on moderate quality evidence
hospital length of stay (relative reduction 10%), based on moderate quality evidence
ED visits (relative reduction 25%), based on moderate quality evidence
There was evidence of no difference in the proportion of patients who experienced a readmission, based on high quality evidence.
Disease-Specific Clinical Outcomes
Following implementation of a variety of eTools with health information exchange capabilities, there was evidence of no difference in the following:
proportion of patients experiencing adverse events, based on high quality evidence
blood pressure, based on low quality evidence
lipid levels, based on low quality evidence
HbA1c, based on very low quality evidence
There was inconclusive evidence of impact on the proportion of patients achieving a previously defined guideline threshold (HbA1c, blood pressure control, lipid levels, smoking status, body mass index, or composite outcomes), based on very low quality evidence.
Process-of-Care Indicators
The evidence did not demonstrate that eTools for health information exchange had an overall positive impact on process-of-care measures, and there was no trend for specific diseases, care coordination aspects, or technologies.
There was evidence of an increase in the number of the following:
foot examinations, based on low quality evidence
fructosamine tests, based on low quality evidence
weight measures, based on low quality evidence
height measures, based on low quality evidence
blood pressure examinations, based on low to very low quality evidence
vaccinations and immunizations, based on low to very low quality evidence
eye examinations, based on very low quality evidence
medication management of beta-blockers, based on very low quality evidence
There was evidence of no difference in the following:
changes in prescribed statins at 1 year, based on moderate quality evidence
blood glucose tests, based on low quality evidence
lipid tests conducted, based on very low quality evidence
medication management, based on very low quality of evidence, of ACE inhibitors, Aspirin, aldosterone antagonists, anticoagulants, or implantable cardioverter and resynchronization devices
There was inconclusive evidence of an impact on the following:
kidney management, based on low to very low quality evidence
behavioural interventions, based on low to very low quality evidence
HbA1c tests, based on very low quality evidence
composite outcomes of process of care indicators, based on very low quality evidence
Measures of Efficiency
The evidence did not demonstrate improved efficiency for care providers upon implementation of eTools for health information exchange.
There was evidence of no difference in the proportion of PCPs receiving discharge summaries within the first week post-discharge, based on high quality evidence.
There was no demonstrated improved impact on the following:
efficiencies related to time, based on very low quality evidence
efficiencies related to communication, based on moderate to very low quality evidence
Acknowledgements
Editorial Staff
Jeanne McKane, CPE, ELS(D)
Medical Information Services
Kaitryn Campbell, BA(H), BEd, MLIS
Kellee Kaulback, BA(H), MISt
Expert Panel for Health Quality Ontario: Optimizing Chronic Disease Management in the Community (Outpatient) Setting
| Name | Title | Organization |
|---|---|---|
| Shirlee Sharkey (chair) | President & CEO | Saint Elizabeth Health Care |
| Theresa Agnew | Executive Director | Nurse Practitioners’ Association of Ontario |
| Onil Bhattacharrya | Clinician Scientist | Li Ka Shing Knowledge Institute, St. Michael’s Hospital, University of Toronto |
| Arlene Bierman | Ontario Women’s Health Council Chair in Women’s Health | Department of Medicine, Keenan Research Centre in the Li Ka Shing Knowledge Institute, St. Michael’s Hospital, University of Toronto |
| Susan Bronskill | Scientist | Institute for Clinical Evaluative Sciences |
| Catherine Demers | Associate Professor | Division of Cardiology, Department of Medicine, McMaster University |
| Alba Dicenso | Professor | School of Nursing, McMaster University |
| Mita Giacomini | Professor | Centre of Health Economics & Policy Analysis, Department of Clinical Epidemiology & Biostatistics |
| Ron Goeree | Director | Programs for Assessment of Technology in Health (PATH) Research Institute, St. Joseph’s Healthcare Hamilton |
| Nick Kates | Senior Medical Advisor | Health Quality Ontario - QI McMaster University Hamilton Family Health Team |
| Murray Krahn | Director | Toronto Health Economics and Technology Assessment (THETA) Collaborative, University of Toronto |
| Wendy Levinson | Sir John and Lady Eaton Professor and Chair | Department of Medicine, University of Toronto |
| Raymond Pong | Senior Research Fellow and Professor | Centre for Rural and Northern Health Research and Northern Ontario School of Medicine, Laurentian University |
| Michael Schull | Deputy CEO & Senior Scientist | Institute for Clinical Evaluative Sciences |
| Moira Stewart | Director | Centre for Studies in Family Medicine, University of Western Ontario |
| Walter Wodchis | Associate Professor | Institute of Health Management Policy and Evaluation, University of Toronto |
Suggested Citation
This report should be cited as follows: Health Quality Ontario. Electronic tools for health information exchange: an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2013 September;13(11):1–76. Available from: http://www.hqontario.ca/en/documents/eds/2013/full-report-OCDM-etools.pdf.
Indexing
The Ontario Health Technology Assessment Series is currently indexed in MEDLINE/PubMed, Excerpta Medica/EMBASE, and the Centre for Reviews and Dissemination database.
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to: EvidenceInfo@hqontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Conflict of Interest Statement
All reports in the Ontario Health Technology Assessment Series are impartial. There are no competing interests or conflicts of interest to declare.
Peer Review
All reports in the Ontario Health Technology Assessment Series are subject to external expert peer review. Additionally, Health Quality Ontario posts draft reports and recommendations on its website for public comment prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
About Health Quality Ontario
Health Quality Ontario (HQO) is an arms-length agency of the Ontario government. It is a partner and leader in transforming Ontario's health care system so that it can deliver a better experience of care, better outcomes for Ontarians and better value for money.
Health Quality Ontario strives to promote health care that is supported by the best available scientific evidence. HQO works with clinical experts, scientific collaborators and field evaluation partners to develop and publish research that evaluates the effectiveness and cost-effectiveness of health technologies and services in Ontario.
Based on the research conducted by HQO and its partners, the Ontario Health Technology Advisory Committee (OHTAC) — a standing advisory sub-committee of the HQO Board — makes recommendations about the uptake, diffusion, distribution or removal of health interventions to Ontario's Ministry of Health and Long-Term Care, clinicians, health system leaders and policy-makers.
This research is published as part of Ontario Health Technology Assessment Series, which is indexed in CINAHL, EMBASE, MEDLINE, and the Centre for Reviews and Dissemination. Corresponding OHTAC recommendations and other associated reports are also published on the HQO website. Visit http://www.hqontario.ca for more information.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, HQO and/or its research partners reviews the available scientific literature, making every effort to consider all relevant national and international research; collaborates with partners across relevant government branches; consults with clinical and other external experts and developers of new health technologies; and solicits any necessary supplemental information.
In addition, HQO collects and analyzes information about how a health intervention fits within current practice and existing treatment alternatives. Details about the diffusion of the intervention into current health care practices in Ontario add an important dimension to the review. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social, and legal issues relating to the intervention assist in making timely and relevant decisions to optimize patient outcomes.
The public consultation process is available to individuals and organizations wishing to comment on reports and recommendations prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Disclaimer
This report was prepared by HQO or one of its research partners for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research. It also incorporates, when available, Ontario data and information provided by experts and applicants to HQO. It is possible that relevant scientific findings may have been reported since completion of the review. This report is current to the date of the literature review specified in the methods section, if available. This analysis may be superseded by an updated publication on the same topic. Please check the HQO website for a list of all publications: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Health Quality Ontario
130 Bloor Street West, 10th Floor
Toronto, Ontario
M5S 1N5
Tel: 416-323-6868
Toll Free: 1-866-623-6868
Fax: 416-323-9261
Email: EvidenceInfo@hqontario.ca
ISSN 1915-7398 (online)
ISBN 978-1-4606-1244-6 (PDF)
© Queen’s Printer for Ontario, 2013
List of Tables
| Table 1: Description and Potential Applications for Various eTools |
| Table 2: Body of Evidence Examined According to Study Design |
| Table 3: Description of Included Studies |
| Table 4: Description of Individual Technologies Applied |
| Table 5: Studies and Outcomes by Chronic Disease Group |
| Table 6: Impact of eTools on Hospitalizations |
| Table 7: Impact of eTools on Length of Stay |
| Table 8: Impact of eTools on Number of ED Visits |
| Table 9: Impact of eTools on Readmissions |
| Table 10: Impact of eTools on HbA1c |
| Table 11: Impact of eTools on Blood Pressure |
| Table 12: Impact of eTools on Lipids |
| Table 13: Impact of eTools on Adverse Events |
| Table 14: Impact of eTools on Achievement of Clinical Guidelines |
| Table 15: Impact of eTools on Blood Pressure Measures Conducted |
| Table 16: Impact of eTools on Lipid Tests Conducted |
| Table 17: Impact of eTools on HbA1c Tests Conducted |
| Table 18: Impact of eTools on Blood Glucose and Fructosamine Tests Conducted |
| Table 19: Impact of eTools on Eye Examinations Conducted |
| Table 20: Impact of eTools on Foot Examinations Conducted |
| Table 21: Impact of eTools on Urine Protein Tests Conducted for Kidney Management |
| Table 22: Impact of eTools on Other Tests Conducted for Kidney Management |
| Table 23: Impact of eTools on Weight Measures Conducted |
| Table 24: Impact of eTools on Height Measures Conducted |
| Table 25: Impact of eTools on Immunizations Administered |
| Table 26: Impact of eTools on Appropriately Prescribed ACE Inhibitors |
| Table 27: Impact of eTools on Appropriately Prescribed Anticoagulation for Atrial Fibrillation |
| Table 28: Impact of eTools on Appropriately Prescribed Aspirin |
| Table 29: Impact of eTools on Other Outcomes of Appropriately Managed Medications |
| Table 30: Impact of eTools on Appropriate Changes Made to Statin Prescriptions |
| Table 31: Impact of eTools on Behavioural Management Interventions |
| Table 32: Impact of eTools on Composite Outcomes of Tests Conducted |
| Table 33: Impact of eTools on Time |
| Table 34: Impact of eTools on Frequency of Communication |
| Table 35: Summary of Health Services Utilization and Disease-Specific Clinical Outcomes |
| Table 36: Summary of Process-of-Care Indicators |
| Table 37: Summary of Measures of Efficiency |
| Table A1: Additional Publications Referenced for Supplementary Details on Included Studies |
| Table A2: GRADE Evidence Profile for Health Services Utilization and Disease-Specific Clinical Outcomes |
| Table A3: GRADE Evidence Profile for Process-of-Care Indicators |
| Table A4: GRADE Evidence Profile for Measures of Efficiency |
| Table A5: Risk of Bias Among Randomized Controlled Trials for the Impact of eTools |
| Table A6: Risk of Bias Among Observational Trials for the Impact of eTools |
List of Figures
| Figure 1: Example of Complex Flow of Information Involved in Care Coordination |
| Figure 2: Citation Flow Chart |
| Figure 3: Pooled Effect Estimate of Foot Examinations Conducted in Observational Studies |
| Figure 4: Subgroup Analysis: Process-of-Care Outcomes By Disease, Care Coordination Aspect, and Technology |
List of Abbreviations
- aDiff
Adjusted risk difference
- ACE
Angiotensin-converting enzyme
- aOR
Adjusted odds ratio
- ARB
Angiotensin receptor blocker
- aRC
Adjusted regression correlation
- BMI
Body mass index
- BP
Blood pressure
- CAD
Coronary artery disease
- CDSS
Clinical decision support system
- CI
Confidence interval
- CPOE
Computerized physician (or provider) order entry
- CRT-D
Cardio-resynchronization therapy with defibrillator
- CRT-P
Cardio-resynchronization therapy with pacemaker
- CT
Computed tomography
- DBP
Diastolic blood pressure
- DEMS
Diabetes electronic management system
- ED
Emergency department
- EDI
Electronic data interchange
- EHR
Electronic health record
- EMR
Electronic medical record
- eTools
Electronic tools
- FRACGP
Fellow of the Royal Australian College of General Practitioners
- GP
General practitioner
- HbA1c
Hemoglobin A1c
- ICD
Implantable cardioverter defibrillator
- LDL-C
Low-density lipoprotein cholesterol
- MRI
Magnetic resonance imaging
- NR
Not reported
- OR
Odds ratio
- PACS
Picture archiving communication system
- PCP
Primary care physician
- PHR
Personal (or patient) health record
- RCT
Randomized controlled trial
- SBP
Systolic blood pressure
Footnotes
Interventions were evaluated based on the application of the eTool, not on the label applied to it. For example, telemedicine was considered for inclusion if a nurse was involved in the transmission of patient data and the eTool was used as a mechanism for care coordination, but it was excluded if the patient was involved in the transmission of data.
Appendices
Appendix 1: Literature Search Strategies
Search date: April 26, 2012
Databases searched: Databases searched: OVID MEDLINE, OVID MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, EBSCO CINAHL, Centre for Reviews and Dissemination.
Ovid MEDLINE(R) <1946 to April Week 3 2012>, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations <April 25, 2012>, Embase <1980 to 2012 Week 16>
Search Strategy:
--------------------------------------------------------------------------------
1 exp Coronary Artery Disease/ (223075)
2 exp Myocardial Infarction/ use mesz (135539)
3 exp heart infarction/ use emez (225793)
4 (coronary artery disease or cad or heart attack).ti. (45983)
5 ((myocardi* or heart or cardiac or coronary) adj2 (atheroscleros* or arterioscleros* or infarct*)).ti. (153984)
6 or/1−5 (559947)
7 exp Atrial Fibrillation/ use mesz (28957)
8 exp heart atrium fibrillation/ use emez (58378)
9 ((atrial or atrium or auricular) adj1 fibrillation*).ti,ab. (77199)
10 or/7−9 (103984)
11 exp heart failure/ (311514)
12 ((myocardi* or heart or cardiac) adj2 (failure or decompensation or insufficiency)).ti,ab. (244313)
13 11 or 12 (396209)
14 exp Stroke/ (184883)
15 exp Ischemic Attack, Transient/ use mesz (16552)
16 exp transient ischemic attack/ use emez (20571)
17 exp stroke patient/ use emez (5818)
18 exp brain infarction/ or exp cerebrovascular accident/ use emez (105144)
19 (stroke or tia or transient ischemic attack or cerebrovascular apoplexy or cerebrovascular accident or cerebrovascular infarct* or brain infarct* or CVA).ti,ab. (294576)
20 or/14–19 (408356)
21 exp Diabetes Mellitus, Type 2/ use mesz (70547)
22 exp non insulin dependent diabetes mellitus/ use emez (108517)
23 exp diabetic patient/ use emez (13718)
24 (diabetes or diabetic* or niddm or t2dm).ti,ab. (799410)
25 or/21–24 (825461)
26 exp Skin Ulcer/ (74421)
27 ((pressure or bed or skin) adj2 (ulcer* or sore* or wound*)).ti,ab. (29783)
28 (decubitus or bedsore*).ti,ab. (8729)
29 or/26–28 (93902)
30 exp Pulmonary Disease, Chronic Obstructive/ use mesz (17882)
31 exp chronic obstructive lung disease/ use emez (57527)
32 (chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) adj (disease* or disorder*)).ti,ab. (57215)
33 (copd or coad).ti,ab. (48215)
34 chronic airflow obstruction.ti,ab. (1086)
35 exp Emphysema/ (38314)
36 exp chronic bronchitis/ use emez (7067)
37 ((chronic adj2 bronchitis) or emphysema).ti,ab. (52038)
38 or/30–37 (165176)
39 exp Chronic Disease/ (352795)
40 ((chronic* adj2 disease*) or (chronic* adj2 ill*)).ti,ab. (230609)
41 39 or 40 (526597)
42 6 or 10 or 13 or 20 or 25 or 29 or 38 or 41 (2710352)
43 exp Medical Informatics/ use mesz (270756)
44 exp Medical Records Systems, Computerized/ use mesz (20862)
45 exp *Data Processing/ use emez (451316)
46 (ehr or ehealth or etool* or eprescri* or (computer* adj2 physician order entry) or CPOE or clinical decision support system* or picture archiving communication* system* or PACS).ti,ab. (13421)
47 ((electronic or e or computer*) adj2 (health or patient or medical) adj record*).ti,ab. (20226)
48 ((electronic or e or computer*) adj2 (management or tool* or system* or prescrib* or decision support or discharge or (medication adj2 reconciliation))).ti,ab. (40980)
49 or/44–48 (515984)
50 exp Intermediate Care Facilities/ use mesz (601)
51 (intermedia* adj2 care).ti,ab. (2483)
52 exp ambulatory care/ (77162)
53 exp Ambulatory Care Facilities/ use mesz (40218)
54 exp ambulatory care nursing/ use emez (9)
55 exp Outpatients/ use mesz (7295)
56 exp Outpatient Department/ use emez (33491)
57 exp outpatient care/ use emez (17984)
58 exp Community Health Services/ use mesz (449731)
59 exp community care/ use emez (88605)
60 exp Community Medicine/ (3920)
61 exp Subacute Care/ use mesz (707)
62 exp General Practice/ (125046)
63 exp Primary Health Care/ (157916)
64 exp Physicians, Family/ or exp general practitioners/ or exp Physicians, Primary Care/ use mesz (63980)
65 exp general practitioner/ use emez (48469)
66 exp family medicine/ use emez (5959)
67 exp Group Practice/ use mesz (22240)
68 exp Team Nursing/ use emez (23)
69 exp Primary Care Nursing/ use mesz (38)
70 exp Patient Care Team/ use mesz (49591)
71 exp Teamwork/ use emez (9370)
72 *Patient Care Management/ use mesz (1271)
73 ((primary or family or community or outpatient* or ambulatory) adj2 (care* or physician* or nurs* or service* or clinic* or facility or facilities)).ti,ab. (342433)
74 ((transitional or multidisciplin* or multifacet* or multi-disciplin* or multi-facet* or cooperat* or co-operat* or interdisciplin* or inter-disciplin* or collaborat* or multispecial* or multi-special* or share or sharing or shared or integrat* or joint or multi-modal or multimodal) adj2 (care or team*)).ti,ab. (43679)
75 (team* or liaison).ti,ab. (185342)
76 ((general or family or primary care or community) adj2 (practic* or clinic* or program* or doctor* or nuse* or physician*)).ti,ab. (212184)
77 or/50–76 (1387096)
78 42 and 49 and 77 (3445)
79 limit 78 to english language (3248)
80 limit 79 to (case reports or comment or editorial or letter) [Limit not valid in Embase; records were retained] (56)
81 Case Report/ use emez (1818833)
82 79 not (80 or 81) (3157)
83 remove duplicates from 82 (2435)
CINAHL
| # | Query | Results |
|---|---|---|
| S56 | S35 and S53 and S54 Limiters - English Language |
478 |
| S55 | S35 and S53 and S54 | 484 |
| S54 | S4 OR S7 OR S10 OR S14 OR S18 OR S21 OR S28 | 110786 |
| S53 | S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 | 218102 |
| S52 | ((general or family or primary care or community) N2 (practic* or clinic* or program* or doctor* or nuse* or physician*)) | 42239 |
| S51 | (team* or liaison) | 51916 |
| S50 | ((transitional or multidisciplin* or multifacet* or multi-disciplin* or multi-facet* or cooperat* or co-operat* or interdisciplin*or inter-disciplin* or collaborat* or multispecial* or multi-special* or share or sharing or shared or integrat* or joint or multi-modal or multimodal) N2 (care or team*)). | 30234 |
| S49 | ((primary or family or community or outpatient* or ambulatory) N2 (care* or physician* or nurs* or service* or clinic* or facility or facilities)) | 120869 |
| S48 | (MH “Team Nursing”) OR (MH “Primary Nursing”) | 1298 |
| S47 | (MH “Multidisciplinary Care Team+”) | 18615 |
| S46 | (MH “Group Practice+”) | 5868 |
| S45 | (MH “Physicians, Family”) | 7237 |
| S44 | (MH “Primary Health Care”) | 25141 |
| S43 | (MH “Family Practice”) | 9219 |
| S42 | (MH “Community Medicine”) | 23 |
| S41 | (MH “Community Programs”) | 3920 |
| S40 | (MM “Community Health Services”) OR (MH “Community Health Nursing+”) OR (MH “Community Networks”) OR (MH “Family Services”) OR (MH “Occupational Health Services+”) | 31826 |
| S39 | (MH “Outpatients”) | 27169 |
| S38 | (MH “Outpatient Service”) | 3017 |
| S37 | (MH “Ambulatory Care”) OR (MH “Ambulatory Care Facilities+”) OR (MH “Ambulatory Care Nursing”) | 13447 |
| S36 | (MH “Subacute Care”) | 976 |
| S35 | S29 or S30 or S31 or S32 or S33 or S34 | 39837 |
| S34 | (electronic or e or computer*) N2 (management or tool* or system* or prescrib* or decision support or discharge or (medication N2 reconciliation)) | 6013 |
| S33 | ((electronic or e or computer*) N2 (health or patient or medical) N1 record*) | 8817 |
| S32 | (ehr or ehealth or etool* or eprescri* or (computer* N2 physician order entry) or CPOE or clinical decision support system* or picture archiving communication* system* or PACS) | 2165 |
| S31 | (MH “Information Technology+”) OR (MH “Systems Development+”) | 13019 |
| S30 | (MH “Computerized Patient Record”) | 7254 |
| S29 | (MH “Health Information Systems+”) OR (MH “Management Information Systems+”) OR (MH “Health Informatics+”) OR (MH “Image Retrieval Systems”) OR (MH “Integrated Advanced Information Management Systems”) OR (MH “Laboratory Automation Systems”) | 25352 |
| S28 | S26 or S27 | 29029 |
| S27 | chronic*N2 disease* or chronic* N2 ill* | 7671 |
| S26 | (MH “Chronic Disease”) | 24387 |
| S25 | chronic N2 bronchitis or emphysema | 1854 |
| S24 | (MH “Emphysema”) | 911 |
| S23 | chronic obstructive N2 disease* or chronic obstructive N2 disorder* or copd or coad | 7697 |
| S22 | (MH “Pulmonary Disease, Chronic Obstructive+”) | 5746 |
| S21 | S19 or S20 | 16558 |
| S20 | pressure N1 ulcer* or bedsore* or bed N1 sore* or skin N1 ulcer* OR pressure N1 wound* OR decubitus | 9821 |
| S19 | (MH “Skin Ulcer+”) | 15161 |
| S18 | S15 or S16 or S17 | 72199 |
| S17 | diabetes or diabetic* or niddm or t2dm | 72199 |
| S16 | (MH “Diabetic Patients”) | 3650 |
| S15 | (MH “Diabetes Mellitus, Type 2”) | 18985 |
| S14 | S19 or S18 or S17 | 71 |
| S13 | stroke or tia or transient ischemic attack or cerebrovascular apoplexy or cerebrovascular accident or cerebrovascular infarct* or brain infarct* or CVA | 38866 |
| S12 | (MH “Cerebral Ischemia, Transient”) | 1954 |
| S11 | (MH “Stroke”) OR (MH “Stroke Patients”) | 26468 |
| S10 | S22 OR S21 | 50 |
| S9 | myocardi*failure OR myocardial decompensation OR myocardial insufficiency OR cardiac failure OR cardiac decompensation or cardiac insufficiency OR heart failure OR heart decompensation OR heart insufficiency | 19373 |
| S8 | (MH “Heart Failure+”) | 14932 |
| S7 | S25 OR S24 | 53 |
| S6 | atrial N1 fibrillation* OR atrium N1 fibrillation* OR auricular N1 fibrillation* | 8361 |
| S5 | (MH “Atrial Fibrillation”) | 6776 |
| S4 | S31 OR S28 OR S27 OR S26 | 76 |
| S3 | TI myocardi* N2 infarct* or TI heart N2 infarct* or TI cardiac N2 infarct* OR TI coronary N2 infarct* or TI arterioscleros* or TI atheroscleros* | 9857 |
| S2 | coronary artery disease OR cad OR heart attack* | 7893 |
| S1 | (MH “Myocardial Infarction+”) or (MH “Coronary Arteriosclerosis”) | 24056 |
CRD
| Line | Search | Hits |
|---|---|---|
| 1 | MeSH DESCRIPTOR coronary artery disease EXPLODE ALL TREES | 300 |
| 2 | (coronary artery disease or cad or heart attack*):TI | 223 |
| 3 | ((myocardi* or heart or cardiac or coronary) adj2 (atheroscleros* or arterioscleros* or infarct*)):TI | 232 |
| 4 | MeSH DESCRIPTOR Atrial Fibrillation EXPLODE ALL TREES | 277 |
| 5 | (((atrial or atrium or auricular) adj1 fibrillation*):TI | 0 |
| 6 | ((atrial or atrium or auricular) adj1 fibrillation*):TI | 181 |
| 7 | MeSH DESCRIPTOR heart failure EXPLODE ALL TREES | 500 |
| 8 | ((myocardi* or heart or cardiac) adj2 (failure or decompensation or insufficiency)):TI | 293 |
| 9 | MeSH DESCRIPTOR stroke EXPLODE ALL TREES | 668 |
| 10 | MeSH DESCRIPTOR Ischemic Attack, Transient EXPLODE ALL TREES | 42 |
| 11 | (stroke or tia or transient ischemic attack or cerebrovascular apoplexy or cerebrovascular accident or cerebrovascular infarct* or brain infarct* or CVA):TI | 640 |
| 12 | MeSH DESCRIPTOR Diabetes Mellitus, Type 2 EXPLODE ALL TREES | 631 |
| 13 | (diabetes or diabetic* or niddm or t2dm):TI | 1276 |
| 14 | MeSH DESCRIPTOR Skin Ulcer EXPLODE ALL TREES | 280 |
| 15 | ((pressure or bed or skin) adj2 (ulcer* or sore* or wound*)):TI | 76 |
| 16 | ( decubitus or bedsore*):TI | 0 |
| 17 | MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive EXPLODE ALL TREES | 291 |
| 18 | (chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) ):TI | 228 |
| 19 | (copd or coad):TI | 116 |
| 20 | (chronic airflow obstruction):TI | 0 |
| 21 | MeSH DESCRIPTOR Emphysema EXPLODE ALL TREES | 11 |
| 22 | ((chronic adj2 bronchitis) or emphysema):TI | 48 |
| 23 | MeSH DESCRIPTOR Chronic Disease EXPLODE ALL TREES | 772 |
| 24 | ((chronic* adj2 disease*) or (chronic* adj2 ill*)):TI | 265 |
| 25 | MeSH DESCRIPTOR Comorbidity EXPLODE ALL TREES | 170 |
| 26 | (comorbid* OR co-morbid* OR multimorbid* OR multi-morbid* OR (complex* adj1 patient*) OR “patient* with multiple” OR (multiple adj2 (condition* OR disease*))):TI | 25 |
| 27 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 | 5010 |
| 28 | MeSH DESCRIPTOR medical informatics EXPLODE ALL TREES | 2338 |
| 29 | MeSH DESCRIPTOR Medical Records Systems, Computerized EXPLODE ALL TREES | 49 |
| 30 | ((ehr or ehealth or etool* or eprescri* or (computer* adj2 physician order entry) or CPOE or clinical decision support system* or picture archiving communication* system* or PACS)) | 64 |
| 31 | (((electronic or e or computer*) adj2 (health or patient or medical) adj record*)) | 86 |
| 32 | ((electronic or e or computer*) adj2 (management or tool* or system* or prescrib* or decision support or discharge or (medication adj2 reconciliation))) | 340 |
| 33 | #28 OR #29 OR #30 OR #31 OR #32 | 2608 |
| 34 | MeSH DESCRIPTOR Intermediate Care Facilities EXPLODE ALL TREES | 4 |
| 35 | (intermedia* adj2 care) | 39 |
| 36 | MeSH DESCRIPTOR ambulatory care EXPLODE ALL TREES | 346 |
| 37 | MeSH DESCRIPTOR Ambulatory Care Facilities EXPLODE ALL TREES | 205 |
| 38 | MeSH DESCRIPTOR Outpatients EXPLODE ALL TREES | 73 |
| 39 | MeSH DESCRIPTOR Community Health Services EXPLODE ALL TREES | 4097 |
| 40 | MeSH DESCRIPTOR Community Medicine EXPLODE ALL TREES | 3 |
| 41 | MeSH DESCRIPTOR Subacute Care EXPLODE ALL TREES | 7 |
| 42 | MeSH DESCRIPTOR Primary Health Care EXPLODE ALL TREES | 673 |
| 43 | MeSH DESCRIPTOR Physicians, Family EXPLODE ALL TREES | 50 |
| 44 | MeSH DESCRIPTOR Group Practice EXPLODE ALL TREES | 65 |
| 45 | MeSH DESCRIPTOR Patient Care Team EXPLODE ALL TREES | 207 |
| 46 | MeSH DESCRIPTOR Patient Care Management EXPLODE ALL TREES | 2512 |
| 47 | (((primary or family or community or outpatient* or ambulatory) adj2 (care* or physician* or nurs* or service* or clinic* or facility or facilities))) OR (((transitional or multidisciplin* or multifacet* or multi-disciplin* or multi-facet* or cooperat* or co-operat* or interdisciplin* or inter-disciplin* or collaborat* or multispecial* or multi-special* or share or sharing or shared or integrat* or joint or multi-modal or multimodal) adj2 (care or team*))) OR (team* or liaison) OR (general or family or primary care or community) adj2 (practic* or clinic* or program* or doctor* or nuse* or physician*))) | 2134 |
| 48 | #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 | 7581 |
| 49 | #27 AND #33 AND #48 | 65 |
Cochrane
| ID | Search | Hits |
|---|---|---|
| #1 | MeSH descriptor Coronary Artery Disease explode all trees | 2250 |
| #2 | MeSH descriptor Myocardial Infarction explode all trees | 7854 |
| #3 | (myocardi* or heart or cardiac or coronary) NEAR/2 (atheroscleros* or arterioscleros* or infarct*):ti or (coronary artery disease or cad or heart attack*):ti | 8562 |
| #4 | MeSH descriptor Atrial Fibrillation explode all trees | 2159 |
| #5 | (atrial NEAR/2 fibrillation* or atrium NEAR/2 fibrillation* or auricular NEAR/2 fibrillation* ):ti | 2357 |
| #6 | MeSH descriptor Heart Failure explode all trees | 4818 |
| #7 | (myocardi* NEAR/2 (failure or decompensation or insufficiency)):ti or (heart NEAR/2 (failure or decompensation or insufficiency)):ti or (cardiac NEAR/2 (failure or decompensation or insufficiency)):ti | 5347 |
| #8 | MeSH descriptor Stroke explode all trees | 4020 |
| #9 | MeSH descriptor Ischemic Attack, Transient explode all trees | 469 |
| #10 | (stroke or tia or transient ischemic attack or cerebrovascular apoplexy or cerebrovascular accident or cerebrovascular infarct* or brain infarct* or CVA):ti | 10009 |
| #11 | MeSH descriptor Diabetes Mellitus, Type 2 explode all trees | 7179 |
| #12 | (diabetes or diabetic* or niddm or t2dm):ti | 16895 |
| #13 | MeSH descriptor Skin Ulcer explode all trees | 1599 |
| #14 | (pressure or bed or skin) NEAR/2 (ulcer* or sore* or wound*):ti | 673 |
| #15 | (decubitus or bedsore*):ti | 100 |
| #16 | MeSH descriptor Pulmonary Disease, Chronic Obstructive explode all trees | 1804 |
| #17 | (chronic obstructive NEAR/2 (lung* or pulmonary or airway* or airflow or respiratory) ):ti | 2436 |
| #18 | (copd or coad):ti | 3352 |
| #19 | (chronic airflow obstruction):ti | 72 |
| #20 | MeSH descriptor Emphysema explode all trees | 92 |
| #21 | (chronic NEAR/2 bronchitis) or emphysema:ti | 1184 |
| #22 | MeSH descriptor Chronic Disease explode all trees | 10019 |
| #23 | (chronic* NEAR/2 disease* or chronic* NEAR/2 ill*):ti | 1702 |
| #24 | MeSH descriptor Comorbidity explode all trees | 1987 |
| #25 | (comorbid* OR co-morbid* OR multimorbid* OR multi-morbid* OR (complex* NEXT patient*) OR “patient* with multiple” OR (multiple NEAR/2 (condition* OR disease*))):ti | 654 |
| #26 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25) | 69160 |
| #27 | MeSH descriptor Medical Informatics explode all trees | 7364 |
| #28 | MeSH descriptor Medical Records Systems, Computerized explode all trees | 287 |
| #29 | ((electronic or e or computer*) NEAR/2 (health or patient or medical) NEAR record*):ti or ((electronic or e or computer*) NEAR/2 (health or patient or medical) NEAR record*):ab | 276 |
| #30 | (ehr or ehealth or etool* or eprescri* or (computer* NEAR/2 physician order entry) or CPOE or clinical decision support system* or picture archiving communication* system* or PACS):ti or (ehr or ehealth or etool* or eprescri* or (computer* NEAR/2 physician order entry) or CPOE or clinical decision support system* or picture archiving communication* system* or PACS):ab | 353 |
| #31 | ((electronic or e or computer*) NEAR/2 (management or tool* or system* or prescrib* or decision support or discharge or (medication NEAR/2 reconciliation))):ti or ((electronic or e or computer*) NEAR/2 (management or tool* or system* or prescrib* or decision support or discharge or (medication NEAR/2 reconciliation))):ab | 889 |
| #32 | (#27 OR #28 OR #29 OR #30 OR #31) | 8363 |
| #33 | MeSH descriptor Intermediate Care Facilities explode all trees | 13 |
| #34 | (intermedia* NEAR/2 care):ti or (intermedia* NEAR/2 care):ab | 95 |
| #35 | MeSH descriptor Ambulatory Care explode all trees | 3189 |
| #36 | MeSH descriptor Ambulatory Care Facilities explode all trees | 1424 |
| #37 | MeSH descriptor Outpatients explode all trees | 692 |
| #38 | MeSH descriptor Community Health Services explode all trees | 19917 |
| #39 | MeSH descriptor Community Medicine explode all trees | 34 |
| #40 | MeSH descriptor Subacute Care explode all trees | 16 |
| #41 | MeSH descriptor General Practice explode all trees | 2113 |
| #42 | MeSH descriptor Primary Health Care explode all trees | 2928 |
| #43 | MeSH descriptor Physicians, Family explode all trees | 445 |
| #44 | MeSH descriptor General Practitioners explode all trees | 31 |
| #45 | MeSH descriptor Physicians, Primary Care explode all trees | 21 |
| #46 | MeSH descriptor Group Practice explode all trees | 378 |
| #47 | MeSH descriptor Primary Care Nursing explode all trees | 1 |
| #48 | MeSH descriptor Patient Care Team explode all trees | 1177 |
| #49 | MeSH descriptor Patient Care Management explode all trees | 13149 |
| #50 | ((primary or family or community or outpatient* or ambulatory) NEAR/2 (care* or physician* or nurs* or service* or clinic* or facility or facilities)):ti and ((primary or family or community or outpatient* or ambulatory) NEAR/2 (care* or physician* or nurs* or service* or clinic* or facility or facilities)):ab | 2110 |
| #51 | (transitional or multidisciplin* or multifacet* or multi-disciplin* or multi-facet* or cooperat* or co-operat* or interdisciplin* or inter-disciplin* or collaborat* or multispecial* or multi-special* or share or sharing or shared or integrat* or joint or multi-modal or multimodal) NEAR/2 (care or team*):ti or (transitional or multidisciplin* or multifacet* or multi-disciplin* or multi-facet* or cooperat* or co-operat* or interdisciplin* or inter-disciplin* or collaborat* or multispecial* or multi-special* or share or sharing or shared or integrat* or joint or multi-modal or multimodal) NEAR/2 (care or team*):ab | 1115 |
| #52 | ((general or family or primary care or community) NEAR/2 (practic* or clinic* or program* or doctor* or nuse* or physician*)):ti or ((general or family or primary care or community) NEAR/2 (practic* or clinic* or | 8087 |
| program* or doctor* or nuse* or physician*)):ab | ||
| #53 | (team* or liaison):ti or (team* or liaison):ab | 3183 |
| #54 | (#50 OR #51 OR #52 OR #53) | 12346 |
| #55 | (#54 AND #32 AND #26) |
Appendix 2: Additional Publications
Table A1: Additional Publications Referenced for Supplementary Details on Included Studies.
| Included Studies | Additional Publications | |||
|---|---|---|---|---|
| Author, Year | Study Design | Description of Intervention | Author, Year | Description of Research Article |
| Khan et al, 2010 (35) | Cluster RCT | Randomized hospital laboratories to use electronic laboratory results management system, which can automatically generate a report for PCPs | MacLean et al, 2004 (43) | Detailed description of planned study protocol |
| Montori et al, 2002 (37) | Cluster controlled trial | Physicians assigned to the intervention group used a diabetes electronic management system compared to control physicians, who maintained usual care with a paper-based patient chart system | Gorman et al, 2000 (44) | Detailed description of intervention technology |
| Walsh et al, 2012 (41) | Prospective case series | EHR use was self-identified through physician surveys; physicians who used EHRs were compared to physicians using paper-based practices—details of individual EHR systems are unknown | Walsh et al, 2010 (45) | Detailed study description and baseline data |
Abbreviations: EHR, electronic health record; PCP, primary care physician; RCT, randomized controlled trial.
Appendix 3: GRADE Tables
Table A2: GRADE Evidence Profile for Health Services Utilization and Disease-Specific Clinical Outcomes.
| No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Hospitalizations | |||||||
| 1 (RCT) | Serious limitations (−1)a | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕⊕ Moderate |
| Length of Stay | |||||||
| 1 (RCT) | Serious limitations (−1)a | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕⊕ Moderate |
| ED Visits | |||||||
| 1 (RCT) | Serious limitations (−1)a | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕⊕ Moderate |
| Readmissions | |||||||
| 1 (RCT) | No serious limitations | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕⊕⊕ High |
| HbA1c | |||||||
| 1 (RCT) | Very serious limitations (−2)b | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| 1 (observational) | Serious limitations (−1)c | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| SBP | |||||||
| 1 (RCT) | Very serious limitations (−2)b | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| DBP | |||||||
| 1 (RCT) | Very serious limitations (−2)b | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| Total Cholesterol | |||||||
| 1 (RCT) | Very serious limitations (−2)b | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| LDL-C | |||||||
| 2 (RCTs) | Very serious limitations (−2)b,d | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| Triglycerides | |||||||
| 1 (RCT) | Very serious limitations (−2)b | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| Adverse Events | |||||||
| 1 (RCT) | No serious limitations | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕⊕⊕ HIgh |
| HbA1c Managed and Below Clinical Guidelines | |||||||
| 2 (observational) | Serious limitations (−1)e,f | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| BP Managed and Below Clinical Guidelines | |||||||
| 2 (observational) | Serious limitations (−1)e,f | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| LDL-C Managed and Below Clinical Guidelines | |||||||
| 2 (observational) | Serious limitations (−1)e,f | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Triglycerides Managed and Below Clinical Guidelines | |||||||
| 1 (observational) | Serious limitations (−1)e | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| BMI < 30 kg/m2 | |||||||
| 1 (observational) | Serious limitations (−1)f | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Nonsmoker | |||||||
| 2 (observational) | Serious limitations (−1)e,f | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Composite Outcomes of Various Targets Met | |||||||
| 3 (observational) | Very serious limitations (−2)e,f,g | No serious limitations | Serious limitations (−1)h | No serious limitations | Undetected | None identified | ⊕ Very low |
Abbreviations: BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; ED, emergency department; EHR, electronic health record; EMR, electronic medical record; HbA1c, hemoglobin A1c; LDL-C, low density lipoprotein cholesterol; No., number; RCT, randomized controlled trial; SBP, systolic blood pressure.
Potential bias as a result of clustering effect.
Physicians to receive intervention were nominated by the study sites through unknown selection methodology. Additional selective reporting bias as authors did not report data for 3 outcomes (hospitalizations, ED visits, and primary care visits).
Physicians with greatest number of referrals were provided with electronic intervention, while the others were considered the control group.
Physicians had patients in both study groups, contaminating blinding.
Unknown methodology for selecting practices involved early versus later in the process of rolling out EHR systems.
Self-selected to use EMRs (or other eTools), and therefore may inherently be different from those who did not.
Intervention was implemented at the level of physician practice, and this resulted in some flux of individual patients within both study groups.
The composite outcomes included different components in the various studies.
Table A3: GRADE Evidence Profile for Process-of-Care Indicators.
| No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| BP Measures | |||||||
| 3 (observational) | Very serious limitations (−2)a,b,c | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Total Cholesterol | |||||||
| 1 (RCT) | Very serious limitations (−2)d | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| 2 (observational) | Serious limitations (−1)a,b | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕Very low |
| Triglycerides | |||||||
| 1 (RCT) | Very serious limitations (−2)d | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| 2 (observational) | Serious limitations (−1)a,b | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| HbA1c | |||||||
| 1 (RCT) | Very serious limitations (−2)d | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| 5 (observational) | Serious limitations (−1)a,b,c | No serious limitations | Serious limitations (−1)e | No serious limitations | Undetected | None identified | ⊕ Very low |
| Blood Glucose | |||||||
| 1 (observational) | Serious limitations (−1)a | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Fructosamine | |||||||
| 1 (observational) | Serious limitations (−1)a | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Eye Examinations | |||||||
| 1 (RCT) | Very serious limitations (−2)d | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| 5 (observational) | Serious limitations (−1)a,b,c | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Foot Examinations | |||||||
| 1 (RCT) | Very serious limitations (−2)d | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| 2 (observational) | Serious limitations (−1)b,c | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Kidney Management: Urine Protein | |||||||
| 1 (RCT) | Very serious limitations (−2)d | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| 3 (observational) | Serious limitations (−1)a,b,c | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Kidney Management: Creatinine | |||||||
| 1 (observational) | Serious limitations (−1)a | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Kidney Management: Composite Outcome | |||||||
| 1 (observational) | Serious limitations (−1)c | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Kidney Management: Urinalysis | |||||||
| 1 (observational) | Serious limitations (−1)b | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Weight | |||||||
| 1 (observational) | Serious limitations (−1)a | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Height | |||||||
| 1 (observational) | Serious limitations (−1)c | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Vaccinations and immunizations | |||||||
| 1 (RCT) | Very serious limitations (−2)d | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| 2 (observational) | Serious limitations (−1)b,c | No serious limitations | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Medications: ACE Inhibitors | |||||||
| 2 (observational) | Serious limitations (−1)c | No serious limitations | Serious limitations (−1)e | No serious limitations | Undetected | None identified | ⊕ Very low |
| Medications: Anticoagulation | |||||||
| 2 (observational) | Serious limitations (−1)c | No serious limitations | Serious limitations (−1)e | No serious limitations | Undetected | None identified | ⊕ Very low |
| Medications: Aspirin | |||||||
| 2 (observational) | Serious limitations (−1)b,c | No serious limitations | Serious limitations (−1)e | No serious limitations | Undetected | None identified | ⊕ Very low |
| Medications: Aldosterone Antagonists | |||||||
| 1 (observational) | Serious limitations (−1)a | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Medications: ICD/CRT-D | |||||||
| 1 (observational) | Serious limitations (−1)c | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Medications: Beta-blocker | |||||||
| 1 (observational) | Serious limitations (−1)c | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Medications: CRT-P/CRT-D | |||||||
| 1 (observational) | Serious limitations (−1)c | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Medications: Changes in Statins (1 month) | |||||||
| 1 (RCT) | Serious limitations (−1)f | Not relevant | No serious limitations | Serious limitations (−1)g | Undetected | None identified | ⊕⊕ Low |
| Medications: Changes in Statins (1 year) | |||||||
| 1 (RCT) | Serious limitations (−1)f | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕⊕ Moderate |
| Behavioural Interventions: Diet Advice | |||||||
| 1 (RCT) | Very serious limitations (−2)d | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| 1 (observational) | Serious limitations (−1)c | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Behavioural Interventions: Smoking Assessment | |||||||
| 1 (RCT) | Very serious limitations (−2)d | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| 1 (observational) | Serious limitations (−1)b | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Behavioural interventions: Exercise Advice | |||||||
| 1 (RCT) | Very serious limitations (−2)d | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| Behavioural interventions: Self-Management Support | |||||||
| 1 (RCT) | Very serious limitations (−2)d | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕ Low |
| Behavioural Interventions: Heart Failure Education | |||||||
| 1 (observational) | Serious limitations (−1)c | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕ Very low |
| Composite Outcomes of Tests Conducted or Recorded | |||||||
| 2 (observational) | Serious limitations (−1)a | No serious limitations | Serious limitations (−1)e | No serious limitations | Undetected | None identified | ⊕ Very low |
Abbreviations: ACE inhibitor, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CRT-D, cardio-resynchronization therapy with defibrillator; CRT-P, cardio-resynchronization therapy with pacemaker; ED, emergency department EHR, electronic health record; EMR, electronic medical record; eTool, electronic tool; HbA1c, hemoglobin A1c; ICD, implantable cardioverter defibrillator; No., number; RCT, randomized controlled trial.
Physicians with the greatest number of referrals were provided with electronic intervention, while the others were considered the control group.
Unknown methodology for selecting practices involved early versus later in the process of rolling out EHR systems.
Physicians self-selected to use EMRs (or other eTools), and therefore may inherently be different from those who did not.
Physicians to receive intervention were nominated by the study sites through unknown selection methodology. Additional selective reporting bias as authors did not report data for 3 outcomes (hospitalizations, ED visits, and primary care visits).
Studies used different measures (e.g., per-patient versus proportion of patients).
Physicians had patients in both study groups, contaminating blinding.
Wide confidence intervals.
Table A4: GRADE Evidence Profile for Measures of Efficiency.
| No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Proportion of PCPs Receiving Discharge Summary Within 1-7 Days | |||||||
| 1 (RCT) | No serious limitations | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕⊕⊕ High |
| Time to First Measure of LDL-C | |||||||
| 1 (RCT) | Serious limitations (−1)a | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕⊕ Moderate |
| Time to Change in Statin Prescription | |||||||
| 1 (RCT) | Serious limitations (−1)a | Not relevant | No serious limitations | No serious limitations | Undetected | None identified | ⊕⊕⊕ Moderate |
| Time Spent by Providers With Patients | |||||||
| 1 (RCT) | Very serious limitations (−2)b | Not relevant | Serious limitations (−1)d | No serious limitations | Undetected | None identified | ⊕ Very low |
| Time Spent by Nurses With Patients | |||||||
| 1 (RCT) | Very serious limitations (−2)b | Not relevant | Serious limitations (−1)d | No serious limitations | Undetected | None identified | ⊕ Very low |
| Number of Letters From GP to Consultant | |||||||
| 1 (observational) | Serious limitations (−1)c | Not relevant | Serious limitations (−1)d | No serious limitations | Undetected | None identified | ⊕ Very low |
| Number of Letters From Consultant to GP | |||||||
| 1 (observational) | Serious limitations (−1)c | Not relevant | Serious limitations (−1)d | No serious limitations | Undetected | None identified | ⊕ Very low |
| Number of Patient Contacts With GP | |||||||
| 1 (observational) | Serious limitations (−1)c | Not relevant | Serious limitations (−1)d | No serious limitations | Undetected | None identified | ⊕ Very low |
| Number of Patient Contacts With Consultant | |||||||
| 1 (observational) | Serious limitations (−1)c | Not relevant | Serious limitations (−1)d | No serious limitations | Undetected | None identified | ⊕ Very low |
Abbreviations: GP, general practitioner; eTool, electronic tool; LDL-C, low-density lipoprotein cholesterol; PCP, primary care physician; No., number; RCT, randomized controlled trial;
Potential bias as a result of cross-contamination of study groups.
Physicians to receive intervention were nominated by the study sites, but with unknown selection methodology. Additionally, while the study design was that of an RCT, this outcome was measured through observational data collected.
Physicians with greatest number of referrals were provided with electronic intervention, while the others were considered the control group.
The correlation between physician time and quality of patient care is unclear. Decrease physician time spent with a patient could be due to improved efficiency or decreased quality of care.
Table A5: Risk of Bias Among Randomized Controlled Trials for the Impact of eTools.
| Author, Year | Allocation Concealment | Blinding | Complete Accounting of Patients and Outcome Events | Selective Reporting Bias | Other Limitations |
|---|---|---|---|---|---|
| Graumlich, 2009 (34) | No limitations | No limitationsa | No limitationsb | No limitations | No limitations |
| Khan et al, 2010 (35) | No limitations | No limitationsa | No limitationsb | No limitations | Serious limitationsc |
| Lester et al, 2005 (33) | No limitations | Serious limitationsd | No limitationsb | No limitations | No limitations |
| Montori et al, 2002 (37) | Very serious limitationse | No limitationsa | No limitationsb | Serious limitationsf | No limitationsg |
Abbreviation: eTools, electronic tools.
Not feasible to blind due to the obvious nature of receiving of an automated electronic report; a possible limitation for subjective outcomes, but not for definitive outcomes such as hospitalizations.
Conducted analyses on an intention-to-treat principle (including studies where no loss to follow-up occurred).
Calculations did not account for potential recruitment bias as a result of clustering effects.
individual physicians had patients in both intervention and control arms and received an email only for patients in the intervention group, causing cross-contamination and potential bias in patient care.
Physicians to receive intervention were nominated by the study sites with unknown selection methodology.
Authors did not report data for 3 outcomes (hospitalizations, ED visits, primary care visits).
Performed multivariate analyses to account for potential baseline differences.
Table A6: Risk of Bias Among Observational Trials for the Impact of eTools.
| Author, Year | Appropriate Eligibility Criteria | Appropriate Measurement of Exposure | Appropriate Measurement of Outcome | Adequate Control for Confounding | Complete Follow-Up |
|---|---|---|---|---|---|
| Branger et al, 1999 (32) | Serious limitationsa | No limitations | No limitations | No limitations | No limitations |
| Cebul et al, 2011 (38) | Serious limitationsb | No limitations | No limitations | No limitationsc | No limitations |
| Crosson et al, 2012 (39) | Serious limitationsb | No limitations | No limitations | No limitationsc | Serious limitationsd |
| Henderson et al, 2010 (36) | Serious limitationsb | No limitations | No limitations | No limitationsc | No limitationse |
| Herrin et al, 2012 (40) | Serious limitationsf | No limitations | No limitations | No limitationsc | No limitationsg |
| Walsh et al, 2012 (41) | Serious limitationsb | No limitations | No limitations | No limitationsc | No limitations |
| Wells et al, 1996 (42) | Serious limitationsb | No limitations | No limitations | No limitations | Serious limitationsd |
Abbreviation: EHR, electronic health record; EMR, electronic medical record; eTools, electronic tools.
Physicians with greatest number of referrals were provided with the electronic intervention, while the others were considered the control group.
Physicians self-selected to use EMRs (or other electronic intervention) and therefore may inherently be different from those who did not.
Statistical modelling was applied to adjust for known or otherwise potential confounding factors.
Intervention was implemented at the level of physician practice, and this resulted in some flux of individual patients within both study groups.
Assessment was conducted at the level of patient encounter; individual patients were not accounted for.
Unknown methodology for selecting practices which were early adopters to EHR and up to 5 years later adoption, introducing potential bias in physician practice type.
Results accounted patient years, not individual patients.
References
- 1.Cabana MD, Jee SH. Does continuity of care improve patient outcomes? J Fam Pract. 2004 Dec;53(12):974–80. [PubMed] [Google Scholar]
- 2.Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007 Feb 28;297(8):831–41. doi: 10.1001/jama.297.8.831. [DOI] [PubMed] [Google Scholar]
- 3.Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007 Sep;2(5):314–23. doi: 10.1002/jhm.228. [DOI] [PubMed] [Google Scholar]
- 4.Bodenheimer T. Coordinating care—a perilous journey through the health care system. N Engl J Med. 2008 Mar 6;358(10):1064–71. doi: 10.1056/NEJMhpr0706165. [DOI] [PubMed] [Google Scholar]
- 5.Brown JB, Lewis L, Ellis K, Stewart M, Freeman TR, Kasperski MJ. Mechanisms for communicating within primary health care teams. Can Fam Physician. 2009 Dec;55(12):1216–22. [PMC free article] [PubMed] [Google Scholar]
- 6.Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, McKendry R. Continuity of care: a multidisciplinary review. BMJ. 2003 Nov 22;327(7425):1219–21. doi: 10.1136/bmj.327.7425.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berner ES, Detmer DE, Simborg D. Will the wave finally break? A brief view of the adoption of electronic medical records in the United States. J Am Med Inform Assoc. 2005 Jan;12(1):3–7. doi: 10.1197/jamia.M1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell CM, Brener SS, Gunraj N, Huo C, Bierman AS, Scales DC, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011 Aug 24;306(8):840–7. doi: 10.1001/jama.2011.1206. [DOI] [PubMed] [Google Scholar]
- 9.Protti D. Comparison of information technology in general practice in 10 countries. Healthc Q. 2007;10(2):107–16. [PubMed] [Google Scholar]
- 10.Protti D, Bowden T, Johansen I. Adoption of information technology in primary care physician offices in New Zealand and Denmark, part 5: final comparisons. Inform Prim Care. 2009;17(1):17–22. doi: 10.14236/jhi.v17i1.710. [DOI] [PubMed] [Google Scholar]
- 11.Kenny C. The use of computers in primary diabetes care. Pract Diabetes Int. 1997;14(5):132–3. [Google Scholar]
- 12.Hsiao C, Hing E, Socey T, Cai B, Division of Health Care Statistic . Bethesda (MD): Centers for Disease Control and Prevention; 2010. [[cited 2013 Jan 28]]. Electronic medical record/electronic health record systems of office-based physicians: United States, 2009 and preliminary 2010 state estimates; p. 6 p. [Internet]. Available from: http://www.cdc.gov/nchs/data/hestat/emr_ehr_09/emr_ehr_09.htm . [Google Scholar]
- 13.eHealth Ontario. Toronto (ON): eHealth Ontario; [[updated 2013; cited 2013 Feb 7]]. What We Do. [Internet] Available from: http://www.ehealthontario.on.ca/en/about . [Google Scholar]
- 14.Ontario Medical Association. A stronger Ontario. Toronto (ON): Ontario Medical Association; 2011. [[cited 2012 Apr 16]]. Better care. Healthier patients; p. 19 p.. [Internet] Available from: https://www.oma.org/Resources/Documents/InsightsAndRecommendations.pdf . [Google Scholar]
- 15.OntarioMD. Toronto (ON): OntarioMD; [[updated 2012; cited 2013 Jan 28]]. EMR Adoption Program. [Internet] Available from: https://www.ontariomd.ca/portal/server.pt/community/emr funding/new emr adopters . [Google Scholar]
- 16.EMRAdvisor. Toronto (ON): EMRAdvisor; [[updated 2012; cited 2013 Jan 28]]. OntarioMD Funding Eligible EMR Offerings: Vendor Market Share. [Internet] Available from: https://www.ontariomd.ca/portal/server.pt/community/emr_offerings/offering_detail . [Google Scholar]
- 17.Review Manager (RevMan) [Computer program]. Version 5.1. Copenhagen (DK): The Nordic Cochrane Centre, The Cochrane Collaboration; 2011. [Google Scholar]
- 18.Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011 Apr;64(4):380–2. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Goodman C. Stockhold, Sweden: Swedish Council on Technology Assessment in Health Care; 1996. Literature searching and evidence interpretation for assessing health care practices; p. 81 p. SBU Report No 119E. [DOI] [PubMed] [Google Scholar]
- 20.Adaji A, Schattner P, Jones K. The use of information technology to enhance diabetes management in primary care: a literature review. Inform Prim Care. 2008 Sep;16(3):229–37. doi: 10.14236/jhi.v16i3.698. [DOI] [PubMed] [Google Scholar]
- 21.Bartoli L, Zanaboni P, Masella C, Ursini N. Systematic review of telemedicine services for patients affected by chronic obstructive pulmonary disease (COPD) Telemed J E Health. 2009;15(9):877–83. doi: 10.1089/tmj.2009.0044. [DOI] [PubMed] [Google Scholar]
- 22.Bryan C, Boren SA. The use and effectiveness of electronic clinical decision support tools in the ambulatory/primary care setting: A systematic review of the literature. Inform Prim Care. 2008;16(2):79–91. doi: 10.14236/jhi.v16i2.679. [DOI] [PubMed] [Google Scholar]
- 23.Costa BM, Fitzgerald KJ, Jones KM, Dunning AT. Effectiveness of IT-based diabetes management interventions: a review of the literature. BMC Fam Pract. 2009;10:72. doi: 10.1186/1471-2296-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorr D, Bonner LM, Cohen AN, Shoai RS, Perrin R, Chaney E, et al. Informatics systems to promote improved care for chronic illness: a literature review. J Am Med Inform Assoc. 2007;14(2):156–63. doi: 10.1197/jamia.M2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson CL, Bolen S, Brancati FL, Batts-Turner ML, Gary TL. A systematic review of interactive computer-assisted technology in diabetes care Interactive information technology in diabetes care. J Gen Intern Med. 2006 Feb;21(2):105–10. doi: 10.1111/j.1525-1497.2005.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lizana FG, Santamera AS. New technologies for chronic disease management and control: a systematic review. J Telemed Telecare. 2007;13:62–8. doi: 10.1258/135763307780096140. [DOI] [PubMed] [Google Scholar]
- 27.Poissant L, Pereira J, Tamblyn R, Kawasumi Y. The impact of electronic health records on time efficiency of physicians and nurses: a systematic review. J Am Med Inform Assoc. 2005 Sep;12(5):505–16. doi: 10.1197/jamia.M1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renders CM, Valk GD, Griffin S, Wagner EH, Eijk JT, Assendelft WJ. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev. 2001;1 doi: 10.1002/14651858.CD001481. CD001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seitz P, Rosemann T, Gensichen J, Huber CA. Interventions in primary care to improve cardiovascular risk factors and glycated haemoglobin (HbA1c) levels in patients with diabetes: a systematic review. Diabetes Obes Metab. 2011;13(6):479–89. doi: 10.1111/j.1463-1326.2010.01347.x. [DOI] [PubMed] [Google Scholar]
- 30.Fontaine P, Ross SE, Zink T, Schilling LM. Systematic review of health information exchange in primary care practices. J Am Board Fam Med. 2010 Sep;23(5):655–70. doi: 10.3122/jabfm.2010.05.090192. [DOI] [PubMed] [Google Scholar]
- 31.van der Kam WJ, Moorman PW, Koppejan-Mulder MJ. Effects of electronic communication in general practice. Int J Med Inform. 2000 Oct;60(1):59–70. doi: 10.1016/s1386-5056(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 32.Branger PJ, Van′T HA, Van Der Wouden JC, Moorman PW, van Bemmel JH. Shared care for diabetes: supporting communication between primary and secondary care. Int J Med Inform. 1999;53(2-3):133–42. doi: 10.1016/s1386-5056(98)00154-3. [DOI] [PubMed] [Google Scholar]
- 33.Lester WT, Grant RW, Barnett GO, Chueh HC. Randomized controlled trial of an informatics-based intervention to increase statin prescription for secondary prevention of coronary disease. J Gen Intern Med. 2006 Jan;21(1):22–9. doi: 10.1111/j.1525-1497.2005.00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graumlich JF, Novotny NL, Nace GS, Kaushal H, Ibrahim-Ali W, Theivanayagam Seal. Patient readmissions, emergency visits, and adverse events after software-assisted discharge from hospital: cluster randomized trial. J Hosp Med. 2009;4(7):E11–E19. doi: 10.1002/jhm.469. [DOI] [PubMed] [Google Scholar]
- 35.Khan S, MacLean CD, Littenberg B. The effect of the Vermont Diabetes Information System on inpatient and emergency department use: results from a randomized trial. Health Outcomes Res Med. 2010;1(1):e61–e66. doi: 10.1016/j.ehrm.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson J, Miller G, Britt H. Effect of computerisation on Australian general practice: does it improve the quality of care? Qual Prim Care. 2010 Feb;18(1):33–47. [PubMed] [Google Scholar]
- 37.Montori VM, Dinneen SF, Gorman CA, Zimmerman BR, Rizza RA, Bjornsen SS, et al. The impact of planned care and a diabetes electronic management system on community-based diabetes care: the Mayo Health System Diabetes Translation Project. Diabetes Care. 2002 Nov;25(11):1952–7. doi: 10.2337/diacare.25.11.1952. [DOI] [PubMed] [Google Scholar]
- 38.Cebul RD, Love TE, Jain AK, Hebert CJ. Electronic health records and quality of diabetes care. N Engl J Med. 2011;365(9):825–33. doi: 10.1056/NEJMsa1102519. [DOI] [PubMed] [Google Scholar]
- 39.Crosson JC, Ohman-Strickland PA, Cohen DJ, Clark EC, Crabtree BF. Typical electronic health record use in primary care practices and the quality of diabetes care. Ann Fam Med. 2012 May;10(3):221–7. doi: 10.1370/afm.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herrin J, da GB, Nicewander D, Fullerton C, Aponte P, Stanek G, et al. The effectiveness of implementing an electronic health record on diabetes care and outcomes. Health Serv Res. 2012 Aug;47(4):1522–40. doi: 10.1111/j.1475-6773.2011.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh MN, Albert NM, Curtis AB, Gheorghiade M, Heywood JT, Liu Y et al. Lack of association between electronic health record systems and improvement in use of evidence-based heart failure therapies in outpatient cardiology practices. Clin Cardiol. 2012 Mar;35(3):187–96. doi: 10.1002/clc.21971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells S, Hill-Smith I. Bridging the communication gap in diabetes care. Pract Diabetes Int. 1996;13(6):174–6. [Google Scholar]
- 43.MacLean CD, Littenberg B, Gagnon M, Reardon M, Turner PD, Jordan C. The Vermont Diabetes Information System (VDIS): study design and subject recruitment for a cluster randomized trial of a decision support system in a regional sample of primary care practices. Clin Trials. 2004;1(6):532–44. doi: 10.1191/1740774504cn051oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorman CA, Zimmerman BR, Smith SA, Dinneen SF, Knudsen JB, Holm D, et al. DEMS-a second generation diabetes electronic management system. Comput Methods Programs Biomed. 2000 Jun;62(2):127–40. doi: 10.1016/s0169-2607(99)00054-1. [DOI] [PubMed] [Google Scholar]
- 45.Walsh MN, Yancy CW, Albert NM, Curtis AB, Stough WG, Gheorghiade M, et al. Electronic health records and quality of care for heart failure. Am Heart J. 2010;159(4):635–42. doi: 10.1016/j.ahj.2010.01.006. [DOI] [PubMed] [Google Scholar]


