Abstract
Traumatic brain injury (TBI) is among the leading causes of death and disability worldwide, with enormous negative social and economic impacts. The heterogeneity of TBI combined with the lack of precise outcome measures have been central to the discouraging results from clinical trials. Current approaches to the characterization of disease severity and outcome have not changed in more than three decades. This prospective multicenter observational pilot study aimed to validate the feasibility of implementing the TBI Common Data Elements (TBI-CDEs). A total of 650 subjects who underwent computed tomography (CT) scans in the emergency department within 24 h of injury were enrolled at three level I trauma centers and one rehabilitation center. The TBI-CDE components collected included: 1) demographic, social and clinical data; 2) biospecimens from blood drawn for genetic and proteomic biomarker analyses; 3) neuroimaging studies at 2 weeks using 3T magnetic resonance imaging (MRI); and 4) outcome assessments at 3 and 6 months. We describe how the infrastructure was established for building data repositories for clinical data, plasma biomarkers, genetics, neuroimaging, and multidimensional outcome measures to create a high quality and accessible information commons for TBI research. Risk factors for poor follow-up, TBI-CDE limitations, and implementation strategies are described. Having demonstrated the feasibility of implementing the TBI-CDEs through successful recruitment and multidimensional data collection, we aim to expand to additional study sites. Furthermore, interested researchers will be provided early access to the Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) data set for collaborative opportunities to more precisely characterize TBI and improve the design of future clinical treatment trials. (ClinicalTrials.gov Identifier NCT01565551.)
Key words: CDEs, human studies, prospective study, TBI
Introduction
Worldwide, traumatic brain injury (TBI) is among the leading causes of death and disability with enormous negative social and economic impacts upon victims, families, and health care systems. Each year in the United States at least 1,700,000 people sustain TBIs. This includes 52,000 deaths, 275,000 hospitalizations, and 1,365,000 patients treated and released from an emergency department. TBI is a contributing factor to 30% of all injury related deaths in the United States.1 An estimated 3,200,000–5,300,000 persons currently live with long-term physical and neuropsychiatric disabilities attributable to TBI.2 Recent studies suggest that published statistics underestimate the burden, and that TBI is underdiagnosed across the spectrum of injuries.3–5 The annual direct and indirect costs of TBI have been estimated at more than $60 billion.6
TBIs are highly heterogeneous in cause, severity, pathology, and clinical course.7 The heterogeneity of TBI combined with the lack of relevant and validated outcome measures have been central to the discouraging results from neuroprotective and therapeutic strategy trials over the last four decades.8 Current approaches to the characterization of disease severity and outcome have not changed in more than three decades. Today, TBI patients are divided into the crude categories of mild, moderate, and severe using the Glasgow Coma Scale (GCS),9 and outcome is measured using the Glasgow Outcome Scale Extended (GOS-E).10 Clinical research in TBI has also been hindered by underpowered trial enrollments, fragmented efforts to collect standardized data, and limited multidisciplinary collaborations.
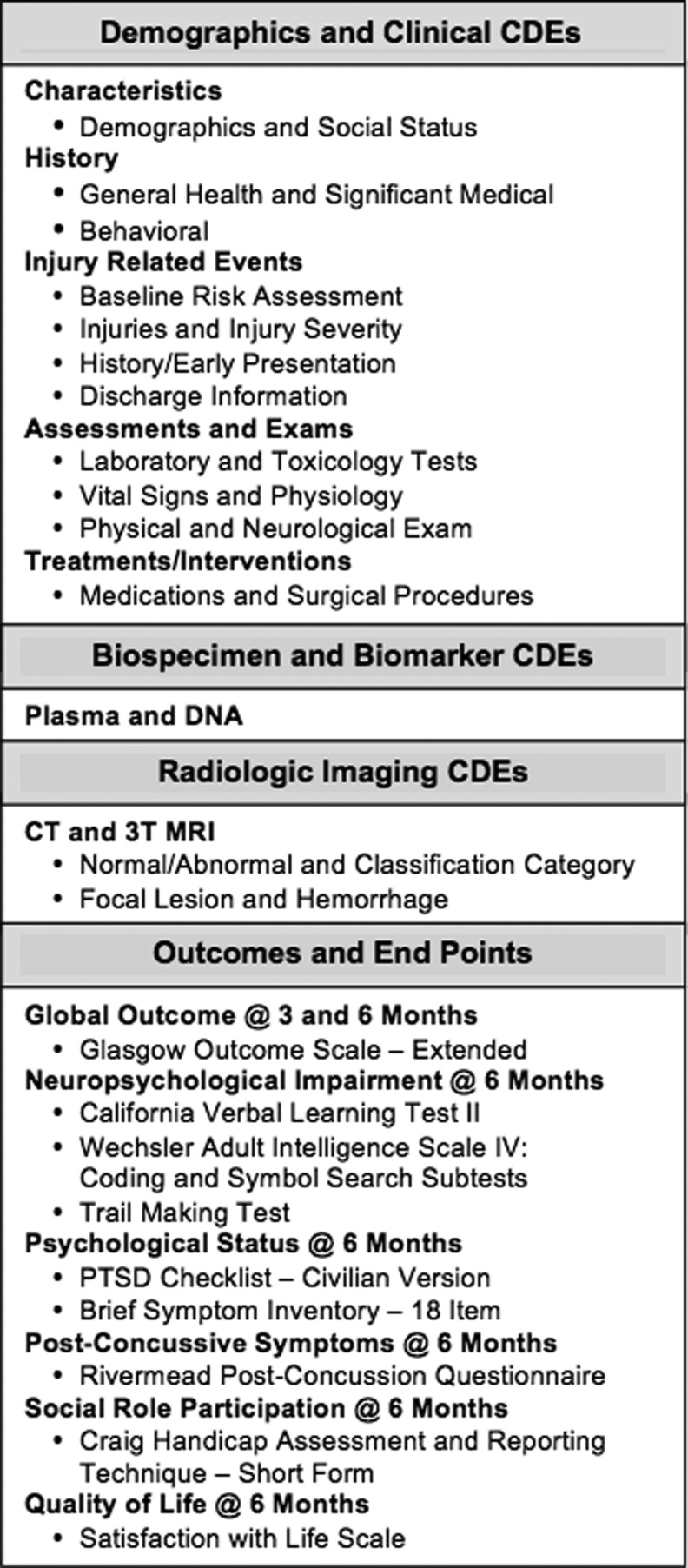
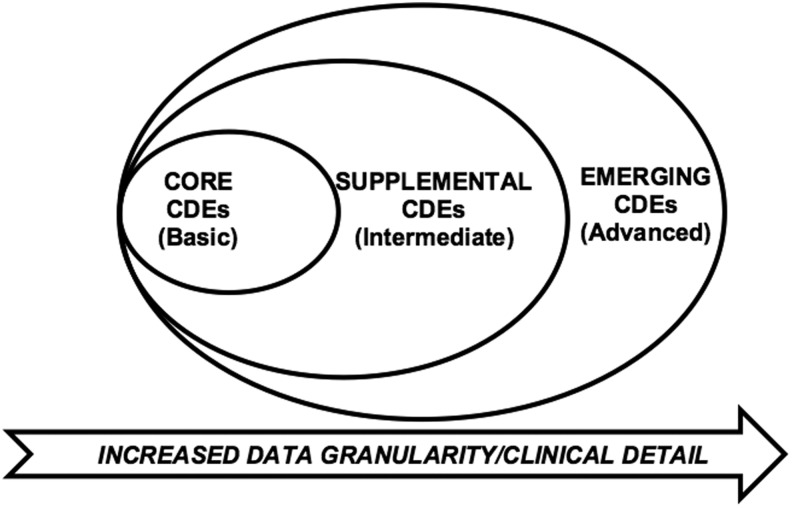
Between 2003 and 2006, the investigators of the International Mission for Prognosis and Analysis of Clinical Trials (IMPACT) combined data from >9000 patients and developed three prognostic models for severe TBI patients.11 With this foundation built, in 2009, the United States initiative to establish Common Data Elements (CDEs) for TBI was led by the National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS) in cosponsorship with the Department of Veterans Affairs, National Institute on Disability and Rehabilitation Research, Defense and Veterans Brain Injury Centers, Defense Centers of Excellence, and others.12 As a result of these conferences, multidisciplinary working groups developed consensus- based recommendations for harmonization of data across clinical trial sites with an emphasis on demographics, clinical care, genetic and proteomic biomarkers, neuroimaging, and a battery of outcome measures suitable for use across the spectrum of TBI (Fig. 1).13,14 Measures were identified according to applicability for the acute, subacute, and chronic phases of TBI care and recovery.15–18 The original (Version 1.0) of the TBI CDE recommendations were composed of three levels of data granularity for each component (Fig. 2): core, supplemental, and emerging, which correspond to the IMPACT CDE levels of basic, intermediate, and advanced, respectively.16 Core CDEs represent data relevant to all TBI patients and studies. Supplemental CDEs capture increased data granularity and clinical detail for examination or for specific types of injuries or care delivery settings. Emerging CDEs represent measures on which consensus has not been achieved, because they are of a more exploratory nature, are not widely available and/or validated and correlated with outcome measures.
FIG. 1.
Traumatic brain injury (TBI) common data elements for Transforming Research and Clinical Knowledge in TBI (TRACK-TBI).
FIG. 2.
Three levels of detail for classification of version 1.0 of the common data elements (CDEs) that correspond to the International Mission for Prognosis and Analysis of Clinical Trials (IMPACT) CDE levels of basic, intermediate, and advanced. Core CDEs represent data relevant to all traumatic brain injury (TBI) patients and studies. Supplemental CDEs capture increased data granularity and clinical detail for examination or for specific types of injuries or care delivery settings. The emerging CDEs represent measures on which consensus has not been achieved because they are of a more exploratory nature, are not widely available, and/or are not validated and correlated with outcome measures.
Under the American Reinvestment and Recovery Act, in 2009, the NIH-NINDS funded the Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) multicenter study. Our aim was to validate the feasibility of implementing the TBI-CDE's. This was accomplished with establishing the infrastructure for data repositories for clinical data, plasma biomarkers, genetics, neuroimaging, and multidimensional outcomes. This approach was aimed at assuring that factors critical to collecting high- quality data were established and providing a robust platform for collection and analysis of patient data for future clinical trials. Here, we report on the TRACK-TBI pilot study design, procedures, patient characteristics, feasibility, and utility of collecting CDE measures from disparate sources, and present recommendations for future efforts.
Methods
TRACK-TBI is a multicenter prospective observational study with patients recruited through convenience sampling at four study sites. The three acute care (Acute) sites are all level I trauma centers and included: San Francisco General Hospital (SFGH), University of Pittsburgh Medical Center (UPMC), and University Medical Center Brackenridge (UMCB) in Austin, Texas. The single rehabilitation center was located at the Mount Sinai Rehabilitation Center (MSRC) in New York City. The SFGH investigators at the University of California San Francisco, Brain and Spinal Injury Center served as the TRACK-TBI Coordinating Center. Institutional review board (IRB) approval was obtained at each site.
Patient screening, inclusion and exclusion
For the acute sites, all patients who presented to the emergency department (ED) and received a head CT within 24 h of a TBI were initially eligible for the study. Patients who were pregnant, in custody, non-English speaking, on a medically evaluated psychiatric hold, and/or in whom potential contraindications existed for MRI, were excluded from the study. Children <8 years of age were also excluded. Informed consent was sought in all patients. For patients who were unable to consent for themselves, the process was pursued with the surrogate next of kin. Consent for patients <18 years of age was obtained from the parent or legal custodian and accompanied by patient assent if the patient was >7 years of age. At the acute sites, patients were compensated $75 for the 2 week MRI but were not compensated at MSRC. For the 6 month in-person outcomes, patients were compensated $77–125 at all sites.
At the three acute sites, 3077 patients were screened and 19% were consented. Patients were screened and excluded for the following reasons: 38% were in the ED during hours that research personnel were not on-site or they were occupied with other patients; 21% could not speak English; 14% had psychological disorders that disturbed the patient sufficiently at baseline to prevent consent; 4% were either pregnant, in custody, or on medical psychiatric hold; 4% had neurological disorders; 3% were inappropriate to approach because of either major trauma, infectious conditions, or late stage cancer; 2.5% could not provide contact information; 2.5% denied that they had sustained head trauma; and 0.5% died before consent could be obtained. Out of the remaining patients with capacity to consent, 8% refused.
Study protocol
Figure 1 lists the CDE subdomain components that were collected including: 1) demographic, social and clinical data; 2) biospecimens from blood samples drawn for genetic and proteomic biomarker analyses; 3) neuroimaging studies using 3T MRI; and 4) outcome assessments performed at 3 and 6 months. The University of California at San Francisco (UCSF) and UMCB IRB's required that patients be allowed to choose to consent to only the study components in which they wanted to participate. UPMC and MSRC patients were enrolled in all four components. At UPMC, screening did not include the ED, and enrollment was limited to patients admitted to the neurosurgical service.
Data collection and development of TRACK-TBI database
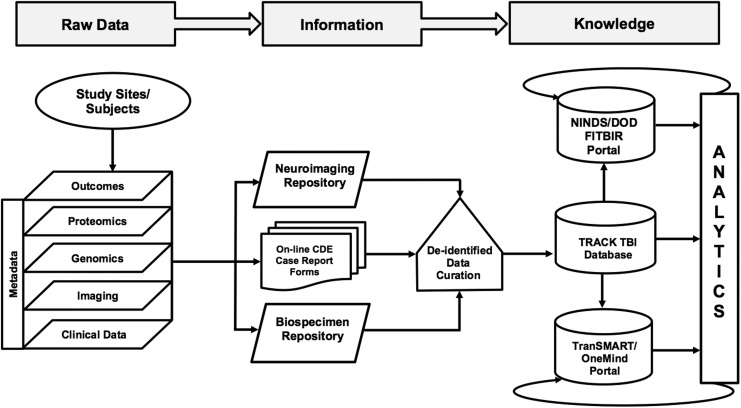
The four domains of CDEs listed in Figure 1 capture demographic, clinical, biospecimen, imaging, and outcome measures. QuesGen Systems, Inc. provided programming services for development of the schematics for the CDEs on a web-based platform. The database was designed and tested over a 6 month period. An overview of the data flow process is described in Figure 3. Data dictionaries applicable to the CDEs were programmed at the “advanced” level. More than 3000 data fields were created when taking into account calculated fields, repeated measures, and date and time stamping of measures. Administrative fields were also created for tracking patient follow-up visits, electronic case report forms (eCRFs) completion status, submission of biospecimens, and transmission of neuroimaging files. Data loaders were used for transfer of clinical laboratory and vital signs for sites that could extract these measures from electronic medical records systems into a spreadsheet format. For data analysis, records were exported to comma-separated or XML files for import into statistical applications. All data communication between the QuesGen browser and secure servers was through an encrypted secure socket layer connection. QuesGen, Inc. has procedures in place for full compliance with Health Insurance Portability and Accountability Act (HIPAA) security standards for protection of personal health information (PHI).
FIG. 3.
Data management flow chart for acquisition and sharing of Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) common data elements (CDEs).
Data quality assurance and analytics
Data collectors were trained in CDE coding and provided with electronic data dictionaries and operational manuals detailing procedures for all domains. Rules for data field range, consistency and missing/not applicable checks were implemented within the QuesGen application. Major and minor logic flags were defined; however, complex relational inconsistency checks are an ongoing effort guided by written curation plan to assure that data was cleaned and CDE compliant prior to submission to Federal Interagency Traumatic Brain Injury Research (FITBIR) repository. At this pilot stage, it was recognized that there was still a need for human eyes to review the data because programming alone cannot identify all errors and inconsistencies. An external team of data curators mapped the QuesGen fields to TranSMART (www.transmartproject.org) which provided the ability to develop more advanced data quality checks for all domains through exploratory queries and review of issues with the database programmers and investigators.19 Ten percent of the records were audited at each site, and feedback was provided for making corrections.
Demographic and clinical data
The following “advanced” CDEs were collected: 1) subject characteristics, 2) subject and family history, 3) disease/injury related events, 4) assessments and examinations, and 5) treatment and intervention data.16 A total of 122 variables were collected on patients in the ED through abstraction of medical records and patient interviews. Upon a patient's admission to a hospital ward, another 124 variables were collected. For each hospital day, 170 additional variables were collected for medications, laboratory test results, vital signs, surgeries, and injury severity classifications. The complete list of data elements and the CRF are available at http://www.brainandspinalinjury.org/research.php?id=189
Biospecimens and biomarkers
The CDE working group guidelines for proteomic preservation of plasma and isolation of DNA were followed.17 Blood samples were collected within 24 h of injury, aliquoted and frozen at −80°C within 1 h of collection. As white blood cell concentrations have been shown to decrease in K3-EDTA, we elected to use 7.2mg K2-EDTA vacutainer tubes. Externally threaded cryovials were selected for storage, as internally threaded cryovials can reduce sample volume. Multiple 250 μl plasma aliquots were prepared to reduce the need for freeze–thaw cycles during analysis. Most single biomarker analytics can be performed in duplicate with a sample volume of 250 μl. An inventory system was implemented for tracking provenance of the samples, including: the time of collection, processing, and storage.20 Samples were shipped in batches to the UCSF DNA Bank, which provides a seismically secure physical environment for round-the-clock temperature control alarm monitoring and emergency backup power for electrical outages. Shipping times were scheduled to avoid weekend and holiday delivery, or days when rain and snowstorms could delay airline departures and arrivals. A preliminary set of single nucleotide polymorphisms (SNPs) were tested via Illumina Bead Array technology on DNA samples with 100% result yield on all 417 acute specimens.
At SFGH, for patients who were identified for enrollment at ≥18 h post-injury, in addition to collecting the study enrollment blood draw, we obtained leftover samples drawn in the ED for the complete blood count (CBC). We coordinated with the clinical laboratory to “add-on” the order for spinning the plasma out of the leftover sample. The plasma and blood pellet for DNA were stored in the −80°C freezer for pickup and processing by research personnel. For the genetic specimens, the “add-on” processing did not impact the assays. However, the “add-on” plasma specimens must be thawed in order to prepare multiple aliquots, and then refrozen. Because the leftover plasma was stored in the clinical laboratory at room temperature, a specimen stability study was undertaken to determine whether this diminished the integrity of the protein biomarkers. This required comparing the plasma biomarker levels measured in the “add-on” samples processed by the clinical laboratory versus samples processed by the research personnel. Blood samples were collected from 10 patients with intracranial lesions of varying degrees of severity. Two standard EDTA vacutainers of blood were drawn from each patient within the first 24 h. One tube was immediately processed by research personnel according to the CDE protocol. The second tube was sent to the clinical laboratory for immediate processing, and then retrieved for a second freeze–thaw cycle in which the plasma was aliquoted. At the same time, an “add-on” order to spin down the specimen was attached to the most recent CBC sample. On average, “add-on” specimens had been at room temperature in the laboratory for 16 h before the “add-on” order was received (range: 11–23 h). Blinded samples were submitted to Banyan Biomarkers, Inc. to perform a sandwich enzyme-linked immunosorbent assay protocol (swELISA) to confirm the neuropeptide level and quality among the three processing methods. Glial fibrillary acidic protein (GFAP) was selected because of its specificity to astrocytes and functional structure of the cytoskeleton. A breakdown product of GFAP (GFAP-BDP) is detectable in blood within 1 h of structural brain injury, and is associated with measures of injury severity including the GCS score, CT lesions, and neurosurgical intervention.21
Imaging studies and repository
All enrolled patients underwent a head CT in the ED. The free text of the dictated reports for the clinical CT and any clinical MRI scans were copied into the database for manual coding by the UCSF neuroradiologist. Each CT and MRI was reviewed by a board-certified neuroradiologist blinded to demographic, socioeconomic, and clinical data except gender and age, and without concurrent access to patients' other imaging studies. Patients who consented for the MRI studies were scheduled for a 3T MRI within 2 weeks from the date of their injury. The CT and MRI findings were coded according to the 26 core CDE's among the 93 CDEs developed by the neuroimaging working group.22 TRACK-TBI neuroradiologists at all sites implemented equivalent imaging protocols for 3T research MRI scanners manufactured by General Electric, Phillips, and Siemens. The MRI protocols complemented the CDE tiers used in the Alzheimer's Disease Neuroimaging Initiative (ADNI). The T1-weighted sequence adapted for non-ADNI studies was adopted for the reason that the magnetization prepared 180 degrees radio-frequency pulses, and rapid gradient-echo (MP RAGE) sampling sequence constituted the heart of the ADNI MRI protocol and increased the probability of obtaining at least one high quality morphometric scan in each examination, to minimize the need for subject rescanning caused by artifact.23
The CT and MRI scans generated large volumes of data that required particular techniques for anonymization, transmission, storage, and visualization by multiple users at each site. We developed a radiology picture archiving system (rPACS) with DCM4chee, Osirix, and Clear Canvas. These are all open-source Digital Imaging and Communications in Medicine (DICOM) software that allow controlled remote access for multiple users at each site for transmission to the UCSF Quantitative Imaging Processing Center (QUIP-C). To comply with HIPAA requirements, the QUIP-C investigators built a multiplatform tool that completely anonymized CT and 3T MRI studies during the transmission process, based on open-source Java-based software available through the RSNA Medical Imaging Resource Center (www.rsna.org=mirc). De-identified images were securely sent to the rPACS via virtual private network. In addition, the rPACS avoided disruption of the clinical PACS with large volumes of research data and allowed authorized users to securely view all study CT and MRI image sequences from any location.
Outcome measures
The TRACK-TBI outcomes battery consisted of all CDE “core” and two “extended” measures that we collected at 3 and 6 months.18 The Rey Auditory Verbal Learning Test (RAVLT) was replaced with the California Verbal Learning Test - Second Edition (CVLT-II) because of the relevant revisions of the second edition as well as higher reported consistency on between-norm sets.24 The Post-Traumatic Stress Disorder Checklist Civilian Version (PCL-C) was added, because it is a widely used self-administered screening tool for identification of patients who need further evaluation for PTSD.25
At 3 months, patients were contacted by telephone to administer the GOS-E, Neurological Symptoms Inventory (NSI), and Post-Discharge Outpatient Care (PDOC) assessment. Both the NSI and PDOC are NIH TBI CDEs for the “Physical/Neurological Examination” module. The NSI is a list of common symptoms compiled from the IMPACT database and coded as “yes” or “no.” The 6 month evaluations were conducted in person by trained personnel and preceded by the Galveston Orientation and Amnesia Test (GOAT) to assess functional capacity.26 In order to control for potential variability among project personnel in the administration and scoring of the CDE outcome measures, the UPMC Neuropsychological Outcomes Coordinator conducted training sessions at each of the study sites. Patients<16 years of age did not receive the neurocognitive tests, because the majority of these measures have been validated only in adults. Instead we administered the GOS-E for Pediatrics.27
Statistical analysis
Contingency tables were constructed to compare patient demographics, and clinical differences among sites were compared with the Pearson χ2 statistic. Descriptive statistics were used to summarize patient characteristics and outcome measures. Student's t test and Pearson÷2 statistics were used for initial comparisons to test for associations between covariates and successful 6 month follow-up by GOS-E for patients alive at discharge. Covariates with statistical significance were then specified for univariable logistical regression analysis. For continuous variables, the coefficient for the intercept was the log odds of a person with a value of zero units for that variable to have successful follow-up, and the coefficient for the variable was the effect of a one unit increase in that variable on the odds ratio (OR). SPSS Statistics (IBM, Chicago, IL) version 19 was used for all analyses.
Results
Study implementation and enrollment
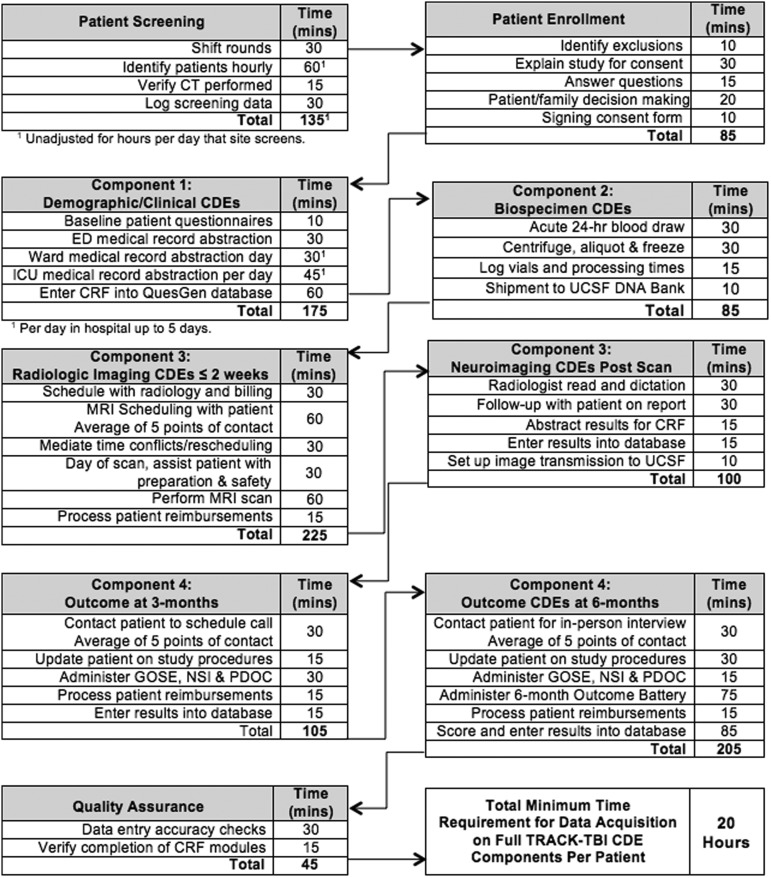
The infrastructure for implementing data collection for the TRACK-TBI CDE domains was established during the first 8 months following receipt of the NINDS funding. This included development of the eCRFs in the TRACK-TBI database; obtaining IRB approvals; recruitment and hiring of personnel; development of training and operational procedure manuals for patient enrollment and follow-up evaluations; implementation of protocols for collection, processing, and banking of biospecimens; and the creation of the neuroimaging research PACS. Figure 4 details the tasks and work flow for each TBI-CDE domain. SFGH research personnel maintained logs of time requirements for completing all tasks. Overall the per-patient time commitment for successful completion of all components from enrollment to follow-up averaged 20 h, not including time for project administrative activities.
FIG. 4.
Workflow and estimated time on task for patient screening, enrollment and collection of data for each common data element (CDE) domain.
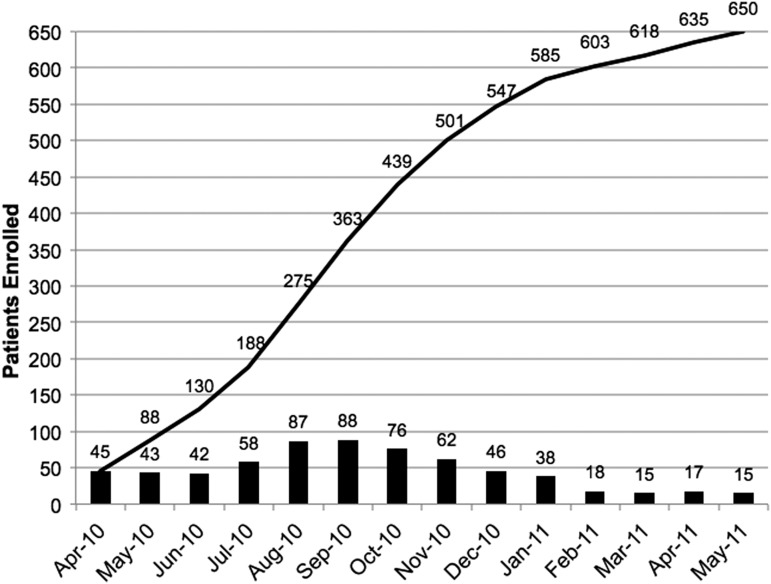
Enrollment began in April 2010, and 650 patients were enrolled over 14 months (Fig. 5). We sought to enroll a more representative TBI population that presents to a level I trauma center by including groups of patients who have often been excluded from clinical trials. Patients were not excluded if they had a history of prior TBI, substance abuse, or mental health issues, or were homeless. Table 1 shows the enrollment by site according to completion rates achieved in each of the TBI-CDE domains. A total of 637 patients >16 years of age completed assessments in at least one of four domains. For the biospecimens, 79% consented and 75% of these had blood samples collected. For the 3T MRIs, 81% consented and 50% of the patients returned for the MRI. For the outcome measures, 98% of patients consented and 77% of these completed 3 month outcome measures. The 6 month outcomes battery was completed to varying degrees: 69% of patients were assessed by GOS-E only and of these, 84% completed all measures that could be administered by telephone interviews, and 59% returned for the in-person full TBI-CDE “Core” battery.
FIG. 5.
Monthly (bars) and cumulative (line) enrollment rates for Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK TBI) patients at all study sites and age ranges (3–94 years old).
Table 1.
Enrollment of Patients >16 Years Old According to Completion Achieved in Each of the CDE Domains
| |
|
|
|
Outcomesa |
|
|---|---|---|---|---|---|
| Site | Demographic/clinical data | Biomarkersa | 3T MRIa | 3-month GOS-E | 6-month GOS-E |
| SFGH | 326 | 256 (79%) | 146 (45%) | 265 (81%) | 248 (76%) |
| UPMC | 180 | 151 (84%) | 52 (29%) | 140 (78%) | 138 (77%) |
| UMCB | 80 | 50 (63%) | 35 (44%) | 49 (61%) | 26 (33%) |
| MSMC | 51 | 22 (43%) | 25 (49%) | 37 (73%) | 30 (57%) |
| Total | 637 (100%) | 479 (75%) | 257 (40%) | 491 (77%) | 431 (69%) |
Percentages calculated from row totals in second column.
CDE, common data elements; GOS-E, Glasgow Outcome Scale – Extended; SFGH, San Francisco General Hospital; UPMC, University of Pittsburgh Medical Center; UMCB, University Medical Center Brackenridge; MSMC, Mount Sinai Medical Center.
Different enrollment strategies were evaluated at different sites. At the SFGH site, 338 patients were enrolled from April 2010 to February 2011. A 20 h/day, 6 day/week screening schedule was implemented with day shift personnel covering the hours of 7 a.m. to 7 p.m., and night shift personnel covering 6:30 p.m. to 5 a.m. Weekend coverage consisted of Saturdays and Sundays from 10 a.m. to 2 p.m., and both nights from 7 p.m. to 7 a.m. Three existing research associates (RAs) each working at 50–75% variable effort were responsible for daytime enrollment. Two full-time RAs were hired to cover four weeknight shifts and two weekend night shifts per week. The UPMC site enrolled 180 TBI patients from April 2010 to June 2011. UPMC had nursing staff 24 h/day, 7-days/week who were trained in all active clinical research protocols. At the UMCB site, 80 patients were enrolled from July 2010 to February 2011. Patient enrollment windows were from 8 a.m. to 8 p.m. every day of the week. The study staff at UMCB consisted of two full-time research associates, one part-time research nurse, and one part-time project manager from a clinical research organization with established experience. The MSRC site enrolled 51 rehabilitation patients from June 2010 to March 2011. Patients presenting to MSRC were self or professional referrals. Patient screening, enrollment, and all study procedures were completed by one full-time research associate with assistance from a part-time co-investigator.
The cost for the implementation of all of the TBI-CDEs varied from site to site, depending upon institutional indirect costs, patient screening, and enrollment strategies that leveraged concurrent TBI research studies, and the volume of patients enrolled. Therefore, the cost per patient was lower at sites with ongoing research, high enrollment, and lower indirect costs. To assist future investigators with budgeting for TBI-CDE implementation, we collected data for the time required for screening, enrollment, and collection of data for each CDE domain (Fig. 4).
Demographics and clinical variables
For reporting on patient demographics and clinical characteristics, we excluded 13 patients who were <16 years of age. We have separately described the 51 MSRC (rehabilitation) patients, leaving 586 adult patients described from the “acute” study sites. Table 2 provides the ED dispositions and associated percentage of TBI-CDE clinical and demographic variables that were available to be collected. Completion rates ranged from 100% for age, gender, race, injury date and time, and psychiatric history. Rates were slightly lower, at 96%, for collection of education and employment status data. For previous TBI, 91% of intensive care unit (ICU) admissions completed the questionnaire, compared with ≥95% for the other dispositions. The loss of consciousness (LOC) and post-traumatic amnesia (PTA) information was also slightly more difficult to complete for patients who were admitted to ICU (95%) than for those who were admitted to an ED or ward (99%, 98%, respectively). ED admission hemoglobin tests were obtained as part of routine care in only 42% of the ED discharges, whereas 90% of ward admissions and 97% of ICU admissions had blood drawn for hemoglobin tests. Data for the admission GCS and laboratory results from the acute ED presentation were rarely available for the rehabilitation patients.
Table 2.
Patient Enrollment Categories and Completion Rates for Collection of Selected Demographic and Clinical CDEs for 637 Patients
| Patient enrollment category(n=637) | ED discharge(n=169)(27%)a | Ward admit(n=213)(34%) | ICU admit(n=204)(32%) | Rehab(n=51)(8%) |
|---|---|---|---|---|
| Common data elements (CDEs) | ||||
| Age and gender | 100% | 100% | 100% | 100% |
| Race | 100% | 99% | 100% | 98% |
| Education | 96% | 99% | 98% | 86% |
| Employment | 96% | 95% | 94% | 98% |
| Date and time of injury | 100% | 100% | 100% | 100% |
| Admission GCS: eye, verbal, motor | 99% | 99% | 99% | 0% |
| Previous TBI | 98% | 95% | 91% | 98% |
| LOC duration | 99% | 98% | 95% | 100% |
| PTA duration | 99% | 98% | 95% | 100% |
| Cause of injury | 95% | 97% | 100% | 100% |
| Psychiatric history | 100% | 100% | 100% | 100% |
| Admit hemoglobin | 42% | 90% | 97% | 8% |
| 6-month GOS-E | 66% | 66% | 78% | 57% |
Shaded cells represent percentages significantly (p<0.05) different from others in the same row.
Totals may not equal 100% because of independent rounding.
ED, emergency department; ICU, intensive care unit; GCS, Glasgow Coma Scale; TBI, traumatic brain injury; LOC, loss of consciousness; PTA, post-traumatic amnesia; GOS-E, Glasgow Outcome Scale-Extended.
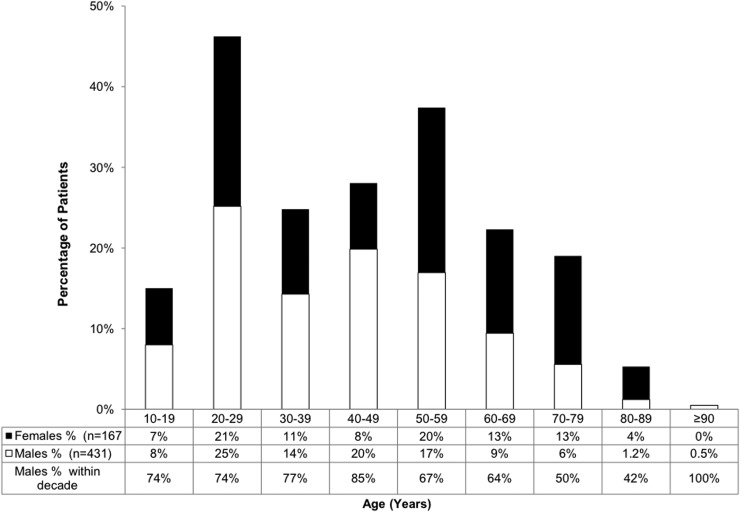
The majority of patients were male (72%) and white (85%). The mean age was 43 years old at the acute sites, ranging from 16 to 99 years. Figure 6 shows age ranges and gender proportions within each decade where males accounted for 62–87% of patients between 16 and 69 years of age. In the age range between 70 and 89 years old, females accounted for 54–71% of injuries. The 51 rehabilitation patients enrolled at MSRC were on average 50 years old and 73% were male. Selected patient demographics and clinical characteristics are described in Table 3, which compares differences among sites. Although study sites were located in four major United States cities, significant differences (all with p<0.05) were found for education levels, race, previous TBI, psychiatric disorders, cause of injury, LOC, PTA, ED admission GCS, and positive admission CT scans. At all sites, the largest proportion of patients had completed high school, with this being their highest level of education (range: 52–69%). For the acute sites, 47% (range: 13–40%) reported on the Ohio State self-administered questionnaire that they had sustained a previous brain injury, of which 28% (range: 12–36%) reported had required hospitalization. For the rehabilitation site, only 10% of the patients reported a previous TBI. For all sites, psychiatric history was reported as present for 28% (range: 13–36%) of the patients.
FIG. 6.
Overall age and gender distributions. Males accounted for 64–85% of patients between 16 and 69 years of age. Among those between 70 and 89 years of age, males accounted for 42–50% of patients. The mean (SD) age was 43±18.
Table 3.
Comparison of Patient Demographics and Clinical Characteristics Among Sites
| CDEs | SFGH | UPMC | UMCB | MSRC | Overall χ2 (p values) |
|---|---|---|---|---|---|
| Race | n=323 | n=178 | n=80 | n=50 | <0.00001 |
| White | 253 (78%) | 167 (94%) | 72 (90%) | 39 (78%) | |
| Education | n=315 | n=173 | n=71 | n=44 | 0.001 |
| Below high school | 35 (11%) | 14 (9%) | 13 (18%) | 3 (7%) | |
| High school graduate | 165 (52%) | 111 (69%) | 42 (59%) | 29 (66%) | |
| Bachelor's and above | 115 (37%) | 36 (22%) | 16 (23%) | 12 (27%) | |
| Previous TBI | n=316 | n=163 | n=74 | n=51 | <0.00001 |
| No | 125 (40%) | 135 (83%) | 32 (43%) | 46 (90%) | |
| Yes without hospitalization | 77 (24%) | 8 (5%) | 18 (24%) | 1 (2%) | |
| Yes with hospitalization | 114 (36%) | 20 (12%) | 24 (32%) | 4 (8%) | |
| Psychiatric history | n=326 | n=180 | n=80 | n=48 | 0.00012 |
| Present | 117 (36%) | 33 (18%) | 20 (25%) | 6 (13%) | |
| ED admission GCS | n=323 | n=178 | n=79 | Unavailable | <0.000001 |
| Severe (3–8) | 16 (5%) | 48 (27%) | 6 (8%) | ||
| Moderate (9–12) | 18 (5%) | 10 (6%) | 3 (4%) | ||
| Mild (13–15) | 289 (90%) | 120 (67%) | 70 (89%) | ||
| LOC | n=324 | n=174 | n=80 | n=49 | 0.002 |
| Yes | 216 (67%) | 123 (71%) | 64 (80%) | 34 (67%) | |
| No | 88 (27%) | 36 (21%) | 6 (8%) | 15 (29%) | |
| Unknown | 20 (6%) | 15 (8%) | 10 (12%) | 2 (4%) | |
| PTA | n=324 | n=175 | n=79 | n=37 | <0.00001 |
| Yes | 192 (59%) | 62 (35%) | 54 (68%) | 12 (24%) | |
| No | 110 (34%) | 43 (25%) | 17 (22%) | 25 (49%) | |
| Unknown | 22 (7%) | 70 (40%) | 8 (10%) | 14 (28%) | |
| Cause of injury | n=325 | n=178 | n=80 | n=51 | <0.00001 |
| Motor vehicle accident | 33 (10%) | 50 (28%) | 22 (28%) | 5 (10%) | |
| MCC/bike accident | 79 (24%) | 13 (7%) | 16 (20%) | 6 (12%) | |
| Pedestrian hit | 35 (11%) | 5 (3%) | 4 (5%) | 9 (18%) | |
| Fall | 92 (28%) | 81 (46%) | 26 (33%) | 20 (39%) | |
| Assault | 69 (21%) | 18 (10%) | 7 (9%) | 6 (12%) | |
| Other | 17 (6%) | 11 (6%) | 5 (6%) | 5 (10%) | |
| ED admission head CT | n=326 | n=180 | n=80 | Unavailable | <0.000001 |
| Positive | 117 (36%) | 173 (96%) | 26 (33%) |
Pearson χ2 p values are displayed for CDE demographic and clinical variables that display statistically significant variation in distribution among the acute care sites and the rehabilitation site.
CDE, common data elements; SFGH, San Fra UPMC, University of Pittsburgh Medical Center; UMCB, University Medical Center Brackenridge; MSRC, Mount Sinai Rehabilitation Center; ED, emergency department; GCS, Glasgow Coma Score; LOC, loss of consciousness; PTA, post-traumatic amnesia; MCC, motorcycle crash.
Nearly all patients (99%) had sustained a closed TBI. The majority (83%) had a mild TBI as defined by a GCS of 13–15 upon admission to the ED; 4% had a moderate TBI (GCS 9–12), and 13% had a severe TBI (GCS 3–8). Accidents involving motor vehicles accounted for 44% of total injuries and 34% of injuries were the result of falls. The patient had been inside a car in 40% of motor vehicle accidents, whereas 41% had been riding a motorcycle or bicycle, and 19% were pedestrians hit by a motor vehicle. The majority of the patients (70%) had LOC and/or PTA (52%), with 43% of patients having both. The prevalence of hypoxia and hypotension was 7% and 6%, respectively.
Biospecimens
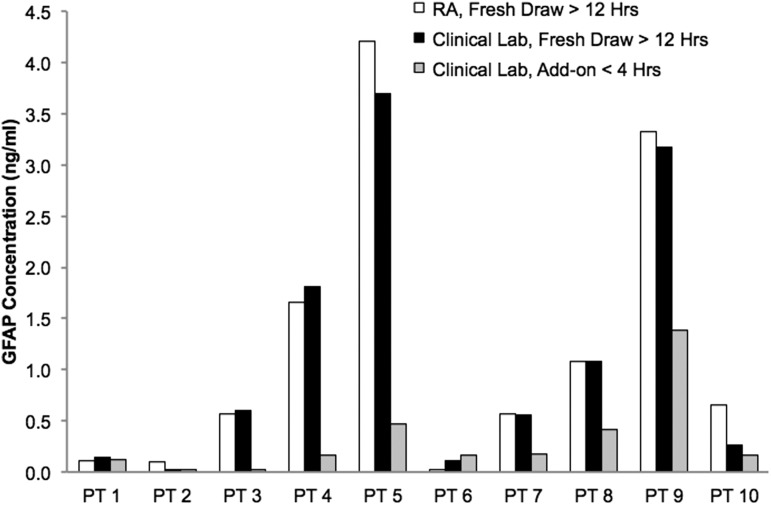
Blood biospecimens were collected from 479 patients. DNA extraction and analysis was successful for all samples. Plasma samples for proteomic biomarker analyses were processed and aliquoted from 447 patients. We examined different methods for biospecimen collection and processing. We compared specimens collected and rapidly processed by research personnel, specimens collected by clinical staff and rapidly processed by clinical laboratory staff, and convenience samples obtained from acute blood samples stored for “add-on” clinical tests. Figure 7 shows the results of these comparisons. For patients with a GFAP concentration of at least 0.5 ng/mL, there were insignificant differences in measured GFAP levels processed by the study staff and clinical laboratory. However, the “add-on” process, in which samples can sit for up to 48 h at room temperature, resulted in substantial reductions in the GFAP concentrations.
FIG. 7.
Effects of different blood specimen processing methods on patient (PT) plasma glial fibrillary acidic protein (GFAP) concentrations. Comparisons were made for fresh blood samples processed immediately after blood was drawm by the research associate (RA) and the clinical laboratory (Lab) versus add-on samples that were collected from ordered blood draws that were kept at room temperature for <4 h and processed by the clinical laboratory.
Imaging
All patients had a CT scan within 24 h of injury, as was required for enrollment. At the acute sites, 232 (36%) patients underwent a 3T research MRI within 12±4 days of injury.28 Rehabilitation patients at MSRC completed 25 (49%) MRIs. Individual site experiences revealed higher rates of participation at SFGH (149/326, 46%) and UMCB (35/80, 44%), and a lower rate at UPMC (52/180, 29%). Issues encountered in getting a 3T research MRI included access and availability of MRI, age, education, and injury severity. The 3T MRI completion rates were 25% for patients ≥60 years of age compared with 44% for patients <60 years. Thirty-one percent of patients with less than a high school education completed 3T MRI compared with 50% of patients with a Bachelor's degree or more. For patients with an Abbreviated Injury Score (AIS) of ≥3 for the head, 33% completed the 3T MRI compared with 47% of patients with an AIS of <3.
Outcomes
Because of our broad inclusion criteria, follow-up and outcome assessment was challenging. At 3 months, the GOS-E could be obtained for 491 (77%) of the patients. Some patients lost to follow-up at 3 months were able to be reached at 6 months. At 6 months, the GOS-E was obtained for 431 (69%) of the patients. Follow-up was related to the degree of injury. Six month GOS-E assessments were completed for 68% of patients with GCS of 13–15 (mild TBI) versus 89% of the patients with GCS of 3–8 (severe TBI). Significant covariates for unsuccessful follow-up are displayed in Table 4 as ORs. Zero years of education has an OR of 0.481, and each additional year of education added 1.134 times the OR of the previous year (p<0.001). Patients with a baseline developmental disorder, as defined by the CDEs to include attention-deficit disorder, attention-deficit hyperactivity disorder (ADHD), and learning disability, had OR of 0.452 for successful follow up (p<0.01). Patients confirmed positive for illicit opioid or cannabis use on ED toxicology results at the time of injury showed a 0.258 OR (p<0.01), and those with homelessness at baseline showed a 0.248 OR, for successful 6 month follow up (p<0.02). Study logs documented, on average, eight attempts to make patient contact per successful 6 month follow-up.
Table 4.
Baseline Variables as Predictors for Successful 6 Month Follow-up
| Baseline variable | Odds ratio | 95% CI | p value |
|---|---|---|---|
| Years of educationa | 0.481 (1.134) | (1.058–1.220) | <0.001 |
| Developmental disorderb | 0.452 | (0.257–0.797) | <0.01 |
| Illicit drug usec | 0.258 | (0.079–0.719) | <0.01 |
| Homelessness | 0.248 | (0.095–0.694) | <0.02 |
Log odds of a person with zero years of education for successful 6 month follow-up is 0.481; each year of education adds 1.134 times the OR of the previous year.
Developmental disorder, as defined by the TBI-CDEs: learning disability, attention-deficit disorder (ADD), or attention-deficit/hyperactivity disorder (ADHD).
Illicit drug use: presence of opioids and cannabis in ED admission toxicology results.
TBI-CDE, traumatic brain injury–common data elements.
Discussion
The TRACK-TBI pilot has successfully demonstrated the feasibility of implementing the TBI-CDEs in a multicenter study that included acute care centers and a rehabilitation facility. The multidisciplinary team established an operational version of TBI-CDEs, developed a web-enabled TBI-CDE database, and created imaging and biospecimen repositories. We were able to collect data at the highest level of granularity (Fig. 2) for demographics and clinical assessment as well as imaging TBI-CDE domains. The study has produced one of the largest prospective multivariate TBI databases that integrate clinical, imaging, proteomic, genomic, and outcome biomarkers from patients across the injury spectrum of concussion to coma. We have worked closely with the FITBIR team, providing our data dictionary, clinical and imaging data, and testing methods for data transfer. We are pleased that the TRACK-TBI Pilot data set is the first to populate FITBIR, providing a new opportunity for collaboration and acceleration of TBI research.
As with most pilot projects, there were lessons learned. Our desire to enroll patients more representative of the TBI population provided important information about these individuals that is often excluded from clinical trials, but also created challenges with follow-up and outcome assessment. We also identified gaps in the TBI-CDEs such as the lack of validated outcome measures for more disabled and non-English-speaking patients, and pediatric-specific CDEs. Pediatric CDEs have subsequently been published, but have yet to be implemented and validated.29–32 The timeline specified by the NIH Grand Opportunity grant did not permit the development of a complete set of best practices, computational tools to facilitate data quality management, and optimal strategies for TBI-CDE curation. Table 5 summarizes the status of important requirements and data quality components that we addressed in the TRACK-TBI Pilot and those that need to be addressed. Most of the limitations are currently being addressed by our public–private partnership with One Mind for Research, QuesGen Inc., and Thomson Reuters. These ongoing efforts along with the infrastructure created by the TRACK-TBI Pilot study provide the foundation for future multidisciplinary, multicenter studies in TBI.
Table 5.
Summary of TRACK-TBI Data Quality Components Comparing the Extent to Which Selected Basic Elements of Good Practice Were Successfully Implemented (White Cells), or Implemented with Limitations (Gray Shaded Cells) to Represent Areas for Future Improvements
| |
CDE subdomains |
|||
|---|---|---|---|---|
| Quality elements | Demographic clinical | Biospecimen biomarkers | Radiologic imaging | Outcomes end-points |
| Standard definitions (NINDS CDEs) | ||||
| Explicit coding rules (developed in QuesGen database) | ||||
| Data collector training | ||||
| Operational manuals | ||||
| Instrument calibration/standardization measures met | ||||
| IRB governance and ethics | ||||
| Protocol standards met for timing the collection of data measures | ||||
| Data curation and analytic plans implemented | ||||
| Completeness of the data collection | ||||
| Subject retention | ||||
| Data privacy and security | ||||
| Achieved desired granularity of data | ||||
| Data are cleaned and validated | ||||
| Audit procedures with discrepancy resolution process | ||||
| Access and data sharing agreements are established | ||||
| Data modifications are tracked and communicated | ||||
| Validated analytical tools are used for main analyses | ||||
| Subjective data prone to bias or inter-rater variations | ||||
| Metadata documented and curated | ||||
| Usability of the data | ||||
| Consistency of findings compared with relevant research | ||||
| Funded resources for data curation and quality assurance | ||||
TRACK-TBI, Transforming Research and Clinical Knowledge in Traumatic Brain Injury; CDE, common data elements; NINDS, National Institute of Neurological Disorders and Stroke; IRB, institutional review board.
Inclusion criteria, screening, and enrollment
The TRACK-TBI pilot study adopted very broad inclusion criteria in order to validate the feasibility of as many TBI-CDEs as possible. Acute patients required a history of TBI within 24 h and clinical indication for a head CT. Rehabilitation patients only needed a history of TBI and an indication for rehabilitation services. Patients were enrolled across the spectrum from concussion to coma. The infrastructure needed for TBI screening and enrollment across the spectrum of injury is challenging, especially for patients seen in the ED. Several screening strategies were explored at different sites. The most comprehensive approach was implemented at SFGH where screening and enrollment were conducted in the ED 20 h/day, 6 days/week. Given the significant volume of TBI seen in this urban level I trauma center, individual research staff frequently exceeded their capacity to screen and enroll patients. This experience demonstrates the large number of potential research subjects along the milder end of the TBI spectra. It also highlights the need for significant resources to achieve consecutive enrollment of TBI patients in the ED. Patients admitted to the hospital or rehabilitation center required fewer personnel and fewer hours of coverage for screening. However, the screening workload was balanced by increased amounts of clinical data collection and data entry. The cost per patient varied from site to site, depending upon existing research infrastructure, indirect costs, and the volume of patient enrollment. This suggests that a per-patient reimbursement model based on the time required for execution of each CDE domain (Fig. 4) and the cost of imaging and biospecimen processing is more cost-effective than the traditional site-based funding model.
For future TBI studies conducted at level I trauma centers, an efficient, systematic sampling framework for subject recruitment will be needed. Given the large volume of TBI patients who present to these centers daily, we recommend that ED patient enrollment be randomized to a limited number of days each week. This would still provide a significant number of ED study subjects and allow more time during the week to enroll patients admitted to the hospital and those admitted to the ICU. These three clinical strata align with the most recent version of the TBI-CDEs (2.0), that provide recommendations for data collection for different types of studies: concussion/mild TBI studies, acute hospitalized studies, moderate/severe TBI: rehabilitation studies, and epidemiology studies.20
Biospecimens
Using the clinical laboratory for biospecimen processing provided similar results as those for specimens processed by research staff. This approach allowed research personnel to spend more time collecting clinical data, especially from patients being discharged from the ED. We also determined that some proteomic biomarkers, such as GFAP, are susceptible to breakdown if stored using standard clinical protocols. Our experience indicates that all blood biospecimens should be collected and processed according to the best practices outlined by the TBI-CDEs, whether research staff or the clinical laboratory processes them. Detailed protocols for collection, processing, and shipping of blood biospecimen, developed for the TRACK-TBI pilot are available on the NINDS TBI-CDE website.20 The TBI-CDE biospecimens protocol was used effectively to collect, process, and store blood biospecimens for proteomic and genetic analyses. We have recently demonstrated the diagnostic accuracy of elevated levels of plasma GFAP in TBI, and candidate-gene allelic association analyses are currently underway.33
Imaging
We were successful in implementing standard imaging protocols for the clinical CT scanners and structural imaging protocols for the 3T MRI units at all study sites. Standardization for more advanced MRI sequences, such as diffusion tensor imaging, will be required for future multicenter studies. A single neuroradiologist read and recorded the pathoanatomical features as recommended by the TBI-CDE Imaging Working Group.15 Given the effort required to extract these data, inter-rater reliability studies are needed, and the utility of these imaging features for diagnostic and prognostic models must be validated.
Site-specific logistics and imaging infrastructure differences contributed to the variations in 3T MRI completion rates. Although 81% of the patients consented to undergo a 3T MRI, only 50% of these were performed. For several sites, the 3T MRI scanner was located off-site, which created access issues for some patients, especially for those too ill for transportation and the elderly. At UPMC, the 3T scanner was located in the hospital; however, access for research was limited to specific block times, preventing some patients from being scheduled. Determining which patients should be enrolled to undergo 3T MRI presented trade-offs between selecting subjects who were likely, or able, to return, and sampling biases. Another significant barrier was the patient's perception of the benefit of a MRI scan, particularly for those with a normal head CT who were told by the treating medical staff that they did not have a TBI. The recent findings by Yuh et al., demonstrating that a significant number of TBI patients with a normal CT have structural abnormalities on a 3T MRI, may serve to help improve follow-up.28
Outcomes
The 6 month “core” TBI-CDE battery included measures of global outcome, neuropsychological impairment, psychological status, post-concussive symptoms, social role participation, health-related quality of life, and post-traumatic stress.18 This required in-person assessment and patient participation. The outcome testing took 90 min on average, with an additional 2 h required for patient contact and scheduling, entering results into the database, and processing patient reimbursements (Fig. 4). We recommend that mobile testing and data entry tools be developed to improve the efficiency of this process.
The GOS-E was graded with respect to pre-injury status; patients who remained at identical levels of homelessness, prior psychological and neurological disorders, and socioeconomics compared with baseline were scored as GOS-E=8, whereas those who sustaind additional or worsened cognitive or psychological burden after TBI were scored GOS-E <8, accordingly. We found that certain core outcome measures could not be given to patients with a poor outcome (GOS-E 2, 3, and 4). We also identified limitations of the core TBI-CDE outcome battery in fully assessing patients at the upper end of recovery (GOS-E 8), particularly with respect to mental health and health economic measures. We recommend that a flexible, or adaptive, assessment battery composed of a broader range of TBI-CDE measures be developed to enable outcome assessment across all phases of recovery and all levels of TBI severity. Another limitation of the core TBI-CDE outcome measures was their inability to be applied to a significant number of non-English-speaking patients. For example, at SFGH 21% of the non-English-speaking patients screened for the TRACK-TBI pilot were excluded. With the exception of the Spanish versions of the Brief Symptom Inventory 18, Satisfaction With Life Scale, and Post-traumatic Stress Disorder Checklist – Civilian version, the Core CDEs are only validated and normed in English.18 With Spanish and Chinese being the second and third leading languages spoken in the United States,34 there should be high priority for funding of research to validate TBI-CDE outcome measures in other languages. Furthermore, the inclusion of more nonverbal memory tests in the outcome CDEs to accommodate patients who do not speak English may expand the applicability of the CDEs both within the United States and with international collaborators.
The requirement of the core outcome battery for in-person assessment and full cooperation of the patient creates a number of challenges. The enrollment of TBI patients with a history of mental health issues, prior TBI, and substance abuse requires significant additional work on the part of the research staff to locate these patients and schedule them for follow-up assessment. Specifically, patients with no post-primary education, baseline learning disorders, illicit drug use, and homelessness have significantly lower ORs for 6 month follow-up. Low education has been associated with lack of return to work, and hence lower stability and traceability in the community after TBI.35 Development of secondary ADHD post-TBI is associated with TBI severity,36 and patients with ADHD have a higher risk of psychoactive substance use,37 suggesting a complicated and potentially compounding effect among predictors of poor follow-up. Impulse control disorders may predispose patients to drug abuse, homelessness, and TBI.38 More than 60% of homeless patients report having experienced more than one TBI. It is traditionally difficult to assess TBI histories of the unsheltered homeless, with few successful studies and inherent variability in sampling.39 In TRACK-TBI, some individuals required nearly 20 attempts to make contact. Follow-up can be improved by not enrolling these patients, but excluding these patients may bias certain studies and reduce the generalizability of the results. Conversely, patients with these risk factors may need to be excluded from more rigorous and expensive prospective longitudinal studies that require comprehensive follow-up. Transportation was a barrier for follow-up and taxi fares had to be provided for some patients. Financial incentives were also provided to increase the likelihood of follow-up. Together, these challenges require well-trained, motivated, and persistent research staff and careful patient selection to achieve acceptable rates of follow-up. In addition, a greater awareness of the importance of TBI research is needed. Researchers need to reach out to TBI patient advocacy organizations and work together to create strategies that are inclusive, raise awareness, and build trust with the community, to enable timely accrual targets and promote retention and follow-up of study subjects.
Data, analytics, and collaboration
The Demographics and Clinical Assessment domains contain the greatest number of TBI-CDEs. For the TRACK-TBI pilot, these data were collected for the highest percentage of patients; however, they were more prone to coding errors, incomplete fields, and relational inconsistencies than other TBI-CDE domains. Some of the data elements are subject to inter-rater variability or rely on patient self-reports, such as history of prior TBI, which are difficult to verify. We are now working closely with QuesGen and our TRACK-TBI investigators to develop information technology solutions to address many of the issues. In addition, clinical trial management software is being developed to facilitate real-time management of patient recruitment/enrollment, data collection, form completion, follow-up, project progress/milestones, and component-based reimbursement for study procedures and outcome assessment. These improvements streamline clinical research workflow and reduce costs.
Over the past 18 months, we have worked with the FITBIR team, sharing our data dictionary and providing test images and data sets. Different methods of data transfer have been explored and we have now successfully transferred the TRACK-TBI pilot data and images to FITBIR. Given that this is the first TBI study in FITBIR, a number of quality control and assurance procedures will be conducted prior to making these data available to the public. FITBIR will ultimately provide a TBI-CDE-compliant environment for data sharing across multiple TBI studies funded by the NIH and Department of Defense (DOD) to gain statistical power and insights that were not possible from smaller studies.
In efforts to accelerate research in TBI, we have initiated a number of collaborations with new investigators that were not part of the TRACK-TBI pilot study. We have also established a public–private partnership with One Mind for Research, a nonprofit organization dedicated to finding cures for TBI. With their support, we have transferred the TRACK-TBI Pilot data to the tranSMART analytical platform. This has provided a new forum for analytics and collaboration. tranSMART is built on the i2b2 platform to enhance data sharing and partnership (http://www.i2b2.org). The de-identified TRACK-TBI Pilot instance is hosted on the Amazon Elastic Compute Cloud (EC2), a cost-effective, secure, and reliable platform that is highly scalable. The analytical capabilities of tranSMART give investigators a data discovery tool to explore the high-content TRACK-TBI pilot data set at a descriptive level, generate hypothesis, and perform complex analyses.
Conclusion
We have demonstrated the feasibility of implementing the TBI-CDEs in a multicenter, prospective observational study. The process of operationalizing the recommendations of the TBI-CDE working groups produced protocols and procedures for collecting standardized, high-quality data. We identified a number of issues and opportunities for improvement in data collection, patient follow-up, quality control, and curation. The successful transfer of the TRACK-TBI pilot data set to FITBIR marks the beginning of an exciting new era in TBI research. Building on the power of standardization with the TBI-CDEs, FITBIR and other informatics tools will promote collaboration and accelerate research in TBI.
Contributor Information
Collaborators: TRACK-TBI Investigators including:
Acknowledgments
This study was supported by NIH/NINDS grants NS069409 (to Dr. Manley) and One Mind for Research. We acknowledge and appreciate the following contributors to the development of the TRACK-TBI database and repositories by organization and alphabetical order by last name.
QuesGen Systems, Inc.: Vibeke Brinck, MS, and Michael Jarrett, MBA
One Mind for Research: General Peter Chiarelli, US Army (Ret), and Magali Haas, MD, PhD
Thomson Reuters: Tatiana Khasanova, PhD, and Sirimon O'Charoen, PhD
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M. Xu L. Wald M.M. Coronado V.G. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta: 2010. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. [Google Scholar]
- 2.Murray C.J. Lopez A.D. Alternative projections of mortality and disability by cause of disease study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 3.Bazarian J.J. Veazie P. Mookerjee S. Lerner E.B. Accuracy of mild traumatic brain injury case ascertainment using ICD-9 codes. Acad. Emerg. Med. 2006;13:31–38. doi: 10.1197/j.aem.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 4.Powell J.M. Ferraro J.V. Dikmen S.S. Temkin N.R. Bell K.R. Accuracy of mild traumatic brain injury diagnosis. Arch. Phys. Med. Rehabil. 2008;89:1550–1555. doi: 10.1016/j.apmr.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Cancelliere C. Cassidy J.D. Cote P. Hincapie C.A. Hartvigsen J. Carroll L.J. Marras C. Boyle E. Kristman V. Hung R. Stalnacke B.M. Rumney P. Coronado V. Holm L.W. Borg J. Nygren-de Boussard C. Af Geijerstam J.L. Keightley M. Protocol for a systematic review of prognosis after mild traumatic brain injury: an update of the WHO Collaborating Centre Task Force findings. Syst. Rev. 2012;1:17. doi: 10.1186/2046-4053-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finkelstein E. Corso P. Miller T. Associates. The Incidence and Economic Burden of Injuries in the United States. Oxford University Press; New York: 2006. [Google Scholar]
- 7.Lingsma H.F. Roozenbeek B. Steyerberg E.W. Murray G.D. Maas A.I. Early prognosis in traumatic brain injury: from prophecies to predictions. Lancet Neurol. 2010;9:543–554. doi: 10.1016/S1474-4422(10)70065-X. [DOI] [PubMed] [Google Scholar]
- 8.Maas A.I. Roozenbeek B. Manley G.T. Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics. 2010;7:115–126. doi: 10.1016/j.nurt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teasdale G. Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir. (Wien). 1976;34:45–55. doi: 10.1007/BF01405862. [DOI] [PubMed] [Google Scholar]
- 10.Wilson J.T. Pettigrew L.E. Teasdale G.M. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J. Neurotrauma. 1998;15:573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 11.Maas A.I.R. Steyerberg E.W. Marmarou A. McHugh G.S. Lingsma H.F. Butcher I. Lu J. Weir J. Roozenbeek B. Murray G.D. IMPACT recommendations for improving the design and analysis of clinical trials in moderate to severe traumatic brain injury. Neurotherapeutics. 2010;7:127–134. doi: 10.1016/j.nurt.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saatman K.E. Duhaime A.C. Bullock R. Maas A.I. Valadka A. Manley G.T. and the Workshop Scientific Team and Advisory Panel Members. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whyte J. Vasterling J. Manley G.T. Common data elements for research on traumatic brain injury and psychological health: current status and future development. Arch. Phys. Med. Rehabil. 2010;91:1692–1696. doi: 10.1016/j.apmr.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 14.Maas A.I.R. Harrison–Felix C.L. Menon D. Adelson P.D. Balkin T. Bullock R. Engel D.C. Gordon W. Langlois–Orman J. Lew H.L. Robertson C. Temkin N. Valadka A. Verfaellie M. Wainwright M. Wright D.W. Schwab K. Standardizing data collection in traumatic brain injury. J. Neurotrauma. 2011;28:177–187. doi: 10.1089/neu.2010.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duhaime A.C. Gean A.D. Haacke E.M. Hicks R. Wintermark M. Mukherjee P. Brody D. Latour L. Riedy G. the Common Data Elements Neuroimaging Working Group Members, and the Pediatric Working Group Members. Common data elements in radiologic imaging of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010;91:1661–1666. doi: 10.1016/j.apmr.2010.07.238. [DOI] [PubMed] [Google Scholar]
- 16.Maas A.I. Harrison–Felix C.L. Menon D. Adelson P.D. Balkin T. Bullock R. Engel D.C. Gordon W. Orman J.L. Lew H.L. Robertson C. Temkin N. Valadka A. Verfaellie M. Wainwright M. Wright D.W. Schwab K. Common data elements for traumatic brain injury: recommendations from the interagency working group on demographics and clinical assessment. Arch. Phys. Med. Rehabil. 2010;91:1641–1649. doi: 10.1016/j.apmr.2010.07.232. [DOI] [PubMed] [Google Scholar]
- 17.Manley G.T. Diaz–Arrastia R. Brophy M. Engel D. Goodman C. Gwinn K. Veenstra T.D. Ling G. Ottens A.K. Tortella F. Hayes R.L. Common data elements for traumatic brain injury: recommendation from the biospecimens and biomarkers working group. Arch. Phys. Med. Rehabil. 2010;91:1667–1672. doi: 10.1016/j.apmr.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Wilde E.A. Whiteneck G.G. Bogner J. Bushnik T. Cifu D.X. Dikmen S. French L. Giacino J.T. Hart T. Malec J.F. Millis S.R. Novack T.A. Sherer M. Tulsky D.S. Vanderploeg R.D. von Steinbuechel N. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch. Phys. Med. Rehabil. 2010;91:1650–1660. doi: 10.1016/j.apmr.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 19.Szalma S. Koka V. Khasanova T. Perakslis E.D. Effective knowledge management in translational medicine. J. Transl. Med. 2010;8:68. doi: 10.1186/1479-5876-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.TBI Standards – NINDS Common Data Elements. http://www.commondataelements.ninds.nih.gov/tbi.aspx. [May 6;2013 ]. http://www.commondataelements.ninds.nih.gov/tbi.aspx Revised April 30, 2013.
- 21.Papa L. Lewis L.M. Falk J.L. Zhang Z. Silvestri S. Giordano P. Brophy G.M. Demery J.A. Dixit N.K. Ferguson I. Liu M.C. Mo J. Akinyi L. Schmid K. Mondello S. Robertson C.S. Tortella F.C. Hayes R.L. Wang K.K. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann. Emerg. Med. 2012;59:471–483. doi: 10.1016/j.annemergmed.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haacke E.M. Duhaime A.C. Gean A.D. Riedy G. Wintermark M. Mukherjee P. Brody D.L. DeGraba T. Duncan T.D. Elovic E. Hurley R. Latour L. Smirniotopoulos J.G. Smith D.H. Common data elements in radiologic imaging of traumatic brain injury. J. Magn. Reson. Imaging. 2010;32:516–543. doi: 10.1002/jmri.22259. [DOI] [PubMed] [Google Scholar]
- 23.Leow A.D. Klunder A.D. Jack C.R., Jr. Toga A.W. Dale A.M. Bernstein M.A. Britson P.J. Gunter J.L. Ward C.P. Whitwell J.L. Borowski B.J. Fleisher A.S. Fox N.C. Harvey D. Kornak J. Schuff N. Studholme C. Alexander G.E. Weiner M.W. Thompson P.M. for the ADNI Preparatory Phase Study. Longitudinal stability of MRI for mapping brain change using tensor-based morphometry. NeuroImage. 2006;31:627–640. doi: 10.1016/j.neuroimage.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stallings G. Boake C. Sherer M. Comparison of the California Verbal Learning Test and the Rey Auditory Verbal Learning Test in head-injured patients. J. Clin. Exp. Neuropsychol. 1995;17:706–712. doi: 10.1080/01688639508405160. [DOI] [PubMed] [Google Scholar]
- 25.Kaloupek D.G. Chard K.M. Freed M.C. Peterson A.L. Riggs D.S. Stein M.B. Tuma F. Common data elements for posttraumatic stress disorder research. Arch. Phys. Med. Rehabil. 2010;91:1684–1691. doi: 10.1016/j.apmr.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 26.Levin H.S. O'Donnell V.M. Grossman R.G. The Galveston Orientation and Amnesia Test. A practical scale to assess cognition after head injury. J. Nerv. Ment. Dis. 1979;167:675–684. doi: 10.1097/00005053-197911000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Beers S.R. Wisniewski S.R. Garcia–Filion P. Tian Y. Hahner T. Berger R.P. Bell M.J. Adelson P.D. Validity of a pediatric version of the Glasgow Outcome Scale–Extended. J. Neurotrauma. 2012;29:1126–1139. doi: 10.1089/neu.2011.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuh E.L. Mukherjee P. Lingsma H.F. Yue J.K. Ferguson A.R. Gordon W.A. Valadka A.B. Schnyer D.M. Okonkwo D.O. Maas A.I. Manley G.T. the TRACK–TBI Investigators. Magnetic resonance imaging improves 3–month outcome prediction in mild traumatic brain injury. Ann. Neurol. 2013;73:224–235. doi: 10.1002/ana.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller A.C. Odenkirchen J. Duhaime A.C. Hicks R. Common data elements for research on traumatic brain injury: pediatric considerations. J. Neurotrauma. 2012;29:634–638. doi: 10.1089/neu.2011.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adelson P.D. Pineda J. Bell M.J. Abend N.S. Berger R.P. Giza C.C. Hotz G. Wainwright M.S. and the Pediatric TBI Demographics and Clinical Assessment Working Group. Common data elements for pediatric traumatic brain injury: recommendations from the working group on demographics and clinical assessment. J. Neurotrauma. 2012;29:639–653. doi: 10.1089/neu.2011.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger R.P. Beers S.R. Papa L. Bell M. and the Pediatric TBI CDE Biospecimens and Biomarkers Workgroup. Common data elements for pediatric traumatic brain injury: recommendations from the biospecimens and biomarkers workgroup. J. Neurotrauma. 2012;29:672–677. doi: 10.1089/neu.2011.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCauley S.R. Wilde E.A. Anderson V.A. Bedell G. Beers S.R. Campbell T.F. Chapman S.B. Ewing–Cobbs L. Gerring J.P. Gioia G.A. Levin H.S. Michaud L.J. Prasad M.R. Swaine B.R. Turkstra L.S. Wade S.L. Yeats K.O. the Pediatric TBI Outcomes Workgroup. Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. J. Neurotrauma. 2012;29:678–705. doi: 10.1089/neu.2011.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okonkwo D.O. Yue J.K. Puccio A.M. Panczykowski D. Inoue T. McMahon P.J. Sorani M.D. Yuh E.L. Lingsma H. Maas A. Valadka A. Manley G.T. the TRACK–TBI Investigators. GFAP–BDP as an acute diagnostic marker in traumatic brain injury: results from the prospective TRACK–TBI study. J. Neurotrauma. 2013 doi: 10.1089/neu.2013.2883. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin H.B. Kominski R.A. U.S. Census Bureau; Washington D.C: 2010. Language Use in the United States: 2007, American Community Survey Reports, ACS–12. [Google Scholar]
- 35.Walker W.C. Marwitz J.H. Kreutzer J.S. Hart T. Novack T.A. Occupational categories and return to work after traumatic brain injury: a multicenter study. Arch. Phys. Med. Rehabil. 2006;87:1576–1582. doi: 10.1016/j.apmr.2006.08.335. [DOI] [PubMed] [Google Scholar]
- 36.Max J.E. Lansing A.E. Koele S.L. Castillo C.S. Bokura H. Schachar R. Collings N. Williams K.E. Attention deficit hyperactivity disorder in children and adolescents following traumatic brain injury. Dev. Neuropsychol. 2004;25:159–177. doi: 10.1080/87565641.2004.9651926. [DOI] [PubMed] [Google Scholar]
- 37.Groenman A.P. Oosterlaan J. Rommelse N. Franke B. Roeyers H. Oades R.D. Sergeant J.A. Buitelaar J.K. Faraone S.V. Substance use disorders in adolescents with attention deficit hyperactivity disorder: a 4–year follow–up study. Addiction. 2013;108:1503–1511. doi: 10.1111/add.12188. [DOI] [PubMed] [Google Scholar]
- 38.Hwang S.W. Colantonio A. Chiu S. Tolomiczenko G. Kiss A. Cowan L. Redelmeier D.A. Levinson W. The effect of traumatic brain injury on the health of homeless people. CMAJ. 2008;179:779–784. doi: 10.1503/cmaj.080341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topolovec–Vranic J. Ennis N. Colantonio A. Cusimano M.D. Hwang S.W. Kontos P. Ouchterlony D. Stergiopoulos V. Traumatic brain injury among people who are homeless: a systematic review. BMC Public Health. 2012;12:1059. doi: 10.1186/1471-2458-12-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]