Abstract
Endothelial-dependent regulation of vascular tone occurs in part via protein kinase G1α-mediated changes in smooth muscle myofilament sensitivity to Ca2+. Tissue-specific differences in PKG-dependent relaxation have been attributed to altered expression of myofilament-associated proteins that are substrates for PKG binding. These include the alternative splicing of the myosin targeting subunit (MYPT1) of myosin light chain phosphatase to yield leucine zipper positive (LZ+) and negative (LZ−) isovariants, with the former being required for PKG-mediated relaxation, and/or altered expressions of telokin, vasodilator-stimulated phosphoprotein (VASP) or heat shock protein Hsp20. During human pregnancy the uterine and placental circulations remain distinct entities and, as such, their mechanisms of vascular tone regulation may differ. Indeed, the sensitivity of myometrial arteries to endothelial-dependent agonists has been suggested to be greater than that of placental arteries. We tested the hypothesis that this was related to tissue-specific changes in PKG-mediated myofilament Ca2+-desensitization and/or the expressions of PKG-interacting myofilament-associated proteins. Permeabilized human placental and myometrial arteries were constricted with maximal activating Ca2+ (pCa 4.5), or sub-maximal Ca2+ (pCa 6.7) and the thrombane mimetic U46619, and exposed to 8-Br-cGMP. In each case, relaxation was significantly greater in myometrial arteries (e.g. relaxation in pCa 4.5 to 8-Br-cGMP was 49 ± 9.7%, n = 7) than placental arteries (relaxation of 23 ± 6.6%, n = 6, P < 0.05). MYPT1 protein levels, or MYPT1 LZ+/LZ− mRNA ratios, were similar for both artery types. Of other proteins examined, only Hsp20 expression was significantly elevated in myometrial arteries than placental arteries. These results demonstrate that the reduced human placental artery relaxation to PKG stimulation lies partly at the level of myofilament (de)activation and may be related to a lower expression of Hsp20 than in myometrial arteries.
Keywords: human placental arteries, human myometrial arteries, PKG, Hsp20
Introduction
The physiological adjustments that occur throughout pregnancy are designed to promote an environment that supports healthy fetal growth while maintaining maternal well-being. The process of hemochorial placentation means that the human placental and uterine circulations remain separated (Benirschke and Kaufmann, 2000) and a tightly regulated exchange between the maternal and fetal circulations across the placental syncytiotrophoblast membrane must develop by which the fetus receives nutrients from, and exports waste products to, the mother. This, in turn, is facilitated by the regulation of blood flow through the uterine and feto-placental circulations. In addition, the placenta lacks neuronal innervations (Reilly and Russell, 1977; Fox and Khong, 1990) suggesting that the tone of the placental vessels is predominantly regulated by local mediatory factors (Poston et al., 1995; Boura et al., 1998). As such, the mechanisms involved in regulating tone, and thereby blood flow, of these two vasculatures may be distinct.
A prominent mechanism of vascular tone regulation is via endothelial cell generation of nitric oxide (NO). The effects of physiologically released NO are in part mediated by activation of soluble guanylate cyclase in underlying smooth muscle cells resulting in an increase in the second messenger cyclic guanosine monophosphate (cGMP), activation of the serine/threonine protein kinase G (PKGIα) and subsequent interaction with downstream effector proteins (Schlossmann et al., 2003; Morgado et al., 2012). The potent vasodilator action of the cGMP/PKG pathway has been ascribed to a decrease in [Ca2+]i through the activation of multiple Ca2+-lowering mechanisms and/or to a decrease in the Ca2+ sensitivity of smooth muscle myofilaments, referred to as Ca2+-desensitization (Wu et al., 1996; Lee et al., 1997; Schlossmann et al., 2003; Morgado et al., 2012). A number of myofilament-associated proteins have been identified as potential downstream targets for PKG actions including Hsp20 (heat shock protein), VASP (vasodilator-stimulated phosphoprotein), telokin and the targeting subunit of myosin phosphatase (MLCP) termed MYPT1 (Rembold et al., 2000; Choudhury et al., 2004; Batts et al., 2005; Khromov et al., 2006; Lu et al., 2008; Fischer, 2010; Ying et al., 2012). In several animal studies, variances in tissue sensitivity of relaxation to PKG stimulants have been attributed to differing expressions of such putative targets and/or of the PKGI isoform (Rembold et al., 2000; Choudhury et al., 2004; Batts et al., 2005; Khromov et al., 2006; Lu et al., 2008; Fischer, 2010; Ying et al., 2012). In particular, considerable support has been given to the notion that alterations in MYPT1 splice variants are important in this regard. The splicing exclusion of a 31-nucleotide 3′ exon in chicken, mouse and rat has been found to induce the expression of an isovariant lacking in amino acids corresponding to a leucine zipper (LZ) motif that would otherwise be present (Karim et al., 2004; Payne et al., 2004; Fischer, 2010). Several studies have suggested that PKG interactions with MYPT1, and its consequent increase in activity to effect relaxation, are dependent upon the expression of a MYPT1 LZ positive (LZ+) isovariant (Surks et al., 1999; Yuen et al., 2011). Indeed, alterations in MYPT1LZ+/LZ− ratios have been correlated with tissue-specific arterial sensitivity to PKG stimulants (Karim et al., 2004; Payne et al., 2004; Lu et al., 2008; Fischer, 2010; Ying et al., 2012). Analogous studies are not available for human placental and uterine arteries, yet a number of separate reports suggest that endothelial-dependent agonists exert more prominent relaxatory influences on isolated uterine than placental arteries (Hull et al., 1994; Amarani et al., 1999; Kublickiene et al., 2000; Spitaler et al., 2002; Wareing et al., 2002; Wimalasundera et al., 2005; Mills et al., 2007; Sweeney et al., 2008).
Therefore, we wished to clarify whether there were differences in the ability of endothelial-dependent agonists to relax human placental and uterine (myometrial) arteries and to establish, for the first time, whether tissue-specific differences may also exist at the level of myofilament deactivation by PKG stimulation. Under similar experimental conditions, we have compared a number of vasodilatory influences between human placental and myometrial arteries. First, we have examined the extent to which two different endothelial-dependent agonists relax preconstricted placental or myometrial arteries. Second, utilizing arteries permeabilized with α-toxin in order to control directly the myofilament activation by Ca2+ alone or by agonists in the presence of a fixed level of Ca2+, we focused on assessing the ability of 8-Bromo-cGMP (8-Br-cGMP), a non-hydrolysable mimetic of cGMP, to induce Ca2+-desensitization of tone in each artery type. Third, we have assessed the expression patterns between human placental and myometrial arteries of MYPT1LZ+/LZ− isovariants and compared this with other putative PKG-interacting myofilament-associated proteins telokin, VASP and Hsp20. Our results demonstrate differences in the extent of relaxation to endothelial-dependent agonists and to PKG-mediated myofilament Ca2+-desensitization between human placental and myometrial arteries. These are not reflected in changes in the expression of MYPT1LZ+/LZ− isovariants but are correlated with changes in the expression of Hsp20.
Materials and Methods
Sample collection and tissue microdissection
The study was approved by Local Research Ethics Committees and all tissue was collected following written informed consent from women with uncomplicated pregnancy delivering a singleton infant at term (38–42 weeks' gestation). Patient details are given in Table I. Placental and myometrial biopsies were immediately placed in ice-cold tissue collection buffer ((TCB); modified Krebs solution −154 mM NaCl/5.4 mM KCl/1.2 mM MgSO4·7H2O/10 mM MOPS/5.5 mM glucose/1.6 mM CaCl2·2H2O; pH 7.4), transported to the lab and, with the use of a stereomicroscope, small artery segments 1–2 mm in length were microdissected, cleaned of all adherent fat and extraneous tissue and placed in TCB. Once microdissected, arteries were either immediately frozen in liquid nitrogen and stored at −80°C until subsequently used for western blotting or prepared for functional myography experiments.
Table I.
Demographic details of the participants used in this study.
| Characteristics | Myometrial biopsies | Placental biopsies |
|---|---|---|
| Number of patients | 62 | 75 |
| Age | 31 ± 9.9 | 30 ± 4.4 |
| Body mass index at booking | 27 ± 3.7 | 27 ± 2.5 |
| Smoker | 12% | 10% |
| Parity | 1.2 ± 0.9 | 1.2 ± 1.1 |
| Gravidity | 2.6 ± 1.2 | 2.4 ± 1.1 |
| Gestational age (weeks) | 39 ± 0.8 | 39 ± 0.9 |
| Birthweight (g) | 3478 ± 23.6 | 3440 ± 21.7 |
| Birthweight centile | 62 ± 7.4 | 61 ± 6.2 |
Tissue preparation for myography
Artery segments were mounted onto a wire myograph (610M; Danish Myotechnologies, Denmark), normalized at 0.9 of L5.1 kPa (placental) or L13.3 kPa (myometrial), as described previously (Corcoran et al., 2012), and equilibrated in 7 ml of physiological salt solution (PSS, composition: 127 mM NaCl, 4.7 mM KCl, 2.4 mM MgSO4·7H2O, 25 mM NaHCO3, 1.18 mM KH2PO4, 0.07 mM EDTA, 1.6 mM CaCl2·2H2O, 6.05 mM glucose at pH 7.4) at 37°C for 20 min. Vessel active wall tension (ΔT in mN/mm) was continuously collected on-line and was transformed to active effective pressure denoted by kPa. Arteries were preconstricted with high K+ solution (KPSS, wherein 55.3 mM NaCl was isosmotically substituted for KCl to give final concentration of 60 mM KCl) to verify contractile viability.
Assessment of endothelial-dependent agonists
Arteries were preconstricted with either the thromboxane mimetic U46619 (1 µM) or high K+ KPSS and, once a plateau tension had been reached, were exposed to the endothelial-dependent agonist bradykinin (1 µM) and any relaxant action monitored. Upon washout with PSS and relaxation to baseline, arteries were again contracted with U46619 or KPSS and exposed to a second endothelial-dependent agonist, histamine (10 µM). Responses to bradykinin or histamine were expressed as percentages of the preceding tone in U46619 or KPSS.
Artery permeabilization and assessment of 8-Br-cGMP-induced myofilament Ca2+-desensitization
Intact human placental and myometrial arteries were equilibrated in a mock intracellular solution (composition in mM: disodium creatine phosphate 30, Na2ATP 5.2, magnesium methanesulphonate 7.3, potassium methanesulphonate 74, K2EGTA 1, PIPES 30, pH 7.1) for 30 min. Solutions of the appropriate pCa (−log[Ca2+]) for subsequent artery permeabilization, and protocols post-permeabilization, were prepared by mixing the required amounts of stock low Ca2+ (composition in mM: disodium creatine phosphate 30, Na2ATP 5.2, magnesium methanesulphonate 7.92, potassium methanesulphonate 46.6, K2EGTA 10, PIPES 30, pH 7.1) and high Ca2+ (composition in mM: disodium creatine phosphate 30, Na2ATP 5.2, magnesium methanesulphonate 7.25, potassium methanesulphonate 47.1, CaEGTA 10, PIPES 30, pH 7.1) solutions (Horiutu, 1998). Arteries were permeabilized with a droplet of 300 units/ml of α-toxin from Staphylococcus aureus in sub-maximal Ca2+-activating pCa 6.7 solution for ∼45 min and then placed in relaxing pCa 9 solution. Contractile viability post-permeabilization was examined by exposure to maximally activating pCa 4.5 solution. Two separate protocols were followed to examine the influence of 8-Br-cGMP:
Protocol 1: Paired permeabilized arteries were maximally constricted in pCa 4.5 solution. 10 µM 8-Br-cGMP was subsequently added to one artery and its effect was monitored until plateau at 30–35 min. The second artery was used as a time-matched control.
Protocol 2: Paired permeabilized arteries were sub-maximally constricted in pCa 6.7 solution (with 2 μM GTP) before the subsequent addition of the G-protein-coupled agonist U46619 (1 µM) and the extent of agonist-induced Ca2+-sensitized force allowed to stabilize. 10 µM 8-Br-cGMP was subsequently added to one artery and the effect monitored for 30–35 min until plateau. The second artery was used as a time-matched control.
Western blotting
Vessels were homogenized as previously described (Corcoran et al., 2012) and then 10–30 µg protein from each sample was resolved by SDS-PAGE and transferred to PVDF membranes for western blotting with antiserum specific for PKGIα (1:400, goat polyclonal, Santa Cruz Biotechnology #sc-10335), Hsp20 (1:7500, rabbit polyclonal, Abcam #ab13491), telokin (1:3000, rabbit polyclonal, Abcam #ab76092), VASP (1:300, rabbit polyclonal, New England Biolabs, #31325) or MYPT1 (1:100, rabbit polyclonal, Santa Cruz Biotechnology, #sc-25618). Immune complexes were visualized by probing membranes with a HRP-goat anti-rabbit-IgG (1:5000; DakoCytomation, #PO448) antibody or horse-radish peroxidase (HRP)-rabbit anti-goat-IgG (1:5000, DakoCytomation, #PO449) followed by chemiluminescence reagents and exposure to X-ray film. The optical densities from the western blot scans were determined using the Intelligent Quantifier Software (BioImage Systems, Inc.). Protein expression was quantified by normalizing the optical density of each band corresponding to a placental or myometrial artery homogenate to the optical density of the positive control following the subtraction of background signal. Membranes were stained with napthol blue black in order to assess actin expression and equal protein loading between lanes.
Quantitative real-time PCR
RNeasy fibrous kit (cat. no. 74704, Qiagen) was used for isolation of RNA from tissue samples according to the manufacturer's instructions. RNA concentration was assessed by measuring optical density in a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) and RNA samples (100 ng) with A260/280 values >1.8, and A260/230 values between 1.8–2.2, were subject to reverse transcription using AffinityScript Multiple Temperature cDNA synthesis Kit 200436 (Stratagene). Information on the human MYPT1 (PPP1R12A) LZ positive gene [NM_002480.1] sequence is publicly available but that of the human MYPT1 LZ negative gene is not. Therefore, to design specific primers against the human LZ negative MYPT1 isovariant, the MYPT1 LZ positive sequence was aligned to the known chicken (NM_205123) and mouse (NM_027892) LZ negative genes. The conserved 31-nucleotide segment ‘GTGTCCGGCAAGAGTCAGTATCTTCTGGGCG’ present in the chicken and mouse MYPT1 LZ negative gene was added to human LZ positive sequence. This shifted the open reading frame to generate a putative human MYPT1 LZ negative read. A sense primer was directed against the 31-nucleotide insert (see Table II). Primer oligonucloetide sequences and the position of forward and reverse primers are given in Table II. Quantitative PCR (50 cycles) with PerfectProbe amplification (Primer Design, UK) was used to measure mRNA expression of MYPT1 LZ+ and MYPT1 LZ− isovariants. Ten microlitres of sample cDNA, run in triplicate, were used as a template for each 25 μl PCR. Standard curves were generated using human reference smooth muscle cDNA (product # 636547, Clontech, France), which also served as in calibrator for fold-change in gene expression assessment. Real-time PCR (50 cycles) with primer specific probes (Perfect Probes, PrimerDesign) was performed using the following parameters: enzyme activation hot start at 95°C for 10 min, denaturing at 95°C for 15 s and extension at 60°C for 60 s. The sample triplicates were within 1 Ct of each other and were >10 Ct values different from no template or water-only controls.
Table II.
Human MYPTLZ+ and LZ− sequences and qPCR primers.
 |
MYPTLZ+ and LZ− sequences with isovariant-specific primer sets (indicated by asterisks) and intended amplicon regions (shaded areas) are displayed next to the summary plots of mRNA expression calculated with respect to internal calibrator control RNA. The underlined sequence of the MYPTLZ− panel indicates the 31-nucleotide insert that results in an open reading frame that encodes for an LZ− isovariant.
Statistics
Data were assessed for normal distribution and analysed in GraphPad Prism 4.0. The temporal effects of 8-Br-cGMP were compared between artery types using two-way ANOVA followed by Bonferroni post hoc test. Unpaired t-tests were used to compare (i) the maximal vasodilatory effects of bradykinin or histamine between preconstricted placental and myometrial arteries; (ii) the t1/2 (time to half maximal relaxation) for 8-Br-cGMP effects in permeabilized placental and myometrial arteries and (iii) the mRNA or protein expression patterns between placental and myometrial arteries. Data are expressed as mean ± SEM, n = number of patients and significance is taken as P < 0.05.
Results
Differing relaxant actions of endothelial-dependent agonists on human placental or myometrial arteries
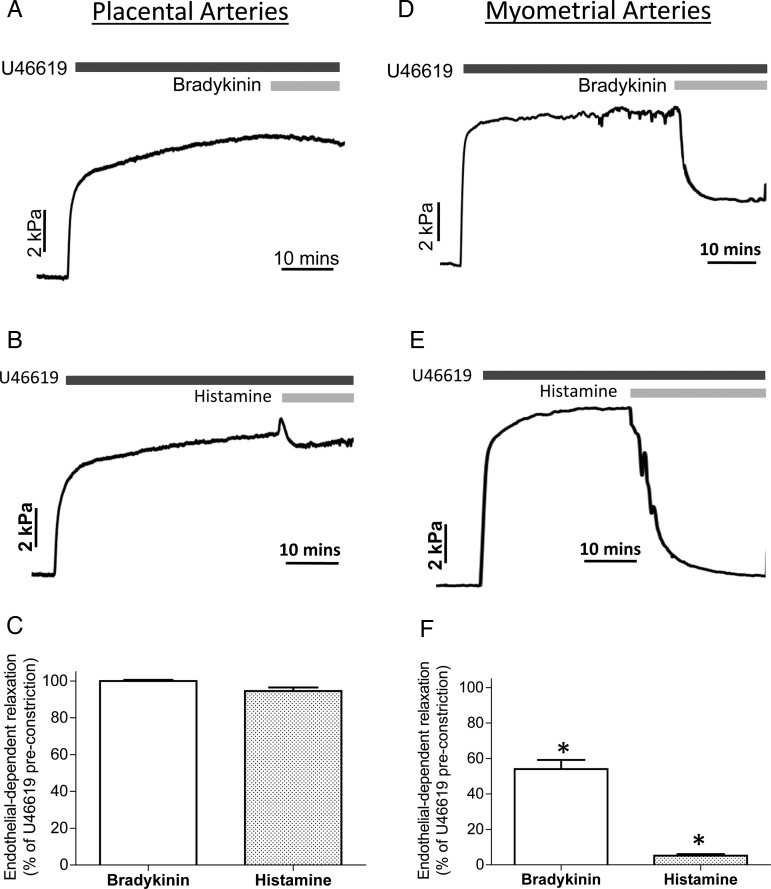
Figure 1 shows original tracings of intact human placental (A and B) and myometrial arteries (D and E) constricted with the thromboxane mimetic U46619 followed by exposure to the endothelial-dependent agonists bradykinin or histamine. In placental arteries, bradykinin or histamine had a negligible effect. This was in contrast to myometrial arteries that relaxed substantially to each agent. Overall, placental arteries (C) did not relax significantly to bradykinin (3.9 ± 1.1%, n = 12) or histamine (8.3 ± 0.9%), whereas myometrial arteries (F) relaxed significantly by 34 ± 1.9% (n = 12) and 95 ± 1.9%, respectively. A similar pattern was seen when arteries were preconstricted with KPSS.
Figure 1.
Differential effects of endothelial-dependent agonists on human placental and myometrial arteries. Placental (A–C) or myometrial (D–F) arteries were preconstricted with U46619 and exposed to the endothelial-dependent agonists bradykinin or histamine. Significant relaxation to either agent was only achieved in myometrial arteries. Raw tracings are displayed in (A)–(D), summary data (n = 6) in (E) and (F).
8-Br-cGMP induces greater myofilament Ca2+-desensitization in human myometrial than placental arteries
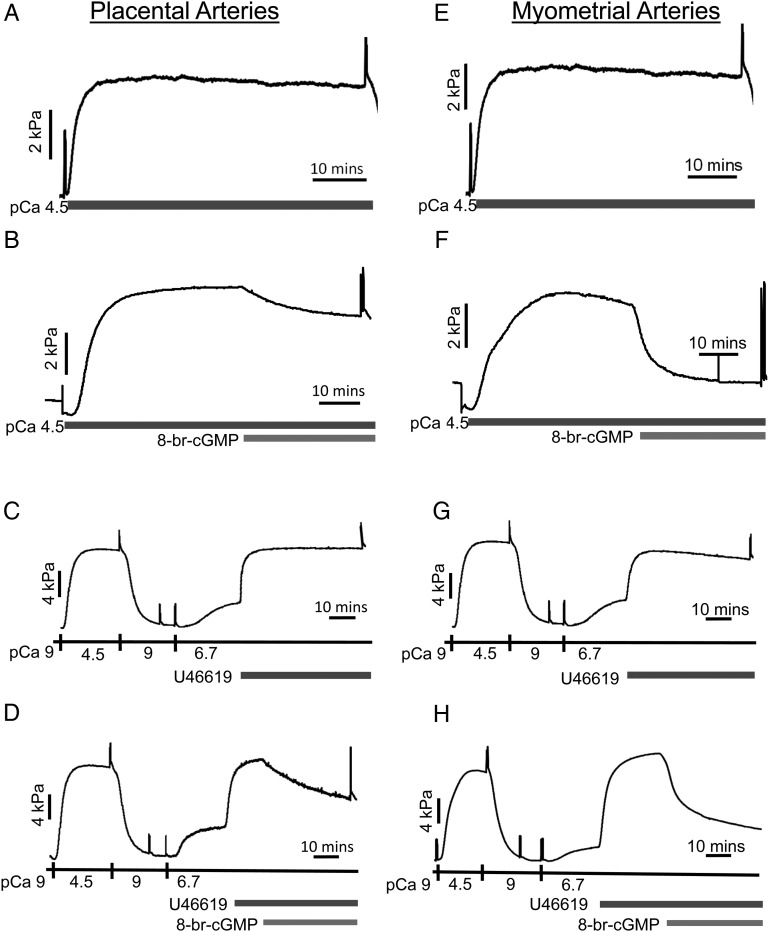
We next investigated whether placental artery myofilament responsiveness to PKG stimulation was different to that of myometrial arteries by examining the effect of 8-Br-cGMP on α-toxin-permeabilized vessels. Figure 2 shows original tracings of individual permeabilized human placental (A–D) and myometrial (E–H) arteries. Those exposed to maximal Ca2+-activating solution (pCa 4.5) produced a sustained constriction. Subsequent addition of 10 µM 8-br-cGMP induced relaxatory responses in placental arteries (B) that were less than those in myometrial arteries (F).
Figure 2.
Differential relaxatory actions of 8-Br-cGMP on force of permeabilized human placental and myometrial arteries. Raw data tracings of permeabilized placental arteries (A–D) and myometrial arteries (E–H) are presented. Permeabilized arteries constricted on exposure to Ca2+-activating pCa 4.5 solution (from basal pCa9 solution). Addition of 8-Br-cGMP relaxed the placental artery of (B) by 20%, yet the same treatment of the myometrial artery of (F) gave a substantially greater relaxation. Time control constrictions to pCa 4.5 solution in the absence of 8-Br-cGMP were maintained (A and E). Addition of pCa6.7 activating solution produced a sub-maximal constriction and addition of U46619 to pCa6.7 solution increased force further, indicative of agonist-induced Ca2+-sensitization of contraction (C and D; G and H). Addition of 8-Br-cGMP relaxed the placental artery of (D) 35%, yet the same treatment gave a markedly greater relaxation of the myometrial artery of (H). Time control constrictions to pCa6.7/U46619 in the absence of 8-Br-cGMP were maintained (C and G).
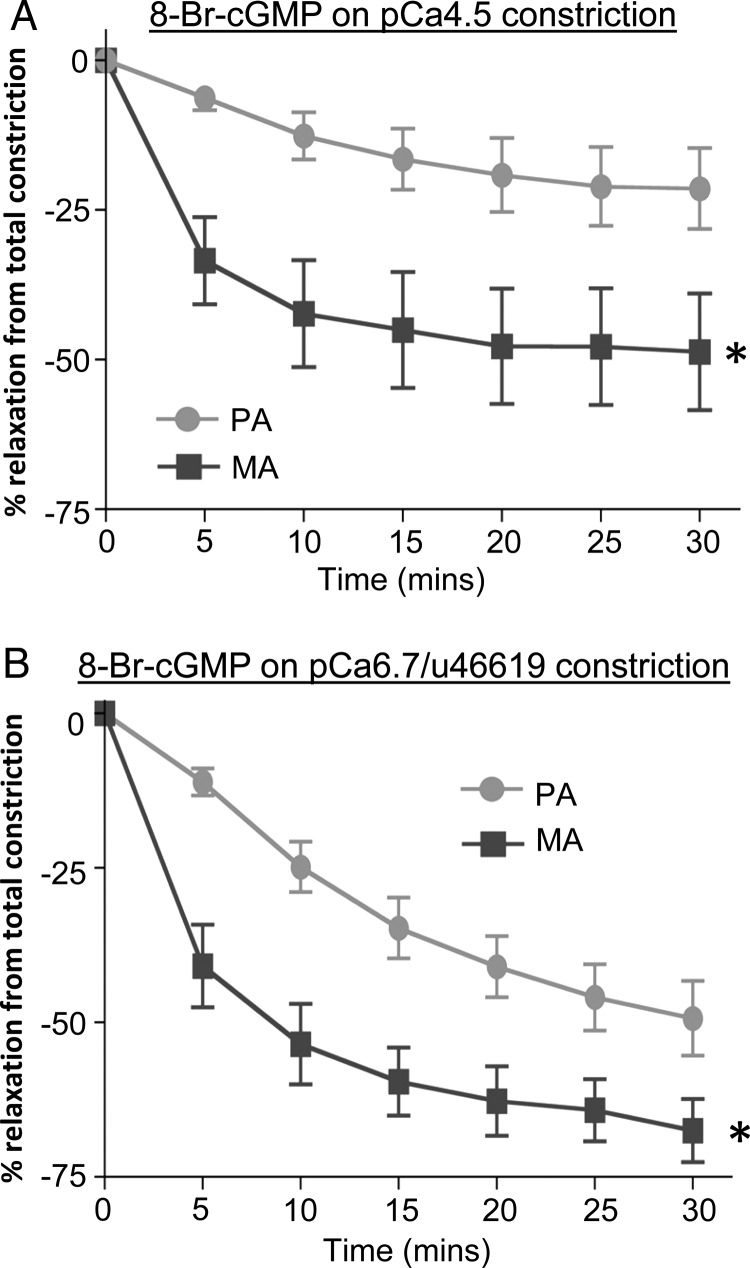
It was of interest to establish whether the phenomenon of cGMP-induced Ca2+-desensitization also occurred in human arteries constricted by a process of G-protein-coupled Ca2+-sensitization. Figure 2 also shows the effect of 10 μM 8-br-cGMP on individual permeabilized placental (D) and myometrial (H) arteries pre-sensitized with the thromboxane mimetic U46619. When exposed to pCa 6.7 activating solution, arteries produced sub-maximal contractions compared with a preceding pCa 4.5 contraction. Subsequent addition of 1 μM U46619 led to further development of force, at the same pCa 6.7 solution, to levels approaching that achieved with the pCa 4.5 solution alone: this indicated Ca2+-sensitization of force. Subsequent addition of 10 µM 8-br-cGMP again induced a relaxation of placental arteries (D) that was less than that of myometrial arteries (H). Overall (Fig. 3A), the relaxation to 8-Br-cGMP in pCa 4.5-constricted placental arteries (23 ± 6.6%, n = 6) was significantly less than that occurring in myometrial arteries (49 ± 9.7%, n = 7). Additionally, the rate of relaxation was slower in placental arteries (t1/2: 8.75 ± 0.33 min) than in myometrial arteries (t1/2: 3.66 ± 0.19 min). Similarly in arteries pre-sensitized to pCa 6.7 and U44619 (Fig. 3B), the mean maximal relaxation to 10 µM 8-br-cGMP in placental arteries (49 ± 6.0%, n = 6) was significantly less than in myometrial arteries (70 ± 5.1%, n = 8). Additionally, the rate of relaxation in placental arteries (t1/2:10.2 ± 1.02 min) was slower than in myometrial arteries (t1/2: 4.67 ± 0.43 min). Similar results were obtained with the use of endothelin-1 as the Ca2+-sensitizing constrictor.
Figure 3.
Summary of the differential relaxations of permeabilized placental and myometrial arteries to 8-Br-cGMP. (A) In pCa 4.5 preconstricted vessels, the magnitude and the rate of relaxation upon 8-Br-cGMP exposure were less for placental than for myometrial arteries. *Significant differences between placental arteries (PA, n = 6) and myometrial arteries (MA, n = 7). (B) In pCa6.7/U46619 preconstricted vessels, the magnitude and the rate of relaxation upon 8-Br-cGMP exposure were less for placental than for myometrial arteries. *Significant differences between placental arteries (PA, n = 6) and myometrial arteries (MA, n = 8).
Expression of total MYPT1 protein, or MYPT1 LZ+/LZ− transcripts, does not vary between human placental and myometrial arteries
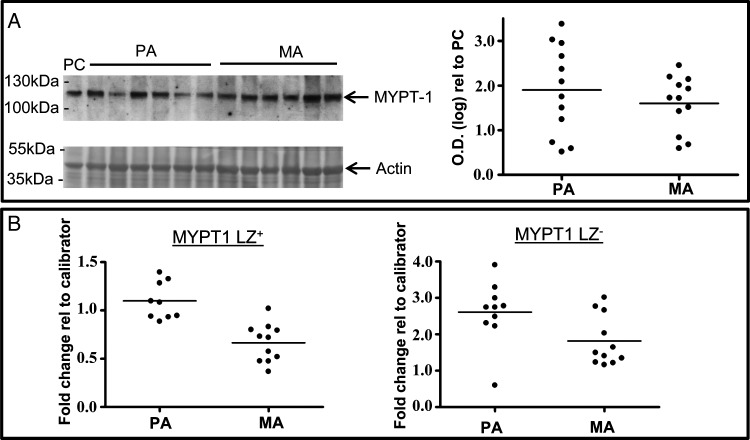
The total expression of MYPT1 protein did not vary between placental and myometrial arteries (Fig. 4A). On examining MYPT1 LZ+/LZ− transcript expression by QPCR it was found that MYPT1LZ− expression (Fig. 4B) did not vary between placental arteries (Ct 25.78 ± 0.08, n = 9) and myometrial arteries (Ct 26.49 ± 0.16, n = 11). Similarly, there was no difference in MYPT1LZ+ expression (Fig. 4B) between placental arteries (Ct 25.68 ± 0.10) and myometrial arteries (Ct 26.42 ± 0.13) nor was there a change in the ratio of MYPT1 LZ−/LZ+ expression between the artery types.
Figure 4.
MYPT1 expression is invariant between human placental and myometrial arteries. (A) Raw data from a western blot comparing the expressions of MYPT1 protein between placental and myometrial arteries are displayed next to the scatter plot of densitometric values for each sample calculated with respect to an internal positive control (PC, human myometrium). Total MYPT protein was similar in both artery types. (B) The expression of MYPTLZ+ or MYPTLZ− isovariants were similar for placental and myometrial arteries. PA, placental arteries; MA, myometrial arteries.
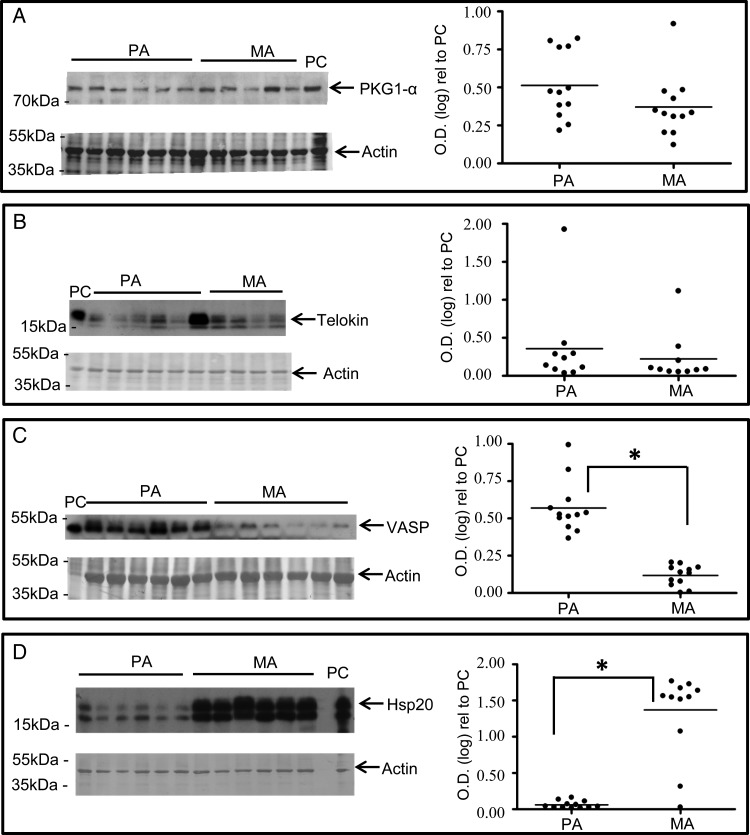
Expression of Hsp20 is less in placental arteries than myometrial arteries
It was thus of interest to investigate whether the expression of other putative PKG-interacting myofilament-associated proteins, or of PKGIα itself, varied between the artery types in a manner that correlated to the altered PKG responsiveness. Figure 5A–C illustrates that the expression of PKGIα, telokin or VASP were not elevated in myometrial arteries compared with placental arteries. Indeed, the expression of VASP was significantly elevated in placental arteries rather than myometrial arteries. Notably, however, the expression of Hsp20 was significantly less in placental arteries than myometrial arteries (Fig. 5D).
Figure 5.
The expression of the Hsp20 is elevated in myometrial arteries compared with placental arteries. (A–D) Raw data from western blots comparing the expressions of PKG1α, telokin, VASP or Hsp20 between placental and myometrial arteries are displayed next to the scatter plots of densitometric values for each sample and protein of interest calculated with respect to an internal positive control (PC). PKG1α (A) or telokin (B) was invariant between the artery types. VASP (C) expression was significantly greater in placental arteries than myometrial arteries. Only Hsp20 (D) expression was significantly higher in myometrial arteries than placental arteries. Positive control: rat aorta (A), rat brain (B), Hela cell lysate (C) and human myometrium (D). PA, placental arteries; MA, myometrial arteries.
Discussion
The mechanisms responsible for controlling vascular tone within the placental and uterine circulations of pregnant women are poorly understood. In this study we report important differences in the manner in which vasodilatory influences affect the relaxation of isolated human placental and myometrial arteries. This contributes to an evolving picture whereby circulatory, or locally produced, factors may regulate human uteroplacental vascular tone in a tissue-specific manner (e.g. Corcoran et al., 2012).
We found that preconstricted human placental arteries evinced markedly less vasodilation to endothelial-dependent agonists than myometrial arteries. This was the case when the arteries were preconstricted with two different stimulants, or exposed separately to two endothelial-dependent agonists, indicating that it is likely to be a generalized phenomenon. Indeed, although this is the first study to compare directly under similar experimental conditions such relaxatory influences, there are a number of reports investigating each artery type separately that supports this finding (Wareing et al., 2005; Hudson et al., 2007). There are a number of possible contributory mechanisms to these findings. The tissue-specific differences persisted when tissues were preconstricted in a manner that negated any possible relaxatory influence of endothelial-dependent hyperpolarizing factor (i.e. with depolarizing KPSS solution). Any endothelial-dependent relaxation was therefore likely to be due to NO-dependent activation of PKG. It could be that the endothelial cell expression of receptors for histamine and bradykinin (and others) that stimulate NO production are reduced in placental than myometrial arteries. In addition, however, we reported previously that permeabilization of human placental and myometrial arteries with α-toxin enabled examination of the mechanisms of myofilament activation by Ca2+ and/or agonists that induced Ca2+-sensitization of force (Wareing et al., 2005; Hudson et al., 2007). Thus, we made use of this technique to pursue our hypothesis that PKG-mediated changes in myofilament activation may differ between human placental and myometrial arteries.
Indeed, permeabilized human myometrial arteries exhibited greater relaxations to 8-Br-cGMP than placental arteries. This was independent of myofilament activation by Ca2+ alone (pCa 4.5 solution), or by agonist-mediated Ca2+-sensitization (sub-maximal pCa 6.7 solution plus U46619 or endothelin-1), indicating, again, that it was likely to be a genuine phenomenon distinguishing both artery types.
This is the first time that PKG-mediated Ca2+-desensitization of force has been reported to differ between human arteries. As these vessels are from two organs whose physiological function is intimately associated, in this case facilitating the matching of blood perfusion between the uterus and placenta to ensure healthy fetal growth and pregnancy outcome, it alerts one to be cautious in extrapolating general mechanisms of vasodilation from one vessel type to another. In several animal studies, there have been suggestions that alterations in PKG-dependent relaxation mechanisms may relate to altered expression of myofilament-associated proteins such as MYPT1 LZ isovariants, telokin, VASP or Hsp20 (Surks et al., 1999; Rembold et al., 2000; Choudhury et al., 2004; Karim et al., 2004; Payne et al., 2004; Batts et al., 2005; Khromov et al., 2006; Lu et al., 2008; Fischer, 2010; Yuen et al., 2011; Ying et al., 2012). It was therefore possible that the differences we observed in this study may be explicable by tissue-dependent changes in the expression of such myofilament-associated proteins especially as human placental artery vasoreactivity has been suggested to be more tonic in nature than myometrial arteries (Sweeney et al., 2008). However, rather surprisingly in light of much data from animal tissues to suggest a correlation between relative MYPT1LZ+ expression and NO/PKG-mediated relaxations, we found no difference in MYPT1LZ+/LZ− isovariants in human placental and myometrial arteries. Nor were the expressions of telokin or PKG1α different between the artery types. The expression of VASP was significantly higher in placental arteries than myometrial arteries but this is the opposite to what would be expected if VASP expression was a predominant determinant of PKG sensitivity in these vessels. Of each of the myofilament-associated proteins tested, only Hsp20 expression correlated with the tissue-specific PKG sensitivity that we observed; that is, Hsp20 expression was elevated in myometrial arteries compared with placental arteries. Batts et al. (2005) have previously noted a correlation between low Hsp20 expression in rabbit bladder smooth muscle and insensitivity to relaxation by PKG stimulation.
Human umbilical artery smooth muscle is also poorly sensitive to stimulation of cyclic nucleotides (Brophy et al., 1997). This has been attributed to an inability of cyclic nucleotides to phosphorylate the ser-16 site thought to be responsible for Hsp20-mediated relaxation. Thus, a determinant of the differing myofilament sensitivities to PKG-mediated stimulation of human placental and myometrial arteries reported herein may not just lie at the level of protein expression but also at the extent of myofilament protein (de)activation by PKG. Indeed, it is noteworthy that the rates of relaxation to 8-Br-cGMP in permeabilized human placental arteries were slower than in myometrial arteries. This points to PKG exerting different influences on myofilament dynamics in each vessel type. Thus, although beyond the scope of this project, it will be of considerable interest in future work to determine whether human placental and myometrial arteries differ in the extent of PKG-mediated phosphorylation of Hsp20 and other myofilament-interacting protein targets. In turn, this may depend upon the resident state of post-translational modification of the PKG-interacting targets. Finally, differences in the spatial arrangement of smooth muscle cells between human myometrial and placental arteries have been noted (Sweeney et al., 2008). It is unclear how this may contribute to the tissue-specific differences in relaxation to 8-Br-cGMP observed in permeabilized arteries unless it influences cell-to-cell mechanical coupling.
Efficient utero-placental blood perfusion is required for a successful pregnancy outcome and, as such, it is imperative to understand the mechanisms by which vascular tone in the placenta and uterus may be regulated. The present study is important for identifying that (i) endothelial-dependent agonists differentially regulate the tone of isolated human placental and uterine arteries; (ii) the extent of PKG-mediated myofilament Ca2+-desensitization may contribute to these responses and (iii) Hsp20 expression levels correlate with the tissue-specific PKG-mediated vasodilatory responses. It is also informative to our future considerations of how the vasodilatory reserve of the placental and/or uterine vasculatures may be challenged in pregnancies compromised by elevated vascular resistance, for example pre-eclampsia, and what new approaches may be developed to correct this.
Authors' roles
A.C.D. and M.S. performed the permeabilized tissue experiments and analysed the data. J.L. and H.W. performed the western blot experiments and analysed the data. J.L. performed the QPCR experiments and analysed the data. S.C.R. and M.J.T. conceived and designed the study. M.J.T. prepared the manuscript and all authors had input to the writing and approved the final version.
Funding
The study was supported by a Wellcome Trust/Academy of Medical Sciences Clinical Lecturer grant to J.L. (SGCL Round2 Lartey) and Newcastle upon Tyne NHS Trust Flexibility and Sustainability Funding.
Conflict of interest
None declared.
Acknowledgements
We are grateful to the patients who kindly took part in this study and for the assistance of the midwifery and theatre staff of Newcastle Royal Victoria Infirmary.
References
- Amarani S, Sangfrat B, Chaudhuri G. Effects of selected endothelium-dependent vasodilators on fetoplacental vasculature: physiological implications. Am J Physiol. 1999;277:H842–H847. doi: 10.1152/ajpheart.1999.277.2.H842. [DOI] [PubMed] [Google Scholar]
- Batts TW, Walker JS, Murphy RA, Rembold CM. Absence of force suppression in rabbit bladder correlates with low expression of heat shock protein 20. BMC Physiol. 2005;5:16. doi: 10.1186/1472-6793-5-16. doi:10.1186/1472-6793-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benirschke K, Kaufmann P. Pathology of the Human Placenta. 4th edn. New York: Springer; 2000. [Google Scholar]
- Boura AL, Leitch IM, Read MA, Walters AW. The control of fetal vascular resistance in the human placenta. Trophoblast Res. 1998;11:299–313. [Google Scholar]
- Brophy CM, Beall A, Lamb S, Dickinson M, Ware DJ. Small heat shock proteins and vasospasm in human umbilical artery smooth muscle. Biol Reprod. 1997;57:1354–1359. doi: 10.1095/biolreprod57.6.1354. doi:10.1095/biolreprod57.6.1354. [DOI] [PubMed] [Google Scholar]
- Choudhury N, Khromov AS, Somlyo AP, Somlyo AV. Telokin mediates Ca2+-desensitization through activation of myosin phosphatase in phasic and tonic smooth muscle. J Muscle Res Cell Motil. 2004;25:657–665. doi: 10.1007/s10974-004-7807-x. doi:10.1007/s10974-004-7807-x. [DOI] [PubMed] [Google Scholar]
- Corcoran J, Charnock JC, Martin J, Taggart MJ, Westwood M. Differential effect of insulin like growth factor-I on constriction of human uterine and placental arteries. J Clin Endocrinol Metab. 2012;97:E2098–E2104. doi: 10.1210/jc.2012-1679. doi:10.1210/jc.2012-1679. [DOI] [PubMed] [Google Scholar]
- Fischer SA. Vascular smooth muscle phenotypic diversity and function. Physiol Genomics. 2010;42A:169–187. doi: 10.1152/physiolgenomics.00111.2010. doi:10.1152/physiolgenomics.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SB, Khong TY. Lack of innervation of human umbilical cord. An immunohistological and histochemical study. Placenta. 1990;11:59–62. doi: 10.1016/s0143-4004(05)80443-6. doi:10.1016/S0143-4004(05)80443-6. [DOI] [PubMed] [Google Scholar]
- Horiutu K. Mechanisms o contracture on cooling of caffeine-treated frog skeletal muscle fibres. J Physiol. 1998;398:131–148. doi: 10.1113/jphysiol.1988.sp017034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson NK, O'Hara M, Lacey HA, Corocoran J, Hemmings DG, Wareing M, Baker P, Taggart MJ. Modulation of human arterial tone during pregnancy: the effect of the bioactive metabolite sphingosine-1-phosphate. Biol Reprod. 2007;77:45–52. doi: 10.1095/biolreprod.107.060681. doi:10.1095/biolreprod.107.060681. [DOI] [PubMed] [Google Scholar]
- Hull AD, White CR, Pearce WJ. Endothelium-derived relaxing factor and cyclic GMP-dependent vasorelaxation in human chorionic plate arteries. Placenta. 1994;15:365–375. doi: 10.1016/0143-4004(94)90004-3. doi:10.1016/0143-4004(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Karim SM, Rhee AY, Given AM, Faulx MD, Hoit BD, Brozovich FV. Vascular reactivity in heart failure: role of myosin light chain phosphatase. Circ Res. 2004;95:612–618. doi: 10.1161/01.RES.0000142736.39359.58. doi:10.1161/01.RES.0000142736.39359.58. [DOI] [PubMed] [Google Scholar]
- Khromov AS, Wang H, Choudhury N, McDuffie M, Herring BP, Nakamoto R, Owens GK, Somlyo AP, Somlyo AV. Smooth muscle of telokin-deficient mice exhibits increased sensitivity to Ca2+ and decreased cGMP-induced relaxation. Proc Natl Acad Sci USA. 2006;103:2440–2445. doi: 10.1073/pnas.0508566103. doi:10.1073/pnas.0508566103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kublickiene KR, Nisell H, Poston L, Kruger K, Lindblom B. Modulation of vascular tone by nitric oxide and endothelin 1 in myometrial resistance arteries from pregnant women at term. Am J Obstet Gynecol. 2000;182:87–93. doi: 10.1016/s0002-9378(00)70495-9. doi:10.1016/S0002-9378(00)70495-9. [DOI] [PubMed] [Google Scholar]
- Lee MR, Li L, Kitazawa T. Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J Biol Chem. 1997;272:5063–5068. doi: 10.1074/jbc.272.8.5063. doi:10.1074/jbc.272.8.5063. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhang H, Gokina N, Mandala M, Sato O, Ikebe M, Osol G, Fisher SA. Uterine artery myosin phosphatase isoform switching and increased sensitivity to SNP in a rat L-NAME model of hypertension of pregnancy. Am J Physiol. 2008;294:C564–C571. doi: 10.1152/ajpcell.00285.2007. doi:10.1152/ajpcell.00285.2007. [DOI] [PubMed] [Google Scholar]
- Mills TA, Taggart MJ, Greenwood SL, Baker PN, Wareing M. Histamine-induced contraction and relaxation of placental chorionic plate arteries. Placenta. 2007;28:1158–1164. doi: 10.1016/j.placenta.2007.05.008. doi:10.1016/j.placenta.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Morgado M, Cairrão E, Santos-Silva AJ, Verde I. Cyclic nucleotide-dependent relaxation pathways in vascular smooth muscle. Cell Mol Life Sci. 2012;69:247–266. doi: 10.1007/s00018-011-0815-2. doi:10.1007/s00018-011-0815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne MC, Zhang H, Shirasawa Y, Koga Y, Ikebe M, Benoit JN, Fisher SA. Dynamic changes in expression of myosin phosphatise in a model of portal hypertension. Am J Physiol. 2004;286:H1801–H1810. doi: 10.1152/ajpheart.00696.2003. [DOI] [PubMed] [Google Scholar]
- Poston L, McCarthy AL, Ritter JM. Control of vascular resistance in the maternal and feto-placental arterial beds. Pharmacol Ther. 1995;65:215–239. doi: 10.1016/0163-7258(94)00064-a. doi:10.1016/0163-7258(94)00064-A. [DOI] [PubMed] [Google Scholar]
- Reilly RD, Russell PT. Neurohistochemical evidence supporting an absence of adrenergic and cholinergic innervation in the human placenta and umbilical cord. Anat Rec. 1977;188:277–286. doi: 10.1002/ar.1091880302. doi:10.1002/ar.1091880302. [DOI] [PubMed] [Google Scholar]
- Rembold CM, Foster DB, Strauss JD, Wingard CJ, Van Eyk JE. cGMP-mediated phosphorylation of heat shock protein 20 may cause smooth muscle relaxation without myosin light chain dephosphorylation in swine carotid artery. J Physiol. 2000;524:865–878. doi: 10.1111/j.1469-7793.2000.00865.x. doi:10.1111/j.1469-7793.2000.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossmann J, Feil F, Hofmann F. Signaling through NO and cGMP-dependent protein kinases. Trends Mol Med. 2003;35:21–27. doi: 10.1080/07853890310004093. [DOI] [PubMed] [Google Scholar]
- Spitaler MM, Hammer A, Malli R, Graier WF. Functional analysis of histamine receptor subtypes involved in endothelium-mediated relaxation of the human uterine artery. Clin Exp Pharmacol Physiol. 2002;29:711–716. doi: 10.1046/j.1440-1681.2002.03704.x. doi:10.1046/j.1440-1681.2002.03704.x. [DOI] [PubMed] [Google Scholar]
- Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelshon ME. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha. Science. 1999;286:1583–1587. doi: 10.1126/science.286.5444.1583. doi:10.1126/science.286.5444.1583. [DOI] [PubMed] [Google Scholar]
- Sweeney M, Wareing M, Mills TA, Baker PN, Taggart MJ. Characterisation of tone oscillations in placental and myometrial arteries from normal pregnancies and those complicated by pre eclampsia and growth restriction. Placenta. 2008;29:356–365. doi: 10.1016/j.placenta.2008.01.007. doi:10.1016/j.placenta.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Wareing M, Crocker IP, Warren A, Taggart MJ, Baker PN. Characterisation of small arteries isolated from human placental chorionic plate. Placenta. 2002;23:400–409. doi: 10.1053/plac.2002.0825. doi:10.1053/plac.2002.0825. [DOI] [PubMed] [Google Scholar]
- Wareing M, O'Hara M, Seghier F, Baker PN, Taggart MJ. The involvement of Rho-associated kinases in agonist-dependent contractions of human maternal and placental arteries at term gestation. Am J Obstet Gynecol. 2005;193:814–824. doi: 10.1016/j.ajog.2005.02.076. [DOI] [PubMed] [Google Scholar]
- Wimalasundera RC, Thom SA, Regan L, Hughes AD. Effects of vasoactive agents on intracellular calcium and force in myometrial and subcutaneous resistance arteries isolated from preeclamptic, pregnant, and nonpregnant woman. Am J Obstet Gynecol. 2005;192:625–632. doi: 10.1016/j.ajog.2004.07.040. doi:10.1016/j.ajog.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Wu X, Somlyo AV, Somlyo AP. Cyclic GMP-dependent stimulation reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphate. Biochem Biophys Res Commun. 1996;220:658–663. doi: 10.1006/bbrc.1996.0460. doi:10.1006/bbrc.1996.0460. [DOI] [PubMed] [Google Scholar]
- Ying L, Xiaojian X, Liu J, Dou D, Yu X, Ye L, He Q, Gao Y. Heterogeneity in relaxation of different sized porcine coronary arteries to nitrovasodilators: role of PKG and MYPT1. Pflugers Arch. 2012;463:257–268. doi: 10.1007/s00424-011-1040-4. doi:10.1007/s00424-011-1040-4. [DOI] [PubMed] [Google Scholar]
- Yuen S, Ogut O, Brozovich FV. MYPT1 protein isoforms are differentially phosphorylated by protein kinase G. J Biol Chem. 2011;286:37274–37279. doi: 10.1074/jbc.M111.282905. doi:10.1074/jbc.M111.282905. [DOI] [PMC free article] [PubMed] [Google Scholar]