Abstract
CD8+ cells can suppress human immunodeficiency virus 1 (HIV-1) replication by releasing soluble factors. In 26 years of intensive research efforts, the identity of the major CD8+ cell antiviral factor has remained elusive. To investigate the mechanism for this antiviral immune response, we performed gene expression analyses on primary CD4+ cells that were exposed to HIV-suppressing CD8+ cells or CD8+ cell-conditioned medium having HIV-suppressing activity. These experiments revealed increased levels of multiple genes stimulated by type I interferons (IFN; eg, IFN-α and IFN-β). Further evaluation revealed that primary CD8+ cells, particularly those from elite controllers and other asymptomatic HIV-1-infected individuals, secrete IFN, and this response directly contributes to the in vitro suppression of HIV replication in CD4+ cells. This novel immune response, likely mediated by memory CD8+ T cells, may play an important role in a wide variety of viral infections, cancers, and autoimmune diseases.
Introduction
In early efforts to isolate the AIDS-associated retrovirus (later named human immunodeficiency virus 1; HIV-1) from the blood of asymptomatic individuals, CD8+ lymphocytes were discovered to inhibit the replication of this virus in vitro (Walker and others 1986). This CD8+ cell antiviral activity was found to suppress the replication of divergent strains of HIV and simian immunodeficiency virus (SIV) (Walker and others 1991b) and did not correlate with cytotoxic T lymphocyte activity (Walker and others 1991a; Mackewicz and others 2003b; Killian and others 2011) or apoptosis-induced cell death (Mackewicz and others 2000). Importantly, this CD8+ cell noncytotoxic antiviral response (CNAR) involved the release of an unidentified soluble CD8+ cell antiviral factor (CAF) (Walker and Levy 1989).
The CD8+ CNAR plays a critical role in controlling HIV-1 replication in vivo (Davenport and Petravic 2010; Killian and others 2011). CNAR becomes detectable during primary HIV-1 infection and is correlated a temporal decline in peak viremia (Killian and others 2009). Strong CNAR activity is a feature of asymptomatic HIV-1-infected individuals (Mackewicz and others 1991; Castelli and others 2002), including those who are long-term survivors (Barker and others 1998). Uninfected individuals and HIV-1-infected persons who progress to AIDS or are receiving antiretroviral therapy generally exhibit little or no CNAR activity (Killian and others 2005). However, CNAR returns upon the discontinuation of antiretroviral therapy and is again temporally associated with a reduced viral load set point (Killian and others 2009). Additionally, the viral replication kinetics after the in vivo depletion of CD8+ cells evidence a vital role for CNAR in SIV-infected rhesus macaques (Klatt and others 2010; Wong and others 2010).
CAF is distinct from the anti-HIV factors that are known to be produced by CD8+ cells, including β-chemokines (Mackewicz and others 1994; Leith and others 1997; Geiben-Lynn and others 2001). Its activity inhibits HIV transcription while having little effect on other stages of the virus life cycle, such as entry into the cell and integration into the host cell genome (Copeland and others 1995; Mackewicz and others 1995). Thus, CAF is not among the most recently described CD8+ cell anti-HIV factors (Cocchi and others 2012). Indeed, the identity of CAF and its precise mechanism for suppressing HIV replication have remained unclear.
We began these studies with the premise that the mechanism of the CD8+ cell anti-HIV response could be revealed by fine analysis of the acted-upon CD4+ target cells. These studies led to the direct identification of a novel immune response having features of both innate and adaptive immunity. Here, we report the finding that CD8+ cells from HIV-infected individuals secrete type I interferons (IFN; eg, IFN-α and IFN-β), and that the release of these cytokines directly contributes to CAF and CNAR activity.
Materials and Methods
Study subjects
The HIV-1-infected subjects in this study were participants in our cohort of long-term survivors at the University of California San Francisco (UCSF) (Castelli and others 2002). These HIV-1-infected individuals were asymptomatic men who were not receiving antiretroviral therapy and had >400 CD4+ T cells/mL of blood. Some of these subjects were elite controllers of HIV-1 infection, who exhibit very low viral loads (<50 HIV RNA copies/mL of plasma) in the absence of antiretroviral therapy (Deeks and Walker 2007). Blood from healthy uninfected individuals was purchased from Blood Centers of the Pacific. Each participant signed informed consent documents, and this study received approval from the UCSF Committee on Human Research.
Cell specimens
All experiments and assays in this report were performed with primary human cells and/or fluids from primary cell cultures. To obtain these cells, whole-blood samples were collected in evacuated blood tubes (BD) containing heparin. Peripheral blood mononuclear cells (PBMC) were isolated by density-gradient separation over Ficoll (Sigma). CD4+ and CD8+ cells were isolated from PBMC by positive selection using immunomagnetic beads (Miltenyi or Dynal) (Killian and others 2005).
Cocultures of CD8+ and CD4+ cells
CD8+ cell noncytotoxic anti-HIV activity was assessed as the ability of CD8+ cells to suppress HIV replication in primary CD4+ cells as previously described (Killian and others 2005, 2011). Briefly, purified CD4+ cells were stimulated with phytohemagglutanin (PHA) (3 μg/mL; Sigma) for 3 days and then acutely infected with the β-chemokine-resistant strain HIV-1SF33 (104 TCID50 per 107 cells/mL) (Mackewicz and others 1997). HIV-1SF33 was used in the coculture experiments to maintain consistency with previous studies (Killian and others 2011). Unstimulated CD8+ cells were cocultured with autologous HIV-infected CD4+ cells at 1:1 cell input ratios at a total cell density of 2×106 cells/mL of a complete medium [RPMI-1640, 10% fetal bovine serum (FBS), antibiotics, 100 U/mL recombinant interleukin-2 (rIL-2), and 2 mM l-glutamine]. HIV replication levels were determined by measuring the reverse transcriptase (RT) activity present in the cell culture supernatants as described previously (Killian and others 2011). CD8+ cell anti-HIV activity (percent suppression) was evaluated as a ratio of the RT activity in wells containing CD4+ cells cultured in the presence and absence of CD8+ cells.
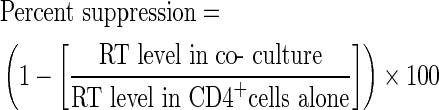 |
Production of CD8+ cell-conditioned medium
CD8+ cell fluids were produced as previously described (Mackewicz and others 2003a). Briefly, CD8+ cells were purified from PBMC using detachable immunomagnetic beads (Dynal), and then stimulated with anti-CD3-coupled beads (4:1 bead/cell ratio). After 3 days of stimulation in a complete medium (see above), the beads were removed, and the CD8+ cells were transferred to a serum-free F12 medium (Gibco) supplemented with 1× insulin-selenium-transferrin (Life Technologies) and 200 U/mL rIL-2 (Invitrogen). During 10 days of culture, CD8+ cell culture fluids were collected every 2 days, and the cells were placed into a fresh medium (2×106 cells/mL). The CD8+ cell fluids were filtered (0.45 μm) and stored (−70°C). To assess the antiviral activity of the CD8+ cell fluids, mitogen-stimulated CD4+ cells were acutely infected (4,000 TCID50 per 107 cells) with a fully infectious molecular clone of HIV-1SF2, a β-chemokine-insensitive virus (Mackewicz and others 1997). The HIV-1SF2 molecular clone was used in experiments to evaluate the anti-HIV activity of CD8+ cell fluids to maintain consistency with previous studies (Mackewicz and others 2003a). After 1 h of spinoculation, the cells were washed and plated at 105 infected CD4+ cells per well in 96-well culture plates in the a complete RPMI medium (see above). The acutely infected CD4+ cells were cultured in the presence of a 50% dilution of a CD8+ cell culture fluid (or F12 control medium) in a final volume of 200 μL. The cultures, in triplicate, were passed every 2 days and monitored for viral RT activity (Hoffman and others 1985) by removing 100 μL of culture fluid for the assay. The medium collected for the RT assay was replaced with an equal volume of CD8+ cell supernatant or control medium (parallel cultures). The anti-HIV activity (percent suppression) of the CD8+ cell fluids was determined by calculating the ratio of the RT activities in CD4+ cell cultures containing CD8+ cell fluids and control medium. CD8+ cell fluids having appreciable anti-HIV activity were identified as those exhibiting ≥50% suppression of RT levels.
Transfection of primary CD8+ cells
Unstimulated primary CD8+ cells from healthy blood bank donors were transfected with plasmids (generously provided by Fiveprime) to overexpress type I (IFN-α) or type II (IFN-γ) IFN genes. Transfection was performed by electropulsing the plasmids into CD8+ cells using a NEON device (Invitrogen) as described (Liu and others 2011). The CD8+ cells were placed at 37°C for 24 h and then washed before their coculture with CD4+ cells at 1:1 cell input ratios. Primary CD8+ cells that were transfected with a control plasmid exhibited <20% suppression of HIV replication as previously described (Liu and others 2011).
Microarrays for gene transcript levels
CD4+ cells (with and without having been acutely infected with HIV-1SF33 as described above) were placed into 24-well tissue culture plates (106 cells/mL of complete growth medium) either alone or at a 1:1 ratio with autologous unstimulated CD8+ cells. The cells were collected after 24 h of culture, and the CD8+ cells were removed from the cocultured CD4+ cells by positive selection using an excess of anti-CD8 immunomagnetic beads (Dynal). The purified CD4+ cells contained <2% residual CD8+ cells as determined by flow cytometry (data not shown). RNA was isolated from the repurified CD4+ cells using RNAeasy kits (Qiagen) and analyzed for concentration and quality on a Nanodrop instrument. The RNA was reverse transcribed and converted to biotinylated cRNA (Ambion) in accordance with standardized Illumina protocols. The resulting cRNA was hybridized to Illumina whole-genome microarrays (HumanRef-8 v.3). Gene expression levels were measured using a BeadStation 500GX system (Illumina).
Flow cytometry
To measure intracellular IFN-α levels in unstimulated CD8+ cells, freshly isolated PBMC were resuspended in phosphate-buffered saline (PBS) containing 2% FBS and stained for 30 min at room temperature with anti-CD8-allophycocyanin (BD). Next, the cells were collected, washed with PBS/2% FBS, and fixed and permeabilized (BD Cytofix/Cytoperm). The cells were then stained with a phycoerythrin (PE)-labeled anti-IFN-α2 antibody (BD clone 7N4-1) or PE-labeled mouse-immunoglobulin G (IgG) at 4°C for 30 min. After the staining procedures, the cells were acquired on a FACSort (BD) cytometer, and analyses were performed using CellQuest (BD) software. Cursor settings were established based on the profiles of the isotype controls.
In separate assays, intracellular staining was performed to measure the levels of activated STAT proteins in CD4+ cells. PHA-stimulated CD4+ cells were incubated at 37°C for 15 min in the presence of human leukocyte interferon (HLI from PBL) or CD8+ cell fluids. Then, the cells were permeabilized, fixed, and stained with monoclonal antibodies specific for phosphorylated STAT1, phosphorylated STAT3, or IgG of the appropriate isotype in accordance with the manufacturer's (BD) protocols. Cell acquisitions and analyses were performed as described above.
Kinetic reverse transcriptase–polymerase chain reaction
To measure the levels of type I and type II IFN transcripts, quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) analysis was performed. RNA was extracted from unstimulated primary CD8+ cells, and analyzed by spectrophotometry for concentration and quality. The RNA was reverse transcribed and amplified using the SYBR® Green RT-PCR Reagents Kit (Applied Biosystems) according to the manufacturer's instructions. Each 25 μL PCR reaction contained oligonucleotide primers (200 nM; synthesized by Eurofins) that were designed to amplify the various IFN genes (primer sequences provided upon request). PCRs and data acquisitions were performed on a StepOne instrument (Applied Biosystems). Cycling conditions were as follows: initial denaturation at 95°C for 10 min, followed by 50 cycles at 95°C for 15 s, and 60°C for 1 min. The quantitative PCR data were analyzed with StepOne software program (Applied Biosystems) using the 2−ΔΔCT method (Livak and Schmittgen 2001).
Enzyme-linked immunosorbent assay and ELISpot assays
IFN-α (multisubtype), IFN-β, and IFN-ω levels in CD8+ cell fluids were measured by enzyme-linked immunosorbent assay (ELISA; PBL) in accordance with the manufacturer's protocols. In other experiments, CD8+ cells were assessed for their ability to secrete IFN-α using ELISpot assays (Mabtech) as directed.
Neutralization and blocking experiments
To neutralize IFN-α, rabbit anti-human IFN-α (PBL; anti-serum) or normal rabbit serum was added along with CD8+ cell fluids to cultures of acutely-HIV-infected CD4+ cells. To block the type I IFN receptor, mouse anti-IFNAR2 (PBL) or mouse-IgG was added along with CD8+ cell fluids to cultures of acutely-HIV-infected CD4+ cells. The HIV replication levels in these cultures were measured as described above.
High-performance liquid chromatography experiments
We performed high-performance liquid chromatography (HPLC) fractionation experiments to compare and assess the contribution of IFN to the anti-HIV activity of CD8+ cell fluids. Samples were concentrated by centrifugation using 10-kDa filters (Millipore) and equilibrated in 20 mM Tris (pH 7). The samples were loaded onto a MonoQ 5/50 GL ion-exchange column (GE Healthcare; equilibrated with 20 mM Tris) and then eluted with a linear NaCl gradient (0–1 M in 20 mM Tris; pH 7). Fractions were collected at 1-min intervals for 15 min at 4°C. The resulting fractions were dialyzed into an F12 medium and sterile-filtered (0.45 μm) before their evaluation for anti-HIV activity (see above). Aliquots of these fractions were also assayed by ELISA for IFN-α as described above.
Data analyses
Data were compiled in an Access database (Microsoft). Total Access Statistics X.8 (FMS) was used to perform the statistical analyses, and SigmaPlot 11.2 (Systat) was used create the graphs. The microarray data analysis approach used in this study followed guidelines for quality control, identification of differentially expressed genes, and pathway determinations (Cordero and others 2007). Microarray data were analyzed using Beadstudio 3.0 (Illumina). The rank-invariant method was used to normalize the array gene expression data. Pathway analyses were performed using DAVID (Dennis and others 2003). Flow cytometric data were analyzed using CellQuest (BD). The quantitative PCR data were analyzed with the StepOne software program (Applied Biosystems). Group compared using Mann–Whitney U tests. Experimental data were defined as P<0.05.
Results
CNAR induces the increased expression of IFN-responsive genes
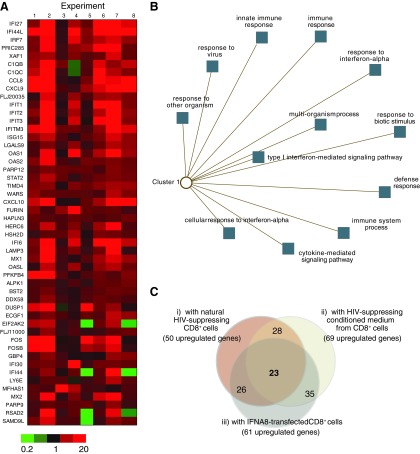
To identify changes in the transcriptomes of target cells caused by the CD8+ cell antiviral response, PHA-stimulated CD4+ cells were cultured alone or in the presence of autologous unstimulated CD8+ cells having high-level (>90% suppression; N=8) or low-level (<30% suppression; N=2) anti-HIV activity (Fig. 1). After 24 h of culture, RNA was extracted from repurified CD4+ cells and then analyzed in gene expression arrays (IlluminaHumanRef-8 BeadChips). The data revealed 50 upregulated genes having at least 62.5% concordance among the 8 experiments with HIV-suppressing CD8+ cells (Fig. 1A). None of these genes were upregulated in the CD4+ cells that had been cocultured with CD8+ cells lacking anti-HIV activity. This gene list was analyzed using the ToppCluster (Kaimal and others 2010) and DAVID (Sherman and others 2007) Web-based bioinformatics programs (Fig. 1B) and found to be significantly enriched for immune response genes (P<0.05, false discovery rate correction method), particularly those associated with type I IFN signaling (eg, OAS1, MX1, and IFI44L). Additional experiments were performed with CD8+ cell-conditioned medium having anti-HIV activity (N=4) and with IFN-transfected (IFNA8) primary CD8+ cells from HIV-negative donors (N=2). CD4+ cells that were cultured with the antiviral CD8+ cell-conditioned medium, and the IFN-transfected CD8+ cells exhibited remarkably similar gene expression profiles (Fig. 1C). Moreover, 23 (∼50%) of the genes upregulated in CD4+ cells by either naturally antiviral CD8+ cells, antiviral-conditioned medium from CD8+ cells, or IFN-transfected CD8+ cells were common to all 3 experimental conditions. The majority of the upregulated genes (19/23=83%) are reported to be stimulated by type I IFNs as revealed using the subtype analysis feature in INTERFEROME (Samarajiwa and others 2009). Together, these experiments and analyses demonstrate the increased presence of gene transcripts stimulated by type I IFNs in CD4+ cells that are exposed to HIV-suppressing CD8+ cells or CD8+ cell-conditioned medium having anti-HIV activity.
FIG. 1.
Upregulation of interferon (IFN)-stimulated genes in CD4+ cells upon exposure to CD8+ cells with anti-HIV activity. (A) CD4+ cells were cultured in the presence (coculture) and absence (alone) of unstimulated CD8+ cells having strong anti-HIV activity. Transcript levels were measured and used to compute a relative gene expression ratio. This ratio is shown in heat map style for the 50 genes found to be upregulated by at least 1.5-fold in the CD4+ cell cocultures in at least 5 of the 8 experiments performed. (B) Gene list enrichment analysis was performed using the 50 upregulated genes. Shown is an abstracted network produced with ToppCluster. (C) A Venn diagram shows the overlap of the genes found to be upregulated in CD4+ cells exposed to primary HIV-suppressing CD8+ cells, to an HIV-suppressing conditioned medium from CD8+ cells, or to primary CD8+ cells that were transfected to overexpress IFN-α.
Low-level expression of IFN in unstimulated CD8+ cells
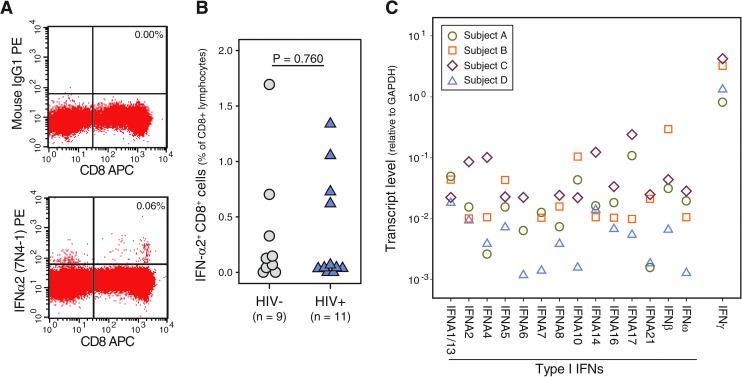
Having observed that unstimulated CD8+ cells can induce the upregulation of IFN-responsive genes, we performed flow cytometry and quantitative RT-PCR to assess IFN levels in unstimulated (ex-vivo) CD8+ cells (Fig. 2). Very few CD8+ T cells (<1.5%) from each of the subjects analyzed (N=20) exhibited positive intracellular staining with an antibody specific for IFN-α2b (BD Pharmingen clone 7N4-1) (Fig. 2A, B). The ex vivo frequencies of IFN+ CD8+ cells from the HIV-1-infected (median=0.05%) and uninfected (median=0.12%) study subjects did not appreciably differ (Mann–Whitney U test, P=0.760). Using quantitative RT-PCR, we measured the RNA transcript levels of all known IFN-α, β, and ω genes (Fig. 2C). The ex vivo CD8+ cells from 3 HIV-infected subjects and 1 uninfected individual contained detectable transcript levels for all 13 unique IFN-α genes, as well as for the IFN-β and IFN-ω genes. Transcript levels for the type I IFNs (Ct=23.6–37.1 cycles) ranged from 0.5 to 3 logs lower than those of the housekeeping gene GAPDH. In comparison, type II IFN (IFN-γ) transcript levels were observed to be equal or slightly elevated (0–1 log10) relative to GAPDH. No-template controls and PCRs containing primers specific for the firefly (Photinus pyralis) luciferase gene exhibited no amplification (Ct>50 cycles, data not shown). These results establish that type I IFN transcripts are present at very low levels in unstimulated CD8+ cells, whereas IFN is narrowly detectable by intracellular cytokine-staining procedures in these ex vivo cells.
FIG. 2.
Low-level IFN in primary CD8+ cells. (A) Dot plots, gated on lymphocytes, show the profiles of unstimulated peripheral blood mononuclear cells after cell-surface staining with anti-CD8 and intracellular staining with an isotype control antibody (upper panel) or anti-IFN-α (lower panel). (B) Shown are the percentages of CD8+ cells staining positive for intracellular IFN-α2 (clone 7N4-1) in 11 human immunodeficiency virus 1 (HIV-1)-infected and 9 uninfected individuals. The Mann–Whitney U test was used to compare the frequencies of IFN+ CD8+ cells among the 2 groups of individuals. (C) Levels of type I and II IFN transcripts were measured in RNA extracted from unstimulated CD8+ cells using quantitative reverse transcriptase (RT)–polymerase chain reaction. Symbols are used to distinguish the transcript levels between the CD8+ cells from the 4 study subjects (see legend; uninfected subject, A; HIV-1-infected subjects, B–D).
Type I IFNs are secreted by CD8+ cells
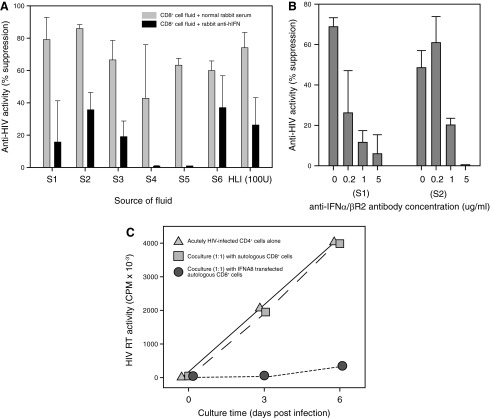
To determine whether or not type I IFNs are secreted by HIV-suppressing CD8+ cells, we performed ELISAs to measure IFN-α, β, and ω levels in supernatants from various cultures of CD8+ cells that were stimulated with anti-CD3 beads (Fig. 3). Upon direct analysis, the ELISA optical density (O.D.) values of the CD8+ cell fluids having anti-HIV activity (ie, >50% suppression) were greater than those of CD8+ cell fluids without anti-HIV activity and the diluent control values, but none of the CD8+ cell fluids contained IFN-α levels within the assay's range of detection (5–500 pg/mL). However, after concentration (10–100-fold) of the fluids using 10-kDa filters, IFN-α levels became measurable. The IFN-α concentration in the CD8+ cell fluids was found to directly correlate with the temporal anti-HIV activity of the fluids collected during the course of cell culture (Fig. 3A, B). IFN-β levels also directly correlated with the anti-HIV activity of the CD8+ cell fluids (Fig. 3B). Further analyses revealed that the large majority (37/44=84%) of CD8+ cell fluids having anti-HIV activity were found to contain detectable IFN-α levels (Fig. 3C). IFN was rarely detected (10/65=15%) in equally concentrated CD8+ cell fluids that lacked anti-HIV activity; when present, the levels were generally lower than those found in active fluids. Moreover, the low frequencies of discrepancies between the IFN-α ELISA and the HIV-1 suppression assay (ie, false-positive and negative IFN-α ELISA results) are consistent with the intraobserver variability of the CD4+ cell-culture-based HIV-1 suppression assay (data not shown).
FIG. 3.
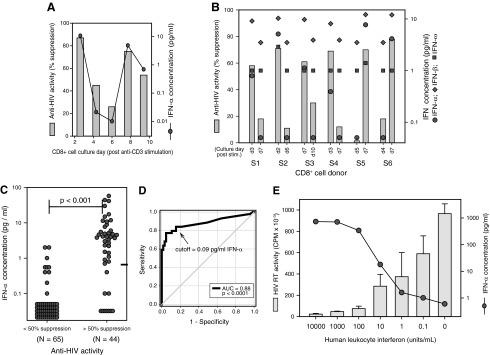
IFN in a CD8+ cell-conditioned medium is directly correlated with the anti-HIV activity. (A) Anti-HIV activity (percent suppression, left axis) and IFN-α levels (right axis) of fluids collected from a single-CD8+-cell culture over the course of 10 days. (B) Shown are the anti-HIV activities (left axis) and IFN levels (right axis) of CD8+ cell fluids from 6 different donors (S1–S6) collected at different days. Symbols differentiate the measurements of IFN-α (circles), IFN-β (diamonds), and IFN-ω (squares). (C) IFN-α levels were measured in 109 separate CD8+ cell fluids that were predetermined to exhibit low (<50%) or high (>50%) anti-HIV activity. (D) A receiver operator curve showing the predictive value of IFN-α levels as related to the anti-HIV activity of CD8+ cell fluids. (E) Effect of decreasing concentrations of human leukocyte interferon (HLI) on HIV replication (bars, left axis) in primary CD4+ cells. IFN-α levels (circles, right axis) in the various HLI dilutions were measured by enzyme-linked immunosorbent assay (ELISA).
After adjustments for the volumetric concentration, the majority of CD8+ cell fluids having anti-HIV activity (>50% suppression) were observed to have IFN-α concentrations in the range of 0.5–55 pg/mL. Receiver operator curve analysis revealed that a cutoff value of 0.09 pg/mL IFN can superbly distinguish CD8+ cell fluids having anti-HIV activity (Fig. 3D). Notably, the HIV-suppressing fluids likely contain higher than measured levels of IFN-α due to expected losses in the concentration procedure. Nonetheless, experiments performed with commercial HLI (HPLC purified to contain primarily IFN-α) confirmed that ∼1 pg/mL of IFN-α was sufficient for a 50% reduction in HIV replication levels in acutely HIV-infected CD4+ T cells (Fig. 3E). These results demonstrate that (1) CD8+ cells can secrete IFN; (2) the anti-HIV activity of CD8+ cell-conditioned medium is directly correlated with the IFN-α concentration and dependent on the presence of IFN-α; and (3) the concentration of IFN-α found in CD8+ cell fluids having anti-HIV activity is sufficient for the appreciable suppression of HIV replication in CD4+ T cells.
CAF activity is dependent on IFN
To evaluate the contribution of IFN to the anti-HIV activity of a conditioned medium from CD8+ cells, we performed experiments using antibodies to neutralize human IFN, and with antibodies to block the IFN receptor (Fig. 4). In 19 experiments, CD8+ cell fluids (from 7 different donors) having anti-HIV activity were treated with rabbit serum containing antihuman-IFN antibodies and with normal rabbit serum (controls). In comparison to the same CD8+ cell fluids that were untreated and to those treated with normal rabbit serum, HIV replication levels were elevated in 19 of 19 (100%) CD4+ cell cultures containing CD8+ cell fluids that were treated with anti-IFN antibodies (Fig. 4A). The HIV replication levels did not appreciably differ between the untreated cultures and those containing normal rabbit serum (mean ratio=1.0). The mean HIV replication ratio of the anti-IFN-α-treated fluids to their respective control-treated fluids was 2.24 (paired t-test, P<0.001). Similarly, when CD8+ cell fluids having antiviral activity were added to acutely HIV-infected CD4+ cells along with medium alone, normal mouse IgG, or a mouse monoclonal antibody specific for the human IFN-α/β receptor chain 2 (IFNAR2, CD118), HIV replication levels were consistently highest in the cultures containing the anti-IFNAR2 antibody (Fig. 4B). Relative to the addition of normal mouse IgG, the addition of anti-IFNAR2 (1 μg/mL) to the cultures resulted in HIV replication levels that were ∼2-fold higher (n=6, mean ratio=2.5, paired t-test P<0.05). Thus, anti-IFN and anti-IFNAR2 antibodies had neutralizing and blocking effects, respectively, on the anti-HIV activity of CD8+ cell fluids.
FIG. 4.
Loss and gain of function of CD8+ cell anti-HIV activity. (A) Shown are representative anti-HIV activity levels of CD8+-cell-conditioned media from 6 different donors (S1–S6) and HLI upon treatment with normal rabbit serum (light bars) or rabbit serum containing anti-human IFN antibodies (dark bars). (B) The effects of increasing amounts of anti-IFN receptor antibody (anti-IFNAR2) on the anti-HIV activity of CD8+-cell-conditioned media from 2 different donors (S1 and S2). (C) Shown are the HIV replication levels (RT activity) in cultures containing acutely infected CD4+ cells alone (circles), cocultured with untreated CD8+ cells from a HIV-negative donor (squares), or cocultured with CD8+ cells from the same donor that were transfected in vitro with an IFN expression vector (triangles).
CNAR activity is gained with IFN transfection
To determine whether or not CD8+ cells lacking anti-HIV activity can be made to suppress HIV replication, we transfected primary unstimulated CD8+ cells from healthy HIV-negative blood donors (N=2) with a plasmid expressing human IFN-α (IFNA8) (Fig. 4C). The untreated CD8+ cells exhibited no appreciable anti-HIV activity, while the IFNA8-transfected primary CD8+ cells were observed to suppress HIV replication by >90%. Consistent with previous reports of CD4+ cells treated with exogenous IFN-γ (Yamamoto and others 1986; Mackewicz and others 1994), CD8+ cells that were transfected with a plasmid expressing human IFN-γ (type II IFN) had no antiviral effects on HIV replication (data not shown). These results demonstrate that CD8+ cells gain anti-HIV function when made to express IFN-α.
IFN and CAF exhibit similar biochemical properties
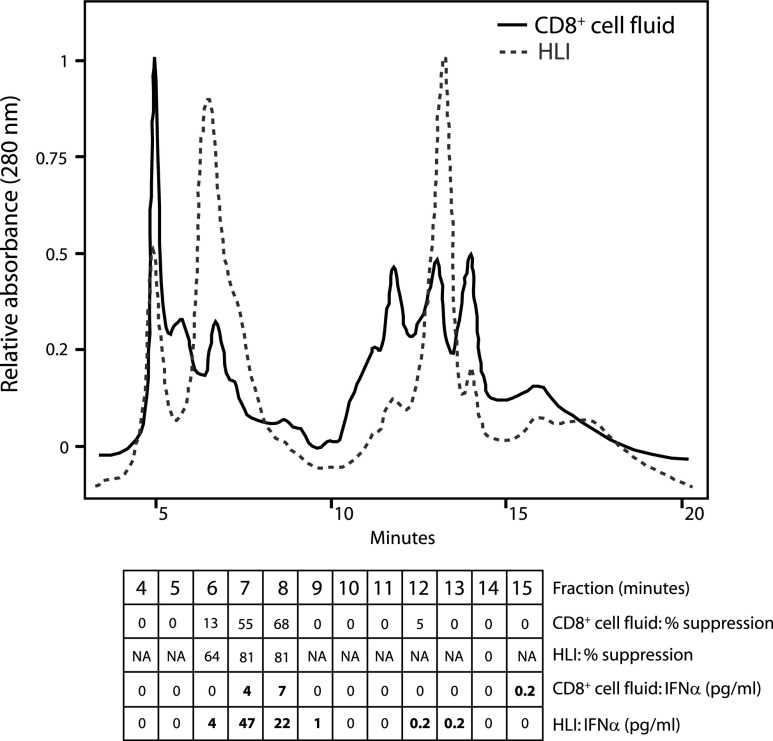
Fractionation of cell culture supernatants using column chromatography procedures has been used to purify and characterize numerous cytokines, including IFN (Pestka and others 2004). To further determine the contribution of IFN to the CD8+ cell anti-HIV activity, we used HPLC procedures to fractionate a commercial HLI and CD8+ cell-conditioned medium having anti-HIV activity (Fig. 5). The resulting fractions were evaluated for anti-HIV activity and assayed for IFN-α by ELISA. Strong agreement was observed between the 2 fluids in terms of the fractions having anti-HIV activity and the fractions containing IFN-α. For both fluids, the fractions eluting at 6–8 min exhibited the greatest anti-HIV activity, and only these fractions contained appreciable IFN-α levels (>1 pg/mL) by ELISA. The confined elution of the CD8+ cell antiviral activity from the anion exchange column is consistent with previous observations (Mackewicz and others 2004, XV International AIDS Conference, Abstract #A10365). These results demonstrate that IFN-α and the active component of HIV-suppressing CD8+ cell fluids similarly bind and elute from an ion-exchange column and therefore share common biochemical properties. Moreover, these results show that IFN-α is present in chromatography fractions of CD8+ cell fluids having anti-HIV activity and largely restricted to those fractions. Thus, these data complement the loss-of-function results described above.
FIG. 5.
IFN and CD8+ cell fluids with anti-HIV activity elute in similar high-performance liquid chromatography (HPLC) fractions. A CD8+ cell fluid having anti-HIV activity and commercial HLI (1,000 U/mL of F12 medium) were fractionated by HPLC using an ion-exchange column. Shown are the chromatograms for each sample. The various fractions were coanalyzed for the ability to suppress HIV replication in primary CD4+ cells and for IFN-α concentration by ELISA. The results are shown below the chromatograms. NA denotes fractions that were not evaluated for anti-HIV activity. Results are representative of 2 separate experiments.
CAF induces STAT phosphorylation
The antiviral activity of IFN is mediated through signaling pathways that involve the phosphorylation of STAT1 and STAT3 (Tanabe and others 2005). To determine whether or not the CD8+ cell-conditioned medium can also induce the phosphorylation of these STAT proteins, phosflow experiments were performed (Fig. 6). PHA-stimulated CD4+ cells were incubated at 37°C for 15 min in the presence of HLI or CD8+ cell fluids and then stained with antibodies specific for phosphorylated STAT1 and STAT3. As expected, dose–response effects were observed between HLI and the levels of phosphorylated STAT1 and STAT3. Similarly, CD8+ cell fluids having low anti-HIV activity (<50% suppression) and high anti-HIV activity (>50% suppression) induced the graded phosphorylation of both STAT1 and STAT3. These data demonstrate that CD8+ cell-conditioned medium having high anti-HIV activity can rapidly induce the phosphorylation of STAT1 and STAT3 in PHA-stimulated CD4+ cells. Also, these observations of conditioned media from cultures of primary anti-CD3-stimulated CD8+ cells are consistent with the reported effects of fluids from CD8+ T cell lines (Chang and others 2002).
FIG. 6.
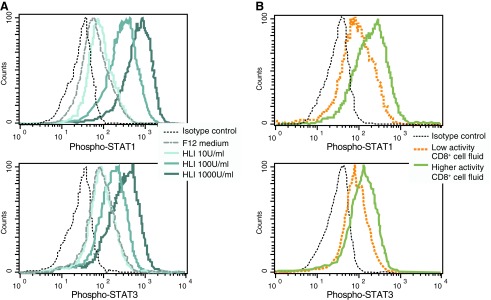
Phosphorylation of STAT proteins in response to IFN and CD8+ cell fluids. Mitogen-stimulated CD4+ T cells were exposed to (A) various concentrations (0, 10, 100, and 1,000 U/mL of F12 medium) of HLI or (B) CD8+ cell fluids (10× concentrated using a 10-kDa filter) having relatively high and low anti-HIV activity. After 15 min of exposure, the cells were processed and stained for measurement of phosphorylated STAT1 (upper) and STAT3 (lower) by flow cytometry. Results are representative of 3 separate experiments.
CD8+ cells secrete IFN upon exposure to HIV-infected CD4+ cells
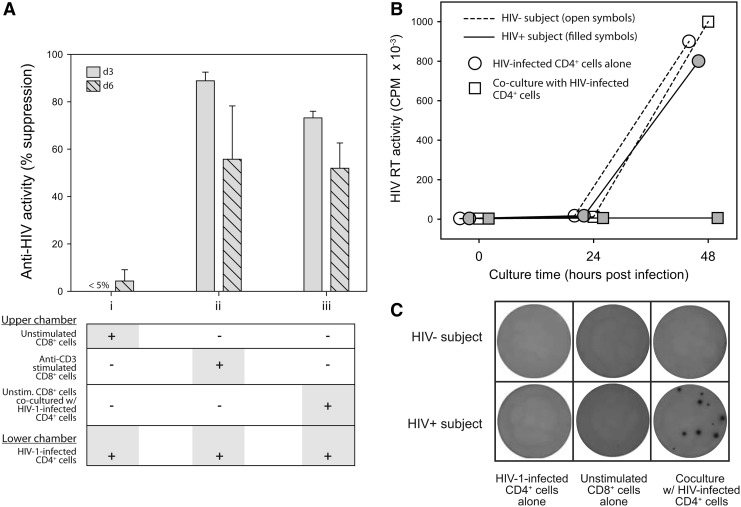
Because the identity of CAF has remained unknown, its relation to the ability of CD8+ cells to suppress HIV-1 replication in cell-to-cell contact assays with CD4+ cells has not been determined. To investigate the potential role of HIV-1-infected CD4+ cells as stimulatory agents for CAF production, we performed parallel cell culture experiments using transwell inserts (Fig. 7A). HIV-1-infected CD4+ cells were placed into the lower chamber of each well. The upper chambers were seeded with (1) unstimulated CD8+ cells, (2) anti-CD3-stimulated CD8+ cells, or (3) unstimulated CD8+ cells and HIV-1-infected CD4+ cells. As expected, the unstimulated CD8+ cells alone were unable to suppress HIV-1 replication (Mackewicz and others 2003b). In comparison, the anti-CD3-stimulated CD8+ cells exhibited diffusible anti-HIV activity (>50% suppression), as did the unstimulated CD8+ cells that were cocultured in the upper chamber with HIV-1-infected CD4+ cells. These results demonstrate that HIV-1-infected CD4+ cells can elicit the secretion of CAF. Thus, these results resolve the outstanding dilemma between unstimulated CD8+ cells that do not secrete CAF and the exquisite suppression of HIV-1 replication that is mediated by unstimulated CD8+ cells in cell-to-cell contact assays with HIV-1-infected CD4+ cells.
FIG. 7.
CD8+ cells suppress HIV replication in CD4+ cells by secreting IFN-α. (A) Transwell assays. Shown is the anti-HIV activity (% suppression measured at culture days 3 and 6) when (i) unstimulated CD8+ cells, (ii) anti-CD3-stimulated CD8+ cells, or (iii) unstimulated CD8+ cells and HIV-1-infected CD4+ cells were placed into the upper chamber of a transwell insert. All wells contained HIV-1-infected CD4+ cells in the lower chamber. Error bars show the standard deviations from duplicate wells, and the results shown are representative of 3 independent experiments. (B) Cell-to-cell contact assays. Shown are line-and-scatter plots of HIV replication levels, measured by RT activity, in the supernatants of CD4+ cell cultures. Acutely HIV-infected CD4+ cells were cultured alone (open symbols) or in the presence of autologous unstimulated CD8+ cells (shaded symbols) at 1:1 cell input ratios. Representative results are shown for cultures established with cells from an HIV-negative (circles) and an HIV-infected individual (squares). (C) ELISpot assays. CD8+ cells from an uninfected donor (upper row) and an HIV-infected donor (lower row) were placed into the wells of an IFN-α (multisubtype) ELISpot plate. Shown are digital images of wells containing 50,000 HIV-1-infected CD4+ cells cultured alone (column 1), 250,000 CD8+ cells cultured alone (column 2), or CD8+ cells that were cocultured at a 5:1 ratio with HIV-1-infected CD4+ cells (column 3). The ELISpot plates were processed after 24 h of cell culture. The results are representative of 3 independent experiments.
In light of the above observations, we investigated the ability of HIV-1-infected CD4+ cells to elicit the secretion of IFN by CD8+ cells. In cell-to-cell contact assays, the unstimulated CD8+ cells from asymptomatic HIV-1-infected individuals, but not those from healthy uninfected blood donors, were able to suppress HIV-1 replication in primary CD4+ T cells (Fig. 7B). Parallel experiments were performed using commercially available IFN-α ELISpot assays (Fig. 7C). These assays that employ the basic principles of ELISA (Czerkinsky and others 1983) can reveal the secretion of IFN-α as visible spots on a semipermeable membrane. No spots were present in wells that contained unstimulated CD8+ cells alone or HIV-1-infected CD4+ cells alone. Also, no spots were present in wells containing unstimulated CD8+ cells from uninfected donors cocultured with acutely HIV-infected CD4+ cells (5:1 cell input ratio). In contrast, the secretion of IFN-α was evidenced by spots in wells containing unstimulated CD8+ cells from HIV-1-infected donors cocultured with HIV-infected CD4+ cells. These results demonstrate that unstimulated CD8+ cells from HIV-1-infected individuals, but generally not those from uninfected individuals, can be induced to secrete IFN by HIV-1-infected CD4+ cells. The results also suggest that the IFN-α ELISpot can be a valuable assay for the assessment of IFN-α-secreting CD8+ cells in research and clinical studies.
Discussion
During the past 26 years, a concerted effort has been made to identify the mechanism by which CD8+ cells are able to suppress HIV replication (Levy 2003); most approaches have focused directly on the CD8+ cells. We began this study by investigating CD4+ cells that were exposed to HIV-suppressing CD8+ cells or CD8+ cell fluids. This approach led to findings, suggesting that the secretion of IFN is the major mechanism for the CD8+ cell-mediated suppression of HIV replication.
Type 1 IFNs have long been known to inhibit HIV replication in vitro (Ho and others 1985; Yamamoto and others 1986; Hartshorn and others 1987; Michaelis and Levy 1989). While the primary antiviral component of CAF seems to be IFN-α, the precise composition of type I IFNs and potentially other synergistic cytokines remains to be determined. IFN subspecies can exhibit differences in their antiviral activities against rhinoviruses (Sperber and others 1993), influenza virus (Moll and others 2011), and HIV (Sperber and others 1992), with specific activities varying by several orders of magnitude. It is possible that IFN secreted by CD8+ cells is unique in its composition of IFN subtypes and/or due to the occurrence of post-translational modifications.
IFN and CAF have several notable similarities. Both are previously reported to be approximately 20 kDa in size, remain stable at high temperatures and low pH, and have the ability to inhibit the replication of divergent viruses (Levy 2003; Valle and others 1975). In addition, we have observed that the human CD8+ cell conditioned medium having anti-HIV activity, like human IFN (Levy-Koenig and others 1970), exhibits heterospecific (cross-species) antiviral activity in vitro (data not shown). Furthermore, the production of both IFN and CAF is greatly decreased in the presence of IL-4 or IL-10 (Levy and others 1996; Payvandi and others 1998; Zhao and others 1998).
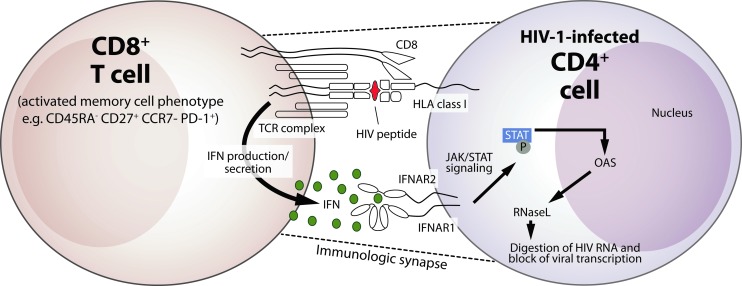
Our studies are the first to establish a mechanism for CNAR that specifies the identity and function of CAF (Fig. 8). We propose that IFN is secreted by CD8+ cells and signals through the IFN-α/β receptor on HIV-infected CD4+ cells to activate the antiviral response and inhibit viral transcription. Consistent with the reported activity of IFN-α (Matikainen and others 1999) and fluids collected from CD8+ cell lines (Chang and others 2002), we observed that the fluids from primary CD8+ cells can activate STAT proteins in CD4+ cells. Downstream effects of IFN-mediated STAT phosphorylation include the activation of oligoadenylate synthetase and RNase L nuclease, leading to the degradation of viral RNA (Maitra and Silverman 1998; Samuel 2001). Via this pathway, the secretion of IFN by CD8+ cells can explain the observed inhibitory effects of CAF and CNAR on HIV transcription (Mackewicz and others 1995). Given the very low levels that are present in unstimulated CD8+ cells and those that are secreted by anti-CD3-stimulated CD8+ cells, it is not surprising that previous studies of CNAR and CAF failed to identify a role for IFN (Brinchmann and others 1991; Mackewicz and others 1994). Nonetheless, the low-level secretion of IFN by CD8+ cells, perhaps in the context of an immunological synapse (Dustin and others 2010), appears to be extremely potent when the target cells are in close contact.
FIG. 8.
Proposed mechanism for the CD8+-cell-mediated suppression of HIV-1 replication. Illustrated is a model for the CD8+ cell noncytotoxic anti-HIV response (CNAR). Upon recognition of a cognate antigen presented in the context of HLA class I by HIV-1-infected CD4+ T cells, a signal is transduced via the T cell receptor (TCR) complex on memory CD8+ T cells (Killian and others 2011) that elicit the secretion of type I IFN. The secreted IFN binds to the IFN receptor (IFNAR2) on the CD4+ target cell, and the subsequent interaction with IFNAR1 activates the JAK/STAT pathway and promotes the phosphorylation of STAT1 and STAT3. This activity induces the upregulation of multiple IFN-responsive genes, including 2′,5′-oligoadenylate synthetase (OAS) and ribonuclease L (RNase L), which can have downstream inhibitory effects on HIV-1 transcription and virus replication.
With respect to HIV-1 pathogenesis, IFN-responsive genes are reported to be upregulated in peripheral blood CD4+ cells from HIV-infected individuals (Hyrcza and others 2007; Rempel and others 2010; Rotger and others 2010). While IFN-producing plasmacytoid dendritic cells (Siegal and others 1999; Killian and others 2006) have been a central focus, the secretion of IFN by CD8+ T cells could also contribute to this observation. Because we are most frequently able to generate CAF+ fluids using CD8+ cells from elite controllers, we hypothesize that the production of IFN by CD8+ cells, possibly involving favorable HLA class I-restricted interactions (Pereyra and others 2010), is a key contributor to the very low viral loads in these HIV-infected subjects. Nonetheless, CD8+ cells from most asymptomatic HIV-1-infected individuals can suppress HIV replication in cell-to-cell contact assays (Killian and others 2005). This can be attributed to our observation that CD8+ cells from both elite controllers and asymptomatic viremic individuals induce the upregulation of IFN-responsive genes in CD4+ cells. Furthermore, the gradual decline in CD4+ T cell numbers that is a hallmark of HIV-1 pathogenesis (Killian and Levy 2011) afflicts many elite controllers (Sauce and others 2011) and could be caused by the subtle cytostatic effects of CD8+ cells that secrete low levels of IFN.
It is important to consider that unstimulated CD8+ cells do not contain appreciable amounts of IFN, do not spontaneously produce fluids having anti-HIV activity (Mackewicz and others 2003b), and they do not freely secrete IFN (see Figs. 2 and 7). However, the fluids from anti-CD3-stimulated CD8+ cells, conditional on the secretion of IFN, have anti-HIV activity. This observation can explain how unstimulated CD8+ cells are able to suppress virus replication in cell-to-cell contact assays with HIV-1-infected CD4+ cells. Similar to the effects of anti-CD3 beads, antigens present on the surfaces HIV-1-infected CD4+ cells interact with the T cell receptors on the unstimulated CD8+ cells and could trigger the release of IFN. This scenario implicates a role for antigen-specific CD8+ cells and is consistent with our recent finding that CNAR is mediated by a small subset of CD8+ T cells having a CD45RA−CD27+CCR7− phenotype (Killian and others 2011). Based on this finding, we expect that memory CD8+ T cells are the primary secretors of type I IFNs. This possibility is supported by the observation that stimulated Epstein-Barr virus-specific CD8+ cells secrete cytokines that suppress HIV replication (Le Borgne and others 2000). Indeed, the role of IFN secretion by memory CD8+ T cells in HIV-infection and other diseases merits further investigation.
Although numerous cell types have been found to produce IFN (Brandt and others 1994), its secretion by CD8+ cells has not been fully appreciated. In this regard, it is possible that cytokines from a contaminating cell type could be designated as a CD8+ CAF, as occurred in a report of α-defensins (Zhang and others 2002). However, this possibility seems unlikely for several reasons. First, our isolated CD8+ cells are routinely >98% pure upon flow cytometric analysis (data not shown), and the CD8+ cell-conditioned fluids found to contain IFN were produced without the use of serum or feeder cells. Second, the CD8+ cell fluids having anti-HIV activity were generated using anti-CD3 stimulation and otherwise would have had no anti-HIV activity, thus implicating the production of IFN by T cells. Third, the ebb and flow nature in which anti-CD3-stimulated CD8+ cells produce fluids having anti-HIV activity over the course of a 12-day culture period has not been described for any other cell type, and lastly, we are unaware of any biases that could have resulted in the differential association of IFN with the CD8+ cell anti-HIV activity in the various experiments; many experiments were performed simultaneously, and all were performed in the same laboratory using standardized procedures.
In summary, the secretion of type 1 IFNs (primarily IFN-α and IFN-β) by CD8+ cells contributes to their anti-HIV activity. Treatment approaches that harness this response could be of clinical benefit to HIV-infected individuals, and the induction of this CD8+ cell activity should be considered in approaches to develop an effective vaccine for HIV infection.
Acknowledgments
This research was funded by grants from the National Institutes of Health (3P30AI027763-19S2) and the California HIV/AIDS Research Program (ID09-SF-058) and was supported in part by the Intramural Research Program of the NIH National Cancer Institute.
Author Contributions
S.K. designed and performed the experiments and data analyses and wrote the article; F.T., R.W., and K.K. assisted with some of the experiments and data analyses; S.K., P.M., and K.K. provided resources for the research; all authors contributed to the final revision of the article.
Author Disclosure Statement
No competing financial interests exist.
References
- Barker E. Mackewicz CE. Reyes-Teran G. Sato A. Stranford SA. Fujimura SH. Christopherson C. Chang SY. Levy JA. Virological and immunological features of long-term human immunodeficiency virus-infected individuals who have remained asymptomatic compared with those who have progressed to acquired immunodeficiency syndrome. Blood. 1998;92(9):3105–3114. [PubMed] [Google Scholar]
- Brandt ER. Linnane AW. Devenish RJ. Expression of IFN A genes in subpopulations of peripheral blood cells. Br J Haematol. 1994;86(4):717–725. doi: 10.1111/j.1365-2141.1994.tb04820.x. [DOI] [PubMed] [Google Scholar]
- Brinchmann JE. Gaudernack G. Vartdal F. In vitro replication of HIV-1 in naturally infected CD4+ T cells is inhibited by rIFN alpha 2 and by a soluble factor secreted by activated CD8+ T cells, but not by rIFN beta, rIFN gamma, or recombinant tumor necrosis factor-alpha. J Acquir Immune Defic Syndr. 1991;4(5):480–488. [PubMed] [Google Scholar]
- Castelli JC. Deeks SG. Shiboski S. Levy JA. Relationship of CD8(+) T cell noncytotoxic anti-HIV response to CD4(+) T cell number in untreated asymptomatic HIV-infected individuals. Blood. 2002;99(11):4225–4227. doi: 10.1182/blood-2001-11-0078. [DOI] [PubMed] [Google Scholar]
- Chang TL. Mosoian A. Pine R. Klotman ME. Moore JP. A soluble factor(s) secreted from CD8(+) T lymphocytes inhibits human immunodeficiency virus type 1 replication through STAT1 activation. J Virol. 2002;76(2):569–581. doi: 10.1128/JVI.76.2.569-581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F. DeVico AL. Lu W. Popovic M. Latinovic O. Sajadi MM. Redfield RR. Lafferty MK. Galli M. Garzino-Demo A. Gallo RC. Soluble factors from T cells inhibiting X4 strains of HIV are a mixture of beta chemokines and RNases. Proc Natl Acad Sci U S A. 2012;109(14):5411–5416. doi: 10.1073/pnas.1202240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland KF. McKay PJ. Rosenthal KL. Suppression of activation of the human immunodeficiency virus long terminal repeat by CD8+ T cells is not lentivirus specific. AIDS Res Hum Retroviruses. 1995;11(11):1321–1326. doi: 10.1089/aid.1995.11.1321. [DOI] [PubMed] [Google Scholar]
- Cordero F. Botta M. Calogero RA. Microarray data analysis and mining approaches. Brief Funct Genomic Proteomics. 2007;6(4):265–281. doi: 10.1093/bfgp/elm034. [DOI] [PubMed] [Google Scholar]
- Czerkinsky CC. Nilsson LA. Nygren H. Ouchterlony O. Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65(1–2):109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- Davenport MP. Petravic J. CD8+ T cell control of HIV—a known unknown. PLoS Pathog. 2010;6(1):e1000728. doi: 10.1371/journal.ppat.1000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG. Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27(3):406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Dennis G., Jr. Sherman BT. Hosack DA. Yang J. Gao W. Lane HC. Lempicki RA. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4(5):3. [PubMed] [Google Scholar]
- Dustin ML. Chakraborty AK. Shaw AS. Understanding the structure and function of the immunological synapse. Cold Spring Harbor Perspect Biol. 2010;2(10):a002311. doi: 10.1101/cshperspect.a002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiben-Lynn R. Kursar M. Brown NV. Kerr EL. Luster AD. Walker BD. Noncytolytic inhibition of X4 virus by bulk CD8(+) cells from human immunodeficiency virus type 1 (HIV-1)-infected persons and HIV-1-specific cytotoxic T lymphocytes is not mediated by beta-chemokines. J Virol. 2001;75(17):8306–8316. doi: 10.1128/JVI.75.17.8306-8316.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorn KL. Neumeyer D. Vogt MW. Schooley RT. Hirsch MS. Activity of interferons alpha, beta, and gamma against human immunodeficiency virus replication in vitro. AIDS Res Hum Retroviruses. 1987;3(2):125–133. doi: 10.1089/aid.1987.3.125. [DOI] [PubMed] [Google Scholar]
- Ho DD. Hartshorn KL. Rota TR. Andrews CA. Kaplan JC. Schooley RT. Hirsch MS. Recombinant human interferon alfa-A suppresses HTLV-III replication in vitro. Lancet. 1985;1(8429):602–604. doi: 10.1016/s0140-6736(85)92144-0. [DOI] [PubMed] [Google Scholar]
- Hoffman AD. Banapour B. Levy JA. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985;147(2):326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- Hyrcza MD. Kovacs C. Loutfy M. Halpenny R. Heisler L. Yang S. Wilkins O. Ostrowski M. Der SD. Distinct transcriptional profiles in ex vivo CD4+ and CD8+ T cells are established early in human immunodeficiency virus type 1 infection and are characterized by a chronic interferon response as well as extensive transcriptional changes in CD8+ T cells. J Virol. 2007;81(7):3477–3486. doi: 10.1128/JVI.01552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaimal V. Bardes EE. Tabar SC. Jegga AG. Aronow BJ. ToppCluster: a multiple gene list feature analyzer for comparative enrichment clustering and network-based dissection of biological systems. Nucleic Acids Res. 2010;38(Web Server issue):W96–W102. doi: 10.1093/nar/gkq418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian MS. Fujimura SH. Hecht FM. Levy JA. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV-1 infection and treatment. AIDS. 2006;20(9):1247–1252. doi: 10.1097/01.aids.0000232231.34253.bd. [DOI] [PubMed] [Google Scholar]
- Killian MS. Johnson C. Teque F. Fujimura S. Levy JA. Natural suppression of human immunodeficiency virus type 1 replication is mediated by transitional memory CD8+ T cells. J Virol. 2011;85(4):1696–1705. doi: 10.1128/JVI.01120-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian MS. Levy JA. HIV/AIDS: 30 years of progress and future challenges. Eur J Immunol. 2011;41(12):3401–3411. doi: 10.1002/eji.201142082. [DOI] [PubMed] [Google Scholar]
- Killian MS. Ng S. Mackewicz CE. Levy JA. A screening assay for detecting CD8(+) cell non-cytotoxic anti-HIV responses. J Immunol Methods. 2005;304(1–2):137–150. doi: 10.1016/j.jim.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Killian MS. Roop J. Ng S. Hecht FM. Levy JA. CD8+ cell anti-HIV activity rapidly increases upon discontinuation of early antiretroviral therapy. J Clin Immunol. 2009;29(3):311–318. doi: 10.1007/s10875-009-9275-y. [DOI] [PubMed] [Google Scholar]
- Klatt NR. Shudo E. Ortiz AM. Engram JC. Paiardini M. Lawson B. Miller MD. Else J. Pandrea I. Estes JD. Apetrei C. Schmitz JE. Ribeiro RM. Perelson AS. Silvestri G. CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog. 2010;6(1):e1000747. doi: 10.1371/journal.ppat.1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne S. Fevrier M. Callebaut C. Lee SP. Riviere Y. CD8(+)-cell antiviral factor activity is not restricted to human immunodeficiency virus (HIV)-specific T cells and can block HIV replication after initiation of reverse transcription. J Virol. 2000;74(10):4456–4464. doi: 10.1128/jvi.74.10.4456-4464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leith JG. Copeland KF. McKay PJ. Richards CD. Rosenthal KL. CD8+ T-cell-mediated suppression of HIV-1 long terminal repeat-driven gene expression is not modulated by the CC chemokines RANTES, macrophage inflammatory protein (MIP)-1 alpha and MIP-1 beta. AIDS. 1997;11(5):575–580. doi: 10.1097/00002030-199705000-00004. [DOI] [PubMed] [Google Scholar]
- Levy JA. The search for the CD8+ cell anti-HIV factor (CAF) Trends Immunol. 2003;24(12):628–632. doi: 10.1016/j.it.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Levy JA. Mackewicz CE. Barker E. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol Today. 1996;17(5):217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- Levy-Koenig RE. Golgher RR. Paucker K. Immunology of interferons. II. Heterospecific activities of human interferons and their neutralization by antibody. J Immunol. 1970;104(4):791–797. [PubMed] [Google Scholar]
- Liu L. Johnson C. Fujimura S. Teque F. Levy JA. Transfection optimization for primary human CD8+ cells. J Immunol Methods. 2011;372(1–2):22–29. doi: 10.1016/j.jim.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mackewicz CE. Barker E. Greco G. Reyes-Teran G. Levy JA. Do beta-chemokines have clinical relevance in HIV infection? J Clin Invest. 1997;100(4):921–930. doi: 10.1172/JCI119608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackewicz CE. Blackbourn DJ. Levy JA. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc Natl Acad Sci U S A. 1995;92(6):2308–2312. doi: 10.1073/pnas.92.6.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackewicz CE. Craik CS. Levy JA. The CD8+ cell noncytotoxic anti-HIV response can be blocked by protease inhibitors. Proc Natl Acad Sci U S A. 2003a;100(6):3433–3438. doi: 10.1073/pnas.0630379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackewicz CE. Ortega HW. Levy JA. CD8+ cell anti-HIV activity correlates with the clinical state of the infected individual. J Clin Invest. 1991;87(4):1462–1466. doi: 10.1172/JCI115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackewicz CE. Ortega H. Levy JA. Effect of cytokines on HIV replication in CD4+ lymphocytes: lack of identity with the CD8+ cell antiviral factor. Cell Immunol. 1994;153(2):329–343. doi: 10.1006/cimm.1994.1032. [DOI] [PubMed] [Google Scholar]
- Mackewicz CE. Ridha S. Levy JA. Fas and Fas ligand are not involved in the suppression of HIV replication by CD8 cells. AIDS. 2000;14(2):204–205. doi: 10.1097/00002030-200001280-00018. [DOI] [PubMed] [Google Scholar]
- Mackewicz CE. Wang B. Metkar S. Richey M. Froelich C. Levy JA. Lack of the CD8+ cell anti-HIV factor in CD8+ cell granules. Blood. 2003b;102(1):180–183. doi: 10.1182/blood-2002-10-3034. [DOI] [PubMed] [Google Scholar]
- Maitra RK. Silverman RH. Regulation of human immunodeficiency virus replication by 2′,5′-oligoadenylate-dependent RNase L. J Virol. 1998;72(2):1146–1152. doi: 10.1128/jvi.72.2.1146-1152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen S. Sareneva T. Ronni T. Lehtonen A. Koskinen PJ. Julkunen I. Interferon-alpha activates multiple STAT proteins and upregulates proliferation-associated IL-2Ralpha, c-myc, and pim-1 genes in human T cells. Blood. 1999;93(6):1980–1991. [PubMed] [Google Scholar]
- Michaelis B. Levy JA. HIV replication can be blocked by recombinant human interferon beta. AIDS. 1989;3(1):27–31. [PubMed] [Google Scholar]
- Moll HP. Maier T. Zommer A. Lavoie T. Brostjan C. The differential activity of interferon-alpha subtypes is consistent among distinct target genes and cell types. Cytokine. 2011;53(1):52–59. doi: 10.1016/j.cyto.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payvandi F. Amrute S. Fitzgerald-Bocarsly P. Exogenous and endogenous IL-10 regulate IFN-alpha production by peripheral blood mononuclear cells in response to viral stimulation. J Immunol. 1998;160(12):5861–5868. [PubMed] [Google Scholar]
- Pereyra F. Jia X. McLaren PJ. Telenti A. de Bakker PI. Walker BD. Ripke S. Brumme CJ. Pulit SL. Carrington M. Kadie CM. Carlson JM. Heckerman D. Graham RR. Plenge RM. Deeks SG. Gianniny L. Crawford G. Sullivan J. Gonzalez E. Davies L. Camargo A. Moore JM. Beattie N. Gupta S. Crenshaw A. Burtt NP. Guiducci C. Gupta N. Gao X. Qi Y. Yuki Y. Piechocka-Trocha A. Cutrell E. Rosenberg R. Moss KL. Lemay P. O'Leary J. Schaefer T. Verma P. Toth I. Block B. Baker B. Rothchild A. Lian J. Proudfoot J. Alvino DM. Vine S. Addo MM. Allen TM. Altfeld M. Henn MR. Le Gall S. Streeck H. Haas DW. Kuritzkes DR. Robbins GK. Shafer RW. Gulick RM. Shikuma CM. Haubrich R. Riddler S. Sax PE. Daar ES. Ribaudo HJ. Agan B. Agarwal S. Ahern RL. Allen BL. Altidor S. Altschuler EL. Ambardar S. Anastos K. Anderson B. Anderson V. Andrady U. Antoniskis D. Bangsberg D. Barbaro D. Barrie W. Bartczak J. Barton S. Basden P. Basgoz N. Bazner S. Bellos NC. Benson AM. Berger J. Bernard NF. Bernard AM. Birch C. Bodner SJ. Bolan RK. Boudreaux ET. Bradley M. Braun JF. Brndjar JE. Brown SJ. Brown K. Brown ST. Burack J. Bush LM. Cafaro V. Campbell O. Campbell J. Carlson RH. Carmichael JK. Casey KK. Cavacuiti C. Celestin G. Chambers ST. Chez N. Chirch LM. Cimoch PJ. Cohen D. Cohn LE. Conway B. Cooper DA. Cornelson B. Cox DT. Cristofano MV. Cuchural G., Jr. Czartoski JL. Dahman JM. Daly JS. Davis BT. Davis K. Davod SM. DeJesus E. Dietz CA. Dunham E. Dunn ME. Ellerin TB. Eron JJ. Fangman JJ. Farel CE. Ferlazzo H. Fidler S. Fleenor-Ford A. Frankel R. Freedberg KA. French NK. Fuchs JD. Fuller JD. Gaberman J. Gallant JE. Gandhi RT. Garcia E. Garmon D. Gathe JC., Jr. Gaultier CR. Gebre W. Gilman FD. Gilson I. Goepfert PA. Gottlieb MS. Goulston C. Groger RK. Gurley TD. Haber S. Hardwicke R. Hardy WD. Harrigan PR. Hawkins TN. Heath S. Hecht FM. Henry WK. Hladek M. Hoffman RP. Horton JM. Hsu RK. Huhn GD. Hunt P. Hupert MJ. Illeman ML. Jaeger H. Jellinger RM. John M. Johnson JA. Johnson KL. Johnson H. Johnson K. Joly J. Jordan WC. Kauffman CA. Khanlou H. Killian RK. Kim AY. Kim DD. Kinder CA. Kirchner JT. Kogelman L. Kojic EM. Korthuis PT. Kurisu W. Kwon DS. LaMar M. Lampiris H. Lanzafame M. Lederman MM. Lee DM. Lee JM. Lee MJ. Lee ET. Lemoine J. Levy JA. Llibre JM. Liguori MA. Little SJ. Liu AY. Lopez AJ. Loutfy MR. Loy D. Mohammed DY. Man A. Mansour MK. Marconi VC. Markowitz M. Marques R. Martin JN. Martin HL., Jr. Mayer KH. McElrath MJ. McGhee TA. McGovern BH. McGowan K. McIntyre D. McLeod GX. Menezes P. Mesa G. Metroka CE. Meyer-Olson D. Miller AO. Montgomery K. Mounzer KC. Nagami EH. Nagin I. Nahass RG. Nelson MO. Nielsen C. Norene DL. O'Connor DH. Ojikutu BO. Okulicz J. Oladehin OO. Oldfield EC., 3rd Olender SA. Ostrowski M. Owen WF., Jr. Pae E. Parsonnet J. Pavlatos AM. Perlmutter AM. Pierce MN. Pincus JM. Pisani L. Price LJ. Proia L. Prokesch RC. Pujet HC. Ramgopal M. Rathod A. Rausch M. Ravishankar J. Rhame FS. Richards CS. Richman DD. Rodes B. Rodriguez M. Rose RC., 3rd Rosenberg ES. Rosenthal D. Ross PE. Rubin DS. Rumbaugh E. Saenz L. Salvaggio MR. Sanchez WC. Sanjana VM. Santiago S. Schmidt W. Schuitemaker H. Sestak PM. Shalit P. Shay W. Shirvani VN. Silebi VI. Sizemore JM., Jr. Skolnik PR. Sokol-Anderson M. Sosman JM. Stabile P. Stapleton JT. Starrett S. Stein F. Stellbrink HJ. Sterman FL. Stone VE. Stone DR. Tambussi G. Taplitz RA. Tedaldi EM. Theisen W. Torres R. Tosiello L. Tremblay C. Tribble MA. Trinh PD. Tsao A. Ueda P. Vaccaro A. Valadas E. Vanig TJ. Vecino I. Vega VM. Veikley W. Wade BH. Walworth C. Wanidworanun C. Ward DJ. Warner DA. Weber RD. Webster D. Weis S. Wheeler DA. White DJ. Wilkins E. Winston A. Wlodaver CG. van't Wout A. Wright DP. Yang OO. Yurdin DL. Zabukovic BW. Zachary KC. Zeeman B. Zhao M. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330(6010):1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Krause CD. Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- Rempel H. Sun B. Calosing C. Pillai SK. Pulliam L. Interferon-alpha drives monocyte gene expression in chronic unsuppressed HIV-1 infection. AIDS. 2010;24(10):1415–1423. doi: 10.1097/QAD.0b013e32833ac623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotger M. Dang KK. Fellay J. Heinzen EL. Feng S. Descombes P. Shianna KV. Ge D. Gunthard HF. Goldstein DB. Telenti A. Genome-wide mRNA expression correlates of viral control in CD4+ T-cells from HIV-1-infected individuals. PLoS Pathog. 2010;6(2):e1000781. doi: 10.1371/journal.ppat.1000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarajiwa SA. Forster S. Auchettl K. Hertzog PJ. INTERFEROME: the database of interferon regulated genes. Nucleic Acids Res. 2009;37(Database issue):D852–D857. doi: 10.1093/nar/gkn732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14(4):778–809. doi: 10.1128/CMR.14.4.778-809.2001. table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauce D. Larsen M. Fastenackels S. Pauchard M. Ait-Mohand H. Schneider L. Guihot A. Boufassa F. Zaunders J. Iguertsira M. Bailey M. Gorochov G. Duvivier C. Carcelain G. Kelleher AD. Simon A. Meyer L. Costagliola D. Deeks SG. Lambotte O. Autran B. Hunt PW. Katlama C. Appay V. HIV disease progression despite suppression of viral replication is associated with exhaustion of lymphopoiesis. Blood. 2011;117(19):5142–5151. doi: 10.1182/blood-2011-01-331306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BT. Huang da W. Tan Q. Guo Y. Bour S. Liu D. Stephens R. Baseler MW. Lane HC. Lempicki RA. DAVID Knowledgebase: a gene-centered database integrating heterogeneous gene annotation resources to facilitate high-throughput gene functional analysis. BMC Bioinform. 2007;8:426. doi: 10.1186/1471-2105-8-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal FP. Kadowaki N. Shodell M. Fitzgerald-Bocarsly PA. Shah K. Ho S. Antonenko S. Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284(5421):1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Sperber SJ. Gocke DJ. Haberzettl C. Kuk R. Schwartz B. Pestka S. Anti-HIV-1 activity of recombinant and hybrid species of interferon-alpha. J Interferon Res. 1992;12(5):363–368. doi: 10.1089/jir.1992.12.363. [DOI] [PubMed] [Google Scholar]
- Sperber SJ. Hunger SB. Schwartz B. Pestka S. Anti-rhinoviral activity of recombinant and hybrid species of interferon alpha. Antiviral Res. 1993;22(2–3):121–129. doi: 10.1016/0166-3542(93)90090-6. [DOI] [PubMed] [Google Scholar]
- Tanabe Y. Nishibori T. Su L. Arduini RM. Baker DP. David M. Cutting edge: role of STAT1, STAT3, and STAT5 in IFN-alpha beta responses in T lymphocytes. J Immunol. 2005;174(2):609–613. doi: 10.4049/jimmunol.174.2.609. [DOI] [PubMed] [Google Scholar]
- Valle MJ. Jordan GW. Haahr S. Merigan TC. Characteristics of immune interferon produced by human lymphocyte cultures compared to other human interferons. J Immunol. 1975;115(1):230–233. [PubMed] [Google Scholar]
- Walker CM. Erickson AL. Hsueh FC. Levy JA. Inhibition of human immunodeficiency virus replication in acutely infected CD4+ cells by CD8+ cells involves a noncytotoxic mechanism. J Virol. 1991a;65(11):5921–5927. doi: 10.1128/jvi.65.11.5921-5927.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CM. Levy JA. A diffusible lymphokine produced by CD8+ T lymphocytes suppresses HIV replication. Immunology. 1989;66(4):628–630. [PMC free article] [PubMed] [Google Scholar]
- Walker CM. Moody DJ. Stites DP. Levy JA. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Walker CM. Thomson-Honnebier GA. Hsueh FC. Erickson AL. Pan LZ. Levy JA. CD8+ T cells from HIV-1-infected individuals inhibit acute infection by human and primate immunodeficiency viruses. Cell Immunol. 1991b;137(2):420–428. doi: 10.1016/0008-8749(91)90090-x. [DOI] [PubMed] [Google Scholar]
- Wong JK. Strain MC. Porrata R. Reay E. Sankaran-Walters S. Ignacio CC. Russell T. Pillai SK. Looney DJ. Dandekar S. In vivo CD8+ T-cell suppression of siv viremia is not mediated by CTL clearance of productively infected cells. PLoS Pathog. 2010;6(1):e1000748. doi: 10.1371/journal.ppat.1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto JK. Barre-Sinoussi F. Bolton V. Pedersen NC. Gardner MB. Human alpha- and beta-interferon but not gamma- suppress the in vitro replication of LAV, HTLV-III, and ARV-2. J Interferon Res. 1986;6(2):143–152. doi: 10.1089/jir.1986.6.143. [DOI] [PubMed] [Google Scholar]
- Zhang L. Yu W. He T. Yu J. Caffrey RE. Dalmasso EA. Fu S. Pham T. Mei J. Ho JJ. Zhang W. Lopez P. Ho DD. Contribution of human alpha-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science. 2002;298(5595):995–1000. doi: 10.1126/science.1076185. [DOI] [PubMed] [Google Scholar]
- Zhao XX. Liao MJ. Rashidbaigi A. Inhibitory effect of interleukin-10 on human leukocyte interferon-alpha production by Sendai virus. Cytokines Cell Mol Ther. 1998;4(1):11–16. [PubMed] [Google Scholar]