Abstract
To accelerate data sharing and research on traumatic brain injury (TBI), several federal agencies have been collaborating to support the development and implementation of common data elements (CDEs). The first recommendations for CDEs were made in 2010, and were well suited for hospital-based studies of acute TBI in adults. To broaden the utility of the TBI CDEs, experts were asked to update the recommendations to make them relevant to all ages, levels of injury severity, and phases of recovery. The second version of the TBI CDEs (v.2) was organized around four major study types: 1) epidemiological research; 2) studies on acute, hospitalized patients; 3) studies of the rehabilitation for moderate/severe TBI; and 4) mild TBI/concussion research. Given the heterogeneity of TBI, only a small set of core CDEs were found to be relevant across all study types. However, within groups, a much larger set of highly relevant CDEs were identified, and these were called basic CDEs. In addition, an expanded number of supplemental CDEs were specified and recommended for use depending upon the study goals. Version 2 provides a rich data dictionary for TBI research with about 900 CDEs. Many of the CDEs overlap across the study types, which will facilitate comparisons and meta-analysis across studies. Further modifications of the CDEs should be based on evaluation of their usefulness following implementation across a range of studies.
Key words: acute, chronic, collaboration, concussion, data standardization, epidemiology, rehabilitation
Introduction
There is an urgent need to accelerate traumatic brain injury (TBI) research, because of the enormous and growing worldwide health burden of this disorder. To address this need, the Interagency Common Data Elements Project was established in 2008 to promote data sharing and collaboration through the standardization of definitions and protocols for TBI research.1 Data elements are basic units of data that have precise definitions, and those that are commonly used are referred to as common data elements (CDEs). The original recommendations for TBI research were developed and scientifically vetted in 2010.1–7 One year later, the CDEs were modified to make them more compatible with the needs of pediatric TBI research.8–14 From the inception of this project, the CDEs were expected to require regular updating to ensure their continued feasibility and utility.
The first version of the CDEs (v.1) was a major advance toward standardization of TBI research, but there were also limitations that needed to be addressed. One limitation was that 242 CDEs were defined as core, meaning that they were recommended for all clinical TBI studies. However, many of the core items, for example, Apgar scores and intracranial pressure measurements, were not relevant to all TBI research, and collecting them was impractical or impossible in studies of certain TBI populations.1,15,16 Another major limitation of v.1 was the lack of elements relevant to studies on milder injuries and on more chronic phases of TBI. Finally, it became apparent that several of the CDEs were redundant, and that one or the other should be eliminated. To address these limitations, the TBI CDEs have been updated in version 2 (v.2). An overview of the updated TBI CDE recommendations, and the process and rationale for the creation of this update, are described in this article.
Methods
After agreeing that there was a need for further modifications to the TBI CDEs, liaisons from the participating agencies nominated scientific experts to serve on new workgroups formed around the following types of TBI studies.
• The workgroup on epidemiology studies addressed two tasks: 1) refining the core CDEs to ensure their relevance to all TBI research; and 2) recommending additional data elements for epidemiological studies. Epidemiology studies tend to have large sample sizes and a small number of data elements in order to characterize a population or examine incidence and prevalence of TBI in a population. The workgroup also considered epidemiological studies that focus on medical record reviews, studies using registries such as trauma registries, and large survey studies.
• The focus of the workgroup on studies of acute, hospitalized subjects was on patients who are admitted to a hospital because of a TBI. The brain injury may have occurred in isolation or in conjunction with systemic injuries. Most patients in this type of study demonstrate acute trauma-related intracranial pathology on CT scans. The spectrum of subjects ranges from the most severely injured patients to those with good neurological status and relatively minor imaging abnormalities. The exact boundary between the least severely injured acute, hospitalized patients and the most severely injured concussion/mild TBI patients is admittedly ambiguous, and drawing it is left to the individual researcher's discretion. The acute, hospitalized domain includes military and civilian populations, as well as both children and adults.
• The workgroup on studies of rehabilitation for moderate–severe TBI reviewed research data elements pertinent to the assessment and rehabilitation of persons who receive a Glasgow Coma Scale (GCS) score of 3–12 within 24 h of injury or demonstrate post-traumatic amnesia for ≥24 h. The workgroup focused on CDEs related to physical and cognitive assessment, treatment interventions, and outcome measures administered to both adult and pediatric populations. The resulting CDE recommendations were intended to apply to rehabilitation research conducted within acute hospital, inpatient rehabilitation, and outpatient settings.
• The workgroup on mild TBI/concussion studies addressed research data elements pertinent to subjects who either require no hospitalization or acute medical care, only a brief visit to the emergency department or physician without hospital admission, or only a brief hospitalization related to the TBI. The workgroup considered both acute and chronic phases of mild TBI.
The composition of the four workgroups was determined by the type of research facility the member represented, their TBI subspecialty, the types of TBI cohorts they had previously studied, and their geographic location; diversity of perspectives was a major consideration. Approximately half of the workgroup members were former members, and the other half were new. Experts in both pediatric and adult TBI research were included, as well as experts in civilian and military TBI. New chairs were also appointed for the workgroups (see Appendix for a list of the workgroup chairs and members). Rather than separating workgroups into “imaging,” “biomarkers,” “outcomes,” and “demographics” as was done in creating v.1, all these topics were considered for respective TBI patient groups within the new structure.
The process for updating the CDEs was similar to that used to develop earlier versions and has been previously described.5,14 Briefly, the CDEs are identified, defined, and vetted by experts in the scientific community, both nationally and internationally. The participating agencies had a “hands off” approach, their staff primarily serving to facilitate the process but not to determine the content of the recommendations. Following an introductory call, the workgroups held conference calls every 3–5 weeks for 4–6 months to reach consensus based on evidence and expert opinion. In addition, the experience and knowledge gained from a pilot study to test the feasibility and utility of collecting the TBI CDEs was incorporated into the workgroup recommendations.17 In cases in which disagreements could not be resolved within the workgroup, the issues were presented to the steering committee, where a decision was agreed upon. The major criteria for inclusion used by all the workgroups were that the v.1 data elements should be preserved and that both new and previously identified data elements should adhere to the updated category definitions for core, basic, and supplemental CDEs (Table 1). After each workgroup had reached consensus, the workgroup chairs met to review all of the recommendations across groups. Next, the draft CDEs were posted on the National Institute of Neurological Disorders and Stroke (NINDS) CDE web site for external review and vetting by the larger research community.18 The process was transparent and inclusive, and collaboration with other agencies and groups that have an interest in CDEs for TBI research was encouraged.
Table 1.
| Tiers | Definition | v.1 | v.2 |
|---|---|---|---|
| Core | A very small set of items relevant to all TBI clinical studies | 242 | 16 |
| Basic | A small set of data elements, beyond the core, recommended for inclusion in specific types of studies | N/A | 224 |
| Supplemental | A large number of optional items for which inclusion depends upon the scope and focus of the research question | 140 | 655 |
| Emerging | Dropped from version 2.0 because the criteria for classifying a CDE as emerging or supplemental were overlapping. | 98 | N/A |
| Total | 480 | 895 |
Outcome measures often include multiple data elements; therefore, they are now reported separately from the individual common data elements (CDEs), and are not included on this table.
Numbers reflect total for both adult and pediatric studies.
CDEs are only counted once; if an item is classified as basic for one study type, and supplemental for another, it is counted as basic.
TBI, traumatic brain injury.
Results
The most significant changes overall between v.1 and v.2 of the TBI CDEs were 1) a marked decrease in the number of core CDEs, 2) an expansion of the total number of CDEs to include more items relevant to milder injuries and the more chronic phases of TBI, 3) reorganization of the categories to include a second tier for items highly relevant to specific types of studies but not to all studies, called basic, 4) dropping the emerging tier for lack of evidence to discriminate it from supplemental CDEs (Table 1), and 5) changing the name to the International TBI Common Data Elements Project. Additional minor changes included alignment of demographic data elements with those endorsed by the National Library of Medicine, to increase their generalizability across other disease areas, and separation of the lists of individual data elements from outcome measures, because the latter often include multiple data elements.
Table 5.
Basic CDEs for Rehabilitation Studies for Moderate–Severe TBIa
| All ages | |
|---|---|
| Education years number | Epidural hematoma indicator |
| Traumatic brain injury mechanism type | Subdural hematoma acute indicator |
| Pupil reactivity to light left eye result | Subarachnoid hemorrhage indicator |
| Pupil reactivity to light right eye result | Midline shift supratentorial indicator |
| Subarachnoid hemorrhage indicator | Contusion indicator |
| Hospital discharge date and time | Intracerebral hemorrhage indicator |
| Hospital discharge destination type | Intraventricular hemorrhage indicator |
| Pupil left eye measurement | Diffuse axonal injury indicator |
| Pupil right eye measurement | Penetrating injury indicator |
| Pupil shape left eye type | Intracranial procedures indicator |
| Pupil shape right eye type | Cervicomedullary junction or brainstem injury indicator |
| Loss of consciousness indicator | Edema indicator |
| Loss of consciousness reporter type | Brain swelling indicator |
| Post-traumatic amnesia indicator | Ischemia or infarction or HI injury indicator |
| Post-traumatic amnesia reporter type | Brain atrophy or encephalomalacia result |
| Alteration of consciousness indicator | Therapy or rehabilitation type |
| Alteration of consciousness reporter type | Therapy or rehabilitation ICD-9-CM code |
| Imaging study date and time | Therapy or rehabilitation frequency |
| Imaging modality type | Therapy or rehabilitation session duration |
| Imaging scanner strength value | Therapy or rehabilitation start date and time |
| Imaging scanner manufacturer name | Therapy or rehabilitation end date and time |
| Imaging scanner model name | Therapy or rehabilitation ongoing indicator |
| Imaging scanner software version number | Residence type |
| Imaging sequence type | Death date and time |
| Adult-specific | Pediatric-specific |
|---|---|
| Marital or partner status | Education/school participation status |
Definitions and guidelines are available on the CDE web site.18
CDE, common data element; TBI, traumatic brain injury; H-I, hypoxia-ischemia; ICD-9-CM, The International Classification of Diseases, Ninth Revision, Clinical Modification.
Revisions to the core CDEs
There was a 15-fold reduction in the number of core CDEs between v.1 and v.2. The smaller number now more accurately reflects “a very small set of items that are relevant to all TBI clinical studies.” Despite their limited number, the core CDEs cover several domains, including demographic characteristics, social status, injury characteristics, etiology, severity indicators, and outcomes (Table 2). Most of the core CDEs pertain to both adults and children, but a few items are age-specific (Table 2).
Table 2.
Core CDEs and Outcome Measures for TBI Researcha
| CDEs – All Ages | |
|---|---|
| Birth date | Traumatic brain injury type |
| Gender | Injury ICD-9-CM external cause code |
| Race (United States category) | Loss of consciousness duration range |
| Ethnicity (United States category) | Post-traumatic amnesia duration range |
| Education level (United States type) | Brain imaging result (if applicable and known) |
| Injury date (and time, if applicable and known) |
| CDEs – adult-specific | CDEs – pediatric-specific |
|---|---|
| GCS motor response | GCS pediatric motor response |
| GCS eye response | GCS pediatric eye response |
| GCS verbal response | GCS pediatric verbal response |
| GCS total score | GCS pediatric total score |
| Employment status | Years of education - primary caregiver |
| Outcome measure – adult specific | Outcome measure – pediatric specific |
|---|---|
| Glasgow Outcome Scale - Extended |
Definitions, codes, permissible values, and other guidelines are available on the CDE web site.18
CDE, common data element; TBI, traumatic brain injury; ICD-9-CM, The International Classification of Diseases, Ninth Revision, Clinical Modification; GCS, Glasgow Coma Scale.
Recommendations for basic and supplemental CDEs
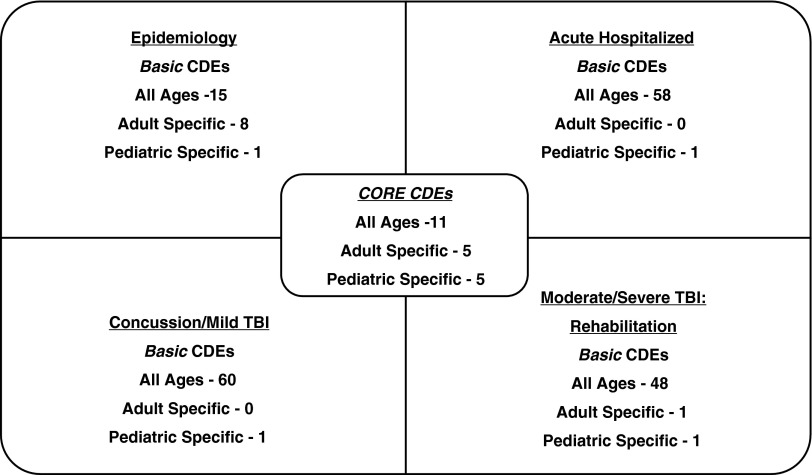
As mentioned, another major revision was that highly relevant items beyond the core were added to meet the needs of four common types of studies in the areas of: epidemiology, acute-hospitalization; rehabilitation for moderate–severe TBI, and mild TBI/concussion. CDEs and outcome measures that are highly relevant or essential for these specific types of studies, but not necessarily relevant to all other types of studies, were called basic (Fig. 1). A summary of the basic CDEs for each study type follows.
FIG. 1.
Core and basic common data elements (CDEs) by study type or population. Note that few basic CDEs are unique to one group; most overlap with one or two other subgroups.
Epidemiology studies
The recommendations for the 24 basic CDEs for epidemiological studies were based on a logical expansion beyond the core, and include: additional subject/participant characteristics; details of educational and job status; marital/partner status; details of the injury, for example, the Ohio State University TBI Identification Method (OSU TBI ID); reliability of injury date; likelihood of abusive head trauma; Abbreviated Injury Scale score; duration of alteration of consciousness; details of types of care received related to the injury, that is, location of definitive clinical care and immediate medical services received after injury, and emergency department and hospital discharge destinations; and outcomes such as return to work/school, type of residence, and date and cause of death.19,20 The epidemiology basic CDEs contained one that was only for pediatric populations (education status), and eight only for adults, including job and marital status and the OSU TBI ID (Table 3).
Table 3.
Basic CDEs for TBI Epidemiology Studiesa
| All ages | |
|---|---|
| Education years number | Emergency room discharge destination type |
| Injury date reliability type | Alteration of consciousness duration range |
| Injury immediate medical services received indicator | Return to work or school status |
| Abusive head trauma likelihood type | Residence type |
| Abbreviated Injury Scale body region category | Death date and time |
| Abbreviated Injury Scale body region score | Death cause text |
| Hospital discharge destination type | Death cause ICD-9-CM code |
| Definitive clinical care location type |
| Adult-specific | Pediatric-specific |
|---|---|
| Job classification status | Education/school participation status |
| Marital or partner status | |
| OSU TBI ID SF Scoring Q1–Q6 |
Definitions, codes, permissible values, and other guidelines are available on the CDE web site.18
CDE, common data element; TBI, traumatic brain injury; ICD-9-CM, The International Classification of Diseases, Ninth Revision, Clinical Modification; OSU TBI ID SF, Ohio State University TBI Identification Method, Short Form.
Studies of acute, hospitalized patients
As mentioned, the acute, hospitalized subgroup refers to studies in which the subjects are admitted to a hospital because of an acute TBI. Patients in this category are commonly described as having “moderate” or “severe” TBI. However, members of this workgroup wished to avoid use of the “mild/moderate/severe” classification scheme, which may blur important distinctions and oversimplify. The name of this category recognizes the immediate post-injury time period during which these patients are seen. It also acknowledges that all of these patients are admitted to the hospital, even if only briefly. The workgroup recommended 58 basic CDEs for all ages, plus 1 pediatric-specific item (Fig. 1 and Table 4).
Table 4.
Basic CDEs for TBI Studies of Acute, Hospitalized Subjectsa
| All ages | |
|---|---|
| Age value | Respiratory rate |
| Education years number | Heart rate |
| Injury date reliability type | Blood pressure systolic measurement |
| Hospital admission date and time | Blood pressure diastolic measurement |
| Hospital discharge date and time | Oxygen saturation measurement |
| Hospital discharge destination type | Traumatic brain injury mechanism type |
| Emergency room discharge destination type | Traffic accident self-alcohol influence likelihood |
| GCS confounders type | Hypoxic episode indicator |
| Loss of consciousness indicator | Hypotensive episode indicator |
| Alteration of consciousness indicator | Pupil reactivity to light left eye result |
| Lab specimen collection date and time | Pupil reactivity to light right eye result |
| Lab panel category | Marshall CT classification code |
| Lab specimen type | Midline shift supratentorial indicator |
| Lab test name | Cisternal compression indicator |
| Lab test LOINC code | Cisternal compression type |
| Lab test result | Contusion indicator |
| Lab test result unit of measure | Intracerebral hemorrhage indicator |
| Lab test result status | Intraventricular hemorrhage indicator |
| Lab test abnormality significance type | Diffuse axonal injury indicator |
| Imaging study date and time | Traumatic axonal injury indicator |
| Imaging modality type | Penetrating injury indicator |
| Imaging scanner software version number | Penetrating injury associated findings type |
| Imaging sequence type | Cervicomedullary junction or brainstem injury indicator |
| Intracranial surgery indicator | Edema indicator |
| Epidural hematoma indicator | Ischemia or infarction or hypoxic-ischemic injury indicator |
| Extra-axial hematoma indicator | Brain atrophy or encephalomalacia result |
| Subdural hematoma acute indicator | Vital status |
| Subdural hematoma mixed density or CSF-like collection indicator | Death date and time |
| Subarachnoid hemorrhage indicator | |
| Post-traumatic amnesia indicator |
| Adult-specific | Pediatric-specific |
|---|---|
| Education/school participation status |
Definitions, codes, permissible values and other guidelines are available on the CDE web site18
CDE, common data element; TBI, traumatic brain injury; LOINC, Logical Observation Identifiers Names and Codes; CSF, cerebrospinal fluid.
Studies of rehabilitation for moderate–severe TBI
The workgroup for these kinds of studies endorsed 48 basic CDEs for all ages, plus 1 adult-specific item and 1 pediatric-specific item (Fig. 1 and Table 5). Of these, ten are unique to rehabilitation research. Four items were selected from the assessments and exams domain to reflect early prognostic indicators (i.e., pupil reactivity, size and shape, alteration of consciousness: reporter type), and are intended to complement the GCS score from the core CDEs. Four items were chosen from the treatment domain to characterize the nature of therapeutic interventions applied (i.e., type of therapy, frequency and duration of sessions, duration of course) (Table 5). The workgroup acknowledged that TBI rehabilitation lacks a coherent taxonomy to adequately characterize treatment approaches and methods. This impedes the development of foundational treatment constructs and places significant constraints on comparative effectiveness research.
Mild TBI/concussion studies
In the recommendations for mild TBI/concussion studies, basic elements included 60 items for all ages, plus 1 item specific to children (Fig. 1 and Table 6). The basic CDEs included items that are relevant to studies of both the acute and chronic phases of injury. An effort was made to incorporate some of the variables and outcome measures that are specifically used in military and sport-related concussion studies as supplemental CDEs. Whereas the outcome measures were otherwise generally retained from v.1, several were added to specifically address research questions that are common within this area of study. For example, the workgroup added symptom validity testing, computerized batteries, and telephone follow-up as these were considered important in many studies in this area.
Table 6.
Basic CDEs for Mild TBI/Concussion Studiesa
| All ages | |
|---|---|
| Age value | Epidural hematoma indicator |
| Language primary ISO 639-2 code | Extra-axial hematoma indicator |
| Language primary text | Subdural hematoma acute indicator |
| Education years number | Subdural hematoma subacute or chronic indicator |
| Concussion prior number | Subdural hematoma mixed density or CSF-like collection indicator |
| Injury date reliability type | Subarachnoid hemorrhage indicator |
| Injury date and time estimation type | Vascular dissection indicator |
| Symptom onset date and time | Traumatic aneurysm indicator |
| Hospital first treated arrival date and time | Venous sinus injury indicator |
| Hospital admission date and time | Midline shift supratentorial indicator |
| Abusive head trauma likelihood type | Cisternal compression indicator |
| TBI mechanism type | Ventricle - fourth shift or effacement indicator |
| Subarachnoid hemorrhage indicator | Contusion indicator |
| Seizure indicator | Contusion findings type |
| Seizure TBI presentation type | Intracerebral hemorrhage indicator |
| Definitive clinical care location type | Intraventricular hemorrhage indicator |
| GCS confounders type | Diffuse axonal injury indicator |
| Loss of consciousness indicator | Subarachnoid hemorrhage indicator |
| Loss of consciousness reporter type | Subdural hematoma acute indicator |
| Post-traumatic amnesia indicator | Subdural hematoma mixed density or CSF-like collection indicator |
| Post-traumatic amnesia reporter type | Subdural hematoma subacute or chronic indicator |
| Alteration of consciousness indicator | Symptom onset date and time |
| TBI symptom or sign type | Traumatic aneurysm indicator |
| TBI symptom or sign indicator | Traumatic axonal injury indicator |
| Imaging study date and time | Traumatic brain injury mechanism type |
| Imaging modality type | Vascular dissection indicator |
| Imaging scanner strength value | Venous sinus injury indicator |
| Imaging scanner manufacturer name | Ventricle - fourth shift or effacement indicator |
| Imaging scanner model name | |
| Imaging scanner software version number | |
| Imaging sequence type | |
| Skull fracture indicator |
| Adult-specific | Pediatric-specific |
|---|---|
| Education school participation status |
Definitions, codes, permissible values and other guidelines are available on the CDE web site.18
CDE, common data element; TBI, traumatic brain injury; GCS, Glasgow Coma Scale; CSF, cerebrospinal fluid.
Supplemental data
In addition to the core and basic CDEs there are hundreds of optional supplemental data elements and outcome measurement tools that may be useful depending upon the aims of the study.20 The list of supplemental items was intended to be large and inclusive, in order to provide a broad range of options, but is expected to gradually narrow as evidence accumulates in favor of specific CDEs. The entire core, many of the basic, and most of the supplemental CDEs are shared across two or more study types, which will facilitate meta-analyses not only within, but also across study types. It is also worth noting that the supplemental data elements are not viewed as comprising an exhaustive list, and, depending upon the purpose of the particular study, additional items may be needed.
Recommendations for outcome measures
The four workgroups also reviewed an extensive list of assessment instruments and scales for use in TBI research. One outcome measure was recommended as a core recommendation, the Glasgow Outcome Score - Extended, but only for older children and adults. The Pediatric Glasgow Outcome Score - Extended was not recommended as a core CDE for pediatric TBI, reflecting differences in expert opinion regarding its utility for studying milder forms of TBI. In addition to the one core outcome measure, a battery of basic outcome measures was also recommended by three of the four workgroups (Table 7). For studies of adults, the battery includes the Motor and Cognitive subscales of the Functional Independence Measure (FIM) to assess motor and cognitive activity limitations, respectively. The Disability Rating Scale (DRS) was chosen for global outcome assessment. To investigate specific neuropsychological functions, the Processing Speed Index from the Wechsler Adult Scale of Intelligence-IV was recommended for evaluating speed of processing, the Trail Making Test for attention and mental control, and the Rey Auditory Verbal Learning Test or the California Verbal Learning Test for memory. For evaluation of quality of life, the Satisfaction with Life Scale was recommended; the Craig Handicap Assessment and Reporting Technique (Short Form) was recommended for measuring societal participation after moderate to severe TBI; and the Rivermead Postconcussive Symptom Questionnaire was recommended for assessing post-concussive symptoms after mild TBI. Finally, the Brief Symptom Inventory-18 was recommended for assessment of psychological status. Most of the measures described have complementary pediatric versions (Table 7).
Table 7.
Basic Outcome Measures by Study Population
| All ages | Acute, hosp. | Mod-severe rehab. | Mild TBI/concussion |
|---|---|---|---|
| Rey Auditory Verbal Learning Test (RAVLT) or California Verbal Learning Test- II (CVLT-II) or (CVLT-C) | X | X | X |
| Adult-specific | |||
| Wechsler Adult Intelligence Scale (WAIS-IV), Processing Speed Index | X | X | X |
| Brief Symptom Inventory - 18 Item (BSI-18) | X | X | X |
| Trail Making Test (TMT) | X | X | X |
| Satisfaction with Life Scale (SWLS) | X | X | |
| Disability Rating Scale (DRS) | X | X | |
| Functional Independence Measure (FIM): Motor Subscale and Cognition Subscale (Cog-FIM) | X | ||
| Craig Handicap and Assessment Reporting Technique, Short Form (CHART-SF) | X | ||
| Rivermead Postconcussive Symptom Questionnaire (RPQ) | X | ||
| Pediatric-specific | |||
| Pediatric Evaluation of Disability Inventory (PEDI) Self Care and Mobility subscales | X | X | X |
| Wechsler Intelligence Scale (WISC-IV), or the Wechsler Preschool and Primary Scale of Intelligence - IV (WPPSI-IV) | X | X | X |
| Pediatric Quality of Life Inventory: Generic core | X | X | X |
| Delis-Kaplan Executive Function System (D-KEFS) Verbal Fluency | X | X | X |
| Pediatric Glasgow Outcome Scale - Extended | X | X | |
| Functional Independence Measure for Children (WeeFIM) | X | ||
| Health and Behavior Inventory (HBI) | X |
TBI, traumatic brain injury.
Discussion
The goal of updating the CDEs was to maintain as much of the v.1 data elements as possible while addressing the need to reduce the core CDEs, fill critical gaps, and eliminate redundancies. This was achieved by creating workgroups in which half of the members were carried over from the v.1 workgroups to ensure continuity of the concepts and process, and half were from new participants chosen to bring in new perspectives. The refinement of the core CDEs, the creation of a new category called basic to target CDE recommendations to specific types of studies, and the expansion of CDEs relevant to milder and/or chronic TBI are all major strengths. At first glance, some data points that are routinely collected in TBI studies, such as GCS score, may seem to have overlooked in compiling the basic CDEs. However, it must be remembered that such elements are classified as core and are recommended to be collected by all of the various types of studies. Other improvements of v.2 are the elimination of redundant CDEs and alignment with the National Library of Medicine standards. Finally, moving the emerging category of CDEs into the supplemental category is another improvement, because it will facilitate data-driven comparisons of their usefulness.
A remaining issue across both versions of the CDEs is that the battery of outcome measures that are highly recommended (basic) includes five to nine assessments or tools depending up the study type, which may be too many for practical use by most research studies. Although a smaller battery of assessment tools would facilitate universal implementation, the workgroups were unable to narrow the recommendations because of the breadth of symptoms associated with TBI, the all-inclusive age range embraced by this project, and the lack of evidence to strongly support one tool over another. However, there are two new computer-adapted testing tools that sound very promising. One is the Neuro-QOL, which is a very comprehensive, patient-report outcome measure that has undergone validation studies on adults with a variety of neurological disorders, including TBI, but still needs pediatric validation studies.21 The other is the National Institutes of Health (NIH) Toolbox for Neurological Function, which consists of a 2 h battery to assess cognitive, emotional, executive, and sensorimotor function.22 The toolbox has been validated in healthy subjects, but not yet in those with TBI. Employing state-of-the-art technology makes it possible for these tools to be very comprehensive, but also brief, because of the adaptive testing utilized. Following validation studies in TBI populations, these two tools may provide brief, inexpensive, and reliable outcome measures for both pediatric and adult TBI research.
One remaining concern with respect to v.2 is the seemingly arbitrary nature by which some data elements are classified, such as basic versus supplemental. This potential weakness is readily acknowledged. This version of the CDEs balanced the evidence-based practice approach with the imperatives of practicality. Future revisions of the TBI CDEs will have a greater emphasis on considering the evidentiary basis for making such categorizations. Before updating to v.3, many workgroup members recommend the real-world use of v.2 to determine which CDEs and outcome measures are most valuable for characterizing patient populations, evaluating tools, and predicting outcomes. Therefore, implementation of the TBI CDEs is the next major challenge. Implementation will be facilitated by the newly developed Federal Interagency TBI Research (FITBIR) Informatics System, which will use the TBI CDEs as its data dictionary.23 FITBIR provides a platform for data sharing, which will accelerate research by allowing individual subject meta-analysis and rigorous comparisons across studies. A further important step toward implementation will be obtaining endorsements by professional organizations. Currently, the American Association of Neurological Surgeons and the Congress of Neurological Surgeons have endorsed v.2 of the TBI CDEs. Translation into other languages to allow use in international studies is also a priority for the future.
Looking beyond implementation, there is the larger question of whether the International TBI Common Data Elements Project will lead to significant advances in knowledge. The concept of harmonization of data elements to enable meta-analysis and collaboration has emerged over the past decade. There are numerous neurological diseases with CDEs, including stroke, epilepsy, and Parkinson's disease, which were created in a manner similar to those for TBI.24 The Alzheimer's Disease Neuroimaging Initiative has demonstrated that data sharing can be very productive, and can accelerate the development of biomarkers and address other questions of high clinical relevance.25 The Institute of Medicine has also described a vision for creating knowledge networks, which are built on CDEs and data sharing, as a platform for personalized medicine and better patient outcomes.26
Conclusion
The International CDE Project for TBI research has been endorsed by numerous Federal agencies and professional organizations and will serve as the data dictionary for the newly developed FITBIR Informatics System. The creation of v.2 was necessary to increase the feasibility and relevance of the TBI CDEs to a wide range of study types and populations. It is anticipated that additional new data elements and small modifications to the current ones may occur in the near term, but that major revisions will be postponed until enough data are in hand to justify the changes.
Appendix
Members of the TBI Common Data Elements (2.0) Workgroups
- • Core CDEs and Epidemiology Studies
- ○ Cindy Harrison-Felix, PhD - Craig Hospital, Chair
- ○ Davida M. Carr, MPH - HJF Contractor for Defense and Veterans Brain Injury Center/Defense Centers of Excellence
- ○ Wayne A. Gordon, PhD, ABPP/Cn - Department of Rehabilitation Medicine, Mount Sinai School of Medicine
- ○ Harvey Levin, PhD - Baylor College of Medicine
- ○ David K. Menon, MD, PhD - Division of Anaesthesia, University of Cambridge
- ○ Jennie Ponsford, MA, PhD - School of Psychology and Psychiatry, Monash University
- ○ Karen Schwab, PhD - Defense and Veterans Brain Injury Center, Walter Reed Army Medical Center
- ○ Grahame Simpson, PhD - Brain Injury Rehabilitation Unit, Sydney, Australia
- ○ Hilaire J. Thompson, PhD, RN, CNRN, FAAN - Biobehavioral Nursing and Health Systems, School of Nursing, University of Washington
- ○ Pieter E. Vos, MD, PhD - Department of Neurology, Radboud University Nijmegen Medical Centre
- ○ Jerry Wright, MS, CBIST - Rehabilitation Research Center, Santa Clara Valley Medical Center
- • Concussion/Mild TBI Studies
- ○ Elisabeth Wilde, PhD - Departments of Physical Medicine and Rehabilitation, Neurology and Radiology, Baylor College of Medicine and Michael E. DeBakey VA Medical Center, Chair
- ○ Vicki Anderson, PhD - Integrated Mental Health Program, Royal Children's Hospital
- ○ Victor G. Coronado, MD - National Center for Injury Prevention and Control, Centers for Disease Control and Prevention
- ○ Gavin Davis, MBBS - Cabrini Medical Centre
- ○ Thomas J. DeGraba, MD, FAHA - National Intrepid Center of Excellence
- ○ Sureyya Dikmen, PhD - Department of Rehabilitation Medicine, University of Washington
- ○ Gerard A. Gioia, PhD - Children's National Medical Center, George Washington University School of Medicine
- ○ Robin A. Hurley, MD - W. G. (Bill) Hefner VA Medical Center
- ○ Grant L. Iverson, PhD - Department of Psychiatry, University of British Columbia
- ○ Andy Jagoda, MD, FACEP - Department of Emergency Department, Mount Sinai School of Medicine
- ○ Michael W. Kirkwood, PhD, ABPP/CN - University of Colorado Denver School of Medicine, Department of Physical Medicine & Rehabilitation
- ○ Russell R. Lonser, MD - Department of Neurological Surgery, The Ohio State University College of Medicine
- ○ Geoffrey T. Manley, MD, PhD - University of California, San Francisco, San Francisco General Hospital, Brain and Spinal Injury Center
- ○ Jeffrey E. Max, MD - Department of Psychiatry, University of California, San Diego & Director, Neuropsychiatric Research, Rady Children's Hospital
- ○ Thomas W. McAllister, MD - Department of Psychiatry, Indiana University School of Medicine
- ○ Michael McCrea, PhD, ABPP - Departments of Neurosurgery and Neurology, Medical College of Wisconsin
- ○ Paul McCrory, MBBS, PhD - Melbourne School of Health Sciences
- ○ Pratik Mukherjee, MD, PhD - Department of Radiology, University of California, San Francisco
- ○ Douglas C. Oberly, MS - BrainScope Company, Inc.
- ○ Ross H. Pastel, PhD - National Intrepid Center of Excellence, National Naval Medical Center
- ○ Leslie S. Prichep, PhD - Brain Research Laboratories, Department of Psychiatry, New York University School of Medicine
- ○ Michael E. Singer, PhD - BrainScope Company, Inc.
- ○ Keith O. Yeates, PhD - The Research Institute at Nationwide Children's Hospital
- • Studies of Acute, Hospitalized Patients
- ○ Alex Valadka, MD, FAANS, FACS - Seton Brain and Spine Institute, Chair
- ○ Rachel P. Berger, MD, MPH - Child Advocacy Center, Children's Hospital of Pittsburgh, University of Pittsburgh Medical Center
- ○ Ann-Christine Duhaime, MD - Massachusetts General Hospital
- ○ Ronald L. Hayes, PhD - Center of Innovative Research, Banyan Biomarkers, Inc.
- ○ Ramona Hicks, PhD - National Institute of Neurological Disorders and Stroke, National Institutes of Health
- ○ Jamie Hutchison, MD - The Hospital for Sick Children
- ○ Andrew I.R. Maas, MD, PhD - Department of Neurosurgery, University Hospital of Antwerp
- ○ Geoffrey T. Manley, MD, PhD - University of California, San Francisco, San Francisco General Hospital, Brain and Spinal Injury Center
- ○ David K. Menon, MD, PhD - Division of Anaesthesia, University of Cambridge
- ○ Jose A. Pineda, MD, MSc - Department of Pediatrics and Neurology, Washington University School of Medicine
- ○ Robert D. Stevens, MD - Departments of Anesthesiology/Critical Care Medicine, Neurology, Neurosurgery, and Radiology, The Johns Hopkins University School of Medicine, Division of Neurosciences Critical Care
- ○ Willie Stewart, MBChB, PhD - Department of Neuropathology, Institute of Neurological Sciences, Southern General Hospital
- ○ Nino Stocchetti, MD - University of Milan
- ○ Monica S. Vavilala, MD - Harborview Injury Prevention and Research Center
- ○ David W. Wright, MD, FACEP - Department of Emergency Medicine, Emory University School of Medicine
- ○ Esther L. Yuh, MD, PhD - University of California, San Francisco
- • Studies of Rehabilitation for Moderate–Severe TBI Rehabilitation
- ○ Joseph T. Giacino, PhD - Spaulding Rehabilitation Hospital, Department of Physical Medicine and Rehabilitation, Harvard Medical School, Chair
- ○ Beth M. Ansel, PhD, CCC-SLP - National Center for Medical Rehabilitation Research, The Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health
- ○ Kathleen R. Bell, MD - Department of Rehabilitation Medicine, University of Washington
- ○ Tamara Bushnik, PhD, FACRM - Department of Rehabilitation Medicine, Rusk Institute for Rehabilitation
- ○ David X. Cifu, MD - Department of Physical Medicine and Rehabilitation, Virginia Commonwealth University, Physical Medicine and Rehabilitation Program Office, U.S. Department of Veterans Affairs
- ○ Linda Ewing-Cobbs, PhD - Dan L Duncan Children's Neurodevelopmental Clinic, Children's Learning Institute
- ○ Tessa Hart, PhD - Moss Rehabilitation Research Institute
- ○ Stuart W. Hoffman, PhD - Office of Research and Development, U.S. Department of Veterans Affairs
- ○ Stephanie A. Kolakowsky-Hayner, PhD, CBIST - Rehabilitation Research Center, Santa Clara Valley Medical Center
- ○ Steven Laureys, MD, PhD - University Hospital of Liège
- ○ A. Cate Miller, PhD - National Institute on Disability and Rehabilitation Research, U.S. Department of Education
- ○ Jennie Ponsford, MA, PhD - School of Psychology and Psychiatry, Monash University
- ○ Louis Puybasset, MD, PhD - The Pitié-Salpêtrière Hospital
- ○ M. Elizabeth Sandel, MD - Kaiser Foundation Rehabilitation Center
- ○ David S. Tulsky, PhD - University of Michigan, North Campus Research Complex
- ○ Shari Wade, PhD - Department of Pediatrics, Cincinnati Children's Hospital Medical Center
Federal Liaisons to TBI Common Data Elements Project
○ Beth M. Ansel, PhD, CCC-SLP - National Center for Medical Rehabilitation Research, The Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health
○ Victor G. Coronado, MD - National Center for Injury Prevention and Control, Centers for Disease Control and Prevention
○ Ken Curley, MD - Combat Casualty Care Directorate, U.S. Army Medical Research and Materiel Command
○ Ramona Hicks, PhD - National Institute of Neurological Disorders and Stroke, National Institutes of Health
○ Stuart W. Hoffman, PhD - Office of Research and Development, U.S. Department of Veterans Affairs
○ Matthew McAuliffe, PhD - Center for Information Technology, National Institutes of Health
○ A. Cate Miller, PhD - National Institute on Disability and Rehabilitation Research, U.S. Department of Education
○ Ross H. Pastel, PhD - National Intrepid Center of Excellence, National Naval Medical Center
○ Wanda Salzer, MD - Combat Casualty Care Directorate, U.S. Army Medical Research and Materiel Command,
○ Karen Schwab, PhD - Defense and Veterans Brain Injury Center, Walter Reed Army Medical Center
NINDS TBI Common Data Elements Project Team
○ Joanne Odenkirchen, MPH – National Institute of Neurological Disorders and Stroke, National Institutes of Health, Lead
○ Alison Garcia - Sapient Government Services
○ Stacie Grinnon, MS - KAI Research, Inc. (An Altarum Company)
○ Amy Price, MS - KAI Research, Inc. (An Altarum Company)
Acknowledgments
The authors gratefully acknowledge the significant contributions of the workgroup members and federal liaisons (see Appendix) to the International TBI CDE Project.
Author Disclosure Statement
No competing financial interests exist. The views expressed are those of the authors and do not necessarily reflect those of the agencies or institutions with which they are affiliated, including the United States Department of Health and Human Services. This work is not an official document, guidance, or policy of the United States government, nor should any official endorsement be inferred.
References
- 1.Thurmond V.A. Hicks R. Gleason T. Miller A.C. Szuflita N. Orman J. Schwab K. Advancing integrated research in psychological health and traumatic brain injury: common data elements. Arch. Phys. Med. Rehabil. 2010;91:1633–1636. doi: 10.1016/j.apmr.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 2.Duhaime A.C. Gean A.D. Haacke E.M. Hicks R. Wintermark M. Mukherjee P. Brody D. Latour L. Riedy G. Common data elements in radiologic imaging of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010;91:1661–1666. doi: 10.1016/j.apmr.2010.07.238. [DOI] [PubMed] [Google Scholar]
- 3.Maas A.I. Harrison–Felix C.L. Menon D. Adelson P.D. Balkin T. Bullock R. Engel D.C. Gordon W. Langlois Orman J. Lew H.L. Robertson C. Temkin N. Valadka A. Verfaellie M. Wainwright M. Wright D.W. Schwab K. Common data elements for traumatic brain injury: recommendations from the Interagency Workgroup on Demographics and Clinical Assessment. Arch. Phys. Med. Rehabil. 2010;91:1641–1649. doi: 10.1016/j.apmr.2010.07.232. [DOI] [PubMed] [Google Scholar]
- 4.Manley G.T. Diaz–Arrastia R. Brophy M. Engel D. Goodman C. Gwinn K. Veenstra T.D. Ling G. Ottens A.K. Tortella F. Hayes R.L. Common data elements for traumatic brain injury: recommendations from the Biospecimens and Biomarkers Working Group. Arch. Phys. Med. Rehabil. 2010;91:1667–1672. doi: 10.1016/j.apmr.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Whyte J. Vasterling J. Manley G.T. Common data elements for research on traumatic brain injury and psychological health: current status and future development. Arch. Phys. Med. Rehabil. 2010;91:1692–1696. doi: 10.1016/j.apmr.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 6.Wilde E.A. Whiteneck G.G. Bogner J. Bushnik T. Cifu D.X. Dikmen S. French L. Giacino J.T. Hart T. Malec J.F. Millis S.R. Novack T.A. Sherer M. Tulsky D.S. Vanderploeg R.D. von Steinbuechel N. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch. Phys. Med. Rehabil. 2010;91:1650–1660. doi: 10.1016/j.apmr.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Haacke E.M. Duhaime A.C. Gean A.D. Riedy G. Wintermark M. Mukherjee P. Brody D.L. DeGraba T. Duncan T.D. Elovic E. Hurley R. Latour L. Smirniotopoulos J.G. Smith D.H. Common data elements in radiologic imaging of traumatic brain injury. J. Magn. Reson. Imaging. 2010;32:516–543. doi: 10.1002/jmri.22259. [DOI] [PubMed] [Google Scholar]
- 8.Adelson P.D. Pineda J. Bell M.J. Abend N.S. Berger R.P. Giza C.C. Holtz G. Wainwright M.S. Pediatric TBI Demographics and Clinical Assessment Workgroup. Common data elements for pediatric traumatic brain injury: recommendations from the Working Group on Demographics and Clinical Assessment. J. Neurotrauma. 2012;29:639–653. doi: 10.1089/neu.2011.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger R.P. Beers S.R. Papa L. Bell M. Pediatric TBI CDE Biospecimens and Biomarkers Workgroup. Common data elements for pediatric traumatic brain injury: recommendations from the Biospecimens and Biomarkers Workgroup. J. Neurotrauma. 2012;29:672–677. doi: 10.1089/neu.2011.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duhaime A.C. Holshouser B. Hunter J.V. Tong K. Common data elements for neuroimaging of traumatic brain injury: pediatric considerations. J. Neurotrauma. 2012;29:629–633. doi: 10.1089/neu.2011.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerring J.P. Wade S. The essential role of psychosocial risk and protective factors in pediatric traumatic brain injury research. J. Neurotrauma. 2012;29:621–628. doi: 10.1089/neu.2011.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter J.V. Wilde E.A. Tong K.A. Holshouser B.A. Emerging imaging tools for use with traumatic brain injury research. J. Neurotrauma. 2012;29:654–671. doi: 10.1089/neu.2011.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCauley S.R. Wilde E.A. Anderson V.A. Bedell G. Beers S.R. Campbell T.F. Chapman S.B. Ewing–Cobbs L. Gerring J.P. Gioia G.A. Levin H.S. Michaud L.J. Prasad M.R. Swaine B.R. Turkstra L.S. Wade S.L. Yeates K.O. Pediatric TBI Outcomes Workgroup. Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. J. Neurotrauma. 2012;29:678–705. doi: 10.1089/neu.2011.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller A.C. Odenkirchen J. Duhaime A.C. Hicks R. Common data elements for research on traumatic brain injury: pediatric considerations. J. Neurotrauma. 2012;29:634–638. doi: 10.1089/neu.2011.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finster M. Wood M. The Apgar score has survived the test of time. Anesthesiology. 2005;102:855–857. doi: 10.1097/00000542-200504000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Steiner L.A. Andrews P.J.D. Monitoring the injured brain: ICP and CBF. Brit. J. Anaesth. 2006;97:26–38. doi: 10.1093/bja/ael110. [DOI] [PubMed] [Google Scholar]
- 17.Yuh E.L. Mukherjee P. Lingsma H.F. Yue J.K. Ferguson A.R. Gordon W.A. Valadka A.B. Schnyer D.M. Okonkwo D.O. Maas A.I.R. Manley G.T. TRACK-TBI Investigators. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann. Neurol. 2013;73:224–235. doi: 10.1002/ana.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institute of Neurological Disorders and Stroke. NINDS common data elements: traumatic brain injury CDE standards. 2013. http://www.commondataelements.ninds.nih.gov/TBI.aspx#tab=Data_Standards. [Jan 8;2013 ]. http://www.commondataelements.ninds.nih.gov/TBI.aspx#tab=Data_Standards
- 19.Corrigan J.D. Bogner J.A. Initial reliability and validity of the Ohio State University TBI Identification Method. J. Head Trauma Rehabil. 2007;22:318–329. doi: 10.1097/01.HTR.0000300227.67748.77. [DOI] [PubMed] [Google Scholar]
- 20.Baker S.P. O'Neill B. Haddon W., Jr. Long W.B. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J. Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 21.Gershon R.C. Lai J.S. Bode R. Choi S. Moy C. Bleck T. Miller D. Peterman A. Cella D. Neuro-QOL: quality of life item banks for adults with neurological disorders: item development and calibrations based upon clinical and general population testing. Qual. Life Res. 2012;21:475–486. doi: 10.1007/s11136-011-9958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gershon R.C. Cella D. Fox N.A. Havlik R.J. Hendrie H.C. Wagster M.W. Assessment of neurological and behavioural function: the NIH Toolbox. Lancet Neurol. 2010;9:138–139. doi: 10.1016/S1474-4422(09)70335-7. [DOI] [PubMed] [Google Scholar]
- 23.Federal Interagency TBI Research (FITBIR) Informatics System. 2013. https://fitbir.nih.gov/jsp/about/index.jsp. [Jan 6;2013 ]. https://fitbir.nih.gov/jsp/about/index.jsp
- 24.National Institute of Neurological Disorders and Stroke. NINDS common data elements. 2013. http://www.commondataelements.ninds.nih.gov/#page=Default. [May 11;2013 ]. http://www.commondataelements.ninds.nih.gov/#page=Default
- 25.Carrillo M.C. Bain L.J. Frisoni B.G. Weiner M.W. Worldwide Alzheimer's disease neuroimaging initiative. Alzheimers Dement. 2012;8:337–342. doi: 10.1016/j.jalz.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 26.National Research Council. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]