This multicenter retrospective review describes 152 cases of histoplasmosis and provides an epidemiologic analysis of timing, patterns of presentation, treatment, and outcome. Although late cases occur, the first year after solid organ transplant is the period of highest risk for histoplasmosis.
Keywords: histoplasmosis, fungal infection, solid organ transplant
Abstract
Background. To improve our understanding of risk factors, management, diagnosis, and outcomes associated with histoplasmosis after solid organ transplant (SOT), we report a large series of histoplasmosis occurring after SOT.
Methods. All cases of histoplasmosis in SOT recipients diagnosed between 1 January 2003 and 31 December 2010 at 24 institutions were identified. Demographic, clinical, and laboratory data were collected.
Results. One hundred fifty-two cases were identified: kidney (51%), liver (16%), kidney/pancreas (14%), heart (9%), lung (5%), pancreas (2%), and other (2%). The median time from transplant to diagnosis was 27 months, but 34% were diagnosed in the first year after transplant. Twenty-eight percent of patients had severe disease (requiring intensive care unit admission); 81% had disseminated disease. Urine Histoplasma antigen detection was the most sensitive diagnostic method, positive in 132 of 142 patients (93%). An amphotericin formulation was administered initially to 73% of patients for a median duration of 2 weeks; step-down therapy with an azole was continued for a median duration of 12 months. Ten percent of patients died due to histoplasmosis with 72% of deaths occurring in the first month after diagnosis; older age and severe disease were risk factors for death from histoplasmosis. Relapse occurred in 6% of patients.
Conclusions. Although late cases occur, the first year after SOT is the period of highest risk for histoplasmosis. In patients who survive the first month after diagnosis, treatment with an amphotericin formulation followed by an azole for 12 months is usually successful, with only rare relapse.
The dimorphic fungus Histoplasma capsulatum is most common in the United States in the Ohio and Mississippi River valleys. Sensitivity to Histoplasma antigens among individuals living in these river valleys may exceed 80% [1]. Infection is often asymptomatic or subclinical. Because of the relationship between an individual's underlying immune function and histoplasmosis severity, the early years of the AIDS epidemic resulted in an increase in the incidence of symptomatic and severe Histoplasma infection [2]. Clinicians thus gained experience regarding the diagnosis, treatment, and outcomes of this life-threatening infection in immunosuppressed populations.
In the field of solid organ transplantation (SOT), T-cell immune dysfunction can also be significant, although the incidence of clinical histoplasmosis is <0.5% in most studies [3–8]. Infection can be difficult to predict with variable clinical presentation, response to therapy, and risk for complications. Symptomatic infection among SOT recipients could occur via primary infection, secondary infection in patients with prior exposure who come in contact with a large inoculum in a now immunosuppressed state, and reactivation of previous latent infection. Rarely, transmission from the allograft itself has been reported [9, 10].
Much of our present knowledge of histoplasmosis after SOT comes from single-center studies from areas of variable endemic rates [3, 5]. Specific data regarding diagnosis, incidence, treatment, and outcomes of this infection are needed. The present study attempts to improve our understanding by performing a large multicenter evaluation of histoplasmosis after SOT.
METHODS
All cases of histoplasmosis in SOT recipients diagnosed between 1 January 2003 and 31 December 2010 at 24 participating institutions were retrospectively identified. Multiple transplant centers in the United States were approached and data were collected by those interested in participating. Diagnosis of histoplasmosis required positive culture, positive serum, or urine Histoplasma antigen (Miravista Diagnostics, Indianapolis, Indiana), histopathology demonstrating yeast-like structures characteristic of H. capsulatum, the presence of H or M precipitin bands by immunodiffusion, or complement fixation titers ≥1:8. Progressive disseminated histoplasmosis was defined as clinical, laboratory, or imaging evidence of extrapulmonary involvement as previously suggested [11]. The site of extrapulmonary involvement was determined based on clinical signs or laboratory evidence. Pulmonary histoplasmosis was defined as respiratory symptoms and chest imaging (radiograph or computed tomography) with infiltrates and/or mediastinal lymphadenopathy in the absence of progressive disseminated histoplasmosis. As has been done in previous studies, disease was characterized as mild, moderate, or severe [12]. Mild disease was treated with outpatient therapy, moderate disease required hospitalization but not critical care, and severe disease was treated in an intensive care unit. Suppressive therapy was defined as treatment continued >12 months after diagnosis except if given for relapse. Death was directly attributed to histoplasmosis if the patient had findings of active histoplasmosis and no other cause of death was identified. Evidence of active histoplasmosis included ≥1 of the following: treatment for <6 weeks, persistent signs and symptoms, failure of antigen to decline, demonstration of organisms by cytology or pathology, or positive culture. Histoplasmosis was considered to have contributed to the death if a patient died of another condition but the investigator felt that the patient had evidence for active histoplasmosis. Clinical and demographic data were collected from investigators. Patient management decisions were made by treating clinicians. Institutional review board approval was obtained by the investigator at each site. At some centers, patients included in this series had been included in previously published series [3, 5, 6, 13]. All patient data were collected independently for this study; no data were extracted from previous publications.
Baseline characteristics were compared using the 2-sample independent t test for continuous variables and χ2 test for categorical variables. For variables with expected cell count of <5, Fisher exact test was used. For variables that are not normally distributed, Mann-Whitney and Wilcoxon rank-sum tests were used. To determine risk factors for death and relapse, a multivariate analysis was used. Initially univariate analysis was conducted based on clinical grounds and available variables. Variables included age, disease severity, fungemia, urine antigen results, immunosuppression reduction, amphotericin treatment, type of transplant, and organ involvement. Variables that were statistically significant (P ≤ .05) were then entered into the multivariate analysis. Appropriateness of the model was assessed using Hosmer and Lemeshow goodness-of-fit test. Two way interactions were tested when appropriate. Casewise diagnostics were performed to detect any outliers. SPSS software version 19 was used.
RESULTS
Patient and Center Characteristics
From 24 SOT centers, we identified 152 cases of posttransplant histoplasmosis. One center contributed 22 cases, 5 centers 10–20 cases, and the remaining centers had ≤10 cases. The location of the centers and the rates of histoplasmin reactivity are in Table 1. Baseline characteristics are described in Table 2. Most patients were on maintenance immunosuppression with a calcineurin inhibitor, mycophenolate mofetil, and a corticosteroid. Ten percent of patients were treated for rejection in the 3 months preceding the diagnosis of histoplasmosis.
Table 1.
Participating Centers and Rates of Histoplasmin Skin Test Reactivity
| City, State | Institution | Skin Test Positive (%) [14] | TransNet Center |
|---|---|---|---|
| Omaha, Nebraska | University of Nebraska | 10.7% | Yes |
| Indianapolis, Indiana | Indiana University | 55.1% | No |
| Birmingham, Alabama | University of Alabama | 13.2% | Yes |
| Cleveland, Ohio | Cleveland Clinic | 4.9% | No |
| Columbus, Ohio | Ohio State University | 44.0% | No |
| Iowa City, Iowa | University of Iowa | 26.6% | Yes |
| Madison, Wisconsin | University of Wisconsin | 10.60% | Yes |
| Minneapolis, Minnesota | University of Minnesota | 9.70% | Yes |
| Rochester, Minnesota | Mayo Clinic | 6.40% | Yes |
| Kansas City, Missouri | University of Kansas | 36.00% | No |
| Ann Arbor, Michigan | University of Michigan | 11.80% | Yes |
| Kansas City, Missouri | Infectious Disease Associates of Kansas City | 36.00% | No |
| Lexington, Kentucky | University of Kentucky | 96.90% | No |
| New York, New York | Mount Sinai | 1.40% | No |
| Wichita, Kansas | University of Kansas | 19.10% | No |
| Chicago, Illinois | University of Chicago | 6.80% | No |
| Dayton, Ohio | Wright-Patterson Medical Center | 67.10% | No |
| Dayton, Ohio | Wright State University | 67.10% | No |
| Joplin, Missouri | St John's Regional Medical Center | 53.60% | No |
| Baltimore, Maryland | Johns Hopkins | 11.10% | Yes |
| New Orleans, Louisiana | Ochsner Medical Foundation | 5.20% | No |
| Pittsburgh, Pennsylvania | University of Pittsburgh | 3.90% | No |
| Fontana, California | Kaiser Permanente | 3.60% | No |
| Miami, Florida | University of Miami | 2.80% | No |
Table 2.
Demographic Characteristics of 152 Patients
| Characteristic | % (No.) |
|---|---|
| Age, y, median (range) | 48.5 (3–80) |
| Male sex | 66% (100/152) |
| Race/ethnicity | |
| White | 80% (121/152) |
| African American | 15% (23/152) |
| Latino | 2% (3/152) |
| Asian | 1% (1/152) |
| Diabetes | 34% (51/152) |
| Cancer | 1% (1/152) |
| Organ transplant | |
| Kidney | 51% (78/152) |
| Liver | 16% (24/152) |
| Kidney/pancreas | 14% (22/152) |
| Heart | 9% (14/152) |
| Lung | 5% (8/152) |
| Pancreas | 2% (3/152) |
| Lung/kidney | 1% (1/152) |
| Small bowel | 1% (1/152) |
| Kidney/heart | 1% (1/152) |
| Immunosuppression | |
| Calcineurin inhibitor | 91% (138/152) |
| Mycophenolate preparation | 82% (124/152) |
| Corticosteroid | 76% (115/152) |
| Azathioprine | 3% (4/152) |
| Sirolimus | 1% (1/152) |
| Rejection within 3 mo before diagnosis | 10% (15/152) |
Clinical Characteristics
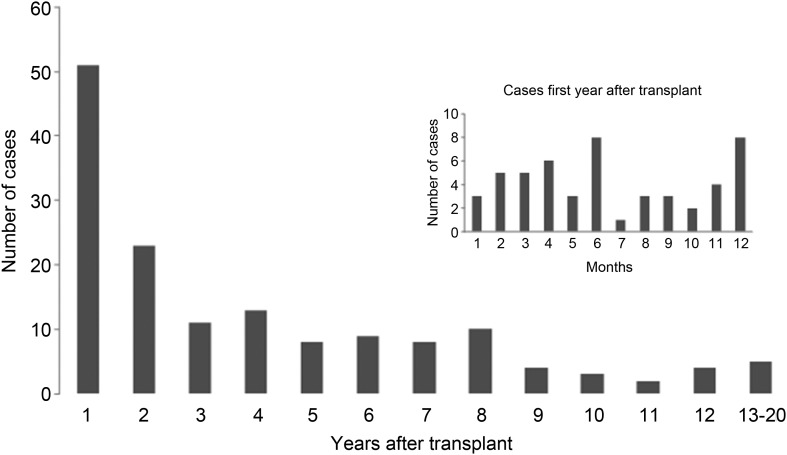
The median time from transplant to diagnosis with histoplasmosis was 27 months, but 34% presented in the first year, with 2% presenting within 1 month of transplant. The longest interval from transplant to diagnosis was 20 years (Figure 1). Most patients had disseminated disease (81%). Table 3 describes specific organ involvement. Fungemia was present in 63% of patients. Twenty-seven percent had severe disease, 63% moderate disease, and 8% mild disease. In multivariate analysis, use of mycophenolate preparation and the presence of fungemia were risk factors for severe disease (Table 3).
Figure 1.
Time in years from transplant to diagnosis with histoplasmosis.
Table 3.
Selected Risk Factors for Severe Disease
| Risk Factor | Mild-Moderatea | Severe | Univariate OR (95% CI) | P Value | Multivariate OR (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Transplant typeb | ||||||
| Heart | 11/105; 10% | 3/43; 7% | 0.71 (.18–2.72) | .76 | ||
| Liver | 15/105; 14% | 9/43; 21% | 1.59 (.63–3.90) | .32 | ||
| Renal | 56/105; 53% | 21/43; 49% | 0.84 (.41–1.70) | .62 | ||
| Pancreas | 1/105; 1% | 2/43; 5% | 5.07 (.56–45.9) | .20 | ||
| Renal/pancreas | 17/105; 16% | 5/43; 12% | 0.68 (.23–1.98) | .48 | ||
| Lung | 5/105; 5% | 3/43; 7% | 1.50 (.34–6.55) | .59 | ||
| Immunosuppression | ||||||
| Calcineurin inhibitor | 99/108; 92% | 40/43; 93% | 1.21 (.28–7.30) | .99 | ||
| Mycophenolate | 83/108; 76% | 39/43; 92% | 2.94 (1.00–8.71) | .05 | 9.41 (1.27–66.1) | .028 |
| Corticosteroid | 86/108; 80% | 28/43; 65% | 0.48 (.22–1.04) | .06 | ||
| Organ involvement | ||||||
| Pulmonary | 85/108; 79% | 37/43; 86% | 2.94 (.99–8.71) | .07 | ||
| Disseminated | 83/108; 77% | 39/43; 91% | 0.37 (.13–1.05) | .06 | ||
| Bone marrow | 23/108; 21% | 16/43; 37% | 2.20 (1.02–4.70) | .04 | 2.62 (.81–12.2) | .098 |
| Liver | 19/108; 18% | 8/43; 19% | 1.07 (.43–2.68) | .89 | ||
| Spleen | 10/108; 9% | 9/43; 21% | 2.50 (.99–6.78) | .06 | ||
| Skin | 4/108; 4% | 1/43; 2% | 0.62 (.07–5.63) | .56 | ||
| Gastrointestinal | 13/108; 12% | 3/43; 7% | 0.50 (.15–2.00) | .56 | ||
| Central nervous system | 6/108; 6% | 4/43; 9% | 1.74 (.47–6.45) | .76 | ||
| Fungemia | 28/57; 49% | 27/30; 90% | 9.32 (2.72–32.1) | .001 | 5.40 (1.24–23.4) | .024 |
| Median urine antigen, ng/mL (No. sent, range) | 10.8 (101c; 0 to >19) | 19 (40c; 0 to >19) | 1.11 (1.05–1.18) | .001 | 1.029 (.87–1.22) | .73 |
| Urine antigen >19 ng/mL | 31% | 56% | 2.9 (1.30–6.20) | .01 | 5.86 (.62–55.7) | .124 |
| Median serum antigen, ng/mL (No. sent, range) | 11.6 (37c; 3.5 to >19) | 10.2 (13c; 0 to >19) | 1.016 (.97–1.07) | .40 | ||
Abbreviations: CI, confidence interval; ng, nanograms; OR, odds ratio.
a Severity of 1 case could not be classified.
b One small bowel and 2 multiorgan nonrenal/pancreas transplants not included.
c No. of patients in whom results of the respective test were available.
Diagnosis
Table 4 describes the overall yield of the diagnostic tests used. Antigen detection provided the highest sensitivity for diagnosis, 93% for antigenuria and 86% for antigenemia. Detection of antibody was the least sensitive diagnostic method, positive in 36% of cases. More than 1 diagnostic test was positive in 115 patients (76%), whereas a single test was positive in 37 (24%). These included antigenuria in 27 (18%), pathology or cytology in 6 (4%), culture in 3 (2%), and antibody detection in 1 (1%). Combined visualization of the organism and detection of antigen was the basis for diagnosis in 69 (45%), whereas antigen was positive but pathology was negative in 20 (13%) and pathology was positive but antigen was negative in 9 patients (6%). Culture provided the sole basis for diagnosis in 3 patients (2%) and antibody detection by complement fixation in 1 patient (1%). In this case, a patient with meningitis demonstrated complement fixation titer of 1:32 in serum and 1:8 in cerebrospinal fluid; antigen was negative in serum using a less sensitive version of the antigen assay as existed in 2003 [15]; urine antigen, cultures, cytology, and pathology were not performed. Antigen detection was more sensitive in patients with disseminated disease compared to those with pulmonary disease alone.
Table 4.
Results of Diagnostic Testing
| Test | Pulmonary | Disseminated | Total | P Value |
|---|---|---|---|---|
| Culture | 17/29 (59%) | 87/118 (74%) | 104/147 (71%) | .11 |
| Lung, respiratory culture | 15/25 (60%) | 56/73 (77%) | 71/98 (72%) | .11 |
| Pathology or cytology | 20/24 (83%) | 65/83 (78%) | 85/107 (79%) | .58 |
| Lung, respiratory pathology or cytology | 18/21 (86%) | 36/49 (74%) | 54/70 (77%) | .26 |
| Culture, pathology, or cytology | 25/29 (86%) | 100/107 (93%) | 125/146 (86%) | .20 |
| Lung, respiratory culture, pathology or cytology | 22/26 (85%) | 60/74 (81%) | 82/100 (82%) | .68 |
| Antigenuria | 19/26 (73%) | 113/116 (97%) | 132/142 (93%) | .01 |
| Antigenemia | 2/4 (59%) | 41/46 (89%) | 43/50 (86%) | .03 |
| Antibody | 5/8 (62%) | 11/36 (28%) | 16/44 (36%) | .08 |
Organisms were visualized in the bone marrow in 12 of 17 patients (71%). In 5 patients, all diagnosed between 7 and 12 months after transplant, granuloma was observed on biopsy of tissue in the transplanted organ.
Treatment and Outcome
The most common management strategy was an amphotericin formulation followed by therapy with an azole (Table 5). For patients with severe disease, 42 (98%) initially received an amphotericin formulation and 1 (2%) initially received an azole. Moderate disease was initially treated with amphotericin in 69% of cases, with 31% receiving initial therapy with an azole. Ninety-two percent of patients with mild disease initially received azole therapy. Seventeen patients (11%) died prior to receiving step-down therapy. In the remaining cases, an azole was given as step-down therapy for a median duration of 12 months (Table 5). Twenty-one percent of patients continued chronic suppressive therapy. Immunosuppression was held or decreased in the majority of patients (Table 5).
Table 5.
Treatment and Immunosuppression Management
| Treatment | % (No.) |
|---|---|
| Initial treatment | |
| Amphotericin product | 72% (110/152) |
| Amphotericin B lipid complex | 39% (60/152) |
| Liposomal amphotericin | 26% (40/152) |
| Amphotericin B deoxycholate | 7% (10/152) |
| Azole | 26% (39/152) |
| Itraconazole | 25% (38/152) |
| Voriconazole/fluconazole/unknown | 1% (1/152) |
| Subsequent treatment | |
| Itraconazole | 74% (113/152) |
| Voriconazole | 6% (9/152) |
| Itraconazole/voriconazole | 9% (14/152) |
| Fluconazole | 1% (1/152) |
| Early death | 10% (15/152) |
| Decrease or stop immunosuppression | |
| Calcineurin inhibitor | 95% (144/152) |
| Mycophenolate preparation | 91% (138/152) |
| Corticosteroid | 71% (108/152) |
Nineteen percent of the patients in the cohort died; histoplasmosis was the cause of death in 10% and 72% of deaths occurred within the first month after diagnosis, resulting in a median interval to death of 2 weeks. No histoplasmosis-related deaths occurred within 2 months of transplant. In univariate analysis, older age, severe disease, fungemia, and higher urine antigen were associated with death from histoplasmosis. In multivariate analysis, age and severe disease remained statistically significant risk factors (Table 6).
Table 6.
Risk Factors for Death Due to Histoplasmosis
| Risk Factor | Death due to Histoplasmosis (n = 15) | Survival (n = 128) | Univariate OR (95% CI) | P Value | Multivariate OR (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Age, y, median (range) | 54 (43–68) | 47 (3–80) | 1.058 (1.009–1.111) | .02 | 1.104 (1.002–1.150) | .046 |
| Severe disease | 94% | 37% | 40 (5–333) | <.001 | 0.044 (0.004–.485) | .011 |
| Fungemia | 92% | 63% | 7.67 (1.0–62.6) | .048 | 3.294 (.156–69.38) | .44 |
| Urine antigen, ng/mL, median (range) | >19 (2.1–19.0) | 12 (0 to >19.0) | 1.112 (1.0–1.26) | .039 | 1.12 (.79–1.59) | .52 |
| Urine antigen >19 ng/mL | 61% | 38% | 3.4 (.9–12) | .06 | .64 | |
| Reduced calcineurin inhibitor | 100% | 75% | a | 1.0 | a | |
| Amphotericin treatment | 87% | 70% | 2.75 (.59–12.7) | .23 |
Abbreviations: CI, confidence interval; ng, nanograms; OR, odds ratio.
a Cannot be calculated with zero count cells.
Relapse occurred in 9 (6%) patients. Six occurred in the first 2 years following the original diagnosis; 1 patient relapsed 9 years later. Follow-up for >1 year after discontinuation of therapy was available for 57 patients; 6 relapsed, 3 received ≤7 months of initial therapy, and 3 were treated for at least 1 year. The other 3 relapses occurred among the 38 patients on chronic maintenance therapy who were followed for at least 1 year (median, 34 months [range, 12–74 months]). Relapse occurred at 5, 14, and 48 months of chronic maintenance antifungal therapy. Relapse occurred in 3 of 38 patients (8%) while receiving therapy, and 6 of 57 (10%) who stopped therapy (P = .67). Information on drug levels was not available. Of the 9 relapsed patients, 2 died of histoplasmosis after relapse. One died 1 month and the other 4 months after beginning treatment for relapse. The only risk factor significantly associated with relapse was failure to reduce calcineurin inhibitor dosage (75% vs 95%; P = .043).
DISCUSSION
This paper describes the largest series (152 cases) of histoplasmosis after SOT. Histoplasma-related mortality was 10%, with most deaths occurring soon after diagnosis. One-third of the cases occurred within 1 year of transplant, and almost half occurred in the first 2 years after transplant. The very early development of disease and presence of granuloma in the transplanted organ suggests that some of these cases were donor-derived. Antigenuria was the most sensitive diagnostic test. Patients were typically treated with polyenes followed by azoles for a median of 1 year, and relapse was rare, suggesting that chronic suppressive treatment is unnecessary in most cases. Nonetheless, 2 deaths occurred after relapse, and clinical and laboratory monitoring after discontinuation of therapy is prudent.
Previous single-center studies have demonstrated an incidence of 0.48% of histoplasmosis among SOT recipients [3–8], with a significant proportion (34%) of cases occurring during the first year after transplant. A recent, large prospective multicenter study of invasive fungal infection after SOT reported 48 cases of histoplasmosis with 43% occurring within 6 months of transplant [13]. These rates are disproportionate compared to the proportion of living transplant patients who were transplanted within the last year. For example, of all living patients in the United Network of Organ Sharing (UNOS) registry as of 31 December 2011, 9.3% were transplanted that year and 89.7% in preceding years (e-mail communication; June 2012, Sarah Taranto, Organ Procurement and Transplantation Network/UNOS). Thus, about one-third of cases occur in approximately 10% of the patients at risk. More intense immunosuppression likely plays a major factor in the increased first-year risk, as could donor-derived infection.
Donor-derived histoplasmosis is rare, but confirmed transmission has been reported [9, 10]. We noted 8 cases that exhibited features suggestive of donor-derived histoplasmosis based either on diagnosis in the first month after transplant or the finding of granuloma in the transplanted organ combined with diagnosis during the first year after transplant. Other possible explanations for early disease include “smoldering” infection in the recipient prior to transplant, and newly acquired disease in the recipient. In either of these circumstances, granuloma might be observed in the transplanted organ. As we do not have clinical information on the donor or other recipients, donor-derived infection cannot be proven. Our study supports continued attention to the possibility of donor-derived histoplasmosis when assessing donors in endemic areas [16].
The significant mortality associated with posttransplant histoplasmosis and the nonspecific clinical presentation emphasizes the importance of maintaining a low threshold for consideration of histoplasmosis, and raises the question whether screening before or during the first year following transplant could identify cases earlier, perhaps reducing mortality. The role of routine screening for antigenuria following transplant has not been studied, but this approach was addressed in patients receiving treatment with tumor necrosis factor inhibitors in which results of a pilot project did not support screening [17]. More information is needed to determine the benefit of screening.
Detection of antigenuria was the most sensitive diagnostic method. Antigenuria was present in all of the cases in 2 other reports [5, 6]. In another study, only 69% of patients exhibited antigenuria, which was attributed to use of older, less sensitive assays [3]. Sensitivity of antigen testing is lower in patients who do not have disseminated disease [12]. Although not demonstrated in this study, other studies and clinical experience show that the highest sensitivity for diagnosis is achieved by testing both urine and serum [18, 19]. Serologic tests for antibodies to H. capsulatum were not sensitive, positive in about one-third of patients, and the sole basis for diagnosis in only 1 patient.
As the majority of patients presented with disseminated disease, treatment usually consisted of a lipid formulation of amphotericin B followed by itraconazole, combined with a reduction in immunosuppression. Despite aggressive treatment, 10% of the patients died of histoplasmosis with most deaths occurring within the first 3 weeks following diagnosis, including 2 who died prior to treatment. In the literature, death during the first few weeks following diagnosis was reported in the 2 other fatal cases for which information was provided [4, 5]. Higher antigen concentration as well as older age and fungemia were associated with higher death rates in univariate analysis. Although most patients with disseminated disease should initially be treated with an amphotericin formulation, patients with these risk factors are particularly likely to require polyene therapy even if renal function is impaired. Although it is common practice and makes intuitive sense to reduce maintenance immunosuppression in SOT recipients with severe infections, we did not find a correlation between reduction of immunosuppression and risk of death. Any benefit of reducing immunosuppression, however, may have been masked by the fact that immunosuppression was reduced in >90% of cases and most deaths occurred soon after diagnosis.
The optimal duration of treatment for histoplasmosis after SOT is unknown. In 2 other reports, suppressive therapy was administered in 23% [6] and 50% [3] of patients; in a third report, none received suppressive therapy with no relapses noted in patients treated for 12 months [5]. Combining the 4 series, 14 of 197 patients (7.1%) relapsed, 9 (64.3%) within the first 2 years, 12 (85.7%) within the first 4 years, and 1 patient each at 6 and 9 years following the original episode. Thus, the greatest risk is during the first 2 years, but 36% occurred later, up to at least 9 years following the original episode. Overall, our findings support the safety of discontinuing antifungal therapy following a 12-month course of treatment. Heightened awareness of findings consistent with relapse should be maintained during the first 2 years following the initial episode, and continued awareness of the risk of relapse for the remainder of the patient's life.
Sequential antigen testing might be useful in determining if a subset of patients is at risk for relapse if azole therapy is discontinued. The Infectious Diseases Society of America guidelines suggest that antigen concentration should be <2 ng/mL prior to stopping therapy and monitored for at least 12 months after treatment is stopped [20]. As patients in our study did not all have antigenuria monitored at routine intervals, our conclusions regarding the utility of this practice are incomplete. However, some observations can be made. Antigenuria progressively declined during therapy. Relapse occurred in 2 of the 7 patients (29%) with antigenuria >2 ng/mL and 1 of 32 patients (3%) with antigen concentrations of ≤1.9 ng/mL (undetectable in 17 of 32). One of the 2 patients who discontinued therapy before the antigen concentration fell below 2 ng/mL died following a relapse of histoplasmosis: the concentration at discontinuation of therapy was 5.9 ng/mL in that patient. In another report, antigenuria persisted in 3 of 9 patients at the time treatment was stopped, and relapse did not occur [5]. Antigen levels in the urine and serum had declined from peak at the time treatment was stopped, and the authors concluded that antigen testing and clinical follow-up should be performed for at least 18–24 months. A significant increase in antigen should prompt very careful clinical monitoring or reinstitution of treatment.
Our study has several important limitations. Data were not collected prospectively, and diagnostic and treatment strategies varied over time and from center to center. The scope of the study and the multicenter nature made a case-control series impractical. Thus, risk factors for the development of histoplasmosis could not be determined. Furthermore, because data from each center providing the number of surviving posttransplant patients stratified by time from transplant were not available, precise incidence rates could not be calculated. A standardized diagnostic protocol was not used at each center, and comparison of performance of diagnostic methods was thus imperfect. Determination of the cause of death by retrospective review of medical records is difficult and impacts the analysis of risk factors. Finally, conclusions regarding the safety of discontinuing chronic maintenance therapy must be tempered by the variable duration of follow-up.
This study describes a large, multicenter cohort of patients with histoplasmosis after SOT. Although a significant number of late cases occur, the first year after transplant is the period of greatest risk. Some of these cases likely represent donor-derived infection. Antigen detection in the serum or urine is the most sensitive diagnostic test. As is true of many infections following transplant, serological tests are of limited sensitivity. Although early deaths occur, treatment with a polyene followed by 12 months of azole therapy is successful in most cases with only rare relapses. Conducting prospective studies to refine the optimal treatment of histoplasmosis would be very challenging. Future prospective studies, however, could focus on universal serological or antigen-based screening of donors and recipients in endemic areas to better inform practices to reduce the incidence of early donor- or recipient-derived disease.
Notes
Acknowledgments. The authors thank Dionissios Neofytos, MD, Jennifer Cuellar-Rodriguez, MD, Steven Mawhorter, MD, Chris Ledtke, MD, and David van Duin, MD, for their work abstracting, collecting, and reviewing data.
Financial support. This work was supported by the National Institutes of Health (grant number K24 A1085118), which supplied funds for the database from which the data were extracted (Johns Hopkins University).
Disclaimer. The views and opinions expressed in this article are those of the authors and do not reflect official policy or position of the US Air Force, Department of Defense, or US government.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Manos NE, Ferebee SH, Kerschbaum WF. Geographic variation in the prevalence of histoplasmin sensitivity. Dis Chest. 1956;29:649–68. doi: 10.1378/chest.29.6.649. [DOI] [PubMed] [Google Scholar]
- 2.Wheat LJ, Connolly-Stringfield PA, Baker RL, et al. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine (Baltimore) 1990;69:361–74. doi: 10.1097/00005792-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Cuellar-Rodriguez J, Avery RK, Lard M, et al. Histoplasmosis in solid organ transplant recipients: 10 years of experience at a large transplant center in an endemic area. Clin Infect Dis. 2009;49:710–6. doi: 10.1086/604712. [DOI] [PubMed] [Google Scholar]
- 4.Davies SF, Sarosi GA, Peterson PK, et al. Disseminated histoplasmosis in renal transplant recipients. Am J Surg. 1979;137:686–91. doi: 10.1016/0002-9610(79)90050-3. [DOI] [PubMed] [Google Scholar]
- 5.Freifeld AG, Iwen PC, Lesiak BL, Gilroy RK, Stevens RB, Kalil AC. Histoplasmosis in solid organ transplant recipients at a large midwestern university transplant center. Transpl Infect Dis. 2005;7:109–15. doi: 10.1111/j.1467-8365.2005.00105.x. [DOI] [PubMed] [Google Scholar]
- 6.Grim SA, Proia L, Miller R, et al. A multicenter study of histoplasmosis and blastomycosis after solid organ transplantation. Transpl Infect Dis. 2012;14:17–23. doi: 10.1111/j.1399-3062.2011.00658.x. [DOI] [PubMed] [Google Scholar]
- 7.Peddi VR, Hariharan S, First MR. Disseminated histoplasmosis in renal allograft recipients. Clin Transplant. 1996;10:160–5. [PubMed] [Google Scholar]
- 8.Wheat LJ, Smith EJ, Sathapatayavongs B, et al. Histoplasmosis in renal allograft recipients. Two large urban outbreaks. Arch Intern Med. 1983;143:703–7. [PubMed] [Google Scholar]
- 9.Ison MG, Nalesnik MA. An update on donor-derived disease transmission in organ transplantation. Am J Transplant. 2011;11:1123–30. doi: 10.1111/j.1600-6143.2011.03493.x. [DOI] [PubMed] [Google Scholar]
- 10.Limaye AP, Connolly PA, Sagar M, et al. Transmission of Histoplasma capsulatum by organ transplantation. N Engl J Med. 2000;343:1163–6. doi: 10.1056/NEJM200010193431605. [DOI] [PubMed] [Google Scholar]
- 11.Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20:115–32. doi: 10.1128/CMR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448–54. doi: 10.1093/cid/cir435. [DOI] [PubMed] [Google Scholar]
- 13.Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50:1101–11. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 14.Edwards LB, Acquaviva FA, Livesay VT, Cross FW, Palmer CE. An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am Rev Respir Dis. 1969;99(suppl):1–132. [PubMed] [Google Scholar]
- 15.Wheat LJ, Connolly P, Durkin M, Book BK, Pescovitz MD. Elimination of false-positive Histoplasma antigenemia caused by human anti-rabbit antibodies in the second-generation Histoplasma antigen assay. Transpl Infect Dis. 2006;8:219–21. doi: 10.1111/j.1399-3062.2006.00151.x. [DOI] [PubMed] [Google Scholar]
- 16.Singh N, Huprikar S, Burdette SD, Morris MI, Blair JE, Wheat LJ. Donor-derived fungal infections in organ transplant recipients: guidelines of the American Society of Transplantation, infectious diseases community of practice. Am J Transplant. 2012;12:2414–28. doi: 10.1111/j.1600-6143.2012.04100.x. [DOI] [PubMed] [Google Scholar]
- 17.Hage CA, Bowyer S, Tarvin SE, Helper D, Kleiman MB, Wheat LJ. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85–92. doi: 10.1086/648724. [DOI] [PubMed] [Google Scholar]
- 18.Swartzentruber S, Rhodes L, Kurkjian K, et al. Diagnosis of acute pulmonary histoplasmosis by antigen detection. Clin Infect Dis. 2009;49:1878–82. doi: 10.1086/648421. [DOI] [PubMed] [Google Scholar]
- 19.Wheat LJ. Approach to the diagnosis of the endemic mycoses. Clin Chest Med. 2009;30:379–89. doi: 10.1016/j.ccm.2009.02.011. viii. [DOI] [PubMed] [Google Scholar]
- 20.Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–25. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]