Blacks had a 40% higher risk of virologic failure on initial antiretroviral therapy compared to whites. This observation was consistent over a broad range of regimens, suggesting it may be driven by unmeasured social factors; biological factors cannot be ruled out.
Keywords: HIV-1, antiretroviral therapy, racial disparity, virologic failure
Abstract
Background. In the United States, black individuals infected with human immunodeficiency virus (HIV) have higher rates of virologic failure on antiretroviral therapy (ART) and of death compared to white individuals. The cause for these disparities is uncertain. We sought to examine differences in virologic outcomes among antiretroviral-naive clinical trial participants starting randomized ART and to investigate factors to explain the differences.
Methods. Individual-level data from participants initiating ART in 5 AIDS Clinical Trials Group studies were analyzed. Included studies were those conducted during 1998–2006 with a primary outcome of virologic failure. The primary outcome measure was time to virologic failure, regardless of ART changes.
Results. A total of 2495 individuals (1151 black; 1344 white) were included with a median follow-up of 129 weeks. Compared to whites, blacks had an increased hazard of virologic failure (hazard ratio [HR]; 1.7; 95% confidence interval [CI], 1.4–1.9; P < .001), with no evidence of heterogeneity across regimens (P = .97); the association remained after adjustment for measured confounders (HR, 1.4; 95% CI, 1.2–1.6; P < .001). Increased hazard of virologic failure was associated with younger age, higher pretreatment HIV type 1 RNA level, lower pretreatment CD4 cell count, hepatitis C antibody, less education, and recent nonadherence to treatment. Sensitivity analyses with different endpoint definitions demonstrated similar results.
Conclusions. In this analysis, blacks had a 40% higher virologic failure risk than whites that was not explained by measured confounders. The observation was consistent over a range of regimens, suggesting that the difference may be driven by social factors; however, biological factors cannot be ruled out. Further research should identify the sources of racial disparities and develop strategies to reduce them.
Racial differences in human immunodeficiency virus (HIV)/AIDS in the United States are well recognized, with nearly half of all new HIV diagnoses occurring among black individuals and an HIV prevalence rate 8-fold higher in blacks compared to whites [1]. The prognosis of HIV infection also is poorer for blacks in the United States, with a 9-fold higher death rate compared to whites. These racial disparities are thought to be multifactorial; possible factors include demographic, clinical, socioeconomic, and other reasons, such as access to and quality of healthcare, individual behaviors, and attitudes, including the level of trust in the healthcare system [2–4].
Data regarding responses to antiretroviral treatment (ART) are varied. Among 435 patients from the southern United States, minority racial/ethnic groups (mostly black) were more likely to discontinue ART earlier and experience virologic failure compared to whites [5]. Other studies have shown similar differences but suggested they may be explained by pretreatment characteristics, medication adherence, and access to care [6–9]. Two reports from settings with ready access to healthcare described persistent differences in virologic responses between minority racial/ethnic groups and whites [10–11], whereas a recent large study found that black race was not associated with decreased virologic responses, but psychosocial factors and place of residence were [12].
Racial/ethnic differences in ART responses in several AIDS Clinical Trials Group (ACTG) studies were previously examined on a study-by-study basis [13–15]. In the current analysis, data from large randomized ACTG studies of ART-naive participants in which virologic outcome was the primary endpoint were combined to examine differences in efficacy between black and white participants and the extent to which these differences can be explained by measured confounders. Randomized assignment to ART in these studies removes the important confounder of access to ART that can affect assessment of race-based outcomes. Given access to all individual-level data, ART outcomes could be examined in an identical way.
METHODS
Study Population
Our study included ART-naive individuals who self-identified as non-Hispanic black or white who initiated randomized ART in 5 ACTG clinical trials conducted between 1998 and 2006 (ACTG 384 [16, 17], ACTG 388 [18], A5073 [19], A5095 [20], and A5142 [21]), with a primary endpoint including virologic failure. We excluded individuals who self-identified as Hispanic (n = 645) due to significant differences in the ART regimens used between men and women; other races (n = 97) due to insufficient numbers; and individuals who never initiated randomized ART. We also excluded individuals randomized to directly observed therapy as part of study A5073 [22] and those randomized to an inferior regimen of 3 nucleoside reverse transcriptase inhibitors (NRTIs) from study A5095 [23].
The ART regimens studied included 2 or 3 NRTIs with a nonnucleoside reverse transcriptase inhibitor (NNRTI); 2 NRTIs with 1–2 protease inhibitors (PIs) with or without ritonavir boosting (PI/r or PI); an NNRTI with PI/r; or a triple-class regimen including 2 NRTIs, an NNRTI, and an unboosted PI (Supplementary Table 1). Study evaluations and antiretroviral drugs were supplied at no cost to participants, with the exception that some medications were not supplied in study A5142 (Supplementary Table 1); however, study eligibility required assurance that access to these drugs was readily available. Participants underwent clinical assessments at least every 8 weeks, including HIV type 1 (HIV-1) RNA levels and CD4 cell count determinations. Self-reported ART adherence and socioeconomic measures were captured using a standardized questionnaire [24] in a subset of individuals (92% and 46%, respectively; Supplementary Table 1). Studies were approved by participating sites' institutional review boards; all participants provided written informed consent. The Supplementary Data include tabular summaries of key design features of the 5 trials as well as definitions and modeling considerations of potential confounding factors.
Study Endpoints
The primary endpoint of the analysis was the time to virologic failure determined regardless of changes in ART (intention-to-treat), defined as the time from study entry to the first of 2 consecutive HIV-1 RNA levels >1000 copies/mL, with the first at or after study week 16 and before study week 24, or >200 copies/mL with the first at or after study week 24. Failure was determined by a single measurement if this was the last sample collected. For study discontinuation prior to week 16, individuals with an HIV-1 RNA level <0.5 log10 lower than baseline and >50 copies/mL at week 4 or <1 log10 lower than baseline and >50 copies/mL at week 8 also were considered virologic failures. All remaining participants had virologic failure time censored at the time of the last measured HIV-1 RNA level.
Secondary endpoints included as-treated virologic failure, where individuals were censored at discontinuation of randomized ART (not including within-class drug substitutions); a combined endpoint of the first of virologic failure or discontinuation of randomized ART (regimen completion); CD4 cell count changes from baseline; and study drug adherence (Supplementary Table 2). As sensitivity analyses, analyses were repeated excluding participants randomized to regimens containing unboosted PIs (including triple-class regimens) and limited to those with full data available for the socioeconomic variables of interest.
Statistical Considerations
Pretreatment characteristics and socioeconomic factors were compared between groups using Wilcoxon rank-sum tests and χ2 tests as appropriate. Failure-time distributions were estimated using Kaplan-Meier methods and compared with log-rank tests. The association of race on the hazard of virologic failure (intention-to-treat and as-treated) and regimen completion was estimated using Cox proportional hazards models stratified by study and ART regimen. Multivariable analysis considered the following factors assessed pretreatment: age, HIV-1 RNA level, CD4 cell count, hepatitis C antibody, self-reported mode of HIV infection, highest education level, Center for Epidemiologic Studies Depression Scale score [25], number of children in household, self-efficacy, perceived social support, perception of ART, alcohol use, and marijuana use. Self-reported medication adherence over the prior 4 days was assessed at approximate 8-week intervals. Specific details of covariate definitions are provided in Supplementary Table 2.
Final model selection used a forward stepwise approach, retaining covariates significant at P < .1. In the event of substantial missing/unavailable categorical data, an “unknown” category was included to retain the overall sample size. Interactions of race with all covariates in the final adjusted model were evaluated sequentially. Proportional hazards were evaluated for all final model covariates sequentially by inclusion of interactions with the natural logarithm of time. In the event of evidence suggesting violation of proportional hazards, the violating covariate was reparameterized to allow for estimation of a time-varying hazard (over the first 6-months, at 6–12 months, and after 1 year).
Differences in CD4 cell count change from baseline were evaluated using Wilcoxon rank-sum tests, linear regression was used to adjust for potential confounding, and failure-time analyses were used to consider the time to CD4 increase ≥250 cells/µL. Ordered categories of self-reported adherence were compared by race using generalized estimating equations [26].
All analyses used SAS software version 9.2 (SAS Institute Inc, Cary, NC). Access to all individual-level study data was available for analysis.
RESULTS
Between 1998 and 2006, 2556 black or white ART-naive individuals were enrolled in 1 of the 5 ACTG studies and 2495 were included in our analysis; 16 who did not start randomized ART and 45 not followed after entry were excluded. ART regimens included NRTI + NNRTI (41%), NRTI + PI (19%), NRTI + PI/r (16%), NNRTI + PI/r (8%), and a triple-class (NRTI + NNRTI + PI) regimen (17%). Median follow-up was 129 weeks and was similarly distributed by race; 43% of participants had >3 years of follow-up (Table 1).
Table 1.
Characteristics and Follow-up of the Study Population
| Characteristic | Total (n = 2495) | Male |
Female |
P Value | ||
|---|---|---|---|---|---|---|
| Whitea (n = 1202) | Blacka (n = 820) | Whitea (n = 142) | Blacka (n = 331) | |||
| Trial characteristics | ||||||
| Treatment-naive study | ||||||
| ACTG 384 | 776 (31%) | 399 (33%) | 237 (29%) | 48 (34%) | 92 (28%) | <.001 |
| ACTG 388 | 362 (15%) | 197 (16%) | 107 (13%) | 26 (18%) | 32 (10%) | |
| A5073 | 200 (8%) | 88 (7%) | 72 (9%) | 7 (5%) | 33 (10%) | |
| A5095 | 573 (23%) | 271 (23%) | 196 (24%) | 36 (25%) | 70 (21%) | |
| A5142 | 584 (23%) | 247 (21%) | 208 (25%) | 25 (18%) | 104 (31%) | |
| Randomized ART regimen class | ||||||
| NNRTI-basedb | 1012 (41%) | 491 (41%) | 340 (41%) | 55 (39%) | 126 (38%) | <.001 |
| PI/r-basedb | 403 (16%) | 170 (14%) | 143 (17%) | 13 (9%) | 77 (23%) | |
| PI-basedb | 479 (19%) | 249 (21%) | 140 (17%) | 36 (25%) | 54 (16%) | |
| PI/r + NNRTI | 188 (8%) | 75 (6%) | 74 (9%) | 11 (8%) | 28 (8%) | |
| Triple-class | 413 (17%) | 217 (18%) | 123 (15%) | 27 (19%) | 46 (14%) | |
| Duration of follow-up, wk, median (Q1-Q3) | 129 (89–168) | 131 (97–161) | 129 (75–154) | 129 (95–155) | 125 (66–152) | … |
| Patient characteristics | ||||||
| Age, y, median | 37 | 37 | 38 | 39 | 36 | .31 |
| HIV-1 RNA level, log10 copies/mL, median (Q1–Q3) | 5.0 (4.5–5.5) | 5.1 (4.6–5.6) | 4.9 (4.4–5.5) | 4.9 (4.3–5.5) | 4.7 (4.2–5.3) | <.001 |
| CD4 countc, cells/µL, median (Q1–Q3) | 210 (65–355) | 244 (81–391) | 178 (37–311) | 209 (81–354) | 199 (78–326) | <.001 |
| Ever hepatitis C antibody positived | 11% | 8% | 16% | 12% | 10% | <.001 |
| Self-reported demographics (% with data)e | 1142 (46%) | 585 (49%) | 352 (43%) | 65 (46%) | 140 (42%) | |
| Mode of HIV infectionf | ||||||
| Shared needles | 8% | 6% | 10% | 17% | 8% | <.001 |
| Sexual contact | 82% | 87% | 75% | 74% | 82% | |
| Needle stick/transfusion | 2% | 2% | 2% | 5% | 2% | |
| Highest education level | ||||||
| Less than high school graduate | 13% | 7% | 17% | 16% | 23% | <.001 |
| High school graduate | 30% | 27% | 31% | 38% | 40% | |
| Some college | 32% | 32% | 35% | 26% | 30% | |
| Bachelor's degree | 16% | 21% | 12% | 14% | 6% | |
| Postgraduate | 8% | 13% | 5% | 5% | 1% | |
| Self-efficacy | ||||||
| Not at all/somewhat sure | 10% | 9% | 12% | 14% | 15% | .22 |
| Very sure | 31% | 31% | 30% | 35% | 35% | |
| Extremely sure | 58% | 61% | 58% | 51% | 51% | |
| Perception that ART will have a positive effect on health | ||||||
| Not at all/somewhat sure | 28% | 24% | 34% | 23% | 27% | <.001 |
| Very sure | 36% | 38% | 30% | 43% | 40% | |
| Extremely sure | 36% | 38% | 35% | 34% | 33% | |
| Perceived social support | ||||||
| Very/somewhat dissatisfied | 12% | 10% | 12% | 11% | 16% | .54 |
| Somewhat satisfied | 29% | 28% | 31% | 34% | 29% | |
| Very satisfied | 58% | 61% | 57% | 55% | 55% | |
| Alcohol useg | ||||||
| ≤20 drinks per month | 41% | 45% | 35% | 45% | 34% | <.001 |
| >20 drinks per month | 22% | 27% | 20% | 16% | 8% | |
| Marijuana useh | ||||||
| Yes | 41% | 44% | 42% | 27% | 24% | .007 |
Data are presented as No. (%) unless otherwise specified. P values are given for test of general differences across the 4 subgroups using a Wilcoxon rank-sum test for continuous outcomes and χ2 test for categorical outcomes. Percentages are taken of the total race/sex cohort.
Abbreviations: ACTG, AIDS Clinical Trials Group; ART, antiretroviral therapy; HIV-1, human immunodeficiency virus type 1; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PI/r, PI taken with low-dose ritonavir for pharmacological boosting; Q1, 1st quartile (25th percentile); Q3,3rd quartile (75th percentile).
a Individuals of self-identified Hispanic ethnicity are excluded.
b Taken in combination with 2–3 nucleoside reverse transcriptase inhibitors.
c Pretreatment CD4 cell count was missing for 3 subjects.
d Due to serology not collected by study at study entry, 675 individuals had unknown hepatitis C exposure status.
e Percentage of individuals with data for at least 1 self-report measure (excluding highest education level). With the exception of highest level of education, self-report data were captured only in a subset of participants of ACTG 388 and ACTG 384 and all participants in A5073; data were not collected in A5095 and A5142. Education status was available for a larger proportion of the study population (n = 2033 [81%]) through their participation in ACTG 5001, a rollover study designed for long-term follow-up [39]. Additional details regarding questioning for self-report outcomes are provided in Supplementary Table 2.
f Not shown; the remaining 8% of participants completing a questionnaire responded “Do not know.”
g Not shown; the remaining 38% of participants completing a questionnaire reported never drinking.
h Marijuana use in the past 6 months.
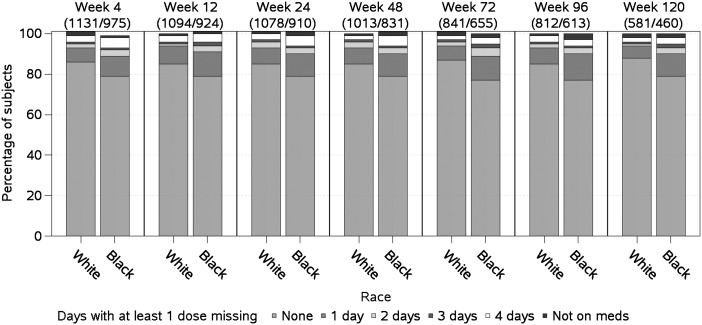
The population was 46% black and 54% white, 19% female and 81% male, with a median age of 37 years, baseline HIV-1 RNA 100 000 copies/mL, and baseline CD4 count 210 cells/µL. At baseline compared to whites, blacks were more likely to be female, to have lower pretreatment HIV-1 RNA levels and CD4 cell counts, and to have hepatitis C antibody (all P < .001). White men were most likely to report education beyond high school (P < .001); women of both races reported lower education than men (Table 1). Although self-reported medication adherence in the cohort was high, whites reported fewer missed doses of medication compared to blacks across all metrics (P ≤ .001). For a metric defined as the number of days reporting at least 1 missed dose, the proportion of blacks reporting in each category (1, 2, 3, or 4 days) was higher compared to whites (Figure 1).
Figure 1.
Self-reported adherence over time. Percentage of subjects reporting 0, 1, 2, 3, or 4 days with at least 1 dose missed over a 4-day recall period by study week. Longitudinal self-reported adherence data were only captured for a subset (92%) of individuals; the numbers of subjects contributing data by study week are given for white/black race, respectively.
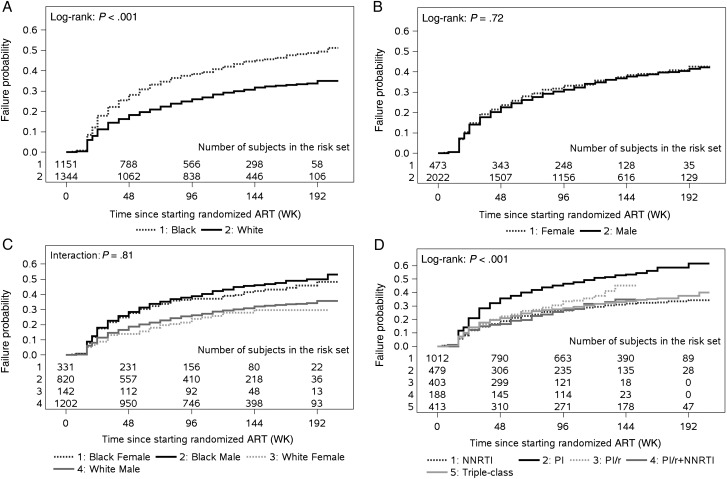
A total of 854 participants (468 black, 386 white) met criteria for virologic failure. The time to virologic failure was significantly shorter for blacks compared to whites (P < .001); the 3-year cumulative probability of virologic failure was 45% (95% confidence interval [CI], 42%–48%) for blacks compared to 32% (95% CI, 29%–35%) for whites (Figure 2A). Virologic failure-time distributions were not different by sex (P = .72; Figure 2B) or race/sex combined (P = .81; Figure 2C). There were differences in failure-time distributions according to ART regimen class; specifically, higher rates of virologic failure were observed for subjects randomized to nonboosted PIs (P < .001; Figure 2D).
Figure 2.
Failure-time distributions. Cumulative probability of time to virologic failure (intention-to-treat) by race (A), sex (B), race and sex (C), and class of randomized antiretroviral treatment (D). Abbreviations: ART, antiretroviral therapy; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PI/r, PI taken with low-dose ritonavir for pharmacological boosting.
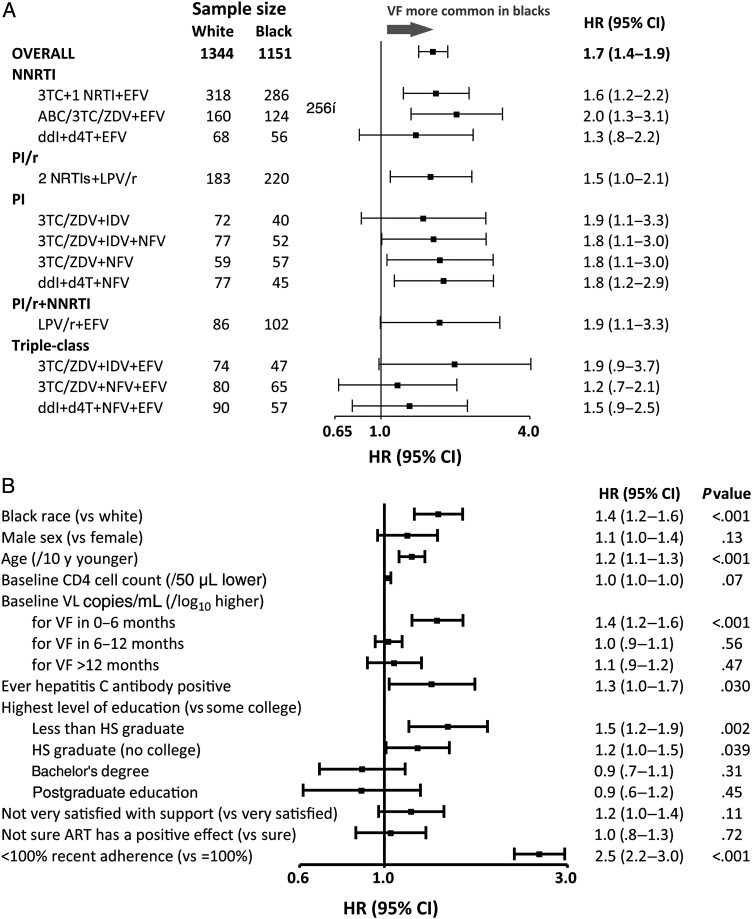
In unadjusted Cox proportional hazards analysis stratified by ART and study, compared to white race, black race was associated with increased hazard of virologic failure (hazard ratio [HR], 1.7 [95% CI, 1.4–1.9], P < .001) without evidence of heterogeneity across the 15 ART regimens (P = .97; Figure 3A). A significant association of black race remained apparent (HR, 1.4 [95% CI, 1.2–1.6], P < .001) following adjustment for measured confounders. Factors also associated with increased hazard of virologic failure in adjusted analyses included younger age, higher pretreatment HIV-1 RNA level, lower pretreatment CD4 cell count, hepatitis C antibody positivity, less education, and recent nonadherence. Of these, all but age and HIV-1 RNA level were more common among blacks (Figure 3B).
Figure 3.
Relative hazard (unadjusted) of virologic failure. Forest plot showing the estimated hazard ratio and 95% confidence interval of virologic failure (intention-to-treat). A, Unadjusted with estimates given overall (stratified by randomized antiretroviral therapy [ART]) and by randomized ART (N = 2495). B, Adjusted for covariates shown (n = 2492). Recent adherence is based on the most recent self-report adherence assessment over a 4-day recall period. Provider choice of nucleoside reverse transcriptase inhibitor (A5073), stavudine extended release (XR) or tenofovir or (A5142) zidovudine, stavudine XR, or tenofovir made prior to randomization. Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; CI, confidence interval; d4T, stavudine; ddI, didanosine; EFV, efavirenz; HR, hazard ratio; HS, high school; IDV, indinavir; LPV, lopinavir; LPV/r, lopinavir-ritonavir; NFV, nelfinavir; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PI/r, PI taken with low-dose ritonavir for pharmacological boosting; VF, virologic failure; ZDV, zidovudine.
Although lower self-reported satisfaction with support and lower perception that ART would have a positive effect on health were significantly associated with an increased hazard of virologic failure in unadjusted analyses, these associations were not evident in adjusted analyses (P = .11 and P = .71, respectively). With the exception of pretreatment HIV-1 RNA level, there was no evidence to suggest changing covariate effect of time (ie, violation of the proportional hazards assumption) for any of the final model covariates (HIV-1 RNA level, P = .08; race, P = .87; other covariates P > .10). Whereas a higher pretreatment HIV-1 RNA level was associated with an increased hazard of virologic failure over the first 6 months of treatment, this effect weakened over time (Figure 3B). There was no evidence of modification of the race effect by any of the final model covariates (all tests for interaction, P > .10). Consistent results were observed for analyses of secondary as-treated virologic failure and regimen completion endpoints (Supplementary Figure 1, adjusted analyses not shown) and for sensitivity analyses.
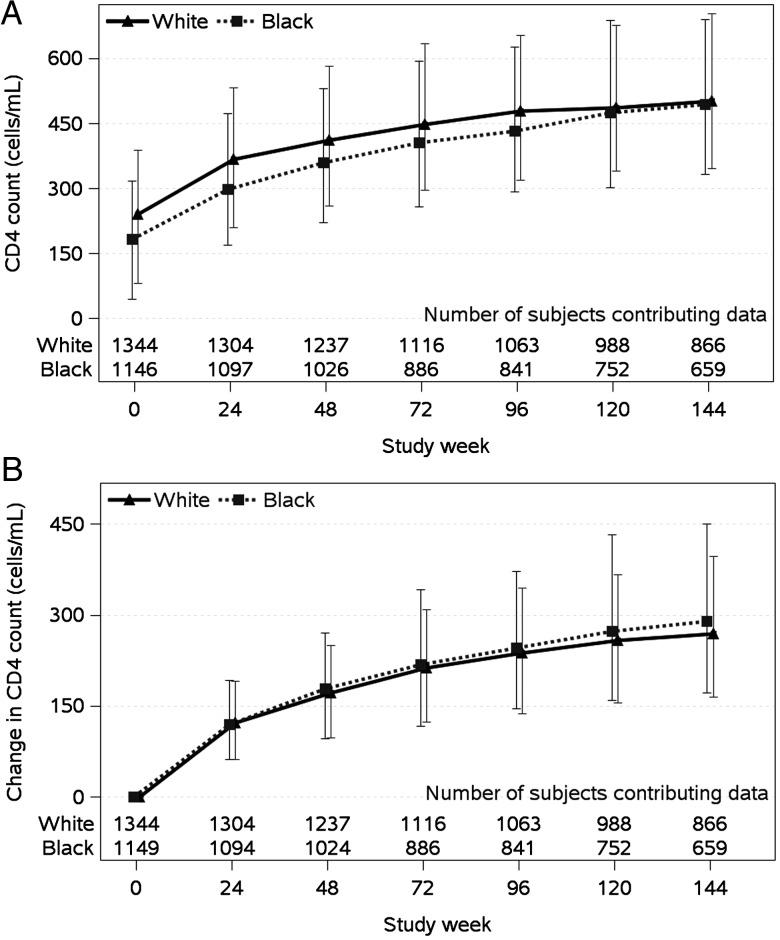
Although no differences by race in CD4 cell count change were detected to weeks 24 or 48, black race was associated with a modestly greater increase from baseline to 96 weeks and beyond (P < .001; Figure 4). In adjusted analyses based on CD4 cell count change from baseline to week 96, blacks had a 33 cells/µL (95% CI, 16–50 cells/µL) larger increase compared to whites. Factors associated with smaller CD4 cell count increases over 96 weeks included male sex (P < .001), older age (P = .03), and hepatitis C antibody (P = .004); lower pretreatment HIV-1 RNA level was associated with larger CD4 cell count changes over 96 weeks (P < .001). Given the differences in the racial subgroups with respect to pretreatment CD4 cell count and sex, interactions of these variables and race were evaluated, but not found (P = .10 and P = .65, respectively). Similar results were obtained in failure-time analyses of time to CD4 count increase >250 cells/µL, although the effect of race in these analyses was not significant (HR, 1.1 [95% CI, .97–1.2], P = .14).
Figure 4.
CD4 cell count changes over time.
DISCUSSION
Our analysis of 2495 HIV-infected ART-naive individuals randomized to 15 different initial ART treatment regimens on 5 ACTG clinical trials and followed for a median of almost 3 years demonstrates a 40% greater hazard of virologic failure for blacks compared to whites. Other factors associated with an increased hazard of virologic failure included younger age, higher pretreatment HIV-1 RNA level, lower pretreatment CD4 cell count, hepatitis C antibody, less education, and recent nonadherence. Notably, most of these factors were more prevalent among blacks (except age and HIV-1 RNA), but the association of black race with increased virologic failure persisted in adjusted analyses. Similar results were seen in sensitivity analyses using alternate endpoints of as-treated virologic failure and regimen completion.
The effect of race was observed consistently across a range of ART regimens despite differences in virologic failure rates across the regimens. These included regimens that were NNRTI-, PI-, and PI/r-based, as well as NRTI-sparing and triple-class (NRTI, NNRTI, and PI) regimens. Given the different mechanisms of action and pharmacokinetic properties of the drugs, this finding adds strength to the hypothesis that racial differences are driven by unmeasured social factors, rather than solely by differences in biological factors, such as metabolism and tolerability.
Access to care may contribute to racial disparities in ART outcomes [9–11, 27]. Although our clinical trials setting provided free and ready access to ART with support from the site staff, it is likely that disparities in other external factors, such as availability of transportation to the clinic, inflexible work schedules, access to childcare facilities, and other social factors, still exist that have an impact on an individual's response to ART [28, 29]. We also assessed self-reported satisfaction with social support in a subset of participants and found that although a lower proportion of black women reported being at least “somewhat satisfied” with their support from family members and friends compared to white women, there was little difference by race among men. Lower satisfaction with support was associated with increased risk of virologic failure; however, this association was not significant in adjusted analyses.
Mistrust of medical establishment and research may disproportionately affect blacks compared to whites [30, 31]. In our cohort, a greater proportion of black men reported being “not at all” or only “somewhat” sure that the study medication they were receiving would have a positive effect on their health compared with white men and all women. Although not significant in multivariable analysis, in unadjusted analysis a lower perception that ART would positively benefit health was associated with increased risk of virologic failure.
In contrast to the observed higher risk of virologic failure, black race also was associated with a greater increase in CD4 cell count over 2 years. This finding is not completely understood, but the effect was modest and of questionable clinical significance. No difference by race was observed in the time to CD4 count increase >250 cells/µL.
Not unexpectedly, lower 4-day self-reported medication adherence was the strongest predictor of virologic failure. The association of adherence and race is controversial. Although some studies have linked race to adherence, others found no association after controlling for confounders such as substance use, low health literacy, and depression [6, 32–36]. Although self-reported rates of medication adherence were high in our cohort, in unadjusted analyses lower adherence was reported by blacks than by whites. Importantly, after adjusting for recent adherence, the disparity by race persisted.
Some associations between black race and virologic failure that were reported in individual trials were not observed in this study. An interaction between race and adherence that suggested a greater effect of nonadherence on virologic failure in blacks than whites was reported in a secondary analyses of one of the studies included in the present analysis (A5095) [14], but not in the current study. Because A5095 was a study of efavirenz-based ART, one hypothesis for the interaction was that it was attributable to a genetic polymorphism that is more common in blacks and results in higher efavirenz concentrations [37] that may increase side effects and medication discontinuation. Although this effect could be masked in our analysis that included non-efavirenz-containing regimens, in an exploratory post hoc analysis, a 3-way interaction between race, adherence, and an indicator for efavirenz-containing regimen was not found (P = .75). In a prior analysis of study ACTG 384, greater racial disparity was associated with lower education [13]. Compared to white men, a lower proportion of all blacks and white women reported education beyond high school in the present study, and although lower education was associated with an increasing risk of virologic failure, an interaction between race and education level was not detected.
Strengths of this study include the average length of follow-up of nearly 3 years; the inclusion of important demographic, clinical, and socioeconomic factors as well as assessments of self-efficacy and adherence; and the structured clinical trial setting that provided comparable data collection across all studies. This study had a large sample size, with significant representation from blacks at 55 sites across the United States, where HIV infection is predominantly with clade B virus [38]. Although 2 international sites, Italy (n = 127, 98% white) and Johannesburg (n = 36, 100% black), were included, the results were unchanged in sensitivity analyses excluding these individuals. Our findings are strengthened by the consistency of results across 3 distinct virologic failure endpoints (intention-to-treat, as-treated, and regimen completion).
One limitation of our study is that our study population may not be representative of the population at large. Clinical trials often attract an adherent, committed, and generally healthier population, and participation in a trial implies a degree of trust in the healthcare system. Racial differences persisted despite this. Other limitations include the exclusion of Hispanics; the inclusion of older regimens that are no longer used (eg, unboosted PIs and triple-class regimens); limited information on socioeconomic factors that could be residual confounders such as income, housing, and life issues; and that documentation of pretreatment confounders by self-report was available for only approximately half of subjects and limited primarily to studies conducted in the late 1990s. Sensitivity analyses restricted to individuals with full data available for the socioeconomic factors of interest and excluding those randomized to unboosted PI regimens yielded conclusions consistent with our main findings.
In summary, in this analysis of randomized ACTG studies, black race was associated with a 40% higher risk of virologic failure on initial ART regimens compared to white race. This finding was not fully explained by demographic, clinical, socioeconomic, and adherence factors that were captured in the study and found to be associated with both race and virologic failure. The consistency of the observation over a range of regimens suggests that it may be driven by unmeasured social factors, although biological factors cannot be ruled out. Future studies should investigate issues contributing to racial disparities in ART outcomes so that effective intervention strategies can be developed to close this gap.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the study volunteers who participated in ACTG 384, ACTG 388, A5073, A5095, and A5142; all the ACTG clinical units that enrolled patients in these ART trials; and Frontier Science Foundation for data management.
The following clinical research sites that participated in these studies are listed based on patient enrollment (highest to lowest) or, in the instance where 2 or more sites have the same number (enrollment), by site number: Judith Feinberg, MD, and Carl J. Fichtenbaum, MD, University of Cincinnati (Site 2401), CTU Grant AI069513; University of Miami AIDS CRS (Site 901), CTU Grant AI069477; Susan L. Koletar, MD, and Mark D. Hite, RN, The Ohio State University (Site 2301), CTU Grant AI069474; Jorge L. Santana Bagur, MD, and Olga Méndez, MD, Puerto Rico AIDS Clinical Trials Unit (Site 5401), CTU Grant 5 U01 AI069415-06; Fred R. Sattler, MD, and Luis M. Mendez, BS, University of Southern California CRS (Site 1201), CTU Grant AI069428; Susan Pedersen, BS, BSN, and David Currin, RN, ACRN, CCRC, UNC AIDS Clinical Trials Unit (Site 3201), CTU Grant 5-U01AI069423-05; Indiana University (Site 2601); Linda Meixner, RN, and Susan Cahill, RN, UCSD Antiviral Research Center (Site 701), CTU Grant AI69432; Steven Johnson and Graham Ray, University of Colorado Hospital (Site 6101), CTU Grant AI069497; Grant RR025780; Nathan M. Thielman, MD, and Martha Silberman, RN, Duke University Medical Center CRS (Site 1601), CTU Grant 5U01 AI069 484-06; Vanderbilt Therapeutics CRS (Site 3652), CTU Grant 1U01AI069439; Judith A. Aberg, MD, and Janet Forcht, RN, New York University/NYC HHC at Bellevue Hospital (Site 401), CTU Grants Al-27665 and Al069532; Robert Murphy, MD, and Baiba Berzins, MPH, Northwestern University CRS (Site 2701), CTU Grant AI069471; Hospital of the University of Pennsylvania CRS (Site 6201), CTU Grant 1U01AI69467; Eric S. Daar and Sadia Shaik, Harbor UCLA Medical Center (Site 603), CTU Grant AI027660; Grant 1RR033176; Washington U CRS (Site 2101), CTU Grant 1U01AI069495; Melinda Robertson, RN, and Rebecca Creamer, University of Alabama Therapeutics CRS (Site 5801), CTU Grant U01 AI069452; University of Minnesota ACTU (Site 1501); University of Texas, Galveston (Site 6301); Johns Hopkins Adult AIDS (Site 201), CRS CTU Grant 1U01AI069465; University of Nebraska Medical Center, Durham Outpatient Center (Site 1505); Benigno Rodriguez, MD, and Barbara Philpotts, RN, Case CRS (Site 2501), CTU Grant AI69501; Melody Palmore, MD, and Jennifer Graham, RN, MSN, Emory University HIV/AIDS Clinical Trials Unit (Site 5802), CTU Grant U01Al69418-01; CFAR Grant P30AI050409; Cook County Hospital CORE Center (Site 2705); Howard University Hospital, Division of Infectious Diseases, ACTU (Site 5301); Karen Tashima, MD, and Helen Patterson, LPN, The Miriam Hospital (Site 2951), CTU Grant 1U01AI069472-01; Christine Hurley, RN, and Roberto Corales, DO-QIDS Care (Site 1108), CTU Grant U01AI069511-02 (as of 12 February 2008); CTSI Grant UL1 RR 024160, Rochester (site 1101); Indiana University School of Medicine, Wishard Memorial (Site 2603); Rush University Medical Center ACTG CRS (Site 2702), CTU Grant 1U01AI069471-01; University of Texas Southwestern Medical Center (Site 3751); Timothy Lane, MD, and Kim Epperson, RN, BSN, CCRC, Regional Center for Infectious Disease (Site 3203), CTU Grant U01AI069423-06; University of Hawaii at Manoa, Leahi Hospital (Site 5201); UCLA CARE Center CRS, CTU Grant 1U01AI069424; Mary Adams RN, MPH, and Amneris Luque, MD, University of Rochester CRS (Site 1101), CTU Grant U01AI069511-02 (as of 12 February 2008); GCRC Grant UL1 RR 024160; Kim Whitely and Traci Davis, MetroHealth CRS (Site 2503), CTU Grant AI-69501; Deborah McMahon, MD, and Barbara Rutecki, MPH, CRNP, Pitt CRS (Site 1001), CTU Grant 1U01AI069494-01; Georgetown University CRS (Site 1008), CTU Grant 1U01AI069494; Ann C. Collier, MD, Christine Jonsson, BS, University of Washington ACTU (Site 1401), CTU Grant AI 069434; CFAR Grant AI27757, University of Washington (Site 1401); Beth Israel Medical Center, ACTU (Site 2851); Debbie Slamowitz, RN, ACRN, and Sandra Valle, PA-C, Stanford AIDS Clinical Trials Unit (Site 501), CTU Grant AI069556; University of Pittsburgh Medical Center (Site 2505); HIV Prevention and Treatment CRS (Site 30329), CTU Grant 1U01AI069470; Massachusetts General Hospital ACTG CRS (Site 101), CTU Grant 1U01AI069472; BMC ACTG CRS (Site 104), CTU Grant 1U01AI069472; St Louis ConnectCare, Infectious Diseases Clinic (Site 2102); Mary Albrecht, MD, and Andrea Kershaw, NP, Beth Israel Deaconess (Partners/Harvard) CRS (Site 103), CTU Grant U01 AI069472-06; UC Davis Medical Center (Site 3852); SUNY–Buffalo, Erie County Medical Center (Site 1102); University of Iowa Healthcare, Division of Infectious Diseases (Site 1504); Brigham and Women's Hospital ACTG CRS (Site 107), CTU Grant 1U01AI069472; Charles Bradley Hare, MD, and Joann Volinski, RN, UCSF AIDS CRS (801), CTU Grant 5UO1 AI069502-06; Carolinas HealthCare System, Carolinas Medical Center (Site 3204); Todd Stroberg, RN, and Valery Hughes, NP, Cornell (Site 7804), CTU Grant AI069419; CTSC Grant RR024996; Wits HIV CRS (Site 11101), CTU Grant 1U01AI069463; Prof Umesh G. Lalloo and Dr Farida C. Amod, Durban Adult HIV CRS (Site 11201), CTU Grant 1U01AI069426-05; Tulane Medical Center, Charity Hospital of New Orleans, ACTU (Site 1702); Beth Israel Medical Center (Mt Sinai) (Site 1802); Robert R. Redfield Jr, MD, and Charles E. Davis Jr, MD, IHV Baltimore Treatment CRS (Site 4651), CTU Grant U01A1069447; McCree McCuller Wellness Center at the Connection Clinic (Site 1107); Dr Massimo Di Pietro and Dr Francesca Vichi, Ospedale S. M. Annunziata (A7904); Santa Clara Valley Medical Center (Site 506); Mary Sarah Dolan, RN, Eileen Chusid, PhD, Henry S. Sacks, PhD, MD, and Israel Lowy, MD, PhD, Mt Sinai (A1801); Cornell, Chelsea Center (Site 2206); Prof Giampiero Carosi and Prof Francesco Castelli, Spedali Civili, Carosi (A7903); San Mateo County AIDS Program (Site 505); Juan J. L. Lertora, MD, PhD, Rebecca Clark, MD, PhD, Mark Beilke, MD, Jeanne Dumestre, RN, FNPC, Tulane University (A1701), Grant IU01AI3844, GCRC grant PHS NCRR M01 RR05096, Tulane/LSU Charity Hospital General Clinical Research Center; Dr Fosca Niero and Dr Amedeo Capetti, Ospedale Luigi Sacco, Milazzo (A7908); Dr Antonella d'Arminio Monforte, Dr Salvatore Sollima and Dr Claudia Balotta, Ospedale Luigi Sacco, Moroni (A7909); Dr Gabriella d'Ettorre and Dr Gabriele Forcina, Universita di Roma, Vullo (A7912); Wake County Department of Health (Raleigh) (Site 3206); Dr Olga Coronado and Dr Giovanni Fasulo, Ospedale Maggiore (A7902); Dr Maria Luisa Soranzo and Dr Antonio Macor, Ospedale Amadeo Di Savoia (A7911); Dr, Gaetana Sterrantino and Dr, Silvia Ambu, Ospedale Careggi (A7905); Spedali Civili Di Brescia (Site 7914); University of Kentucky, Lexington A2405 CRS (Site 2405); Methodist Hospital of Indiana (Site 2602); SSTAR, Family Healthcare Center (Site 2954); Weill Medical College of Cornell University, The Cornell CTU (Site 7803); Prof Antonietta Cargnel and Dr Paola Meraviglia, Ospedale Luigi Sacco, Cargnel (A7907); Willow Clinic A0507 CRS (Site 507); Marin Country Department of Health, San Rafael, California (Site 809); University of California, Davis Medical Center, ACTU (Site 3851); University of Pennsylvania Health System, Presbyterian Medical Center (Site 6206); Prof Florio Ghinelli and Dr Laura Sighinolfi, Archispedale S. Anna (A7920); Dr Domenico Santoro and Dr Enrico Rinaldi, Ospedale Sant'Anna (A7922); Kaiser Permanente LAMC (Site 606); Philadelphia Veterans Administration Medical Center A6205 CRS (Site 6205); Prof Dante Bassetti and Dr Antonio Di Biagio, Universita di Genova (A7915); Cleveland Clinic Foundation (Site 2508); Rhode Island Hospital (Site 2953); Azienda Ospedaliera, Padova (Site 7916); Prof Carlo Ferrari and Dr Giancarlo Pasetti, Azienda Ospedaliera di Parma (A7925); Prof Salvatore Delia and Dr Maria Ciardi, Universita di Roma, Delia (A7910); Dr Giacomo Magnani and Dr Giuliana Zoboli, Arcispedale S. Maria Nuova (A7931); Cornell University A2201 (Site 2201); Weiss Memorial Hospital (Site 2708); Rockefeller University (Site 7801); Immunologia Clinica E Allergologia, Universita Di Roma (Site 7913); Dr Giovanni Guaraldi and Prof Roberto Esposito, Universita delgi Studi di Modena (A7924); Prof Antonio Chirianni and Dr Miriam Gargiulo, Azienda Opedaliera D. Cotugno, Chirianni (A7928); Prof Francesco Alberici and Dr Daria Sacchini, Azienda U.S.L. of Piacenza, Ospedale Civile (A7930); Prof Lorenzo Minoli and Dr Renato Maserati, IRCCS Policlinico S. Matteo, Minoli (A7918); Dr Alessandra Riva and Prof Giorgio Scalise, Azienda Ospedaliera Umberto I (A7921); Prof Francesco Chiodo and Dr Marco Borderi, Policlinico S. Orsola (A7923); Prof Nicola Abrescia and Dr Annunziata Busto, Azienda Ospedaliera D. Cotugno, Abrescia (A7927); Prof Crescenzo M. Izzo and Costanza Sbreglia, Azienda Ospedaliera D. Cotugno, Izzo (A7929); Georgetown University Hospital (Site 9327); Emory University Hemophilia Program Office (Site 9413); Tulane Hemophilia Treatment Center (Site 9426).
Author contributions. All authors contributed to the origination and completion of this work, as well as preparation, review, and approval of the final manuscript. H. J. R. and Y. C had full access to the study data and take responsibility for the integrity of the data and the accuracy of the data analysis. Additionally, G. K. R., M. A. F., C. F., R. M. G., R. H., and S. A. R. were protocol chairs for ACTG 384, ACTG 388, A5073, A5095, and A5142, respectively.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health, the National Institute of Dental and Craniofacial Research, and the National Center for Research Resources at the National Institutes of Health (grant numbers UM1-AI068636, AI38858, AI51966, AI069465, AI062435, AI069432, AI69472, AI68634, AI69419, AI38855, AI069474, AI069434, AI27670, AI36214, AI064086, and UL1 RR024996).
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Potential conflicts of interest. K. Y. S. has received consulting fees for advisory board participation from Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, and ViiV. G. K. R. has received research support from Gilead Sciences. C. F. has received research grant support from Boehringer-Ingelheim and GlaxoSmithKline for research unrelated to this study and honoraria for presentations at meetings sponsored in part by Abbott Laboratories, and has served as a consultant to Bristol-Myers Squibb, Boehringer-Ingelheim, GlaxoSmithKline, Merck, Roche, Schering-Plough, Vertex, and Tibotec. R. H. has received honoraria or consultant fees from Bristol-Myers Squibb, Gilead Sciences, Tibotec, and Vertex and research support (to UCSD) from Abbott, GlaxoSmithKline, Pfizer, and Merck. M. A. F. has received research grant support from Abbott Laboratories, GlaxoSmithKline, DuPont Pharmaceuticals, and Merck. R. M. G. has received research grant support (to Weill Cornell) from Merck/Schering, Pfizer/Wyeth, and ViiV/GlaxoSmithKline and has served as an ad hoc consultant to Bristol-Myers, Gilead, GlaxoSmithKline, Janssen, Koronis, Merck, ViiV, and Virostatics. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Diagnoses of HIV infection and AIDS in the United States and dependent areas. 2010. Available at: http://www.cdc.gov/hiv/surveillance/resources/reports/2010report/index.htm. Accessed 18 December, 2012.
- 2.Kraut-Becher J, Eisenberg M, Voytek C, Brown T, Metzger DS, Aral S. Examining racial disparities in HIV: lessons from sexually transmitted infections research. J Acquir Immune Defic Syndr. 2008;47(uppl 1):S20–7. doi: 10.1097/QAI.0b013e3181605b95. [DOI] [PubMed] [Google Scholar]
- 3.Stone VE. Optimizing the care of minority patients with HIV/AIDS. Clin Infect Dis. 2004;38:400–4. doi: 10.1086/380969. [DOI] [PubMed] [Google Scholar]
- 4.Oramasionwu CU, Brown CM, Lawson KA, Ryan L, Frei CR. Evaluating HIV/AIDS disparities for blacks in the United States: a review of antiretroviral and mortality studies. J Natl Med Assoc. 2009;101:1221–9. doi: 10.1016/s0027-9684(15)31133-0. [DOI] [PubMed] [Google Scholar]
- 5.Pence BW, Ostermann J, Kumar V, Whetten K, Thielman N, Mugavero MJ. The influence of psychosocial characteristics and race/ethnicity on the use, duration, and success of antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:194–201. doi: 10.1097/QAI.0b013e31815ace7e. [DOI] [PubMed] [Google Scholar]
- 6.van den Berg JB, Hak E, Vervoort SC, et al. Increased risk of early virological failure in non-European HIV-1-infected patients in a Dutch cohort on highly active antiretroviral therapy. HIV Med. 2005;6:299–306. doi: 10.1111/j.1468-1293.2005.00304.x. [DOI] [PubMed] [Google Scholar]
- 7.Yazdanpanah Y, Sissoko D, Egger M, Mouton Y, Zwahlen M, Chene G. Clinical efficacy of antiretroviral combination therapy based on protease inhibitors or non-nucleoside analogue reverse transcriptase inhibitors: indirect comparison of controlled trials. BMJ. 2004;328:249. doi: 10.1136/bmj.37995.435787.A6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anastos K, Schneider MF, Gange SJ, et al. The association of race, sociodemographic, and behavioral characteristics with response to highly active antiretroviral therapy in women. J Acquir Immune Defic Syndr. 2005;39:537–44. [PubMed] [Google Scholar]
- 9.Silverberg MJ, Wegner SA, Milazzo MJ, et al. Effectiveness of highly-active antiretroviral therapy by race/ethnicity. AIDS. 2006;20:1531–8. doi: 10.1097/01.aids.0000237369.41617.0f. [DOI] [PubMed] [Google Scholar]
- 10.Hartzell JD, Spooner K, Howard R, Wegner S, Wortmann G. Race and mental health diagnosis are risk factors for highly active antiretroviral therapy failure in a military cohort despite equal access to care. J Acquir Immune Defic Syndr. 2007;44:411–6. doi: 10.1097/QAI.0b013e31802f83a6. [DOI] [PubMed] [Google Scholar]
- 11.Weintrob AC, Grandits GA, Agan BK, et al. Virologic response differences between African Americans and European Americans initiating highly active antiretroviral therapy with equal access to care. J Acquir Immune Defic Syndr. 2009;52:574–80. doi: 10.1097/QAI.0b013e3181b98537. [DOI] [PubMed] [Google Scholar]
- 12.Hanna DB, Buchacz K, Gebo KA, et al. Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-Infected individuals in North America, 2001–2009. Clin Infect Dis. 2013;56:1174–82. doi: 10.1093/cid/cit003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marc LG, Testa MA, Walker AM, et al. Educational attainment and response to HAART during initial therapy for HIV-1 infection. J Psychosom Res. 2007;63:207–16. doi: 10.1016/j.jpsychores.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Schackman BR, Ribaudo HJ, Krambrink A, Hughes V, Kuritzkes DR, Gulick RM. Racial differences in virologic failure associated with adherence and quality of life on efavirenz-containing regimens for initial HIV therapy: results of ACTG A5095. J Acquir Immune Defic Syndr. 2007;46:547–54. doi: 10.1097/qai.0b013e31815ac499. [DOI] [PubMed] [Google Scholar]
- 15.Bosch RJ, Bennett K, Collier AC, Zackin R, Benson CA. Pretreatment factors associated with 3-year (144-week) virologic and immunologic responses to potent antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44:268–77. doi: 10.1097/QAI.0b013e31802c7e20. [DOI] [PubMed] [Google Scholar]
- 16.Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349:2293–303. doi: 10.1056/NEJMoa030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shafer RW, Smeaton LM, Robbins GK, et al. Comparison of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349:2304–15. doi: 10.1056/NEJMoa030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischl MA, Ribaudo HJ, Collier AC, et al. A randomized trial of 2 different 4-drug antiretroviral regimens versus a 3-drug regimen, in advanced human immunodeficiency virus disease. J Infect Dis. 2003;188:625–34. doi: 10.1086/377311. [DOI] [PubMed] [Google Scholar]
- 19.Flexner C, Tierney C, Gross R, et al. Comparison of once-daily versus twice-daily combination antiretroviral therapy in treatment-naive patients: results of AIDS clinical trials group (ACTG) A5073, a 48-week randomized controlled trial. Clin Infect Dis. 2010;50:1041–52. doi: 10.1086/651118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA. 2006;296:769–81. doi: 10.1001/jama.296.7.769. [DOI] [PubMed] [Google Scholar]
- 21.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross R, Tierney C, Andrade A, et al. Modified directly observed antiretroviral therapy compared with self-administered therapy in treatment-naive HIV-1-infected patients: a randomized trial. Arch Intern Med. 2009;169:1224–32. doi: 10.1001/archinternmed.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350:1850–61. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 24.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 25.Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 26.Gameroff M. Using the proportional odds model for health related outcomes: why, when, and how with various SAS procedures. Proceedings of the 30th Annual SAS Users Group International Conference. Cary,; NC. SAS Institute Inc; Available at: http://www2.sas.com/proceedings/sugi30/205-30.pdf. Accessed 5 January 2012. [Google Scholar]
- 27.Jensen-Fangel S, Pedersen L, Pedersen C, et al. The effect of race/ethnicity on the outcome of highly active antiretroviral therapy for human immunodeficiency virus type 1-infected patients. Clin Infect Dis. 2002;35:1541–8. doi: 10.1086/344769. [DOI] [PubMed] [Google Scholar]
- 28.Wong MD, Sarkisian CA, Davis C, Kinsler J, Cunningham WE. The association between life chaos, health care use, and health status among HIV-infected persons. J Gen Intern Med. 2007;22:1286–91. doi: 10.1007/s11606-007-0265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holzemer WL, Corless IB, Nokes KM, et al. Predictors of self-reported adherence in persons living with HIV disease. AIDS Patient Care STDS. 1999;13:185–97. doi: 10.1089/apc.1999.13.185. [DOI] [PubMed] [Google Scholar]
- 30.Saha S, Jacobs EA, Moore RD, Beach MC. Trust in physicians and racial disparities in HIV care. AIDS Patient Care STDS. 2010;24:415–20. doi: 10.1089/apc.2009.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saha S, Sanders DS, Korthuis PT, et al. The role of cultural distance between patient and provider in explaining racial/ethnic disparities in HIV care. Patient Educ Couns. 2011;85:e278–84. doi: 10.1016/j.pec.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleeberger CA, Buechner J, Palella F, et al. Changes in adherence to highly active antiretroviral therapy medications in the multicenter AIDS cohort study. AIDS. 2004;18:683–8. doi: 10.1097/00002030-200403050-00013. [DOI] [PubMed] [Google Scholar]
- 33.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis. 2002;34:1115–21. doi: 10.1086/339074. [DOI] [PubMed] [Google Scholar]
- 34.Sherr L, Lampe FC, Clucas C, et al. Self-reported non-adherence to ART and virological outcome in a multiclinic UK study. AIDS Care. 2010;22:939–45. doi: 10.1080/09540121.2010.482126. [DOI] [PubMed] [Google Scholar]
- 35.Kacanek D, Jacobson DL, Spiegelman D, Wanke C, Isaac R, Wilson IB. Incident depression symptoms are associated with poorer HAART adherence: a longitudinal analysis from the nutrition for healthy living study. J Acquir Immune Defic Syndr. 2010;53:266–72. doi: 10.1097/QAI.0b013e3181b720e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleeberger CA, Phair JP, Strathdee SA, Detels R, Kingsley L, Jacobson LP. Determinants of heterogeneous adherence to HIV-antiretroviral therapies in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2001;26:82–92. doi: 10.1097/00126334-200101010-00012. [DOI] [PubMed] [Google Scholar]
- 37.Haas DW, Ribaudo HJ, Kim RB, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18:2391–400. [PubMed] [Google Scholar]
- 38.Junqueira DM, de Medeiros RM, Matte MC, et al. Reviewing the history of HIV-1: spread of subtype B in the Americas. PLoS One. 2011;6:e27489. doi: 10.1371/journal.pone.0027489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smurzynski M, Collier AC, Koletar SL, et al. AIDS Clinical Trials Group Longitudinal Linked Randomized Trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials. 2008;9:269–82. doi: 10.1310/hct0904-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.