Abstract
Background. Few studies have examined the relationship of human immunodeficiency virus (HIV) monoinfection and its associated perturbations with liver fibrosis.
Methods. Using multivariable linear regression, we examined the demographic, behavioral, metabolic and viral factors associated with transient elastography–measured liver stiffness in 314 participants (165 HIV positive/hepatitis C virus [HCV] negative, 78 HIV positive/HCV positive, 14 HIV negative/HCV positive, 57 HIV negative/HCV negative) in the Women's Interagency HIV Study.
Results. Compared with HIV negative/HCV negative women, HIV positive/HCV positive women had higher median liver stiffness values (7.1 vs 4.4 kPa; P < .001); HIV positive/HCV negative and HIV negative/HCV negative women had similar liver stiffness values (both 4.4 kPa; P = .94). HIV/HCV coinfection remained associated with higher liver stiffness values (74% higher; 95% confidence interval [CI], 49–104) even after multivariable adjustment. Among HCV positive women, waist circumference (per 10-cm increase) was associated with 18% (95% CI, 7.5%–30%) higher liver stiffness values after multivariable adjustment; waist circumference showed little association among HIV positive/HCV negative or HIV negative/HCV negative women. Among HIV positive/HCV negative women, history of AIDS (13%; 95% CI, 4% –27%) and HIV RNA (7.3%; 95% CI, 1.59%–13.3%, per 10-fold increase) were associated with greater liver stiffness.
Conclusions. HCV infection but not HIV infection is associated with greater liver stiffness when infected women are compared with those with neither infection. Our finding that waist circumference, a marker of central obesity, is associated with greater liver stiffness in HIV/HCV-coinfected but not HIV-monoinfected or women with neither infection suggests that in the absence of HCV-associated liver injury the adverse effects of obesity are lessened.
Keywords: HIV, HCV, liver fibrosis, transient elastography, obesity, women
Liver disease is a leading cause of morbidity and mortality in human immunodeficiency virus (HIV)–infected persons [1, 2]. Coinfection with hepatitis C virus (HCV) is commonly implicated and has been associated with accelerated progression of liver fibrosis and cirrhosis compared with HCV infection alone [3–5]. Some studies show that HIV-monoinfected adults have greater liver fibrosis than those with neither HIV nor HCV infection [6, 7].
Possible reasons for the reported increase in liver fibrosis in HIV-monoinfected adults include both immunosuppression and uncontrolled HIV viral replication, factors that have been associated with fibrosis in studies of predominantly HIV-monoinfected patients [6–9]. Perturbations in fat and metabolic parameters, including increased adiposity, insulin resistance, diabetes, and dyslipidemia, have also been postulated as possible causes of fibrosis in the HIV setting, but few studies have examined these factors.
Furthermore, the few studies that have examined the association of HIV infection (in the presence or absence of HCV infection) with fibrosis have used indirect serum markers, such as liver enzyme elevations [10] or indirect markers of liver fibrosis [6, 7]. Although liver biopsy remains the clinical reference standard to assess fibrosis, it is generally not clinically indicated in HIV-infected persons. Transient elastography (TE) is an alternative, noninvasive imaging technique that can directly visualize the liver to estimate the degree of fibrosis.
In the current study, we examined the association of HIV, HCV, fat and metabolic parameters with liver fibrosis estimated using ultrasound-based TE in a geographically and ethnically diverse cohort of US women with HIV monoinfection, HCV infection (with or without HIV infection), or neither infection from the Women's Interagency HIV Study (WIHS). We hypothesized that both HCV and HIV infection would be associated with increased fibrosis compared with the absence of both infections and that metabolic perturbations would be independently associated with fibrosis in these groups.
METHODS
Study Population
The WIHS is a multicenter prospective cohort study that was established in 1994 to investigate the progression of HIV in women with and at risk for HIV. A total of 4114 women (2791 HIV infected and 975 HIV uninfected) were enrolled in either 1994–1995 (n = 2623), 2001–2002 (n = 1143), or 2011–2012 (n = 348) from 6 United States cities (Bronx, Brooklyn, Chicago, Los Angeles, San Francisco, and Washington, DC). Baseline sociodemographic characteristics and HIV risk factors were similar between HIV-infected and HIV-uninfected women [11, 12]. An institutional review board approved study protocols and consent forms, and each study participant gave written informed consent.
Every 6 months, participants complete a comprehensive physical examination, provide biological specimens for CD4 cell count and HIV RNA viral load determination, and complete an interviewer-administered questionnaire, which collects information on demographics, disease characteristics, and specific antiretroviral therapy (ART) use.
From November 2010 through April 2012, a total of 368 women from the San Francisco, Chicago, and Washington, DC, sites between the ages of 25 and 65 years agreed to participate in a TE substudy. Women who were pregnant, receiving interferon-based therapy, or positive for hepatitis B surface antigen were excluded. Women who had a body mass index (BMI) >35 kg/m2 or had evidence of decompensated cirrhosis (ascites, hepatic encephalopathy, or esophageal varices), acute hepatitis, hepatitis flare, or cholestasis were also excluded from the study owing to reported interference with the TE measurement [13].
Ascertainment of Liver Stiffness by TE
All TE operators were trained and certified. Measurements were obtained via an ultrasonic probe (placed in the intercostal space at the level of the xiphoid process) perpendicular to the skin overlying the right lobe of the liver. The TE-measured liver stiffness was expressed in kilopascals and determined from the median value of 10 “valid” measurements (ie, elastic waves that are propagated through the liver according to the machine software). A liver stiffness value >7.1 kPa was used to define significant fibrosis (which corresponds to fibrosis stage ≥2 in validated studies of HCV-infected patients) [14].
Of the 368 women who underwent TE, 44 (11 HIV positive/HCV negative, 2 HIV positive/HCV positive, 18 HIV negative/HCV positive, 13 HIV negative/HCV negative) were excluded because their liver stiffness values did not meet established criteria [15, 16]: (1) ≥10 “valid” scan data points were not acquired, according to the machine software (n = 15); (2) >60% of the total scan data points acquired were not valid (n = 5); or (3) the interquartile range was not ≤30% of the median liver stiffness value (n = 24). The 44 excluded women had a higher median BMI than those included in the analysis (29 vs 25 kg/m2; P < .001). Another 10 women were excluded because HIV and HCV status were not available, leaving 314 women (165 HIV positive/HCV negative 78 HIV positive/HCV positive, 14 HIV negative/HCV positive, 57 HIV negative/HCV negative) included in the analysis.
For comparison, serum biomarkers of fibrosis were calculated from the most recent laboratory measurements in all participants included in the analysis. The aspartate aminotransferase (AST) platelet ratio index (APRI) was calculated as AST/(upper limit normal)/platelet count (109/L), and the FIB-4 score as [AST (UI/L) × age (years)/platelet count (109/L)] × [ALT (UI/L) × ½] , where ALT indicates alanine aminotransferase [17, 18]. Because HIV itself (and not liver injury) may alter the laboratory parameters used to calculate the APRI and FIB-4 score, our analysis of factors associated with liver fibrosis in HCV-infected women and HIV-monoinfected women is focused on TE measurement of liver stiffness.
Covariates
The following primary predictors and covariates of liver stiffness were included in the analyses: HIV infection (defined as documentation of a positive HIV enzyme immunonassay result confirmed by Western blot); chronic HCV infection (defined as documentation of a positive HCV enzyme immunonassay result confirmed by detectable HCV RNA); demographic factors (age and ethnicity); menopausal status; lifestyle factors, including history of injection drug use, alcohol use (none, light [1–15 g/d], moderate [15–30 g/d], or heavy [>30 g/d]); marijuana use (none, occasional, or daily), smoking (none, current, or past); body composition, including waist circumference, waist-to-hip ratio, and BMI (calculated as weight [in kilograms] divided by height [in meters] squared); and metabolic factors, including diabetes mellitus (defined as a confirmed elevated fasting glucose level, elevated hemoglobin A1C level, or self-report of antidiabetes medications), insulin resistance estimated using the homeostasis model assessment, fasting lipid levels (high-density lipoprotein, low-density lipoprotein, triglycerides, and total cholesterol), use of lipid-lowering therapy, and use of antidiabetes medications. In analyses among HIV-infected women, current CD4, nadir CD4, current HIV RNA, history of clinical AIDS, and current use of highly active ART and ART by class were assessed. In analyses among HCV-infected women, HCV RNA level and HCV genotype were also assessed. In exploratory analyses, we controlled for liver enzyme levels (ALT and AST). Multiple imputation with the Markov chain Monte Carlo method was used to impute missing covariates [19].
Statistical Analysis
We compared demographic and clinical characteristics among 4 groups: women with HIV monoinfection, HIV/HCV coinfection, HCV monoinfection, or neither infection; we used the Kruskal-Wallis test for continuous variables and Fisher's exact test for categorical variables. We used multivariable linear regression with robust standard errors [20, 21] to evaluate the association of HIV and HCV infection and metabolic parameters with liver stiffness. Liver stiffness was found to be right skewed and was therefore log-transformed for analysis; results were back-transformed to produce estimated percentage differences. We used relative risk regression with a robust variance estimator [22] to investigate the association of HIV and HCV status with significant fibrosis.
To determine whether HIV and HCV infection were independently associated with liver stiffness, multivariable models were sequentially adjusted for (1) demographics, (2) lifestyle factors, and (3) metabolic parameters. Factors forced in the full model included age and race or ethnicity. We then constructed separate models in participants with (1) HIV infection alone, (2) HIV/HCV coinfection and HCV monoinfection, and (3) neither infection, to examine the associations of demographic, lifestyle, and metabolic risk factors with liver stiffness within each group. HIV-related factors were included in models of HIV-infected women, and HCV-related factors in models of HCV-infected women. Stepwise regression with a cutoff at P ≤ .05 was used for entry and retention; the candidate variables were selected based on their hypothesized associations with fibrosis.
We used Bayesian model averaging as an alternative model building approach; predictors with posterior probabilities >35% were retained in the model [23]. Models constructed using the 2 approaches were very similar. Bayesian model averaging was performed using the BMA package for the R statistical computing language (R Development Core Team). All analyses were conducted using the SAS system, version 9.2 (SAS Institute).
RESULTS
The demographic, behavioral and clinical characteristics of the 314 women included in the analysis are shown in Table 1, stratified by HIV and HCV status. HCV-infected women were older than HIV-monoinfected women, and both groups were older than women with neither infection. More than half of all women were African American, with the largest proportion among the HCV-infected group. Compared with HIV-infected women and those with neither infection, HCV-infected women were more likely to report being in menopause, having a history of injection drug use, and smoking currently and also more likely to have diabetes and to have a higher homeostasis model assessment–estimated insulin resistance value but a lower low-density lipoprotein level. HIV-infected women (with or without HCV) had lower high-density lipoprotein levels and higher triglyceride levels than HIV-uninfected women. Compared with HIV-monoinfected women, HIV/HCV-coinfected women had slightly lower CD4 counts and higher current HIV viral loads.
Table 1.
Demographic and Clinical Characteristics of Study Participants by HIV and HCV Statusa
| Characteristic | HCV Positive/HIV Positive (n = 78) | HCV Positive/HIV Negative (n = 14) | HCV Negative/HIV Positive (n = 165) | HIV Negative/HCV Negative (n = 57) | P Value |
|---|---|---|---|---|---|
| Age, median (IQR), y | 53 (50–57) | 55 (41–57) | 46 (40–52) | 42 (35–49) | <.001 |
| Race | .19 | ||||
| White | 8 (10) | 2 (14) | 35 (21) | 8 (14) | |
| African American | 60 (77) | 12 (86) | 99 (60) | 37 (65) | |
| Hispanic | 8 (10) | 0 | 21 (13) | 7 (12) | |
| Other | 2 (3) | 0 | 10 (6) | 5 (9) | |
| Menopausal | 75 (96) | 10 (71) | 97 (59) | 21 (37) | <.001 |
| Alcohol use, g/d | .06 | ||||
| None | 41 (53) | 3 (21) | 83 (50) | 30 (53) | |
| Light (<1 to 15) | 19 (24) | 6 (43) | 48 (29) | 16 (28) | |
| Moderate (16–30) | 14 (18) | 1 (7) | 24 (15) | 5 (9) | |
| Heavy (>30) | 4 (5) | 4 (29) | 10 (6) | 6 (11) | |
| Current smoking | 54 (69) | 10 (71) | 57 (35) | 27 (47) | <.001 |
| Any marijuana use | 31 (40) | 10 (71) | 49 (30) | 20 (35) | .42 |
| BMI, median, (IQR), kg/m2 | 25 (22–28) | 25 (22–29) | 25 (22–28) | 27 (24–29) | .24 |
| Waist circumference, cm | 92 (80–100) | 86 (81–96) | 87 (80–96) | 86 (81–97) | .51 |
| Metabolic related | |||||
| Diabetes | 15 (19) | 4 (29) | 13 (8) | 8 (14) | .02 |
| HOMA-IR, median (IQR) | 1.7 (0.8–3.7) | 2.4 (0.8–4.1) | 0.9 (0.4–2.0) | 0.6 (0.4–2.0) | <.001 |
| Current lipid-lowering treatment | 6 (8) | 0 | 27 (16) | 4 (7) | .07 |
| Lipid level, median (IQR), mg/dL | |||||
| Total cholesterol | 153.5 (126.0–182.5) | 176.0 (127.0–183.0) | 176.0 (150.0–207.0) | 186.0 (170.0–211.0) | <.001 |
| HDL | 52.0 (37.5–62.0) | 62.0 (48.0–73.0) | 54.0 (46.0–69.0) | 64.5 (48.0–78.0) | .01 |
| LDL | 76.5 (61.0–98.5) | 77.0 (57.0–97.0) | 97.0 (80.0–121.0) | 99.0 (81.0–128.0) | <.001 |
| Triglycerides | 113.0 (87.0–149.0) | 75.0 (72.0–95.0) | 89.0 (70.0–132.0) | 75.0 (54.0–114.0) | <.001 |
| HIV related | |||||
| Current CD4 count, median (IQR), cells/mm3 | 505 (318–700) | 911 (685–1101) | 587 (373–772) | 962 (715–1137) | <.001 |
| HIV RNA <50 copies/mL | 31 (40) | … | 90 (55) | … | .06 |
| History of AIDS | 41 (53) | … | 55 (33) | … | <.001 |
| No ART | 14 (18) | … | 25 (15) | … | .81 |
| ART duration, median (IQR), y | 14 (9–15) | … | 12 (8–15) | … | .30 |
| Liver related | |||||
| AST level, median (IQR), U/L | 39.5 (30.0–60.0) | 28.0 (19.0–49.0) | 20.0 (18.0–26.0) | 17.0 (15.0–20.0) | <.001 |
| ALT level, median (IQR), U/L | 30.0 (21.0–46.0) | 25.0 (14.0–36.0) | 17.0 (12.0–23.0) | 13.0 (10.0–19.0) | <.001 |
| HCV genotype 1 | 52 (67) | 10 (71) | … | … | .56 |
Abbreviations: ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; BMI, body mass index; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; HOMA-IR, homeostasis model assessment–estimated insulin resistance; IQR, interquartile range; LDL, low-density lipoprotein.
a Unless otherwise indicated, data represent No. (%) of women.
Association of HIV and HCV With Liver Stiffness
HIV/HCV-coinfected and HCV-monoinfected women had higher liver stiffness values than those with neither HIV nor HCV infection (Figure 1). There was little difference in liver stiffness values between HIV-monoinfected women and those with neither infection. We also examined the relationship of HIV and HCV status with serum markers of fibrosis, APRI, and FIB-4. Similar to our TE findings, we found that HIV/HCV-coinfected and HCV-monoinfected women had greater APRI (median, 0.5 and 0.4, respectively, vs 0.2; P < .001) and FIB-4 (median, 2.0 and 1.6, respectively, vs 0.7; P < .001) scores than those with neither HIV nor HCV infection. In contrast to our TE findings, HIV-monoinfected women had higher APRI (median, 0.3 vs 0.2; P < .001) and FIB-4 (median, 1.0 vs 0.7; P < .001) scores than those with neither infection.
Figure 1.
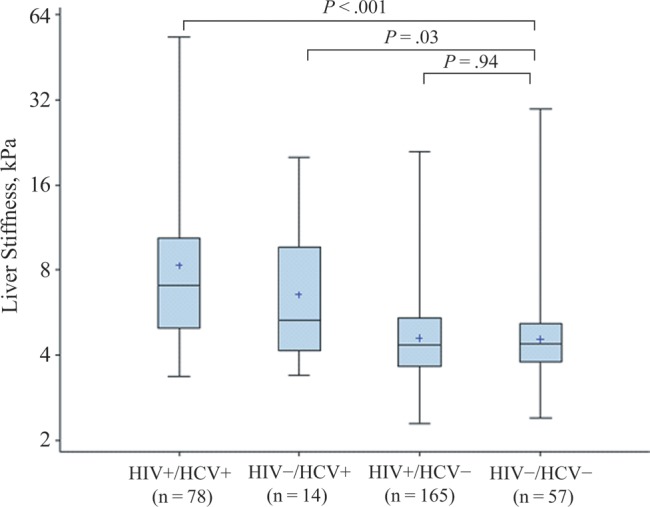
Median transient elastography–measured liver stiffness values by human immunodeficiency virus (HIV) and hepatitis C virus (HCV) status. Abbreviations: HCV+, HCV positive; HCV−, HCV negative; HIV+, HIV positive; HIV−, HIV negative.
After adjustment for demographic, behavioral, and metabolic factors, HIV/HCV coinfection remained strongly associated with higher liver stiffness values (74.2% higher relative to uninfected women; 95% confidence interval [CI], 49%–104%; P < .001). By contrast, HIV monoinfection did not seem to be associated with greater liver stiffness (−0.25%; 95% CI, -11% to 12%; P = .97). HIV/HCV-coinfected and HCV-monoinfected women had a greater prevalence of significant fibrosis (liver stiffness value >7.1 kPa) than women with neither infection (47% and 36%, respectively, vs 8.8%; both P < .001). HIV monoinfected women and those with neither infection had similar prevalences of significant fibrosis (10.3% vs 8.8%; P = .74). After adjustment for demographic, behavioral, and metabolic factors, HIV/HCV coinfection remained strongly associated with a greater prevalence of significant fibrosis (relative risk, 4.8; 95% CI, 2.5–9.1; P < .001). Among the HIV-monoinfected women, the median ALT level was higher in those with significant fibrosis (n = 17) than in those without significant fibrosis (n = 148), but the difference was not statistically significant (23 vs 20 U/L; P = .07). Among women with neither HIV nor HCV infection, the median ALT was the same in those with significant (n = 5) and those without (n = 52) significant (17 U/L in both groups; P = .34).
Factors Associated With Liver Stiffness by HIV and HCV Status
Greater waist circumference was positively associated with liver stiffness in both HIV/HCV-coinfected and HCV-monoinfected women (Figure 2) but showed little association with liver stiffness in the HIV-monoinfected group and the group with neither infection. Waist circumference remained associated with greater liver stiffness after adjustment for age and race/ethnicity in HCV-infected women (+18.2% per 10-cm increase; 95% CI, 7.5%–30.1%; P < .001). Although BMI was associated with greater liver stiffness in unadjusted analysis (+3.2%; 95% CI, .96%–5.5%; P = .005), after adjustment for waist circumference the association was attenuated and in a negative direction (−0.74%; 95% CI, −5.9 to 4.7; P = .78). Waist circumference did not seem to be associated with greater liver stiffness in HIV-monoinfected women after multivariable adjustment (−3.6% per 10-cm increase; 95% CI, −8.3 to 1.35; P = .15).
Figure 2.
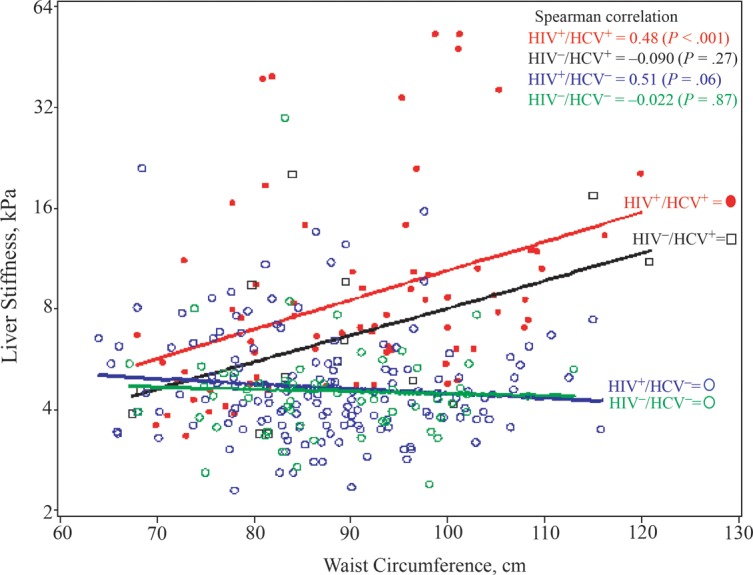
Association of waist circumference with liver stiffness values by human immunodeficiency virus (HIV) and hepatitis C virus (HCV) status. Abbreviations: HCV+, HCV positive; HCV−, HCV negative; HIV+, HIV positive; HIV−, HIV negative.
Among the HIV/HCV-coinfected women, waist circumference and a history of AIDS were associated with greater liver stiffness (Table 2). There was little association of any other metabolic and lifestyle factor with liver stiffness. Among the HIV-monoinfected women, history of AIDS and greater HIV RNA remained associated with greater liver stiffness after adjustment, as did Hispanic race, whereas marijuana use was associated with less liver stiffness (Table 3). In women with neither infection, there was little association of any factor with liver stiffness (data not shown).
Table 2.
Factors Associated With Liver Stiffness in HIV/HCV-Coinfected and HCV-Monoinfected Women
| Factor | Estimated Difference (95% CI), %a |
|||
|---|---|---|---|---|
| Unadjusted | P Value | Adjusted | P Value | |
| Age (per decade) | −4.4 (−22.2 to 17.6) | .67 | −14.8 (−29.3 to 2.7) | .09 |
| Black vs white (not Hispanic) | 28.8 (−2.2 to 69.7) | .07 | 22.3 (−10.4 to 66.9) | .20 |
| Hispanic vs white | 54.9 (−21.9 to 207.5) | .21 | 26.6 (−37.0 to 154.5) | .51 |
| Waist circumference (per 10 cm)b | 17.6 (6.5 to 29.9) | .001 | 18.7 (6.8 to 32.0) | .001 |
| History of clinical AIDS | 31.7 (−.53 to 74.5) | .054 | 35.5 (3.7 to 76.9) | .03 |
Abbreviations: CI, confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
a Liver stiffness, measured with transient elastography, was log-transformed, and results were back-transformed to produce estimated percentage differences in stiffness attributable to each factor. Results are shown for all 78 HCV-infected women, including both those with HIV/HCV coinfection and those with HCV monoinfection
b A 10-cm increase is equivalent to a 3.94-inch increase.
Table 3.
Factors Associated With Liver Stiffness in HIV-Monoinfected Women
| Factor | Estimated Difference (95% CI), %a |
|||
|---|---|---|---|---|
| Unadjusted | P Value | Adjusted | P Value | |
| Age (per decade) | 2.6 (−4.1 to 9.8) | .45 | 3.3 (−3.1 to 10.2) | .32 |
| Black vs white (not Hispanic) | 7.1 (−5.1 to 20.8) | .27 | 9.7 (−2.3 to 23.2) | .12 |
| Hispanic vs white | 29.3 (2.1 to 63.7) | .03 | 31.4 (6.4 to 62.4) | .01 |
| Marijuana (daily vs none) | −16.5 (−26.8 to −4.8) | .007 | −14.8 (−25.9 to −1.96) | .02 |
| HIV RNA level (per 10-fold increase) | 6.8 (.91 to 13.0) | .02 | 7.3 (1.59 to 13.3) | .01 |
| History of clinical AIDS | 14.2 (.82 to 29.4), | .04 | 12.9 (.40 to 27.0) | .04 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
a Liver stiffness, measured with transient elastography, was log-transformed, and results were back-transformed to produce estimated percentage differences in stiffness attributable to each factor. Results are shown for 165 HIV-monoinfected women.
DISCUSSION
To our knowledge, our study is the first to examine liver fibrosis using ultrasound-based TE measurement of liver stiffness in a geographically and ethnically diverse cohort of US women (with HIV monoinfection, HIV/HCV coinfection, HCV monoinfection, or neither infection) with well-characterized metabolic parameters. We found that HIV/HCV-coinfected women but not HIV-monoinfected women had higher liver stiffness values than those with neither infection. Greater waist circumference, a marker of central obesity, was associated with liver stiffness in HCV-infected women, but not HIV-monoinfected women. Among HIV-infected women (with or without HCV Infection), a history of AIDS was associated with greater liver stiffness.
Our finding that HIV-monoinfected women did not seem to have higher liver stiffness values than uninfected women was unexpected. HIV infection per se has been shown to infect hepatic stellate cells (HSCs), which in turn can lead to hepatic injury [24]. The authors of that study [24] suggested that HSC activation (through HCV replication or possibly through HIV replication) would be required in order to infect HSCs and stimulate expression of collagen and proinflammatory cytokines that promote fibrosis. In our study, >50% of our women had undetectable HIV RNA levels. Furthermore, in analyzing HIV-moninfected women only, we observed an association of liver stiffness with higher HIV RNA levels and a history of clinical AIDS (which could serve as a marker of prolonged viremia and immunosuppression).
There are 2 possible reasons for the difference between our finding of a lack of association between HIV monoinfection and liver fibrosis and findings of other studies [6, 7] directly comparing HIV-monoinfected adults with adults with neither HIV nor HCV infection. First, Price et al [7] studied HIV-monoinfected men not receiving highly active ART, and Blackard et al [6] studied HIV-monoinfected women seen in the HERS cohort from 1993 to 2000. These studies therefore included patients that were more likely to have uncontrolled viral replication and lower CD4 counts and to be receiving antiretroviral agents associated with hepatic steatosis or liver injury, such as stavudine, didanosine, and zidovudine. In both studies, when the analysis was limited to those with HIV monoinfection only, greater immunosuppression was associated with greater fibrosis.
Second, those studies used indirect serum markers of fibrosis APRI and FIB-4, which are derived from clinical laboratory values that might be altered by HIV infection itself. Vermehren et al [25] found that whereas 37% of HIV-monoinfected patients had significant liver fibrosis or cirrhosis, as indicated by the serum fibrosis marker Fibrotest (derived from 6 markers, including bilirubin), only 21% had significant fibrosis as predicted by TE. That study concluded that Fibrotest estimated much higher levels of fibrosis than TE in HIV-monoinfected patients [25]. Examination of the association of HIV and HCV status with indirect serum markers of fibrosis and direct serum markers (eg, enhanced liver fibrosis marker) relative to TE-measured liver stiffness are currently underway.
It is noteworthy that waist circumference was associated with liver stiffness in our HCV-infected patients but not in HIV-monoinfected patients. Studies in HCV-monoinfected persons have shown that central obesity (as determined by waist circumference) is a strong predictor of fibrosis, a stronger predictor than BMI, consistent with our findings [26–28]. In HIV-monoinfected patients, DallaPiazza et al [8] did not find an association of obesity with liver fibrosis estimated by the APRI. That study did not examine the association of waist circumference with liver fibrosis. Taken together, our findings and others suggest that central obesity further worsens underlying HCV-associated liver injury. By contrast, in the setting of little injury to the liver, the adverse effects of obesity may be less apparent. One question that needs study is whether hepatic steatosis, thought to be a consequence of excess visceral adipose tissue, is a mechanism by which rapid fibrosis progression occurs in the setting of HCV infection.
In our study we found that daily marijuana use was associated with less liver fibrosis, with associations reaching statistical significance in HIV-monoinfected and control participants. By contrast, previous studies in HCV-monoinfected patients found daily marijuana use to be a strong risk factor for fibrosis progression [29, 30]. This discrepancy may be partly explained by the fact that our study included few women with significant fibrosis, and marijuana use was more common in women without HCV infection. Findings of prior studies have suggested that cannabis may have little or no influence on the initiation of fibrosis but may only be an important cofactor in fibrosis progression when fibrosis is already present [29, 30].
Although we also found that Hispanic race was associated with greater liver stiffness in HIV-monoinfected women, the number of women of Hispanic race in our cohort was low, and therefore our findings should be interpreted with caution. Nevertheless, studies in the general population have shown that, compared with African Americans and whites, Hispanics have a greater prevalence of hepatic steatosis and are at greater risk of nonalcoholic fatty liver disease–associated cirrhosis [31–33].
Our study has some important limitations. First, the cross-sectional design limits our study to evaluation of exposures and disease status at one point in time. It is theoretically possible that liver fibrosis preceded our studied exposures; this is especially true of metabolic markers. Second, the proportion of patients with significant fibrosis in our HIV-monoinfected patients was small (10%) and limits our statistical power for identifying potential risk factors. However, the advantage of TE in providing a continuous measure of liver stiffness allows for broader study of fibrosis progression, producing a more robust evaluation of the factors associated with liver disease progression. Finally, the use of TE in our cohort has some limitations, including the exclusion of women that were more often obese. The exclusion of women with a BMI >35 kg/m2 may also have limited our ability to detect an effect of central obesity with fibrosis in the HIV-monoinfected and control women. A detailed analysis comparing serum biomarkers of fibrosis with TE in the same study population is underway.
Using TE to estimate fibrosis in a large cohort of women with HIV monoinfection, HIV/HCV coinfection, HCV monoinfection, and neither infection, we conclude that HCV but not HIV infection is associated with increased liver fibrosis. Central obesity further worsens liver fibrosis in HCV-infected women, but its effect in the absence of HCV-associated liver injury seems to be lessened. The mechanisms by which central obesity may worsen liver fibrosis in the setting of underlying HCV-associated liver injury need further investigation, including study of the contribution of hepatic steatosis and inflammation (both potential consequences of central obesity) to liver fibrosis.
Notes
Acknowledgments. Data for this work were collected by the WIHS Collaborative Study Group with centers (principal investigators) at the New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); the Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); the Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); and the Data Coordinating Center (Stephen Gange).
Financial support. The WIHS is funded by the National Institute of Allergy and Infectious Diseases (grants UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and the National Institute of Child Health and Human Development (grant UO1-HD-32632). It is cofunded by the National Cancer Institute, the National Institute on Drug Abuse, the National Institute on Deafness and Other Communication Disorders, and the National Center for Research Resources (grants MO1-RR-00071, MO1-RR-00079, MO1-RR-00083). The current study was also supported by the University of California, San Francisco, Liver Center, the National Institutes of Health (grant P30 DK026743), the National Institute of Allergy and Infectious Diseases (grant R01 AI 087176 [PCT], administered by the Northern California Institute for Research and Education), and the Veterans Affairs Medical Center, San Francisco, California.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Salmon-Ceron D, Lewden C, Morlat P, et al. Liver disease as a major cause of death among HIV infected patients: role of hepatitis C and B viruses and alcohol. J Hepatol. 2005;42:799–805. doi: 10.1016/j.jhep.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Weber R, Sabin C, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D: A: D study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 3.Bani-Sadr F, Carrat F, Bedossa P, et al. Hepatic steatosis in HIV-HCV coinfected patients: analysis of risk factors. AIDS. 2006;20:525. doi: 10.1097/01.aids.0000210606.63138.f5. [DOI] [PubMed] [Google Scholar]
- 4.Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. Hepatology. 1999;30:1054–8. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Guardado A, Maradona JA, Asensi V, Cartón JA, Casado L. Hepatitis C virus in patients with HIV infection and lipodystrophy. J Acquir Immune Defic Syndr. 2003;32:348–9. doi: 10.1097/00126334-200303010-00018. [DOI] [PubMed] [Google Scholar]
- 6.Blackard JT, Welge JA, Taylor LE, et al. HIV mono-infection is associated with FIB-4—a noninvasive index of liver fibrosis—in women. Clin Infect Dis. 2011;52:674–80. doi: 10.1093/cid/ciq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price JC, Seaberg EC, Badri S, Witt MD, D'Acunto K, Thio CL. HIV monoinfection is associated with increased aspartate aminotransferase-to-platelet ratio index, a surrogate marker for hepatic fibrosis. J Infect Dis. 2012;205:1005–13. doi: 10.1093/infdis/jir885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DallaPiazza M, Amorosa VK, Localio R, Kostman JR, Lo Re V., III Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC Infect Dis. 2010;10:116. doi: 10.1186/1471-2334-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forrester JE, Rhee MS, McGovern BH, Sterling RK, Knox TA, Terrin N. The association of HIV viral load with indirect markers of liver injury. J Viral Hepat. 2012;19:e202–11. doi: 10.1111/j.1365-2893.2011.01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tien PC, Kotler DP, Overton ET, et al. Regional adipose tissue and elevations in serum aminotransferases in HIV-infected individuals. J Acquir Immune Defic Syndr. 2008;48:169–76. doi: 10.1097/QAI.0b013e3181685700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacon MC, Von Wyl V, Alden C, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–19. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barkan SE, Melnick SL, Preston-Martin S, et al. The women's interagency HIV study. Epidemiology. 1998;9:117–25. [PubMed] [Google Scholar]
- 13.Cohen EB, Afdhal NH. Ultrasound-based hepatic elastography: origins, limitations, and applications. J Clin Gastroenterol. 2010;44:637–45. doi: 10.1097/MCG.0b013e3181e12c39. [DOI] [PubMed] [Google Scholar]
- 14.Vergara S, Macías J, Rivero A, et al. The use of transient elastometry for assessing liver fibrosis in patients with HIV and hepatitis C virus coinfection. Clin Infect Dis. 2007;45:969–74. doi: 10.1086/521857. [DOI] [PubMed] [Google Scholar]
- 15.Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968–73. doi: 10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucidarme D, Foucher J, Le Bail B, et al. Factors of accuracy of transient elastography (Fibroscan) for the diagnosis of liver fibrosis in chronic hepatitis C. Hepatology. 2009;49:1083–9. doi: 10.1002/hep.22748. [DOI] [PubMed] [Google Scholar]
- 17.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 18.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 19.Gilks WR, Richardson S, Spiegelhalter DJ. Markov chain Monte Carlo in practice. London: Chapman & Hall; 1996. [Google Scholar]
- 20.Huber P. The behavior of maximum likelihood estimates under nonstandard conditions; 1967. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probablility, University of California Press, Berkeley, CA. [Google Scholar]
- 21.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–30. [Google Scholar]
- 22.Lumley T, Kronmal R, Ma S. Relative risk regression in medical research: models, contrasts, estimators, and algorithms. UW Biostatistics Working Paper Series; 2006. Berkeley Electronic Press, Seattle, WA. [Google Scholar]
- 23.Hoeting JA, Madigan D, Raftery AE, Volinsky CT. Bayesian model averaging: a tutorial. Stat Sci. 1999;14:382–401. [Google Scholar]
- 24.Tuyama AC, Hong F, Saiman Y, et al. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus–induced liver fibrosis. Hepatology. 2010;52:612–22. doi: 10.1002/hep.23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermehren J, Vermehren A, Mueller A, et al. Assessment of liver fibrosis and associated risk factors in HIV-infected individuals using transient elastography and serum biomarkers. BMC Gastroenterol. 2012;12:27. doi: 10.1186/1471-230X-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adinolfi LE, Gambardella M, Andreana A, Tripodi M, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358–64. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- 27.Lo Iacono O, VENEZIA G, Petta S, et al. The impact of insulin resistance, serum adipocytokines and visceral obesity on steatosis and fibrosis in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2007;25:1181–91. doi: 10.1111/j.1365-2036.2007.03309.x. [DOI] [PubMed] [Google Scholar]
- 28.Stranges S, Dorn JM, Muti P, et al. Body fat distribution, relative weight, and liver enzyme levels: a population-based study. Hepatology. 2004;39:754–63. doi: 10.1002/hep.20149. [DOI] [PubMed] [Google Scholar]
- 29.Hezode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42:63–71. doi: 10.1002/hep.20733. [DOI] [PubMed] [Google Scholar]
- 30.Ishida JH, Peters MG, Jin C, et al. Influence of cannabis use on severity of hepatitis C disease. Clin Gastroenterol Hepatol. 2008;6:69–75. doi: 10.1016/j.cgh.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Browning JD, Kumar KS, Saboorian MH, Thiele DL. Ethnic differences in the prevalence of cryptogenic cirrhosis. Am J Gastroenterol. 2004;99:292–8. doi: 10.1111/j.1572-0241.2004.04059.x. [DOI] [PubMed] [Google Scholar]
- 32.Mohanty SR, Troy TN, Huo D, O'Brien BL, Jensen DM, Hart J. Influence of ethnicity on histological differences in non-alcoholic fatty liver disease. J Hepatol. 2009;50:797–804. doi: 10.1016/j.jhep.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372–9. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]


