Abstract
Background. In 2010, Zambia had a large measles outbreak, providing an opportunity to measure changes in measles serostatus following highly active antiretroviral therapy (HAART), exposure to measles virus, and revaccination among children infected with human immunodeficiency virus (HIV).
Methods. A prospective cohort study of 169 HIV-infected Zambian children aged 9–60 months with a history of measles vaccination was conducted to characterize the effects of HAART and revaccination on measles immunoglobulin G (IgG) serostatus by enzyme immunoassay.
Results. Prior to the measles outbreak, only 23% of HIV-infected children were measles IgG seropositive at HAART initiation. After adjusting for 6-month changes in baseline age and 5% changes in nadir CD4+ T-cell percentage, HAART was not associated with measles IgG seroconversion. However, 18 of 19 children seroconverted after revaccination. Eight children seroconverted during the outbreak without revaccination and were likely exposed to wild-type measles virus, but none were reported to have had clinical measles.
Conclusions. Immune reconstitution after HAART initiation did not restore protective levels of measles IgG antibodies, but almost all children developed protective antibody levels after revaccination. Some previously vaccinated HIV-infected children had serological evidence of exposure to wild-type measles virus without a reported history of measles.
Keywords: measles, HIV, antiretroviral therapy, immune reconstitution, antibody, outbreak
Measles remains an important cause of child mortality despite an effective vaccine that protects approximately 85% of recipients after a single dose at 9 months of age [1]. Over the past decade, the Measles and Rubella Initiative and partners dedicated resources to reduce measles incidence and mortality. This effort proved largely successful in Africa where the number of reported measles cases declined >90% between 2001 and 2008 [2]. In 2010, however, large measles outbreaks occurred throughout sub-Saharan Africa that continued through 2011 [3, 4], underscoring the importance of maintaining high levels of population immunity and surveillance activities.
Children born to women infected with human immunodeficiency virus (HIV) have lower concentrations of maternal antibodies to measles virus [5, 6] and are at increased risk of measles at younger ages [7]. Measles-containing vaccine is recommended at 9 months of age in countries with circulating measles virus and may be administered as young as 6 months of age to HIV-infected children who are not severely immunosuppressed [8]. HIV infection, however, can adversely impact immune responses to measles vaccine [9]. A meta-analysis indicated that fewer HIV-infected children responded to measles vaccination at 9 and 12 months of age compared to uninfected children [10], and antibody levels to measles virus waned over 2–3 years in children infected with HIV [11]. In addition to quantitative differences, the quality of antibody responses to measles virus is impaired in HIV-infected children. Antibody avidity, a functional measure of a mature B-cell response that is correlated with the development of long-lived antibody-secreting plasma cells, decreased 3 months following measles vaccination in HIV-infected Zambian children [12].
Prior to the availability of highly active antiretroviral therapy (HAART), high rates of HIV-related mortality maintained a low proportion of HIV-infected children susceptible to measles virus infection. However, with the rapid scale-up of HAART, HIV-related mortality has declined [13], allowing for accumulation of measles-susceptible HIV-infected children [14]. Antibodies to multiple vaccine-preventable diseases, including measles, are frequently below protective levels in children receiving HAART [15], suggesting that revaccination may be needed to maintain high levels of population immunity. Additionally, cellular reconstitution of HIV-infected children after HAART initiation likely occurs via a predominance of naive T lymphocytes, with only a small contribution through expansion of memory lymphocytes [16].
To evaluate the effect of HAART and revaccination on measles immunity, we measured immunoglobulin G (IgG) antibody levels to measles virus in HIV-infected Zambian children initiating HAART before and after the 2010 measles outbreak and supplemental immunization activities (SIAs).
MATERIALS AND METHODS
Study Design and Population
We conducted a prospective, observational cohort study among HIV-infected children initiating HAART in Lusaka, Zambia. HIV-infected children between 9 months and 10 years of age with a documented history of measles vaccination and initiating HAART at 1 of 2 public clinics were eligible for enrollment. Both clinics provide care to HIV-infected individuals in urban, low-income communities within Lusaka [17]. Children were enrolled during a routine clinic visit 2 weeks after initial evaluation for HAART eligibility. The decision to initiate HAART was made by the child's healthcare provider. Written informed consent was obtained in English, Nyanja, or Bemba from the accompanying caregiver. The study was approved by the Research Ethics Committee of the University of Zambia, the Institutional Review Board of the University of Alabama, and the Institutional Review Board at the Johns Hopkins University Bloomberg School of Public Health.
At the baseline visit, a peripheral blood sample was obtained from the child and a questionnaire was administered to the child's caregiver to collect demographic and clinical information, including immunization history from the Under-5 Immunization Card, signs and symptoms of previous and current acute and chronic infections, and physical examination findings. Children returned to the clinic every 3 months for routine clinical follow-up during which a study questionnaire was administered and a blood sample collected. The questionnaire administered at the 3-month follow-up visits collected additional information via interview about measles revaccination, illnesses between study visits, and changes in physical examination findings.
A comparison group of children presenting to the same clinics for routine clinical care was enrolled for a single study visit, during which a peripheral blood sample was collected and a questionnaire was administered to the child's caregiver.The HIV infection status of comparison children was not confirmed but was presumed to be negative based on clinical assessment and self-reported maternal HIV infection status.
In 2010, 817 measles cases were reported to the Zambian Ministry of Health by 15 June, and SIAs were implemented in July 2010 for children between 9 and 47 months of age [18, 19]. Despite these actions, 15 736 measles cases were reported in 2010 [3] and 13 150 in 2011 [20].
Laboratory Assays
Peripheral blood was collected via venipuncture in Vacutainer tubes containing EDTA or heparin sulfate (BD Biosciences). The number and proportion of CD4+ T cells were measured by immunophenotyping. In brief, whole blood was mixed with CD3-allophycocyanin and CD4-peridinin chlorophyll-A protein. Red blood cells were lysed with FACS/Lyse solution, and samples were washed with phosphate-buffered saline and fixed with 1% paraformaldehyde. Fixed cells were analyzed on a FACSCalibur Flow Cytometer.
The Enzygnost Anti-Measles Virus IgG Combipack enzyme immunoassay (Dade Behring) was used to measure measles virus IgG antibody levels in plasma samples according to the manufacturer's instructions. This enzyme immunoassay allows conversion of optical density readings to approximate concentrations in milli–international units per milliliter (mIU/mL). Children with antibody concentrations >120 mIU/mL were considered to be seropositive [21], although results were unchanged when 200 mIU/mL was used as the threshold value. We performed repeat testing of samples with qualitative differences at adjacent visits when antibody concentrations substantially decreased or antibody concentrations increased without report of clinical measles or revaccination. Discrepant results were resolved with a third test.
Data Analysis
Children were excluded from analysis if they were aged >60 months at study entry due to small sample size in this age category. Study visits were excluded if caregivers reported or were unsure whether the child had measles in the intervening period. In July 2007, an SIA was conducted in Zambia during which children aged 6–59 months were eligible for measles vaccination. Children whose caregivers reported or were unsure of revaccination in the 2007 campaign were excluded from analysis to ensure inclusion of children who received only a single dose of measles vaccine prior to the 2010 SIA. Weight-for-age z score (WAZ) and height-for-age z score (HAZ) were calculated using the World Health Organization's (WHO) child growth standards [22]. A z score of less than −2.00 was considered underweight (WAZ) or stunted (HAZ).
Study visits occurred from 20 January 2009 to 28 February 2012 and were divided into 4 periods: (1) prior to the measles outbreak (visits before 1 June 2010), (2) during the outbreak and prior to revaccination (visits between 1 June 2010 and 1 July 2011 and revaccination not reported), (3) during the outbreak and after revaccination (visits after 19 July 2010 with reported revaccination), and (4) after the peak of the outbreak (visits after 1 July 2011 and revaccination not reported). The period 1 June 2010 to 1 July 2011 was considered to be that during which children were at increased risk of exposure to wild-type measles virus as determined by the number of hospital admissions for measles at the University Teaching Hospital and reported cases [19, 23]. Supplemental immunization activities were conducted from 20 to 24 July 2010. Parents or guardians received a revaccination card indicating the date of revaccination, which was recorded during study visits. If the date of revaccination was illegible or the card was unavailable but the child's caregiver reported revaccination, the midpoint of the week-long SIA was used as the date of revaccination.
To determine if HAART exposure affected measles IgG seroprevalence, discrete time proportional hazards models were constructed to estimate the conditional probability of incident seroconversion at each 3-month visit after HAART initiation among children who were seronegative at enrollment, adjusting for 6-month changes in baseline age (centered at 18 months) and 5% changes in nadir CD4+ T-cell percentage (centered at 10%). A child potentially contributed person-time to multiple periods if he or she enrolled during an earlier period (eg, prior to the outbreak), was seronegative at the enrollment visit, and remained at risk for seroconversion during a later period (eg, during the outbreak and prior to revaccination). Children were censored at the previous study visit if their caregiver reported signs and symptoms of measles or if they were diagnosed with measles. Observations were administratively censored on the date of the child's final visit if they were lost to follow-up or on 28 February 2012. Person-time due to missed visits was interval censored. Visits beyond 18 months after HAART initiation were excluded from analysis due to small sample size.
Log-binomial generalized linear models were used to estimate prevalence ratios (PrRs) to determine the effect of the measles outbreak on measles IgG serostatus at enrollment, adjusting for 6-month changes in baseline age and 5% change in CD4+ T-cell percentage at HAART initiation. Additional potential covariates evaluated in the models were sex, illness during the prior 4 weeks, illness at the current study visit, and WAZ and HAZ scores.
Continuous covariates were compared using Kruskal–Wallis test, and categorical covariates were compared using χ2test. Statistical analyses were conducted using Stata software, version 10.1 and R version 2.13. The R packages “lattice,” “fields,” and “colorRamps” were used to generate lasagna plots [24–27].
RESULTS
Characteristics of the Study Population
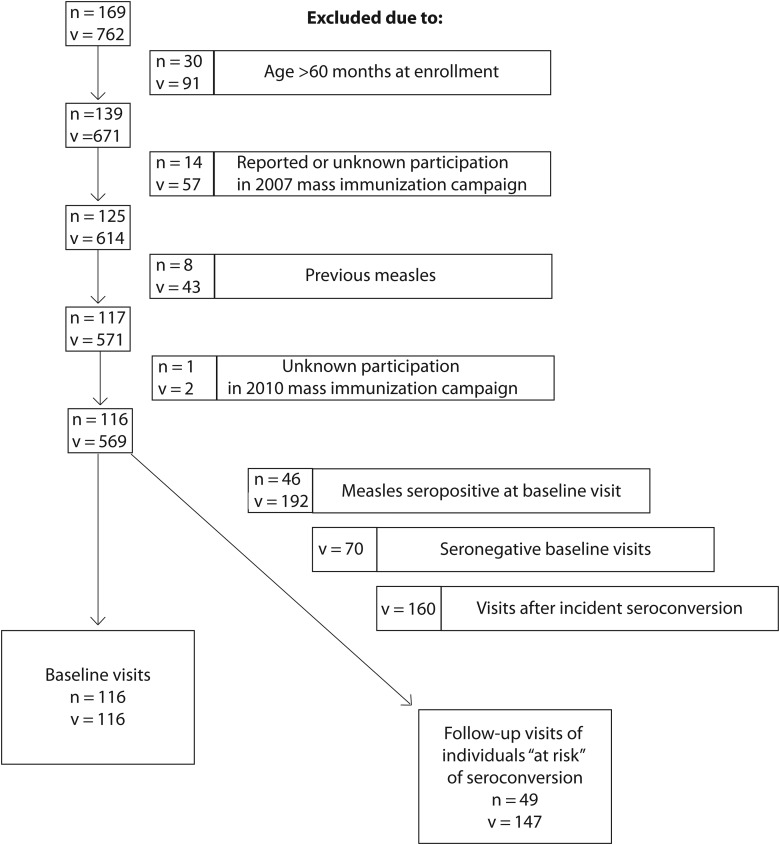
Between January 2009 and February 2012, measles IgG antibody levels were measured in 169 HIV-infected Zambian children during 762 study visits. After excluding children aged >60 months at HAART initiation (n = 30), children with reported or unknown participation in the 2007 SIA (n = 15), and children with a history of prior measles (n = 8), a total of 116 HIV-infected children evaluated at 569 study visits were included in the analysis (Figure 1). These 116 children represented the subgroup of children with a single exposure to measles vaccine at a median age of 10.0 months (interquartile range [IQR], 9.40–12.2) and a median of 10.5 months (IQR, 4.30–21.6) before HAART initiation. At enrollment, this group of children had a median CD4+ T-cell percentage of 11.0% (IQR, 7.4–16.6), which increased to 21.9% (IQR, 16.4%–28.2%) among the 70 children observed 6 months after HAART initiation.
Figure 1.
Flow diagram of study visit exclusions. The number of observations is denoted as “v” and the number of individuals as “n.”
Twenty-five comparison children were enrolled after the outbreak began and had similar characteristics to the HIV-infected children except that their percentage of CD4+ T cells was significantly higher, with a median of 34.2% (IQR, 29.8–37.2; Table 1).
Table 1.
Characteristics of HIV-Infected Zambian Children at Initiation of Highly Active Antiretroviral Therapy and Controls
| Characteristic | Before Outbreak | During Outbreak, Prior to Revaccination | During Outbreak, After Revaccination | After Outbreak, No Revaccination | Control Group Children | P Valuea |
|---|---|---|---|---|---|---|
| No. | 61 | 21 | 16 | 18 | 25 | |
| Female sex | 31 (51%) | 9 (43%) | 8 (50%) | 7 (39%) | 12 (48%) | .795, .958 |
| Age, mo | 23.2 (17.9–32.3) | 19.7 (13.8–32.8) | 26.0 (21.3–33.9) | 23.5 (15.7–26.6) | 21.2 (17.4–32.1) | .728, .564 |
| % CD4+ T cells | 9.74 (7.51–17.6) [n = 57] |
12.3 (10.1–15.5) [n = 21] |
10.2 (7.36–18.1) [n = 14] |
12.4 (7.43–17.0) [n = 15] |
34.2 (29.8–37.2) [n = 12] |
.915, <.001 |
| Illness in the previous 4 wk | 47 (77%) | 15 (71%) | 14 (88%) | 14 (78%) | 0 | .712, <.001 |
| Ill at enrollment visit | 20 (33%) | 8 (38%) | 7 (44%) | 3 (17%) | 0 | .353, .017 |
| WAZ | −2.37 (−3.51 to −1.31) | −2.32 (−2.83 to −1.25) | −2.92 (−3.75 to −1.78) | …b | .608 | |
| HAZ | −3.71 (−4.69 to −2.55) [n = 54] |
−3.73 (−4.87 to −1.16) | −2.95 (−4.50 to −2.00) | −2.99 (−3.44 to −1.62) | …b | .645 |
| Age at measles vaccination, mo | 10.0 (9.37–12.1) [n = 54] |
10.5 (9.67–12.3) [n = 14] |
10.4 (8.87–23.9) [n = 15] |
9.80 (9.48–10.2) [n = 16] |
9.50 (8.93–9.80) | .499, .022 |
| Months since measles vaccination | 10.1 (3.73–21.5) [n = 57] |
4.37 (0.47–18.8) [n = 15] |
11.9 (1.28–22.3) | 12.0 (5.72–19.8) [n = 16] |
11.6 (5.10–21.7) | .484, .296 |
| Measles seropositive | 14 (22%) | 10 (48%) | 9 (56%) | 13 (72%) | 22 (88%) | .001, <.001 |
Categorical characteristics are expressed as No. (%). Continuous characteristics are expressed as median (interquartile range).
Abbreviations: HAZ, height-for-age z score; HIV, human immunodeficiency virus; WAZ, weight-for-age z score.
a P values are from χ2 or Kruskal–Wallis tests comparing HIV-infected children between periods of enrollment (before comma) or control children vs all HIV-infected children (after comma).
b Weight and height measurements were not collected for control children.
Measles Seropositivity at HAART Initiation
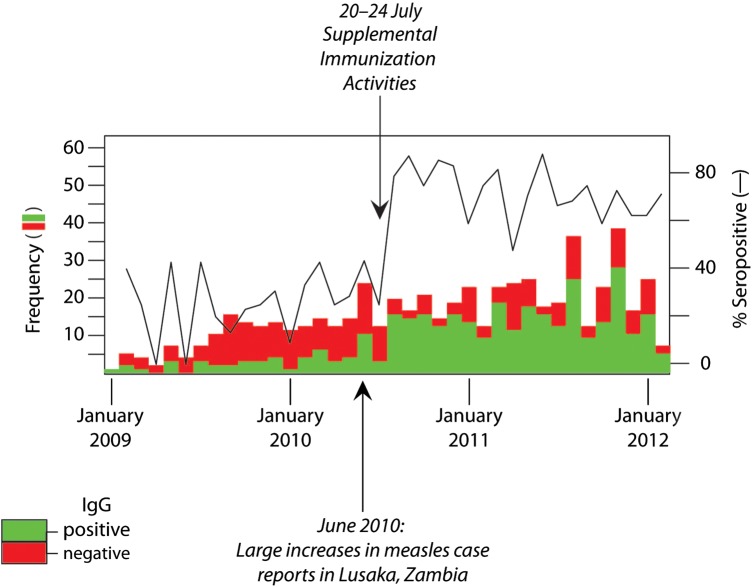
Despite a documented history of previous measles vaccination, only 46 of 116 children (40%) were measles IgG seropositive at HAART initiation over the study period (Figure 2). However, an increase in measles IgG seroprevalence was observed among study visits occurring after June 2010 (Figure 2), corresponding to an increase in hospital admissions for measles, announcement of a measles outbreak, and implementation of the SIA [18]. Study visits were consequently stratified by calendar time based on the dates of these events.
Figure 2.
Measles immunoglobulin G (IgG) serostatus time series of 3-month study visits for human immunodeficiency virus–infected children initiating highly active antiretroviral therapy in Lusaka, Zambia. Increased reports of wild-type measles cases began in June 2010 and were followed by supplemental immunization activities in July 2010.
Sixty-one of the 116 children (53%) initiated HAART before the 2010 measles outbreak, 21 children (18%) were enrolled during the outbreak but prior to the SIA, 16 children (14%) were enrolled after revaccination during the SIA, and 18 children (15%) were enrolled after the outbreak and were not revaccinated. Of the 61 children enrolled prior to the measles outbreak, 51% were girls and the median age was 23.2 months, which was not significantly different from the 21 children enrolled during the outbreak, the 16 children enrolled after revaccination, or the 18 children enrolled after the outbreak (Table 1). At enrollment, the majority (63%) of children had a percentage of CD4+ T cells <15%, and most children met the WHO criteria for being underweight (63%) or stunted (75%) (Table 1).
Among the 61 children enrolled prior to the outbreak, only 23% were measles IgG seropositive at enrollment compared to 48%, 56%, and 72% of the children enrolled during the outbreak, after revaccination, and after the outbreak, respectively (P = .001 by Kruskal–Wallis test; Table 1). After adjusting for age and CD4+ T-cell percentage, increased proportions of HIV-infected children were measles IgG seropositive at enrollment during the outbreak but prior to revaccination (PrR = 2.23 [95% confidence interval {CI}, 1.14–4.41]), after revaccination (PrR = 2.99 [95% CI, 1.58–5.68]), and after the peak of the outbreak without revaccination (PrR = 3.40 [95% CI, 1.79–6.48]) compared to the proportion of seropositive children enrolled prior to the outbreak (Table 2).
Table 2.
Baseline Prevalence Ratios of Measles Immunoglobulin G Seropositivity Among Zambian Children Prior to the Initiation of Highly Active Antiretroviral Therapy
| Prevalence Ratio (95% CI) |
||
|---|---|---|
| Characteristic | Univariable | Multivariable |
| Enrollment perioda | ||
| Before outbreak | Reference | Reference |
| During outbreak, prior to revaccination | 2.26 (1.15–4.44) | 2.23 (1.14–4.41) |
| During outbreak, after revaccination | 3.05 (1.62–5.77) | 2.99 (1.58–5.68) |
| After outbreak, no revaccination | 3.48 (1.93–6.27) | 3.40 (1.79–6.48) |
| 6-mo increase in age | 0.93 (.83–1.04) | 0.97 (.89–1.06) |
| 5% increase in CD4+ T cells | 1.08 (.92–1.27) | 0.99 (.82–1.19) |
| Female sex | 0.96 (.60–1.54) | … |
| Illness in the previous 4 wk | 0.76 (.46–1.25) | … |
| Ill at current study visit | 1.03 (.62–1.69) | … |
| Weight-for-age z score | 1.00 (.84–1.18) | … |
| Height-for-age z score [n = 74] | 1.05 (.93–1.18) | … |
Abbreviation: CI, confidence interval.
a Enrollment periods were assessed as a categorical variable.
All 25 control children were enrolled after the beginning of the outbreak. Nine of 12 children (75%) who were not revaccinated were measles IgG seropositive and all of the 13 children revaccinated before enrollment were measles IgG seropositive.
Seroconversion During Follow-up
Of 81 children with follow-up visits, 32 (40%) children maintained their original measles IgG serostatus following initiation of HAART: 11 (14%) remained seronegative and 21 (26%) remained seropositive (Figure 3). The serostatus of the remaining 49 (60%) children changed (Figure 3). Thirty-eight children who were measles seronegative at baseline seroconverted during follow-up, although 13 of these children again became seronegative. Eleven children who were measles seropositive at baseline seroreverted during follow-up, although 3 of these children subsequently became seropositive (Supplementary Table 1).
Figure 3.
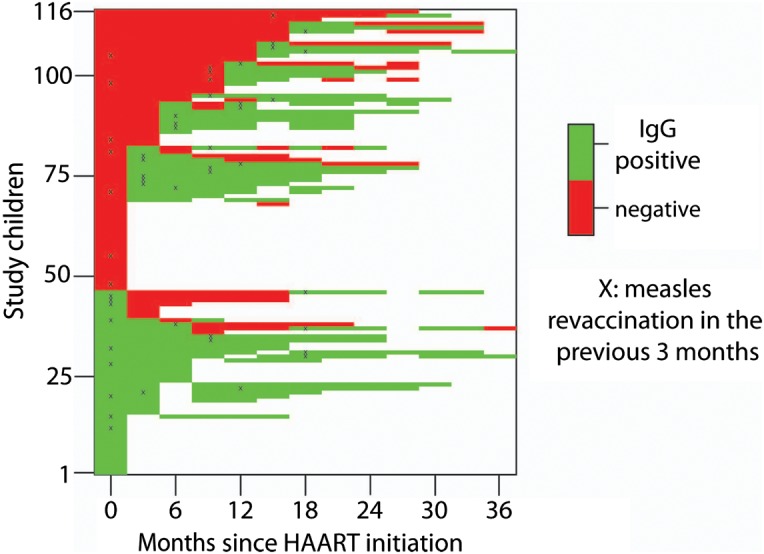
Measles immunoglobulin G (IgG) serostatus trajectories of 116 Zambian children initiating highly active antiretroviral therapy (HAART). The visit after revaccination is denoted with an “X.”
Effect of HAART on Measles Seroconversion
Of 130 follow-up visits from 46 children who were seronegative at enrollment, HAART exposure prior to the outbreak was not associated with seroconversion at any visit, nor was there a trend indicating that seroconversion was more likely with increased time on HAART after adjusting for baseline age and CD4+ T-cell percentage (Table 3).
Table 3.
Risk of Measles Immunoglobulin G Seroconversion After Initiation of Highly Active Antiretroviral Therapy Before and During the Measles Outbreak and After Measles Revaccination Among HIV-Infected Seronegative Children at Study Enrollment
| Characteristic | Hazard Ratio (95% CI) |
|---|---|
| Months since HAART initiation | |
| 3 | Reference |
| 6 | 1.08 (.43–2.71) |
| 9 | 0.13 (.02–.98) |
| 12 | 0.96 (.35–2.69) |
| 15 | 0.86 (.27–2.69) |
| 18 | 0.99 (.25–3.89) |
| During outbreak, prior to revaccination | 2.48 (.86–7.11) |
| After revaccination | 9.85 (3.78–25.7) |
| 6-mo increase in baseline age | 1.08 (.90–1.29) |
| 5% change in baseline CD4+ T cells | 1.09 (.83–1.43) |
Abbreviations: CI, confidence interval; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus.
Effect of Revaccination on Measles Serostatus
Nineteen children enrolled before the SIA were seronegative at HAART initiation and were revaccinated during the SIA. Of these, 1 child had an increase in measles IgG antibody concentration but failed to reach a concentration of 120 mIU/mL. The remaining 18 children (95%) seroconverted by the subsequent study visit following revaccination, with a median time from revaccination to the seropositive study visit of 68 days (IQR, 28–80; Supplementary Table 1). Neither the absolute nor relative change in measles IgG concentrations were associated with CD4+ T-cell percentages at the visit before seroconversion, duration of HAART exposure, age at revaccination, or age at HAART initiation (Supplementary Table 2). Among 13 study visits after the outbreak among children who were not revaccinated, no seroconversions occurred.
In the subset of children who were seronegative at enrollment, study visits occurring during the outbreak were not significantly associated with seroconversion (hazard ratio [HR] = 2.48 [95% CI, .86–7.11]), but revaccination during the SIA was strongly associated with seroconversion (HR = 9.85 [95% CI, 3.78–25.7]) (Table 3). Changes in measles antibody concentrations were greatest among those who were revaccinated (Table 4).
Table 4.
Measles Immunoglobulin G Antibody Concentrations Among HIV-Infected Children Who Seroconverted During Study Follow-up
| Period of Seroconversion |
P Value | ||||
|---|---|---|---|---|---|
| Characteristic | Before Outbreak | During Outbreak, Prior to Revaccination | During Outbreak, After Revaccination | Remained Seronegative | |
| No. | 8 | 8 | 22 | 7 | |
| HAART initiation, mIU/mL | 32 (25–81) | 38 (32–59) | 38 (23–59) | 38 (21–51) | .785 |
| Study visit prior to seroconversion, mIU/mL | 76 (51–88) | 61 (23–80) | 45 (29–68) | … | .204 |
| Change from HAART initiation to seroconversion, mIU/mL | 181 (114–289) | 253 (126–381) | 620 (177–1029) | … | .075 |
| Change from visit prior to seroconversion, mIU/mL | 129 (105–286) | 253 (95–396) | 603 (177–973) | … | .036 |
Data are presented as median (interquartile range). P values are from Kruskal–Wallis test.
Abbreviations: HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; mIU/mL, milli–international units per milliliter.
Seroreversion During Follow-up
Among the 46 children who were measles IgG seropositive at enrollment, 32 (70%) had 146 follow-up visits, of whom 11 (24%) seroreverted at some point during follow-up. Seven of these children were enrolled prior to the SIA, 5 of whom were revaccinated during the SIA and were seropositive at the subsequent study visit (as indicated by a black “X” in Figure 3); however, 3 (60%) seroreverted again within 6 months of revaccination. The 2 remaining children were not revaccinated but were followed for only 2 study visits.
Clinical and Subclinical Measles
The caregivers of 5 children reported a history of measles at the enrollment visit, and prior history of measles for 3 children was unknown (Figure 1). Three additional children developed measles during follow-up but none were reported to have died of measles.
Eight children seroconverted during the outbreak without revaccination and were likely exposed to wild-type measles virus, but none were reported to have had clinical measles. These children with possible subclinical measles did not differ from the 3 children who developed clinical measles during follow-up with respect to age or time since measles vaccination. Measles IgG concentrations at HAART initiation was slightly higher among children who did not develop clinical measles (median, 37.5 mIU/mL [IQR, 31.5–59.0]) compared to children who developed measles (median, 21.0 mIU/mL [IQR, 6.0–32.0]) (P = .052). The percentage of CD4+ T cells was slightly higher at HAART initiation in children who reported measles (median, 13.7% [IQR, 9.74%–16.2%]) compared to those with subclinical measles (median, 9.23% [IQR, 6.36%–12.5%]) (P = .066).
DISCUSSION
Despite a history of documented measles vaccination, most HIV-infected Zambian children were measles IgG seronegative by enzyme immunoassay at the time of HAART initiation. Immune reconstitution following treatment with HAART failed to restore protective antibody levels to measles virus, and the proportion of children with protective antibody levels remained low preceding the measles outbreak and revaccination during the SIA. Almost all measles seronegative children seroconverted after revaccination. These findings provide further evidence that HIV-infected children in sub-Saharan Africa should be revaccinated against measles [28, 29]. However, some HIV-infected children had serological evidence of exposure to wild-type measles virus during the outbreak without a reported history of measles, suggesting protection against clinical disease in the absence of protective antibody levels as measured by enzyme immunoassay.
We hypothesized that measles seroconversion in this cohort could be due to 3 factors: immune reconstitution after HAART initiation, antibody boosting following exposure to wild-type measles virus during the outbreak, or revaccination during the SIA. Given the lack of stimulation by measles virus antigens and immune reconstitution with naive T lymphocytes in young children, seroconversion due solely to HAART was considered unlikely. However, seroconversion after 6 months of HAART was reported in 40% of 43 Kenyan children without a history of clinical measles and prior to revaccination [30]. Failure to reconstitute IgG antibodies to measles virus after HAART initiation also was observed among HIV-infected Thai children [31]. Our findings provide evidence that HAART does not restore protective antibody levels to measles virus among a cohort of HIV-infected children in sub-Saharan Africa.
Although other studies have shown increased IgG antibody responses to measles virus after revaccination of children receiving HAART, most were conducted in developed countries [32–34] or measured immune responses at only 1 or 2 time points [30, 35].The largest study, involving HIV-infected children receiving antiretroviral therapy in the United States, measured neutralizing antibody concentrations to measles virus in 193 children before and after receipt of an additional dose of measles-mumps-rubella vaccine [34]. Only 52% of children had protective antibody concentrations at the time of starting antiretroviral therapy; however, 89% had protective concentrations 8 weeks after revaccination and 80% maintained protective concentrations at 80 weeks. Our findings support recommendations to revaccinate HIV-infected children against measles in sub-Saharan Africa.
The observation that some HIV-infected children developed serological evidence of exposure to wild-type measles virus in the absence of clinical disease was unexpected. There are several reasons why HIV-infected children may not develop clinical measles despite low antibody levels to measles virus. Enzyme immunoassays detect a large proportion of nonneutralizing IgG antibodies directed toward the nucleoprotein, but circulating high-affinity IgG antibodies to the H glycoprotein are responsible for neutralizing measles virus and conferring protection [36]. A plaque reduction neutralization assay may have been more sensitive in detecting protective antibody levels in these children. Cell-mediated immunity also is important in protecting against disease, as previous investigations have demonstrated high levels of lymphoproliferative responses in vaccine recipients with low or undetectable IgG concentrations [37], and individuals with agammaglobulinemia have been shown to successfully recover from measles infection and remain protected after reexposure [38]. Thus, high-affinity IgG antibodies against the hemagglutinin protein and strong, anamnestic cellular immune responses may explain why protection was conferred to the HIV-infected children with low levels of preexisting antibodies to measles virus. A less likely alternative explanation is that these children had measles without rash as a consequence of severe immunosuppression [39].
This study had several limitations. First, although it is possible that revaccination during the SIA was underreported, we believe recall bias is unlikely considering the time and effort required by parents or guardians to have their child revaccinated. Moreover, vaccination cards provided to parents or guardians during the SIA were evaluated at study visits. Second, enzyme immunoassays quantify circulating IgG antibodies against measles virus but do not measure the ability of these antibodies to neutralize measles virus. A functional assay such as the plaque reduction neutralization assay would provide a better measure of protective immunity and reduce misclassification. Third, we did not identify associations between CD4+ T-cell percentages and measles IgG antibody levels or seroconversion as observed by others [30, 35] and were unable to assess correlations with HIV load, which was associated with seroprotection following measles revaccination in the United States [34].
Most HIV-infected children initiating HAART lacked protective antibody concentrations to measles virus despite prior vaccination. Immune reconstitution after HAART initiation did not restore protective levels of measles antibodies, but most children developed protective antibody levels after revaccination. Our findings support recommendations to revaccinate HIV-infected children receiving HAART to ensure individual protection and achieve measles elimination goals.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the study participants and their caregivers. We also thank Patricia Bwalya, Peter Chisenga, Casey Kabaso, Martha Mponda, Priscilla Mukongolwa, Brenda Muluka, Julius Mwanza, and Hendrix Ndhlovu, who collected data and cared for the participating children.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (R01AI070018).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cutts FT, Grabowsky M, Markowitz LE. The effect of dose and strain of live attenuated measles vaccines on serological responses in young infants. Biologicals. 1995;23:95–106. doi: 10.1016/1045-1056(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 2.Progress toward measles control—African region, 2001–2008. MMWR Morb Mortal Wkly Rep. 2009;58:1036–41. [PubMed] [Google Scholar]
- 3.Measles outbreaks and progress toward measles preelimination—African region, 2009–2010. MMWR Morb Mortal Wkly Rep. 2011;60:374–8. [PubMed] [Google Scholar]
- 4.Moss WJ, Griffin DE. Measles. Lancet. 2012;379:153–64. doi: 10.1016/S0140-6736(10)62352-5. [DOI] [PubMed] [Google Scholar]
- 5.Scott S, Cumberland P, Shulman CE, et al. Neonatal measles immunity in rural Kenya: the influence of HIV and placental malaria infections on placental transfer of antibodies and levels of antibody in maternal and cord serum samples. J Infect Dis. 2005;191:1854–60. doi: 10.1086/429963. [DOI] [PubMed] [Google Scholar]
- 6.Scott S, Moss WJ, Cousens S, et al. The influence of HIV-1 exposure and infection on levels of passively acquired antibodies to measles virus in Zambian infants. Clin Infect Dis. 2007;45:1417–24. doi: 10.1086/522989. [DOI] [PubMed] [Google Scholar]
- 7.Embree JE, Datta P, Stackiw W, et al. Increased risk of early measles in infants of human immunodeficiency virus type 1-seropositive mothers. J Infect Dis. 1992;165:262–7. doi: 10.1093/infdis/165.2.262. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO position on measles vaccines. Vaccine. 2009;27:7219–21. doi: 10.1016/j.vaccine.2009.09.116. [DOI] [PubMed] [Google Scholar]
- 9.Lowther SA, Curriero FC, Kalish BT, Shields TM, Monze M, Moss WJ. Population immunity to measles virus and the effect of HIV-1 infection after a mass measles vaccination campaign in Lusaka, Zambia: a cross-sectional survey. Lancet. 2009;373:1025–32. doi: 10.1016/S0140-6736(09)60142-2. [DOI] [PubMed] [Google Scholar]
- 10.Scott P, Moss WJ, Gilani Z, Low N. Measles vaccination in HIV-infected children: systematic review and meta-analysis of safety and immunogenicity. J Infect Dis. 2011;204(uppl 1):S164–78. doi: 10.1093/infdis/jir071. [DOI] [PubMed] [Google Scholar]
- 11.Moss WJ, Scott S, Mugala N, et al. Immunogenicity of standard-titer measles vaccine in HIV-1-infected and uninfected Zambian children: an observational study. J Infect Dis. 2007;196:347–55. doi: 10.1086/519169. [DOI] [PubMed] [Google Scholar]
- 12.Nair N, Moss WJ, Scott S, et al. HIV-1 infection in Zambian children impairs the development and avidity maturation of measles virus-specific immunoglobulin G after vaccination and infection. J Infect Dis. 2009;200:1031–8. doi: 10.1086/605648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutcliffe CG, Scott S, Mugala N, et al. Survival from 9 months of age among HIV-infected and uninfected Zambian children prior to the availability of antiretroviral therapy. Clin Infect Dis. 2008;47:837–44. doi: 10.1086/591203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott S, Mossong J, Moss WJ, Cutts FT, Cousens S. Predicted impact of the HIV-1 epidemic on measles in developing countries: results from a dynamic age-structured model. Int J Epidemiol. 2008;37:356–67. doi: 10.1093/ije/dyn007. [DOI] [PubMed] [Google Scholar]
- 15.Sutcliffe CG, Moss WJ. Do children infected with HIV receiving HAART need to be revaccinated? Lancet Infect Dis. 2010;10:630–42. doi: 10.1016/S1473-3099(10)70116-X. [DOI] [PubMed] [Google Scholar]
- 16.Rainwater-Lovett K, Moss WJ. Immunologic basis for revaccination of HIV-infected children receiving HAART. Future Virol. 2011;6:59–71. doi: 10.2217/fvl.10.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–99. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 18.Gilliam E. Responding to a measles outbreak in Zambia. 2010. http://www.unicefusa.org/news/news-from-the-field/responding-to-a-measles-outbreak-in-zambia.html. Accessed 21 September 2011. [Google Scholar]
- 19.United Nations. Funding gap leads to major measles outbreak in eastern and southern Africa. http://www.un.org/apps/news/story.asp?NewsID=35073&Cr=measles&Cr1. Accessed 21 September 2011. [Google Scholar]
- 20.World Health Organization. Reported measles cases and incidence rates by member states. 2012. http://www.who.int/immunization_monitoring/diseases/measles_monthlydata/en/index1.html. Accessed 31 May 2012.
- 21.World Health Organization. Geneva, Switzerland: WHO; 2009. Immunological basis for immunization series, module 7: Measles, update 2009. [Google Scholar]
- 22.World Health Organization. 2011. Child growth standards. http://www.who.int/childgrowth/en/ Accessed 19 May 2010. [Google Scholar]
- 23.Measles Initiative. 2012. Measles in 2011: devastating, surprising outbreaks and some success. http://stopmeaslesrubella.org/2012/02/20/measles-in-2011-devastating-and-surprising-outbreaks-and-some-success/ Accessed 4 May 2012. [Google Scholar]
- 24.Swihart BJ, Caffo B, James BD, Strand M, Schwartz BS, Punjabi NM. Lasagna plots: a saucy alternative to spaghetti plots. Epidemiology. 2010;21:621–5. doi: 10.1097/EDE.0b013e3181e5b06a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkar D. New York: Springer; 2008. Lattice: multivariate data visualization with R. [Google Scholar]
- 26.Tools for Spatial Data. Boulder, CO: National Center for Atmospheric Research; 2006. [Google Scholar]
- 27.Keitt TH. Coherent ecological dynamics induced by large scale disturbance. Nature. 2008;454:331–4. doi: 10.1038/nature06935. [DOI] [PubMed] [Google Scholar]
- 28.Rainwater-Lovett K, Moss WJ. The urgent need for recommendations on revaccination of HIV-infected children after successful antiretroviral therapy. Clin Infect Dis. 2010;51:634–5. doi: 10.1086/655769. [DOI] [PubMed] [Google Scholar]
- 29.Maldonado Y. Measles vaccine, HIV infection, and antiretroviral therapy—a window of opportunity. J Infect Dis. 2012;206:466–8. doi: 10.1093/infdis/jis392. [DOI] [PubMed] [Google Scholar]
- 30.Farquhar C, Wamalwa D, Selig S, et al. Immune responses to measles and tetanus vaccines among Kenyan human immunodeficiency virus type 1 (HIV-1)-infected children pre- and post-highly active antiretroviral therapy and revaccination. Pediatr Infect Dis J. 2009;28:295–9. doi: 10.1097/INF.0b013e3181903ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aurpibul L, Puthanakit T, Siriaksorn S, Sirisanthana T, Sirisanthana V. Prevalence of protective antibody against measles in HIV-infected children with immune recovery after highly active antiretroviral therapy. HIV Med. 2006;7:467–70. doi: 10.1111/j.1468-1293.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 32.Berkelhamer S, Borock E, Elsen C, Englund J, Johnson D. Effect of highly active antiretroviral therapy on the serological response to additional measles vaccinations in human immunodeficiency virus-infected children. Clin Infect Dis. 2001;32:1090–4. doi: 10.1086/319591. [DOI] [PubMed] [Google Scholar]
- 33.Melvin AJ, Mohan KM. Response to immunization with measles, tetanus, and Haemophilus influenzae type b vaccines in children who have human immunodeficiency virus type 1 infection and are treated with highly active antiretroviral therapy. Pediatrics. 2003;111:e641–4. doi: 10.1542/peds.111.6.e641. [DOI] [PubMed] [Google Scholar]
- 34.Abzug MJ, Qin M, Levin MJ, et al. Immunogenicity, immunologic memory, and safety following measles revaccination in HIV-infected children receiving highly active antiretroviral therapy. J Infect Dis. 2012;206:512–22. doi: 10.1093/infdis/jis386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aurpibul L, Puthanakit T, Sirisanthana T, Sirisanthana V. Response to measles, mumps, and rubella revaccination in HIV-infected children with immune recovery after highly active antiretroviral therapy. Clin Infect Dis. 2007;45:637–42. doi: 10.1086/520651. [DOI] [PubMed] [Google Scholar]
- 36.Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–42. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 37.Ward BJ, Boulianne N, Ratnam S, Guiot MC, Couillard M, De SG. Cellular immunity in measles vaccine failure: demonstration of measles antigen-specific lymphoproliferative responses despite limited serum antibody production after revaccination. J Infect Dis. 1995;172:1591–5. doi: 10.1093/infdis/172.6.1591. [DOI] [PubMed] [Google Scholar]
- 38.Good RA, Zak SJ. Disturbances in gamma globulin synthesis as “experiments of nature”: E. Mead Johnson Award . Pediatrics. 1956;18:109–49. [PubMed] [Google Scholar]
- 39.Markowitz LE, Chandler FW, Roldan EO, et al. Fatal measles pneumonia without rash in a child with AIDS. J Infect Dis. 1988;158:480–3. doi: 10.1093/infdis/158.2.480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.