Abstract
Background:
In pursuit of innovative approaches for the management of severe infections in young infants, which is a major cause of mortality, a multipartner research program was conceptualized to provide right care in the right place. The primary objective was to generate evidence and identify a simple, safe and effective treatment regimen for young infants with severe infections that can be provided closer to home by trained health workers where referral is not possible.
Research:
Published and nonpublished data on community-based approaches for the management of neonatal sepsis were critically reviewed by an independent expert panel convened in 2007 by the World Health Organization in collaboration with the United States Agency for International Development and Save the Children/Saving Newborn Lives. These stakeholders agreed to 1) undertake research to improve the specificity of a diagnostic algorithm and revise World Health Organization/United Nations International Children’s Emergency Fund Integrated Management of Childhood Illness guidelines to identify sick young infants for referral, 2) develop research studies with common research designs (1 site in each Bangladesh and Pakistan and a multicentre site in Democratic Republic of Congo, Kenya and Nigeria) and oversight mechanisms to evaluate antibiotic regimens (when referral is not accepted by the family) that are safe and efficacious, appropriate to the severity of infection, and deployable on a large scale and 3) utilize existing program delivery structures incorporating community health workers, skilled health workers to deliver simple antibiotic treatment when referral is not possible.
Conclusions:
This research program facilitated innovative research in different geographical, cultural and administrative milieus to generate recommendations for policy.
Keywords: research, innovation, neonates, young infants, antibiotic treatment, infection
Recognizing a unique opportunity to address a major unmet need, namely high neonatal mortality, a multistakeholder collaborative initiative was conceptualized around a set of guiding principles. The primary objective of this initiative was to generate evidence for best practices in neonatal and young infant care at various levels—including home, community and health facility—according to the philosophy of “the right care in the right place.”
Severe infections are among the major causes of neonatal and young infant (0–59 days) deaths in low- and middle-income countries.1,2 For suspected bacterial sepsis in newborns, the World Health Organization (WHO) recommends hospitalization and injectable antibiotics for at least 10 days.3 Delays in recognition of signs of illness and in care-seeking by families and lack of access to appropriately trained health workers and treatment are all contributing factors to high neonatal mortality.4–6
Bang and colleagues7 proposed a home-based neonatal care approach, emphasizing delivery of essential newborn care, early recognition of signs of illness and delivery of care for possible neonatal sepsis at or close to home. Although the delivery of essential newborn care and simplified assessment are now well-accepted practices, scaling up care through the use of community health workers (CHWs), as is proposed in the Bang et al model, has encountered challenges. Good insight often raises new questions and identifies challenges that need to be addressed in order to create workable models. It invites further innovation in the classification of sick neonates, in treatment regimens and in the way that sick neonates are managed. Home visitation,8 coupled with a simplified yet reliable assessment and classification approach for possible serious bacterial infection (PSBI) in neonates and young infants, creates an opportunity to achieve high impact by improving treatment regimens and optimizing both place and mode of care delivery.
APPROACH
Expert Consultation on Community-based Approaches for Neonatal Sepsis Management
The unique prospect for innovation and the opportunity to contribute to a reduction in neonatal and young infant mortality by improving treatment regimens has brought together multiple partners. The objective was to generate decisive evidence that builds consensus toward an efficacious yet deployable regimen, tailored to the severity of infections in a health facility closer to home. In 2007, the WHO co-organized an expert consultation in collaboration with the United States Agency for International Development (USAID) and Save the Children/Saving Newborn Lives (SC/SNL) and considered several options for treatment regimens as well as place and mode of delivery.9 An independent expert panel reviewed published and nonpublished evidence from several available studies on the effectiveness of community-based interventions for the prevention and treatment of neonatal infections. The group also reviewed 2 differing perspectives of an ethical and equity conundrum: Where there is no or highly limited access to facility care and infants will die due to lack of options for treatment, should the standard of care recognize this void and provide treatment to families at the community- and household-level? Or rather, is it unethical to recommend treatment other than the gold standard of care (ie, daily administration of penicillin/gentamicin in a hospital setting)?
Based on the expert review, different models to manage neonatal sepsis were identified, including:
Identification of signs of illness by CHWs and provision of treatment at home without any referral7;
Identification of signs of illness by CHWs and provision of treatment at home when referral was refused10;
Identification of signs of illness by CHWs and referral to outpatient treatment when hospital referral was refused11; and
Identification of signs of illness by a clinician, followed by treatment when hospital referral was refused.6,12
The expert group concluded that at the time, there was insufficient evidence to recommend an antibiotic treatment regime for treating PSBI in young infants at the community or outpatient level.
In response to the findings of the expert panel, the following recommendations were made:
Research and program experience is needed on shorter-course injectable antibiotic treatment, and on switch therapy of initial injectable antibiotic therapy followed by oral antibiotics;
Research is essential in order to develop a simple diagnostic algorithm to identify sick young infants in the community and in outpatient settings; and
Optimal delivery strategies need to be incorporated into essential newborn care, encompassing both preventive and treatment approaches in various health system scenarios.
It was recognized that research to evaluate simplified antibiotic regimens was possible when referral was not accepted by the families. Ideally, regimens should be efficacious, appropriate to the severity of infection and deployable on a large scale to reach all in need. Similar approaches to promote short stays, as well as day care approaches, have been successfully introduced in inpatient eye care and surgery; the consultation concluded that providing care “closer to home” needs to be similarly scaled up for neonatal infections. Therefore, WHO, USAID and SC/SNL agreed on a plan of action to address the aforementioned research issues on a priority basis.
Follow-up Actions
Based on the findings of the 2007 consultation, a series of follow-up actions were defined and carried out by the partners.
Improving the Specificity of a Diagnostic Algorithm to Recognize PSBI at Outpatient Level.
The algorithm of assessment for young infants with PSBI was further refined.13–16 Seven clinical signs and symptoms were included in the revised WHO/United Nations International Children’s Emergency Fund Integrated Management of Childhood Illness (IMCI) for young infants, which performed well in both 0- to 6-day-old and 7- to 59-day-old infants.17 The newly incorporated clinical signs include not feeding well, convulsions, respiratory rate of >60 breaths per minute, severe chest indrawing, temperature of ≥37.5°C or <35.5°C and movement only when stimulated or no movement at all.
Research Studies to Manage PSBI in Young Infants With Simplified Antibiotic Regimens.
Following the expert consultation, there was a lack of consensus among partners on the optimal study design. Initially, SC/SNL, in consultation with WHO, began planning a study to evaluate simplified management for PSBI in Pakistan, and USAID began planning support for a study in Bangladesh. Consequently, both protocols were initially prepared as individual studies with somewhat different designs. It became obvious to all stakeholders that there was a need to harmonize these studies in order to achieve policy-relevant results. The Bill and Melinda Gates Foundation (BMGF), with technical support from WHO and in collaboration with USAID and SC/SNL, supported activities in conjunction with the Pakistan and Bangladesh site investigators to harmonize the 2 studies and to develop a common oversight mechanism. This included establishing a common technical steering committee as well as a Data Safety Monitoring Board and determining criteria for monitoring, implementation and quality.18 By late 2009, the study objectives, research questions, screening, inclusion and exclusion criteria, enrolment, interventions, outcomes and procedures were synchronized at both sites.19,20
Realizing that the lack of African data on management of newborn infections would ultimately limit the global policy relevance of the research, BMGF agreed to a proposal from WHO and USAID to support a large community-based trial in Africa as a complement to the studies being conducted in Pakistan and Bangladesh. After a call for proposals from African countries, WHO selected 11 of 18 applications, which fulfilled the required criteria. The 11 research teams were subsequently invited to a research proposal development workshop in Abuja, Nigeria, in July 2009. Study objectives, research questions, study outcomes and procedures were shared with the potential African research groups by the Asia trial teams, and a common protocol was discussed and developed rapidly. Additionally, all teams prepared their implementation plans and the budgets required to carry out the common protocol at each of their respective sites. Five research teams and sites were selected after external review: 1 site each in the Democratic Republic of Congo and Kenya and 3 sites in Nigeria. Over the following 3 months, a multicentre protocol to address the effectiveness of simplified antibiotic regimens for treatment of severe infections in young infants in Africa was finalized. The study received funding by BMGF in October 2009.21,22
The protocols and methods for the 3 studies (in Bangladesh, Pakistan and in the African sites) are aligned. Each study has a population under surveillance, consisting of all pregnant mothers and young infants with PSBI (identified and referred at the time of birth). The population composition at each of the research sites is diverse: the Bangladesh site includes both urban and rural populations, the Nigerian sites include peri-urban and rural populations, the Pakistan site is composed of peri-urban populations and the Kenya and Democratic Republic of Congo sites include only rural populations. This diversity promises to facilitate the generalizability of the findings.
In each site, careful attention is paid to ethical issues: before being offered enrolment and treatment, the parent/caregiver of any neonate or young infant with PSBI must have first refused referral to a higher-level facility and consent must have been obtained. In order to assure quality, both internal and external validity have been taken into account in the design and conduct of the trials. The technical steering committee for the Asian studies and the Technical Advisory Group for the African trials have common external experts and representatives from WHO, USAID, SC/SNL and BMGF. Data Safety Monitoring Boards of the trials also had common independent experts. Data management supervision is provided by the London School of Hygiene and Tropical Medicine for each of the 3 studies. There is a common Strategic Planning Committee that is responsible for coordinating implementation and analysis plans and for discussing policy implications as well as dissemination of research results. Standardized training was conducted at all sites with robust internal and external monitoring. It is anticipated that the data will be broadly generalizable as Bangladesh and Pakistan took their inspiration from India, and are thus representative of Southeast Asia, which has very high burden of neonatal infections. The African trial includes sites in east, central and West Africa (Kenya, Democratic Republic of Congo and Nigeria, respectively), which will enable generalizability of the findings in the sub-Saharan African region.
Developing a Study Within the Context of an Existing Program-based Framework.
One of the key considerations in the development of this multipartner research initiative was to nest it within the context of an existing program-based framework.9 It was not approached as a stand-alone intervention, but rather was intended to be integrated with other newborn and young infant care initiatives. Therefore, aspects of home-based newborn care, IMCI for infants, and treatment of serious infections in young infants where referral is not possible were combined into the approach (Fig. 1). Elements such as home visits, pregnancy surveillance, antenatal visits, preparation for birth, CHW home care training packages, IMCI for young infants, refusal of referral and treatment by a trained health worker at home or at a health facility are cornerstones of the approach used in this work. It is anticipated that the incorporation of the study into an existing program-based framework will facilitate successful execution of the study, and perhaps more importantly, ensure sustainability and institutionalization of ensuing newborn infectious disease policy changes within the national ministries of health.
FIGURE 1.
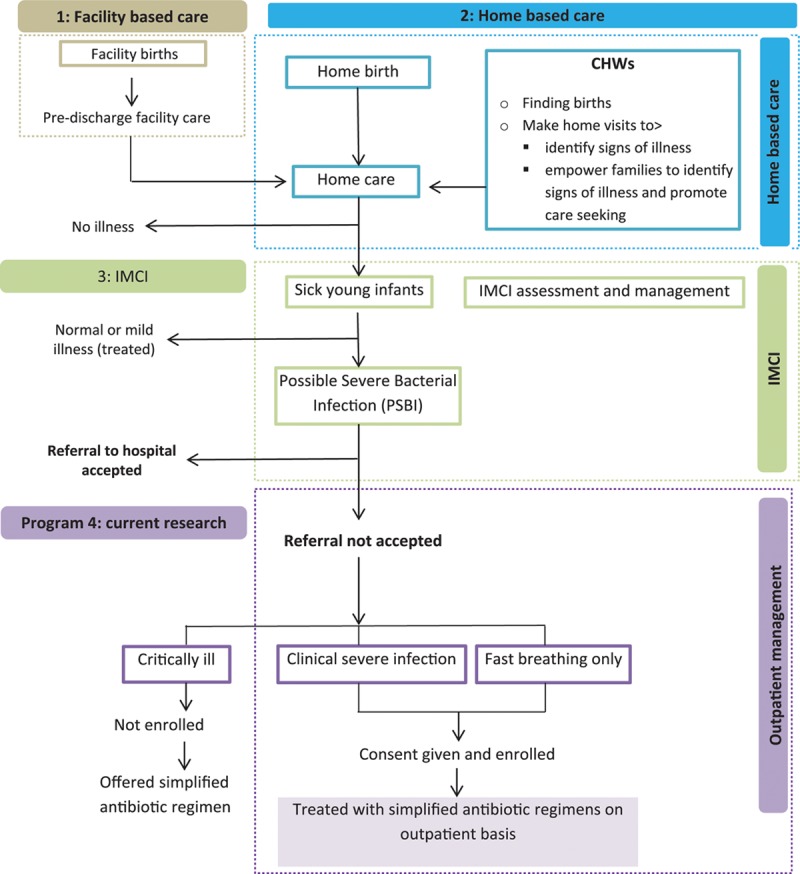
Program framework and context of research.
IMPLICATIONS AND LESSONS LEARNED
A number of key lessons were learned from the process of initiating, designing, implementing and coordinating this multipartner research program. One of the key factors for success was the exceptional willingness to collaborate and use the synergies among stakeholders like WHO, SC/SNL, USAID and BMGF. Among the partners, there was a common understanding and agreement that in order to realize a reduction in infant mortality, early recognition and management of infections in newborn and young infants is critical. Furthermore, there was recognition of an unmet need for unique and innovative treatment regimens, as well as the need to create and connect treatment regimens to existing efforts of home care, IMCI and first-level newborn units.
This multipartner research program provided a chance to collaborate and facilitate research in different geographical, cultural and administrative milieus, to pool resources, expertise, and credibility of researchers, and to generate recommendations for policy. Using an integrated model of young infant care based on home care detection, simplified assessment and relevant treatment regimens could pave the way for scaling up neonatal care programs. Although this research was ethically challenging, the partners shared a common vision for a reduction in neonatal mortality, and thus had a common understanding of the importance of this research. Moreover, several aspects of these trials resulted in increased supply of demand for relevant interventions. On the demand side, increased recognition of PSBI by parents, an increased number of trained CHWs for surveillance of young infants and an increase in the number of families trained in better care have all been observed. Similarly, the trials also increased the supply and provision of services for families with newborns and young infants suffering from PSBI, for example, training health workers, providing medicines and initiating robust supervision systems.
These studies had some limitations. Birth care where referral was not possible was excluded (as it was expected to be treated in hospital), as was the management of critically ill neonates and young infants. Furthermore, financial as well as other aspects of demand creation were not addressed.
In conclusion, this research program represents a multistakeholder collaborative process—based in careful evidence review, dialogue and discussion—that is being used to address an issue of great public health importance. The approach can be used as a model for conducting research in other areas. It is envisaged that the results of these studies will lead to a revised policy framework, and ultimately, to a reduction in the neonatal mortality burden due to PSBI.
ACKNOWLEDGMENTS
We acknowledge the contribution of Jose Martines, Sachiyo Yoshida and Rachel MacLean in the preparation of this article.
Footnotes
Accepted for publication June 5, 2013.
The views and opinions expressed in this article are those of the author(s) and not necessarily the views and opinions of the World Health Organization or United States Agency for International Development.
This journal article was supported with funds provided by Save the Children’s Saving Newborn Lives Program through a grant from the Bill and Melinda Gates Foundation. Its contents are solely the responsibility of the authors and do not necessarily reflect the views of Save the Children or the Bill and Melinda Gates Foundation.
This work was supported by World Health Organization through grants from United States Agency for International Development and Bill and Melinda Gates Foundation.
S.A.Q. and R.B. are staff members of the World Health Organization, and N.B. is a staff member of United States Agency for International Development. The authors have no other funding or conflicts of interest to disclose.
Address for correspondence: Shamim Ahmad Qazi, MB BS, DCH, MSc, MD, Department of Maternal, Newborn, Child and Adolescent Health, World Health Organization, 20 Avenue Appia, Geneva 27, 1211 Switzerland. E-mail: qazis@who.int.
Copyright © 2013 by Maharaj Kishan Bhan. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License, where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially.
REFERENCES
- 1.Lawn JE, Cousens S, Zupan J Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? where? why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Johnson HL, Cousens S, et al. Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Pocket Book of Hospital Care for Children. Guidelines for the Management of Common Illnesses. Second edition. Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 4.Waiswa P, Kallander K, Peterson S, et al. Using the three delays model to understand why newborn babies die in eastern Uganda. Trop Med Int Health. 2010;15:964–972. doi: 10.1111/j.1365-3156.2010.02557.x. [DOI] [PubMed] [Google Scholar]
- 5.Engmann C, Adongo P, Akawire Aborigo R, et al. Infant illness spanning the antenatal to early neonatal continuum in rural northern Ghana: local perceptions, beliefs and practices. J Perinatol. 2013;33:476–481. doi: 10.1038/jp.2012.151. [DOI] [PubMed] [Google Scholar]
- 6.Zaidi AK, Tikmani SS, Warraich HJ, et al. Community-based treatment of serious bacterial infections in newborns and young infants: a randomized controlled trial assessing three antibiotic regimens. Pediatr Infect Dis J. 2012;31:667–672. doi: 10.1097/INF.0b013e318256f86c. [DOI] [PubMed] [Google Scholar]
- 7.Bang AT, Bang RA, Baitule SB, et al. Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet. 1999;354:1955–1961. doi: 10.1016/S0140-6736(99)03046-9. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization, UNICEF. WHO/UNICEF Joint Statement - Home Visits for the Newborn Child:A Strategy to Improve Survival. WHO/FCH/CAH/09.02. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 9.Save the Children; /Saving Newborn Lives, USAID World Health Organization. Expert Consultation on Community-Based Approaches for Neonatal Sepsis Management September 26–28, 2007. Meeting Report. London, UK: Save the Children/Saving Newborn Lives, USAID, World Health Organization; 2008. Available at: http://www.harpnet.org/doc/london_sepsis_mtg09-07.pdf. Accessed July 10, 2013. [Google Scholar]
- 10.Baqui AH, El-Arifeen S, Darmstadt GL, et al. Projahnmo Study Group. Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: a cluster-randomised controlled trial. Lancet. 2008;371:1936–1944. doi: 10.1016/S0140-6736(08)60835-1. [DOI] [PubMed] [Google Scholar]
- 11.Khanal S, Sharma J, GC VS, et al. Community health workers can identify and manage possible infections in neonates and young infants: MINI–a model from Nepal. J Health Popul Nutr. 2011;29:255–264. doi: 10.3329/jhpn.v29i3.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhandari N, Bahl R, Bhatnagar V, et al. Treating sick young infants in urban slum setting [letter]. Lancet. 1996;347:1774–1775. [PubMed] [Google Scholar]
- 13.The Young Infants Clinical Signs Study Group. Clinical signs that predict severe illness in children under age 2 months: a multicentre study. Lancet. 2008;371:135–142. doi: 10.1016/S0140-6736(08)60106-3. [DOI] [PubMed] [Google Scholar]
- 14.Darmstadt GL, Baqui AH, Choi Y, et al. Bangladesh Projahnmo-2 (Mirzapur) Study Group. Validation of community health workers’ assessment of neonatal illness in rural Bangladesh. Bull World Health Organ. 2009;87:12–19. doi: 10.2471/BLT.07.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darmstadt GL, Baqui AH, Choi Y, et al. Bangladesh Projahnmo-2 (Mirzapur) Study. Validation of a clinical algorithm to identify neonates with severe illness during routine household visits in rural Bangladesh. Arch Dis Child. 2011;96:1140–1146. doi: 10.1136/archdischild-2011-300591. [DOI] [PubMed] [Google Scholar]
- 16.Bang AT, Bang RA, Reddy MH, et al. Simple clinical criteria to identify sepsis or pneumonia in neonates in the community needing treatment or referral. Pediatr Infect Dis J. 2005;24:335–341. doi: 10.1097/01.inf.0000157094.43609.17. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization, UNICEF. Integrated Management of Childhood Illness (IMCI) Chart Booklet. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 18.Wall SN, Mazzeo CI, Adejuigbe EA, et al. Ensuring quality in the AFRINEST and SATT trials: clinical standardization and monitoring. Pediatr Infect Dis J. 2013;32(suppl):S39–S45. doi: 10.1097/INF.0b013e31829ff801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baqui AH, Saha SK, Ahmed ASMN, et al. Safety and efficacy of simplified antibiotic regimens for outpatient treatment of clinical serious infection among neonates and young infants aged 0–59 days in Bangladesh: design of a randomized controlled trial. Pediatr Infect Dis J. 2013;32(suppl):S12–S18. doi: 10.1097/INF.0b013e31829ff790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaidi AKM, Tikmani SS, Sultana S, et al. Simplified antibiotic regimens for the management of clinically diagnosed severe infections in newborns and young infants in first-level facilities in Karachi, Pakistan: study design for an outpatient randomized controlled equivalence trial. Pediatr Infect Dis J. 2013;32(suppl):S19–S25. doi: 10.1097/INF.0b013e31829ff7aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AFRINEST (AFRIcan NEonatal Sepsis Trial) Group. Simplified regimens for management of neonates and young infants with clinical severe infection in situations when hospital admission is not possible: study protocol for a randomized, open-label equivalence trial. Pediatr Infect Dis J. 2013;32(suppl):S26–S32. doi: 10.1097/INF.0b013e31829ff7d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AFRINEST (AFRIcan NEonatal Sepsis Trial) Group. Treatment of fast breathing in neonates and young Infants with oral amoxicillin compared to injectable penicillin-gentamicin combination: Study protocol for a randomized, open-label equivalence trial. Pediatr Infect Dis J. 2013;32(suppl):S33–S39. doi: 10.1097/INF.0b013e31829ff7eb. [DOI] [PMC free article] [PubMed] [Google Scholar]


