Abstract
The world is naturally radioactive and approximately 82% of human-absorbed radiation doses, which are out of control, arise from natural sources such as cosmic, terrestrial, and exposure from inhalation or intake radiation sources. In recent years, several international studies have been carried out, which have reported different values regarding the effect of background radiation on human health. Gamma radiation emitted from natural sources (background radiation) is largely due to primordial radionuclides, mainly 232Th and 238U series, and their decay products, as well as 40K, which exist at trace levels in the earth's crust. Their concentrations in soil, sands, and rocks depend on the local geology of each region in the world. Naturally occurring radioactive materials generally contain terrestrial-origin radionuclides, left over since the creation of the earth. In addition, the existence of some springs and quarries increases the dose rate of background radiation in some regions that are known as high level background radiation regions. The type of building materials used in houses can also affect the dose rate of background radiations. The present review article was carried out to consider all of the natural radiations, including cosmic, terrestrial, and food radiation.
Keywords: Background radiation, cosmic, human's health, terrestrial
INTRODUCTION
More than sixty radionuclides can be found in the environment, which can be divided into three general categories: Primordial (which formed before the earth creation), cosmogenic (which formed as a consequence of cosmic ray interactions), and human produced (which formed due to human actions; they are minor amounts compared to natural). Radionuclides are found naturally in air, soil, water, and food.
Natural radioactivity is common in the rocks and soil that constitute planet earth, in water and oceans, and in building materials and homes. There is no place on earth that has no natural radioactivity.[1]
Some radioactive nuclides are detectable in soil. They belong to natural radionuclides such as the members of the uranium and thorium decay series. More specifically, natural environment radioactivity and the associated external exposure due to gamma radiation depend on the geological and geographical conditions and appear at different levels in the soils of each region in the world.[2,3] The specific levels of terrestrial radiation are related to the geological composition of each lithologically separated area and to the content of the rock from which the soils originated in each area in the radioactive elements of thorium (232Th), uranium (238U), and potassium (40K).
All building materials contain various amounts of radioactivity. For example, materials derived from rock and soil contains natural radionuclides of the uranium and thorium series and the radioactive isotope of potassium. Artificial radionuclides can also be present, such as cesium (137Cs), resulting from the fallout from weapons testing and the Chernobyl accident. All these can be sources of both internal and external radiation exposures. Internal exposure occurs through the inhalation of radon gas, and external exposure occurs through the emission of penetrating gamma rays.[4]
Considering that about 50% of natural exposure of people is from radon gas, it is the leading cause of cancer patients suffering from respiratory and gastrointestinal system problems, and the highest percentage of radon that enters the human body is from drinking water and breathing. Once radon in water supplies reaches consumers, it may result in human exposure via inhalation and direct digestion. Radon in water transfers into the air during the rains, flushing toilets, washing dishes, and washing clothes. The aerosols tend to deposit in the lungs, where they release radiation that has been shown to increase the likelihood of lung cancer. Radon can also reach other body tissues through ingestion, resulting in radiation exposure to the internal organs. Ingestion of radon is believed to increase the risk of stomach cancer.[5,6] Besides the effect of soils in population exposure by using them as building material, they can affect the human body by taking the food containing radionuclide, which enters the food chain from deeper soil layers and also tainting the ground water.
Owing to the inevitable effects of radiations and health risk from these exposures, it is necessary to investigate all reported data in the last few years. Hence, this review article aims to consider all natural radiations, including cosmic, terrestrial, and food radiation.
DISCUSSION
Cosmic rays
Cosmic radiation originates from the sun, stars, collapsed stars (such as neutron stars), quasars, and in the hot galactic and intergalactic plasma. It has many components, such as X-rays, gamma rays, and particles, which may be mesons, electrons, protons, neutrons, or hyperons. The initial energy of the individual particles constitutes a broad spectrum from a few electron volts (eV) to about 1,020 eV. Cosmic radiation loses energy as it penetrates the atmosphere. The protective shield of the atmosphere and the earth's magnetic field prevent the soft energy radiation components from penetrating the atmosphere. The hardest components, the mesons, dominate at sea level. Above about 5 km from the sea level, the electrons are equal or dominate the mesons, whereas above 25 km protons dominate. Cosmic radiation produces X-rays and neutrons as it penetrates through the atmosphere. All researchers believe that the contents of natural radionuclides (uranium, potassium, and thorium) as well as the thin layer of atmosphere in the higher altitude regions (mountains) are reasons why they have high levels of human exposure.[4]
Generally, the natural dose rates from cosmic rays depend strongly on the altitude and slightly on the latitude. The latitude effect is due to the charged particle nature of the primary cosmic rays, and the effect of the Earth's magnetic field, which tends to direct ions away from the equator and toward the poles.[4]
In addition, the primary particles often transform to new particles. Penetration of charged particles depends strongly on the magnetic field. The radiation they produce, including neutrons, depends on the magnetic field.[4,7,8,9,10]
During and after slowing down in the atmosphere, the neutrons may in turn produce radioactive isotopes, such as 14C and 3H. The thickness of the atmosphere corresponds to about 10 m of water or about 4 m of concrete.[7] Nevertheless, at the sea level the cosmic radiation contributes on the average of 0.27 mSv/year to the human body. At the ground level, only a small fraction of that is due to neutrons. Cosmic radiation dose increases with altitude. At 2.5 km, it is about 0.55 mSv/year on the order of 60 times greater (17 mSv/year).[4,7,11] At a slightly higher altitude of 15 km and 60° magnetic latitude, it levels off and reaches a maximum of 30 mSv/year.
Cosmic radiation increases with magnetic latitude, especially at higher elevations. For example, at 12.5 km altitude, the dose rate from neutrons alone increases from 8 mSv/year at a magnetic latitude of 25° to 19 mSv/year at a magnetic latitude of 50°.[7] Figure 1 shows how the cosmic neutron dose rate at sea level and at 12.5 km altitude depends on the magnetic latitude.[7] The neutron dose rate at sea level and magnetic latitude of 43° is seen to be approximately 300 times smaller than that at 12.5 km over sea level. At a magnetic latitude of 50°, the cosmic neutron dose at a height of 12.5 km over sea level is about 20 mSv/year, whereas at the ground level and at the same magnetic latitude, the cosmic ray neutron dose is about 19/300 = 0.063 mSv/year.[7]
Figure 1.
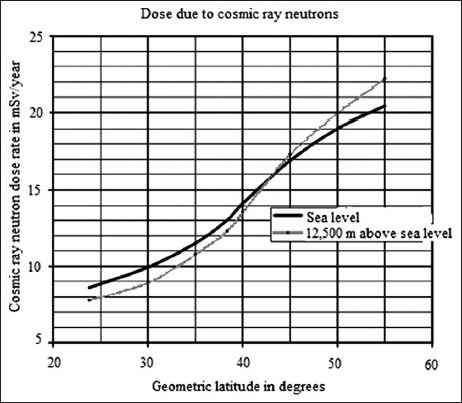
The ordinate shows the neutron dose rate. The curve with the lower slope shows the cosmic neutron dose rate at sea level multiplied by 300 as a function of the magnetic latitude on the abscissa. The steeper curve shows the cosmic neutron dose rate at 12.5 km altitude as a function of the magnetic latitude
Terrestrial rays
Terrestrial radiations from natural radioactive elements in the ground, stones, trees, and walls of houses contribute on the average about 0.28 m Sv/year. The terrestrial sources vary significantly from place to place. These are categorized into building materials and soils surface.
Radioactivity in buildings
Determining population's exposure to radiation from building materials is important, because almost 80% of human life is spent indoors.[12] All building materials mostly constitute rock and soil; these two raw materials include natural radioactive isotopes such as 232Th and 238U decay series and 40K.[13]
The activity concentration of natural radionuclides in construction materials has been estimated in various countries and areas of the world such as Australia,[14] Bangladesh,[15] Pakistan,[16,17] Tanzania,[18] Eastern Europe,[19] Syria,[20] Kuwait,[21] China,[22] Egypt,[23,24,25,26] and Cyprus.[27]
Because of the importance of this investigation, a survey has been carried out in Portland cement industry. The results showed that all the measured values were commensurable with the worldwide data reported in United Nations Scientific Committee of the Effect of Atomic Radiation (UNSCEAR publications).[28] A study carried out in Turkey, determining the natural radioactivity levels of granites used in constructions, have showed that the presence of a large amount orthoclase and radiogenic accessory minerals are the sources of high activity congregation levels in the country.[29]
A survey carried out in Iran on natural radioactivity in buildings examined five popular construction materials, namely cement, gypsum, cement blocks, brick, and gravel.[12] The survey results showed that cement specimens had maximum values of the mean 226Ra and 232Th congregation, whereas the lowest value for average congregation of these two radionuclides was found in gypsum specimens. The highest and lowest values of 40K mean concentration was found in brick and gypsum samples, respectively. The calculated radium equivalent activities were below the admissible level of 370 Bq/kg for all construction materials. The values of hazard indexes were found below the exhorted levels; therefore, buildings constructed from such materials are considered secure for its inhabitants. These survey results are in agreement with other results of other investigations in carried out in various parts of the world.[12] The radioactivity content of some building materials in some countries is shown in Table 1.[12]
Table 1.
Radioactivity content of building materials in some countries
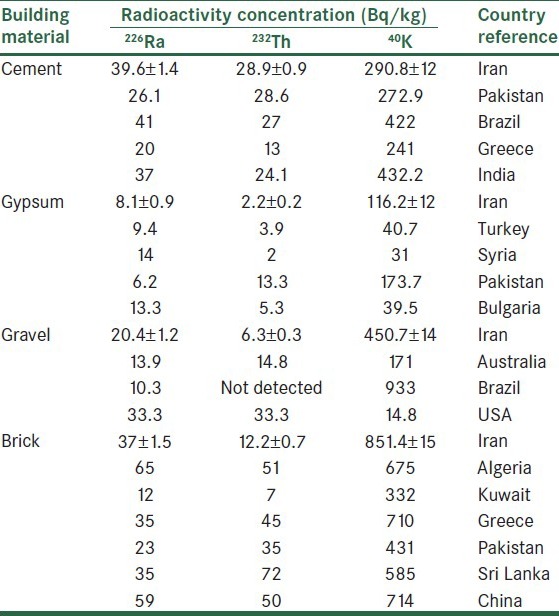
In the last few decades, several surveys have calculated the concentration of indoor radionuclides in countries such as Canada and India.[30,31]
A survey conducted in public elementary schools in Canada showed, on average, targeted schools occupants are exposed to radon concentrations of 56 Bq/m3, which is generally below the Federal guideline levels (i.e., 200 Bq/m3).[31]
In the estimated 222Rn and 220Rn concentrations in dwellings of south-western Punjab, a state in India, results have showed that the values of 222Rn differed from 21 to 79 Bq/m3, with a geometric mean of 45 Bq/m3.[6] In another study carried out in India, 222Rn and 220Rn levels were calculated in 200 various kinds of dwellings at 10 diverse locations in and around Bangalore Metropolitan, India. Overall, not much significant radiological risk for inhabitants was observed and the 222Rn levels were found to be well within the limits of the global average concentration of 40 Bq/m3. However, the observed 220Rn levels were found to be higher than the global average of 10 Bq/m3.[5]
Radioactivity in soils surface
It is important that the natural radioactivity, which exists in the soils surface, must be investigated because to determine the population's exposure to radiation from building materials, such as soils.
The concentration of potassium usually ranges from 1,000 to 30,000 ppm. It is usually lower but more variable in the basaltic rock region (1,500-20,000 ppm) than in acidic (high concentration of SiO2) rock regions. For example, in granite rock, the concentration is often about 29,000 ppm.
Radium (226Ra) is the most important radionuclide in the 238U decay chain from the radiobiological viewpoint; therefore, the measurements of 226Ra concentration in building materials are considered as reference in all investigations. Natural radionuclides in building materials may cause both external exposure, caused by their direct gamma radiation, and internal exposure from radon gas.
The concentration of rubidium (which is chemically similar to potassium) is often about 1% of that of potassium. Accordingly, the concentration of radioactivity of rubidium is often about 60% of that of potassium. Rubidium (87Rb), similar to 14C and 3H, emits only soft β-rays and contributes to internal radiation but not to the external radiation exposure.
Most of the terrestrial background radiation is due to potassium and to elements of the uranium series (238U to 206Pb), thorium series (232Th to 209Pb), and actinium series (235U to 207Pb). Each of these series consists of many α, β, and γ emitters. The concentration of these radioactive isotopes in the soil and water varies greatly. In certain areas, such as in the coastal areas of Kerala in India, the average dose was found to be 11 mSv/year. In certain areas of southwestern France, in Guarapari in Brazil, and in Ramsar in Iran, the dose rate may be about 17 mSv/year, and in small places within these areas the dose rate may be as high as 170-430 mSv/year. These levels are caused by the higher than usual natural background levels of uranium and thorium isotopes in the soil.[4]
Several studies have been carried out in countries such as Vietnam[32] and Turkey.[33] In Vietnam, the estimated outdoor and indoor annual effective doses to the population were found to be higher than the corresponding values in the rest of the world. The results showed that the radium equivalent activity and the external hazard index of the Vietnam soils surface are lower than the corresponding admissible limits of 370 Bq/kg and 1, respectively. Therefore, Vietnam soil, which is being used as a building material, is secure for the human population. In another study, the natural gamma radioactivity levels of the Samsun city center soil specimens, in Turkey, were calculated. The calculated external hazard index showed the radiation hazard in Samsun to be trivial.
It is important to mention that soil, by being used as building material, can affect a population's exposure to radionuclides; they can also affect the human body by taking food that contains radionuclides; these radionuclides enter the food chain from the deeper soil layers, besides tainting the ground water.
Because of this effect, several surveys have estimated the distribution of natural and synthetic radionuclides in soil profiles and in the surface layer of the soil.
In one survey, the activity-depth profiles of 137Cs were determined in soil specimens from 20 sites in and around the city of Istanbul, Turkey. It was found that the activity concentrations of 40K, 232Th, and 226Ra were distributed uniformly with regard to soil depth and the depth distribution of 137Cs generally fitted a linear function.[34]
Radioactivity in foods
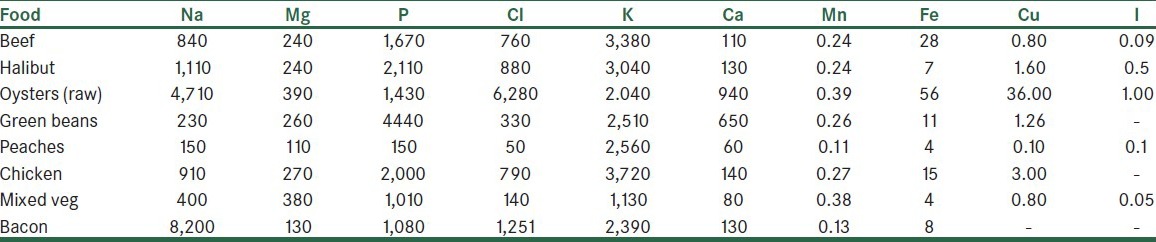
Food, water, and air usually contain trace amounts of alpha emitters from the uranium, thorium, and actinium series. Some of the radon (222Rn, and to a lesser extent 220Rn and 219Rn) gas diffuses into the food supply. For example, the radon in the ground and in the water, and its many decay products, precipitate onto the field and onto the vegetation in the field. Because these radioactive elements perfuse into the food chain and affect the human body, several investigations were carried out to determine the concentration of the major trace elements in food. Table 2 shows examples of concentrations of major trace elements in actual food samples.
Table 2.
Typical concentration of some of the major trace elements in foods
A study has investigated the radiology of natural and mineral drinking waters in Slovenia. In this survey, miscellaneous genres of water were accumulated for three different age groups of the population. It was observed in all cases that the calculated median committed effective dose from the investigated radionuclides was well below the recommended value of 100 μSv/year. Results have shown that children are the most exposed with the highest absolute dose. It is important to mention that the contribution of each specific radionuclide to total doses varied among diverse water types and within each genre, as well as between various age groups.[35]
In the human body, the concentration of activity of potassium (40K), carbon (14C), tritium (3H), polonium (210Po), and 226Ra is 63, 66, 133, 0.0002, and 2.7 × 10−5 Bq/kg, respectively.
The concentration of natural radioactivity in food is often in the range of 40-600 Becquerel per kilogram of food. For example, the radioactivity from potassium alone may be typically 50 Bq/kg in milk, 420 Bq/kg in milk powder, 165 Bq/kg in potatoes, and 125 Bq/kg in beef. Investigation on the radioactivity in foods are reported by Ramachandran and Mishra.[36] They found the concentration of 40K radioactivity in different foods varies from 45.9 to 649.0 Bq/kg; that of 226Ra varies from 0.01 to 1.16 Bq/kg; and that of 228Th varies from 0.02 to 1.26 Bq/kg. To derive the corresponding dose in mSv/year, it is necessary to take into account the energy and fraction deposited in the body, besides taking into account not only the radioactive lifetime but also the biological lifetime of the isotopes in the human body.
To analyze natural and induced radioactivity in food, it is necessary to consider the elemental composition of food.[37,38] The natural radioactivity from the 40K isotope, which is a constant fraction (0.0117%) of the potassium content in food, varies significantly with potassium concentration from food to food. Usually, the concentration of potassium is in the range of 1,000-6,000 ppm. The concentration of potassium in a reference food was found to be 4,000 ppm. The average concentration in the human body is about 2,000 ppm. Of the daily intake, about 90% is excreted in the urine and 10% in the stool. The concentrations of many other trace elements also show great variations. Table 2 shows concentration of some of the major trace elements in foods.[37] As shown in Table 2, the concentration of sodium may vary from 150 to 8,200 ppm, that of magnesium from 110 to 390, and that of phosphorus from 150 to 2,110 ppm.
CONCLUSIONS
Humans are always exposed to a background radiation spread of radioactive nuclei in the air, soil, rock, water, and building materials. The amount of background radiation is different in terms of height, the amount of the nuclei present in the soil, and the geographical conditions of different regions. Radioactivity is common in rocks, soil, beach sand, sediment and riverbed soil, in rivers and oceans, and even in building materials and homes. The concentration of radioactive isotopes in soil is an indicator of radioactive accumulation in the environment, which impacts humans, plants, and animals. They are typically long lived, with half-lives often about hundreds of millions of years. Another point is that the number of radionuclides such as uranium and thorium that exist in the region's soil can change the dose rate of background radiation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ramachandran TV. Background radiation people and the environment. Iran J Radiat Res. 2011;9:63–76. [Google Scholar]
- 2.Tzortzis M, Svoukis E, Tsertos H. A comprehensive study of natural gamma radioactivity levels and associated dose rates from surface soils in cyprus. Radiat Prot Dosimetry. 2004;109:17–24. doi: 10.1093/rpd/nch300. [DOI] [PubMed] [Google Scholar]
- 3.United Nations Scientific Committee of the Effect of Atomic Radiation (UNSCEAR) 2000. New York: USA: Report on General Assembly; Sources and Effects of Ionizing Radiation. [Google Scholar]
- 4.Shahbazi-Gahrouei D. Natural background radiation dosimetry in the highest altitude region of Iran. J Radiat Res. 2003;44:285–7. doi: 10.1269/jrr.44.285. [DOI] [PubMed] [Google Scholar]
- 5.Sathish LA, Nagaraja K, Ramachandran TV. Indoor 222Rn and 220Rn concentrations and doses in Bangalore, India. Radiat Prot Dosimetry. 2012;151:344–53. doi: 10.1093/rpd/ncs015. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Singh S, Bajwa BS, Singh B, Sabharwal AD, Eappen KP. Indoor inhalation dose estimates due to radon and thoron in some areas of South-Western Punjab, India. Radiat Prot Dosimetry. 2012;151:112–6. doi: 10.1093/rpd/ncr461. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura T, Uwamino Y, Ohkubo T, Hara A. Altitude variation of cosmic-ray neutrons. Health Phys. 1987;53:509–17. doi: 10.1097/00004032-198711000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Stone JM, Whicker RD, Ibrahim SA, Whicker FW. Spatial variations in natural background radiation: Absorbed dose rates in air in Colorado. Health Phys. 1999;76:516–23. doi: 10.1097/00004032-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Kam E, Bozkurt A. Environmental radioactivity measurements in Kastamonu region of northern Turkey. Appl Radiat Isot. 2007;65:440–4. doi: 10.1016/j.apradiso.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Bozkurt A, Yorulmaz N, Kam E, Karahan G, Osmanlioglu AE. Assessment of environmental radioactivity for Sanliurfa region of southeastern Turkey. Radiat Meas. 2007;42:1387–91. [Google Scholar]
- 11.Shahbazi-Gahrouei D. Annual background radiation in Chaharmahal and Bakhtiari Province. Iranian J Radiat Res. 2003;1:87–91. [Google Scholar]
- 12.Mehdizadeh S, Faghihi R, Sina S. Natural radioactivity in building material in Iran, Iran. Nukleonika. 2011;56:363–8. [Google Scholar]
- 13.Faghihi R, Mehdizadeh S, Sina S. Natural and artificial radioactivity distribution in soil of Fars Province, Iran. Radiat Prot Dosimetry. 2010;138:1–9. doi: 10.1093/rpd/ncq367. [DOI] [PubMed] [Google Scholar]
- 14.Beretka J, Matthew PJ. Natural radioactivity of Australian building materials, industrial wastes and by-products. Health Phys. 1985;48:87–95. doi: 10.1097/00004032-198501000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Alam MN, Miah MM, Chowdhury MI, Kamal M, Ghose S, Rahman R. Attenuation coefficients of soils and some building materials of Bangladesh in the energy range 276-1332 keV. Appl Radiat Isot. 2001;54:973–6. doi: 10.1016/s0969-8043(00)00354-7. [DOI] [PubMed] [Google Scholar]
- 16.Faheem M, Mujahid SA, Matiullah M. Assessment of radiological hazards due to the natural radioactivity in soil and building material samples collected from six districts of the Punjab province, Pakistan. Radiat Meas. 2008;43:1443–7. [Google Scholar]
- 17.Khan K, Khan HM. Natural gamma-emiting radionuclides in Pakistani Portland cement. Appl Radiat Isot. 2001;54:861–5. doi: 10.1016/s0969-8043(00)00327-4. [DOI] [PubMed] [Google Scholar]
- 18.Msaki P, Banzi FP. Radioactivity in products derived from gypsum in Tanzania spectrometry. Radiat Prot Dosimetry. 2000;91:409–12. [Google Scholar]
- 19.Krstic D, Nikezic D, Stevanovic N, Vucic D. Radioactivity of some domestic and imported building materials from South Eastern Europe. Radiat Meas. 2007;2:1731–6. [Google Scholar]
- 20.Othman I, Mahrouka M. Radionuclide content in some building materials in Syria and their indoor gamma dose rate. Radiat Prot Dosimetry. 1994;55:299–304. [Google Scholar]
- 21.Bou-Rabee F, Bem H. Natural radioactivity in building materials utilized in the state of Kuwait. J Radioanal Nucl Chem. 1996;213:143–9. [Google Scholar]
- 22.Yang YX, Wu XM, Jiang ZY, Wang WX, Lu JG, Lin J, et al. Radioactivity concentrations in soils of the Xiazhuang granite area, China. Appl Radiat Isot. 2005;63:255–9. doi: 10.1016/j.apradiso.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad F. Natural radioactivity in building materials in Egypt. Radiat Eff Defects Solids. 2007;162:43–52. [Google Scholar]
- 24.Arafa W. Specific activity and hazards of granite samples collected from the Eastern Desert of Egypt. J Environ Radioact. 2004;75:315–27. doi: 10.1016/j.jenvrad.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 25.El-Arabi AM, Abbady AG, El-Hussein A. Gamma-ray measurements of natural radioactivity in sedimentary rocks from Egypt. Nucl Sci Tech. 2006;17:123–8. [Google Scholar]
- 26.Higgy RH, El-Tahawy MS, Abdel-Fattah AT, Al-Akabawy UA. Radionuclide content of building materials and associated gamma dose rates in Egyptian dwellings. J Environ Radioact. 2000;50:253–61. [Google Scholar]
- 27.Michaell F, Parpottas Y, Tsertos H. Gamma radiation measurements and dose rates in commonly used building materials in Cyprus. Radiat Prot Dosimetry. 2010;142:282–91. doi: 10.1093/rpd/ncq193. [DOI] [PubMed] [Google Scholar]
- 28.Aslam M, Gul R, Ara T, Hussain M. Assessment of radiological hazards of naturally occurring radioactive materials in cement industry. Radiat Prot Dosimetry. 2012;151:483–8. doi: 10.1093/rpd/ncs018. [DOI] [PubMed] [Google Scholar]
- 29.Cetin E, Altinsoy N, Orgün Y. Natural radioactivity levels of granites used in Turkey. Radiat Prot Dosimetry. 2012;151:299–305. doi: 10.1093/rpd/ncs007. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed JU. High levels of natural radiation: Report of an international conference in Ramsar. IAEA Bulletin. 1991;33:36–8. [Google Scholar]
- 31.Poulin P, Leclerc JM, Dessau JC, Deck W, Gagnon F. Radon measurement in schools located in three priority investigation areas in the province of Quebec, Canada. Radiat Prot Dosimetry. 2012;151:278–89. doi: 10.1093/rpd/ncr483. [DOI] [PubMed] [Google Scholar]
- 32.Huy NQ, Hien PD, Luyen TV, Hoang DV, Hiep HT, Quang NH, et al. Natural radioactivity and external dose assessment of surface soils in Vietnam. Radiat Prot Dosimetry. 2012;151:522–31. doi: 10.1093/rpd/ncs033. [DOI] [PubMed] [Google Scholar]
- 33.Mustafa CT, Selma B. Radioactivity concentrations in soil and dose assessment for Samsun City Centre, Turkey. Radiat Prot Dosimetry. 2012;151:532–6. doi: 10.1093/rpd/ncs034. [DOI] [PubMed] [Google Scholar]
- 34.Belivermiş M. Vertical distributions of 137Cs, 40K, 232Th and 226Ra in soil samples from Istanbul and its environs, Turkey. Radiat Prot Dosimetry. 2012;151:511–21. doi: 10.1093/rpd/ncs023. [DOI] [PubMed] [Google Scholar]
- 35.Benedik L, Jeran Z. Radiological of natural and mineral drinking waters in Slovenia. Radiat Prot Dosimetry. 2012;151:306–13. doi: 10.1093/rpd/ncs009. [DOI] [PubMed] [Google Scholar]
- 36.Ramachandran TV, Mishra UC. Measurement of natural radioactivity levels in Indian foodstuffs by gamma spectrometry. Appl Radiat Isot. 1989;40:723–6. doi: 10.1016/0883-2889(89)90085-3. [DOI] [PubMed] [Google Scholar]
- 37.Vienna: IAEA, TECDOC; 2002. IAEA, International Atomic Energy Agency. Natural and induced radioactivity in food; p. 1287. [Google Scholar]
- 38.Shahbazi-Gahrouei D, Saeb M. Dose assessment and radioactivity of the mineral water resources of Dimeh springs in Chaharmahal and Bakhtiari Province, Iran. Nukleonika. 2008;53:31–4. [Google Scholar]


