Abstract
Objective
Previous studies have reported an association between endometrial cancer and the risk of metabolic syndrome; however, the pattern of endometrial cancer-associated dyslipidemia is not well understood. The standard therapy for endometrial cancer is total abdominal hysterectomy and bilateral salpingo-oophorectomy. Premenopausal bilateral salpingo-oophorectomy may cause adverse events, including dyslipidemia. Gynecologists have to care dyslipidemia in endometrial cancer survivors at cancer follow-up clinic.
Methods
This study included 693 patients who had undergone bilateral salpingo-oophorectomy, and included 412 women with incident endometrial cancer and 281 controls. We divided the patients into two categories according to whether they had a premenopausal or postmenopausal bilateral oophorectomy. Serum lipid levels were measured and statistically analyzed.
Results
Hypertriglyceridemia was statistically more frequent in patients who had undergone bilateral salpingo-oophorectomy both before and after menopause than in the corresponding non-endometrial cancer controls. High levels of low-density lipoprotein cholesterol and a high low-density lipoprotein cholesterol/high-density lipoprotein cholesterol ratio were statistically more frequent in patients who had undergone bilateral salpingo-oophorectomy before menopause than in non-endometrial cancer controls.
Conclusions
Our report highlights the importance of the relationship between endometrial cancer and lipid metabolism, which may aid in preventing cerebrovascular or cardiovascular diseases due to dyslipidemia and improving the quality of life in endometrial cancer survivors.
Keywords: dyslipidemia, endometrial cancer, hypertriglycemia, cancer survivor
INTRODUCTION
Endometrial cancer (EC) is the most common invasive neoplasm of the female genital tract in Europe and North America. Worldwide, ∼150 000 cases are diagnosed each year, making EC the fifth most common cancer in women (1). Although the highest rates of EC are seen in Europe and North America, reports from many other countries have shown an increased incidence of EC http://ganjoho.jp/public/statistics/backnumber/2011_en.html. While the incidence rates in developing countries and Japan are four to five times lower (1), the age-adjusted incidence rate of EC in Japan has been increasing since the 1970s.
Like breast cancer, EC is an estrogen-dependent tumor. The predominant treatment for EC is total abdominal hysterectomy and bilateral salpingo-oophorectomy (BSO), and this operation should be performed in all cases whenever feasible (2). Due to the rise in the onset of EC at a young age in many countries, the number of premenopausal EC survivors who will undergo BSO will increase in the near future. BSO places premenopausal women at a high risk for multiple adverse events, including early postmenopausal symptoms (hot flashes, fatigue, shoulder stiffness and palpitations), and can lead to further presentation of symptoms such as coital pain, atrophic (senile) vaginitis, urethritis, urinary incontinence, rough and dull skin accompanying skin atrophy and obesity. In addition, other long-term conditions, such as osteoporosis or osteopenia, dyslipidemia, atherosclerosis, and subsequent cerebrovascular and cardiovascular diseases may manifest. These illnesses can be difficult to diagnose and have a direct effect on the quality of life (QOL) when they progress, necessitating management from an early stage. Management of postmenopausal symptoms should be carried out in addition to surveillance for recurrence.
The incidence and mortality rates of coronary heart disease are lower in Japanese populations than in Caucasian populations (3–6); however, the increasingly Westernized lifestyle in Japan is the cause for rapidly increasing rates of dyslipidemia or coronary heart diseases (7–10).
The proposed risk factors for EC are obesity, diabetes and hormonal stimulation caused by an early age at menarche, advanced age at menopause, nulliparity, estrogen replacement therapy and tamoxifen treatment, among other risk factors (1). A few studies have reported an association between EC and the risk of metabolic syndrome (MetS) (11,12), including the pattern of dyslipidemia, hyper-low-density lipoprotein (LDL) cholesterolemia, hypertriglyceridemia or hypo-high-density lipoprotein (HDL) cholesterolemia. Furthermore, the LDL- cholesterol (LDL-C)/HDL-C ratio may represent the effects of both LDL-C and HDL-C. The LDL-C/HDL-C ratio has been reported to correlate with ischemic heart disease (13), but the patterns of dyslipidemia associated with EC are not well understood.
As the incidence rates of dyslipidemia and EC are simultaneously increasing, we hypothesize that serum lipids play a role in endometrial carcinogenesis. Currently, effective diagnostic serum markers for EC have not been identified, and mass screening of the population for EC is not practical (2). In this study, we report the serum lipid characteristics of patients with EC compared with those of non-EC cases following BSO to establish a QOL surveillance system for EC survivors. This attempt to determine the biological characteristics of EC, in addition, might enable the development of preventive or therapeutic targets and biomarkers associated with serum lipids in the future.
PATIENTS AND METHODS
This study was conducted using data from 693 patients who had undergone BSO [412 women with incident EC and 281 without incident EC (non-EC controls)], who visited the menopausal clinic or cancer follow-up clinic of the Department of Obstetrics and Gynecology, Keio University Hospital (Tokyo, Japan), from 2007 to 2011 (Table 1). Because patients experienced dyslipidemia thereafter due to ovarian dysfunction caused by premenopausal oophorectomy (14), they were divided into two groups depending on whether they had undergone BSO before (premenopausal BSO group) or after (postmenopausal BSO group) menopause. The premenopausal BSO group included 169 non-EC patients and 181 EC patients, and the postmenopausal BSO group included 112 non-EC patients and 231 EC patients. Age, time interval from surgical menopause in the premenopausal BSO group, time interval from natural menopause in the postmenopausal BSO group and body mass index (BMI) were analyzed (Table 1). This study was conducted with the approval from the ethics committee of the School of Medicine, Keio University (approval number: 20070081).
Table 1.
Characteristics of endometrial cancer patients and non-endometrial cancer controls by oophorectomy status
| Total |
Premenopausal bilateral oophorectomy |
Postmenopausal bilateral oophorectomy |
||||
|---|---|---|---|---|---|---|
| Non-EC | EC | Non-EC | EC | Non-EC | EC | |
| Number | 281 | 412 | 169 | 181 | 112 | 231 |
| Ovarian tumor: 146 | Ovarian tumor: 68 | Ovarian tumor: 78 | ||||
| Cervical cancer: 69 | Cervical cancer: 53 | Cervical cancer: 16 | ||||
| Myoma or endometriosis: 51 | Myoma or endometriosis: 43 | Myoma or endometriosis: 8 | ||||
| Others: 15 | Others: 5 | Others: 10 | ||||
| Age, years | ||||||
| Average | 54.1 | 59.9 | 48.1 | 51.6 | 62.4 | 66.3 |
| P (F test) | 0.89 | 0.68 | 0.92 | |||
| Interval from surgical menopause | ||||||
| Average | 5.2 | 7.0 | NA | |||
| P (F test) | 0.86 | |||||
| Interval from natural menopause | ||||||
| Average | NA | 11.6 | 15.1 | |||
| P (F test) | 0.27 | |||||
| Body mass index, kg/m2 | ||||||
| Average | 21.3 | 23.2 | 21.2 | 23.7 | 21.4 | 22.8 |
| P (Mann–Whitney U test) | 8.3 × 10−11 | 3.6 × 10−8 | 3.9 × 10−5 | |||
BMI, body mass index; EC, endometrial cancer; non-EC, non-endometrial cancer; NA, not applicable.
Serum lipid, triglyceride (TG), LDL-C and HDL-C levels and the LDL-C/HDL-C ratio were measured. Blood collected after fasting was used to measure serum lipids levels, thereby avoiding any dietary influences that would cause conflicting results among the studies. The Japan Atherosclerosis Society Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases were used to diagnose dyslipidemia (15). To be eligible for participation, patients were required to meet the following criteria: patients (i) who had undergone BSO, (ii) aged ≥20 years and (iii) not deemed to be ineligible for participation for any other reason. Exclusion criteria for this study included the presence of non-epithelial tumors, multiple primary cancers accompanying EC, hypothyroidism, diabetes mellitus, familial hypercholesterolemia and the administration of antihyperlipidemic drugs.
Statistical analysis was performed with Excel: Mac 2011 (Microsoft, USA) with the add-in software Statcel 3 (OMS, Japan) and Prism 6 (GraphPad Software, USA) using the indicated tests. The F-test was used to verify the heterogeneity of variances. Normally distributed variables were compared by Student's t-test, and non-parametric distributed variables compared by the Mann-Whitney U test. The χ2 for independence test was carried out for 2 × 2 contingency tables. The results with P < 0.05 were considered significant.
RESULTS
Table 1 lists the patient characteristics of the EC cases and non-EC controls by oophorectomy status. In the overall patient population, patients who received premenopausal BSO and patients who received postmenopausal BSO, the EC and non-EC groups did not differ with regard to age (P = 0.89, 0.68, 0.92, respectively). The interval from surgical menopause and the interval from natural menopause were not significantly different between the EC and non-EC groups in patients who received premenopausal BSO and those who received postmenopausal BSO, respectively (P = 0.86 and 0.27, respectively). The BMI was significantly higher in the EC group than in the non-EC group in the overall patient population, in those who received premenopausal BSO and in those who received postmenopausal BSO (P = 8.3 × 10−11, 3.6 × 10−8 and 3.9 × 10−5, respectively).
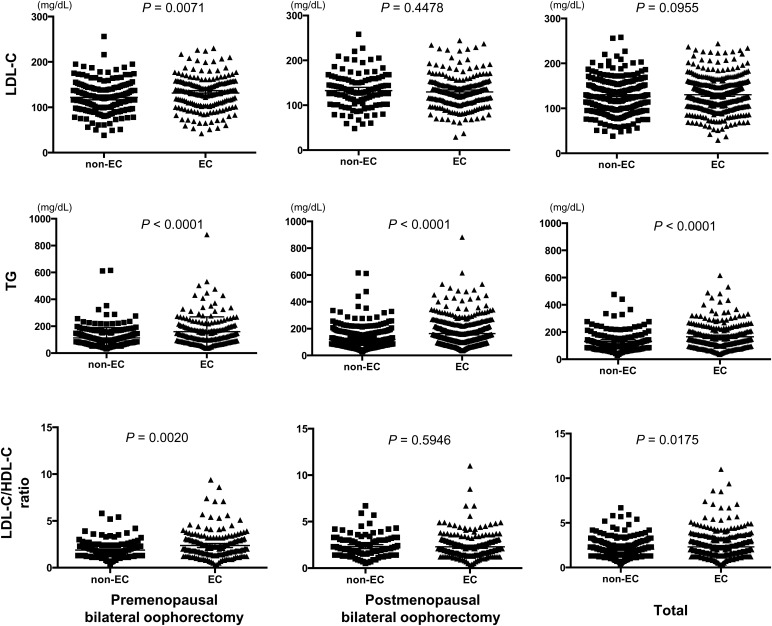
Differences between the serum lipid levels of each group were analyzed using the Mann–Whitney U test (Fig. 1). TG levels were significantly higher in all EC groups (P < 0.0001). LDL-C levels (P = 0.0071) and the LDL-C/HDL-C ratio (P = 0.0020) were significantly higher in the EC patients of the premenopausal BSO group.
Figure 1.
Mann–Whitney U test for low-density lipoprotein cholesterol (LDL-C) and triglyceride (TG) levels and the LDL-C/high-density lipoprotein cholesterol (HDL-C) ratio in endometrial cancer and non-endometrial cancer groups.
Table 2 shows the relationship between EC and serum lipid levels. Hyper-LDL cholesterolemia was significantly more frequent in EC than in non-EC patients in the premenopausal BSO group [P = 0.0261, odds ratio (OR) = 1.655]. Hypertriglyceridemia was significantly more frequent in EC patients in all groups (total, premenopausal BSO and postmenopausal BSO; P < 0.0001, <0.0001 and 0.00154, respectively). This was particularly evident in the premenopausal BSO group, where the OR was 2.259. The LDL-C/HDL-C ratio was significantly higher in EC than that in non-EC patients in the premenopausal BSO group (P = 0.0121, OR = 1.719).
Table 2.
EC and serum lipid levels
| Total |
Premenopausal bilateral oophorectomy |
Postmenopausal bilateral oophorectomy |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-EC (n = 281) | EC (n = 412) | Odds ratio (95% CI) | P (χ2 test) | Non-EC (n = 169) | EC (n = 181) | Overall confidence interval) | P (χ2 test) | Non-EC (n = 112) | EC (n = 231) | OR (95% CI interval) | P (χ2 test) | |
| LDL-C (mg/dl) | ||||||||||||
| <140 | 186 | 262 | 1.121 (0.8152–1.541) | 0.4821 | 120 | 108 | 1.655 (1.060–2.585) | 0.0261 | 66 | 154 | 0.7174 (0.4504–1.143) | 0.1611 |
| ≥140a | 95 | 150 | 49 | 73 | 46 | 77 | ||||||
| TG (mg/dl) | ||||||||||||
| <150 | 204 | 225 | 2.202 (1.589–3.051) | <0.0001 | 128 | 100 | 2.529 (1.600–3.997) | <0.0001 | 76 | 125 | 1.790 (1.115–2.875) | 0.0154 |
| ≥150b | 77 | 187 | 41 | 81 | 36 | 106 | ||||||
| LDL-C/HDL-C ratio | ||||||||||||
| <2 | 150 | 193 | 1.299 (0.9587–1.761) | 0.0911 | 102 | 85 | 1.719 (1.125–2.629) | 0.0121 | 48 | 108 | 0.8542 (0.5420–1.346) | 0.4958 |
| ≥2 | 131 | 219 | 67 | 96 | 64 | 123 | ||||||
TG, triglyceride; CI, confidence interval.
aHigh-LDL-cholesterolemia.
bHypertriglyceridemia.
DISCUSSION
Hypertriglyceridemia is a marker of MetS and is assuming an increasingly important role in the assessment and management of cardiovascular disease risk (16). MetS, initially defined as a risk factor for cardiovascular disorders, has recently been associated with various cancers (11,17–22). Lipoprotein abnormalities in MetS include hypertriglyceridemia, high remnant lipoproteinemia, small dense LDL particles and low-HDL cholesterolemia. Cases of MetS are increasingly observed during the postmenopausal period.
Several studies have reported a direct association between the EC risk and MetS (11,23), and some have reported that diabetes is a risk factor for EC, independent of obesity (24–27). A case–control study nested within the European Prospective Investigation into Cancer and Nutrition on 284 women with EC showed that the presence of MetS was associated with EC risk (relative risk = 2.12, 95% confidence interval: 1.51–2.97), and there was a positive trend in risk with an increasing number of MetS components (28). These findings suggest that metabolic abnormalities may act to increase EC risk, but data on dyslipidemia, with details on each type of serum lipid, are limited (29,30).
Some studies attempted to investigate whether serum dyslipidemia influences EC risk, but these results are contradictory (28–31). Lindemann et al. (29) examined the association of serum total cholesterol (TC) levels, LDL-C, non-HDL and HDL-C with EC risk in 100 EC cases. The results showed a positive correlation between serum TG levels and EC risk and no association between TC, LDL-C or HDL-C. Cust et al. (28) reported that TG and HDL-C levels were positively and negatively associated with EC risk, respectively, but TC and LDL-C were not. Swanson et al. (30) analyzed serum lipid levels in 256 EC cases and 185 controls <75 years old, and indicated that the EC risk was reduced by 25% in women with the lowest serum TG compared with women in the highest quartile. Zhang et al. reported positive correlations of EC risk with TC, TG and LDL-C, but a negative correlation with HDL-C using fasting blood to measure serum lipid levels (31). We also have used fasting blood in our study, as we consider it to be the most accurate.
The relationship between EC and lipid metabolism is important for some reasons. First, even if the patient is cancer-free and does not die of EC, the cause of death may be cerebrovascular or cardiovascular disease due to dyslipidemia. Therefore, the prevention of these diseases may lead to an improved QOL for EC survivors. TG levels were elevated in EC patients irrespective of age at BSO, and hyper-LDL cholesterolemia or a high LDL-C/HDL-C ratio was observed in EC survivors who had undergone premenopausal BSO. It is important to prevent cerebrovascular or cardiovascular events in these patients by treating dyslipidemia during follow-up.
Second, serum lipid profiles may be a useful EC biomarker. Mass screening of the population for EC is not practical and no blood test with sufficient sensitivity and specificity has been developed (1,2). Screening for EC or its precursors is justified for certain high-risk populations, such as postmenopausal women on exogenous estrogens without progestins, women from families with Lynch syndrome and premenopausal women with anovulatory cycles such as those with polycystic ovarian disease (2). On the basis of our findings, we propose that women with dyslipidemia may belong to a high-risk EC group. Clinicians should be advised to refer women with dyslipidemia to a gynecologist for endometrial surveillance. Thus, hypertriglycemia is a potential EC biomarker and could be used for screening of individuals with a high risk for EC.
It is notable that this is the first report to use an optimal study design, including non-EC control patients who had undergone BSO. Moreover, we emphasize that we used fasting blood to measure serum lipid levels, while previous studies used either non-fasting or a mixture of both fasting and non-fasting blood samples.
Malignancy, cerebrovascular disease and heart disease accounted for ∼54% of all mortality in Japan in 2011 http://www.mhlw.go.jp/english/database/db-hw/vs01.html. Our report highlights the importance of the relationship between endometrial cancer and lipid metabolism, which may aid in preventing cerebrovascular or cardiovascular diseases due to dyslipidemia and improving the QOL of endometrial cancer survivors.
Funding
This work was supported in part by the Foundation for the Promotion of Cancer Research in Japan (2009–2011), and JMWH Bayer Grant (2011) from The Japan Society for Menopause and Women's Health. Funding to pay the Open Access publication charges for this article was provided by Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research (KAKENHI) (C) (25462609).
Conflict of interest statement
None declared.
Acknowledgements
We thank the patients and the supporting medical staff for making this study possible. We are grateful to Ms Keiko Abe and Ms Tomomi Noda for their secretarial assistance.
References
- 1.Ellenson LH, Ronnett BM, Soslow RA, Zaino RJ, Kurman RJ. Endometrial cancer. In: Kurman RJ, Ellenson LH, Ronnett BM, editors. Blaustein's Pathology of the Female Genital Tract. 6th edn. New York: Springer; 2011. pp. 394–452. [Google Scholar]
- 2.Hacker NF. Uterine Cancer. In: Berek JS, Hacker NF, editors. Practical Gynecologic Oncology. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 397–442. [Google Scholar]
- 3.Saito I, Folsom AR, Aono H, Ozawa H, Ikebe T, Yamashita T. Comparison of fatal coronary heart disease occurrence based on population surveys in Japan and the USA. Int J Epidemiol. 2000;29:837–44. doi: 10.1093/ije/29.5.837. [DOI] [PubMed] [Google Scholar]
- 4.Menotti A, Blackburn H, Kromhout D, et al. Changes in population cholesterol levels and coronary heart disease deaths in seven countries. Eur Heart J. 1997;18:566–71. doi: 10.1093/oxfordjournals.eurheartj.a015298. [DOI] [PubMed] [Google Scholar]
- 5.Verschuren WM, Jacobs DR, Bloemberg BP, et al. Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. JAMA. 1995;274:131–6. [PubMed] [Google Scholar]
- 6.Hirobe K, Terai T, Fujioka S, Goto K, Dohi S 3M-Study Project Committee of the Japan Association of Occupational Physicians ‘San-yu-kai’. Morbidity of Myocardial Infarction Multicenter Study in Japan (3M study): study design and event rates for myocardial infarction and coronary death by age category in Japanese workers. Circ J. 2005;69:767–73. doi: 10.1253/circj.69.767. [DOI] [PubMed] [Google Scholar]
- 7.Okamura T, Kokubo Y, Watanabe M, et al. Low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban Japanese cohort study: the Suita study. Atherosclerosis. 2009;203:587–92. doi: 10.1016/j.atherosclerosis.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura A, Sato S, Kiyama M, et al. Trends in the incidence of coronary heart disease and stroke and their risk factors in Japan, 1964 to 2003: the Akita-Osaka study. J Am Coll Cardiol. 2008;52:71–9. doi: 10.1016/j.jacc.2008.02.075. [DOI] [PubMed] [Google Scholar]
- 9.Takii T, Yasuda S, Takahashi J, et al. Trends in acute myocardial infarction incidence and mortality over 30 years in Japan: report from the MIYAGI-AMI Registry Study. Circ J. 2010;74:93–100. doi: 10.1253/circj.cj-09-0619. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura M, Koyama I, Iso H, et al. Ten-year evaluation of homogeneous low-density lipoprotein cholesterol methods developed by Japanese manufacturers. Application of the Centers for Disease Control and Prevention/Cholesterol Reference Method Laboratory Network lipid standardization protocol. J Atheroscler Thromb. 2010;17:1275–81. doi: 10.5551/jat.5470. [DOI] [PubMed] [Google Scholar]
- 11.Rosato V, Zucchetto A, Bosetti C, et al. Metabolic syndrome and endometrial cancer risk. Ann Oncol. 2011;22:884–9. doi: 10.1093/annonc/mdq464. [DOI] [PubMed] [Google Scholar]
- 12.Friedenreich CM, Biel RK, Lau DC, et al. Case–control study of the metabolic syndrome and metabolic risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2384–95. doi: 10.1158/1055-9965.EPI-11-0715. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi T, Araki A, Kawashima S, et al. Metabolic predictors of ischemic heart disease and cerebrovascular attack in elderly diabetic individuals: difference in risk by age. Cardiovasc Diabetol. 2013;12:10. doi: 10.1186/1475-2840-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirasawa A, Masuda K, Akahane T, et al. Experience of risk-reducing salpingo-oophorectomy for a BRCA1 mutation carrier and establishment of a system performing a preventive surgery for hereditary breast and ovarian cancer syndrome in Japan: our challenges for the future. Jpn J Clin Oncol. 2013;43:515–9. doi: 10.1093/jjco/hyt036. [DOI] [PubMed] [Google Scholar]
- 15.Teramoto T, Sasaki J, Ueshima H, et al. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb. 2007;14:45–50. doi: 10.5551/jat.14.45. [DOI] [PubMed] [Google Scholar]
- 16.Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 17.Cowey S, Hardy RW. The metabolic syndrome: a high-risk state for cancer? Am J Pathol. 2006;169:1505–22. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JS, Cho SI, Park HS. Metabolic syndrome and cancer-related mortality among Korean men and women. Ann Oncol. 2010;21:640–5. doi: 10.1093/annonc/mdp344. [DOI] [PubMed] [Google Scholar]
- 19.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836–42. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 20.Inoue M, Noda M, Kurahashi N, et al. Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev. 2009;18:240–7. doi: 10.1097/CEJ.0b013e3283240460. [DOI] [PubMed] [Google Scholar]
- 21.Pothiwala P, Jain SK, Yaturu S. Metabolic syndrome and cancer. Metab Syndr Relat Disord. 2009;7:279–88. doi: 10.1089/met.2008.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo A, Autelitano M, Bisanti L. Metabolic syndrome and cancer risk. Eur J Cancer. 2008;44:293–7. doi: 10.1016/j.ejca.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Cook L, Weiss NS, Doherty JA, Chen C. Endometrial Cancer. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. 3rd edn. New York: Oxford University Press; 2006. pp. 1027–43. [Google Scholar]
- 24.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–64. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50:1365–74. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 26.Lindemann K, Vatten LJ, Ellstrøm-Engh M, Eskild A. Body mass, diabetes and smoking, and endometrial cancer risk: a follow-up study. Br J Cancer. 2008;98:1582–5. doi: 10.1038/sj.bjc.6604313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucenteforte E, Bosetti C, Talamini R, et al. Diabetes and endometrial cancer: effect modification by body weight, physical activity and hypertension. Br J Cancer. 2007;97:995–8. doi: 10.1038/sj.bjc.6603933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cust AE, Kaaks R, Friedenreich C, et al. Metabolic syndrome, plasma lipid, lipoprotein and glucose levels, and endometrial cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC.) Endocr Relat Cancer. 2007;14:755–67. doi: 10.1677/ERC-07-0132. [DOI] [PubMed] [Google Scholar]
- 29.Lindemann K, Vatten LJ, Ellstrøm-Engh M, Eskild A. Serum lipids and endometrial cancer risk: results from the HUNT-II study. Int J Cancer. 2009;124:2938–41. doi: 10.1002/ijc.24285. [DOI] [PubMed] [Google Scholar]
- 30.Swanson CA, Potischman N, Barrett RJ, et al. Endometrial cancer risk in relation to serum lipids and lipoprotein levels. Cancer Epidemiol Biomarkers Prev. 1994;3:575–81. [PubMed] [Google Scholar]
- 31.Zhang Y, Liu Z, Yu X, et al. The association between metabolic abnormality and endometrial cancer: a large case–control study in China. Gynecol Oncol. 2010;117:41–6. doi: 10.1016/j.ygyno.2009.12.029. [DOI] [PubMed] [Google Scholar]