Abstract
Objective
To retrospectively analyze and compare the outcomes of patients with hepatocellular carcinoma treated with either surgical excision or CyberKnife® from September 2006 to August 2011.
Materials and methods
Local control and toxicity were the primary endpoints, followed by local progression-free survival, progression-free survival, and overall survival as the secondary endpoints. Response Evaluation Criteria In Solid Tumors were the evaluation criteria for efficacy; Common Toxicity Criteria 3.0 were the evaluation criteria for adverse events. Local control was calculated using the direct method (nonactuarial). The survival curves were drawn using the Kaplan–Meier method along with log-rank test analysis.
Results
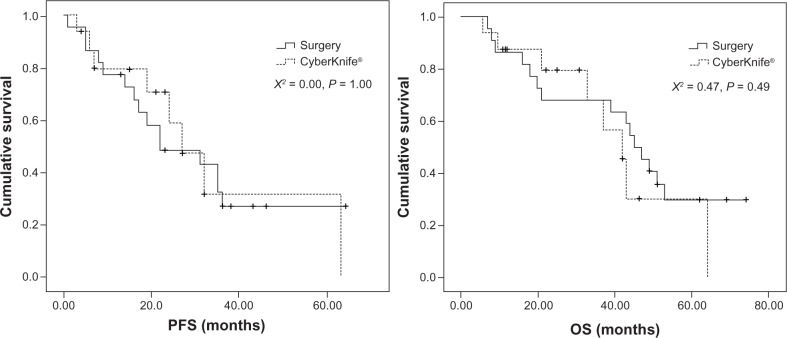
The research included 26 patients treated with tumor-free cutting edge (R0) surgical excision and 22 patients treated with CyberKnife treatment. The results showed that the adverse effects of CyberKnife were milder, with 1-, 2-, and 3-year local control rates of 92.9%, 90.0%, and 67.7%, respectively. The overall survival rates of the surgical treatment were 88.5%, 73.1%, and 69.2% for the same periods, while those of CyberKnife treatment were 72.7%, 66.7%, and 57.1%, respectively. In this study, surgical excision appeared to prolong overall survival to a greater extent, but with no statistical significance; no statistical difference was observed in the tumor-specific overall survival and progression-free survival between the two cohorts.
Conclusion
According to this preliminary study, with its mild toxicity, the efficacy of CyberKnife treatment for early hepatocellular carcinoma was on par with that of surgical resection.
Keywords: hepatic carcinoma, stereotactic body radiation therapy, hepatectomy
Introduction
Respiratory tracking, hypofractionated dosing, and short-course irradiation regimens are among the attractive features of CyberKnife® (Accuray Incorporated, Sunnyvale, CA, USA) stereotactic body radiotherapy (SBRT).1–4 The recent recognition of the radiosensitivity of hepatocellular carcinoma also helped to extend the application of radiotherapy to this indication.5–8 However, at present surgery is still the main treatment for early hepatocellular carcinoma, with SBRT being used only for recurrent or metastatic lesions and lesions that are difficult to resect. The study of the differences in efficacy of the CyberKnife SBRT and surgical excision for Stage I hepatocellular carcinoma would exert significant clinical influence on the clarification of the advantages and disadvantages of the two treatments.
Materials and methods
Clinical data
This is a retrospective analysis of hepatocellular carcinoma cases treated either with surgical excision or CyberKnife treatment from June 2006 to August 2011. The patients in the surgery group all had tumor-free cutting edge (RO) resection. Eighteen men and four women between the ages of 23 and 83 (median: 57) were in the CyberKnife group. Tumor diameters ranged from 1.6–9.5 cm (median: 4.3 cm). According to the Child-Pugh classification, ten cases were Grade A, ten were Grade B, and two were Grade C. The surgery arm included 24 men and two women between the ages of 37 and 77 (median: 55). Tumor diameters ranged from 1.8–13.7 cm (median: 4.6 cm) (Table 1). The volume of the damaged liver tissue due to the tumor was less than 30% in both groups. Nine patients in the CyberKnife arm and three patients in the surgery arm had systemic diseases. Six patients had coronary heart disease (CyberKnife arm); four patients had chronic obstructive pulmonary diseases (two patients in the CyberKnife arm and two patients in the surgery arm), and two patients had hypertension (one patient in the CyberKnife arm and one patient in the surgery arm). Pretreatment evaluations included a complete patient history, physical examination, electrocardiogram, upper abdomen and neck ultrasound, bone scan, liver magnetic resonance imaging (MRI), and intensified Computed Tomography (CT) (patients who could not undergo an MRI due to internal metal objects were not examined with MRI). Laboratory evaluations included hematology, renal and hepatic function analysis, and measurements of blood glucose, electrolytes, and α-fetoprotein (AFP). All images were studied by two radiologists to identify the tumor stage.
Table 1.
Baseline characteristics
| Characteristics | CyberKnife® (n = 22) | Surgery (n = 26) |
|---|---|---|
| Sex | ||
| Male (n) | 18 | 24 |
| Female (n) | 4 | 2 |
| Age | ||
| Range | 43–80 | 37–77 |
| Median | 57 | 55 |
| Tumor diameters (cm) | ||
| Range | 1.6–9.5 | 1.8–13.7 |
| Median | 4.3 | 4.6 |
| Lesion site (n) | ||
| Left medial lobe | 6 | 5 |
| Left lateral lobe | 3 | 8 |
| Right anterior lobe | 8 | 3 |
| Right posterior lobe | 3 | 7 |
| Caudate lobe | 2 | 3 |
| AFP (before treatment) (ug/L) | ||
| Range | 2.8–7589 | 0.9–9840 |
| Median | 318.3 | 84.6 |
| Child-Pugh score | ||
| A | 10/22* | 23/26* |
| B | 10/22* | 3/26* |
| C | 2/22 | 0/26 |
| Systemic disease | ||
| Yes | 9/22* | 3/26* |
| No | 13/22* | 23/26* |
| Following treatment | ||
| No | 11/22 | 14/26 |
| Yes | 11/22 | 12/26 |
Notes:
P < 0.05. The remaining differences between the corresponding data were not statistically significant CyberKnife® (Accuray Incorporated, Sunnyvale, CA, USA).
Abbreviations: AFP, α-fetoprotein; n, number.
The study was approved by the Tianjin Cancer Institute and Hospital ethics committee. All patients gave consent for their information to be stored in the hospital database and used for research. Because the study is a retrospective study, we were unable to obtain written consent from all patients; some patients were incapacitated, some had died, and many lived far from the hospital. All living participants provided written consent to participate in this study; patients unable to provide written consent provided verbal informed consent with the written consent of a family member. A treating physician, a hospital ethics committee member, and a medical records department employee were present at follow-up to document the process. During this process, the hospital ethics committee member and medical records department employee did not have access to the patients’ medical records; the confidentiality of records has been respected throughout.
Treatment methods
Surgical approaches included left lateral hepatic lobectomy, left hepatic trisectionectomy, right hepatectomy, lobectomy of the caudate lobe, and irregular partial hepatectomy, according to the tumor location. Patients in the CyberKnife arm were immobilized by a vacuum mattress in the supine position with hands on each side. We used a conventional contrast-enhanced CT scan (1.5 mm slice thickness), which covered 15 cm above and below the lesion to include the target area and the organs at risk. All patients were treated with Synchrony® (Accuray Incorporated) respiratory tracking. The dose was prescribed to a median 79% isodose line (range, 72%–82%) that covered the planning target volume (PTV) and irradiated a 3–6 mm margin based on the gross tumor volume. The prescription dose was 3900–5400 cGy (median: 4500 cGy), and the biologically equivalent dose was 8550–14450 cGy (median: 10080 cGy α/β = 10). The dose was delivered in three to eight fractions (median: five) on consecutive days, taking into consideration the effect on normal tissue.
Follow-up and endpoints
The follow-up program for the two arms was the same. All patients underwent local and systemic examinations to evaluate local control and overall progression 2 months after treatment. The post-treatment evaluations also included a complete patient history, physical examination, electrocardiogram, upper abdomen and neck ultrasound B, bone scan, and liver MRI and intensified CT (patients who could not undergo an MRI due to internal metal objects were not examined with MRI). Laboratory evaluations included hematology, renal and hepatic function analysis, and measurements of blood glucose, electrolytes, and AFP. The later examination period was determined according to local efficacy, primary disease, and the presence of metastasis before treatment and efficacy evaluation. In the first year post-treatment, follow-ups were conducted every 3 months. After 1 year, the follow-up frequency for patients exhibiting complete response (CR) was reduced to every 6 months. Local control and toxicity were the primary endpoints, followed by local progression-free survival (LPFS), progression-free survival (PFS), and overall survival (OS) as the secondary endpoints. The efficacy and CR, partial response, stable disease, and progressive disease states were established according to Response Evaluation Criteria In Solid Tumors.9,10 LPFS was defined as the period from the beginning of the treatment to the hepatic local progression or death; PFS was the period from the beginning of the treatment to overall progression or death, and OS was the period from the beginning of the treatment to the death of the patient. Adverse events were evaluated according to Common Toxicity Criteria 3.0.11,12
Statistical methods
Local control rate was calculated using SPSS 17.0 software (IBM Corporation, Armonk, NY, USA). The Kaplan–Meier technique was used to calculate survival rate along with log-rank test. P < 0.05 was defined as the statistical significance standard.
Results
Local efficacy of CyberKnife
The CR, partial response, and stable disease rates were 50% (eleven cases), 41% (nine cases), and 9% (two cases), respectively. Positive response rate was 91%. Six–month, 1-, 2-, and 3-year local control rates were 94.4% (17/18), 92.9% (13/14), 90.0% (9/10), and 67.7% (4/6), respectively.
Toxicity analysis
The most common Grade ≥2 toxicities in the CyberKnife group were hypodynamia, nausea, vomiting, and epigastric discomfort. No acute Grade ≥4 toxicities were observed. One patient suffered a late-developing stenosis of the esophagus, because the lesion was on the left lobe and near the esophagus. Hypodynamia, hydrothorax, and ascites were also common in the surgery group. Meanwhile, many patients in the surgery group suffered transient elevations of liver function tests, while this symptom was seldom seen in the CyberKnife treatment cohort (Table 2).
Table 2.
Toxicity between the two groups (grade ≥2)
| Toxicity | CyberKnife® | Surgery |
|---|---|---|
| Fatigue | 7/22 (31.8%)* | 4/26 (15.4%) |
| Anorexia | 6/22 (27.3%)* | 2/26 (7.7%) |
| Epigastric discomfort | 7/22 (31.8%)* | 1/26 (3.8%) |
| Nausea | 7/22 (31.8%)* | 1/26 (3.8%) |
| Vomiting | 4/22 (18.2%)* | 1/26 (3.8%) |
| Esophagitis | 3/22 (13.6%)* | 0/26 (0%) |
| Anemia | 1/22 (4.5%) | 2/26 (7.7%) |
| Stenosis of esophagus | 1/22 (4.5%) | 0/26 (0%) |
| Enterostenosis | 0/22 (0%) | 0/26 (0%) |
| Intestinal fistula | 0/22 (0%) | 0/26 (0%) |
| ALT/AST | 1/22 (4.5%) | 17/26 (65.4%)* |
| Hemorrhage | 0/22 (0%) | 2/26 (7.7%) |
| Ascites | 0/22 (0%) | 4/26 (15.4%)* |
| Hydrothorax | 0/22 (0%) | 3/26 (11.5%)* |
Note:
P < 0.05.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Survival analysis
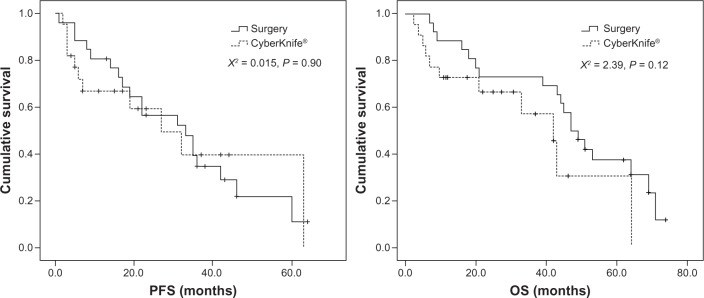
At follow-up (median: 53.4 months; range: 7.5–79.3 months), the 1-, 2-, and 3-year survival rates of the surgery arm were 88.5%, 73.1%, and 69.2%, respectively, while those of the CyberKnife group were 72.7%, 66.7%, and 57.1%, respectively (median follow-up time: 23.5 months; range: 2.5–69.2 months). According to log-rank analysis, the survival curves of the two cohorts showed that there was no statistical difference between the PFS of the patients in the two groups. Although no statistical difference was observed in the OS of the two study groups, the surgery group fared a little better than the CyberKnife-treated group (Figure 1). Because of the myriad of heterogeneities between the two study arms, we carried out a cause-of-death analysis in the two groups (Table 3), which showed that more patients died from nondisease-related causes in the CyberKnife treatment group than in the surgery group. When these patients were excluded, no statistical differences were observed in PFS and OS on the tumor-specific survival analysis (Figure 2).
Figure 1.
The comparison of PFS and OS between Cyberknife® and surgery.
Note: CyberKnife® (Accuray Incorporated, Sunnyvale, CA, USA).
Abbreviations: PFS, progression-free survival; OS, overall survival.
Table 3.
Cause of death analysis in the two groups
| Characteristics | CyberKnife® (cases) | Surgery (cases) |
|---|---|---|
| Cases of death | 14 | 19 |
| Cause of death | ||
| Tumor-related causes | 8* | 16* |
| Nontumor-related causes | 6* | 3* |
Note:
P < 0.05. CyberKnife® (Accuray Incorporated, Sunnyvale, CA, USA).
Figure 2.
Tumor special PFS and tumor special OS between CyberKnife® group and surgery group.
Note: CyberKnife® (Accuray Incorporated, Sunnyvale, CA, USA).
Abbreviations: OS, overall survival; PFS, progression-free survival.
Conclusion
In this study, the results appear to show that the efficacy of the CyberKnife treatment for Stage I hepatocellular carcinoma is equivalent to that of surgical excision, with milder adverse events.
The promising efficacy of the CyberKnife treatment in Stage I hepatocellular carcinoma can be attributed to its solid technical capabilities. Local control is the main focus of the approach to Stage I hepatocellular carcinoma, free of local advancement or metastasis. CyberKnife has several advantages for the treatment of hepatocellular carcinoma. Among these, the Synchrony Respiratory Tracking System (Accuray Incorporated) guarantees the centering of the treatment beam on the PTV despite the movement of the tumor with the breathing cycle of the patient.13 Early attempts at treating liver malignancies with radiation were largely unsatisfactory due to the extraordinary sensitivity of normal liver tissue to the effects of radiation. Therefore, the secret of appropriate radiotherapy for liver applications lies not in the nature of the radiation, but rather in the delivery method. The hypofractionated, short-course irradiation pattern of CyberKnife delivers precise, high, biologically equivalent doses, which makes it a powerful weapon against inoperable hepatocellular carcinoma. A typical CyberKnife dose distribution is characterized by a very high dose concentrating on the tumor with a rapid dose fall-off around the PTV, which protects normal tissue and organs from the detrimental effects.14–16 This distribution pattern, in theory, should maximize local efficacy while minimizing adverse effects.
The results of the present study appear to support this theory. It can be speculated that the lack of obvious PFS differences in the two cohorts can be attributed to intrahepatic metastasis of hepatocellular carcinoma and distant metastasis. Both CyberKnife SBRT and surgical resection are, after all, local treatments. Biological behavior of hepatocellular carcinoma is characterized by its tendency for intrahepatic and distant metastases. Therefore, the two treatment methods cannot help with the PFS17–19 even when they have excellent local efficacy. According to the comparison of OS rates, the surgery has the advantage in prolonging survival over CyberKnife, though with no statistical significance. However, no difference exists between the two groups if we consider the complications, performance statuses, and causes of death of the patients, and the disease-specific survival. This study provides further evidence of the efficacy of CyberKnife treatment in Stage I hepatocellular carcinoma from the aspect of survival.
It should be noted that, unlike stereotactic radiotherapy for lung cancer, there are many methods of categorization of hepatocellular carcinoma stages, with great differences and distinctive features for different stages. At present, it’s very difficult to find a generally accepted and completely prognosis-related categorization method. We adapted the application of the AJCC Cancer Staging Atlas20 (based on the 6th edition) in this research, because it is in line with the international standards.
Most of the early research focused only on the efficacy of CyberKnife, without regard to the staging and comparison of the results to those of surgical excision. Through comparison with surgical excision, this study does not only showcase the efficacy of CyberKnife treatment for hepatocellular carcinoma, but also offers an alternative treatment choice for Stage I hepatocellular carcinoma, which affords valuable lessons for clinical hepatocellular carcinoma treatment and application of CyberKnife in hepatocellular carcinoma treatment.
In summary, through a comparative study on the efficacy of CyberKnife and surgical excision, this study has made a preliminary exploration in the selection of new treatments for Stage I hepatocellular carcinoma and an investigation on the efficacy of CyberKnife treatment. Although it has not become the main treatment for hepatocellular carcinoma yet, we believe that stereotactic radiotherapy, such as the CyberKnife treatment, will find more widespread acceptance in the future.
Acknowledgments
The authors wish to thank Maobin Meng and the staff of the Tianjin Cancer Hospital and all patients who were associated with this study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Martin A, Gaya A. Stereotactic body radiotherapy: a review. Clin Oncol (R Coll Radiol) 2010;22(3):157–172. doi: 10.1016/j.clon.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Ernst F, Schlaefer A, Schweikard A. Smoothing of respiratory motion traces for motion-compensated radiotherapy. Med Phys. 2010;37(1):282–294. doi: 10.1118/1.3271684. [DOI] [PubMed] [Google Scholar]
- 3.Goyal K, Einstein D, Yao M, et al. Cyberknife stereotactic body radiation therapy for nonresectable tumors of the liver: preliminary results HPB Surg 20102010309780.Available from: http://dx.doi.org/10.1155/2010/309780Accessed August 22, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis C, Dewas S, Mirabel X, et al. Stereotactic radiotherapy of hepatocellular carcinoma: preliminary results. Technol Cancer Res Treat. 2010;9(5):479–487. doi: 10.1177/153303461000900506. [DOI] [PubMed] [Google Scholar]
- 5.Seong J. Recent developments in radiotherapy of hepatocellular carcinoma. Korean J Hepatol. 2004;10(4):241–247. Korean. [PubMed] [Google Scholar]
- 6.Zeng ZC, Tang ZY, Fan J, et al. Consideration of the role of radiotherapy for unresectable intrahepatic cholangiocarcinoma: a retrospective analysis of 75 patients. Cancer J. 2006;12(2):113–122. [PubMed] [Google Scholar]
- 7.Zeng ZC, Fan J, Tang ZY, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005;61(2):432–443. doi: 10.1016/j.ijrobp.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Zeng ZC, Tang ZY, Yang BH, et al. Radiation therapy for the locoregional lymph node metastases from hepatocellular carcinoma, phase I clinical trial. Hepatogastroenterology. 2004;51(55):201–207. [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe H, Okada M, Kaji Y, et al. New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1) Gan To Kagaku Ryoho. 2009;36(13):2495–2501. Japanese. [PubMed] [Google Scholar]
- 11.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 12.Palazzi M, Tomatis S, Orlandi E, et al. Effects of treatment intensification on acute local toxicity during radiotherapy for head and neck cancer: prospective observational study validating CTCAE, version 3.0, scoring system. Int J Radiat Oncol Biol Phys. 2008;70(2):330–337. doi: 10.1016/j.ijrobp.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Dieterich S, Gibbs IC. The CyberKnife in clinical use: current roles, future expectations. Front Radiat Ther Oncol. 2011;43:181–194. doi: 10.1159/000322423. [DOI] [PubMed] [Google Scholar]
- 14.Dewas S, Mirabel X, Kramar A, et al. Radiothérapie stéréotaxique hépatique par CyberKnife®: l’expérience lilloise. [Stereotactic body radiation therapy for liver primary and metastases: the Lille experience.] Cancer Radiother. 2012;16(1):58–69. doi: 10.1016/j.canrad.2011.06.005. French. [DOI] [PubMed] [Google Scholar]
- 15.Huang WY, Jen YM, Lee MS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84(2):355–361. doi: 10.1016/j.ijrobp.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 16.Lanciano R, Lamond J, Yang J, et al. Stereotactic body radiation therapy for patients with heavily pretreated liver metastases and liver tumors. Front Oncol. 2012;2:23. doi: 10.3389/fonc.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi GH, Choi SH, Kim SH, et al. Robotic liver resection: technique and results of 30 consecutive procedures. Surg Endosc. 2012;26(8):2247–2258. doi: 10.1007/s00464-012-2168-9. [DOI] [PubMed] [Google Scholar]
- 18.Mostaedi R, Milosevic Z, Han HS, Khatri VP. Laparoscopic liver resection: Current role and limitations. World J Gastrointest Oncol. 2012;4(8):187–192. doi: 10.4251/wjgo.v4.i8.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232(1):10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edge Stephen B, Byrd David R, Compton Carolyn C, et al. AJCC Cancer Staging Manual. 6th edition. Springer; 2006. pp. 191–201. [Google Scholar]