Supplemental Digital Content is available in the text.
Keywords: newborn, neonatal, infant, sepsis, infection, case management, community, clinic, outpatient, Pakistan
Abstract
Background:
Infection in young infants is a major cause of morbidity and mortality in low-middle income countries, with high neonatal mortality rates. Timely case management is lifesaving, but the current standard of hospitalization for parenteral antibiotic therapy is not always feasible. Alternative, simpler antibiotic regimens that could be used in outpatient settings have the potential to save thousands of lives.
Methods:
This trial aims to determine whether 2 simplified antibiotic regimens are equivalent to the reference therapy with 7 days of once-daily (OD) intramuscular (IM) procaine penicillin and gentamicin for outpatient management of young infants with clinically presumed systemic bacterial infection treated in primary health-care clinics in 5 communities in Karachi, Pakistan. The reference regimen is close to the current recommendation of the hospital-based intravenous ampicillin and gentamicin therapy for neonatal sepsis. The 2 comparison arms are (1) IM gentamicin OD and oral amoxicillin twice daily for 7 days; and (2) IM penicillin and gentamicin OD for 2 days, followed by oral amoxicillin twice daily for 5 days; 2250 “evaluable” infants will be enrolled. The primary outcome of this trial is treatment failure (death, deterioration or lack of improvement) within 7 days of enrollment. Results are expected by early 2014.
Discussion:
This trial will determine whether simplified antibiotic regimens with fewer injections in combination with high-dose amoxicillin are equivalent to 7 days of IM procaine penicillin and gentamicin in young infants with clinical severe infection. Results will have program and policy implications in countries with limited access to hospital care and high burden of neonatal deaths.
Infection in the young infant (0–59 days) is a major cause of morbidity and mortality in low-middle income countries, with high neonatal mortality rates.1,2 The World Health Organization (WHO) estimates that globally, approximately 40% of all child deaths are in the neonatal (0–27 day) period and a third are attributable to serious bacterial infections.2 Timely case management could save thousands of lives, and thus is an important strategy for improving child survival and meeting UN Millennium Development Goal 4 targets for reduction of child mortality in developing countries.
Most neonatal deaths in developing countries occur at home, with few coming to formal medical attention.1,3 Moreover, sick newborns who are hospitalized experience very high case fatality rates (20–40%), most commonly from multidrug-resistant hospital-acquired infections related to inadequate infection control standards in newborn nurseries in developing countries, as well as due to delays in care-seeking.4 WHO recommends hospitalization and 10 or more days of parenteral therapy with penicillin/ampicillin and gentamicin for neonates with sepsis.5 This recommendation is often difficult to put in practice as most families are unable or unwilling to use such care for a variety of sociocultural and economic reasons.6 Thus, there is a strong rationale for the development of simple management and referral guidelines for common life-threatening infections that can be applied in community and first-level facility settings and scaled up for use by national maternal and child health programs.
In his pioneering work, Dr. Abhay Bang in Gadchiroli, India, demonstrated a 76% reduction in sepsis-specific neonatal mortality through home-based neonatal care and infection management.7 Baqui et al8 recently showed the effectiveness of a community-based package of newborn health interventions in rural Bangladesh, including home-based diagnosis and treatment of infection with a 7- to 10-day course of intramuscular (IM) penicillin and gentamicin if referral for facility-based treatment was not accepted. This “package” of interventions, implemented through home visits by community health workers (CHWs), resulted in a 34% reduction in neonatal mortality rate compared with baseline. In Karachi, Pakistan, an open-label trial in 434 young infants with possible serious bacterial infection (PSBI) whose families refused hospitalization compared 2 study regimens [IM ceftriaxone once daily (OD) and IM gentamicin OD combined with oral trimethoprim-sulfamethoxazole twice daily] with reference therapy of 7 days of IM procaine penicillin and gentamicin OD in primary health-care clinics and found the penicillin and gentamicin combination to be the most effective with a 91% success rate.9 Ceftriaxone had a success rate of 85%. The success rate with trimethoprim-sulfamethoxazole and gentamicin regimen was 82%, with significantly higher death rate associated with this regimen. Investigators noted that programmatic implementation of a 7-day injectable penicillin and gentamicin regimen may be hampered by the difficulty of giving 2 daily injections to very small infants. In the Pakistan study, 10% of infants receiving penicillin and gentamicin injections withdrew after the first day, the families refusing all further injections.9 Extensive family counseling was needed for the completion of 7 days of therapy with 2 injections daily. In addition, physicians reported that procaine penicillin injections were difficult to mix, viscous and hard to administer with narrow bore needles.
Meta-analyses of use of oral antibiotics for neonatal pneumonia management in community settings also suggest an effect on overall and pneumonia-specific mortality.10,11 A recent review of the impact of case management strategies on mortality among newborns with sepsis or pneumonia found 4 trials evaluating oral antibiotics for neonatal pneumonia in nonrandomized, concurrently controlled designs. A meta-analysis of these studies suggested reductions in all-cause neonatal mortality [relative risk 0.75; 95% confidence interval (CI): 0.64–0.89; 4 studies] and neonatal pneumonia-specific mortality (relative risk 0.58; 95% CI: 0.41–0.82; 3 studies).11
A consultation of WHO, Save the Children/Saving Newborn Lives, United States Agency for International Development, Bill and Melinda Gates Foundation and newborn health experts convened to discuss on newborn infection case management guidelines for community settings in London in 2007. The group recommended future research to evaluate different combination oral and IM antibiotic regimens, or injectable to oral “switch” regimens that would be feasible in weak health systems for use in first-level facility and home-based management algorithms, would be acceptable to families and show treatment success rates as close as possible to the current standard regimen of injectable gentamicin and penicillin for 7–10 days.
MATERIALS AND METHODS
Study Design
This is a randomized controlled open-label equivalence trial in young infants with clinically diagnosed severe infections (CSI) seen at primary health-care clinics in Karachi, Pakistan. The trial aims to evaluate if (1) IM gentamicin OD and oral amoxicillin twice daily for 7 days; and (2) IM penicillin and gentamicin OD for 2 days followed by oral amoxicillin twice daily for 5 days are equivalent to 7 days of IM procaine penicillin and gentamicin (reference therapy). We expect that the proportion of babies who fail therapy at or before day 8 will be about 10% in each group. The difference in failure rates comparing each of the experimental treatments with the reference treatment will be calculated along with 95% CIs. An experimental treatment will be considered equivalent to the reference treatment if the upper bound on the 95% CI is <5%. The secondary objectives are to identify clinical predictors of treatment failure and to determine the proportion of relapses (young infants who were considered cured by day 7, but developed any of the signs of suspected severe infection by day 14), and compliance with therapy.
Study Setting
The study areas are in low-income settlements in coastal Karachi [Rehri Goth, Ibrahim Hyderi, Ali Akbar Shah Goth and Bhains (Cattle) colony] and in Bilal Colony located approximately an hour’s drive from the Aga Khan University’s (AKU) Stadium Road campus in Karachi (Fig. 1). The coastal areas were originally fishing villages, but with the rapid expansion of Karachi have acquired a peri-urban character, with multiple ethnic groups and mixed livelihoods including fishing, cattle farming for diary production and small retail businesses serving the local population. Bilal Colony is an urban squatter settlement located in an industrial area of Karachi where the leather industry is the dominant business activity.
FIGURE 1.
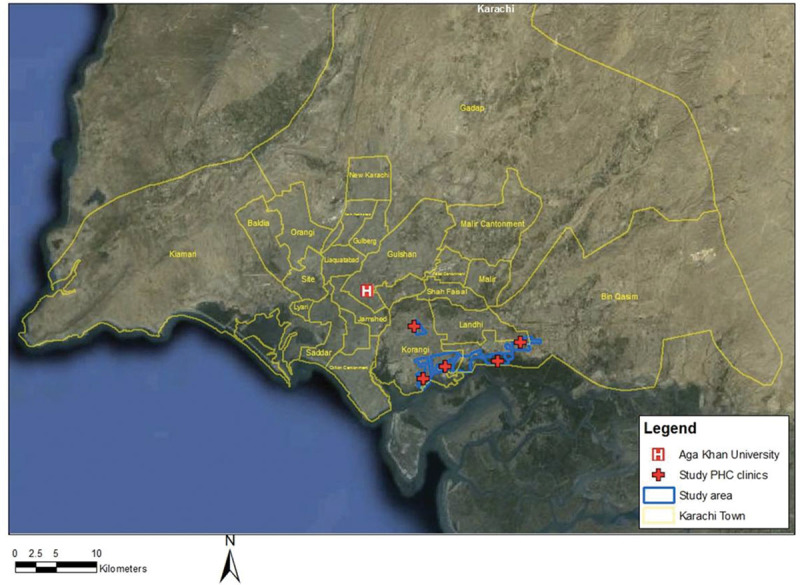
Map of study area in Karachi, Pakistan.
The Department of Paediatrics and Child Health, AKU, has been maintaining demographic surveillance and running primary care clinics in these areas for several years. Household surveillance for pregnancy and newborns was established in 2003. The total population of the catchment area under surveillance is about 370,000, with a crude birth rate of 33 per 1000. Annually, approximately 10,000 pregnancies are actively followed at home by locally recruited and trained women working as CHWs. At baseline, that is, before the study began, 58% of births occurred at home; almost all attended by unskilled attendants; the neonatal mortality rate was 35 per 1000 live births.
CHWs refer sick young infants to the primary care clinic for management. The primary health clinics (PHCs) are staffed by several (at least 2) physicians, 1 female health visitor and CHWs. The PHCs provide free health care to children younger than 5 years, including medications, and facilitated referral for hospitalization.
Enrollment
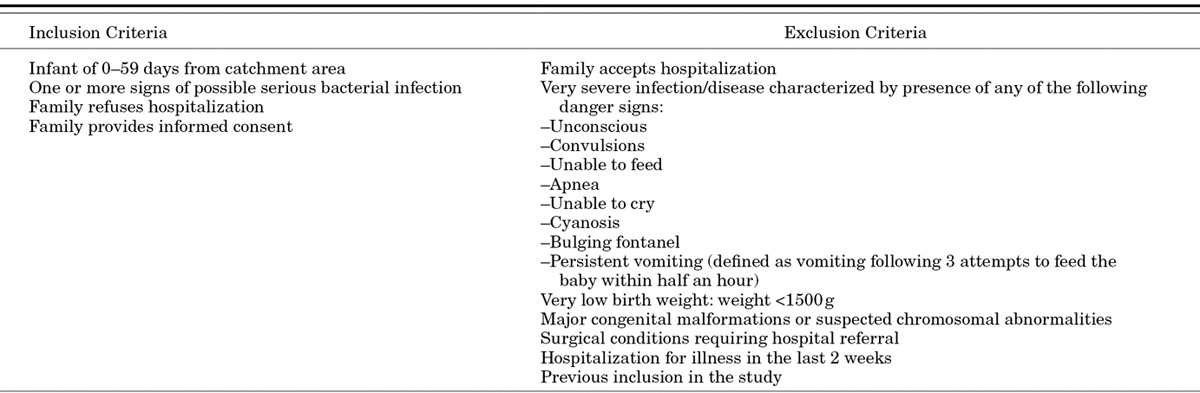
A study physician at the PHC examines all young infants referred by the CHWs and any brought directly by the family. The recruitment procedure is summarized in Figure 2. Eligible infants with signs of CSI (Table 1) meeting the case definition are enrolled in the study if caretakers refuse hospitalization despite provision of free facilitated transport and medical care costs and provide consent to participate in the trial (Table 2). The case definition of CSI used represents a modification of the 7-sign clinical algorithm for the identification of sick young infants needing referral developed by WHO in which the Pakistan trial site also participated.12 To increase the specificity of diagnosis of the possible severe infection, the fast respiratory rate sign has been dropped and the higher than normal temperature cutoff increased to 38°C instead of 37.5°C. All clinical signs are elicited by physicians performing a standardized physical examination. Definitions of clinical signs are provided in Appendix (Supplemental Digital Content 1, http://links.lww.com/INF/B620). Criteria for exclusion from the trial include acceptance of hospitalization, infants with danger signs indicating very severe infection/disease, weight <1500 g, hospitalization for illness in the last 2 weeks, previous inclusion in the study or refusal to provide consent to participate (Table 2). For families providing consent for blood sampling, 1–3 mL of blood is obtained from the infant for culture using aseptic precautions and the sample processed in a BACTEC Peds Plus blood culture bottle using a BD 9050 automated blood culture system (Becton Dickensen, Franklin Lakes, NJ). Any bacterial growth is identified using standard microbiologic procedures. Families refusing blood sampling are not excluded from participating in the trial. All enrollment information is recorded on eligibility and enrollment case record forms. For caretakers providing consent, a video recording of the clinical presentation is also made.
FIGURE 2.
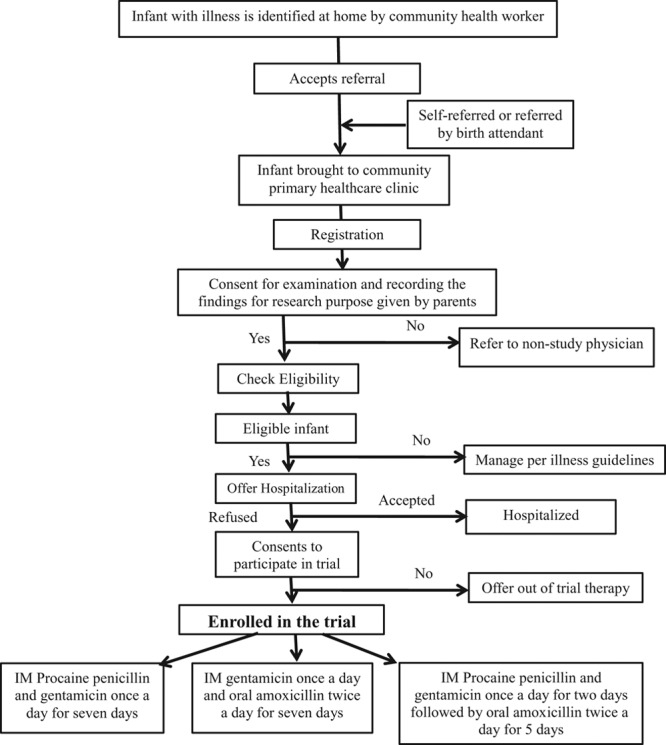
Trial profile.
TABLE 1.
Case Definition of CSI in Young Infants

TABLE 2.
Study Inclusion and Exclusion Criteria
Randomization and Allocation Concealment
Randomization is performed via computer-generated random numbers. A block randomization strategy with individual blocks of varying multiples of 3 is used to periodically balance the assignment in the 3 study groups. Randomization lists are stratified by site and by age categories 0–6 and 7–59 days to maintain similar composition of ages in all 3 study arms. The group assignment for each unique ID is placed in a sealed opaque envelope by a study administrative assistant located centrally (at AKU). The study assistants are not involved in any clinical care decisions in the field sites. These envelopes are maintained in sequential order at each study site in batches of 20–30. Different colored envelopes for 0–6 (white) and 7–59 days (brown) old infants are used to minimize the chances of error in opening the wrong envelope. Once a child’s family has consented to participate in the study, the study physician obtains the treatment assignment by opening the sealed envelope.
Drug Dosages and Administration
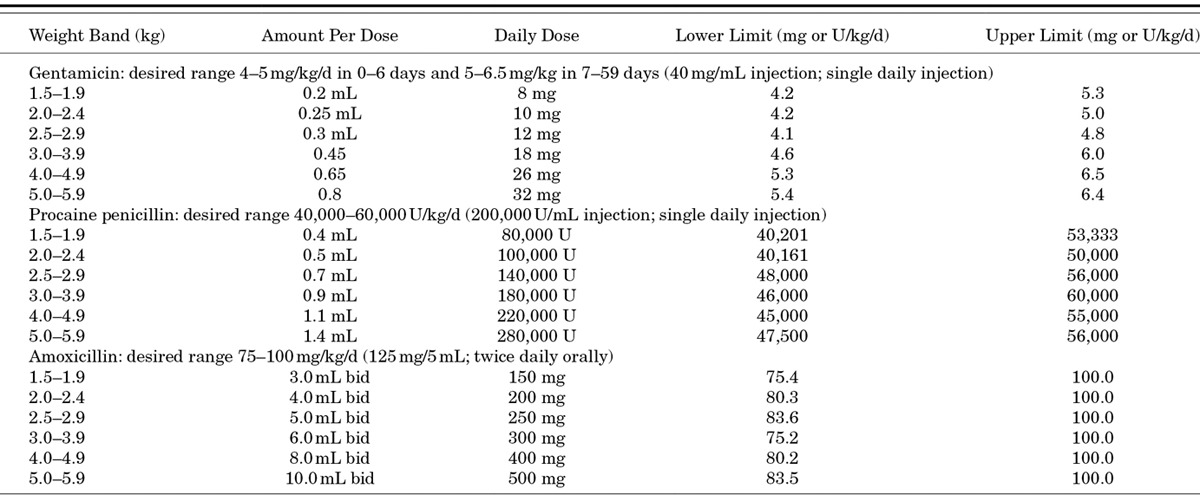
Doses were selected to optimize efficacy, safety and feasibility for eventual programmatic use. The safety and efficacy of once-daily dosing of gentamicin in newborns are well-established.13 Procaine penicillin is a long-acting penicillin that has been used in a dose of 50,000 U/kg for the treatment of congenital syphilis.13 High-dose amoxicillin of 80–100 mg/kg is currently recommended for the management of childhood pneumonia, in areas where pneumococci with reduced sensitivity to penicillin are common.14 The medications are provided by the AKU pharmacy and stored at room temperature away from direct sunlight at the study clinics. Oral amoxicillin once reconstituted is kept refrigerated at the PHC. The morning dose is administered by study personnel in the clinic, and the evening dose is administered by a CHW at home. Residual medication in the study bottle is measured by study supervisors to ascertain compliance with oral therapy. Gentamicin injections are given by physicians or paramedical staff. The dosage chart of 6 weight bands is shown in Table 3. Because of ethical considerations relating to the use of placebo injections in the infants randomized to simplified regimens using oral amoxicillin and the need to assess differences in compliance with regimens using fewer injections, blinding of therapeutic arms was not possible in this study.
TABLE 3.
Antibiotic Dosage by Weight
Follow-up and Determination of Treatment Failure and Relapse
Enrolled infants are followed daily at the PHCs for 7 days by study physicians who perform a repeat standardized physical examination to assess clinical signs of treatment failure. The primary outcome of this trial is treatment failure within 7 days of enrollment. The criteria for the determination of treatment failure are listed in Table 4.
TABLE 4.
Definition of Treatment Failure
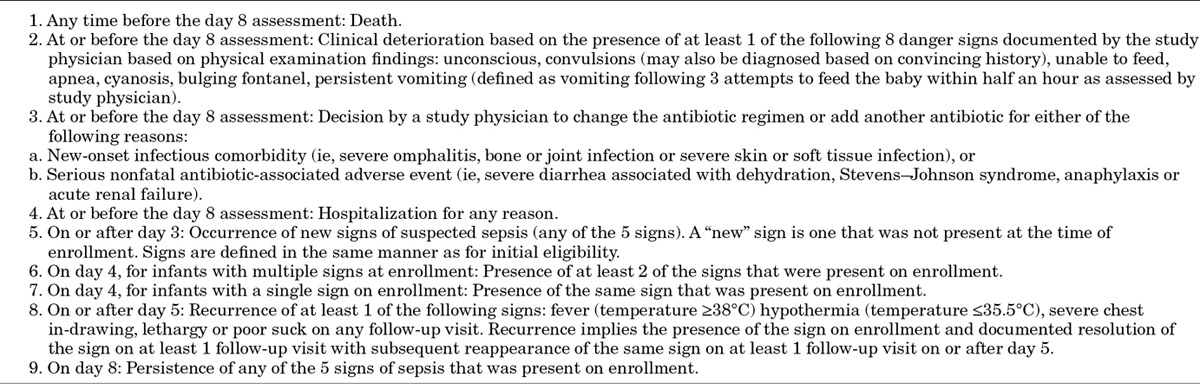
As this is an unblinded trial, careful attention to determine the clinical signs associated with treatment failure has been built into study procedures. All infants who are judged to have treatment failure by study physicians and a 10% random sample of infants not meeting treatment failure criteria are examined by a second physician on-site, who is blinded to the findings of the study physician located in a different room. The second physician records his/her findings on a designated case record form. A study lady health visitor/midwife reviews the forms of the study physicians and second physician for agreement. If there is agreement regarding treatment failure, the infant is classified as a treatment failure. If there is disagreement, a supervising physician is asked to evaluate the infant. The supervisor’s decision is recorded and considered final. If families consent, a video recording of the signs of treatment failure is also made. Infants with treatment failure are counseled to accept hospitalization. Those who still refuse referral are managed at the PHCs with ceftriaxone and gentamicin injection therapy. Infants whose families refuse to visit the PHC are evaluated at home by study physicians to maximize the number of infants who are “per protocol.” Enrolled infants are also evaluated at the PHCs on study days 11 and 14 to determine whether clinical relapse has occurred. Relapse is defined as death, development of at least 1 of the 5 signs of CSI and/or development of any of the 8 danger signs after the day 8 assessment (Tables 1–3) up to day 14 follow-up.
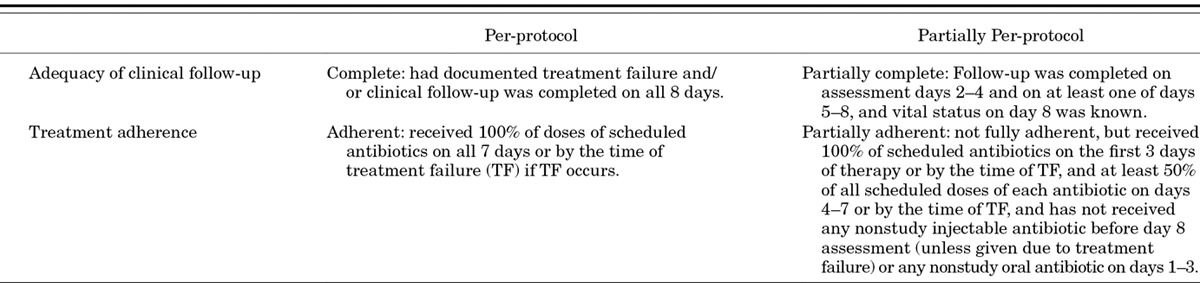
Determination of Per-protocol Status
Stringent criteria for the determination of per-protocol status have been developed, which take into account both adequacy and completeness of clinical assessments and adherence to therapy. These are presented in Table 5.
TABLE 5.
Definitions of Per-Protocol Standards
Sample Size and Data Analysis
Table 6 shows the expected power of different sample size choices to demonstrate the similarity of 2 treatments (with a similarity margin of 5%) over the entire 7-day time period from randomization and initiation of treatment. It is assumed that the true failure rate in arm A (the gold standard) will be 10% over this time period and that the true failure rate in the experimental arm will be identical (10%) or only slightly worse (11%). A sample size of 750 children per arm will provide the study with a power of at least 70% to demonstrate “similarity” of the treatments if the assumptions about failure rates are roughly correct.
TABLE 6.
Power of Different Effective Sample Sizes to Demonstrate the Similarity of 2 Treatments (with a Similarity Margin of +5%) Under Different Assumptions About True Similarity of the 2 Treatments (Identical or Difference of 1% in True Failure Rates)
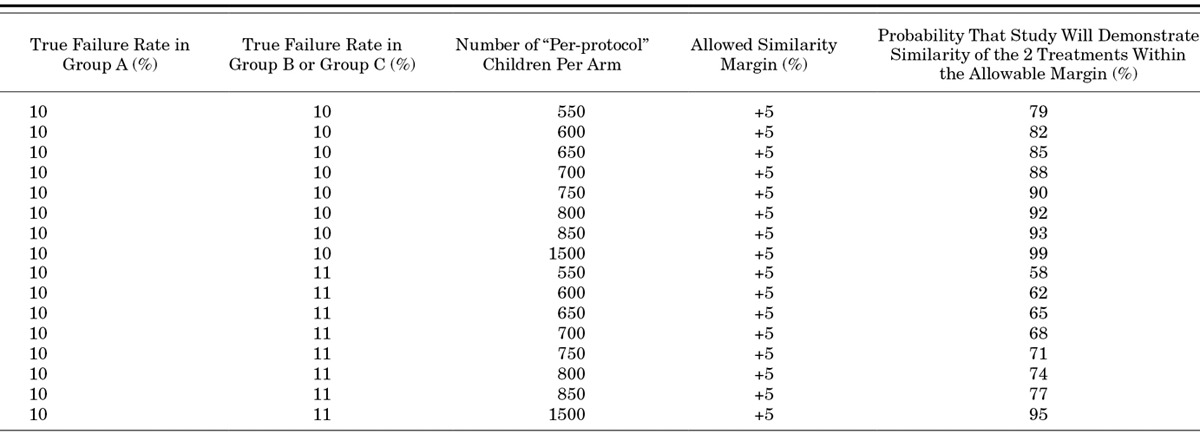
Statistical analysis will be based on a comparison of treatment failure rates observed in arms B and C compared with the failure rate in arm A providing injection penicillin and gentamicin for 7 days. For each comparison, the point estimate of the failure rate difference (experimental – gold standard) between the 2 treatment arms will be calculated together with a 2-sided 95% CI. The alternative treatment will be judged to be “of similar effectiveness” to the gold standard treatment (A) if the upper bound of the 95% CI lies below the allowed “similarity margin” in the Table 6. Both intention-to-treat and modified per-protocol analyses will be reported.
Data Management and Quality Assurance
All completed CRFs are transferred from PHCs to the central Data Management Unit of AKU on a weekly basis. Upon entry into the log register, forms are stored at secure location under the supervision of dedicated data management staff. Access to all CRFs is limited to ensure security and confidentiality. The data are entered in customized software designed using Microsoft Visual Basic 6.0 as front-end and Microsoft SQL Server 2005 as back-end database for storing all the data. The data entry program has built-in system checks for implausible, inconsistent or missing values. To ensure data quality and minimize data entry errors, all CRFs are dual entered by 2 different data input operators. A dual check report is included in the data entry software that reports the differences in the 2 entries. Dual check reports are generated and printed once the data entry of each batch of CRFs is complete. Conflicting entries are verified from the hard copies and the correct ones are highlighted on the report. After carrying out these corrections, the report is stored for future reference. Once the 2 entries are 100% matched, an error checking report is generated to identify any possible inconsistent or out of range entries, which could not be picked up at the time of data entry. The data entry program maintains the audit trails of any change made to the entered data at any stage. The entered database with the removal of identifying information is transferred to the London School of Hygiene and Tropical Medicine on a quarterly basis for checking and analysis of treatment adherence, clinical follow-up, and treatment failure rates. Quarterly reports for the 3 arms combined are produced for the Trial Steering Committee (see below). Quarterly reports, broken down by arm, are produced for the trial data safety monitoring board (see below).
Monitoring for Study Quality and Safety
Ensuring good clinical practice and a high standard of compliance with study procedures is a priority. For day-to-day monitoring, 2 dedicated clinician supervisors observe the enrollment process from screening to enrollment, randomization, to the delivery of therapy and archiving of medicine. These supervisors also check case files for errors, inconsistencies and missing values. Refresher training and clinical sign interpretation standardization exercises using video recordings from the field sites are held monthly to ensure consistency in evaluating infants for signs of CSI and treatment failure across all the sites.
A Trial Steering Committee comprising independent experts appointed by the sponsors of this study is charged with study oversight. The Trial Steering Committee reviews quarterly reports, and members make annual visits to the field sites where they spend a week in the field observing study procedures. An independent data safety monitoring board monitors study results to ensure safety of study participants.
Approvals
This study was reviewed and approved by the Research Ethics Review Committees of Aga Khan University, London School of Hygiene and Tropical Medicine, and the WHO. The trial has been registered at Clinicaltrials.gov before enrolment was initiated (NCT01027429).
DISCUSSION
This trial is designed to determine whether simplified antibiotic therapy for young infants with CSI is effective and safe in first-level facility settings in an environment of high neonatal mortality. Trials with similar design are also being done in Bangladesh and 3 African countries with location-specific adaptations. Their study designs are reported elsewhere in this supplement.15,16 The results of these trials will provide much-needed information to policy makers on the most appropriate regimen to include when scaling up community-based newborn or young infant care programs.
Several considerations underlie the design of this trial in Karachi and are discussed in detail elsewhere in this supplement.17 This study was undertaken in primary care clinic settings rather than homes to deliver injectable antibiotic therapy to young infants with PSBI with a view to address ease of potential programmatic uptake in Pakistan. Recently, the Pakistan government has provided funding to revitalize basic health units for curative service delivery and a combination of early identification of sick young infants at home by the government’s lady health workers and referral to the nearest basic health unit for the management of those refusing referral. This should minimize concerns about indiscriminate gentamicin use, injection safety and antimicrobial resistance.
We have chosen to exclude young infants with very severe disease (Table 2) because of ethical considerations regarding the use of oral medication in these infants when we have the capability of giving parenteral therapy, which will limit generalizability of study findings. Such infants are either hospitalized or if families refuse hospitalization, receive daily IM procaine penicillin and gentamicin injections, or oral amoxicillin if injectable therapy is refused. Therefore, we will have availability of the data and outcome of the entire cohort of young infants with CSI whether or not they participated in the trial, as well as those with very severe disease, which will be reported separately, and will provide additional valuable information on the impact of case management for all young infants with clinical illness compatible with serious bacterial infections.
The trial will report results for treatment failure outcomes for young infants 0–59 days of age. Although we recognize that the pathophysiology of early-onset sepsis is distinct from late-onset sepsis, and therefore, treatment failure rates with simplified regimens may be different, sample size limitations preclude sufficient power to detect differences between these therapies for 0- to 6-day and 7- to 59-day-old infants separately. However, because 3 trials using similar regimens are currently underway, pooled analyses of infants in these age groups may have sufficient power to detect, if any, or exclude differences.
The main risk in this trial is therapy failure and potential death in young infants with CSI PSBI. From previous experience, we know that infants receiving a procaine penicillin- and gentamicin-based regimen in our clinic settings experience a case fatality rate of about 2%, whereas infants whose families refuse any antibiotic therapy have a case fatality rate of 20%.9 Every effort is being made to prevent adverse outcomes and death among trial participants, including home visits in the evening and 24-hour hotline availability to address concerns and arrange referrals, if needed. Two percent case fatality has been set as a benchmark to trigger an additional safety review if the observed death rate at 1 week exceeds this figure.
Finally, we believe that the findings from this trial will also be of interest to the global pediatric community if we can identify young infants with CSI who are at low risk of therapy failure with outpatient management, potentially preventing many hospital admissions.
ACKNOWLEDGMENTS
The authors thank the field staff, physicians and families who participated in these studies.
Footnotes
Accepted for publication June 5, 2013.
The Trial Registration number was Clinicaltrials.gov NCT01027429.
This research was funded by the Saving Newborn Lives program of Save the Children Federation, Inc, through a grant from the Bill and Melinda Gates Foundation and the World Health Organization. S.S.T., S.S. and F.J. received training support from the Fogarty International Center, National Institute of Health, grant D43TW007585. A.K.M.Z. designed the study and drafted the manuscript, S.S.T. developed detailed study protocols and manuscript figures, S.S., B.B. and K.K. are study supervisors, M.K. wrote the study site description, H.R. and I.A. wrote the data management description, F.J. assisted with editing and S.C. wrote the analysis plan. The authors have no other funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Address for correspondence: Anita K. M. Zaidi, MB BS, SM, Department of Paediatrics and Child Health, Aga Khan University, Stadium Road, PO Box 3500, Karachi, Pakistan. E-mail: anita.zaidi@aku.edu.
Copyright © 2013 by Maharaj Kishan Bhan. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License, where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially.
REFERENCES
- 1.Lawn JE, Cousens S, Darmstadt GL, et al. Why are 4 million newborn babies dying every year? Lancet. 2004;364:2020. doi: 10.1016/S0140-6736(04)17511-9. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 3.Darmstadt GL, Black RE, Santosham M. Research priorities and postpartum care strategies for the prevention and optimal management of neonatal infections in less developed countries. Pediatr Infect Dis J. 2000;19:739–750. doi: 10.1097/00006454-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Zaidi AK, Huskins WC, Thaver D, et al. Hospital-acquired neonatal infections in developing countries. Lancet. 2005;365:1175–1188. doi: 10.1016/S0140-6736(05)71881-X. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Illnesses with Limited Resources. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 6.Owais A, Sultana S, Stein AD, et al. Why do families of sick newborns accept hospital care? A community-based cohort study in Karachi, Pakistan. J Perinatol. 2011;31:586–592. doi: 10.1038/jp.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bang AT, Bang RA, Baitule SB, et al. Effect of home-based neonatal care and management of sepsis on neonatal mortality: Field trial in rural India. Lancet. 1999;354:1955–1961. doi: 10.1016/S0140-6736(99)03046-9. [DOI] [PubMed] [Google Scholar]
- 8.Baqui AH, El-Arifeen S, Darmstadt GL, et al. Projahnmo Study Group. Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: A cluster-randomised controlled trial. Lancet. 2008;371:1936–1944. doi: 10.1016/S0140-6736(08)60835-1. [DOI] [PubMed] [Google Scholar]
- 9.Zaidi AK, Tikmani SS, Warraich HJ, et al. Community-based treatment of serious bacterial infections in newborns and young infants: A randomized controlled trial assessing three antibiotic regimens. Pediatr Infect Dis J. 2012;31:667–672. doi: 10.1097/INF.0b013e318256f86c. [DOI] [PubMed] [Google Scholar]
- 10.Sazawal S, Black RE Pneumonia Case Management Trials Group. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: A meta-analysis of community-based trials. Lancet Infect Dis. 2003;3:547–556. doi: 10.1016/s1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- 11.Zaidi AK, Ganatra HA, Syed S, et al. Effect of case management on neonatal mortality due to sepsis and pneumonia. BMC Public Health. 2011;11(suppl 3):S13. doi: 10.1186/1471-2458-11-S3-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Group YICSS. Clinical signs that predict severe illness in children under age 2 months: A multicentre study. Lancet. 2008;12:8. doi: 10.1016/S0140-6736(08)60106-3. [DOI] [PubMed] [Google Scholar]
- 13.Darmstadt GL, Batra M, Zaidi AK. Parenteral antibiotics for the treatment of serious neonatal bacterial infections in developing country settings. Pediatr Infect Dis J. 2009;28(1 suppl):S37–S42. doi: 10.1097/INF.0b013e31819588c3. [DOI] [PubMed] [Google Scholar]
- 14.Hale KA, Isaacs D. Antibiotics in childhood pneumonia. Paediatr Respir Rev. 2006;7:145–151. doi: 10.1016/j.prrv.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Baqui A, et al. Safety and efficacy of simplified antibiotic regimens for outpatient treatment of clinical serious infection among neonates and young infants aged 0–59 days in Bangladesh: Design of a randomized controlled trial. Pediatr Infect Dis J. 2013;32(suppl):S12–S18. doi: 10.1097/INF.0b013e31829ff790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qazi S. Simplified regimens for management of neonates and young infants with clinical severe infection in situations when hospital admission is not possible: Study protocol for a randomized, open-label equivalence trial. Pediatr Infect Dis J. 2013;32(suppl):S19–S25. doi: 10.1097/INF.0b013e31829ff7d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaidi AK. Scientific rationale for study design of community-based simplified antibiotic therapy trials in newborns and young infants with clinically diagnosed severe infections or fast breathing in South Asia and sub-Saharan Africa. Pediatr Infect Dis J. 2013;32(suppl):S7–S11. doi: 10.1097/INF.0b013e31829ff5fc. [DOI] [PMC free article] [PubMed] [Google Scholar]


