Abstract
Background:
The World Health Organization recommends hospitalization and injectable antibiotic treatment for young infants (0–59 days old), who present with signs of possible serious bacterial infection. Fast breathing alone is not associated with a high mortality risk for young infants and has been treated with oral antibiotics in some settings. This trial was designed to examine the safety and efficacy of oral amoxicillin for young infants with fast breathing compared with that of an injectable penicillin–gentamicin combination. The study is currently being conducted in the Democratic Republic of Congo, Kenya and Nigeria.
Methods/Design:
This is a randomized, open-label equivalence trial. All births in the community are visited at home by trained community health workers to identify sick infants who are then referred to a trial study nurse for assessment. The primary outcome is treatment failure by day 8 after enrollment, defined as clinical deterioration, development of a serious adverse event including death, persistence of fast breathing by day 4 or recurrence up to day 8. Secondary outcomes include adherence to study therapy, relapse, death between days 9 and 15 and adverse effects associated with the study drugs. Study outcomes are assessed on days 4, 8, 11 and 15 after randomization by an independent outcome assessor who is blinded to the treatment being given.
Discussion:
The results of this study will help inform the development of policies for the treatment of fast breathing among neonates and young infants in resource-limited settings.
Keywords: neonates, young infants, antibiotic treatment, fast breathing, severe infection
Neonatal sepsis, meningitis and pneumonia are major causes of morbidity and mortality in developing countries, resulting in an estimated 700,000–800,000 deaths per year.1 As it is clinically difficult to differentiate between these infections, they are often identified together as a clinical syndrome of possible serious bacterial infection (PSBI). Signs used to diagnose this syndrome include poor feeding, convulsions, fast breathing, severe chest indrawing, fever or hypothermia and movement only when stimulated or no movement at all.2 The World Health Organization (WHO) currently recommends transfer to a referral facility, hospitalization and injectable antibiotic therapy with a combination of gentamicin and penicillin/ampicillin for 7-10 days for the management of this clinical syndrome in young infants up to 2 months of age.2,3
Concerns have been raised about these recommendations. First, many infants experiencing PSBI are not referred for hospital care, either due to limited access or refusal on the part of families.4–7 Second, while fast breathing is the most common clinical sign suggesting pneumonia or sepsis among children with PSBI, it is less severe than the other signs included in the syndrome. A multicountry study reported that fast breathing was seen in up to one-fifth of sick young infants presenting to a health facility.8 Furthermore, fast breathing was not predictive of mortality in studies from Bangladesh and India, unlike other more serious signs such as lethargy or unconsciousness, convulsions, inability to breastfeed well, hypothermia and chest indrawing.9–11
In some settings, neonates and young infants with fast breathing have been successfully managed with oral antibiotics in the community, resulting in low case fatality rates and reductions in neonatal and infant mortality.12–14 However, these studies were all conducted in Asia and none compared oral antibiotic treatment with the standard therapy of penicillin and gentamicin injections. Therefore, to better inform public health policy for the management of fast breathing, there is a need to evaluate whether young infants with fast breathing alone can be safely and effectively treated in the community with oral antibiotics alone, particularly in previously unstudied African settings.
In 2009, WHO requested proposals from African sites interested in conducting a randomized, open-label equivalence trial to address this issue. After an external review process, 5 sites in Africa were selected to participate in this study using a common protocol. The sites include 1 site each in the Democratic Republic of Congo (DRC) and Kenya, and 3 sites in Nigeria (Ibadan, Ile-Ife and Zaria). This article describes the protocol for this trial.
OBJECTIVES
The overall goal of the study was to inform policy on the use of oral amoxicillin for young infants with fast breathing. Our underlying hypothesis is that young infants with “fast breathing” alone can be safely and effectively managed with an oral antibiotic.
Primary objective: To evaluate the use of oral amoxicillin compared with intramuscular procaine penicillin and gentamicin for the provision of safe and effective treatment at first-level facilities and in the community for 0- to 59-day-old young infants with fast breathing alone, whose families do not accept or cannot access referral level care.
Secondary objectives: To assess family acceptance of and compliance with an oral antibiotic treatment regimen compared with intramuscular antibiotic injections and to document and compare the health worker time costs and other logistical requirements of the 2 treatment regimens.
METHODS
Study Settings
This study is being carried out at 5 sites, 1 each in DRC and Kenya and 3 sites in Nigeria; Ibadan, Ile-Ife and Zaria. Of note, another study is being performed concomitantly in the same study sites for young infants with clinical severe infection (one or more of the following 5 signs: poor feeding, movement only when stimulated, severe chest indrawing, axillary temperature >38.0°C or <35.5°C), who are treated with different experimental antibiotic regimens, although the reference treatment arm is the same for both studies. As some components of the methods are common for both studies, we have reported the commonalities such as the description of study sites and details of data management only in the clinical severe infection methods paper, also published in this supplement.15
Study Design
The study approach is outlined in Figure 1. This is an individually randomized, open-label equivalence trial. All 5 study sites will contribute to the overall sample size. Sites in the DRC, Kenya and Nigeria are following the same study design, with similar inclusion criteria, the same intervention, comparison and outcomes, as well as quality control and coordination mechanisms.
FIGURE 1.
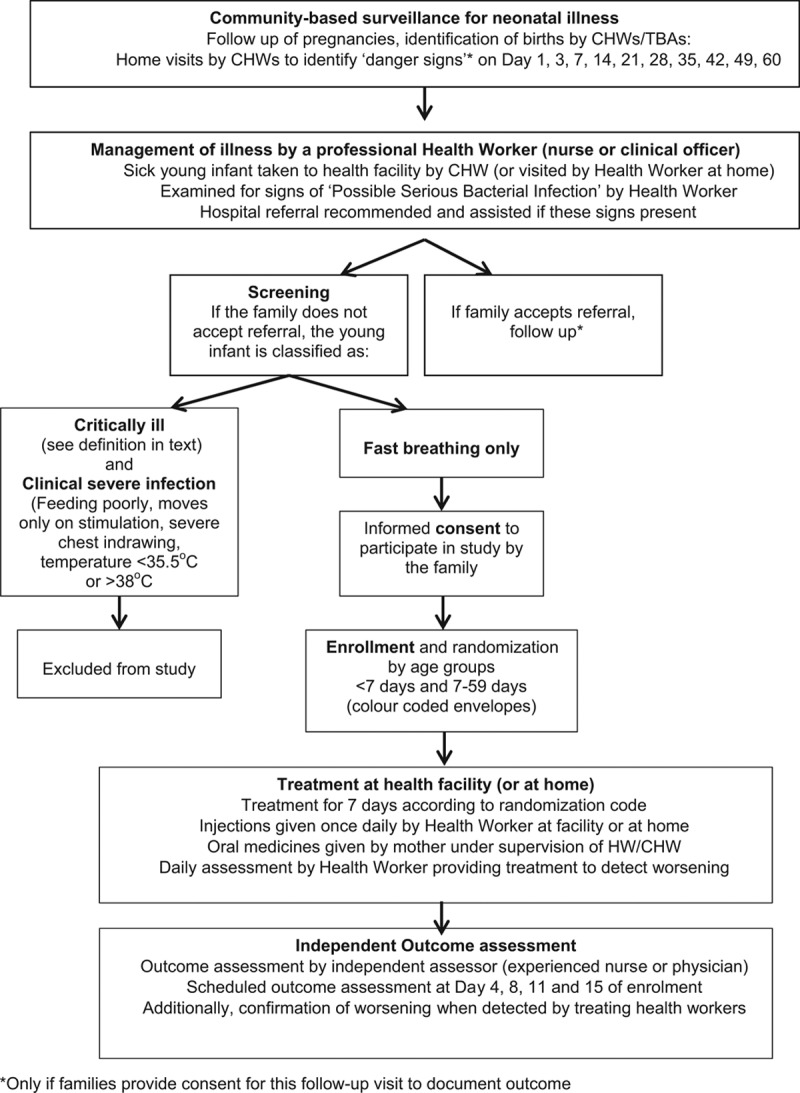
Overall study approach.
Participants
Identification of Newborns
Births are identified by community health workers (CHWs), community health extension workers (CHEWs) and traditional birth attendants and are reported within 24 hours of delivery. CHWs and traditional birth attendants cannot give injections; in contrast, CHEWs (only present in Nigeria) receive 2 years of professional training and thus can give injections. In the DRC, birth information is also transmitted by health center nurses directly to the study enrollment/treatment nurse or the study community coordinator. CHWs/CHEWs transmit information to the community coordinator/supervisor/cluster coordinator as soon as possible. The CHWs/CHEWs record details of the household and the place where the birth occurred; they also perform a postnatal home visit on the day of birth (day 1) and on days 3, 7, 14, 21, 28, 35, 42, 49 and 60 to check the newborn’s condition. CHWs/CHEWs provide families with contact details and counsel parents on the need to report any concerns they have about their newborn. The CHWs/CHEWs alert an enrollment nurse if there are any young infants with fast breathing or other symptoms and signs suggestive of PSBI whose parents refuse referral. In DRC and Kenya, the CHW accompanies the family to the health center if the family agrees, whereas in Nigeria the study enrollment/treatment nurse is called to infant’s home.
Screening and Enrollment
Screening and enrollment are conducted by enrollment/treatment nurses. In DRC and Kenya, this takes place at the health center/dispensary. In the Nigerian sites, it takes place at the child’s home. All young infants with suspected PSBI are referred by the enrollment nurse. Infants with PSBI whose parents/caretakers refuse referral for treatment are evaluated for inclusion/exclusion criteria and if found eligible, the study is explained to the parents. The mother/caregiver is counseled on the implications of refusing referral. If the parents agree to participate in the study, consent is obtained from the mother/father. If he/she is illiterate, she/he will affix a thumbprint on the form in the presence of a literate witness who signs the same form.
Young infants are included in the study if they fulfill the following criteria:
Age 0–59 days of age.
Fast breathing, defined as respiratory rate of >60 breaths per minute.
Do not have any of the following exclusion criteria:
○Signs of severe infection (defined as poor feeding on observation, movement only when stimulated, severe chest indrawing and axillary temperature ≥38.0°C or <35.5°C);
○Critically ill (characterized by the presence of any of the following signs: unconsciousness, convulsions, unable to feed at all, apnea, unable to cry, cyanosis, dehydration, bulging fontanel, major congenital malformations inhibiting oral antibiotic intake, active bleeding requiring transfusion, surgical conditions needing hospital referral and persistent vomiting defined as vomiting following 3 attempts to feed the baby within one-half hour);
○Very low weight (<1500 g at the time of presentation);
○Hospitalized for illness in the last 2 weeks or previously enrolled in the study.
Parents do not accept or cannot access referral level care.
Parents give consent to participate in the study.
At the time of enrollment, infants are stratified into 2 age groups: 0–6 days and 7–59 days. They are individually randomized to treatment regimens within each site and age stratum. The reference treatment is injectable gentamicin once daily and injectable procaine penicillin once daily for 7 days (treatment regimen A). The intervention arm consists of oral amoxicillin twice daily for 7 days (dosages described below).
Randomization schemes with a block size of 8 were computer-generated off-site at WHO using STATA version 10.0 (STATA Corp., College Station, TX) by a person not involved with the study. For allocation concealment, treatment codes are printed on small pieces of card folded once and sealed in an opaque envelope (2 sets of color-coded envelopes are used, one for each age group).
Treatments are given at a health facility or at home. Injections are given once daily by a health worker at a facility or at home, whereas oral medicines are given at home by the mother under the supervision of CHWs. A daily assessment by a health worker (treatment nurses in DRC and Kenya and CHEW in Nigeria) is conducted to identify possible treatment failures.
Allocation of Treatment Arm
In the Kenyan and Nigerian sites, the randomization list is held centrally and the treatment allocation is provided to the enrollment nurse, according to the child’s age category, by phone and is confirmed by SMS. In the DRC where mobile phone communication is generally unavailable across the study catchment area, each facility where enrollment occurs is given a block of 8 envelopes for each age group, and used blocks are regularly replaced so that a sufficient number of envelopes are always available at the facility. When the first young infant is enrolled at a facility in a stratum, the first envelope of the block for that stratum and age category at the facility is opened and the infant is treated according to the treatment code inside. When the next infant is enrolled, the next envelope of the appropriate stratum and age category block is opened. A record of all randomizations is kept by the study supervisors or study community coordinators.
Provision of Treatment
In the DRC and Kenya, all injectable treatment is provided at the health facility, by the enrollment/treatment nurse who also provides the first dose of oral therapy. Mothers observe the first dose being given and are instructed on provision of oral amoxicillin at home. In Nigeria, the first dose of injectable and of oral treatment is provided at home by the enrollment/treatment nurse after randomization. The rest of the injectable treatments are provided by CHEWs. Oral treatments are given to the mother of the enrolled infant and are administered in the home, under direct observation of the CHW/CHEW.
Outcome Assessment of Enrolled Patients
An independent outcome assessment nurse visits enrolled infants on days 4, 8, 11 and 15 after the day of enrollment to assess outcome. All assessments are conducted at the young infant’s home.
Choice of Antibiotic Regimens
A combination of penicillin and gentamicin injections is currently the WHO recommended treatment for PSBI in young infants. Thus, single daily injections of procaine penicillin and gentamicin were chosen as the reference treatment. A detailed rationale for the selection of antibiotics and study outcomes is described in another article in this supplement.16
Oral amoxicillin has been shown to be superior to oral trimethoprim-sulfamethoxazole for the treatment of pneumonia17,18 and has been shown to be effective in treating severe pneumonia in children 2–59 months of age.19–22 The most frequent pathogens associated with pneumonia are generally similar in neonates and young children and are frequently susceptible to amoxicillin.17,23 Oral amoxicillin was therefore chosen as the experimental treatment for this study.
The antibiotic dosages for young infants enrolled in the study are injection procaine penicillin in a dose of 50,000 units/kg once-daily IM; injection gentamicin in the range 4.0–7.5 mg/kg/d once-daily dose IM (depending on age of the young infant) and oral amoxicillin in suspension form in a dose of 100 mg/kg/d (<2 kg are given 75 mg/kg/d), divided in 2 equal doses. Both treatment regimens are being used for 7 days.
Study Outcomes
Primary Outcome
The primary outcome for the study is treatment failure by the day 8 postenrollment visit. Treatment failure is defined as any one of the following:
Death;
Clinical deterioration, defined as the emergence of any sign of severe infection or critical illness at any time after enrollment (as defined in exclusion criteria), or hospitalization any time after enrollment;
Persistence of fast breathing on day 4 or recurrence after day 4 up to day 8;
Development of a serious adverse event (other than death) that is related to the study antibiotics, eg, organ failure, anaphylactic reaction, severe diarrhea, disseminated and severe rash;
Secondary Outcomes
Death occurring 9–15 days after enrollment;
Relapse, defined as fast breathing that disappears on day 8 of enrollment and reemerges between days 9 and 15, or development of any sign of severe infection or critical illness signs between days 9 and 15 after enrollment;
Adherence to the study therapy between days 1 and 8.
Sample Size
In the absence of population-based incidence data, the incidence of fast breathing was conservatively estimated to be about 3.5% in Africa. The figure is based on a large multicenter study, where 5–18% of all sick young infants brought for care had fast breathing.8 Community-based studies in India and Bangladesh reported that 4.6–8.1% of babies had fast breathing at some point during the neonatal period.4,24
The sample size calculations assumed that the statistical analysis will be based on a comparison between the failure rate observed with the reference treatment regimen of injection penicillin and gentamicin for 7 days (assumed treatment failure rate of 8%) and the experimental regimen of oral amoxicillin for 7 days. A point estimate of the failure rate difference (experimental—reference treatment) between the 2 treatment regimens will be calculated together with a 2-sided 95% confidence interval. The alternative treatment will be judged to be “of similar effectiveness” to the reference treatment if the upper bound of the 95% confidence interval lies below the allowed “similarity margin” of +4%. A power of 90% to demonstrate the similarity of 2 treatments over the 7-day period following randomization was required, assuming that the true failure rates with the reference treatment and the experimental treatment regimens will be identical (assumed to be 8%).
Using the above assumptions, the required sample size was determined to be 1150 infants for each treatment group, which is likely to yield 970 “analyzable” infants per treatment arm.
Data Collection, Management and Analysis
Data are collected either at home or in the health facility on paper-based standard case report forms; forms are completed by CHWs/CHEWs, enrollment and treatment nurses, and independent outcome assessors. All completed case report forms are checked by study supervisors before the data entry. Data are entered into a Structured Query Language database specifically developed for the study. Double data entry is carried out by data entry clerks at each site; logical checks are performed by the data manager in consultation with the study supervisor and if necessary by the principal investigator. The cleaned database is sent monthly to the central data coordination center at the London School of Hygiene and Tropical Medicine (LSHTM), which developed the data management system. Quality checks are carried out both at the site and at LSHTM. LSHTM assists in quality control through database monitoring and preparing necessary reports for the Data Safety Monitoring Board (DSMB) and the trial Technical Advisory Group (TAG); they also liaise with the WHO study coordination team. Further details are described in the accompanying article on severe infections, as the trials were conducted concurrently.15
Analysis Plan
The primary analysis will be a combined analysis across all the sites. Simple comparisons of means and proportions by treatment group will be used to check whether the randomization scheme resulted in baseline comparability of the treatment groups. The primary analyses will be for equivalence between the reference treatment and the experimental (intervention) arm. It will consist of the comparison of proportions of infants with treatment failure in each treatment arm.
The primary analysis will be conducted on a per-protocol basis; an intention-to-treat analysis will also be conducted. For an enrolled infant to be included in the per-protocol analysis, she/he should have received: (1) all antibiotic doses due to be received for the first 3 days of treatment or by the time of treatment failure; and (2) at least 50% of all scheduled doses of each antibiotic on days 4–7 or by the time of treatment failure. Furthermore, for inclusion in the per-protocol analysis, follow-up must be completed on assessment days 2–4 and on at least 1 of days 5–8, and vital status on day 8 must be known.
Analyses will be performed with data from all sites, adjusted for any baseline covariates that were unbalanced at baseline and with addition of dummy variables for site and age group. The primary and secondary outcomes, the proportion of young infants who received treatment for the entire duration and costs of such treatment will be compared across the 2 study arms. The difference in the risk of treatment failure together with 95% confidence intervals will be calculated. Secondary analyses will be performed to investigate the effect on adverse events including death and other serious outcomes such as diarrhea with severe dehydration, disseminated and severe rash, anaphylactic reaction, stopped passing urine for >12 hours, cellulitis or abscess at injection site. Univariate and multivariate regression analyses will be undertaken to identify predictors of treatment failure for young infants with fast breathing.
Ethical Issues
Study Approvals
The trial protocol and all associated data collection instruments and consent forms were submitted for ethical review to the local institutional review boards at each site as well as to the WHO Ethical Review Committee and to LSHTM. The trial was registered as ACTRN12610000286044 with Australian New Zealand Clinical Trials Registry. The trial follows the Council for International Organizations of Medical Sciences (CIOMS) and Good Clinical Practice guidelines.
Informed Consent
The study was explained to members of the community at all sites before study enrollment; this was achieved through meetings with community leaders and community groups. Informed written consent is obtained for the home visits for pregnancy and birth, enrollment and treatment, as well as for the follow-up visit of the nonenrolled infants. Safety of young infants enrolled in this trial was ensured by close monitoring and follow-up. Health workers were trained to facilitate referral through counseling using integrated management of childhood illness guidance for assisting referral. Only babies whose families refused referral and were willing to document this by witnessed signature/thumbprint were enrolled.
Monitoring of Potential Adverse Events
The families are asked to contact the CHW/CHEW if any adverse events occur (as defined in the study protocol). In the case of an adverse event, CHWs contact the supervising CHW and CHEWs call the outcome assessment nurse, who then confirms and documents the adverse event and conveys the information to the community coordinator/supervisor. All serious adverse events (including death, unable to pass urine for >12 hours, anaphylactic reaction, severe dehydration due to diarrhea, disseminated or severe rash) are reported to WHO within 48 hours of occurrence. This information is also provided on a regular basis to DSMB, institutional review boards and the WHO Ethical Review Committee.
Rescue Therapy
Should the condition of the infant worsen and he/she requires further medical care, the infant may be given rescue therapy as determined by the study protocol. This includes intramuscular ceftriaxone for 7 days if the families still refuse referral to a higher level of care.
Data Safety Monitoring Board
The DSMB is responsible for monitoring and assessing the safety of the trial and consists of an epidemiologist, a statistician and a clinician scientist/researcher from each of the 3 countries. The DSMB convenes at least once a year in a face-to-face meeting, which consists of both an open and a closed session. Two interim analyses will be conducted, first when one-third of participants and second when two-thirds of the participants have been enrolled and treated. The interim analysis is conducted on a blinded basis.
The DSMB recommended that the trial be stopped or modified if an interim analysis of the safety endpoints of neonatal deaths and/or serious adverse events demonstrated a difference between treatment and control of at least 2 standard errors (P = 0.01) for effectiveness or futility. Furthermore, termination or modification may be recommended for any other perceived safety concern based on clinical judgment, including but not limited to a higher than anticipated rate for any component of the primary endpoint or unexpected serious adverse events. For this purpose, the serious adverse event forms are sent to the DSMB on the quarterly basis.
Quality Assurance
Training
Training courses were held for master trainers from all sites; these master trainers then trained local study personnel. Training covered the following courses: WHO/UNICEF Home Care for Newborns, WHO/UNICEF Young Infant integrated management of childhood illness (for study nurses) and a study-specific procedures course. All CHWs/CHEWs and enrollment, treatment and outcome assessment nurses employed by the study were trained at their respective sites by the master trainers. All supervisors and site coordinators were also trained in all courses. Further details of training are given in the accompanying methods paper on severe infection.15
Standardization, Quality Assurance of Training and Quality Control
Before starting study enrollment, standardization exercises are conducted on clinical and other study procedures for the appropriate cadres of workers, including CHW/CHEWs, enrollment and treatment nurses, independent outcome assessors, supervisors and study coordinators. Standardization exercises are repeated every 6 months over the course of the study. Data-based monitoring is also carried out with the help of the DMC in London and the WHO coordination team in Geneva. Further details on quality assurance are given in the accompanying articles.15,25
Supervision
Supervisors make accompanied and unaccompanied visits regularly to oversee field activities. Investigators made random visits to check quality.
Site Monitoring
All sites prepare and submit monthly progress reports, which are reviewed by the WHO coordination team on a monthly basis. Regular conference calls are held to review progress. WHO monitors conduct site visits twice a year to review progress in the field. Further details on site monitoring are given in the accompanying methods paper.15
Coordination Mechanisms
The WHO Department of Maternal, Newborn, Child and Adolescent Health is coordinating this study. Technical advice is provided by a TAG, which includes all principal investigators, the WHO coordination team and external experts. The TAG convenes at least once a year in a face-to-face meeting. LSHTM assists in quality control through database monitoring and preparation of reports for the DSMB, the TAG and the WHO study coordination team.
Timelines
The enrollment of the study participants started in April 2011 at all study sites and is likely to be complete by May 2013.
DISCUSSION
The current WHO algorithm for identification of PSBI in young infants includes a range of signs from milder signs such as fast breathing to very serious signs like convulsions and no movement on stimulation. WHO recommends that sick infants with any of these signs be referred for hospitalization and treated with up to 50 injections with a penicillin (benzyl penicillin or ampicillin) plus gentamicin for at least 10 days. This is not always feasible for a variety of reasons, including families’ lack of access to appropriate health facilities and the cost of such treatment. Young infants with fast breathing are at lower risk of serious adverse events compared with sick young infants with other more serious signs.10,11 Evidence also suggests that fast breathing in young infants can be treated with oral antibiotics in the community.13 Moreover, the safety, feasibility and cost of managing severe neonatal infections in community settings to address high neonatal mortality have been identified as a high research priority.26 If this study demonstrates that young infants with fast breathing can be treated safely and effectively with oral amoxicillin at home, it would greatly benefit both the families and health system and would facilitate increased coverage of treatment.
Blinding of therapy was not possible in this trial because of the differences in delivery of the 2 experimental regimens. Although the assessment of treatment failure is to some degree subjective, based on the presence or absence of clinical signs, an independent outcome assessor who is blinded to the treatment regimen assesses the study outcomes.
The ongoing trial described in this article is a multicenter, multicountry study involving 5 sites. The primary health care system is operational in all study areas, but referral systems are weak or unavailable in most of the study areas. This study is the largest community-based study evaluating a simplified antibiotic regimen for fast breathing in neonates and young infants in Africa and will provide important information to help formulate local and global policies to better manage PSBI in young infants.
ACKNOWLEDGMENTS
The following other members of the study group mentioned below have contributed to the study and we therefore acknowledge their substantial contributions: Democratic Republic of Congo: Serge Ngaima, Justin Ga’do, Daniel Ishoso, Kalonji Michel, John Otomba; Kenya: David Muyodi, Millsort Robins, Judith Sitti, Robin Achoki, Evelyn Etemesi, Lydia Chebet, Enock Nyambane, Sylvia Nyabera, Moses Sitati, Edwi Kirwa, Peter Nandikove, Prisca Mosol, Francisca Lagat; Ibadan, Nigeria: Akintunde Sowunmi, Elijah Afolabi Bamgboye, Ayotunde Olajide, Seye Idris, Olumide Ovigwe, Nathanael Afolabi, Joseph Aderibigbe, Bukola Adeniji, Ronke Egunjobi and Olaide Olakehinde; Ile-Ife, Nigeria: Chineme Henry Anyabolu, Olapeju Esimai, Olufolake Akano, Christiana Olufunke Olagoke, Tosin Ogunsola, Aminat Subair, Jumoke Awoyemi and Olusanjo Oyedokun; Zaria, Nigeria: Clara L. Ejembi.
Footnotes
Accepted for publication late April 2013.
AFRINEST GROUP: Antoinette Tshefu, MD, MPH, PhD,* Adrien Lokangaka, MB BS,* Cyril Engmann, MD, FAAP,†‡ Fabian Esamai, MB ChB, MMed, MPH, PhD,§ Peter Gisore, MB ChB,§ Adejumoke Idowu Ayede, MB BS, MSc, FMCPaed,¶ Adegoke Gbadegesin Falade, MB BS, FMCPaed, MD, FRCP (Edin),¶ Ebunoluwa A. Adejuyigbe, MB ChB, FMCPaed,‖ Chineme Henry Anyabolu, MB BS, FWACP,‖ Robinson D. Wammanda, MB BS, FWACP Paed,‖ William N. Ogala, MB BS, FMCPaed, FWACPaed,** Lu Gram, MSc, MPhil,†† Simon Cousens, MA, DipMathStat,†† Rajiv Bahl, MB BS, MD, PhD,‡‡ Nigel Rollins, MB BS, PhD,‡‡ and Sachiyo Yoshida, MPH,‡‡ Shamim Ahmed Qazi, MB BS, DCH, MSc, MD‡‡.
From the *Department of Community Health, Kinshasa School of Public Health, Kinshasa, Demographic Republic of Congo; †Departments of Pediatrics and Maternal Child Health, Schools of Medicine and Public Health, University of North Carolina, Chapel Hill, North Carolina; ‡Bill and Melinda Gates Foundation, Seattle, Washington; §Department of Child Health and Paediatrics, School of Medicine, Moi University, Eldoret, Kenya; ¶Department of Pediatrics, College of Medicine, University of Ibadan, Ibadan, Nigeria; ‖Department of Paediatrics and Child Health, Obafemi Awolowo University, Ile-Ife, Nigeria; **Department of Paediatrics, Ahmadu Bello University Teaching Hospital, Ahmadu Bello University, Zaria, Nigeria; ††Department of Infectious Disease Epidemiology, Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, United Kingdom; and ‡‡Department of Maternal, Newborn, Child and Adolescent Health, World Health Organization, Geneva, Switzerland.
The trial registration number was Australian New Zealand Clinical Trials Registry (ANZCTR) ACTRN12610000286044.
The views and opinions expressed in this article are those of the authors and not necessarily the views and opinions of the World Health Organization.
The study proposal was developed by all the authors of the article. Site authors along with the study group from each site are implementing the study. WHO staff is providing technical support, monitoring and coordination for the trials. The article was prepared jointly by all authors during a workshop and has the final approval of all authors.
The study is funded by a grant from the Bill and Melinda Gates Foundation to the World Health Organization. S.A.Q., R.B., N.R. and S.Y. are staff members of the World Health Organization. The authors have no other funding or conflicts of interest to disclose.
Address for correspondence: Shamim Qazi, MB BS, DCH, MD Paediatrics, Department of Maternal, Newborn, Child and Adolescent Health, World Health Organization, 20 Avenue Appia, Geneva 27, 1211, Switzerland. E-mail: qazis@who.int.
Copyright © 2013 by Maharaj Kishan Bhan. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License, where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially.
REFERENCES
- 1.Liu L, Johnson HL, Cousens S, et al. Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization and UNICEF. Integrated Management of Childhood Illness (IMCI) Chart Booklet. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 3.World Health Organization. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Illnesses With Limited Resources. 2nd edition. Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 4.Darmstadt GL, Baqui AH, Choi Y, et al. Bangladesh Projahnmo-2 (Mirzapur) Study Group. Validation of community health workers’ assessment of neonatal illness in rural Bangladesh. Bull World Health Organ. 2009;87:12–19. doi: 10.2471/BLT.07.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baqui AH, El-Arifeen S, Darmstadt GL, et al. Projahnmo Study Group. Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: A cluster-randomised controlled trial. Lancet. 2008;371:1936–1944. doi: 10.1016/S0140-6736(08)60835-1. [DOI] [PubMed] [Google Scholar]
- 6.Bhandari N, Bahl R, Bhatnagar V, et al. Treating sick young infants in urban slum setting [letter]. Lancet. 1996;347:1774–1775. [PubMed] [Google Scholar]
- 7.Zaidi AK, Tikmani SS, Warraich HJ, et al. Community-based treatment of serious bacterial infections in newborns and young infants: A randomized controlled trial assessing three antibiotic regimens. Pediatr Infect Dis J. 2012;31:667–672. doi: 10.1097/INF.0b013e318256f86c. [DOI] [PubMed] [Google Scholar]
- 8.Young Infant Clinical Signs Study Group. Clinical signs that predict severe illness in children under age 2 months: A multicentre study. Lancet. 2008;371:135–142. doi: 10.1016/S0140-6736(08)60106-3. [DOI] [PubMed] [Google Scholar]
- 9.Reddy MH, Bang AT. How to identify neonates at risk of death in rural India: Clinical criteria for the risk approach. J Perinatol. 2005;25(suppl 1):S44–S50. doi: 10.1038/sj.jp.7211272. [DOI] [PubMed] [Google Scholar]
- 10.Bang AT, Bang RA, Reddy MH, et al. Simple clinical criteria to identify sepsis or pneumonia in neonates in the community needing treatment or referral. Pediatr Infect Dis J. 2005;24:335–341. doi: 10.1097/01.inf.0000157094.43609.17. [DOI] [PubMed] [Google Scholar]
- 11.Baqui AH, Arifeen SE, Williams EK, et al. Effectiveness of home-based management of newborn infections by community health workers in rural Bangladesh. Pediatr Infect Dis J. 2009;28:304–310. doi: 10.1097/INF.0b013e31819069e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bang AT, Bang RA, Morankar VP, et al. Pneumonia in neonates: Can it be managed in the community? Arch Dis Child. 1993;68(5 Spec No):550–556. doi: 10.1136/adc.68.5_spec_no.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sazawal S, Black RE Pneumonia Case Management Trials Group. Effect of pneumonia case management on mortality in neonates, infants and preschool children: A meta-analysis of community-based trials. Lancet Infect Dis. 2003;3:547–556. doi: 10.1016/s1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- 14.Theodoratou E, Al-Jilaihawi S, Woodward F, et al. The effect of case management on childhood pneumonia mortality in developing countries. Int J Epidemiol. 2010;39(suppl 1):i155–i171. doi: 10.1093/ije/dyq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AFRINEST (AFRIcan NEonatal Sepsis Trial) Group. Simplified regimens for management of neonates and young infants with severe infection in situations when hospital admission is not possible: Study protocol for a randomized, open-label equivalence trial. Pediatr Infect Dis J. 2013;32(suppl):S26–S32. doi: 10.1097/INF.0b013e31829ff7d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaidi AKM, Baqui AH, Qazi SA, et al. Scientific rationale for study design of community-based simplified antibiotic therapy trials in newborns and young infants with clinically diagnosed severe infections in South Asia and sub-Saharan Africa. Pediatr Infect Dis J. 2013;32(suppl):S7–S11. doi: 10.1097/INF.0b013e31829ff5fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabra SK, Lodha R, Pandey RM. Antibiotics for community-acquired pneumonia in children. Cochrane Database Syst Rev. 2010:CD004874. doi: 10.1002/14651858.CD004874.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Grant GB, Campbell H, Dowell SF, et al. World Health Organization Department of Child and Adolescent Health and Development. Recommendations for treatment of childhood non-severe pneumonia. Lancet Infect Dis. 2009;9:185–196. doi: 10.1016/S1473-3099(09)70044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atkinson M, Lakhanpaul M, Smyth A, et al. A multicentre randomised controlled equivalence trial comparing oral amoxicillin and intravenous benzyl penicillin for community acquired pneumonia in children PIVOT Trial. Thorax. 2007;62:1102–1106. doi: 10.1136/thx.2006.074906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Addo-Yobo E, Chisaka N, Hassan M, et al. Oral amoxicillin versus injectable penicillin for severe pneumonia in children aged 3 to 59 months: A randomised multicentre equivalency study. Lancet. 2004;364:1141–1148. doi: 10.1016/S0140-6736(04)17100-6. [DOI] [PubMed] [Google Scholar]
- 21.Hazir T, Fox LM, Nisar YB, et al. New Outpatient Short-Course Home Oral Therapy for Severe Pneumonia Study Group. Ambulatory short-course high-dose oral amoxicillin for treatment of severe pneumonia in children: A randomised equivalency trial. Lancet. 2008;371:49–56. doi: 10.1016/S0140-6736(08)60071-9. [DOI] [PubMed] [Google Scholar]
- 22.Addo-Yobo E, Anh DD, El-Sayed HF, et al. Multicenter Amoxicillin Severe pneumonia Study (MASS) Group. Outpatient treatment of children with severe pneumonia with oral amoxicillin in four countries: The MASS study. Trop Med Int Health. 2011;16:995–1006. doi: 10.1111/j.1365-3156.2011.02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott JA, Brooks WA, Peiris JS, et al. Pneumonia research to reduce childhood mortality in the developing world. J Clin Invest. 2008;118:1291–1300. doi: 10.1172/JCI33947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bang AT, Bang RA, Stoll BJ, et al. Is home-based diagnosis and treatment of neonatal sepsis feasible and effective? Seven years of intervention in the Gadchiroli field trial (1996 to 2003). J Perinatol. 2005;25(suppl 1):S62–S71. doi: 10.1038/sj.jp.7211273. [DOI] [PubMed] [Google Scholar]
- 25.Wall SN, Mazzeo CI, Adejuyigbe EA, et al. Ensuring quality in the AFRINEST and SATT trials. Pediatr Infect Dis J. 2013;32(suppl):S39–S45. doi: 10.1097/INF.0b013e31829ff801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahl R, Martines J, Ali N, et al. Research priorities to reduce global mortality from newborn infections by 2015. Pediatr Infect Dis J. 2009;28(1 suppl):S43–S48. doi: 10.1097/INF.0b013e31819588d7. [DOI] [PubMed] [Google Scholar]


