Abstract
Late-life depression (LLD) and amnestic mild cognitive impairment (aMCI) are associated with medial temporal lobe structural abnormalities. However, the hippocampal functional connectivity (HFC) similarities and differences related to these syndromes when they occur alone or coexist are unclear. Resting-state functional connectivity MRI (R-fMRI) technique was used to measure left and right HFC in 72 elderly participants (LLD [n = 18], aMCI [n = 17], LLD with comorbid aMCI [n = 12], and healthy controls [n = 25]). The main and interactive relationships of LLD and aMCI on the HFC networks were determined, after controlling for age, gender, education and gray matter volumes. The effects of depressive symptoms and episodic memory deficits on the hippocampal functional connections also were assessed. While increased and decreased left and right HFC with several cortical and subcortical structures involved in mood regulation were related to LLD, aMCI was associated with globally diminished connectivity. Significant LLD–aMCI interactions on the right HFC networks were seen in the brain regions critical for emotion processing and higher-order cognitive functions. In the interactive brain regions, LLD and aMCI were associated with diminished hippocampal functional connections, whereas the comorbid group demonstrated enhanced connectivity. Main and interactive effects of depressive symptoms and episodic memory performance were also associated with bilateral HFC network abnormalities. In conclusion, these findings indicate that discrete hippocampal functional network abnormalities are associated with LLD and aMCI when they occur alone. However, when these conditions coexist, more pronounced vulnerabilities of the hippocampal networks occur, which may be a marker of disease severity and impending cognitive decline. By utilizing R-fMRI technique, this study provides novel insights into the neural mechanisms underlying LLD and aMCI in the functional network level.
Keywords: Depression, Mild cognitive impairment, Hippocampus, Functional connectivity, MRI, Episodic memory, Depressive symptoms, Elderly
Highlights
-
•
Effects of late-life depression and MCI on hippocampal memory network using R-fMRI
-
•
Distinct hippocampal functional network correlates in late-life depression and MCI.
-
•
Late-life depression and MCI interact on hippocampal functional network level.
-
•
Hippocampal functional network correlates with neuropsychological measures.
-
•
Novel insights into functional network vulnerability underlying depression and MCI
1. Introduction
Late-life depression (LLD) and amnestic mild cognitive impairment (aMCI) are highly prevalent neuropsychiatric syndromes that often coexist in the elderly (Steffens et al., 2006). LLD is associated with poorer treatment response and outcomes of comorbid medical disorders, increased mortality risk, caregiver burden and functional disability (Alexopoulos, 2005). LLD and aMCI, alone or in combination, also increase the risk of developing Alzheimer's disease (AD) (Gauthier et al., 2006; Modrego and Ferrandez, 2004; Ownby et al., 2006). Although LLD and aMCI are considered distinct clinical phenotypes, it is unclear if shared or distinct pathogenic mechanisms are involved in these disorders when they occur alone versus coexists. Neuroimaging investigations have provided unique insights into the hippocampal involvement in LLD and aMCI. The hippocampus is central to the episodic memory formation and has been the focus of AD research for several decades (Dickerson and Eichenbaum, 2010). This brain region is also vital to the dorsal-cognitive system of the mood regulation circuitry (MRC) and is implicated in the neuroanatomical model of LLD (Alexopoulos, 2005). Hippocampal structural abnormalities were evident in individuals with LLD and aMCI, and those with smaller volumes may be at a greater risk for subsequent cognitive decline (Benjamin and Steffens, 2011; Leung et al., 2010; Sexton et al., 2013; Shi et al., 2009; Steffens et al., 2011a).
Positron emission tomography and functional magnetic resonance imaging (fMRI) methods have unraveled the functional neuroanatomy of LLD and aMCI. The frontostriatal dysfunction commonly described in LLD is persistent and may only partially improve with antidepressant treatment (Aizenstein et al., 2005, 2009). However, the extent of MTL-based neural circuit abnormalities associated with LLD remains unclear. In contrast, altered hippocampal activation to various memory paradigms has been demonstrated in aMCI (Dickerson and Sperling, 2009; Miller et al., 2008). These fMRI experiments have clarified task-dependent MTL activations or deactivations in LLD and aMCI, but important challenges remain. First, differences in task performance between cognitively healthy and patients with LLD and/or aMCI may complicate data interpretation. Second, neuroimaging investigations have failed to examine the mechanistic similarities and differences between LLD and aMCI, when these conditions occur alone or in combination. Third, while traditional fMRI studies provide critical information into the memory task-dependent differences of specific brain regions, they offer limited conclusions as to how different functionally related structures are interconnected in healthy and diseased states. This is pertinent because LLD and aMCI have been hypothesized as disconnection syndromes affecting the distributed brain networks (Dickerson and Sperling, 2009; Weisenbach et al., 2012).
The resting-state functional connectivity MRI (R-fMRI) technique is based on the observation that temporal interregional correlations of spontaneous low-frequency blood oxygenation level-dependent fluctuations exist between functionally coupled but spatially segregated brain regions in the absence of a task (Biswal et al., 1995, 2010; Fox and Raichle, 2007). The brain's organization into multiple, functionally connected networks that subserve various behavioral functions have been identified using the R-fMRI method in normal and pathological conditions, including depression, and preclinical and clinical AD (Agosta et al., 2012; Alexopoulos et al., 2012; Buckner et al., 2009; Chen et al., 2011; Goveas et al., 2011a,b; Greicius et al., 2007; Kenny et al., 2010; Menon and Uddin, 2010; Seeley et al., 2007; Sheline et al., 2010b; Steffens et al., 2011b). The hippocampus is central to the episodic memory network (EMN) and is densely interconnected with functionally related limbic and neocortical regions (Dickerson and Eichenbaum, 2010). The hippocampus is also critical for the brain's interactive and integrative potential to bring together emotion and memory functions (LaBar and Cabeza, 2006). A left–right asymmetry in the hippocampal volumetric and functional connectivity alterations have been reported in LLD, aMCI and/or early AD (Shi et al., 2009; Steffens et al., 2011a; Wang et al., 2006). Greater decreases in hippocampal subregional functional connectivity were associated with episodic memory declines in persons with aMCI (Bai et al., 2011). In contrast, others have demonstrated increased and decreased MTL connectivity with aMCI (Das et al., 2013; Wang et al., 2011). Furthermore, predominantly diminished functional connectivity in the hippocampal networks has been demonstrated in AD (Allen et al., 2007; Wang et al., 2006). Regardless, how the disruption in the episodic memory system unfolds in LLD and aMCI, when these disorders appear independently or co-occur remains to be understood.
The primary objective of this study was to investigate the main and interactive effects of LLD and aMCI on the left and right hippocampal functional connectivity in the elderly. We hypothesized that distinct hippocampal functional connectivity (HFC) abnormalities will be seen in LLD and aMCI groups, whereas significant interactive effects will be found between LLD and aMCI involving regions critical for emotional regulation and multidomain cognitive functions. Furthermore, we hypothesize that the comorbid group will predominantly drive the HFC disruptions in the interactive regions. As a secondary objective, we also determined the neural correlates of the main and interactive effects of depressive symptoms and episodic memory performance on the HFC networks across all subjects.
2. Material and methods
2.1. Participants
Seventy-two subjects aged 60 and older (cognitively healthy, LLD [n = 18]; aMCI alone [n = 17]; LLD with aMCI [n = 12]; and cognitively normal, nondepressed controls [n = 25]) participated in this study (Xie et al., 2012c). All patients diagnosed as having clinically significant depression and/or aMCI were recruited from the Geriatric Psychiatry and Memory Disorders Clinics at the Medical College of Wisconsin. Control subjects were recruited from the community through advertisements. All participants provided written informed consent according to the institution's guidelines for human subject protection.
Study participants received detailed clinical and neuropsychiatric assessments. The core neuropsychological battery administered to all participants included the (1) Mini-Mental State Examination (MMSE) (all subjects had to score ≥ 24); (2) Mattis Dementia Rating Scale-2 (MDRS-2) (age- and education-corrected MOANS-scaled score of ≥ 5) (Lucas et al., 1998); (3) education-adjusted Logical Memory II Delayed paragraph recall (LMII-DR) subscale from the Wechsler Memory Scale-Revised (Wechsler, 1987); (4) Physical Self Maintenance Scale/Instrumental Activities of Daily Living (PSMS/IADL) (Lawton and Brody, 1969); (5) 30-item Yesavage Geriatric Depression Scale (GDS) (Yesavage et al., 1982); (6) Diagnostic assessment for Axis 1 disorders including the depression module from the Structured Clinical Interview for DSM IV (First et al., 2002); and (7) Hamilton Anxiety Scale (HAM-A). All participants scored ≤ 4 on the modified Hachinski ischemic scale (HIS). GDS was repeated again on the day of the imaging scan visit. Clinical assessment findings were reviewed during the weekly multidisciplinary consensus conferences.
Thirty participants met criteria for clinically significant depression according to the Structured Clinical Interview for DSM-IV TR Axis 1 disorders, research version, non-patient edition [SCID]. Eighteen participants with clinically significant depression were included in the cognitively normal LLD group (major depression: n = 17, and minor depression: n = 1). All participants had a GDS score of 10 or above, MMSE ≥ 26, PSMS ≤ 6 and IADL ≤ 9, score above the education-adjusted cutoff on the LMII-DR (Delayed recall score > 8 for 16 or more years of education or score > 4 for 8–15 years of education).
All participants who also met aMCI criteria with significant depression (n = 12) were included in the comorbid LLD and aMCI group (major depression: n = 11, dysthymic disorder: n = 1). Antidepressant doses in both depressed groups had to be at stable doses for at least two weeks before study enrollment and until completion of the study procedures.
Amnestic mild cognitive impairment (aMCI) was operationally defined according to the established criteria (Winblad et al., 2004): (1) subjective report of cognitive decline; (2) objective cognitive impairment that includes scoring 1.5 SD below on memory measures; (3) intact ADLs and relatively preserved IADLs; and (4) no dementia. For meeting criteria for objective cognitive impairment, participants had to score below the education-adjusted cutoff on the LMII-DR (i.e., ≤ 8 for 16 or more years of education, and ≤ 4 for 8–15 years of education), and score below 1.5 SD below the mean on one or more subscales (one of the impairments had to be memory) of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph, 1998), Behavioral Dyscontrol scale or the Boston Naming Test. Subjects who met the aMCI criteria and did not have clinically significant depression (n = 17) were included in the nondepressed aMCI group.
The control group consisted of participants who did not meet criteria for cognitive or Axis 1 psychiatric disorders and were not on psychoactive medications (n = 25).
Exclusion criteria included past or current history of concurrent Axis 1 psychiatric disorders, such as psychotic or bipolar disorders; alcohol or substance abuse/dependence during the past five years; active suicidality; MMSE scores < 24; history of neurological diseases, including Parkinson's disease, dementia, multiple sclerosis, seizures, or stroke; head injury with loss of consciousness; MRI contraindications and unstable medical conditions.
2.2. MRI acquisition
Imaging was performed using a whole-body 3T Signa GE scanner (Waukesha, WI) with a standard transmit-receive head coil. During the resting-state acquisitions, no cognitive tasks were performed and the participants were instructed to close their eyes, relax and stay awake. Sagittal R-fMRI data sets of the whole brain were obtained in 8 min with a single-shot gradient echo–echo planar imaging pulse sequence. The R-fMRI parameters were: TE = 25 ms, TR = 2000 ms, flip angle of 90°; number of slices = 36; slice thickness = 4 mm, matrix size = 64 × 64 and field of view (FOV) = 240 × 240 mm. High-resolution 3D spoiled gradient-recalled echo (SPGR) axial images were acquired for anatomical reference. The parameters were: TE/TR/TI of 4/10/450 ms, flip angle of 12°, number of slices = 144, slice thickness = 1 mm, matrix size = 256 × 192 and FOV = 240 × 240 mm.
2.3. Structural and functional connectivity MRI data processing
2.3.1. R-fMRI preprocessing
R-fMRI data analyses were carried out by using AFNI software (http://afni.nimh.nih.gov/afni) and MATLAB programs (The MathWorks Inc., Natick, MA). The preprocessing steps are identical to the one followed in our previous publications (Goveas et al., 2011a,b; Li et al., 2012; Xie et al., 2012a,b). The spikes in the raw R-fMRI data were removed (3dDespike); motion correction was performed by volume registration on the R-fMRI data (3dvolreg); and detrending was carried out to remove Legendre polynomials (3dDetrend). Possible contamination from the signals in white matter, cerebrospinal fluid, the six-motion vectors, physical noise (cardiac and respiratory signals) and global signal were regressed out from each voxel time series (Birn et al., 2008; Cox, 1996; Fox et al., 2009; Glover et al., 2000; Orfanidis, 1996; Rombouts et al., 2003). A band-pass filter was applied to isolate spontaneous low-frequency fluctuations within the range of 0.015 Hz and 0.1.
2.3.2. Hippocampal functional connectivity analysis
The left and the right hippocampus regions of interest (ROI) were manually drawn on the coronal slices, with reference to the sagittal and axial slices, and on the structural images of individual subject, as described previously (Rombouts et al., 2003), using AFNI software, after being blinded to the participants' demographics and clinical characteristics. The various processing steps were described previously (Goveas et al., 2011a,b, 2013; Xie et al., 2012b). Briefly, the time courses of these hippocampal voxels were cross-correlated to the time courses of all the voxels in the brain mask, and the Pearson correlation coefficients (r) were obtained, The cross-correlation coefficients (r) were converted to m scores according to Fisher's z transformation for normal distribution [m = 0.5ln(1 + r) / (1 − r)] (Zar, 1996). These voxelwise m values were spatially transformed to the Talairach template coordinates (adwarp), resampled to 2-mm isotropic voxels, and smoothed with a Gaussian kernel (6-mm full-width half-maximum) using AFNI software (3dmerge). The end result was the left (L) and the right (R) HFC network maps for each participant.
2.3.3. Structural image processing
Optimized voxel-based morphometry (VBM) analysis was performed, using the VBM8 toolbox in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). The individual T1-weighted images for all subjects were segmented into gray matter (GM), white matter (WM) and cerebral spinal fluid (CSF) and the summation of them comprised the intracranial volume (ICV). The segmented GM volumes were then normalized, smoothed at 6-mm full-width half-maximum, and resampled to 2-mm isotropic voxels for further analyses. The voxelwise GM volume was controlled as a covariate in the statistical analyses.
2.4. Statistical analysis
2.4.1. Subject characteristics
Group comparisons for demographic information (age and education), except gender (χ2 test) were compared using analysis of variance (ANOVA) (significance set at p < 0.05 to avoid false negative rate). Neuropsychological measurements were compared by ANCOVA, after controlling for age, gender and education (significance threshold at p < 0.0045, Bonferroni corrected) (SPSS 18.0; SPSS Inc., Chicago, IL). The sources of the differences between the means of the four groups were examined by post-hoc Fisher's least significant difference test for demographics; and the post-hoc Bonferroni-corrected tests were performed when appropriate for neuropsychological measures.
2.4.2. Group-level HFC analysis
Individual HFC maps for each subject were analyzed with a random-effects one-sample t test to identify voxels, showing a significant positive or negative correlation with the seed time series, and the patterns of left and right HFC network for four groups were separately obtained after controlling the effects of age, gender, education and voxelwise GM volume (3dttest++, p < 0.01, corrected with AlphaSim, cluster size > 1042 mm3). To examine the group difference of the LHFC and RHFC networks across all subjects, voxelwise 2 (depression) × 2 (cognitive impairment) ANCOVA was separately performed after controlling for age, gender, education and GM volume (3dRegAna, AFNI) (p < 0.05, corrected with AlphaSim, cluster size > 4048 mm3).
2.4.3. Behavioral significance
To investigate the behavioral significance of the left and right HFC networks, multiple linear regression analyses were employed (3dRegAna, AFNI) between the individual HFC maps and the GDS and LMII-DR scores across the four groups, using the following equation:
where mi is the m value of ith voxel across group subjects and β0i is the intercept of straight line fit of the ith group in the model. β1, β2, and β3 are the effects of GDS scores, memory scores (LMII-DR), and interaction of GDS and memory scores on the functional connectivity strength of ith voxel within HFC network, respectively. β4, β5, β6, and β7 are the effects of age, gender, education, and GM volume as covariates of no interest in the above model. G is the group variable in the model; specifically, β8G0 = 1 if not LLD and not aMCI, 0 otherwise; β9G1 = 1 if LLD and not aMCI, 0 otherwise; β10G2 = 1 if aMCI and not LLD, 0 otherwise; β11G3 = 1 if LLD and aMCI, 0 otherwise. The voxelwise multiple linear regression map was also generated to examine the main and interactive effects of GDS and LMII-DR on the bilateral HFC networks (AlphaSim correction, p < 0.05, cluster size > 4048 mm3).
3. Results
3.1. Demographic and neuropsychological characteristics
Significant age and neuropsychiatric measure differences were found; post-hoc analyses revealed the source of differences (Table 1). One-sample patterns of the left and right HFC networks in the four groups are presented in Figs. S1 and S2.
Table 1.
Demographic and neuropsychiatric characteristics.
| CN (n = 25) |
Dep (n = 18) |
aMCI (n = 17) |
aMCI/Dep (n = 12) |
p-Value |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age (year) | 74.28 | 8.25 | 68.61a | 6.81 | 75.12d | 6.62 | 68.33f | 13.87 | 0.043Ϯ |
| Education (years) | 15.32 | 2.87 | 14.61 | 2.57 | 13.47 | 2.07 | 14.25 | 2.99 | 0.175 |
| Gender (F/M) | 12/13 | 14/4 | 11/6 | 7/5 | 0.135 | ||||
| Neuropsychological measures | |||||||||
| MMSE | 28.92 | 1.22 | 28.06 | 1.21 | 27.29 | 1.83 | 26.92c | 1.93 | 0.001* |
| DRS-2 raw scores | |||||||||
| Attention | 36.56 | 0.51 | 36.39 | 0.70 | 35.71b | 0.77 | 36.00 | 1.21 | 0.004* |
| INIT/PERS | 36.32 | 1.38 | 36.22 | 2.10 | 34.88 | 3.62 | 32.50 | 5.55 | 0.005 |
| Construct | 6.00 | 0.00 | 5.94 | 0.24 | 5.94 | 0.24 | 5.83 | 0.58 | 0.436 |
| Conceptual | 37.76 | 1.33 | 37.61 | 1.24 | 35.88b | 2.45 | 35.41ce | 3.12 | 0.001* |
| Memory | 23.64 | 1.04 | 23.89 | 1.13 | 19.06bd | 2.68 | 19.50ce | 3.60 | < 0.001* |
| Total | 140.36 | 2.53 | 140.11 | 3.20 | 130.88bd | 5.49 | 129.67ce | 6.70 | < 0.001* |
| Recall scores | |||||||||
| IMMED | 14.36 | 3.73 | 14.17 | 3.96 | 8.41bd | 3.59 | 7.17ce | 3.76 | < 0.001* |
| DELAYED | 12.92 | 4.03 | 12.06 | 4.24 | 2.47bd | 3.30 | 3.25ce | 3.14 | < 0.001* |
| HAMA | 1.16 | 1.06 | 10.88a | 5.07 | 2.18d | 1.55 | 8.67cf | 4.19 | < 0.001* |
| GDS | 1.88 | 2.11 | 17.28a | 3.29 | 4.35d | 2.67 | 14.25cf | 6.43 | < 0.001* |
| Current antidepressants (%) | |||||||||
| No antidepressant | – | 2 (11.1) | 16 (94.1) | 2 (16.7) | |||||
| SSRI monotherapy | – | 2 (11.1) | 1 (5.9) | 2 (16.7) | |||||
| SNRI monotherapy | – | 4 (22.2) | – | 2 (16.7) | |||||
| Other | – | 2 (11.1) | – | 1 (8.3) | |||||
| Combination therapy | – | 8 (44.5) | – | 5 (41.6) | |||||
| Current cognitive enhancers (%) | |||||||||
| No cognitive enhancers | – | – | 8 (47.1) | 8 (66.6) | |||||
| ChEI monotherapy | – | – | 7 (41.1) | 2 (16.7) | |||||
| Memantine monotherapy | – | – | 1 (5.9) | 2 (16.7) | |||||
| Combination therapy | – | – | 1 (5.9) | – | |||||
Notes: In demographic information, age showed significant across the subject groups without corrections for multiple comparisons (Ϯ: p < 0.05). In addition, significant differences (*: p < 0.0045, Bonferroni correction for multiple comparison) were found in MMSE, DRS-2 raw scores (except for INIT/PERS and Construct), memory recalled scores (IMMED and Delayed) and emotional scores (HAMA and GDS) among four groups. a–f: post-hoc analysis further revealed the source of ANOVA difference (a: CN vs Dep; b: CN vs aMCI; c: CN vs aMCI/Dep; d: Dep vs aMCI; e: Dep vs aMCI/Dep; f: aMCI vs aMCI/Dep). Unless otherwise indicated, data are presented as mean ± SD. Abbreviation: CN, cognitive normal or controls; Dep, depression; aMCI, amnestic mild cognitive impairment; aMCI/Dep, amnestic mild cognitive impairment with depression; M, mean; SD, standard deviation; F/M: female/male; MMSE, mini-mental state examination; DRS-2: dementia rating scale-2; INIT/PERS: Initiation/Preservation; IMMED, immediate recall scores; DELAYED, delayed recall scores; HAMA, Hamilton anxiety scales scores; GDS, geriatric depression scale scores; SSRI: selective serotonin re-uptake inhibitors; SNRI: serotonin norepinephrine re-uptake inhibitors; ChEI: cholinesterase inhibitors. Other antidepressants include: bupropion, mirtazapine, and trazodone.
Fig. S1.
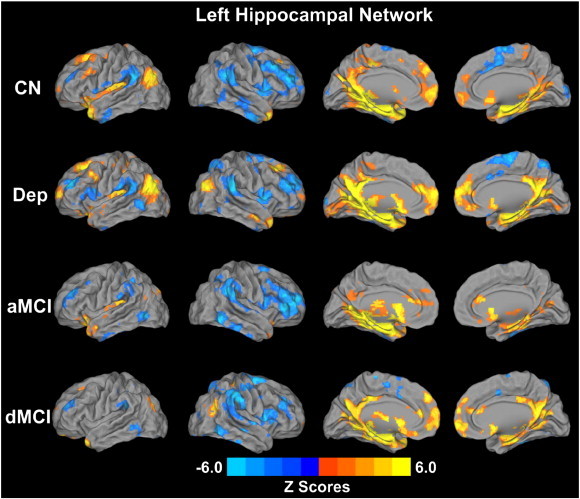
Whole-brain voxelwise pattern of the left hippocampal functional connectivity (LHFC) networks in the four group subjects (p < 0.01, AlphaSim correction). Bright color indicates positive connectivity and blue color indicates negative or anticorrelated connectivity. Color bar is presented with z scores. Abbreviation: CN, control; Dep, depression; aMCI, amnestic mild cognitive impairment; dMCI, depressed mild cognitive impairment.
Fig. S2.
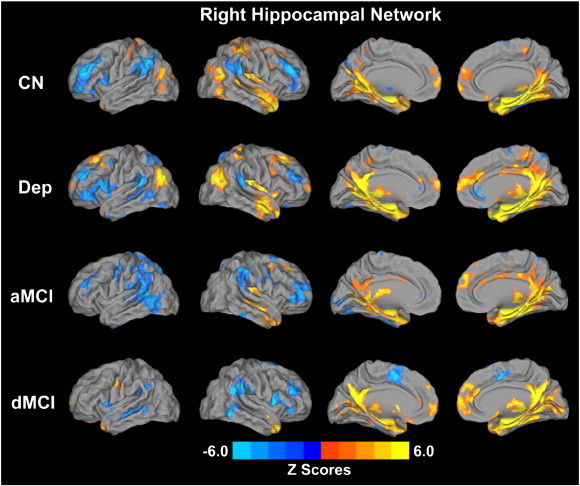
Whole-brain voxelwise pattern of the right hippocampal functional connectivity (RHFC) networks in the four subject groups (p < 0.01, AlphaSim correction). Bright color indicates positive connectivity and blue color indicates negative or anticorrelated connectivity. Color bar is presented with z scores. Abbreviation: CN, cognitively normal; Dep, depression; aMCI, amnestic mild cognitive impairment; dMCI, depressed mild cognitive impairment.
Significant age and neuropsychiatric measure differences were found; post-hoc analyses revealed the source of differences (Table 1). One-sample patterns of the left and right HFC networks in the four groups are presented in Figs. S1 and S2.
3.2. Neuroimaging data
3.2.1. Main effects of LLD and cognitive impairment on the HFC networks
3.2.1.1. Left HFC network
The depressed groups demonstrated increased left HFC in the bilateral PCC, and right DMPFC, and decreased anticorrelation (with a reversal of pattern to positive) in the right DLPFC, relative to the nondepressed groups. The mild cognitive impairment (CI) groups showed consistently decreased positive and negative HFC in the bilateral dorsolateral prefrontal cortex (DLPFC), dorsomedial prefrontal cortex (DMPFC), inferior parietal cortex (IPC), retrosplenial cortex (RSC); postcentral gyrus and posterior middle temporal gyrus (pMTG) on the left; and right dorsal anterior cingulate cortex (dACC) and superior parietal cortex (SPC). In bilateral DMPFC, and left postcentral gyrus, IPC and pMTG, there is a reversal of pattern from positive to negative correlation in the CI group, relative to non-CI group. Similarly, there is a reversal of negative to positive correlation pattern in the CI group in the right IPC, dACC and SPC compared to the non-CI subjects (Fig. 1).
Fig. 1.
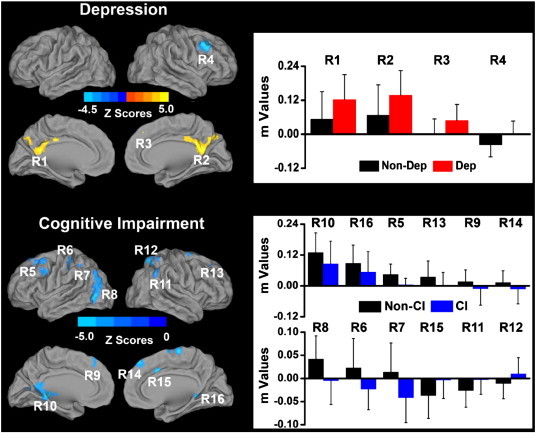
Main effects of depression and mild cognitive impairment on the left hippocampal functional connectivity (LHFC) networks across all subjects (p < 0.05, AlphaSim correction). Left: Brain regions with significant main effects of depression and cognitive impairment on the LHFC network. Bright color indicates increased connectivity and blue color indicates decreased connectivity. Color bar is presented with z scores. Right: Numerical representation of significant main effects of depression and mild cognitive impairment on the LHFC network (m is z value from the cross-correlation coefficient after Fisher's z transformed, same below). Abbreviations: Non-Dep: no depression; Dep: depression; Non-CI: no amnestic mild cognitive impairment; CI: amnestic mild cognitive impairment. R1: left posterior cingulate cortex/precuneus (LPCC/Pcu); R2: right posterior cingulate cortex/precuneus (RPCC/Pcu); R3: right dorsomedial prefrontal cortex (RDMPFC); R4: right dorsolateral prefrontal cortex (RDLPFC); R5: left dorsolateral prefrontal cortex (LDLPFC); R6: left postcentral gyrus; R7: left inferior parietal cortex (LIPC); R8: left posterior middle temporal gyrus (LpMTG); R9: left dorsomedial prefrontal cortex (LDMPFC); R10: left retrosplenial cortex (LRSC); R11: right inferior parietal cortex (RIPC); R12: right superior parietal cortex (RSPC); R13: right dorsolateral prefrontal cortex (RDLPFC); R14: right dorsomedial prefrontal cortex (RDMPFC); R15: right dorsal cingulate cortex (RdACC); R16: right retrosplenial cortex (RRSC). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.1.2. Right HFC network differences
The main effects of depression on the RHFC network included increased HFC in the bilateral thalamus, left hippocampus and right lentiform nucleus; and decreased anticorrelated hippocampal connectivity (with a reversal of pattern to positive correlation) in the bilateral middle occipital gyrus (MOG), and in the left DLPFC and dorsal striatum (caudate and putamen). The main effects of the CI (vs. the non-CI) groups on the RHFC network included consistently diminished positive and negative HFC in bilateral anterior temporal pole (aTP) and IPC; left ventrolateral prefrontal cortex (vlPFC), parahippocampal gyrus (PHG), and pMTG; and right inferior temporal cortex (ITC). The patterns are reversed in all but aTP in the CI groups, relative to non-CI subjects (Fig. 2).
Fig. 2.
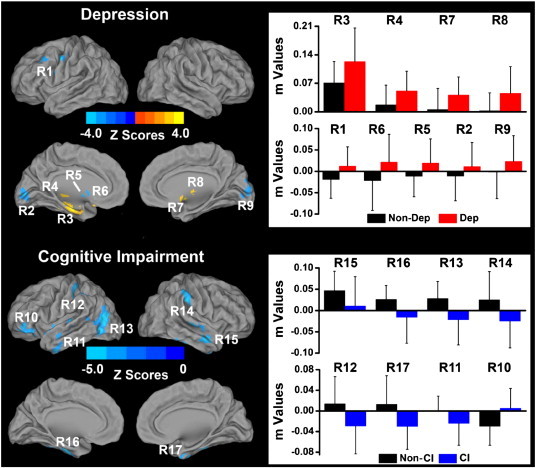
Main effects of depression and mild cognitive impairment on the right hippocampal functional connectivity (RHFC) networks across all subjects (p < 0.05, AlphaSim correction). Left: Brain regions with significant main effects of depression and mild cognitive impairment on the RHFC network. Bright color indicates increased connectivity and blue color indicates decreased connectivity. Color bar is presented with z scores. Right: Numerical representation of significant main effects of depression and mild cognitive impairment on the RHFC network. Abbreviations: Non-Dep: no depression; Dep: depression; Non-CI: no amnestic mild cognitive impairment; CI: amnestic mild cognitive impairment. R1: left dorsolateral prefrontal cortex (LDLPFC); R2: left middle occipital gyrus (LMOG); R3: left hippocampus; R4: left thalamus; R5: left putamen; R6: left caudate; R7: right lentiform nucleus; R8: right thalamus; R9: right middle occipital gyrus (RMOG); R10: left ventrolateral prefrontal cortex (LvlPFC); R11: left anterior temporal pole (LaTP); R12: left inferior parietal cortex (LIPC); R13: left posterior middle temporal gyrus (LpMTG); R14: right supramarginal gyrus/inferior parietal cortex; R15: right anterior temporal pole (RaTP); R16: left parahippocampal gyrus (LPHG); R17: right inferior temporal cortex (RITG). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.2. Interactive effects of LLD and cognitive impairment on the HFC networks
The interactive effects of LLD and CI were only identified on the RHFC network and included the ventromedial prefrontal cortex (vmPFC) and MOG bilaterally, left dACC and right DLPFC/dACC cluster (Fig. 3).
Fig. 3.
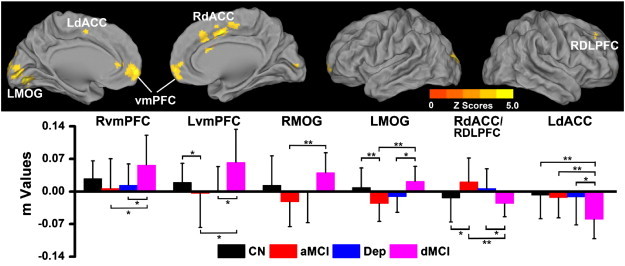
Interactive effects of late-life depression and amnestic mild cognitive impairment on the right hippocampal functional connectivity (RHFC) networks (p < 0.05, AlphaSim correction). Top: Brain regions with significant interactive effects between late-life depression and mild cognitive impairment on the RHFC network. Bottom: Numerical representation of significant interactive effects of depression and cognitive impairment on the RHFC network. *: p < 0.05; **: p < 0.01. Abbreviations: LMOG, left middle temporal gyrus; LdACC, left dorsal cingulate cortex; vmPFC, ventromedial prefrontal cortex; RdACC, right dorsal cingulate cortex; RMOG, right middle temporal gyrus; RDLPFC, right dorsolateral prefrontal cortex; RvmPFC, right vmPFC; LvmPFC, left vmPFC; CN, cognitively normal; aMCI, amnestic mild cognitive impairment; Dep, Late-life depression; dMCI, comorbid late-life depression and amnestic mild cognitive impairment.
3.2.3. The effects of depressive symptoms and memory deficits on the hippocampal networks
The GDS scores positively correlated with the left HFC network in the DMPFC and inferior temporal cortex (ITC) bilaterally; negatively correlated with the left HFC network in the hippocampus/PHG bilaterally, and left DLPFC. The GDS scores positively correlated with the right HFC in the bilateral DMPFC and ITC, right pMTG, and negatively correlated in the bilateral DLPFC, vmPFC, and hippocampus/PHG, left IFG, and right IPC and anterior insula/frontal operculum (aI/fO) (Fig. 4 top row).
Fig. 4.
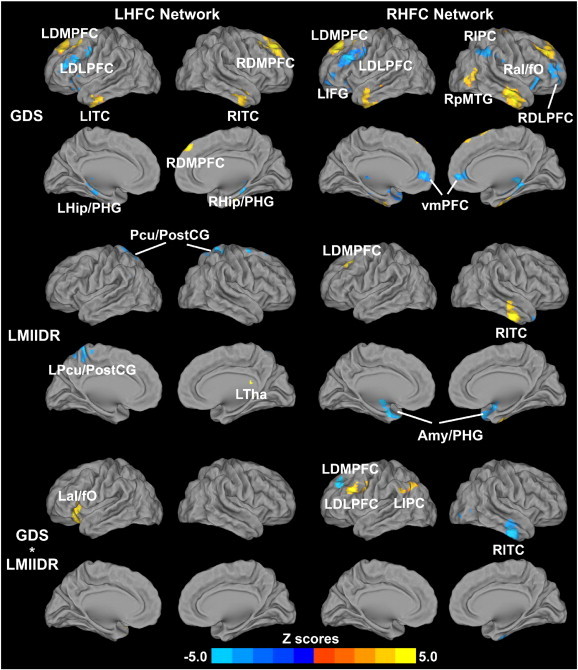
Behavioral significance of the bilateral hippocampal functional connectivity networks across all subjects (p < 0.05, AlphaSim correction). Top row: Main effects of the depressive symptoms on the bilateral HFC networks; Middle row: Main effects of the episodic memory scores (LMIIDR scores) on the bilateral HFC networks. Bright color indicates positive correlation and blue color indicates negative correlation; Bottom row: Interaction of depressive symptoms and memory function on the bilateral HFC networks. Bright color indicates that the interactive effects are positively correlated with bilateral HFC; blue color demonstrates that the interactive effects are negatively correlated with bilateral HFC. Color bar is presented with z scores.
Abbreviations: GDS, geriatric depression scale; LHFC, left hippocampal functional connectivity; RHFC, right hippocampal functional connectivity; LDLPFC, left dorsolateral prefrontal cortex; RDLPFC, right dorsolateral prefrontal cortex; LDMPFC, left dorsomedial prefrontal cortex; RDMPFC, right dorsomedial prefrontal cortex; LITC, left inferior temporal cortex; RITC, right inferior temporal cortex; LHip/PHG, left hippocampus/parahippocampal gyrus; RHip/PHG, right hippocampus/parahippocampal gyrus; LIPC, left inferior parietal cortex; RIPC, right inferior parietal cortex; LaI/fO, left anterior insula/frontal operculum; RaI/fO, right anterior insula/frontal operculum; LIFG, left inferior frontal gyrus; LTha, left thalamus; L Pcu/PCG, precuneus/postcentral gyrus; Pcu/PCG, precuneus/postcentral gyrus; vmPFC, ventromedial prefrontal cortex; Amy/PHG, amygdala/parahippocampal gyrus; RpMTG, right posterior middle temporal gyrus. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
LMII-DR scores positively correlated with the left HFC network in the left thalamus, and negatively correlated in the bilateral precuneus/posterior central gyrus (Pcu/PostCG). LMII-DR scores positively correlated with the RHFC network in the left DMPFC and right ITC and negatively correlated in the amygdala/PHG (Amy/PHG) (Fig. 4 middle row).
The GDS–LMII-DR interactions on the left HFC network were found in the left aI/fO, and in the right HFC network were demonstrated in DMPFC, DLPFC and IPC on the right ITC (Fig. 4 bottom row).
4. Discussion
This study demonstrates that the main and interactive effects of LLD and aMCI are present in the hippocampus-centered EMN in older adults free of dementia. Both increased and decreased HFC with several cortical and subcortical structures involved in emotion processing were related to LLD. aMCI was associated with globally diminished connectivity in cortical and MTL regions. Significant LLD and aMCI interactions on the right HFC networks were seen in regions critical for mood regulation and higher-order cognitive functions. We also demonstrated that main and interactive effects of depressive symptoms and episodic memory deficits on the HFC networks exist in the elderly, which is consistent with our prior observations.
4.1. Main effects of depression on the hippocampal functional brain networks
In the left hippocampal networks, we found increased functional connections with the default mode network (DMN) regions. These findings are consistent with the recent observations in LLD of increased cortical glucose metabolism and DMN functional connectivity (Alexopoulos et al., 2012; Andreescu et al., 2011; Smith et al., 2009). Similar functional connectivity patterns and hyperactivation in the DMN and MTL structures to an emotional regulation paradigm have been demonstrated in younger depressed adults (Sheline et al., 2009, 2010b). Sustained increased activation within the DMN and EMN structures has been related to greater ruminative thought patterns and severe depressive episodes in major depression.
While LLD was associated with increased right hippocampal network connections with the thalamus, basal ganglia and MTL, diminished hippocampal-DLPFC connectivity was seen. These regions are crucial components of the limbic-cortical-striato-pallido-thalamic circuits implicated in the neurobiological model of depression. The prolonged processing of negative emotions seen in depression is maintained by the impairments of the top-down cognitive control by the DLPFC over the subcortical and limbic structures. Individuals with depression show higher awareness to the negative emotional stimuli, which is first relayed to the thalamus, and subsequently transferred and interpreted by the MTL structures (Mayberg, 2003; Phillips et al., 2003). The increased hippocampal connections with the subcortical structures, and decreased HFC-DLPFC functional connectivity found here are consistent with this theory. Moreover, decreased DLPFC connectivity and increased subcortical connectivity are seen in LLD (Alexopoulos et al., 2012). We also identified diminished right hippocampal connections in the dorsal striatum with a reversal from negative (or anticorrelated) to positive connectivity values in the LLD groups (Fig. 2). The increased periventricular and basal ganglia white matter hyperintensities and decreased structural integrity of the adjacent tracts that is reported in LLD may have contributed to these findings (Aizenstein et al., 2011; Steffens et al., 2011b). On the contrary, increased dorsal striatal connectivity is reported in LLD, though it is unclear whether contamination from the nuisance variables (white matter, CSF, cardiac, respiratory and global signals) was regressed out in that study (Kenny et al., 2010). A few other possibilities may also explain the discrepant findings. The selection of different predefined ROIs and R-fMRI methods in LLD studies makes direct comparisons difficult. Also, several seed-based R-fMRI studies have limited the analyses to only positive connectivity differences, which may have failed to identify the shifts of connectivity values from negative to positive patterns. While some argue that the negative correlations in R-fMRI analyses are artificially induced by global signal removal, recent evidence has demonstrated a biological basis to the anticorrelated connectivity (Fox et al., 2009; Goveas et al., 2011b; Seeley et al., 2007; Sheline et al., 2010a; Xie et al., 2012b).
4.2. Main effects of cognitive impairment on hippocampal functional brain networks
Our findings of diminished HFC in the frontal, parietal, temporal and MTL regions in aMCI patients are consistent with the literature (Bai et al., 2011). The hippocampus is extensively interconnected with neocortical association areas and temporal lobe structures. These regions are essential for episodic memory, semantic processing, self-monitoring and related social cognitive functions, mood regulation and attentional switching, which are often impaired in aMCI. Previously, decreased hippocampal functional connections with similar brain regions have been reported in patients with aMCI and early AD, and MTL functional disruptions may be indicative of the incipient AD (Sorg et al., 2007; Wang et al., 2006, 2011). Also, the left and right HFC differences did not mirror each other, which are similar to the previous aMCI and early AD studies (Wang et al., 2006, 2011). On the contrary, we found no regions with increased functional connections to the hippocampus as previously reported in one study (Wang et al., 2011). Interestingly, baseline increases were followed by longitudinal decreases in the left and right HFC in aMCI patients, suggesting that the initially observed enhanced connections are a compensatory process (Wang et al., 2011). Moreover, while increased functional connectivity within the MTL subregions was seen in aMCI patients, the MTL connections with the nodes in the DMN and fronto-parietal cortical system were decreased in aMCI in another recent investigation, the latter findings are consistent with our data (Das et al., 2013). Additional cross-sectional and longitudinal studies are needed to gain in-depth understanding of the dynamic hippocampal functional connectivity alterations in the normal elderly and aMCI patients.
4.3. Interactive effects of depression and cognitive impairment on HFC networks
Significant LLD–aMCI interactions in the hippocampal functional network level were found in the default mode, cognitive control and salience network regions critical for emotion processing, episodic memory and executive function processes. The effects of LLD and aMCI comorbidity on the HFC were greater than the independent effects of these disorders, suggesting that the effects of depression were significantly magnified by comorbid CI, and vice versa. VMPFC is responsible for context-appropriate emotional evaluation of events and stimuli, and reward processing (Brassen et al., 2008; Teasdale et al., 1999). The DLPFC and the dorsal ACC play key roles in conflict monitoring, error detection, response selection and mediating executive functions (Alexopoulos et al., 2008). While the findings in the MOG are intriguing, hypoperfusion in the primary visual cortices is seen in aMCI, and is one of the brain areas that accurately discriminate AD from controls in R-fMRI studies (Caroli et al., 2007; Chen et al., 2011). In these interactive brain regions, when LLD and nondepressed aMCI subjects demonstrated decreased functional connectivity, patients with coexisting LLD and aMCI showed significantly enhanced connections. These findings were present despite the fact that the comorbid group demonstrated comparable levels of depression severity as the LLD-only group and a similar degree of cognitive deficits as the nondepressed aMCI subjects.
The coexistence of subclinical and syndromal LLD with aMCI is associated with greater gray and white matter atrophy, cognitive decline and conversion to AD (Lee et al., 2012; Modrego and Ferrandez, 2004; Xie et al., 2012c). In individuals at-risk for AD, increased functional connectivity and increased activation to memory tasks in the prefrontal and MTL regions have been hypothesized as compensatory recruitment of neural resources to maintain adequate cognitive performance or a transient phase that represent impending failure of neuronal networks (Sperling et al., 2010). For instance, greater hippocampal activation predicted those individuals with aMCI at highest risk for subsequent cognitive decline in one study (Miller et al., 2008). Major depression also has been associated with increased DMN and affective network functional connectivity (Alexopoulos et al., 2012; Greicius et al., 2007; Sheline et al., 2010b). Taken together, our data may reflect a compensatory process whereby the comorbid group is recruiting additional neural resources to improve cognitive functions, a transient response of a failing functional network and/or an effect of the depressed state.
4.4. Neural correlates of the effects of depressive symptoms and episodic memory on HFC networks
Greater depressive symptom severity was associated with mostly increased positive and anticorrelated HFC bilaterally in several frontal, parietal and temporal areas, which are consistent with our previous results (Goveas et al., 2011a) as well as the LLD group findings. The DLPFC, inferior parietal, frontal opercular and insular cortices, which are regions associated with executive control, attentional and salience processing, were functionally anticorrelated to the hippocampus (i.e., blue color in these regions represent increased anticorrelations being related to greater depressive symptom severity) (Fig. 4A top row). In contrast, increased hippocampal-DLPFC connectivity was associated with greater depressive symptom severity, after regressing out the subject group effects. These results suggest that the diminished hippocampal-DLPFC functional connections seen in LLD may be a disorder-specific phenomenon.
We also found positive correlations between HFC and LMII-DR in the medial prefrontal and inferior temporal cortices, and thalamus (i.e., increased hippocampal connections were related to better episodic memory performance) (Fig. 4A middle row). However, we found an opposite relationship where poorer memory was associated with increased hippocampal connections in the sensorimotor, precuneus and the MTL regions, which are contrary to the aMCI group findings. It is possible that older adults with poorer episodic memory compensate by enhancing functional connections in these regions. An alternative explanation is that decreased MTL and posterior default mode functional connections are specific to the neurodegenerative disease-state. The differences in sample sizes, inclusion/exclusion criteria and the HFC assessments separately (versus combined in the previous study (Goveas et al., 2011a) may also have contributed to the discrepancies.
The GDS and LMII-DR interactive effects on the HFC in the default mode, fronto-parietal and salience regions (Fig. 4A bottom row) are similar to our prior findings (Goveas et al., 2011a). Future studies that examine the HFC alterations specific to different affective and cognitive disorders versus dimensional measures should be conducted.
4.5. Potential pathophysiological mechanisms
LLD may share common pathophysiologic mechanisms with aMCI and AD. Patients with remitted geriatric depression that met aMCI criteria had cortical amyloid accumulation comparable to that seen in AD (Butters et al., 2008). Significantly higher amyloid and tau deposition in the PCC and temporal lobes in symptomatic individuals with LLD were recently reported (Kumar et al., 2011). Other mechanisms may be shared between LLD and aMCI, including subclinical cerebrovascular disease, genetic variations, proinflammatory cytokines and oxidative stress (Kumar et al., 2008; Mufson et al., 2012). Separate mechanisms may differentially contribute to the abnormal hippocampal functional integrity when LLD and aMCI occur alone. However, a multiple neurobiological vulnerabilities may be present in those with comorbidities that may lead to more pronounced HFC abnormalities and subsequent cognitive decline.
4.6. Limitations
First, we are unable to make firm conclusions if the increased HFC in the comorbid group reflects a compensatory process of a failing network and/or an effect of the depressed state. Longitudinal studies are essential to elucidate this hypothesis. Second, the sample in the comorbid group is relatively small, and our results need to be replicated in larger studies. Third, an ideal study design would be to include antidepressant-free participants to eliminate potential confounding effects. However, since the majority of our depressed participants had failed at least one therapeutic antidepressant trial and had moderate-to-severe depression (LLD only: N = 9; comorbid group: N = 10), the ethical aspects restricted us from performing such a study. Similarly, while acetylcholinesterase inhibitors (ChEI) and memantine are not approved for treatment of aMCI, physicians commonly prescribe these medications off-label to those with aMCI with and without coexisting LLD (McClendon et al., 2009; Reynolds et al., 2011; Roberts et al., 2010). Therefore, as long as aMCI patients were on stable doses and the dosages were not expected to change during the study participation, cognitive enhancer use was allowed. Fourth, although we excluded subjects with unstable medical conditions and all participants had to score within the normal range on the modified HIS, varying levels of cerebrovascular and chronic medical disease burden across groups may have influenced our findings.
5. Conclusions
In conclusion, discrete HFC abnormalities were associated with LLD and aMCI, whereas interplay between these syndromes was present in the hippocampal network level involving hubs of the commonly described intrinsic resting-state networks. Interestingly, these differences were predominantly driven by differences between the comorbid and other subject groups. Future R-fMRI studies should determine changes in the hippocampal functional connectivity over time in LLD patients with and without aMCI, as it relates to the risk of future cognitive decline and incident dementia.
The following are the supplementary data related to this article.
Supplementary material.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2013.09.002.
Acknowledgments
We thank all the study volunteers for their participation. We are also grateful to Ms. Carrie M. O'Connor, M.A., for editorial assistance, Ms. Stacy Claesges for subject recruitment, and Ms. Judi Zaferos-Pylant and Mr. Yu Liu, M.S. for MRI technical support.
This work was supported by Alzheimer's Association New Investigator Research Grant NIRG-11-204070 (Dr. Goveas); Advancing Healthier Wisconsin Endowment for Research to MCW (Dr. Goveas); Extendicare Foundation grant (Drs. Goveas and Antuono); the Brain and Behavior Research Foundation (formerly NARSAD; Dr. Goveas); NIH grant R01 AD20279 (Dr. S-J Li); and 1UL1RR031973 from the Clinical and Translational Science Award Program of the National Center for Research Resources (Drs. Goveas and S-J Li).
Disclosure
Drs. Xie, W. Li, Chen and Goveas, and Mr. Ward and Ms. Jones report no potential conflicts of interest. Within the past 5 years, Dr. Antuono has served on the speaker bureau of Novartis and Pfizer. Dr. Antuono has received research support from Myriad, Glaxo Smith Kline, Pfizer, ICON, Premier Rach, Octa Pharma, Eisai, Bristol-Myers Squibb, Janssen, Baxter and Elan. Dr. Franczak has received research funding from Genentech, Novartis and Forest Pharmaceuticals; and Dr. Shi-Jiang Li has received research grants from Pfizer and has served as a consultant for Bristol-Myers Squibb and Brainsymphonics, LLC. The authors disclose no potential conflicts of interest, financial or otherwise, related directly or indirectly to this work. All authors have made substantial intellectual contribution to this manuscript in one or more of the following areas: design or conceptualization of the study, analysis or interpretation of the data, or drafting and revising the manuscript. All authors have given final approval of this manuscript.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Chunming Xie, Email: cxie@mcw.edu.
Wenjun Li, Email: wli@mcw.edu.
Gang Chen, Email: gachen@mcw.edu.
B. Douglas Ward, Email: ward@mcw.edu.
Malgorzata B. Franczak, Email: MFranczak@mcw.edu.
Jennifer L. Jones, Email: JlJones@mcw.edu.
Piero G. Antuono, Email: PAntuono@mcw.edu.
Shi-Jiang Li, Email: sjli@mcw.edu.
Joseph S. Goveas, Email: jgoveas@mcw.edu.
References
- Agosta F., Pievani M., Geroldi C., Copetti M., Frisoni G.B., Filippi M. Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol. Aging. 2012;33:1564–1578. doi: 10.1016/j.neurobiolaging.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Aizenstein H.J., Butters M.A., Figurski J.L., Stenger V.A., Reynolds C.F., III, Carter C.S. Prefrontal and striatal activation during sequence learning in geriatric depression. Biol. Psychiatry. 2005;58:290–296. doi: 10.1016/j.biopsych.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Aizenstein H.J., Butters M.A., Wu M., Mazurkewicz L.M., Stenger V.A., Gianaros P.J., Becker J.T., Reynolds C.F., III, Carter C.S. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am. J. Geriatr. Psychiatry. 2009;17:30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein H.J., Andreescu C., Edelman K.L., Cochran J.L., Price J., Butters M.A., Karp J., Patel M., Reynolds C.F., III fMRI correlates of white matter hyperintensities in late-life depression. Am. J. Psychiatry. 2011;168:1075–1082. doi: 10.1176/appi.ajp.2011.10060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos G.S. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- Alexopoulos G.S., Gunning-Dixon F.M., Latoussakis V., Kanellopoulos D., Murphy C.F. Anterior cingulate dysfunction in geriatric depression. Int. J. Geriatr. Psychiatry. 2008;23:347–355. doi: 10.1002/gps.1939. [DOI] [PubMed] [Google Scholar]
- Alexopoulos G.S., Hoptman M.J., Kanellopoulos D., Murphy C.F., Lim K.O., Gunning F.M. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J. Affect. Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G., Barnard H., McColl R., Hester A.L., Fields J.A., Weiner M.F., Ringe W.K., Lipton A.M., Brooker M., McDonald E., Rubin C.D., Cullum C.M. Reduced hippocampal functional connectivity in Alzheimer disease. Arch. Neurol. 2007;64:1482–1487. doi: 10.1001/archneur.64.10.1482. [DOI] [PubMed] [Google Scholar]
- Andreescu C., Wu M., Butters M.A., Figurski J., Reynolds C.F., III, Aizenstein H.J. The default mode network in late-life anxious depression. Am. J. Geriatr. Psychiatry. 2011;19:980–983. doi: 10.1097/JGP.0b013e318227f4f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F., Xie C., Watson D.R., Shi Y., Yuan Y., Wang Y., Yue C., Teng Y., Wu D., Zhang Z. Aberrant hippocampal subregion networks associated with the classifications of aMCI subjects: a longitudinal resting-state study. PLoS One. 2011;6:e29288. doi: 10.1371/journal.pone.0029288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin S., Steffens D.C. Structural neuroimaging of geriatric depression. Psychiatr. Clin. North Am. 2011;34:423–435. doi: 10.1016/j.psc.2011.02.001. (ix) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M., Murphy K., Bandettini P.A. The effect of respiration variations on independent component analysis results of resting state functional connectivity. Hum. Brain Mapp. 2008;29:740–750. doi: 10.1002/hbm.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal B.B., Mennes M., Zuo X.N., Gohel S., Kelly C., Smith S.M., Beckmann C.F., Adelstein J.S., Buckner R.L., Colcombe S., Dogonowski A.M., Ernst M., Fair D., Hampson M., Hoptman M.J., Hyde J.S., Kiviniemi V.J., Kotter R., Li S.J., Lin C.P., Lowe M.J., Mackay C., Madden D.J., Madsen K.H., Margulies D.S., Mayberg H.S., McMahon K., Monk C.S., Mostofsky S.H., Nagel B.J., Pekar J.J., Peltier S.J., Petersen S.E., Riedl V., Rombouts S.A., Rypma B., Schlaggar B.L., Schmidt S., Seidler R.D., Siegle G.J., Sorg C., Teng G.J., Veijola J., Villringer A., Walter M., Wang L., Weng X.C., Whitfield-Gabrieli S., Williamson P., Windischberger C., Zang Y.F., Zhang H.Y., Castellanos F.X., Milham M.P. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassen S., Kalisch R., Weber-Fahr W., Braus D.F., Buchel C. Ventromedial prefrontal cortex processing during emotional evaluation in late-life depression: a longitudinal functional magnetic resonance imaging study. Biol. Psychiatry. 2008;64:349–355. doi: 10.1016/j.biopsych.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Sepulcre J., Talukdar T., Krienen F.M., Liu H., Hedden T., Andrews-Hanna J.R., Sperling R.A., Johnson K.A. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J. Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters M.A., Klunk W.E., Mathis C.A., Price J.C., Ziolko S.K., Hoge J.A., Tsopelas N.D., Lopresti B.J., Reynolds C.F., III, DeKosky S.T., Meltzer C.C. Imaging Alzheimer pathology in late-life depression with PET and Pittsburgh Compound-B. Alzheimer Dis. Assoc. Disord. 2008;22:261–268. doi: 10.1097/WAD.0b013e31816c92bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroli A., Testa C., Geroldi C., Nobili F., Barnden L.R., Guerra U.P., Bonetti M., Frisoni G.B. Cerebral perfusion correlates of conversion to Alzheimer's disease in amnestic mild cognitive impairment. J. Neurol. 2007;254:1698–1707. doi: 10.1007/s00415-007-0631-7. [DOI] [PubMed] [Google Scholar]
- Chen G., Ward B.D., Xie C., Li W., Wu Z., Jones J.L., Franczak M., Antuono P., Li S.J. Classification of Alzheimer disease, mild cognitive impairment, and normal cognitive status with large-scale network analysis based on resting-state functional MR imaging. Radiology. 2011;259:213–221. doi: 10.1148/radiol.10100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Das S.R., Pluta J., Mancuso L., Kliot D., Orozco S., Dickerson B.C., Yushkevich P.A., Wolk D.A. Increased functional connectivity within medial temporal lobe in mild cognitive impairment. Hippocampus. 2013;23:1–6. doi: 10.1002/hipo.22051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson B.C., Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson B.C., Sperling R.A. Large-scale functional brain network abnormalities in Alzheimer's disease: insights from functional neuroimaging. Behav. Neurol. 2009;21:63–75. doi: 10.3233/BEN-2009-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV TR Axis 1 disorders, research version, non-patient edition (SCID-I/NP) [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Zhang D., Snyder A.Z., Raichle M.E. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier S., Reisberg B., Zaudig M., Petersen R.C., Ritchie K., Broich K., Belleville S., Brodaty H., Bennett D., Chertkow H., Cummings J.L., de Leon M., Feldman H., Ganguli M., Hampel H., Scheltens P., Tierney M.C., Whitehouse P., Winblad B. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- Glover G.H., Li T.Q., Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Goveas J., Xie C., Wu Z., Douglas Ward B., Li W., Franczak M.B., Jones J.L., Antuono P.G., Yang Z., Li S.J. Neural correlates of the interactive relationship between memory deficits and depressive symptoms in nondemented elderly: resting fMRI study. Behav. Brain Res. 2011;219:205–212. doi: 10.1016/j.bbr.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goveas J.S., Xie C., Ward B.D., Wu Z., Li W., Franczak M., Jones J.L., Antuono P.G., Li S.J. Recovery of hippocampal network connectivity correlates with cognitive improvement in mild Alzheimer's disease patients treated with donepezil assessed by resting-state fMRI. J. Magn. Reson. Imaging. 2011;34:764–773. doi: 10.1002/jmri.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goveas J.S., Xie C., Chen G., Li W., Ward B.D., Franczak M.B., Jones J.L., Antuono P.G., Li S.J. Functional network endophenotypes unravel the effects of apolipoprotein E epsilon 4 in middle-aged adults. PLoS One. 2013;8:e55902. doi: 10.1371/journal.pone.0055902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Flores B.H., Menon V., Glover G.H., Solvason H.B., Kenna H., Reiss A.L., Schatzberg A.F. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny E.R., O'Brien J.T., Cousins D.A., Richardson J., Thomas A.J., Firbank M.J., Blamire A.M. Functional connectivity in late-life depression using resting-state functional magnetic resonance imaging. Am. J. Geriatr. Psychiatry. 2010;18:643–651. doi: 10.1097/JGP.0b013e3181cabd0e. [DOI] [PubMed] [Google Scholar]
- Kumar A., Ajilore O., Kepe V., Barrio J.R., Small G. Mood, cognition and in vivo protein imaging: the emerging nexus in clinical neuroscience. Int. J. Geriatr. Psychiatry. 2008;23:555–563. doi: 10.1002/gps.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Kepe V., Barrio J.R., Siddarth P., Manoukian V., Elderkin-Thompson V., Small G.W. Protein binding in patients with late-life depression. Arch. Gen. Psychiatry. 2011;68:1143–1150. doi: 10.1001/archgenpsychiatry.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar K.S., Cabeza R. Cognitive neuroscience of emotional memory. Nat. Rev. Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- Lee G.J., Lu P.H., Hua X., Lee S., Wu S., Nguyen K., Teng E., Leow A.D., Jack C.R., Jr., Toga A.W., Weiner M.W., Bartzokis G., Thompson P.M. Depressive symptoms in mild cognitive impairment predict greater atrophy in Alzheimer's disease-related regions. Biol. Psychiatry. 2012;71:814–821. doi: 10.1016/j.biopsych.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K.K., Barnes J., Ridgway G.R., Bartlett J.W., Clarkson M.J., Macdonald K., Schuff N., Fox N.C., Ourselin S. Automated cross-sectional and longitudinal hippocampal volume measurement in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2010;51:1345–1359. doi: 10.1016/j.neuroimage.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Antuono P.G., Xie C., Chen G., Jones J.L., Ward B.D., Franczak M.B., Goveas J.S., Li S.J. Changes in regional cerebral blood flow and functional connectivity in the cholinergic pathway associated with cognitive performance in subjects with mild Alzheimer's disease after 12-week donepezil treatment. Neuroimage. 2012;60:1083–1091. doi: 10.1016/j.neuroimage.2011.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J.A., Ivnik R.J., Smith G.E., Bohac D.L., Tangalos E.G., Kokmen E., Graff-Radford N.R., Petersen R.C. Normative data for the Mattis Dementia Rating Scale. J. Clin. Exp. Neuropsychol. 1998;20:536–547. doi: 10.1076/jcen.20.4.536.1469. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br. Med. Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- McClendon M.J., Hernandez S., Smyth K.A., Lerner A.J. Memantine and acetylcholinesterase inhibitor treatment in cases of CDR 0.5 or questionable impairment. J. Alzheimers Dis. 2009;16:577–583. doi: 10.3233/JAD-2009-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.L., Fenstermacher E., Bates J., Blacker D., Sperling R.A., Dickerson B.C. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J. Neurol. Neurosurg. Psychiatry. 2008;79:630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrego P.J., Ferrandez J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch. Neurol. 2004;61:1290–1293. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- Mufson E.J., Binder L., Counts S.E., DeKosky S.T., de Toledo-Morrell L., Ginsberg S.D., Ikonomovic M.D., Perez S.E., Scheff S.W. Mild cognitive impairment: pathology and mechanisms. Acta Neuropathol. 2012;123:13–30. doi: 10.1007/s00401-011-0884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfanidis S.J. Prentice Hall; Upper Saddle River, NJ: 1996. Introduction to Signal Processing. [Google Scholar]
- Ownby R.L., Crocco E., Acevedo A., John V., Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Randolph C. The Psychological Corporation; San Antonio: 1998. Repeatable Battery for the Assessment of Neuropsychological Status. [Google Scholar]
- Reynolds C.F., III, Butters M.A., Lopez O., Pollock B.G., Dew M.A., Mulsant B.H., Lenze E.J., Holm M., Rogers J.C., Mazumdar S., Houck P.R., Begley A., Anderson S., Karp J.F., Miller M.D., Whyte E.M., Stack J., Gildengers A., Szanto K., Bensasi S., Kaufer D.I., Kamboh M.I., DeKosky S.T. Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Arch. Gen. Psychiatry. 2011;68:51–60. doi: 10.1001/archgenpsychiatry.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J.S., Karlawish J.H., Uhlmann W.R., Petersen R.C., Green R.C. Mild cognitive impairment in clinical care: a survey of American Academy of Neurology members. Neurology. 2010;75:425–431. doi: 10.1212/WNL.0b013e3181eb5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts S.A., Stam C.J., Kuijer J.P., Scheltens P., Barkhof F. Identifying confounds to increase specificity during a “no task condition”. Evidence for hippocampal connectivity using fMRI. Neuroimage. 2003;20:1236–1245. doi: 10.1016/S1053-8119(03)00386-0. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton C.E., Mackay C.E., Ebmeier K.P. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am. J. Geriatr. Psychiatry. 2013;21:184–195. doi: 10.1016/j.jagp.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., Rundle M.M., Vaishnavi S.N., Snyder A.Z., Mintun M.A., Wang S., Coalson R.S., Raichle M.E. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Morris J.C., Snyder A.Z., Price J.L., Yan Z., D'Angelo G., Liu C., Dixit S., Benzinger T., Fagan A., Goate A., Mintun M.A. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J. Neurosci. 2010;30:17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Price J.L., Yan Z., Mintun M.A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Liu B., Zhou Y., Yu C., Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: meta-analyses of MRI studies. Hippocampus. 2009;19:1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- Smith G.S., Kramer E., Ma Y., Kingsley P., Dhawan V., Chaly T., Eidelberg D. The functional neuroanatomy of geriatric depression. Int. J. Geriatr. Psychiatry. 2009;24:798–808. doi: 10.1002/gps.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg C., Riedl V., Muhlau M., Calhoun V.D., Eichele T., Laer L., Drzezga A., Forstl H., Kurz A., Zimmer C., Wohlschlager A.M. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R.A., Dickerson B.C., Pihlajamaki M., Vannini P., LaViolette P.S., Vitolo O.V., Hedden T., Becker J.A., Rentz D.M., Selkoe D.J., Johnson K.A. Functional alterations in memory networks in early Alzheimer's disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens D.C., Otey E., Alexopoulos G.S., Butters M.A., Cuthbert B., Ganguli M., Geda Y.E., Hendrie H.C., Krishnan R.R., Kumar A., Lopez O.L., Lyketsos C.G., Mast B.T., Morris J.C., Norton M.C., Peavy G.M., Petersen R.C., Reynolds C.F., Salloway S., Welsh-Bohmer K.A., Yesavage J. Perspectives on depression, mild cognitive impairment, and cognitive decline. Arch. Gen. Psychiatry. 2006;63:130–138. doi: 10.1001/archpsyc.63.2.130. [DOI] [PubMed] [Google Scholar]
- Steffens D.C., McQuoid D.R., Payne M.E., Potter G.G. Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. Am. J. Geriatr. Psychiatry. 2011;19:4–12. doi: 10.1097/JGP.0b013e3181d6c245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens D.C., Taylor W.D., Denny K.L., Bergman S.R., Wang L. Structural integrity of the uncinate fasciculus and resting state functional connectivity of the ventral prefrontal cortex in late life depression. PLoS One. 2011;6:e22697. doi: 10.1371/journal.pone.0022697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale J.D., Howard R.J., Cox S.G., Ha Y., Brammer M.J., Williams S.C., Checkley S.A. Functional MRI study of the cognitive generation of affect. Am. J. Psychiatry. 1999;156:209–215. doi: 10.1176/ajp.156.2.209. [DOI] [PubMed] [Google Scholar]
- Wang L., Zang Y., He Y., Liang M., Zhang X., Tian L., Wu T., Jiang T., Li K. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. Neuroimage. 2006;31:496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Wang Z., Liang P., Jia X., Qi Z., Yu L., Yang Y., Zhou W., Lu J., Li K. Baseline and longitudinal patterns of hippocampal connectivity in mild cognitive impairment: evidence from resting state fMRI. J. Neurol. Sci. 2011;309:79–85. doi: 10.1016/j.jns.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; San Antonio: 1987. Manual for the Wechsler Memory Scale-Revised. [Google Scholar]
- Weisenbach S.L., Boore L.A., Kales H.C. Depression and cognitive impairment in older adults. Curr. Psychiatry Rep. 2012;14:280–288. doi: 10.1007/s11920-012-0278-7. [DOI] [PubMed] [Google Scholar]
- Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L.O., Nordberg A., Backman L., Albert M., Almkvist O., Arai H., Basun H., Blennow K., de Leon M., DeCarli C., Erkinjuntti T., Giacobini E., Graff C., Hardy J., Jack C., Jorm A., Ritchie K., van Duijn C., Visser P., Petersen R.C. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Xie C., Bai F., Yu H., Shi Y., Yuan Y., Chen G., Li W., Zhang Z., Li S.J. Abnormal insula functional network is associated with episodic memory decline in amnestic mild cognitive impairment. Neuroimage. 2012;63:320–327. doi: 10.1016/j.neuroimage.2012.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Goveas J., Wu Z., Li W., Chen G., Franczak M., Antuono P.G., Jones J.L., Zhang Z., Li S.J. Neural basis of the association between depressive symptoms and memory deficits in nondemented subjects: resting-state fMRI study. Hum. Brain Mapp. 2012;33:1352–1363. doi: 10.1002/hbm.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Li W., Chen G., Douglas Ward B., Franczak M.B., Jones J.L., Antuono P.G., Li S.J., Goveas J.S. The co-existence of geriatric depression and amnestic mild cognitive impairment detrimentally affect gray matter volumes: voxel-based morphometry study. Behav. Brain Res. 2012;235:244–250. doi: 10.1016/j.bbr.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage J.A., Brink T.L., Rose T.L., Lum O., Huang V., Adey M., Leirer V.O. Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatr. Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zar J. 3rd ed. Prentice-Hall, Inc.; Upper Saddle River, NJ: 1996. Biostatistical Analysis. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.


