Abstract
Although brain plasticity in the form of gray matter increases and decreases has been observed in chronic pain, factors determining the patterns of directionality are largely unknown. Here we tested the hypothesis that fibromyalgia interacts with age to produce distinct patterns of gray matter differences, specifically increases in younger and decreases in older patients, when compared to age-matched healthy controls. The relative contribution of pain duration was also investigated. Regional gray matter was measured in younger (n = 14, mean age 43, range 29–49) and older (n = 14; mean age 55, range 51–60) female fibromyalgia patients and matched controls using voxel-based morphometry and cortical thickness analysis of T1-weighted magnetic resonance images. To examine their functional significance, gray matter differences were compared with experimental pain sensitivity. Diffusion-tensor imaging was used to assess whether white matter changed in parallel with gray matter, and resting-state fMRI was acquired to examine whether pain-related gray matter changes are associated with altered functional connectivity. Older patients showed exclusively decreased gray matter, accompanied by compromised white matter integrity. In contrast, younger patients showed exclusively gray matter increases, namely in the basal ganglia and insula, which were independent of pain duration. Associated white matter changes in younger patients were compatible with gray matter hypertrophy. In both age groups, structural brain alterations were associated with experimental pain sensitivity, which was increased in older patients but normal in younger patients. Whereas more pronounced gray matter decreases in the posterior cingulate cortex were related to increased experimental pain sensitivity in older patients, insular gray matter increases in younger patients correlated with lower pain sensitivity, possibly indicating the recruitment of endogenous pain modulatory mechanisms. This is supported by the finding that the insula in younger patients showed functional decoupling from an important pain-processing region, the dorsal anterior cingulate cortex. These results suggest that brain structure and function shift from being adaptive in younger to being maladaptive in older patients, which might have important treatment implications.
Abbreviations: VBM, voxel-based morphometry; CTA, cortical thickness analysis; FA, fractional anisotropy; aINS, anterior insula; NAc, nucleus accumbens; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; MPFC, medial prefrontal cortex; DLPFC, dorsolateral prefrontal cortex; PMC, premotor cortex
Keywords: Chronic pain, Age, MRI, Insula, Cingulate, VBM
1. Introduction
The human brain displays a high degree of plasticity, and gray matter and white matter change throughout the life span. In addition to undergoing pre-determined maturation and aging processes (Ziegler et al., 2011), the brain's structure responds to environmental stimuli (Lederbogen et al., 2011), causing use-dependent plasticity (May, 2011). Long-term pain is interesting in the context of brain plasticity: continuous afferent nociceptive activation provides a recurring stimulus input similar to that shown to produce structural brain changes during sensory learning (Golestani et al., 2002; Schneider et al., 2002). Also, there is evidence that the aging brain responds differently to disease states than a younger brain (Lado et al., 2000), leading potentially to interactions between the impacts of age and disease on brain structure.
Neuroimaging studies provide evidence for structural brain changes resulting from prolonged pain as well as some suggestion for interactions between chronic pain and age (Moayedi et al., 2012a). Decreased gray matter has been observed in various chronic pain conditions, including fibromyalgia (Burgmer et al., 2009; Kuchinad et al., 2007; Puri et al., 2010; Robinson et al., 2011; Schmidt-Wilcke et al., 2007; Wood et al., 2009), back pain, and irritable bowel syndrome (Davis and Moayedi, 2012; Schweinhardt and Bushnell, 2010). Such decreases primarily affect the insular, cingulate and prefrontal cortices (Schweinhardt and Bushnell, 2010). Several studies have reported more pronounced gray matter decreases with prolonged pain duration (Absinta et al., 2012; Apkarian et al., 2004; Baliki et al., 2011b; Geha et al., 2008; Jin et al., 2013; Kim et al., 2008; Kuchinad et al., 2007; Obermann et al., 2013; Rocca et al., 2006; Schmidt-Wilcke et al., 2005; Seminowicz et al., 2010; Wartolowska et al., 2012) or reversal with pain resolution (Gwilym et al., 2010; Rodriguez-Raecke et al., 2009; Seminowicz et al., 2011), indicating that gray matter decreases might be a consequence of prolonged pain. Albeit less frequently, gray matter increases have also been observed in chronic pain, including fibromyalgia, and are located mostly in the basal ganglia, insula and the hippocampal complex (Davis and Moayedi, 2012). In a study showing exclusively gray matter increases in young women with chronic vulvar pain (Schweinhardt et al., 2008), we first proposed that the relationship between chronic pain and gray matter may be affected by the patients' age and might follow a bi-directional trajectory resulting in increased gray matter in younger patients and decreased gray matter in older patients.
This study tested the hypothesis that patients' age influences the effect of fibromyalgia on the brain, specifically that younger patients show gray matter increases and older patients have gray matter decreases.
2. Materials and methods
2.1. Subjects
All procedures were approved by the McGill University Institutional Review Board and written informed consent was obtained from all participants according to the Declaration of Helsinki. Exclusion criteria included male gender (because fibromyalgia displays a female-to-male ratio of 10:1 (Clauw and Crofford, 2003)), smoking, use of recreational drugs, use of opioid medications, alcohol consumption of > 10 UK units per week, pregnancy or breastfeeding, chronic pain conditions other than fibromyalgia, major medical, neurological, or current psychiatric conditions, including severe depression and generalized anxiety disorder, and MRI contraindications. Patients were on stable medication (Table 1). Patients met the American College of Rheumatology criteria for fibromyalgia (Wolfe et al., 1990, 2010), as confirmed by an experienced rheumatologist (MAF). The total study sample consisted of 28 female fibromyalgia patients (mean age ± SD: 48.7 ± 7.8 years, range 29–60 years) and 28 individually age-matched (± 3 years) healthy female controls (48.8 ± 7.7 years, range 30–63 years; t = 0.03, p = 0.973). The groups were further matched for handedness (Edinburgh Handedness Inventory (Oldfield, 1971)), years of education, individual annual income, level of physical activity (International Physical Activity Questionnaire, IPAQ (Craig et al., 2003)), phase of menstrual cycle and menopausal status (Randolph et al., 2006) (Table 1).
Table 1.
Sample clinical characteristics.
| All subjects |
Younger (Y) subjects (age < 50) |
Older (O) subjects (age > 50) |
Patients Y vs. O |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls n = 28 Mean (SD) range |
Patients n = 28 Mean (SD) range |
p value | Controls n = 15 Mean (SD) range |
Patients n = 14 Mean (SD) range |
p value | Controls n = 13 Mean (SD) range |
Patients n = 14 Mean (SD) range |
p value | p value | |
| Age | 48.8 (7.7) 30–63 |
48.7 (7.8) 29–60 |
.973 | 43.1 (5.3) 30–49 |
42.4 (5.9) 29–49 |
.761 | 55.4 (3.7) 51–63 |
55.0 (2.9) 51–60 |
.764 | < .001 |
| BMI | 24.1 (3.6) 18.5–31.8 |
26.8 (4.3) 17.5–35.2 |
.014 | 24.4 (3.8) 18.5–31.8 |
27.0 (3.6) 22.0–35.1 |
.073 | 23.8 (3.5) 19.6–31.8 |
26.7 (5.1) 17.7–35.3 |
.098 | .876 |
| Right–handed (Edinburgh) | 26 | 27 | .553a | 13 | 14 | .157a | 13 | 13 | .310a | .327a |
| Years of education | 16.6 (3.7) 11–23 |
15.3 (2.8) 11–20 |
.137 | 16.7 (3.7) 11–23 |
15.8 (3.1) 11–20 |
.459 | 16.5 (3.9) 11–23 |
14.9 (2.5) 11–20 |
.189 | .350 |
| Individual annual income | .310a | .145a | .829a | .900a | ||||||
| < $30,000 | 8 | 7 | 4 | 4 | 4 | 3 | ||||
| $30,000–600,000 | 18 | 15 | 11 | 7 | 7 | 8 | ||||
| > $60,000 | 2 | 6 | 0 | 3 | 2 | 3 | ||||
| Level of physical activity | .835a | .516a | .289a | .974a | ||||||
| Low | 2 | 2 | 0 | 1 | 2 | 1 | ||||
| Mid | 17 | 19 | 12 | 10 | 5 | 9 | ||||
| High | 9 | 7 | 3 | 4 | 6 | 3 | ||||
| Menopausal status | .546a | .290a | .955 | < .001a | ||||||
| Pre | 16 | 14 | 15 | 13 | 1 | 1 | ||||
| Post | 12 | 12 | 0 | 1 | 12 | 11 | ||||
| Peri | 0 | 1 | 0 | 0 | 0 | 1 | ||||
| Menstrual phase; “younger” subjects | ||||||||||
| Follicular phase | 8 | 6 | .724a | 1 | 1 | |||||
| Luteal phase | 5 | 3 | ||||||||
| No mensesb | 2 | 1 | ||||||||
| Missing data | 2 | 1 | ||||||||
| Clinical pain | ||||||||||
| Intensity (VAS) | 0.05 (0.2) 0–1 |
2.6 (2.7) 0–9 |
< .001 | 0 (0) 0 |
2.8 (3.0) 0–9 |
.001 | 0.1 (0.3) 0–1 |
2.41 (2.3) 0–6.5 |
.002 | .679 |
| Duration (years) | / | 11.5 (8.7) 2–35 | / | / | 8.8 (7.1) 2–30 |
/ | / | 12.1 (9.0) 2–35 |
/ | .099 |
| Medication (n of patients) | ||||||||||
| NSAIDS | 24 | / | 12 | / | 12 | / | .932a | |||
| Antidepressants | 13 | 6 | 7 | |||||||
| Muscle relaxants | 5 | 3 | 2 | |||||||
| Anticonvulsants | 7 | 3 | 4 | |||||||
| Cannabinoids | 1 | 0 | 1 | |||||||
| Triptans | 2 | 1 | 1 | |||||||
| Affective disturbances | ||||||||||
| Anxiety (HADS) |
5.0 (3.0) 0–10 | 10.3 (4.5) 0–20 |
< .001 | 5.7 (3.1) 0–10 |
9.8 (5.4) 0–20 |
.019 | 4.0 (2.6) 1–9 |
10.8 (3.3) 5–15 |
< .001 | .591 |
| Depression (HADS) | 2.1 (2.1) 0–9 | 5.7 (4.3) 1–16 |
< .001 | 2.3 (2.3) 0–9 |
5.6 (4.1) 1–13 |
.013 | 1.8 (1.6) 0–5 |
5.8 (4.7) 1–16 |
.011 | .902 |
| General fatigue (MFI-20) | 9.1 (2.8) 4–15 | 15.8 (3.0) 9–20 |
< .001 | 10.1 (2.5) 7–15 |
15.8 (2.2) 12–20 |
< .001 | 7.9 (2.7) 4–12 |
15.8 (3.7) 9–20 |
< .001 | .999 |
| Mental fatigue (MFI-20) | 8.3 (3.2) 4–15 | 13.1 (3.6) 4–20 |
< .001 | 9.7 (3.0) 5–15 |
13.6 (3.5) 10–20 |
.004 | 6.6 (2.5) 4–11 |
12.7 (3.7) 4–18 |
< .001 | .534 |
| Catastrophizing (PCS) | 10.6 (7.1) 0–27 |
22.1 (11.8) 8–52 |
< .001 | 13.2 (6.3) 0–23 |
20.6 (12.0) 8–52 |
.044 | 7.6 (7.2) 0–27 |
23.6 (11.8) 8–42 |
< .001 | .521 |
χ2 test.
Partial hysterectomy (in two patients); mechanical contraceptive (in one healthy control).
2.2. General design
Each participant underwent two sessions: one behavioral and one MRI session (4 ± 3 days apart). All questionnaire data as well as pressure pain sensitivity were obtained in the behavioral session.
2.3. Behavioral measures
2.3.1. Clinical assessment
Pain duration was recorded as time in years since onset of widespread body pain. Clinical pain intensity was measured on an 11-point numerical pain intensity rating scale from 0 to 10 (0 — no pain, 1 — pain threshold, 10 — worst bearable pain). The final score was an average of two ratings: one from the behavioral and one from the MRI session. General and mental fatigue were assessed with the Multidimensional Fatigue Inventory (MFI-20) (Smets et al., 1995), catastrophic thinking related to pain with the Pain Catastrophizing Scale (PCS) (Sullivan et al., 1995), and depressive symptoms and anxiety with the Hospital Anxiety and Depression Score (HADS) (Zigmond and Snaith, 1983).
2.3.2. Pressure sensitivity
Using a calibrated hand-held algometer with a 1 cm diameter tip (Force Dial FDK/FDN Series Push Pull Force Gauge, Wagner Instruments, USA) pressure was applied to subjects' thumbnail because the thumbnail has previously been shown to reflect a person's overall pressure pain sensitivity (Petzke et al., 2001). Following familiarization with the testing procedure, three trials of predetermined intensity and duration (4 kg/cm2 for 3 s) were performed on each thumb (alternating between left and right), with inter-stimulus intervals of approximately 10 s. Participants were instructed to rate the intensity and hedonic quality (pleasantness/unpleasantness) of each pressure stimulus immediately after its presentation. Ratings were expressed verbally, while observing Visual Analogue Scales (VAS) as reference. To help participants distinguish sensory and affective aspects of the sensations evoked by the pressure stimuli, we stressed differences between intensity and pleasantness/unpleasantness of the sensation using explanations adapted from Price et al. (1983). The 200-mm intensity scale was anchored with 0 (no sensation) and 200 (most intense pain tolerable) with a mid-point of 100 defined as the pain threshold. The 200-mm hedonic scale was anchored with − 100 (extremely unpleasant) and 100 (extremely pleasant) with a mid-point of 0 labeled neutral. These scales have been used successfully to differentiate between sensory and hedonic aspects of pain sensations (Loggia et al., 2008; Villemure et al., 2003). Data were missing from two controls (one could not follow instructions; one refused the test). Ratings in response to pressure stimuli did not differ between left and right thumbs (intensity: p = 0.94, unpleasantness: p = 0.99) and were therefore pooled for further analysis.
2.4. MRI acquisition
The MRI scanning session included a 10-min anatomical MRI scan, a 15-min DTI scan, and an 8-min resting state functional MRI scan. Throughout the session, participants wore earplugs and their heads were immobilized. Brain images were acquired using a 3 T Siemens TIM-Trio scanner (Siemens, Erlangen, Germany) with a standard 12-channel head coil. Anatomical images were acquired using a T1-weighted 3D magnetization prepared rapid acquisition by gradient echo (MP-RAGE) sequence (repetition time (TR) 2300 ms, echo time (TE) 2.98 ms, flip angle 9°, field of view 256 mm, resolution 1 × 1 × 1 mm). DTI data were acquired using a diffusion-weighted single shot spin-echo echoplanar imaging (SE-EPI) sequence (TR 8300 ms, TE 89 ms, resolution 2 × 2 × 2 mm, b0 = 1000 s/mm2, 99 directions). Additionally, ten images with no diffusion weighting (b = 0 s/mm2) were acquired. Resting state functional MRI data were acquired using a blood oxygenation level-dependent (BOLD) protocol with a T2*-weighted gradient echo planar imaging (EPI) sequence (TR 2260 ms, TE 30 ms, flip angle 90°, resolution 3.5 × 3.5 × 3.5 mm). Axial slices were oriented 30° from the line between the anterior and posterior commissures, covering the entire brain, and excluding the eyes. After discarding the first three volumes to allow for steady-state magnetization, 190 volumes were acquired. Immediately after the MRI scanning session, subjects were asked to rate the intensity of their clinical pain during the resting state scan.
2.5. Behavioral data analysis
All data are expressed as means ± standard deviations. Variables were compared between groups using independent samples two-tailed t-tests in SPSS 19 and a significance level of p < 0.05 was used in all analyses. Because pain intensity and unpleasantness ratings of the pressure stimulus were highly correlated (r = − 0.76, p < 0.001), we corrected for multiple comparisons using an adjustment method recommended for highly correlated endpoints (Sankoh et al., 1997; Tukey et al., 1985).
2.6. MRI data analysis
2.6.1. Voxel-based morphometry (VBM)
Anatomical MRI data were preprocessed with the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) for SPM8 (http://www.fil.ion.ucl.ac.uk/spm/), running on Matlab R2010. Images were bias corrected and tissue classified [gray matter, white matter, cerebrospinal fluid (CSF)], and partial volumes were calculated in each voxel. Images were then spatially normalized to the MNI space using linear (12-parameter affine) and non-linear transformations. The non-linear transformation parameters were calculated via the high dimensional DARTEL algorithm (Ashburner, 2007) with the supplied standard template. Subsequently, the voxel values were multiplied by the non-linear components derived from the spatial normalization (modulation) to allow for comparison of absolute tissue volumes corrected for individual brain sizes. Finally, the modulated images were smoothed with a Gaussian kernel of 8 mm full width at half maximum (FWHM).
Total native gray matter, white matter, and CSF volumes were extracted with the VBM8 toolbox and group differences as well as group by age interactions were tested using SPSS 19. Voxel-wise whole-brain gray matter analyses (excluding the cerebellum) were performed using a general linear model (GLM) in SPM8. In order to avoid possible edge effects between gray matter and other tissue types, all voxels with gray matter values of less than 0.1 were excluded from the analysis (absolute threshold masking). VBM cluster sizes were corrected for non-stationarity using the NS toolbox in SPM5 (Hayasaka and Nichols, 2003; Worsley et al., 1999) (http://fmri.wfubmc.edu/cms/software).
2.6.1.1. Method for defining ‘younger’ and ‘older’ age groups
We first performed a group by age interaction analysis of gray matter, with and without controlling for pain duration and menopausal status (voxel-wise threshold p < 0.05, cluster-corrected for multiple comparisons across the whole brain at p < 0.05 using Gaussian random field theory (RFT) (Worsley et al., 1996)), to examine whether the relationship between age and gray matter differs for patients and controls. The interaction between group and age was significant, indicating that the relationships between age and gray matter were indeed different for patients compared to controls. We then used the age corresponding to the intersection point of the two regression lines to divide subjects into the ‘younger’ and ‘older’ groups, in order to further investigate the impact of age on brain differences and to relate age-dependent brain differences to pain sensitivity (Fig. 1B). In addition to conducting separate analyses for younger and older subjects, we report group comparisons between patients and controls for the whole sample to allow comparison with previous studies.
Fig. 1.
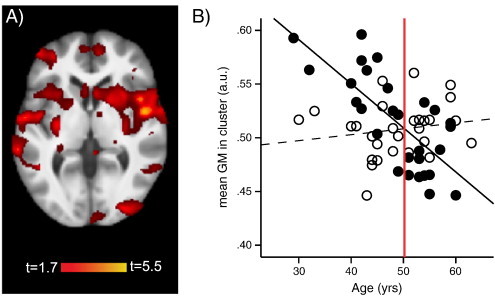
VBM group by age interaction analysis of gray matter. A) Significant gray matter cluster included bilaterally the insula, basal ganglia, superior temporal cortex, and ventrolateral prefrontal cortex (VLPFC) (t = 5.12, p < 0.001, cluster size 27,944 voxels; MNI coordinates at peak − 57, − 42, 21). B) The amount of gray matter was extracted for each participant from the cluster (average value over entire cluster) that was significant in the group by age interaction analysis and plotted against age. The regression lines of patients (r = − 0.73, p < 0.001) and controls (r = 0.168, p = 0.393) crossed at age 50.2 (red line). Therefore, age 50 was chosen to split the study sample into “younger” (< 50) and “older” (> 50) subjects. Controlling for pain duration and/or menopausal status did not change the age where the regression lines crossed. Controls, open circles, dotted line; patients, full circles, full line; a.u., arbitrary unit. Please note that the gray matter plotted against age is derived from a cluster consisting of subcortical and cortical brain regions, with a significant contribution from the basal ganglia. This is important because while it is correct that the volume of the neocortex usually decreases with age (except possibly the ventral ACC (Engvig et al., 2012)), recent studies have shown a different trajectory (mostly gray matter increases with age) for some subcortical regions and in fact primarily for the basal ganglia and hippocampal regions (Peelle et al., 2012; Ziegler et al., 2011).
2.6.1.2. Separate voxel-wise analyses in younger and older subjects
Whole brain statistical maps were compared between younger patients (n = 14) and matched controls (n = 15), and between older patients (n = 14) and matched controls (n = 13), controlling for age or age and pain duration (voxel-wise threshold p < 0.01, cluster-corrected for multiple comparisons at p < 0.05 using RFT).
2.6.1.3. Regression analysis with pain duration
Across all patients (n = 28), we performed a whole-brain regression analysis with pain duration as predictor, controlling for age or age and menopausal status (voxel-wise threshold p < 0.01, cluster-corrected for multiple comparisons at p < 0.05 using RFT).
2.6.1.4. Regression analysis with pain sensitivity
For each patient group separately, we performed a whole-brain VBM regression analysis controlling for age or age and pain duration with the unpleasantness ratings of the pressure stimulus as predictor. Because this was an exploratory analysis, we set a voxel-wise threshold of p < 0.001 cluster-corrected for multiple comparisons across the whole brain at p < 0.05 using RFT.
Cortical gray matter was also compared between groups using cortical thickness analysis (CTA). Details can be found in the Supplementary methods.
2.6.2. DTI analysis
DTI data were not acquired in one control subject due to technical issues. The DTI data of 55 subjects were pre-processed in FSL 4.1 (http://www.fmrib.ox.ac.uk/fsl/fdt/index.html). Raw data were corrected for eddy-currents and head motion, followed by skull and non-brain tissue removal using BET (Smith, 2002). Fractional anisotropy (FA) images, reflecting the degree of water diffusion anisotropy in each voxel, and eigenvalue (L) images, describing the direction of water diffusivity, were created by fitting a tensor model to the diffusion data using FSL's Diffusion Toolbox (FDT). All subjects' FA data were aligned into a common space via the supplied FMRIB58_FA standard-space image using FSL's nonlinear registration tool FNIRT, followed by affine registration into MNI space. Next, a mean FA image was built and thinned to create a mean FA skeleton representing the centers of all tracts common to the study sample. Finally, each subject's aligned FA image was projected onto this skeleton. Maps of mean diffusivity [MD = (L1 + L2 + L3) / 3)], axial diffusivity (AD = L1; parallel to principal diffusion direction), and radial diffusivity [RD = (L2 + L3) / 2; perpendicular to principal diffusion direction] were generated by applying the transformation matrices obtained with the FA images.
Statistical analysis of the FA data was carried out using TBSS (Tract-Based Spatial Statistics) (Smith et al., 2006) in FSL. FA values were compared between groups, first across the whole brain's white matter, and then in a region of interest (ROI) mask encompassing the significant gray matter clusters, dilated to include the adjacent white matter. TBSS was performed using randomise, a permutation-based inference tool for nonparametric statistical thresholding, with the number of permutations set at 10,000. For group comparisons we used GLMs controlling for age, with cluster correction for multiple comparisons at p < 0.05 using TFCE (threshold-free cluster enhancement). Mean MD, AD and RD values were extracted from significant FA clusters and compared between groups with univariate ANOVAs controlling for age in SPSS 19.
2.6.2.1. Tractography
To visualize the connectivity profile of white matter with significantly altered FA, we performed probabilistic tractography (Behrens et al., 2007) on each subject's DTI image. We used FSL's Diffusion Toolbox (FDT) to produce an estimate of the most likely pathway passing through the clusters with significant FA differences. Briefly, a local model of fiber orientation, capable of resolving crossing fibers, was calculated, followed by building up distributions on the diffusion parameters at each voxel in the individual subject's space by repetitive sampling. The seed (FA cluster) was transformed from MNI standard space into DTI space of each subject and 5000 sample tracts were generated from each seed voxel. Individual subjects' connectivity distributions were back-transformed to MNI standard space, thresholded at 5 out of 5000 samples (0.1%) to eliminate spurious connections, and binarized. For visualization, a group map was built by summing the binarized subject connectivity maps and overlaid on the study average brain.
2.6.3. Resting state functional MRI analysis
Resting state functional MRI data were not acquired in one control subject and one patient due to technical issues, and were excluded in one control subject due to scanner artifacts. The remaining 53 data sets were pre-processed in SPM8. Briefly, pre-processing involved six-parameter rigid body correction for head motion, co-registration to the T1-weighted anatomical image and spatial normalization to MNI space [linear (12-parameter affine) and non-linear], followed by smoothing with an 8 mm Gaussian kernel.
We performed seed-based functional connectivity analysis using the CONN toolbox in SPM8 (http://web.mit.edu/swg/software.htm). Because functional connectivity analysis in this study served to better understand the functional significance of gray matter alterations, brain regions (seeds) were defined based on the whole brain VBM regression analyses of gray matter and experimental pain sensitivity. Specifically, seeds corresponded to spheres of 4 mm radius centered on the MNI coordinates of the peak voxels within significant clusters of the regression analysis. To control for non-neuronal noise, we included as nuisance variables six parameters derived from head motion correction, and seven parameters representing the time-course data from three ventricular and four white matter ROIs (Cordes et al., 2000; Fox et al., 2005). We did not remove the average whole-brain signal because such a correction might introduce artificial anti-correlations (Fox et al., 2009; Saad et al., 2012). We applied a temporal band-pass filter of 0.009 Hz to 0.08 Hz to reduce the effects of low-frequency scanner drift and high-frequency cardiac and respiratory signals (Biswal et al., 1995; Cordes et al., 2000; Fox et al., 2005; Fransson, 2005; Lowe et al., 1998). For each subject the mean time series in each seed was correlated with the time series of each gray matter voxel. Correlation coefficient (r) maps were then compared between groups using GLMs in SPM8 controlling for age. The resting state analysis was exploratory and a voxel-wise threshold of p < 0.001 across the whole brain cluster-corrected for multiple comparisons at p < 0.05 was used.
2.6.3.1. Correlation analysis with current pain in patients
Mean connectivity values from significant clusters were extracted with MarsBaR and correlated in SPSS 19 with the level of pain patients experienced during the resting state scan.
3. Results
3.1. Relationship between gray matter differences and age
Total gray matter volume showed a significant interaction between group and age, controlling for pain duration (ANOVAGroup ∗ Age(− PD) F = 4.38, p = 0.041). Age and pain duration were correlated in fibromyalgia patients (r = 0.37, p = 0.049), but the interaction between group and age was similar when pain duration was not controlled for (p = 0.061). Total gray matter volume was negatively correlated with age in patients (rpartial = − 0.48, p = 0.010), but not in controls (r = − 0.09, p = 0.649). The interaction between age and group remained significant after controlling for the effect of menopausal status (ANOVAGroup ∗ Age(− PD, − Meno) F = 4.31, p = 0.043). No differences between groups were observed for any total brain tissue volume, i.e. total gray matter (t = 0.53, p = 0.601), white matter (t = − 0.16, p = 0.872), or CSF (t = 0.92, p = 0.362).
Whole brain voxel-wise group by age interaction analysis of gray matter controlling for pain duration revealed a large cluster of cortical and subcortical structures including bilaterally the insula, basal ganglia, superior temporal cortex, and ventrolateral prefrontal cortex (VLPFC) (t = 5.12, p < 0.001, cluster size 27,944 voxels; MNI coordinates at peak − 57, − 42, 21) (Fig. 1A). Results were virtually identical when pain duration was not controlled for or when menopause was controlled for in addition to pain duration. Following a significant interaction between group and age, the study sample was split into “younger” and “older” individuals based on the age where the regression lines of the two study groups crossed, which was age 50 (Fig. 1B), to examine group differences that were driving the results of the interaction analysis. The split sample comprised 29 younger (14 patients, age 42.4 ± 5.9 years and 15 controls, age 43.1 ± 5.3 years; t = 0.31; p = 0.761) and 27 older (14 patients, age 55.0 ± 2.9 years and 13 controls, age 55.4 ± 3.7 years; t = 0.30; p = 0.764) participants. Importantly, the younger and older patient groups were matched to the respective control groups on all matching criteria (Table 1).
Older fibromyalgia patients showed pronounced gray matter decreases compared to their controls: they had less gray matter in the bilateral anterior cingulate cortex (ACC)/medial prefrontal cortex (MPFC)/frontal pole (FP), right premotor cortex (PMC), VLPFC, right dorsolateral prefrontal cortex (DLPFC), and right posterior cingulate cortex (PCC) (Fig. 2A, Table 2). No region showed increased gray matter in older patients. Age and pain duration were significantly correlated in older patients (r = 0.60, p = 0.024) and after controlling for pain duration in addition to age, gray matter decreases were no longer significant except for the cluster in the DLPFC/PMC. Total native gray matter, white matter, and CSF did not differ significantly between older patients and controls.
Fig. 2.
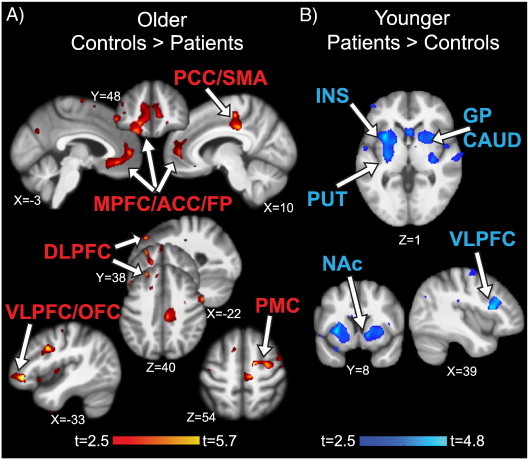
Gray matter findings in fibromyalgia patients. A) Older patients had gray matter decreases compared to matched controls in the MPFC/ACC/FP (medial prefrontal cortex/anterior cingulate cortex/frontal pole), PCC/SMA (posterior cingulate cortex/supplementary motor area), VLPFC/OFC (ventrolateral prefrontal cortex/orbitofrontal cortex), DLPFC (dorsolateral prefrontal cortex), and PMC (premotor cortex); colorbar shows t-values for the contrast older controls > older patients. There were no significant clusters for the opposite contrast. B) Younger patients had gray matter increases compared to matched controls in the INS (insula), PUT (putamen), GP (globus pallidus), CAUD (caudate), NAc (nucleus accumbens), and VLPFC; colorbar shows t-values for the contrast younger patients > younger controls. There were no significant clusters for the opposite contrast. Significant clusters controlling for age (p < 0.05 corrected) are displayed on the study average brain; left side of the brain is on the left.
Table 2.
VBM statistics.
| Brain region | MNI coordinates (x, y, z) | Cluster size (voxels) | p value of cluster | Peak t value |
|---|---|---|---|---|
| Older: Controls > patients | ||||
| ACC/MPFC/FP [L] | − 18, 44, − 8 | 3674 | < 0.001 | 3.66 |
| ACC/MPFC [L] | − 15, 48, − 11 | 3.45 | ||
| ACC/MPFC [R] | 12, 50, 3 | 3.54 | ||
| PMC [R] | 16, − 6, 55 | 1633 | 0.001 | 4.55 |
| PMC/DLPFC [R] | 32, − 1, 48 | 4.33 | ||
| Temporooccipital [R] | 62, − 40, − 14 | 1476 | 0.019 | 4.12 |
| Temporooccipital [R] | 72, − 36, − 0 | 3.56 | ||
| PCC [R] | 9, − 28, 33 | 1088 | 0.048 | 4.21 |
| PCC/SMA/M1 [R] | 10, − 24, 52 | 4.13 | ||
| PCC [R] | 18, − 24, 42 | 3.14 | ||
| VLPFC/OFC [L] | − 42, 38, − 6 | 488 | 0.034 | 5.70 |
| DLPFC [L] | − 24, 38, 42 | 295 | 0.012 | 4.58 |
| DLPFC [L] | − 15, 36, 25 | 4.27 | ||
| DLPFC [L] | − 15, 27, 36 | 3.88 | ||
| Older: Patients > controls | ||||
| No significant regions | ||||
| Younger: Patients > controls | ||||
| aINS [L] | − 30, 9, 1 | 3490 | 0.002 | 4.60 |
| Putamen [L] | − 28, − 12, 10 | 4.35 | ||
| mINS [L] | − 32, − 4, 6 | 4.01 | ||
| GP/Putamen [R] | 14, 8, − 5 | 1778 | 0.011 | 4.22 |
| NAc/GP/Putamen [R] | 18, 8, 3 | 3.67 | ||
| aINS [R] | 32, 9, 0 | 3.55 | ||
| VLPFC [R] | 39, 18, 15 | 1391 | 0.005 | 4.81 |
| VLPFC [R] | 36, 26, 24 | 4.51 | ||
| VLPFC [R] | 50, 20, 21 | 3.84 | ||
| Younger: Controls > patients | ||||
| No significant regions | ||||
PMC, premotor cortex; ACC/MPFC anterior cingulate/medial prefrontal cortex; DLPFC, dorsolateral prefrontal cortex; FP, frontal pole; M1, primary motor cortex; VLPFC, ventrolateral prefrontal cortex; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; SMA, supplementary motor area; aINS, anterior insula; mINS, mid insula; GP, globus pallidus; NAc, nucleus accumbens.
In contrast, younger fibromyalgia patients showed exclusively gray matter increases compared to their controls in the left insula/putamen, right putamen/globus pallidus (GP)/nucleus accumbens (NAc), and right VLPFC (Fig. 2B, Table 2). No region showed decreased gray matter in younger patients. Age and pain duration were not correlated in younger patients (r = − 0.01, p = 0.99) and controlling for pain duration in addition to age did not change the significant gray matter increases in younger patients. Total native gray matter, white matter, and CSF did not differ significantly between younger patients and controls.
Cortical thickness differences between groups largely confirmed the VBM findings. Please refer to the Supplementary material (Fig. S2) for details.
Because previous studies typically did not subdivide patients into “younger” and “older”, we performed a whole-brain voxel-wise analysis of regional gray matter on the total study sample. This analysis revealed that fibromyalgia patients had gray matter decreases compared to controls in the right PMC, right anterior ACC/MPFC, and left DLPFC (Supplementary Fig. 1, Supplementary Table 1). There were no regions in which patients had more gray matter than controls.
3.2. Effect of pain duration on gray matter
We performed a separate whole brain gray matter voxel-wise analysis in all fibromyalgia patients with pain duration as a predictor. We observed a significant negative effect of pain duration on gray matter (more pronounced gray matter decrease with longer fibromyalgia pain) in a large cluster including bilateral dorsal ACC (dACC)/paracingulate (t = 4.01, p = 0.004, cluster size 2461 voxels; MNI coordinates at peak 2, 29, 40), and an additional small cluster in the right middle occipital gyrus (t = 4.48, p = 0.037, cluster size 407 voxels; MNI coordinates at peak 28, − 88, 27). The dACC/paracingulate cluster was no longer significant when age with or without menopause was controlled for.
3.3. White matter differences
Whole-brain analysis yielded no significant group differences in white matter FA in the older fibromyalgia patients compared to matched controls. In the ROI analysis focusing on white matter adjacent to the regions with gray matter decreases (bilateral ACC/MPFC, left VLPFC, right DLPFC, right PMC, right PCC), older patients had significantly decreased FA compared to their controls adjacent to the PCC (corpus callosum, t = 4.11, p = 0.041, cluster size 43 voxels, MNI coordinates at peak 17, − 16, 35, Fig. 3A). The FA decrease was caused by a significant decrease in axial diffusivity (F = 5.85, p = 0.024) and increase in radial diffusivity (F = 7.72, p = 0.011) (Fig. 3D). Mean diffusivity did not differ significantly between groups (F = 0.31, p = 0.581). We next used probabilistic tractography to determine the connectivity of the corpus callosum region with decreased FA, and identified connections to motor/premotor cortex, an area where older patients had decreased gray matter. Here, the identified white matter pathway merged with the corticospinal tract (Fig. 3C, left). No region showed a significant FA increase in older patients.
Fig. 3.
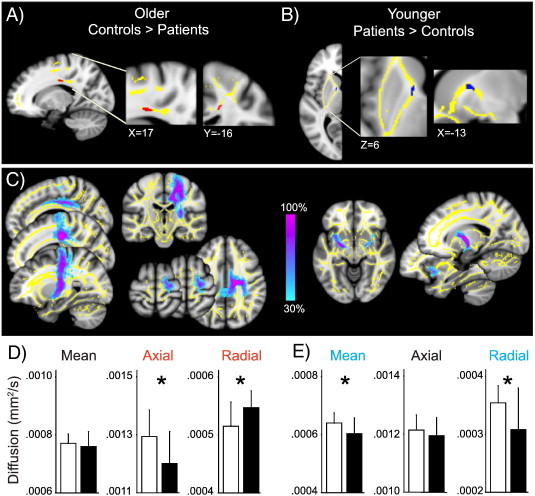
White matter findings in fibromyalgia patients. A) Older patients had decreased FA compared to matched controls in the corpus callosum adjacent to the right PCC (posterior cingulate cortex). The significant FA cluster controlling for age (older patients < older controls; red, p < 0.05 corrected) is displayed in the ROI mask (yellow). There were no significant clusters for the opposite contrast. B) Younger patients had marginally increased FA compared to matched controls in the anterior thalamic radiation/anterior limb of the internal capsule medial to the left putamen. The FA cluster controlling for age (younger patients > younger controls; blue, p = 0.058 corrected) is displayed in the ROI mask (yellow). There were no significant (or near significant) clusters for the opposite contrast. C) Affected white matter tracts in older (left) and younger (right) patients. Colorbar shows the percentage of subjects who share a common pathway. D) Axial diffusivity was significantly lower in older patients compared to matched controls (ANOVA controlling for age; F = 5.85, p = 0.024), radial diffusivity was significantly higher (F = 7.71, p = 0.011) and there was no significant group difference in mean diffusivity (F = 0.32, p = 0.581). E) Mean and radial diffusivities were significantly lower in younger patients compared to matched controls (ANOVA controlling for age; mean diff., F = 5.05, p = 0.033; radial, F = 5.31, p = 0.029), with no significant group difference in axial diffusivity (F = 0.832, 0.370). Results are displayed on the study average brain; left side of the brain is on the left. Bars represent mean in controls (white) and patients (black), error bars represent SD.
Similarly, whole-brain analysis in younger fibromyalgia subjects did not reveal significant differences in white matter FA between patients and matched controls. ROI analysis of FA of white matter adjacent to the left insula/putamen, right putamen/globus pallidus (GP)/nucleus accumbens (NAc), and right VLPFC showed marginally increased FA in younger patients compared to controls in the white matter medial to the left putamen (anterior thalamic radiation/anterior limb of the internal capsule, t = 3.50, p = 0.058, cluster size 35 voxels, MNI coordinates at peak − 16, 1, 8; Fig. 3B). In the area of FA increase, radial diffusivity (F = 5.31, p = 0.029) and mean diffusivity (F = 5.05, p = 0.033) were decreased (Fig. 3E). Axial diffusivity did not differ between groups (F = 0.83, p = 0.370). No region showed significantly decreased FA values in younger patients. Probabilistic tractography revealed that the region of increased FA is part of a white matter pathway between the insula and basal ganglia (Chikama et al., 1997), more specifically the putamen and GP, with some of the white matter pathway reaching the contralateral basal ganglia (Fig. 3C, right).
3.4. Pain sensitivity in older and younger patients compared to age-matched controls
Fibromyalgia patients were hypersensitive to a standardized pressure stimulus applied to the thumbnail, rating the stimulus as significantly more unpleasant [patients mean (SD) − 25.89 (24.78), controls − 11.28 (14.86); t = 2.56, padjusted = 0.02], with a trend for higher intensity ratings [patients 99.93 (47.71), controls 75.73 (42.13), t = 1.97, padjusted = 0.074]. This finding was driven by the older patients who rated the pressure stimulus as significantly more unpleasant compared to their matched controls [older patients − 32.74 (22.94), older controls − 12.46 (12.72), t = 2.72, padjusted = 0.016; Fig. 4A, top], whereas younger patients did not [younger patients − 19.05 (25.43), younger controls − 10.20 (17.26), t = 1.05, padjusted = 0.4; Fig. 4A, bottom]. Ratings of unpleasantness were strongly correlated with ratings of intensity (r = − 0.759, p < 0.001), and thus, results for the perceived intensity of the pressure stimulus were similar to those for the unpleasantness ratings. Older patients on average rated the pressure stimulus as painful with a trend for higher ratings compared to their controls [older patients 111.83 (37.25), older controls 78.61 (45.79), t = 2.04, padjusted = 0.072; Fig. 4B, top], while younger patients on average rated the stimulus below the pain threshold and not different from their controls [younger patients 88.02 (55.10), younger controls 73.26 (40.32), t = 0.81, padjusted = 0.54; Fig. 4B, bottom]. Please note that because the psychophysical differences were more pronounced for unpleasantness ratings we used those for further analyses. However, any relationship reported between unpleasantness ratings and gray matter volumes was largely similar when intensity ratings were used as expected with two measures that are highly correlated. Also, when intensity ratings were controlled for, brain measures no longer correlated with unpleasantness (and vice versa), again indicating the shared variance of the two measures.
Fig. 4.
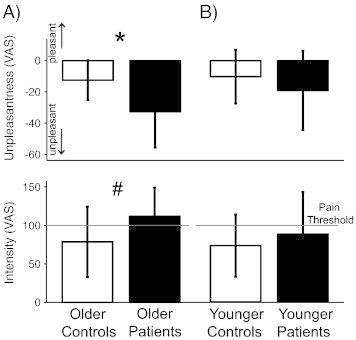
Pain sensitivity in fibromyalgia patients. A) Older patients (full bars) were more sensitive to pressure stimuli applied on the thumb than matched controls (open bars) *p = 0.012 (unpleasantness), #p = 0.052 (intensity). B) Younger patients (full bars) were not more sensitive to pressure than matched controls (open bars; p = 0.304 (unpleasantness), p = 0.426 (intensity)).
Despite displaying different experimental pain sensitivities, older and younger patients did not differ significantly with respect to the extent of affective disturbances (anxiety, depressive symptoms, catastrophizing), fatigue, duration or intensity of clinical pain, or medication use (Table 1).
3.5. In older patients, gray matter in the PCC is inversely related to pain sensitivity and shows altered resting state correlations with MPFC
A voxel-wise whole brain regression of gray matter with unpleasantness ratings of the pressure stimuli revealed that of the areas that showed gray matter decreases in older patients, PCC gray matter volume was negatively correlated with pain sensitivity, i.e. patients with the least amount of gray matter were most sensitive to the experimental pressure stimuli (t = 5.32, p = 0.014, cluster size 157 voxels, MNI coordinates 3, − 19, 42; Fig. 5A). This gray matter cluster showed no significant correlation with pain catastrophizing scores (r = − 0.165, p = 0.57). To better understand the implications of reduced gray matter in the PCC, we conducted a functional connectivity analysis seeding in the PCC. This revealed decreased resting state correlations with the MPFC in older patients compared to their controls (t = 4.87, p = 0.010, cluster size 177 voxels, MNI coordinates 4, 38, − 12; Fig. 5B). No region showed significantly increased resting state correlations with the PCC in older patients. The PCC–MPFC connectivity in patients was not related to pain catastrophizing scores (r = − 0.04, p = 0.902) or the level of their pain during the resting state scan (r = − 0.04, p = 0.896).
Fig. 5.
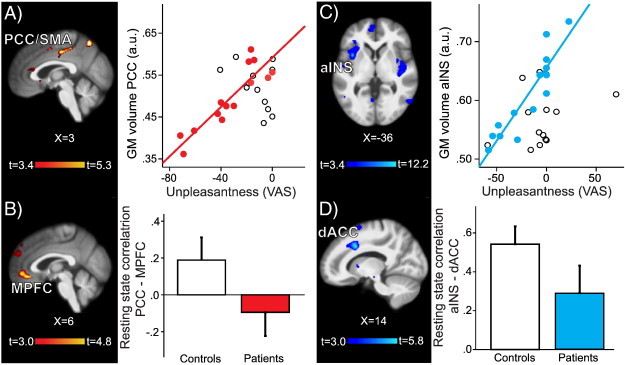
Relationship between pain sensitivity and structural and functional gray matter findings in older and younger patients. A) Left: In older patients the gray matter of the right PCC/SMA (posterior cingulate cortex/supplementary motor area) was negatively correlated with pain unpleasantness. Right: Extracted mean gray matter of the PCC/SMA cluster plotted against pain unpleasantness scores in older patients (red circles) and older controls (open circles). B) Left: Functional connectivity analysis of the PCC/SMA seed showed decreased resting state correlations with MPFC (medial prefrontal cortex) in older patients compared to matched controls; colorbar shows t-values for the contrast older patients < older controls; there were no significant clusters for the opposite contrast. Right: Parameter estimates (correlation values) for older controls (white bar) and older patients (red bar). C) Left: In younger patients the gray matter of the left aINS (anterior insula) was negatively correlated with pain unpleasantness (colorbar shows t-values for the whole brain VBM regression with pain unpleasantness score. Right: Extracted mean gray matter of the aINS cluster plotted against pain unpleasantness scores in younger patients (blue circles) and younger controls (open circles). D) Left: Functional connectivity analysis of the aINS seed showed decreased resting state correlations to dACC (dorsal anterior cingulate cortex) in younger patients compared to matched controls; colorbar shows t-values for the contrast younger < younger controls; there were no significant clusters for the opposite contrast. Right: Parameter estimates (correlation values) for younger controls (white bar) and younger patients (blue bar). Results are displayed on study average brain, left side of the brain is on the left; a.u., arbitrary unit.
3.6. In younger patients, insular gray matter is inversely related to pain sensitivity and pain catastrophizing, and has decreased resting-state correlations with dorsal ACC
Of the regions in which younger fibromyalgia patients had more gray matter than their controls, the left anterior insula (aINS) showed an inverse relationship between the amount of gray matter and patients' unpleasantness ratings of the pressure stimuli (t = 12.14, p < 0.001, cluster size 1053 voxels, MNI coordinates − 36, 9, 0; Fig. 5C), as well as pain catastrophizing scores (r = − 0.54, p = 0.044; Supplementary Fig. 4A). We next examined resting state connectivity of this region and found that in younger patients compared to their controls the left aINS had significantly decreased connectivity (weaker correlations) to dACC (t = 5.75, p = 0.021, cluster size 160 voxels, MNI coordinates 14, 14, 36; Fig. 5D), and this connectivity was in patients inversely related to pain catastrophizing scores (r = 0.59, p = 0.025; Supplementary Fig. 4B). The aINS–dACC connectivity in patients was not related to patients' pain during the resting state scan (r = − 0.29, p = 0.310). The left aINS also showed a decreased connectivity to the temporal cortex (t = 6.11, p = 0.013, cluster size 189 voxels, MNI coordinates 66, − 46, 12), also unrelated to patients' pain during the resting state scan (r = 0.02, p = 0.943). Finally, left aINS showed increased connectivity to primary somatosensory/motor cortex (S1M1; t = 4.75, p = 0.010, cluster size 206 voxels, MNI coordinates 30, − 26, 50), which was positively correlated with patients' pain during the resting state scan (r = 0.54, p = 0.047).
3.7. In younger patients, nucleus accumbens gray matter is inversely related to pain sensitivity and has increased resting-state correlations with the lateral prefrontal cortex
The only other region showing an inverse relationship between gray matter and experimental pain sensitivity in the younger fibromyalgia patients was the nucleus accumbens (NAc) (t = 6.06, p = 0.001, cluster size 280 voxels, MNI coordinates 10, 12, − 9). This cluster showed a trend for negative correlation with pain catastrophizing scores (r = − 0.49, p = 0.074; Supplementary Fig. 4C). Resting-state analysis from this area revealed increased connectivity (stronger correlations) to a cluster located in DLPFC and PMC compared to their controls (t = 4.91, p = 0.006, cluster size 222, MNI coordinates 38, 8, 34), and this connectivity was unrelated to pain catastrophizing scores (r = 0.174, p = 0.55) or patients' pain during the resting state scan (r = 0.44, p = 0.107). No region showed significantly decreased connectivity to the NAc in younger patients.
4. Discussion
Here we show fibromyalgia and age interact in that younger and older patients show distinct gray matter differences compared to controls. These structural gray matter differences were accompanied by differences in white matter and functional connectivity that also depended on patients' age. Perhaps most importantly, brain differences were related to patients' pain sensitivity, and functional disengagement between two important pain-processing regions might explain why younger patients did not show increased experimental pain sensitivity.
4.1. Age-dependent structural brain differences in chronic pain
Confirming our hypothesis, gray matter differences in fibromyalgia patients showed a significant interaction with age: while patients over 50 years old had decreased gray matter in several brain regions, patients under 50 showed exclusively gray matter increases. It should be noted that previous studies in fibromyalgia typically investigated patients with a mean age around 50 (range across studies 42 to 54, median of the means 51) without subdividing into different age groups. These studies (Burgmer et al., 2009; Kuchinad et al., 2007; Lutz et al., 2008; Puri et al., 2010; Robinson et al., 2011; Schmidt-Wilcke et al., 2007; Wood et al., 2009), as also studies in other chronic pain conditions (Davis and Moayedi, 2012; Schweinhardt and Bushnell, 2010), have mostly reported gray matter decreases, which is in line with our investigation of the whole study sample. It has been suggested that gray matter decreases are a consequence of long-term nociceptive input (Apkarian et al., 2004; Kim et al., 2008; Schmidt-Wilcke et al., 2005; Schmitz et al., 2008) also because gray matter decreases have been reported to be related to pain duration. Pain duration is likely to be correlated with age in many patient samples, as was the case in the present investigation, and it can therefore be difficult to tease out the exact contribution of each factor to brain changes (Moayedi et al., 2012a). In the present study, as well as in a previous investigation (Wartolowska et al., 2012), gray matter decreases related to pain duration across the whole patient sample were no longer significant when age was controlled for. Nevertheless, several studies suggest that gray matter decreases are more pronounced with longer pain duration independent of age effects (Apkarian et al., 2004; Geha et al., 2008; Obermann et al., 2013; Rocca et al., 2006). Interestingly, when we controlled for pain duration in the older patients, most of the gray matter decreases were no longer significant, indicating that they might indeed be related to prolonged pain. In contrast, the observed gray matter increases in the younger patients were independent of pain duration, in line with previous reports on gray matter increases in chronic pain (Absinta et al., 2012; As-Sanie et al., 2012; Blankstein et al., 2010; Moayedi et al., 2011; Rocca et al., 2006; Schmidt-Wilcke et al., 2006, 2007; Seminowicz et al., 2010; Younger et al., 2010). Thus, we propose that prolonged pain might only lead to decreased gray matter in an older or otherwise vulnerable brain.
In contrast to gray matter decreases in older patients, younger patients showed increased gray matter in the insula, basal ganglia, and the PFC, regions previously reported as larger in fibromyalgia (Schmidt-Wilcke et al., 2007) and other chronic pain conditions (Gwilym et al., 2010; Moayedi et al., 2011; Schmidt-Wilcke et al., 2006; Schweinhardt et al., 2008; Seminowicz et al., 2010; Wartolowska et al., 2012; Younger et al., 2010). Examining the literature suggests that gray matter increases are more commonly observed in younger compared to older patients, including temporomandibular disorder (TMD) (Moayedi et al., 2011; Younger et al., 2010), IBS (Blankstein et al., 2010; Seminowicz et al., 2010), pelvic (As-Sanie et al., 2012), menstrual (Tu et al., 2010), or vulvar pain (Schweinhardt et al., 2008). The notion of bi-directional gray matter changes in chronic pain is supported by closely examining two studies of total brain gray matter in patients with fibromyalgia (Kuchinad et al., 2007) and TMD (Moayedi et al., 2012a), in which steeper slopes of an increased age-related decline of gray matter appear to be driven by greater decreases in older patients as well as increases in younger patients. Interestingly, bi-directional structural brain changes are not unique to chronic pain, and have been observed in other stress- or mood-related disorders, including bipolar (Adler et al., 2007; Lyoo et al., 2004), obsessive compulsive (Atmaca et al., 2007), and post-traumatic stress (Tupler and De Bellis, 2006) disorders. The observed patterns resemble the results from the present study: younger patients present with gray matter increases while older patients show gray matter decreases.
Similar to what we observed for gray matter, white matter differences in fibromyalgia patients were age-dependent. In the younger patients, the white matter tract connecting the insula and basal ganglia showed increased FA caused by a decrease in radial diffusivity. This could possibly reflect a hindrance to water diffusion caused by the adjacent gray matter hypertrophy. In contrast, older patients had decreased FA in the white matter adjacent to their gray matter decrease, supporting several reports of compromised white matter in pain-related areas in chronic pain (Chen et al., 2011; Geha et al., 2008; Moayedi et al., 2012b), including fibromyalgia (Lutz et al., 2008; Sundgren et al., 2007). This decrease was driven by decreased axial and increased radial water diffusivity, which might indicate damaged axonal membranes, axonal swelling, or weakened myelin (Beaulieu, 2002).
4.2. Functional significance of structural brain differences
The divergent structural brain findings in younger and older patients are likely not explained by intensity or duration of clinical pain or by affective disturbances, because these variables did not differ between younger and older patients of the present study. However, only older patients showed hypersensitivity to experimental pain. Hypersensitivity is considered a hallmark of fibromyalgia, reported in many previous studies (Geisser et al., 2003; Geisser et al., 2008; Giesecke et al., 2004; Gracely et al., 2002; Kosek et al., 1996; Petzke et al., 2003), and clinically assessed in the ‘tender point count’ (Wolfe et al., 1990). It is important to note that patients in these studies are typically around the age of 50, and two studies, in addition to the present one did not find hypersensitivity in younger patients (Cook et al., 2004; Harris et al., 2009). Therefore, age might contribute to hypersensitivity to external stimuli in fibromyalgia, rather than to spontaneous widespread pain, which is the chief diagnostic criterion for the condition (Wolfe, 2010). Because pressure sensitivity was related to gray matter changes in both age groups, structural brain differences might underlie the differential experimental pain sensitivity in older and younger patients. Older patients with the highest pain sensitivity had the least amount of gray matter in the PCC, a structure implicated in skeletomotor orientation to noxious stimuli (Vogt, 2005; Vogt et al., 1996) and in evaluating the valence of potentially threatening stimuli (Maddock et al., 2003), including pain (Ochsner et al., 2006). White matter connectivity from the PCC to motor areas was compromised and the PCC of older patients showed decreased resting state correlation with the MPFC. PCC/precuneus and MPFC form central nodes of the brain's default mode network (DMN), which is disrupted by multiple chronic pain states (Baliki et al., 2008; Baliki et al., 2011a; Cauda et al., 2009), including fibromyalgia (Cifre et al., 2012; Napadow et al., 2010). Because a faulty DMN has been associated with impaired cognition (Fassbender et al., 2009; Greicius et al., 2004), the reduced PCC–MPFC connectivity reported here might be related to commonly reported cognitive deficits in fibromyalgia (Glass, 2009; Moriarty et al., 2011). In summary, older fibromyalgia patients showed evidence for maladaptive brain structure and function.
Although several previous studies have described increased regional gray matter in chronic pain, the functional significance of such increases remains unclear. The increases we observed in the younger fibromyalgia patients might reflect hypertrophy caused by over-engagement of endogenous pain modulatory systems because insula, basal ganglia, and prefrontal cortex are all important pain modulatory regions (reviewed in Schweinhardt and Bushnell, 2010). In support of this notion, the NAc, an area involved in motivation, showed increased resting-state correlation to an important pain inhibitory region, i.e. the DLPFC. In addition, experimental pain sensitivity was inversely related to gray matter volume in the aINS and the younger patients' aINS showed decreased resting-state correlation to dACC. Importantly, the altered resting-state correlation was independent of pain during the scan, suggesting a clinically relevant reorganization of the resting-state network, rather than a transient situation-related change of connectivity. The dACC is implicated in processing emotional salience and unpleasantness of painful stimuli (Rainville et al., 1999; Villemure and Bushnell, 2009; Vogt, 2005), and the aINS and dACC form the central nodes of the resting-state salience network (Seeley et al., 2007; Taylor et al., 2009), proposed to evaluate the biological relevance of internal states and incoming stimuli. Thus, decoupling of aINS and ACC in younger fibromyalgia patients could result in attenuated negative valence of nociceptive stimuli. Such adaptations could underlie the finding that younger patients had normal perception of pressure stimuli despite having chronic widespread pain that is characteristic of fibromyalgia.
4.3. Adaptive and maladaptive brain plasticity in response to environmental demand might depend on age
Based on the preceding discussion, we suggest the following model for brain changes in fibromyalgia: the brains of young fibromyalgia patients might be over-engaging endogenous pain modulatory systems in an attempt to attenuate the clinical condition. This situation leads to hypertrophy in relevant brain structures. Indeed, gray matter hypertrophy in response to stressors has been described in humans upon repeated exposure to experimental pain (Teutsch et al., 2008) and in rodents that show persisting hypertrophy of the amygdala in response to prolonged behavioral stress, mediated by increases in dendritic length and branching complexity (Vyas and Chattarji, 2004; Vyas et al., 2003). One mechanism by which the overactive pain modulatory systems might try to attenuate pain is by disconnecting brain structures that contribute to the processing and perception of pain. Consequently, the insula and the ACC would be no longer acting in concert, with the result of keeping sensitivity to external stimuli at normal levels. Thus, we propose that the anatomical brain changes in younger fibromyalgia patients are adaptive and reflect successful coping. But as the brain ages, these compensatory mechanisms and other effective pain coping mechanisms might exhaust, supported by our finding that insular gray matter showed a significant decline with age across all patients but not controls (Supplementary Fig. 3). Now, detrimental effects of prolonged pain manifest, which in turn contribute to enhanced hypersensitivity. As a result, increased hypersensitivity, the hallmark of fibromyalgia, is observed in older patients in addition to chronic widespread pain. Brain structure and function and pain coping mechanisms (Perrot et al., 2008) would have shifted from being adaptive in younger patients to being maladaptive in older patients. This age-dependent brain plasticity could have important implications for treatment considerations when faced with patients of different age groups, affected by pain or potentially other disease states.
4.4. Limitations
In any cross-sectional study, causal relationships are difficult to infer. An alternative interpretation to the one discussed above is that gray matter differences in fibromyalgia represent pre-existing structural brain differences, potentially contributing to individuals' vulnerability to develop fibromyalgia. However, because younger and older fibromyalgia patients of our study belong to the same population with respect to diagnosis, level of clinical pain, and affective disturbances, it seems improbable that the younger and older patient group should present with different pre-existing brain differences. Nevertheless, one potentially important difference between the younger and the older subjects in the present investigation is that the large majority of older subjects (patients and controls) were post-menopausal, and almost all younger subjects were pre-menopausal (Table 1). Therefore, it might be that menopausal status contributes to the differential findings in younger and older patients. However, because gray matter did not only differ between groups but also showed a relationship with age (Fig. 1), it seems unlikely that differences between younger and older patients are solely caused by menopausal status.
Because of the known effects of opioids on brain structure and function (Upadhyay et al., 2010; Younger et al., 2011), we did not include any patient using opioids. In addition, antidepressants have been shown to have a positive effect on brain gray matter (Bremner and Vermetten, 2004; Lai and Hsu, 2011; Lai and Wu, 2013). In our study, 13 of 28 patients were taking antidepressants and could have potentially influenced our findings. However, we think this is highly unlikely for several reasons. First, the use of antidepressants was equally distributed across older and younger patients while the gray matter differences of these two groups to matched control subjects were of opposite directions (decreased in the older patients, increased in the younger). Second, the regional gray matter differences observed in our study do not correspond to the areas associated with the reported effects of antidepressants. Similar arguments as for antidepressants apply to a potential confounding effect of NSAIDs, taken by a majority of patients in our study. Again, the proportion did not differ between older and younger patients and the brain areas identified in our study do not match the areas reported to be influenced by NSAIDs (Bendlin et al., 2010; Walther et al., 2011). In addition, NSAIDs have been shown to partially counteract accelerated age-related decline of gray matter volume (Bendlin et al., 2010; Walther et al., 2011), which might suggest that the observed age-related decline we observed in gray matter might have been more pronounced had the patients not been taking NSAIDs.
Acknowledgments
We acknowledge the American Fibromyalgia Syndrome Association (AFSA) for the financial support. This research was supported in part by the Intramural Research Program of the NIH, National Center for Complementary and Alternative Medicine. We thank the team at the McConnell Brain Imaging Centre of the Montreal Neurological Institute for the expert MRI data acquisition. We further thank Valerie Cotton, Marissa Lapedis, and Carl Frechette for the subject recruitment. Finally, we thank David Seminowicz, Brian Walitt, and Hakan Olausson for the valuable comments on the manuscript.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest: The authors declare no competing financial interests.
Appendix A. Supplementary data
Supplementary material.
References
- Absinta M., Rocca M.A., Colombo B., Falini A., Comi G., Filippi M. Selective decreased grey matter volume of the pain-matrix network in cluster headache. Cephalalgia. 2012;32:109–115. doi: 10.1177/0333102411431334. [DOI] [PubMed] [Google Scholar]
- Adler C.M., DelBello M.P., Jarvis K., Levine A., Adams J., Strakowski S.M. Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biol. Psychiatry. 2007;61:776–781. doi: 10.1016/j.biopsych.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Apkarian A.V., Sosa Y., Sonty S., Levy R.M., Harden R.N., Parrish T.B., Gitelman D.R. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- As-Sanie S., Harris R.E., Napadow V., Kim J., Neshewat G., Kairys A., Williams D., Clauw D.J., Schmidt-Wilcke T. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153:1006–1014. doi: 10.1016/j.pain.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmaca M., Yildirim H., Ozdemir H., Tezcan E., Poyraz A.K. Volumetric MRI study of key brain regions implicated in obsessive-compulsive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:46–52. doi: 10.1016/j.pnpbp.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Baliki M.N., Geha P.Y., Apkarian A.V., Chialvo D.R. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Baria A.T., Apkarian A.V. The cortical rhythms of chronic back pain. J. Neurosci. 2011;31:13981–13990. doi: 10.1523/JNEUROSCI.1984-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Schnitzer T.J., Bauer W.R., Apkarian A.V. Brain morphological signatures for chronic pain. PLoS One. 2011;6:e26010. doi: 10.1371/journal.pone.0026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system — a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Behrens T.E., Berg H.J., Jbabdi S., Rushworth M.F., Woolrich M.W. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendlin B.B., Newman L.M., Ries M.L., Puglielli L., Carlsson C.M., Sager M.A., Rowley H.A., Gallagher C.L., Willette A.A., Alexander A.L., Asthana S., Johnson S.C. NSAIDs may protect against age-related brain atrophy. Front. Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blankstein U., Chen J., Diamant N.E., Davis K.D. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138:1783–1789. doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Vermetten E. Neuroanatomical changes associated with pharmacotherapy in posttraumatic stress disorder. Ann. N. Y. Acad. Sci. 2004;1032:154–157. doi: 10.1196/annals.1314.012. [DOI] [PubMed] [Google Scholar]
- Burgmer M., Pogatzki-Zahn E., Gaubitz M., Wessoleck E., Heuft G., Pfleiderer B. Altered brain activity during pain processing in fibromyalgia. NeuroImage. 2009;44:502–508. doi: 10.1016/j.neuroimage.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Cauda F., Sacco K., Duca S., Cocito D., D'Agata F., Geminiani G.C., Canavero S. Altered resting state in diabetic neuropathic pain. PLoS One. 2009;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.Y., Blankstein U., Diamant N.E., Davis K.D. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res. 2011;1392:121–131. doi: 10.1016/j.brainres.2011.03.069. [DOI] [PubMed] [Google Scholar]
- Chikama M., McFarland N.R., Amaral D.G., Haber S.N. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J. Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifre I., Sitges C., Fraiman D., Munoz M.A., Balenzuela P., Gonzalez-Roldan A., Martinez-Jauand M., Birbaumer N., Chialvo D.R., Montoya P. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom. Med. 2012;74:55–62. doi: 10.1097/PSY.0b013e3182408f04. [DOI] [PubMed] [Google Scholar]
- Clauw D.J., Crofford L.J. Chronic widespread pain and fibromyalgia: what we know, and what we need to know. Best Pract. Res. Clin. Rheumatol. 2003;17:685–701. doi: 10.1016/s1521-6942(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Cook D.B., Lange G., Ciccone D.S., Liu W.C., Steffener J., Natelson B.H. Functional imaging of pain in patients with primary fibromyalgia. J. Rheumatol. 2004;31:364–378. [PubMed] [Google Scholar]
- Cordes D., Haughton V.M., Arfanakis K., Wendt G.J., Turski P.A., Moritz C.H., Quigley M.A., Meyerand M.E. Mapping functionally related regions of brain with functional connectivity MR imaging. Am. J. Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Craig C.L., Marshall A.L., Sjostrom M., Bauman A.E., Booth M.L., Ainsworth B.E., Pratt M., Ekelund U., Yngve A., Sallis J.F., Oja P. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Davis K.D., Moayedi M. Central mechanisms of pain revealed through functional and structural MRI. J. Neuroimmune Pharmacol. 2012 doi: 10.1007/s11481-012-9386-8. [DOI] [PubMed] [Google Scholar]
- Engvig A., Fjell A.M., Westlye L.T., Moberget T., Sundseth O., Larsen V.A., Walhovd K.B. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum. Brain Mapp. 2012;33:2390–2406. doi: 10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C., Zhang H., Buzy W.M., Cortes C.R., Mizuiri D., Beckett L., Schweitzer J.B. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Zhang D., Snyder A.Z., Raichle M.E. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum. Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha P.Y., Baliki M.N., Harden R.N., Bauer W.R., Parrish T.B., Apkarian A.V. The brain in chronic CRPS pain: abnormal gray–white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisser M.E., Casey K.L., Brucksch C.B., Ribbens C.M., Appleton B.B., Crofford L.J. Perception of noxious and innocuous heat stimulation among healthy women and women with fibromyalgia: association with mood, somatic focus, and catastrophizing. Pain. 2003;102:243–250. doi: 10.1016/S0304-3959(02)00417-7. [DOI] [PubMed] [Google Scholar]
- Geisser M.E., Glass J.M., Rajcevska L.D., Clauw D.J., Williams D.A., Kileny P.R., Gracely R.H. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J. Pain. 2008;9:417–422. doi: 10.1016/j.jpain.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Giesecke T., Gracely R.H., Grant M.A., Nachemson A., Petzke F., Williams D.A., Clauw D.J. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- Glass J.M. Review of cognitive dysfunction in fibromyalgia: a convergence on working memory and attentional control impairments. Rheum. Dis. Clin. N. Am. 2009;35:299–311. doi: 10.1016/j.rdc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Golestani N., Paus T., Zatorre R.J. Anatomical correlates of learning novel speech sounds. Neuron. 2002;35:997–1010. doi: 10.1016/s0896-6273(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Gracely R.H., Petzke F., Wolf J.M., Clauw D.J. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Srivastava G., Reiss A.L., Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwilym S.E., Filippini N., Douaud G., Carr A.J., Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum. 2010;62:2930–2940. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- Harris R.E., Sundgren P.C., Craig A.D., Kirshenbaum E., Sen A., Napadow V., Clauw D.J. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S., Nichols T.E. Validating cluster size inference: random field and permutation methods. NeuroImage. 2003;20:2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Jin C., Yuan K., Zhao L., Zhao L., Yu D., von Deneen K.M., Zhang M., Qin W., Sun W., Tian J. Structural and functional abnormalities in migraine patients without aura. NMR Biomed. 2013;26:58–64. doi: 10.1002/nbm.2819. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Suh S.I., Seol H.Y., Oh K., Seo W.K., Yu S.W., Park K.W., Koh S.B. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008;28:598–604. doi: 10.1111/j.1468-2982.2008.01550.x. [DOI] [PubMed] [Google Scholar]
- Kosek E., Ekholm J., Hansson P. Sensory dysfunction in fibromyalgia patients with implications for pathogenic mechanisms. Pain. 1996;68:375–383. doi: 10.1016/s0304-3959(96)03188-0. [DOI] [PubMed] [Google Scholar]
- Kuchinad A., Schweinhardt P., Seminowicz D.A., Wood P.B., Chizh B.A., Bushnell M.C. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J. Neurosci. 2007;27:4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lado F.A., Sankar R., Lowenstein D., Moshe S.L. Age-dependent consequences of seizures: relationship to seizure frequency, brain damage, and circuitry reorganization. Ment. Retard. Dev. Disabil. Res. Rev. 2000;6:242–252. doi: 10.1002/1098-2779(2000)6:4<242::AID-MRDD3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Lai C.H., Hsu Y.Y. A subtle grey-matter increase in first-episode, drug-naive major depressive disorder with panic disorder after 6 weeks' duloxetine therapy. Int. J. Neuropsychopharmacol. 2011;14:225–235. doi: 10.1017/S1461145710000829. [DOI] [PubMed] [Google Scholar]
- Lai C.H., Wu Y.T. Changes in gray matter volume of remitted first-episode, drug-naive, panic disorder patients after 6-week antidepressant therapy. J. Psychiatr. Res. 2013;47:122–127. doi: 10.1016/j.jpsychires.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Lederbogen F., Kirsch P., Haddad L., Streit F., Tost H., Schuch P., Wust S., Pruessner J.C., Rietschel M., Deuschle M., Meyer-Lindenberg A. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- Loggia M.L., Mogil J.S., Catherine Bushnell M. Empathy hurts: compassion for another increases both sensory and affective components of pain perception. Pain. 2008;136:168–176. doi: 10.1016/j.pain.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Lowe M.J., Mock B.J., Sorenson J.A. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lutz J., Jager L., de Q.D., Krauseneck T., Padberg F., Wichnalek M., Beyer A., Stahl R., Zirngibl B., Morhard D., Reiser M., Schelling G. White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis Rheum. 2008;58:3960–3969. doi: 10.1002/art.24070. [DOI] [PubMed] [Google Scholar]
- Lyoo I.K., Kim M.J., Stoll A.L., Demopulos C.M., Parow A.M., Dager S.R., Friedman S.D., Dunner D.L., Renshaw P.F. Frontal lobe gray matter density decreases in bipolar I disorder. Biol. Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Maddock R.J., Garrett A.S., Buonocore M.H. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum. Brain Mapp. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. Experience-dependent structural plasticity in the adult human brain. Trends Cogn. Sci. 2011;15:475–482. doi: 10.1016/j.tics.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Moayedi M., Weissman-Fogel I., Crawley A.P., Goldberg M.B., Freeman B.V., Tenenbaum H.C., Davis K.D. Contribution of chronic pain and neuroticism to abnormal forebrain gray matter in patients with temporomandibular disorder. NeuroImage. 2011;55:277–286. doi: 10.1016/j.neuroimage.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Moayedi M., Weissman-Fogel I., Salomons T.V., Crawley A.P., Goldberg M.B., Freeman B.V., Tenenbaum H.C., Davis K.D. Abnormal gray matter aging in chronic pain patients. Brain Res. 2012;1456:82–93. doi: 10.1016/j.brainres.2012.03.040. [DOI] [PubMed] [Google Scholar]
- Moayedi M., Weissman-Fogel I., Salomons T.V., Crawley A.P., Goldberg M.B., Freeman B.V., Tenenbaum H.C., Davis K.D. White matter brain and trigeminal nerve abnormalities in temporomandibular disorder. Pain. 2012;153:1467–1477. doi: 10.1016/j.pain.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Moriarty O., McGuire B.E., Finn D.P. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog. Neurobiol. 2011;93:385–404. doi: 10.1016/j.pneurobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Napadow V., LaCount L., Park K., As-Sanie S., Clauw D.J., Harris R.E. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann M., Rodriguez-Raecke R., Naegel S., Holle D., Mueller D., Yoon M.S., Theysohn N., Blex S., Diener H.C., Katsarava Z. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage. 2013;74:352–358. doi: 10.1016/j.neuroimage.2013.02.029. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ludlow D.H., Knierim K., Hanelin J., Ramachandran T., Glover G.C., Mackey S.C. Neural correlates of individual differences in pain-related fear and anxiety. Pain. 2006;120:69–77. doi: 10.1016/j.pain.2005.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peelle J.E., Cusack R., Henson R.N. Adjusting for global effects in voxel-based morphometry: gray matter decline in normal aging. NeuroImage. 2012;60:1503–1516. doi: 10.1016/j.neuroimage.2011.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot S., Poiraudeau S., Kabir M., Bertin P., Sichere P., Serrie A., Rannou F. Active or passive pain coping strategies in hip and knee osteoarthritis? Results of a national survey of 4,719 patients in a primary care setting. Arthritis Rheum. 2008;59:1555–1562. doi: 10.1002/art.24205. [DOI] [PubMed] [Google Scholar]
- Petzke F., Khine A., Williams D., Groner K., Clauw D.J., Gracely R.H. Dolorimetry performed at 3 paired tender points highly predicts overall tenderness. J. Rheumatol. 2001;28:2568–2569. [PubMed] [Google Scholar]
- Petzke F., Clauw D.J., Ambrose K., Khine A., Gracely R.H. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105:403–413. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- Price D.D., McGrath P.A., Rafii A., Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- Puri B.K., Agour M., Gunatilake K.D., Fernando K.A., Gurusinghe A.I., Treasaden I.H. Reduction in left supplementary motor area grey matter in adult female fibromyalgia sufferers with marked fatigue and without affective disorder: a pilot controlled 3-T magnetic resonance imaging voxel-based morphometry study. J. Int. Med. Res. 2010;38:1468–1472. doi: 10.1177/147323001003800429. [DOI] [PubMed] [Google Scholar]
- Rainville P., Carrier B., Hofbauer R.K., Bushnell M.C., Duncan G.H. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain. 1999;82:159–171. doi: 10.1016/S0304-3959(99)00048-2. [DOI] [PubMed] [Google Scholar]
- Randolph J.F., Jr., Crawford S., Dennerstein L., Cain K., Harlow S.D., Little R., Mitchell E.S., Nan B., Taffe J., Yosef M. The value of follicle-stimulating hormone concentration and clinical findings as markers of the late menopausal transition. J. Clin. Endocrinol. Metab. 2006;91:3034–3040. doi: 10.1210/jc.2006-0243. [DOI] [PubMed] [Google Scholar]
- Robinson M.E., Craggs J.G., Price D.D., Perlstein W.M., Staud R. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J. Pain. 2011;12:436–443. doi: 10.1016/j.jpain.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca M.A., Ceccarelli A., Falini A., Colombo B., Tortorella P., Bernasconi L., Comi G., Scotti G., Filippi M. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke. 2006;37:1765–1770. doi: 10.1161/01.STR.0000226589.00599.4d. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Raecke R., Niemeier A., Ihle K., Ruether W., May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J. Neurosci. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad Z.S., Gotts S.J., Murphy K., Chen G., Jo H.J., Martin A., Cox R.W. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoh A.J., Huque M.F., Dubey S.D. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat. Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T., Leinisch E., Straube A., Kampfe N., Draganski B., Diener H.C., Bogdahn U., May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65:1483–1486. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T., Leinisch E., bauer S., Draganski B., Bogdahn U., Altmeppen J., May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T., Luerding R., Weigand T., Jurgens T., Schuierer G., Leinisch E., Bogdahn U. Striatal grey matter increase in patients suffering from fibromyalgia — a voxel-based morphometry study. Pain. 2007;132:S109–S116. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Schmitz N., dmiraal-Behloul F., Arkink E.B., Kruit M.C., Schoonman G.G., Ferrari M.D., van Buchem M.A. Attack frequency and disease duration as indicators for brain damage in migraine. Headache. 2008;48:1044–1055. doi: 10.1111/j.1526-4610.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- Schneider P., Scherg M., Dosch H.G., Specht H.J., Gutschalk A., Rupp A. Morphology of Heschl's gyrus reflects enhanced activation in the auditory cortex of musicians. Nat. Neurosci. 2002;5:688–694. doi: 10.1038/nn871. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P., Bushnell M.C. Pain imaging in health and disease—how far have we come? J. Clin. Invest. 2010;120:3788–3797. doi: 10.1172/JCI43498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinhardt P., Kuchinad A., Pukall C.F., Bushnell M.C. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140:411–419. doi: 10.1016/j.pain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz D.A., Labus J.S., Bueller J.A., Tillisch K., Naliboff B.D., Bushnell M.C., Mayer E.A. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48–57. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz D.A., Wideman T.H., Naso L., Hatami-Khoroushahi Z., Fallatah S., Ware M.A., Jarzem P., Bushnell M.C., Shir Y., Ouellet J.A., Stone L.S. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci. 2011;31:7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets E.M., Garssen B., Bonke B., De Haes J.C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Sullivan M.J., Bishop S.R., Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol. Assess. 1995;7:524–532. [Google Scholar]
- Sundgren P.C., Petrou M., Harris R.E., Fan X., Foerster B., Mehrotra N., Sen A., Clauw D.J., Welsh R.C. Diffusion-weighted and diffusion tensor imaging in fibromyalgia patients: a prospective study of whole brain diffusivity, apparent diffusion coefficient, and fraction anisotropy in different regions of the brain and correlation with symptom severity. Acad. Radiol. 2007;14:839–846. doi: 10.1016/j.acra.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Taylor K.S., Seminowicz D.A., Davis K.D. Two systems of resting state connectivity between the insula and cingulate cortex. Hum. Brain Mapp. 2009;30:2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teutsch S., Herken W., Bingel U., Schoell E., May A. Changes in brain gray matter due to repetitive painful stimulation. NeuroImage. 2008;42:845–849. doi: 10.1016/j.neuroimage.2008.05.044. [DOI] [PubMed] [Google Scholar]
- Tu C.H., Niddam D.M., Chao H.T., Chen L.F., Chen Y.S., Wu Y.T., Yeh T.C., Lirng J.F., Hsieh J.C. Brain morphological changes associated with cyclic menstrual pain. Pain. 2010;150:462–468. doi: 10.1016/j.pain.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Tukey J.W., Ciminera J.L., Heyse J.F. Testing the statistical certainty of a response to increasing doses of a drug. Biometrics. 1985;41:295–301. [PubMed] [Google Scholar]
- Tupler L.A., De Bellis M.D. Segmented hippocampal volume in children and adolescents with posttraumatic stress disorder. Biol. Psychiatry. 2006;59:523–529. doi: 10.1016/j.biopsych.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Upadhyay J., Maleki N., Potter J., Elman I., Rudrauf D., Knudsen J., Wallin D., Pendse G., McDonald L., Griffin M., Anderson J., Nutile L., Renshaw P., Weiss R., Becerra L., Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemure C., Bushnell M.C. Mood influences supraspinal pain processing separately from attention. J. Neurosci. 2009;29:705–715. doi: 10.1523/JNEUROSCI.3822-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemure C., Slotnick B.M., Bushnell M.C. Effects of odors on pain perception: deciphering the roles of emotion and attention. Pain. 2003;106:101–108. doi: 10.1016/s0304-3959(03)00297-5. [DOI] [PubMed] [Google Scholar]
- Vogt B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B.A., Derbyshire S., Jones A.K. Pain processing in four regions of human cingulate cortex localized with co-registered PET and MR imaging. Eur. J. Neurosci. 1996;8:1461–1473. doi: 10.1111/j.1460-9568.1996.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Vyas A., Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behav. Neurosci. 2004;118:1450–1454. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- Vyas A., Bernal S., Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Walther K., Bendlin B.B., Glisky E.L., Trouard T.P., Lisse J.R., Posever J.O., Ryan L. Anti-inflammatory drugs reduce age-related decreases in brain volume in cognitively normal older adults. Neurobiol. Aging. 2011;32:497–505. doi: 10.1016/j.neurobiolaging.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Wartolowska K., Hough M.G., Jenkinson M., Andersson J., Wordsworth B.P., Tracey I. Structural changes of the brain in rheumatoid arthritis. Arthritis Rheum. 2012;64:371–379. doi: 10.1002/art.33326. [DOI] [PubMed] [Google Scholar]
- Wolfe F. New American College of Rheumatology criteria for fibromyalgia: a twenty-year journey. Arthritis Care Res. 2010;62:583–584. doi: 10.1002/acr.20156. [DOI] [PubMed] [Google Scholar]
- Wolfe F., Smythe H.A., Yunus M.B., Bennett R.M., Bombardier C., Goldenberg D.L., Tugwell P., Campbell S.M., Abeles M., Clark P. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- Wolfe F., Clauw D.J., Fitzcharles M.A., Goldenberg D.L., Katz R.S., Mease P., Russell A.S., Russell I.J., Winfield J.B., Yunus M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- Wood P.B., Glabus M.F., Simpson R., Patterson J.C., 2nd Changes in gray matter density in fibromyalgia: correlation with dopamine metabolism. J. Pain. 2009;10:609–618. doi: 10.1016/j.jpain.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Worsley K.J., Marrett S., Neelin P., Vandal A.C., Friston K.J., Evans A.C. A unified statistical approach for determining significant signals in images of cerebral activation. Hum. Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Worsley K.J., Andermann M., Koulis T., MacDonald D., Evans A.C. Detecting changes in nonisotropic images. Hum. Brain Mapp. 1999;8:98–101. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<98::AID-HBM5>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger J.W., Shen Y.F., Goddard G., Mackey S.C. Chronic myofascial temporomandibular pain is associated with neural abnormalities in the trigeminal and limbic systems. Pain. 2010;149:222–228. doi: 10.1016/j.pain.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger J.W., Chu L.F., D'Arcy N.T., Trott K.E., Jastrzab L.E., Mackey S.C. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152:1803–1810. doi: 10.1016/j.pain.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler G., Dahnke R., Jancke L., Yotter R.A., May A., Gaser C. Brain structural trajectories over the adult lifespan. Hum. Brain Mapp. 2011;33:2377–2389. doi: 10.1002/hbm.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.


