Abstract
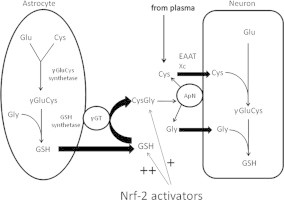
Neurons rely on the release and subsequent cleavage of GSH to cysteinylglycine (CysGly) by astrocytes in order to maintain optimal intracellular GSH levels. In neurodegenerative diseases characterised by oxidative stress, neurons need an optimal GSH supply to defend themselves against free radicals released from activated microglia and astroglia. The rate of GSH synthesis is controlled largely by the activity of γ-glutamyl cysteine ligase. Expression of γ-glutamyl cysteine ligase and of the Xc- system, which facilitates cystine uptake, is regulated by the redox-sensitive transcription factor, nuclear factor erythroid-2-related factor 2 (Nrf2). Compounds that can activate the Nrf2-ARE pathway, referred to as ‘Nrf2 activators’ are receiving growing attention due to their potential as GSH-boosting drugs.
This study compares four known Nrf2 activators, R-α-Lipoic acid (LA), tert-butylhydroquinone (TBHQ), sulforaphane (SFN) and Polygonum cuspidatum extract containing 50% resveratrol (PC-Res) for their effects on astroglial release of GSH and CysGly. GSH levels increased dose-dependently in response to all four drugs. Sulforaphane produced the most potent effect, increasing GSH by up to 2.4-fold. PC-Res increased GSH up to 1.6-fold, followed by TBHQ (1.5-fold) and LA (1.4-fold). GSH is processed by the ectoenzyme, γ-glutamyl transpeptidase, to form CysGly. Once again, SFN produced the most potent effect, increasing CysGly by up to 1.7-fold, compared to control cells. TBHQ and PC-Res both induced fold increases of 1.3, followed by LA with a fold increase of 1.2. The results from the present study showed that sulforaphane, followed by lipoic acid, resveratrol and Polygonum multiflorum were all identified as potent “GSH and Cys-Gly boosters”.
Abbreviations: ARE, antioxidant response elements; CysGly, cysteinylglycine; DMEM, Dulbeccos's Modified Eagle Medium; GSH, glutathione; HCys, homocysteine; Nrf2, nuclear factor erythroid-2-related factor 2; LA, α-lipoic acid; PC, Polygonum cuspidatum; ROS, reactive oxygen species; SFN, sulforaphane; TBHQ, Tert-butylhydroquinone
Keywords: Astroglia, Nrf2 activators, Glutathione, Cysteinylglycine
Graphical abstract
Highlights
-
•
R-α-Lipoic acid (LA), tert-butylhydroquinone (TBHQ), sulforaphane (SFN) and Polygonum cuspidatum extract containing 50% resveratrol (PC-Res) increase astroglial release of GSH.
-
•
Sulforaphane produced the most potent effect, increasing GSH by up to 2.4-fold. PC-Res increased GSH up to 1.6-fold, followed by TBHQ (1.5-fold) and LA (1.4-fold). GSH is processed by the ectoenzyme, γ-glutamyl transpeptidase, to form CysGly.
-
•
Once again, SFN produced the most potent effect, increasing CysGly by up to 1.7-fold, compared to control cells. TBHQ and PC-Res both induced fold increases of 1.3, followed by LA with a fold increase of 1.2.
Introduction
Oxidative stress, defined as an imbalance between the production and detoxification of reactive oxygen species (ROS), is thought to play a significant role in the neurodegeneration evident in Alzheimer's disease (AD) [15].
Glutathione (GSH), a tripeptide consisting of glutamate, cysteine and glycine is the key regulator of the intracellular redox state. It can non-enzymatically detoxify ROS, such as superoxide and hydroxyl radicals, as well as act as an electron donor for the reduction of peroxides, catalysed by glutathione peroxidase [4]. GSH is synthesised from its constituent amino acids by the sequential action of two enzymes, the rate-limiting enzyme γ-glutamyl cysteine ligase and glutathione synthetase [24].
In the brain, neurons rely on the release and subsequent cleavage of GSH by astrocytes in order to maintain optimal intracellular GSH levels [9]. Extracellular GSH released from astrocytes is metabolised by γ-glutamyl transpeptidase to form the dipeptide, cysteinylglycine (CysGly), which is then processed by the neuronal ectopeptidase, aminopeptidase N, allowing neurons to immediately take up the resultant cysteine and glycine.
In neurodegenerative diseases such as AD, neurons need an optimal GSH supply to defend themselves against free radicals, such as superoxide and nitric oxide, released from activated microglia and astrocytes [2,32]. Therapeutic strategies enabling astrocytes to provide neurons with sufficient substrates for GSH synthesis is of particular interest as reductions in neuronal GSH levels may contribute to neuronal cell death in a pro-oxidative, pro-inflammatory environment.
The rate of GSH synthesis is controlled largely by the activity of γ-glutamyl cysteine ligase, the first enzyme required for GSH synthesis, and by the availability of cysteine/cystine [10,12,3]. Expression of the catalytic and modulatory subunits of γ-glutamyl cysteine ligase and of the Xc-system, which facilitates cystine uptake, are regulated by the redox-sensitive transcription factor, nuclear factor erythroid-2-related factor 2 (Nrf2) [8]. Under basal conditions, Nrf2 interacts with Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm and undergoes ubiquitin-mediated proteasomal degradation [25]. Upon oxidative modification of cysteine residues within Keap1, Keap1 dissociates from Nrf2, permitting Nrf2 translocation into the nucleus. Once in the nucleus, Nrf2 binds to antioxidant response elements (ARE) present in the regulatory regions of a range of phase II antioxidant defence genes, including numerous GSH related genes. In addition to γ-glutamate cysteine ligase and the Xc-system, Nrf2 also regulates the expression of glutathione synthetase, the second enzyme required for synthesis of GSH, multidrug resistance protein transporters which export GSH from the cell and γ-glutamyl transpeptidase, the ectoenzyme responsible for cleavage of glutamate from GSH to form CysGly [28,18].
Therefore, compounds that can activate the Nrf2-ARE pathway, referred to as ‘Nrf2 activators’ are receiving growing attention due to their potential as GSH-boosting drugs for application to a wide range of oxidative stress related diseases [6,33,34].
This study compares four known Nrf2 activators, R-α-lipoic acid, tert-butylhydroquinone, sulforaphane and Polygonum cuspidatum extract containing 50% resveratrol for their effects on astroglial release of GSH and production of CysGly. R-α-lipoic acid (LA) is a naturally occurring dithiol compound with a broad neuroprotective capacity that appears to slow cognitive decline in AD patients [13]. Sulforaphane (SFN) is an organosulphur compound that is extracted from edible plants such as broccoli, brussels sprouts and cabbage and has been described as possessing anticarcinogenic, anti-inflammatory and antioxidant properties [21]. The roots of Polygonum cuspidatum (PC), commonly known as Japanese knotweed, are used in Traditional Chinese medicine for treatment of inflammatory diseases, hepatitis and tumours [7]. Polygonum cuspidatum contains resveratrol (trans-3,5,4′-trihydroxystilbene), a polyphenolic compound that has gained wide attention due to its purported anti-cancer properties [16]. Tert-butylhydroquinone (TBHQ) is an aromatic organic compound, which is used as a synthetic food grade antioxidant.
Although all four of these compounds have previously been shown to increase intracellular GSH synthesis via the Nrf2-ARE pathway [17,23,27,29], this is the first study to investigate the effect of Nrf2 activators on astrocytic production of the neuronal GSH substrate, CysGly. Furthermore, we also monitored the effect of Nrf2-activation on the levels of homocysteine (HCys), a thiol compound that is metabolically related to GSH, but is toxic to neurons at elevated levels [20,35].
Materials and methods
Cell culture and experimental protocols
The U373-MG human astrocytoma cell line was kindly provided by Dr. Peter Lock (The Royal Melbourne Hospital, Australia). U373 cells were maintained in Dulbeccos's Modified Eagle Medium (DMEM) containing 25 mM glucose, supplemented with 200 U/ml penicillin, 200 µg/ml streptomycin, 2.6 μg/ml fungizone, 200 mM glutamine and 5% foetal bovine serum (FBS). Cells were grown in 175 cm2 tissue culture flasks and incubated at 37 °C in 5% CO2. Cells were harvested with a solution containing 0.05% trypsin and 0.02% EDTA in phosphate buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) and seeded into 96 well, flat-bottom, tissue culture plates at a density of 9×103 cells/well. FBS concentration was reduced to 3% to minimise the proliferation and the total volume of media in each well was 100 µl. After 24 h incubation, the media was replaced with fresh media containing different concentrations of potential GSH booster drugs for 48 h. All cell culture materials were from Invitrogen (Mulgrave, Australia).
Preparation of “GSH booster” drug solutions
LA, TBHQ and SFN were dissolved in 10% ethanol to produce stock solutions of 1 mg/ml. Polygonum cuspidatum extract (standardised to contain 50% resveratrol: PA-Res) was dissolved in dimethylsulphoxide to produce a stock solution of 100 mg/ml. Stock solutions were diluted in DMEM to give concentrations of 12.5–50 µM, 2.5–10 µM, 2.5–10 µM and 1–3.9 µg/ml of LA, TBHQ, SFN and PA-Res, respectively.
Analysis of cell viability
Cell viability was assessed in terms of the metabolic capability of cells to convert the fluorogenic redox indicator, resazurin, into its highly fluorescent product, resorufin. A modified version of the resazurin-reduction assay was used [5]. Resazurin was dissolved in PBS to give a concentration of 0.001% (w/v). This solution was sterile filtered (0.22 µm), protected from light with aluminium foil and stored at 4 °C for up to six months. To determine cell viability, incubation media was removed from wells and replaced with 100 µl of resazurin solution. Plates were incubated at 37 °C, with 5% CO2 for 45 min and then fluorescence was measured with excitation at 530 nm and emission at 590 nm in a POLARstar Omega microplate reader (BMG Labtech). For every plate, background fluorescence determined in cell-free wells was subtracted from all wells, and values were expressed as a percentage of untreated control cells.
Determination of extracellular GSH and related thiols by high performance liquid chromatography and fluorescence detection
A Dionex HPLC system consisting of an ASI-100 automated sample injector, a P680 solvent pump, a TCC-100 thermostatted column compartment and an RF-2000 fluorescence detector was used for all chromatographic analyses. The system was equipped with a Luna C18(2) column (150 mm×4.6 mm id, 3 µm) protected by a SecurityGuardC18 Cartridge (4.0 mm×3.0 mm) in a SecurityGuardCartridge Holder supplied by Phenomenex. The Chromeleon 6.8 Chromatography Data System from Dionex was used to control instruments, acquire data and quantify peak areas.
Detection of thiols was performed as described previously [31]. Briefly, media taken from U373 cells treated for 48 h with LA, TBHQ, SFN or PC-Res was centrifuged at 200g for 5 min at 4 °C to pellet cellular debris. Following centrifugation, the supernatant was mixed with an equal volume of 1% 5-sulfosalicylic acid containing 1 mM EDTA, centrifuged at 14,000g for 10 min at 4 °C to precipitate protein, and the resulting supernatant placed in fresh tubes and stored at −80 °C until analysis. Upon thawing of samples and standard solutions, fresh microcentrifuge vials were placed in a heating block at 35 °C and 50 µl of sample or standard added. To reduce all disulphide bonds, 30 µl of a 1 mM solution of the reducing agent Tris(2-carboxyethyl)phosphine hydrochloride was added. For the derivatisation reaction, vials were incubated for 5 min at 35 °C before the addition of 100 µl of borate buffer (0.1 M, pH 9.3, with 1 mM EDTA) and 30 µl of the derivatising agent 4-fluoro-7-aminosulfonylbenzofurazan (ABD-F; Novachem) (1 mg/ml in 0.1 M borate buffer). Samples were incubated at 35 °C for 10 min, before the reaction was stopped by addition of 50 µl of 2 M hydrochloric acid. Vials were then centrifuged at 14,000g for 5 min at 4 °C in order to pellet any particulates that could potentially damage the HPLC system. Supernatants were placed into fresh vials and loaded into an autosampler. The autosampler maintained sample temperatures at 8 °C to prevent evaporation and injected 10 µl aliquots for analysis. The mobile phase used for separation of ABD-F-derivatised thiols was 0.1 M acetate buffer (pH 4)-methanol [86:14]. An isocratic programme with a flow rate of 1 ml/min was used and column temperature was maintained at 35 °C. The fluorescence detector was set to an excitation and emission wavelength of 390 nm and 510 nm, respectively, with high level sensitivity.
Statistics
Data presented are the mean of three independent experiments and error bars denote standard deviation (SD). Significant differences were assessed by one-way ANOVA with Dunnett's post hoc tests and shown as ⁎p<0.05, ⁎⁎p<0.01, and ⁎⁎⁎p<0.001.
Results
U373 cell viability was assessed using the resazurin assay after 48 h treatment with increasing concentrations of LA, TBHQ, SFN and PC-Res to enable selection of non-toxic concentrations for further experiments (data not shown). To test the effect of Nrf2 activation on the release of total extracellular GSH and its derivatives, U373 astroglial cells were treated with 12.5–50 µM LA, 2.5–10 µM TBHQ, 2.5–10 µM SFN or 1–3.9 µg/ml of PC-Res for 48 h. The concentrations of total extracellular GSH, CysGly and HCys in the media were then determined by HPLC with fluorescence detection.
As shown in Fig. 1, GSH levels increased dose-dependently in response to all four drugs. SFN produced the most potent effect, increasing GSH by up to 2.4-fold compared to control cells. PC-Res, the second most potent drug, increased GSH up to 1.6-fold, followed by TBHQ (1.5-fold) and LA (1.4-fold).
Fig. 1.
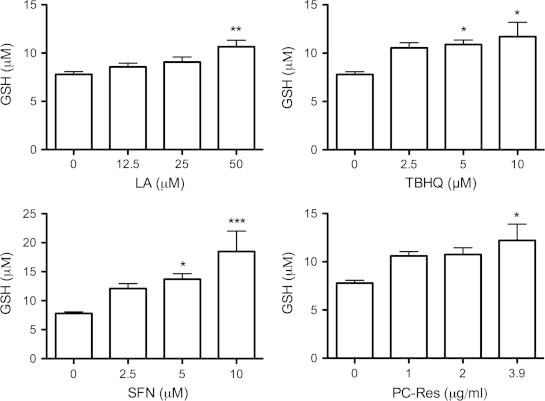
Nrf2 activators increased extracellular GSH levels. U373 cells were treated with R-lipoic acid (LA), tert-butylhydroquinone (TBHQ), sulforaphane (SFN) or Polygonum cuspidatum containing 50% resveratrol (PC-Res) for 48 h. The concentration of glutathione (GSH) in the media was then determined by HPLC with fluorescence detection. Data points represent mean values from three independent experiments, while error bars represent SD. ⁎ and ⁎⁎⁎ designate significant differences (p<0.05 and p<0.001) to the non-treated control.
Extracellular GSH is processed by the ectoenzyme, γ-glutamyl transpeptidase, to form CysGly, an important substrate for neuronal GSH synthesis. Therefore, we tested whether Nrf2 activation of U373 cells resulted in elevated CysGly in the media. Once again SFN produced the most potent effect, increasing CysGly by up to 1.7-fold, compared to control cells. TBHQ and PC-Res both induced fold increases of 1.3, followed by LA with a fold increase of 1.2 (Fig. 2).
Fig. 2.
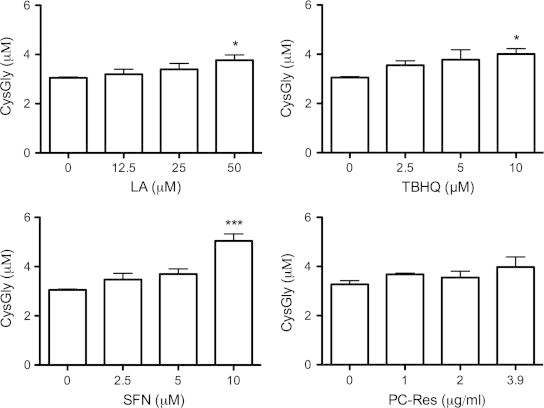
Nrf2 activators increase extracellular CysGly levels. U373 cells were treated with R-lipoic acid (LA), tert-butylhydroquinone (TBHQ), sulforaphane (SFN) or Polygonum cuspidatum containing 50% resveratrol (PC-Res) for 48 h. The concentration of cysteinylglycine (CysGly) in the media was then determined by HPLC with fluorescence detection. Data points represent mean values from three independent experiments, while error bars represent SD. ⁎ and ⁎⁎⁎ designate significant differences (p<0.05 and p<0.001) to the non-treated control.
While the order of drug potency remained almost the same for boosting GSH and CysGly, all drugs were able to increase GSH levels between 1.2 and 1.4 times more than they were able to increase CysGly (Table 1).
Table 1.
Fold differences of thiol concentrations in media between cells treated with highest, non-toxic concentrations of drugs and non-treated control cells.
| Drugs | GSH | CysGly | Hcys | Ratio GSH:CysGlya |
|---|---|---|---|---|
| LA | 1.4⁎⁎ | 1.2⁎ | 1.6 | 1.2 |
| TBHQ | 1.5⁎⁎ | 1.3⁎ | 0.9 | 1.2 |
| SFN | 2.4⁎⁎ | 1.7⁎⁎ | 1.0 | 1.4 |
| PC-Res | 1.6⁎⁎ | 1.3 | 1.0 | 1.2 |
Concentrations of lipoic acid (LA), tert-butylhydroquinone (TBHQ), sulforaphane (SFN) and Polygonum cuspidatum extract containing 50% resveratrol (PC-Res) used to treat cells were 50 µM, 10 µM, 10 µM and 3.9 µg/ml.
Ratio represents fold change in glutathione (GSH) divided by fold change in cysteinylglycine (CysGly).
Designate significant differences (p<0.05) to the non-treated control.
Designate significant differences (p<0.001) to the non-treated control.
HCys is another thiol compound that is metabolically related to GSH, however it has been shown to be toxic to neurons at elevated concentrations [20,35]. We have previously shown that inflammation-stressed astroglial cells increase their release of HCys [30]. Therefore, we were also interested in whether Nrf2 activation resulted in increased HCys in the media. When HCys was measured in the media of cells treated with 12.5–50 µM LA, 2.5–10 µM TBHQ, 2.5–10 µM SFN or 1–3.9 µg/ml PC-Res, and in non-treated control cells. Although a trend for HCys increase for LA was observed, no significant differences were found between any of the conditions (data not shown, but fold-changes are summarised in Table 1).
Discussion
The main aim of this study was to determine whether activation of Nrf2 in astrocytes results in increased GSH release by astrocytes as well as elevated levels of extracellular CysGly. Treatment of U373 cells with four known Nrf2 activators, LA, TBHQ, SFN and PA-Res, resulted in elevated levels of both GSH and CysGly (not significant for PC-Res) in the media. For both thiols, SFN followed by TBHQ, produced the most potent effects. When comparing the extent of the increase in GSH compared to the extent of the increase in CysGly, a larger increase was seen for GSH compared to CysGly in all four cases. It showed that, although in total more CysGly is produced by Nrf2 activated cells, a smaller percentage of extracellular GSH is being converted to CysGly in the Nrf2 activated cells compared to control cells. This finding is supported by a publication from 1996, which showed that γ-glutamyl transpeptidase (GGT) and γ-glutamate cysteine ligase (GCL) are differentially enhanced by TBHQ in rat lung epithelial L2 cells. The highest mRNA level of GGT occurred after 12 h treatment with 50 μM TBHQ, after which it decreased back to the control level by 24 h. On the other hand, GCL-mRNA level peaked after 6 h treatment with 50 μM TBHQ but was still significantly elevated after 24 h. Under the same conditions, GCL activity increased significantly after 6 h, whereas an increase in GGT was not observed until after 16 h. From nuclear run-on experiments that confirmed that the increase in GCL-mRNA, but not GGT-mRNA, was due to increased transcription, the authors suggested that the increase in GGT-mRNA probably results from a decreased degradation rate [21].
Importantly, despite a reduced rate of increase compared to extracellular GSH, significant increases in total CysGly were observed for SFN, TBHQ and LA, therefore suggesting their suitability for use as GSH booster drugs, capable of increasing delivery of GSH precursors to neurons.
When levels of HCys were analysed in media collected from cultures of U373 cells treated with LA, TBHQ, SFN and PC-Res, no changes in HCys levels were observed. HCys, a sulphydrul-containing amino acid that is metabolically linked to GSH via the transulfuration pathway is recognised as an independent risk factor for a variety of chronic diseases, including AD [11,19,22,26]. It has previously been shown that HCys exported from astrocytes is harmful to adjacent neurons through the activation of neuronal NMDA-type glutamatergic receptors and induction of oxidative stress and apoptosis [1,14,20]. The absence of an increase in HCys levels observed in response to the selected Nrf2 activators can therefore be considered as a positive outcome.
Conclusions
Based on the results presented here, LA, TBHQ, and especially SFN increase astroglial provision of GSH and CysGly, without rising extracellular levels of HCys. They therefore represent promising candidates as Nrf2-activation based drugs (if therapeutic concentration can be achieved in the target tissue) for the treatment of AD and related diseases. Additionally, based on our observation that Nrf2 activation in astroyctes increased CysGly in the media, but at a slightly reduced rate compared to GSH, co-application of direct neuronal GSH precursors such as CysGly or other cysteine homologues might also be useful.
Submission declaration
This work has not been published previously or submitted elsewhere.
This work was carried out in accordance with the Code of Ethics of the World Medical Association.
Acknowledgements
We thank the University of Western Sydney for financial support through the College Research Grant scheme and Alzheimer's Australia for their financial support through the Dementia Research grants programme.
Megan L. Steele was supported financially by the Hunter Postgraduate Research Scholarship from Alzheimer's Australia.
The sponsor(s) had no input in the study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Althausen S., Paschen W. Homocysteine-induced changes in mRNA levels of genes coding for cytoplasmic- and endoplasmic reticulum-resident stress proteins in neuronal cell cultures. Molecular Brain Research. 2000;84:32–40. doi: 10.1016/s0169-328x(00)00208-4. [DOI] [PubMed] [Google Scholar]
- 2.Aschner M. Neuron–astrocyte interactions: implications for cellular energetics and antioxidant levels. Neurotoxicology. 2000;21:1101–1107. [PubMed] [Google Scholar]
- 3.Bender A.S., Reichelt W., Norenberg M.D. Characterization of cystine uptake in cultured astrocytes. Neurochemistry International. 2000;37:269–276. doi: 10.1016/s0197-0186(00)00035-8. [DOI] [PubMed] [Google Scholar]
- 4.Brannan T.S., Maker H.S., Weiss C., Cohen G. Regional distribution of glutathione peroxidase in the adult rat brain. Journal of Neurochemistry. 1980;35:1013–1014. doi: 10.1111/j.1471-4159.1980.tb07102.x. [DOI] [PubMed] [Google Scholar]
- 5.Buranrat B., Prawan A., Kukongviriyapan V. Optimization of resazurin-based assay for cytotoxicity test in cholangiocarcinoma cells. KKU Research Journal. 2008;8:73–80. [Google Scholar]
- 6.Chen X.L., Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Current Pharmaceutical Design. 2004;10:879–891. doi: 10.2174/1381612043452901. [DOI] [PubMed] [Google Scholar]
- 7.Chu X., Sun A., Liu R. Preparative isolation and purification of five compounds from the Chinese medicinal herb Polygonum cuspidatum Sieb. et Zucc by high-speed counter-current chromatography. Journal of Chromatography A. 2005;1097:33–39. doi: 10.1016/j.chroma.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Correa F., Ljunggren E., Mallard C., Nilsson M., Weber S.G., Sandberg M. The Nrf2-inducible antioxidant defense in astrocytes can be both up- and down-regulated by activated microglia:involvement of p38 MAPK. Glia. 2011;59:785–799. doi: 10.1002/glia.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dringen R., Hamprecht B. N-acetylcysteine, but not methionine or 2-oxothiazolidine-4-carboxylate, serves as cysteine donor for the synthesis of glutathione in cultured neurons derived from embryonal rat brain. Neuroscience Letters. 1999;259:79–82. doi: 10.1016/s0304-3940(98)00894-5. [DOI] [PubMed] [Google Scholar]
- 10.Dringen R., Pfeiffer B., Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. Journal of Neuroscience. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dwyer B.E., Raina A.K., Perry G., Smith M.A. Homocysteine and Alzheimer's disease: a modifiable risk? Free Radical Biology and Medicine. 2004;36:1471–1475. doi: 10.1016/j.freeradbiomed.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Griffith O.W., Mulcahy R.T. The enzymes of glutathione synthesis: gamma-glutamylcysteine synthetase. Advances in Enzymology and Related Areas of Molecular Biology. 1999;73:209–267. doi: 10.1002/9780470123195.ch7. (xii) [DOI] [PubMed] [Google Scholar]
- 13.Hager K., Kenklies M., McAfoose J., Engel J., Munch G. Alpha-lipoic acid as a new treatment option for Alzheimer's disease-a 48 months follow-up analysis. Journal of Neural Transmission Supplementa. 2007:189–193. doi: 10.1007/978-3-211-73574-9_24. [DOI] [PubMed] [Google Scholar]
- 14.Ho P.I., Ortiz D., Rogers E., Shea T.B. Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. Journal of Neuroscience Research. 2002;70:694–702. doi: 10.1002/jnr.10416. [DOI] [PubMed] [Google Scholar]
- 15.Karelson E., Bogdanovic N., Garlind A., Winblad B., Zilmer K., Kullisaar T., Vihalemm T., Kairane C., Zilmer M. The cerebrocortical areas in normal brain aging and in Alzheimer's disease: noticeable differences in the lipid peroxidation level and in antioxidant defense. Neurochemical Research. 2001;26:353–361. doi: 10.1023/a:1010942929678. [DOI] [PubMed] [Google Scholar]
- 16.Kimura Y., Okuda H. Resveratrol isolated from Polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor-induced neovascularization in Lewis lung carcinoma-bearing mice. Journal of Nutrition. 2001;131:1844–1849. doi: 10.1093/jn/131.6.1844. [DOI] [PubMed] [Google Scholar]
- 17.Kode A., Rajendrasozhan S., Caito S., Yang S.R., Megson I.L., Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. American journal of physiology. Lung Cellular and Molecular Physiology. 2008;294:L478–488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 18.Kraft A.D., Johnson D.A., Johnson J.A. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. Journal of Neuroscience. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z., Sun L., Zhang H., Liao Y., Wang D., Zhao B., Zhu Z., Zhao J., Ma A., Han Y., Wang Y., Shi Y., Ye J., Hui R. Elevated plasma homocysteine was associated with hemorrhagic and ischemic stroke, but methylenetetrahydrofolate reductase gene C677T polymorphism was a risk factor for thrombotic stroke: a multicenter case-control study in China. Stroke. 2003;34:2085–2090. doi: 10.1161/01.STR.0000086753.00555.0D. [DOI] [PubMed] [Google Scholar]
- 20.Lipton S.A., Kim W.K., Choi Y.B., Kumar S., D’Emilia D.M., Rayudu P.V., Arnelle D.R., Stamler J.S. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5923–5928. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu R.M., Hu H., Robison T.W., Forman H.J. Differential enhancement of gamma-glutamyl transpeptidase and gamma-glutamylcysteine synthetase by tert-butylhydroquinone in rat lung epithelial L2 cells. American Journal of Respiratory Cell and Molecular Biology. 1996;14:186–191. doi: 10.1165/ajrcmb.14.2.8630269. [DOI] [PubMed] [Google Scholar]
- 22.Pietrzik K. Homocysteine as a cardiovascular marker and risk factor. Clinical Research in Cardiology. 2006;95(Suppl. 6):VI28–33. doi: 10.1007/s00392-006-1806-4. [DOI] [PubMed] [Google Scholar]
- 23.Rababah T.M., Hettiarachchy N.S., Horax R. Total phenolics and antioxidant activities of fenugreek, green tea, black tea, grape seed, ginger, rosemary, gotu kola, and ginkgo extracts, vitamin E, and tert-butylhydroquinone. Journal of Agricultural and Food Chemistry. 2004;52:5183–5186. doi: 10.1021/jf049645z. [DOI] [PubMed] [Google Scholar]
- 24.Rahman I., Antonicelli F., MacNee W. Molecular mechanism of the regulation of glutathione synthesis by tumor necrosis factor and dexamethasone in human alveolar epithelial cells. Journal of Biological Chemistry. 1999;274:5088. doi: 10.1074/jbc.274.8.5088. [DOI] [PubMed] [Google Scholar]
- 25.Schonhusen U., Kuhla S., Zitnan R., Wutzke K.D., Huber K., Moors S., Voigt J. Effect of a soy protein-based diet on ribonucleic acid metabolism in the small intestinal mucosa of goat kids. Journal of the American Dairy Science. 2007;90:2404–2412. doi: 10.3168/jds.2006-502. [DOI] [PubMed] [Google Scholar]
- 26.Seshadri S., Beiser A., Selhub J., Jacques P.F., Rosenberg I.H., D’Agostino R.B., Wilson P.W., Wolf P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. New England Journal of Medicine. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 27.Shay K.P., Michels A.J., Li W., Kong A.T., Hagen T.M. Cap-independent Nrf2 translation is part of a lipoic acid-stimulated detoxification stress response. Biochimica et Biophysica Acta. 2012;1823:1101–1109. doi: 10.1016/j.bbamcr.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shih A.Y., Johnson D.A., Wong G., Kraft A.D., Jiang L., Erb H., Johnson J.A., Murphy T.H. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. Journal of Neuroscience. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinkai Y., Sumi D., Fukami I., Ishii T., Kumagai Y. Sulforaphane, an activator of Nrf2, suppresses cellular accumulation of arsenic and its cytotoxicity in primary mouse hepatocytes. FEBS Letters. 2006;580:1771–1774. doi: 10.1016/j.febslet.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 30.Steele M.L., Fuller S., Maczurek A.E., Kersaitis C., Ooi L., Munch G. Chronic inflammation alters production and release of glutathione and related thiols in human U373 astroglial cells. Cellular and Molecular Neurobiology. 2013;33:19–30. doi: 10.1007/s10571-012-9867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steele M.L., Ooi L., Munch G. Development of a high-performance liquid chromatography method for the simultaneous quantitation of glutathione and related thiols. Analytical Biochemistry. 2012;429:45–52. doi: 10.1016/j.ab.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Steele M.L., Robinson S.R. Reactive astrocytes give neurons less support: implications for Alzheimer's disease. Neurobiology of Aging. 2012;33(423):e421–413. doi: 10.1016/j.neurobiolaging.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Surh Y.J. NF-kappa B and Nrf2 as potential chemopreventive targets of some anti-inflammatory and antioxidative phytonutrients with anti-inflammatory and antioxidative activities. Asia Pacific Journal of Clinical Nutrition. 2008;17(Suppl. 1):269–272. [PubMed] [Google Scholar]
- 34.Vargas M.R., Johnson J.A. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Reviews in Molecular Medicine. 2009;11:e17. doi: 10.1017/S1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vitvitsky V., Thomas M., Ghorpade A., Gendelman H.E., Banerjee R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. Journal of Biological Chemistry. 2006;281:35785–35793. doi: 10.1074/jbc.M602799200. [DOI] [PubMed] [Google Scholar]


