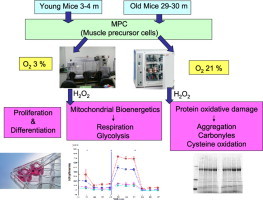
Abstract
In the majority of studies using primary cultures of myoblasts, the cells are maintained at ambient oxygen tension (21% O2), despite the fact that physiological O2 at the tissue level in vivo is much lower (~1–5% O2). We hypothesized that the cellular response in presence of high oxygen concentration might be particularly important in studies comparing energetic function or oxidative stress in cells isolated from young versus old animals. To test this, we asked whether oxygen tension plays a role in mitochondrial bioenergetics (oxygen consumption, glycolysis and fatty acid oxidation) or oxidative damage to proteins (protein disulfides, carbonyls and aggregates) in myoblast precursor cells (MPCs) isolated from young (3–4 m) and old (29–30 m) C57BL/6 mice. MPCs were grown under physiological (3%) or ambient (21%) O2 for two weeks prior to exposure to an acute oxidative insult (H2O2). Our results show significantly higher basal mitochondrial respiration in young versus old MPCs, an increase in basal respiration in young MPCs maintained at 3% O2 compared to cells maintained at 21% O2, and a shift toward glycolytic metabolism in old MPCs grown at 21% O2. H2O2 treatment significantly reduced respiration in old MPCs grown at 3% O2 but did not further repress respiration at 21% O2 in old MPCs. Oxidative damage to protein was higher in cells maintained at 21% O2 and increased in response to H2O2 in old MPCs. These data underscore the importance of understanding the effect of ambient oxygen tension in cell culture studies, in particular studies measuring oxidative damage and mitochondrial function.
Keywords: Myoblasts, Mitochondrial respiration, Protein damage, Hypoxia, Oxidative stress
Graphical abstract
Highlights
-
•
Myoblast precursor cells (MPC) were isolated from old and young mice.
-
•
The effect of ambient (21%) or physiological (3%) O2 tension on MPCs was addressed.
-
•
Mitochondrial bioenergetics after H2O2 insult was determined regards to age.
-
•
3% O2 induces old MPCs to shift from glycolysis toward oxidative phosphorylation.
-
•
Protein oxidative damage was higher in old MPCs at 21% O2.
Introduction
Oxygen is an essential element for life. It is a critical signaling molecule and a key intermediate for a diverse array of events that control normal cellular function. However, oxygen is also the substrate for the generation of potentially harmful reactive oxygen species (ROS). Therefore, changes in oxygen concentration could contribute to cell dysfunction, disease and premature aging in a number of ways. The majority of cell culture studies in vitro are conducted using cells cultured at ambient oxygen tension (21% O2), despite the fact that physiological oxygen pressure at the organ and cellular level is known to be much lower, and existing data suggest that 21% oxygen tension is itself an oxidative stress for cells [1–4]. In the current study, we asked whether high levels of oxygen tension can impact pathways related to mitochondrial function and oxidative stress in a cell culture model of myoblast precursor cells (MPCs), the proliferating progenitor cell population resident in skeletal muscle. MPCs have been frequently used as a model to study oxygen tension in cell culture [5,6], but almost nothing is known with respect to the effect of O2 levels in cells from older mice. Previous studies modifying O2 in tissue cultures have shown an effect on cell growth and differentiation [7,8] as well as the induction of cellular senescence [9,10], although the biochemical and molecular mechanisms leading to these outcomes are still poorly understood.
Because oxygen supply to respiring tissue, cells, and mitochondria regulates metabolism, gene expression, and cell fate [11], and mitochondrial oxygen consumption also contributes to the production of free radicals, we asked whether oxygen tension is an effector of mitochondrial bioenergetics in cell culture and whether this is altered by aging. We also asked whether changes in oxygen tension impact cellular oxidative damage by monitoring changes in protein oxidation. Protein oxidation is an important contributor to the progressive loss of functional cellular processes that lead to aging. Oxidation of specific amino acids induces changes in structure and function, resulting in protein mis-folding and aggregation that can lead to cellular disorders and age-related diseases. It has been shown that cells grown under low O2 tension display lower levels of oxidative damage to DNA [10,12], but the effect of low O2 tension on protein oxidation, in particular during the formation of oxidized/cross-linked protein aggregates, has not been studied.
We found that young MPCs at 3% O2 proliferate faster than at 21% O2, yet there was not a significant difference in mitochondrial oxygen consumption at the different oxygen tensions. Old MPCs cultured at 21% O2 showed a glycolytic metabolism in comparison with young MPCs, however they showed a shift toward oxidative phosphorylation at 3% O2. Also, cells cultured at 21% presented higher protein damage. Our findings suggest that higher oxygen tension not only can impact protein oxidation, but also mitochondrial bioenergetics and cell metabolism. Further experiments are needed to fully understand the physiological role of oxygen tension conditions for aging studies.
Materials and methods
MPC cell culture
MPCs were obtained according to the method described by Lee et al. [6]. Briefly, cells were isolated from the hind limb muscles from young mice (3–4 months) and old mice (29–30 months) of male C57BL/6 J mice (The Jackson Laboratory, Bar Harbor, ME). Muscles were minced into a coarse slurry and digested for 1 h with pronase (650 U/mL, Calbiochem, San Diego, CA) in DMEM (American Type Culture Collection, Manassas, VA) containing 25 mM HEPES at 37 °C with gentle agitation. The digest was mechanically dissociated by triturating the muscle slurry repeatedly and filtered through a 100 µm filter (Millipore, Bedford, MA). The filtered digest was centrifuged through an isotonic Percoll gradient (60% overlaid with 20%, GE Healthcare, Piscataway, NJ). Cells were collected from the interface of the Percoll gradient and resuspended in a primary growth medium (GM) containing Ham's F-10 (Invitrogen, Carlsbad, CA), 20% FBS (VWR, West Chester, PA), 10 ng/mL fibroblast growth factor-2 (FGF-2; Promega, Madison, WI),100 U/mL penicillin G, and 100 µg/mL streptomycin (Invitrogen) and grown on collagen 0.1 mg/mL, (PureCol, INAMED Biometarials, The Netherlands)-coated tissue culture plates.
MPC expansion and prevention of differentiation were accomplished by culture in the growth medium (GM, mentioned above) and collagen-coated plates. Cultures were 96±2.3% positive for MyoD by immunocytochemistry, confirming their myoblast origin. The cells were expanded under ambient oxygen tension and frozen at passage-five and then thawed under high or low oxygen tension respectively. Cells were cultured for 2 weeks at 3% O2 and 21% O2 with CO2 kept at a constant 5% in both conditions. Cells at 3% O2 were maintained under strict low O2 conditions throughout the study in a customized gas-controlled ex vivo incubator (Coy Labs, Grass Lake, MI). Although we acknowledge that the addition of 5% CO2 to room air gas may decrease the O2 tension to less than 21% O2, in this work we will refer to those cultures maintained to ambient oxygen tension as 21% O2.
H2O2 treatment
In order to study cellular response to oxidative stress in relation to mitochondrial bioenergetics and proteostasis during low oxygen tension, cells where challenged with an acute and sub-lethal oxidative insult of 100 μM H2O2 for 19 h. Of note, we used this time point because our cell viability assay showed that the best time was between 18–24 h post H2O2 treatment. Because the Seahorse Bioscience XF24 Extracellular Flux Analyzer experiments need extra time (~4 h) for setting up the cells for the experiment itself, we decided to incubate for 19 h with H2O2 and then start the analysis in the Extracellular Flux Analyzer. After that time, mitochondrial function and protein damage were assayed.
Proliferation rate
After two weeks at 3% O2 or 21% O2, MPCs were trypsinized and seeded at a cell density of 2.5×103 cells per well on a 24-well multi-chamber plate (Corning, Acton MA, USA). The entire population of cells in a well was quantified and scored in order to measure the proliferation rate as described elsewhere [13]. Determinations were performed every 2 days in triplicate in three independent cultures. The number of living cells in 10 μl of this suspension was scored using five fields of a hemocytometer under a phase-contrast optical microscope. All determinations were always performed with sub-confluent cultures.
Measurement of mitochondrial bioenergetics
To measure mitochondrial function in intact MPC, a Seahorse Bioscience XF24 Extracellular Flux Analyzer was used (North Billerica, MA. USA). The optimal seeding density needed to obtain a measurable OCR (oxygen consumption rate) and ECAR (extracellular acidification rate) was established as described elsewhere [14]. The seeding density used was 25,000 cells per well. A standard running medium (DMEM medium+4.5 g/L glucose and l-glutamine, with 1 mM sodium pyruvate) was used (Invitrogen, Carlsbad, CA). The mitochondrial function assay employed is known as BOFA: Basal respiration followed by sequential injections of Oligomycin, FCCP and Antimycin A (1 µM). The BOFA assay is used to define a number of mitochondrial parameters such as basal OCR, maximal respiratory capacity, reserve respiratory capacity, non-mitochondrial oxygen consumption, etc [15]. The BOFA assay was performed at 3% O2 and 21% O2 to evaluate the cellular bioenergetics at these two conditions. All the experiments were done with the same cellular number, after each BOFA assay, cells were lysed to quantify total protein by Bradford assay [16]. Using these data, the Seahorse Bioscience XF24 Extracellular Flux Analyzer software normalized the results considering the protein content, and expressed them as pmole/min.
Fatty acid oxidation
Cellular oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were used to monitor fatty acid oxidation (FAO) in real time using the Seahorse XF24 Extracellular Flux Analyzer [17]. MPCs were plated into 24-well plates at a cellular density of 25,000 cells per well (Seahorse Bioscience, Billerica, MA, USA) The assay media used was the low-buffered KHB buffer consisting of 110 mM NaCl, 4.7 mM KCl, 2 mM MgSO4 1.2 mM Na2HPO4, 2.5 mM glucose adjusted to pH7.4 supplemented with 0.5 mM carnitine. OCR and ECAR were measured at basal rates and after palmitateconjugated to BSA (0.2 mM) or BSA alone was injected.
Measurement of cysteine oxidation
Cysteine oxidation was measured by detection of disulfides [18]. Briefly, 2×106 cells were homogenized in 50 mM potassium phosphate buffer (pH 8.0) containing 0.5 mM MgCl2, 1 mM EDTA, 150 mM iodoacetamide, and protease inhibitor mixture [500 µM 4-(2-aminoethyl) benzenesulfonyl fluoride, 150 nM aprotinin, 0.5 mM EDTA, and 1 µM leupeptinhemisulfate] and centrifuged for 30 min at 16,000g at 4 °C. For the disulfide assay, samples were incubated in phosphate buffer (pH 8.0) also containing 150 mM iodoacetamide, which reacts with free thiol groups; the free iodoacetamide was removed by protein precipitation with 10%trichloroacetic acid and washed 3 times with 100% ethanol/ethyl acetate (1:1). Samples were resuspended in 8 M urea and incubated with 1 mM DTT for 30 min at 37 °C to reduce disulfide bonds in the samples. Free thiol groups (–SH) arising from the reduced disulfides were labeled with 1 mM 6-IAF, a fluorescent-tagged iodoacetamide. For the sulfenic assay (reversible cysteine oxidation), cytosolic samples were incubated with 6 M Urea and 2 mM DTT for 1 hat 37 °C. Then free thiol groups arising from these reducing conditions were blocked with 200 mM iodoacetmide. AsO3 (5 mM) was added to reduce the reversible oxidation in cysteines, followed by 1 mM DTT. Free thiol groups arising from these reducing conditions were labeled with 1 mM 6-IAF. For determination of irreversible cysteine oxidation, we measured the total amount of cysteine indirectly (thiol and reversible oxidation) by incubating with 6 M Urea and 2 mM DTT for 1 h at 37 °C. Then 5 mM of AsO3 was added to reduce all reversible oxidation. Free thiol groups arising from these reducing conditions were labeled with 1 mM 6-IAF. Protein concentration was measured by using the Bradford method [16], and 10 µg of protein was subjected to gel electrophoresis. After electrophoresis, the image of the fluorescent protein and the total amount of protein (measured by Sypro Ruby staining) were captured by using the Typhoon 9400 scanner (emission filter 526 and 620 nm, respectively; Amersham Bioscience). The intensities of fluorescence and Sypro Ruby were calculated for each protein by using Image Quant 5.0 software (Molecular Dynamics). Data were expressed as nmol of cystine oxidation/mg of protein.
Protein carbonyls in cytosolic fraction
Protein carbonyls were measured as previously described [19]. Cells were homogenized in deaerated 20 mM phosphate buffer pH 6.0 containing 0.5 mM MgCl2, 1 mM EDTA and a cocktail of protease inhibitors, centrifuged at 100,000g for 1 h at 4 °C to obtain the cytosolic soluble fraction. The supernatant was treated with 1% streptomycin sulfate and centrifuged at 11,000g for 10 min at room temperature followed by treatment with 0.3 M guanidine HCl for 1 h at 37 °C. The cytosolic fraction was treated with fluorescein-5-thiocarbazide (1 mM) (FTC) under anaerobic conditions for 2 h in the dark followed by treatment with 20% TCA (1:1) (v/v) and centrifugation at 11,000g for 10 min at room temperature. The pellets were washed with ethanol/ethyl acetate mixture (1:1) at least 4 times to remove the free FTC. The pellets were dissolved in 50 mM phosphate buffer pH 7.9 containing 6 M urea and 1 mM DTT, and the protein concentration was measured by the Bradford protein assay [16]. Equal amounts (15 µg) of FTC-labeled proteins were resolved by SDS-PAGE. Gel images were acquired on a Typhoon 9400 variable mode imager (GE Healthcare/Amersham Biosciences) to determine the fluorescence of FTC labeled proteins. After capturing the fluorescence data, gels were stained overnight in the dark with SYPRO Ruby (Invitrogen) to assess protein content and, in addition, to calculate the FTC to SYPRO Ruby fluorescence intensity ratio.
Protein aggregates
Protein aggregation was measured in cells cultured at 21 and 3% oxygen. Cells were harvested in 50 mM potassium phosphate (pH 6.9) containing 0.5 mM MgCl2 and 1 mM EDTA. The homogenates were centrifuged at 16,000g for 15 min at 4 °C, and the supernatant (cytosol) was used in the assays of protein aggregation. To obtain protein aggregates, the cytosolic extracts were centrifuged further at 100,000g for 30 min, and the resulting pellets were dissolved in an equal volume of 50 mM phosphate buffer (pH 7.9) containing 2% SDS, 0.5% NP-40, 0.5% sodium deoxycholate and 1 mM DTT. Equal amounts of volume obtained from each pellet were resolved by SDS-PAGE.
Data analysis
Data are reported as the means±SD or standard error, for at least four independent experiments performed in triplicate. The Kruskal–Wallis analysis was used to compare the bioenergetics results, because the data did not have a normal distribution, and the media were different, along with two tests for multiple comparisons in order to determine the differences between groups, the Bonferroni and the Z test. The ANOVA test was used for the protein data. A 0.05 level of probability was used as a minimum criterion of significance.
Results
Cell proliferation rate under low oxygen tension
The proliferation rate of MPCs derived from old (OM) and young mice (YM), at 3% and 21% O2 was quantified. The results in Fig. 1 show that low oxygen (3%) increases the cell proliferation rate in both young and old MPCs starting from the fifth day of culture. The insert in Fig. 1A was used as an example to compare cell proliferation, in this case at day eight of culture. At day eight, cell proliferation was significantly higher in both young (3-fold higher) and old (4.4-fold higher) MPCs at 3% versus 21% O2. The proliferation was also higher in young versus old MPCs at 3% O2, but the increase was smaller than that observed at 21%. The proliferation rate was also higher in young versus old MPCs at 21% O2 (3-foldhigher). Old MPCs cultured at 3% O2 more than doubled their proliferation compared to the proliferation at 21% O2. It is interesting to note that there was a slower and less robust differentiation of MPCs at 21% O2 for both YM and OM, which is illustrated by the representative photographs in Fig. 1B. Furthermore, cell proliferation observed for young MPCs at 21% O2 was almost the same as the old MPCs proliferation rate at 3% O2. Similar results were observed at the quantified time points. Together these data confirm the toxicity of high oxygen conditions, implying that higher oxygen tension decreases cell proliferation.
Fig. 1.
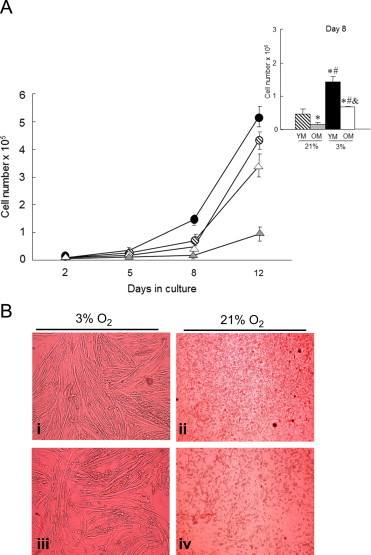
Low oxygen tension (3%) increases cell proliferation rate in both young and old MPCs. (A) Cell proliferation rate was measured in young and old MPCs under 3 and 21% O2 conditions. Young MPCs are represented by circles: black for 3% O2 and pinstriped for 21% O2. Old MPCs are represented by triangles: white for 3% O2 and gray for 21% O2. The insert in this figure shows the quantization of the cell proliferation rate at the eighth day of the culture. Each point represents the mean±SE of 3 determinations performed in three independent experiments. Statistical significance is marked as follows: ⁎Different from YM 21% O2; p<0.05; #Different from OM 21% O2; p<0.05; &Different from YM 3% O2; p<0.05. (B) High oxygen conditions (21%) decrease MPC differentiation. After 2 weeks at 3% or 21% O2, FBS 10% was replaced for horse serum 2% for 6 days to induce myotube differentiation. Representative photographs were obtained with a Zeiss microscope and a color camera Nikon Ds (magnification 4×); i: YM 3%; ii: YM 21%; iii: OM 3%; iv: OM 21%. n=3.
Mitochondrial bioenergetics
Cellular bioenergetics in intact MPCs was determined using the BOFA protocol in the Seahorse Bioscience XF24 Extracellular Flux Analyzer as described in materials and methods. The basal and the maximal oxygen consumption rates (OCR) as well as maximal mitochondrial function or maximal OCR were determined. The basal OCR is referred to as basal mitochondrial respiration without the addition of respiratory uncouplers or inhibitors, while the maximal OCR represents maximal mitochondrial respiration and is assessed by adding the proton ionophore FCCP (a mitochondrial uncoupler) to the cells, since respiratory chain uncoupling stimulates oxygen consumption, forcing the system to its maximum capacity.
Shown in Fig. 2A (21% O2) and 2B (3% O2) are two complete representative BOFA experiments (total 97 min). Fig. 2C shows the quantification of both basal and maximal OCR under different oxygen tension conditions (arrows in Fig. 2A and B indicate the time points that were used to construct Fig. 2C). At 21% O2, old MPCs have lower basal and maximal OCR (64% and 72% respectively, in comparison to young MPCs). It is interesting to note that old MPCs cultured at 3% O2 respire at a rate similar to young MPCs (at both 21 and 3% O2) (white bars in Fig. 2C). When cells were treated with H2O2to induce a sublethal oxidative stress, there was an approximate 30% decrease in both basal and maximal OCR in young MPCs at 21% O2. In contrast, there was no decline in OCR in OM cells after oxidative insult, potentially because the OCR had already reached minimal threshold. These results concur with the proliferation data and suggest that old MPCs are more robust at physiological (3% O2) than at ambient air (21% O2), and although H2O2 treatment decreased basal (51%) and maximal (59%) OCR in OM cells at 3%, their performance was still significantly better than their homolog cells at 21% O2.
Fig. 2.
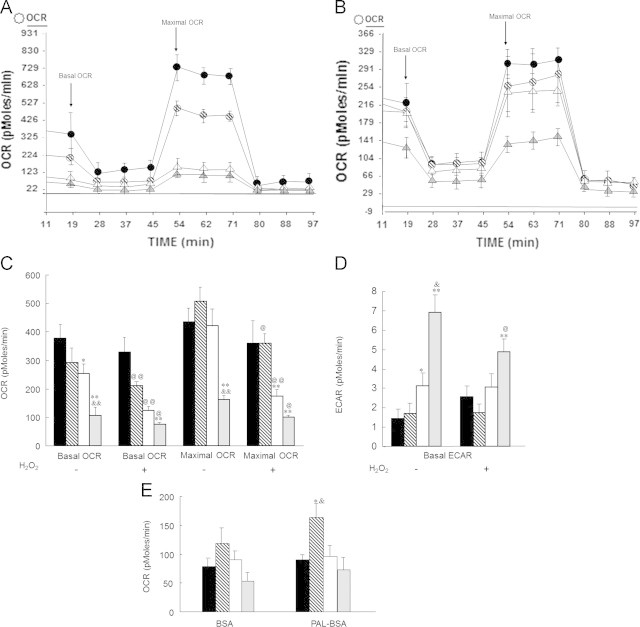
Low oxygen tension (3%) induces old MPCs to shift from glycolytic metabolism toward oxidative phosphorylation. Cellular bioenergetics in intact MPC was determined using the BOFA protocol in the Seahorse Bioscience XF24 Extracellular Flux Analyzer. Fig. 2A shows a representative BOFA assay performed at 21% O2; Fig. 2B shows a representative BOFA assay performed at 3% O2. Young MPCs are represented by circles: black for 3% O2 and hatched for 21% O2. Old MPCs are represented by triangles: white for 3% O2 and gray for 21% O2. Fig. 2C shows the basal and maximal oxygen consumption rate (OCR) under different O2 tensions; Fig. 2D shows the extracellular acidification rate (ECAR) under different O2 tensions. Fig. 2E shows the OCR due to fatty acid oxidation (FAO). Young MPCs are represented by black bars for 3% O2 and hatched bars for 21% O2. Old MPCs are represented by white bars for 3% O2 and gray bars for 21% O2. The assays were performed with and without oxidative treatment (100 μM H2O2 for 19 h). Each point represents the mean±SE of four independent experiments. Statistical significance is marked as follows: &represents difference between different O2, i.e. OM 3% O2 vs. OM 21% O2; &p<0.05; &&p<0.005. ⁎represents difference between the same O2, i.e. YM vs. OM at 3% or YM vs. OM at 21%. ⁎p<0.05; ⁎⁎p<0.005. @represents difference between control and H2O2 treated, @p<0.05; @@p<0.005.
In summary, these experiments show that there is no significant difference in basal or maximal respiration rate in young MPCs as a result of the oxygen tension where they were grown, and that neither is there a decrease in their OCR after H2O2 treatment. However, old MPCs were susceptible to H2O2 treatment, and they decreased their respiration rate at both O2 levels studied. As expected, when old MPCs were grown at 21% O2, their basal and maximal OCR was significantly lower than basal and maximal OCR in young MPCs, but when old MPCs were grown at 3% O2, those differences were abrogated, and respiration was equal to the value determined for young MPCs. The measurement of extracellular acidification rate (ECAR) is a surrogate marker of glycolysis. The data in Fig. 4D show that the rate of glycolysis in young MPCs is independent of the oxygen tension, and although there was a slight increase in glycolysis after H2O2 treatment, the increase was not statistically significant. However, when we measured old MPCs, there was a significant difference in ECAR data between old MPCs at 21% O2 versus old MPCs at 3% and young MCPs at both O2 levels. For example, the ECAR determined for old MPCs at 21% O2 was between 3 and 5 times higher than the ECAR measured in young MPCs (at both oxygen tensions) and old MPCs at 3%. Our results on fatty acid oxidation (FAO, Fig. 2E) show that only young MPC at 21% O2 increased their OCR due to FAO activity after palmitate was added. It is possible that high oxygen concentration in the environment induced these cells to employ both bioenergetic pathways simultaneously.
Fig. 4.
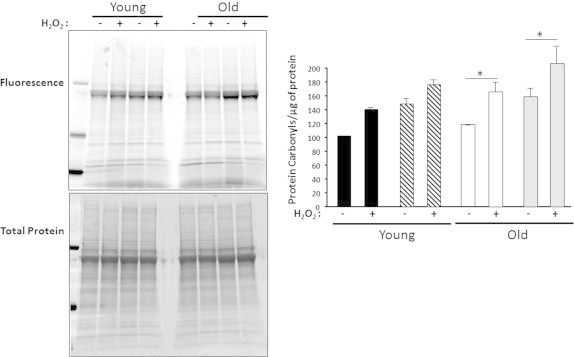
Low oxygen conditions (3%) induceminor levels of protein carbonyls in old MPCs. Protein carbonyls were measured in the cytosolic fraction isolated from MPCs with and without oxidative treatment (100 μM H2O2 for 19 h). Young MPCs are represented by black bars for 3% O2 and hatched bars for 21% O2. Old MPCs are represented by white bars for 3% O2 and gray bars for 21% O2. Each point represents the mean±SE of three independent experiments. Statistical significance with respect to untreated cells, ⁎p<0.05.
Considering all the above, our data suggest that glycolysis probably plays a major role in energy generation when the MPCs derived from old animals are grown at room air oxygen tension but not at the tissue oxygen level (3% O2).
Protein disulfides, carbonyls and aggregates
In order to determine whether oxygen tension has an effect on the level of protein oxidation (protein disulfides and protein carbonyls) and protein aggregates, we measured these parameters in the cytosolic fraction isolated from MPCs grown at 3 or 21% O2, with or without oxidative treatment (100 μM H2O2 for 19 h). As shown in Fig. 3, we found no difference in the levels of protein disulfides between young and old MPCs at the two O2 levels. In addition, even though there was a mild increment in the protein disulfides in the H2O2-treated samples, the difference was not statistically significant. With respect to protein carbonyls (Fig. 4), we found no difference in carbonyl levels in proteins isolated from young MPCs at 21 and 3% O2 and no effect from H2O2 treatment in young MPCs. In contrast, there was a 30 to 40% increase in carbonyls in the proteins isolated from H2O2-treated old MPCs compared to untreated old MPCs, concurring with the results in Fig. 2 that show a decrease in mitochondrial respiration after H2O2 treatment and suggesting oxidative damage. In addition, old treated MPCs grown at 21% O2 had significantly higher carbonyl levels (~40%) than old treated MPCs grown at 3%. Protein aggregates increased in response to oxidative treatment in all cell types (Fig. 5), but the effect of O2 was only evident in the old MPCs, whereas cells cultured at 21% O2 showed more protein aggregates. These findings suggest that old cells are in general more sensitive to oxidative treatment.
Fig. 3.
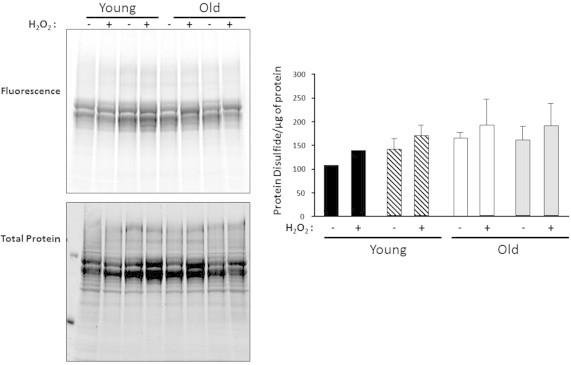
No differences in protein disulfides content in both age nor oxygen conditions. Protein disulfides were measured in the cytosolic fraction isolated from MPCs with and without oxidative treatment (100 μM H2O2 for 19 h). Young MPCs are represented by black bars for 3% O2 and hatched bars for 21% O2. Old MPCs are represented by white bars for 3% O2 and gray bars for 21% O2. Each point represents the mean±SE of three independent experiments.
Fig. 5.
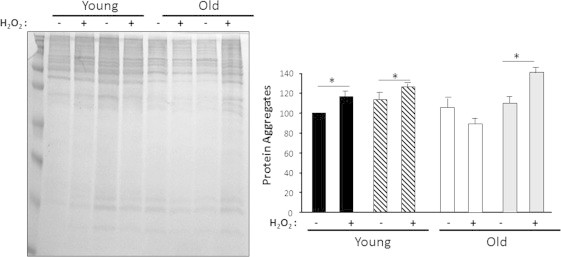
High oxygen conditions (21%) induce elevated levels of protein aggregates in old MPCs. Protein aggregates were measured in the cytosolic fraction isolated from MPCs with and without oxidative treatment (100 μM H2O2 for 19 h). Young MPCs are represented by black bars for 3% O2 and hatched bars for 21% O2. Old MPCs are represented by white bars for 3% O2 and gray bars for 21% O2. Each point represents the mean±SE of three independent experiments. Statistical significance with respect to untreated cells, ⁎p<0.05.
Discussion
It is known that modifications in oxygen tension can modulate cellular signaling cascades, which in turn can affect important physiological pathways during life span, from embryogenesis and development to the maintenance of normal function, dysfunction, disease and aging [20]. Standard (atmospheric) cell culture conditions do not reflect the in vivo physiological environment, and thus they may compromise or obfuscate interpretations of cell based studies. The goal of this study was to determine the effect of O2 on young and old MPCs mitochondrial respiratory capacity and bioenergetics before and after an acute oxidative insult, as well as its effect on protein oxidation and aggregation in relation to age. MPCs were isolated from young (3–4 m) and old (29–30 m) C57BL/6 mice, and cultured under physiological (3% O2) or ambient (21% O2) prior to treatment with an acute oxidative insult (100 μM H2O2 for 19 h).
The effect of lowering oxygen tension on cell proliferation in culture has been previously reported to be either beneficial or detrimental, depending on the cell line studied. For example, cultures under low physiological oxygen conditions have been employed studies using stem cells, because they have been proved to improve stemness and quality of pluripotent stem cells [21,22]. A different scenario has been shown in transformed cells, where the metabolic response depended on the cancer cell model used [23,24]. Conversely, there is an adverse effect on the proliferative capacity of the kidney rat cell lines, NRK and INS-1 grown at 3% O2 [25]; whereas, mouse C2C12 myoblasts grown at 3% O2 have higher proliferation rates than myoblasts grown at 20% O2 [26]. Murine satellite cells [5,27] and human skeletal muscle precursor cells derived from elderly donors show enhanced proliferation and differentiation when grown at 3% O2 [8]. Oxygen concentrations ranking from 1–5% have been used, however, most of myoblast studies have been done using 3% O2 [2,7]. All the above point toward the importance of considering oxygen tension effects as a critical factor in cell culture studies of aging, especially with respect to the significance of redox and O2-based signaling in mammalian cells [28–30].
Redox status affects virtually all cellular processes including DNA synthesis, transcriptional activation, enzyme kinetics, and protein folding and trafficking [31–33]. Cell proliferation and cellular response to damage are inevitably impacted by redox potential changes, and it is likely that cells derived from old animals would be more susceptible to these changes. Recently, Duguez et al. [34] showed the importance of mitochondrial regulation and ROS production in the myoblast response to oxygen tension, highlighting the importance of studying mitochondrial function in MPCs. Hence, using technology that permits studying cellular bioenergetics in intact cells, we were able to demonstrate here for the first time that there is a significant difference in the mitochondrial metabolism of MPCs derived from old animals in comparison to MPCs derived from young animals. Although young MPCs at 3% O2 proliferate faster than at 21% O2, there was not a significant difference in mitochondrial respiration. Moreover, a completely opposite response was observed in old MPCs, which showed a significant reduction in mitochondrial respiration when grown at 21% O2, along with increased glycolytic function and null fatty acid oxidation, suggesting that despite a higher O2 need, these cells probably meet their energetic needs through anaerobic respiration. This low energetic flux might partially explain the poor proliferation rate and differentiation. It is also important to note that in a previous study we found a significant reduction in mitochondrial mass as measured by mitochondrial DNA content in the old MPCs [35]. It is possible that reduced mitochondrial number contributes to the reduction in mitochondrial respotration measured in the current study in old MPCs.
When old MPCs are grown at physiological O2 (3%), glycolysis decreased and mitochondrial respiration rose to a level that was indistinguishable from the one determined for MPCs derived from young animals and grown at 3 or 21% O2. This suggests that cells at physiological oxygen tension within the muscle have the capacity to respire and proliferate quite efficiently despite their age. Because the cells were isolated at ambient conditions, some damage from the culturing in high oxygen before freezing might have occurred. However, the bioenergetics and oxidative effect addressed in this paper is related to the cellular response after their adaptation to low or high oxygen concentrations.
It is known that the intracellular redox status in aged skeletal muscle shifts toward a more oxidizing milieu, permitting local persistence of reactive oxygen species (ROS), progressive oxidative damage [36–39]. Based on our results, cells from older mice exhibit a shift toward a glycolytic and less efficient energetic metabolism. In addition, MPCs subjected to a mild oxidative insult confirm that cells derived from old animals, even if they are grown at 3% O2, are more susceptible to redox changes, and their mitochondrial respiration declined.
Protein damages (oxidation, misfolding and aggregation) are important contributors to age-related muscle deterioration [40,41]. In that respect, our results show that OM cells grown at 21% have an increased level of protein damage as measured by the level of aggregates and carbonyls, and the levels of these markers increased further after oxidative treatment. It is very important to note the fact that the intracellular redox milieu may differ depending on the age of the organism from which MPCs came from, and that the redox state could have been affected differently in the young and old MPCs incubated under 3% or 21% O2. Our results indicate that cells grown at ambient O2 (21%), and probably aged cells living in a more oxidizing milieu, are exposed to a higher oxidative stress that might be damaging cellular macromolecules, in this particular case, proteins. However, the exact intracellular redox milieu of the different groups, i.e., young and old MPCs incubated under 3% or 21% O2, is an important question that should be addressed in further experiments.
Another explanation for the slow proliferation rate of old MPCs at an oxidizing milieu is that cells growing at this higher oxygen tension might be at a crossroad between using their resources in repairing oxidative damage or for proliferating; it is important to consider that besides this “dilemma”, oxidative damage might be so large that it might also impair cellular functions like mitochondrial respiration, DNA and protein repair and clearance (autophagy) and ultimately affecting cellular proliferation and function.
This is the first study on mitochondrial bioenergetics in real time performed in MPCs derived from old and young mice muscle and cultured under different oxygen tensions; therefore no other data are available in the literature for comparison. It is interesting to note that the in vitro results at 21% would suggest impairment in mitochondrial function in the cells from old mice; yet, the results at 3% indicate no deficit in the old cells. These results emphasize that the manipulations of the oxygen microenvironment or the intracellular redox potential can have profound effects on growing old cells at 21% O2, which might over estimate the aging decline.
Acknowledgments
This work was supported by Ellison Medical Foundation AG-NS-0705-10/PEREZ and by NIH P01 AG20591/VAN REMMEN. MK was a Fulbright and a CONACyT scholar (147827).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Morrison S.J., Csete M., Groves A.K., Melega W., Wold B., Anderson D.J. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. Journal of Neuroscience. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Csete M. Oxygen in the cultivation of stem cells. Annals of the New York Academy of Sciences. 2005;1049:1–8. doi: 10.1196/annals.1334.001. [DOI] [PubMed] [Google Scholar]
- 3.Biressi S., Rando T.A. Heterogeneity in the muscle satellite cell population. Seminars in Cell & Developmental Biology. 2010;21:845–854. doi: 10.1016/j.semcdb.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lees S.J., Childs T.E., Booth F.W. p21(Cip1) expression is increased in ambient oxygen, compared to estimated physiological (5%) levels in rat muscle precursor cell culture. Cell Proliferation. 2008;41:193–207. doi: 10.1111/j.1365-2184.2008.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakravarthya M.V., Spangenburg E.E., Booth F.W. Culture in low levels of oxygen enhances in vitro proliferation potential of satellite cells from old skeletal muscles. Cellular and Molecular Life Sciences. 2001;58:1150–1158. doi: 10.1007/PL00000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S.H., Shin S., Shireman P.K., Vasilaki A., Van Remmen H., Csete M.E. Glutathione-peroxidase-1 null muscle progenitor cells are globally defective. Free Radical Biology and Medicine. 2006;41:1174–1184. doi: 10.1016/j.freeradbiomed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Li X., Zhu L., Chen X., Fan M. Effects of hypoxia on proliferation and differentiation of myoblasts. Medical Hypotheses. 2007;69:629–636. doi: 10.1016/j.mehy.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 8.Martin S.D., Collier F.M., Kirkland M.A., Walder K., Stupka N. Enhanced proliferation of human skeletal muscle precursor cells derived from elderly donors cultured in estimated physiologic (5%) oxygen. Cytotechnology. 2009;61(3):93–107. doi: 10.1007/s10616-009-9247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parrinello S., Samper E., Krtolica A., Goldstein J., Melov S., Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nature Cell Biology. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho H.Y., Cheng M.L., Cheng P.F., Chiu D.T. Low oxygen tension alleviates oxidative damage and delays cellular senescence in G6PD-deficient cells. Free Radical Research. 2007;41:571–579. doi: 10.1080/10715760601184819. [DOI] [PubMed] [Google Scholar]
- 11.Dmitriev R.I., Zhdanov A.V., Jasionek G., Papkovsky D.B. Assessment of cellular oxygen gradients with a panel of phosphorescent oxygen-sensitive probes. Analytical Chemistry. 2012;84:2930–2938. doi: 10.1021/ac3000144. [DOI] [PubMed] [Google Scholar]
- 12.Estrada J.C., Albo C., Benguría A., Dopazo A., López-Romero P., Carrera-Quintanar L., Roche E., Clemente E.P., Enríquez J.A., Bernad A., Samper E. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death & Differentiation. 2012;19:743–755. doi: 10.1038/cdd.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López-Diazguerrero N.E., López-Araiza H., Conde-Pérezprina J.C., Bucio L., Cárdenas M.C., Ventura J.L., Covarrubias L., Gutiérrez-Ruiz M.C., Zentella A., Königsberg M. Bcl-2 protects against oxidative stress while inducing premature senescence. Free Radical Biology and Medicine. 2006;40:1161–1169. doi: 10.1016/j.freeradbiomed.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Dranka B.P., Benavides G.A., Diers A.R., Giordano S., Zelickson B.R., Reily C., Zou L., Chatham J.C., Hill B.G., Zhang J., Landar A., Darley-Usmar V.M. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radical Biology and Medicine. 2011;51:1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diers A.R., Higdon A.N., Ricart K.C., Johnson M.S., Agarwal A., Kalyanaraman B., Landar A., Darley-Usmar V.M. Mitochondrial targeting of the electrophilic lipid 15-deoxy-Delta12,14-prostaglandin J2 increases apoptotic efficacy via redox cell signaling mechanisms. Biochemical Journal. 2010;426:31–41. doi: 10.1042/BJ20091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradford M.M. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 17.Fang Y.H., Piao L., Hong Z., Toth P.T., Marsboom G., Bache-Wiig P., Rehman J., Archer S.L. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: exploiting Randle's cycle. Journal of Molecular Medicine. 2012;90:31–43. doi: 10.1007/s00109-011-0804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez V.I., Buffenstein R., Masamsetti V., Leonard S., Salmon A.B., Mele J., Andziak B., Yang T., Edrey Y., Friguet B., Ward W.F., Richardson A., Chaudhuri A. Protein stability and resistance to oxidative stress are determinants of longevity in the longest living rodent, the naked mole-rat. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhuri A.R., de Waal E.M., Pierce A., Van Remmen H., Ward W.F., Richardson A. Detection of protein carbonyls in aging liver tissue: a fluorescence-based proteomic approach. Mechanisms of Ageing and Development. 2006;127:849–861. doi: 10.1016/j.mad.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular Signalling. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Ippolito G., Diabira S., Howard G.A., Roos B.A., Schiller P.C. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513–522. doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 22.Guo C.W., Kawakatsu M., Idemitsu M., Urata Y., Goto S., Ono Y., Hamano K., Li T.S. Culture under low physiological oxygen conditions improves the stemness and quality of induced pluripotent stem cells. Journal of Cellular Physiology. 2013;228:2159–2166. doi: 10.1002/jcp.24389. [DOI] [PubMed] [Google Scholar]
- 23.Higgins L.H., Withers H.G., Garbens A., Love H.D., Magnoni L., Hayward S.W., Moyes C.D. Hypoxia and the metabolic phenotype of prostate cancer cells. Biochimica et Biophysica Acta. 2009;1787:1433–1443. doi: 10.1016/j.bbabio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Vaapil M., Helczynska K., Villadsen R., Petersen O.W., Johansson E., Beckman S., Larsson C., Påhlman S., Jögi A. Hypoxic conditions induce a cancer-like phenotype in human breast epithelial cells. PLoS One. 2012;7:e46543. doi: 10.1371/journal.pone.0046543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J.H., Jones R.H., Tarry-Adkins J., Smith N.H., Ozanne S.E. Adverse effects of reduced oxygen tension on the proliferative capacity of rat kidney and insulin-secreting cell lines involve DNA damage and stress responses. Experimental Cell Research. 2008;314:3075–3080. doi: 10.1016/j.yexcr.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yun Z., Lin Q., Giaccia A.J. Adaptive myogenesis under hypoxia. Molecular and Cellular Biology. 2005;25:3040–3055. doi: 10.1128/MCB.25.8.3040-3055.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Csete M., Walikonis J., Slawny N., Wei Y., Korsnes S., Doyle J.C., Wold B. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. Journal of Cellular Physiology. 2001;189:189–196. doi: 10.1002/jcp.10016. [DOI] [PubMed] [Google Scholar]
- 28.Suna Q.A., Hessa D.T., Nogueirad L., Yonge S., Bowlesf D.E., Eud J., Lauritae K.R., Meissnerg G., Stamler J.S. Oxygen-coupled redox regulation of the skeletalmuscle ryanodine receptor-Ca2+ release channel by NADPH oxidase. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16098–16103. doi: 10.1073/pnas.1109546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Ippolito G., Howard G.A., Roos B.A., Schiller P.C. Sustained stromal stem cell self-renewal and osteoblastic differentiation during aging. Rejuvenation Research. 2006;9:10–19. doi: 10.1089/rej.2006.9.10. [DOI] [PubMed] [Google Scholar]
- 30.Diabira S., Howard G.A., Roos B.A., Schiller P.C. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513–522. doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 31.Sen C.K., Packer L. Antioxidant and redox regulation of gene transcription. FASEB Journal. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 32.CotgreaveIA Gerdes R.G. Recent trends in glutathione biochemistry—glutathione-protein interactions: a molecular link between oxidative stress and cell proliferation? Biochemical and Biophysical Research Communications. 1998;242:1–9. doi: 10.1006/bbrc.1997.7812. [DOI] [PubMed] [Google Scholar]
- 33.Krüger A., Grüning N.M., Wamelink M.M., Kerick M., Kirpy A., Parkhomchuk D., Bluemlein K., Schweiger M.R., Soldatov A., Lehrach H., Jakobs C., Ralser M. The pentose phosphate pathway is a metabolic redox sensor and regulates transcription during the antioxidant response. Antioxidants & Redox Signaling. 2011;15:311–324. doi: 10.1089/ars.2010.3797. [DOI] [PubMed] [Google Scholar]
- 34.Duguez S., Duddy W.J., Gnocchi V., Bowe J., Dadgar S., Partridge T.A. Atmospheric oxygen tension slows myoblast proliferation via mitochondrial activation. PLoS One. 2012;7(8):e43853. doi: 10.1371/journal.pone.0043853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S., Van Remmen H., Csete M. Sod2 overexpression preserves myoblast mitochondrial mass and function, but not muscle mass with aging. Aging Cell. 2009;8:296–310. doi: 10.1111/j.1474-9726.2009.00477.x. [DOI] [PubMed] [Google Scholar]
- 36.Hagiwara M., Nagata K. Redox-dependent protein quality control in the endoplasmic reticulum: folding to degradation. Antioxidants & Redox Signaling. 2012;16(10):1119–1128. doi: 10.1089/ars.2011.4495. [DOI] [PubMed] [Google Scholar]
- 37.Jang Y.C., Lustgarten M.S., Liu Y., Muller F.L., Bhattacharya A., Liang H., Salmon A.B., Brooks S.V., Larkin L., Hayworth C.R., Richardson A., Van Remmen H. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB Journal. 2010;24:1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson M.J., McArdle A. Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. Journal of Physiology. 2011;589:2139–2145. doi: 10.1113/jphysiol.2011.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lustgarten M.S., Jang Y.C., Liu Y., Qi W., Qin Y., Dahia P.L., Shi Y., Bhattacharya A., Muller F.L., Shimizu T., Shirasawa T., Richardson A., Van Remmen H. MnSOD deficiency results in elevated oxidative stress and decreased mitochondrial function but does not lead to muscle atrophy during aging. Aging Cell. 2011;10:493–505. doi: 10.1111/j.1474-9726.2011.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth D.M., Balch W.E. Modeling general proteostasis: proteome balance in health and disease. Current Opinion in Cell Biology. 2010;23:1–9. doi: 10.1016/j.ceb.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dillin A., Cohen E. Ageing and protein aggregation-mediated disorders: from invertebrates to mammals. Philosophical Transactions of the Royal Society B. 2011;366:94–98. doi: 10.1098/rstb.2010.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]


