Abstract
Sex steroids affect cognitive function as well as emotion processing and regulation. They may also play a role in the pathophysiology of schizophrenia. However, the effects of sex steroids on cognition and emotion-related brain activation in schizophrenia are poorly understood. Our aim was to determine the extent to which circulating testosterone relates to brain activation in men with schizophrenia compared to healthy men during cognitive-emotional processing. We assessed brain activation in 18 men with schizophrenia and 22 age-matched healthy men during an emotional go/no-go task using fMRI and measured total serum testosterone levels on the same morning. We performed an ROI analysis to assess the relationship between serum testosterone and brain activation, focusing on cortical regions involved the emotional go/no-go task. Slower RT and reduced accuracy was observed when participants responded to neutral stimuli, while inhibiting responses to negative stimuli. Healthy men showed a robust increase in activation of the middle frontal gyrus when inhibiting responses to negative stimuli, but there was no significant association between activation and serum testosterone level in healthy men. Men with schizophrenia showed a less pronounced increase in activation when inhibiting responses to negative stimuli; however, they did show a strong inverse association between serum testosterone level and activation of the bilateral middle frontal gyrus and left insula. Additionally, increased accuracy during inhibition of response to negative words was associated with both higher serum testosterone levels and decreased activation of the middle frontal gyrus in men with schizophrenia only. We conclude that endogenous hormone levels, even within the normal range, may play an enhanced modulatory role in determining the neural and behavioural response during cognitive-emotional processing in schizophrenia.
Introduction
Many observations support the relationship between sex steroid hormones and the development and course of schizophrenia. Sex differences have been demonstrated in the onset and severity of schizophrenia with men being more severely affected [1]. Estrogen may act as a neuroprotective factor in women with schizophrenia [2], but little is known about the role of testosterone in men with schizophrenia. The peak in age-of-onset during adolescence suggests a link between exposure to increased androgens and development of schizophrenia in at-risk men. However, a number of studies, including first episode and ultra-high risk, have reported either low or normal circulating testosterone levels in men with schizophrenia [3]–[6]. The evidence for a relationship with testosterone is strengthened by reports of increased negative symptoms and worse cognitive function in association with low endogenous testosterone levels in men with schizophrenia [7]–[11]. Furthermore, exogenous testosterone supplementation can reduce negative symptoms in men with schizophrenia [12].
Variation in testosterone level has been shown to influence aspects of affective [13] and social behaviour [14]–[16], as well as cognitive performance in healthy men [17], and those with schizophrenia [10]. Evidence from neuroimaging studies in healthy adults indicates that fronto-limbic activation correlates with testosterone level during emotion-related processing [18]–[21]. However, little is known about the relationship between endogenous testosterone levels and brain activation associated with cognitive or affective processes in schizophrenia. One recent study assessing brain activation associated with visual-spatial ability reported a positive correlation with testosterone in healthy men but no correlation in men with schizophrenia [22]. However, this study also reported lower testosterone levels in the patient group. We predict that testosterone levels may affect other neurocognitive processes that are more central to the illness, such as executive control, attention [23] and emotion processing and regulation [24].
The aim of the current study was to test whether circulating testosterone levels were correlated with brain activation during cognitive-emotional processing in men with schizophrenia. We used an emotional go/no-go paradigm, which activates dorsal prefrontal executive control brain regions in addition to insular and limbic cortex associated with emotion regulation [25], [26]. Brain activation during this task was previously shown to be sensitive to sex steroid modulation of prefrontal and cingulate activity in healthy adults [27]. We focused on negative valence, since negative emotion has been shown to have stronger interference effects on cognitive processes in schizophrenia [28] and because our own work indicated that positive emotional go/no-go conditions did not engage the same frontal network as robustly [26].
Based on existing evidence of a relationship between endogenous testosterone and brain activity during emotion-related tests, as well as testosterone’s putative involvement in the pathophysiology and course of schizophrenia, we predicted that the relationship between circulating testosterone and task-related brain activation during an emotional go/no-go task would differ between men with schizophrenia and healthy men. We tested this hypothesis in an a priori-defined cortical network known to be involved in controlling and integrating cognitive and emotional processing.
Materials and Methods
Ethics Statement
All procedures were approved by the University of New South Wales and the South Eastern Sydney and Illawarra Area Health Service Human Research Ethic Committees and the study was conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from all participants prior to entry into the study. Ability to provide informed consent was assessed first by the participant’s referring clinician who was not associated with the study and an additional study physician prior to inclusion in the study. None of the participants had significant cognitive impairment (FSIQ<75) which would interfere with their ability to provide informed consent. All potential participants who declined to participate or otherwise did not participate were eligible for treatment and were not disadvantaged in any other way by not participating in the study.
Participants
Eighteen right-handed men with schizophrenia or schizoaffective disorder and twenty-two right-handed healthy men took part in the fMRI experiment. Patients were recruited via information leaflets distributed within the local area health service and through a national television documentary. Healthy men were recruited via advertisements in newspapers and on community notice boards. Data from a subset of these participants (15 men with schizophrenia and 11 healthy men) were included in a previous report from our lab [26]. General exclusion criteria for the study were the presence of a concurrent Axis I diagnosis other than schizophrenia/schizoaffective disorder for the patient group and a personal history of psychiatric illness or a first degree relative with schizophrenia/schizoaffective disorder for the healthy control group. For both participant groups further exclusion criteria were a history of recent (within 5 years) substance abuse or dependency, uncontrolled diabetes or cardiovascular disease including hypertension, a history of neurological disorders, head injury with loss of consciousness, epileptic seizures, and structural brain abnormalities. All men with schizophrenia received out-patient care and had been receiving treatment with antipsychotics for at least one year prior to inclusion in the study. Psychiatric diagnosis was obtained from medical record review and confirmed by means of the Structured Clinical Interview for DSM-IV-TR, (SCID) [29]. Current symptom severity was assessed with the Positive and Negative Syndrome Scale (PANSS) [30]. A trained psychologist or psychiatrist conducted all clinical and diagnostic assessments. All participants were assessed with a four subtest version of the Wechsler Adult Intelligence Scale, 3rd edition (WAIS-III) [31] to estimate current intellectual functioning.
Hormonal Assays
Fasting peripheral blood was obtained by venepuncture between 9 and 11 am to control for circadian variation in hormone levels. Immediately following collection, clotted and heparinised blood samples were delivered on ice to the Prince of Wales Hospital South Eastern Area Laboratory Services Pathology unit. Sex steroid levels were measured from serum and provided a measure of “total” circulating hormone levels. Testosterone and estradiol were assayed using a solid-phase competitive chemiluminescent immunometric assay and prolactin was assayed using a solid-phase two-site chemiluminescent immunometric assay (Siemens Healthcare Diagnostics Products Ltd.). Reference ranges were set at 5.6–23.6 nmol/L for testosterone and 0–206 pmol/L for estradiol, sensitivity of the assay was 0.7 nmol/L for testosterone and 73 pmol/L for estradiol and the interassay coefficients of variation (CV) were ≈9.7% and ≈13.5% for testosterone and estradiol, respectively. For prolactin, reference ranges were set at 0–372 mlU/L, sensitivity of the assay was 11.0 mlU/L, the intra-assay CV was 6 and the interassay CV was ≈6.5%.
Emotional Go/no-go Task
The stimuli used in the emotional go/no-go task were selected from the Affective Norms for English Words (ANEW) [32] stimulus set, which provides normative valence and arousal ratings. We selected 40 neutral, 20 positive and 20 negative words from the normative lists and matched them for word length and frequency. Positive and negative words also matched for arousal. All participants rated the stimuli in a pen-and-paper forced choice questionnaire, indicating for each of the stimuli whether they were perceived as positive, neutral or negative. These subjective ratings were used to define response accuracy in the emotional go/no-go task on an individual basis.
All participants received detailed verbal instructions prior to scanning. The emotional go/no-go task includes four conditions: 1) inhibit neutral/respond to negative: responding to negative words while inhibiting responses to neutral ones and 2) inhibit negative/respond to neutral: responding to neutral words while inhibiting responses to negative ones. The two remaining task conditions involve responding to/inhibiting responses to positive words compared to neutral words. For the purposes of the current study, we selectively focused on the task conditions with negative valence, i.e., conditions 1 and 2 above, based on previous work showing that these conditions produced robust activation of a prefrontal network, whereas contrasting positive and neutral words elicited much weaker activation [26].
Participants were instructed to respond as quickly as possible to stimuli of the specified valence (targets), but to ignore stimuli of other valences (distracters). A single trial consisted of a fixation cross for 600 ms, a word stimulus presented in the center of the screen for 600 ms, and an inter-stimulus response interval of 1800 ms. Stimuli were presented in blocks of 10 trials. Each block was preceded by an instruction: the word “NEGATIVE,” “POSITIVE” or “NEUTRAL” appeared in capital letters to indicate the target valence. There were 4 different blocks with 5 negative and 5 neutral stimuli. Each of these blocks was presented twice, once with a “NEGATIVE” instruction and once with a “NEUTRAL” instruction. The two conditions were thus identical with respect to stimuli and differed only in terms of the instruction given at the start of each block. Block order was pseudo-randomized such that the same condition could not appear in consecutive blocks. A 30 second fixation crosshair was presented at the initiation and conclusion of the scan session. The total duration was 10 minutes. Stimuli were presented via a computer screen that participants viewed via a mirror and responses were recorded via a fiber optic response pad (Lumina Systems), which collected accuracy (percent correct) and reaction time (RT) in ms.
fMRI Scanning Procedure
Magnetic resonance imaging was performed using a 3 Tesla Phillips Achieva MRI scanner with an 8 channel bird-cage type head coil at Neuroscience Research Australia, Randwick, NSW, Australia. A T1-weighted high-resolution anatomical scan was obtained for each participant for registration purposes and to screen for anatomical abnormalities (TR, 5.4 ms; TE, 2.4 ms; FOV, 256 mm; matrix, 256×256; sagittal plane; slice thickness, 1 mm, no gap; 180 slices). Functional T2* weighted images were obtained using a gradient echo-planar imaging sequence, TR/TE = 3000/30; 32 interleaved slices, covering the whole brain, thickness = 3 mm, gap = 1 mm; voxel size 3×3×3 mm; scan repetitions = 212; flip angle = 90°; field of view = 24 cm.
Data Analysis
Hormone assays
Independent sample t-tests were performed to assess group differences in testosterone, estradiol and prolactin levels. Pearson product-moment correlation coefficients were obtained among hormone levels, demographic and clinical characteristics of the sample, including age, medication dose and PANSS symptom scores.
Emotional go/no-go task performance
Task performance measures included reaction times (RTs) for correct responses, response accuracy (% correct responses), the number of false alarm errors and omissions. Correct responses were defined as a button press to a target and the withholding of a button press to a distracter. False alarms were defined as a button press to a distracter, whereas omissions refer to the withholding of a button press to a target stimulus. Mixed ANOVAs were performed with group as between-subjects and condition as within-subjects factors. In addition, Pearson product-moment correlation coefficients were obtained between hormone levels and task performance.
fMRI analysis
All analyses were performed with SPM8 (Wellcome Trust Centre for Neuroimaging), running under Matlab2010b. Four dummy scans were obtained before each fMRI data acquisition to allow for the equilibration of the MRI signal. Functional images were realigned to the first image in the time series and co-registered with the anatomical image. The images were manually reoriented to optimize the normalization process and normalized to the Montreal Neurological Institute (MNI) anatomical template using a non-linear 12 parameter affine transformation. Images were smoothed with a 10 mm FWHM Gaussian Kernel. All data sets were screened for movement artifacts (subjects were excluded if movement parameters exceeded 3 mm translation along x, y or z axes or 3 degrees of rotation) and successful normalization. To further control for motion effects, the motion parameters were also included as regressors in the first level analysis. At the first level of analysis, contrasts were created for each subject to assess the magnitude of difference in blood oxygen level-dependent (BOLD) signal between conditions of interest: inhibit negative/respond to neutral versus inhibit neutral/respond to negative, to arrive at the relative activation during inhibition of responses to negative versus neutral stimuli. At the second level, we first constructed single sample T-test models for the group of healthy men and the group of men with schizophrenia separately to assess the main task effect for the contrast of interest (inhibit negative versus neutral). Next, to assess the relationship between serum testosterone and BOLD response, the first level contrasts (inhibit negative versus neutral) were entered into a regression model with testosterone levels as a continuous predictor, for the group of healthy men and men with schizophrenia separately. In addition, we performed a control analysis to examine the relationship between testosterone and BOLD response when controlling for the level of estradiol, as the two hormones may interact.
The inhibit negative versus neutral contrast compares conditions that differ only in the subjective valence of the stimuli to which responses are inhibited, producing relatively subtle effects when tested at the whole brain level [26]. In order to maximize our statistical power, we confined the analyses to a set of regions of interest (ROIs), based on previous results comparing the same task conditions (inhibit negative versus neutral) [26]. The selected regions are known to be involved in emotional go/no-go conditions [25], [33]. The following bilateral structural ROIs were selected from the Anatomical Automatic Labeling (AAL) Atlas [34]: middle frontal gyrus, posterior cingulate, precuneus and insula. In addition, we selected a control region, the postcentral gyrus, which was not previously associated with the emotional inhibition task and for which we expected no testosterone effects [25], [27]. A structural ROI analysis was performed using the MarsBar toolbox implemented in SPM [35]. All voxels within the ROIs are treated as many samples of the same signal and a summary value, based on the mean, is calculated across all voxels in the ROI. Results are reported with FDR corrections for multiple comparisons, unless stated otherwise. Parameter estimates for the inhibit negative versus neutral task contrast were extracted for each of the ROIs and Pearson product-moment correlations were determined between BOLD response, task performance (accuracy), medication dosage, and symptom severity (PANSS scores).
Results
Demographic, Clinical and Neuropsychological Characteristics
Table 1 summarizes the demographic, clinical and neuropsychological characteristics of the sample. Statistical comparison using t-tests confirmed that the groups were well matched on age. As expected, healthy men had a significantly higher estimated full scale IQ and education level compared to the men with schizophrenia. PANSS scores for the men with schizophrenia were within the mild to moderately severe range.
Table 1. Demographic and clinical characteristics of the sample, comprising 18 males with schizophrenia and 22 healthy males.
| Males with schizophrenia | Healthy males | Statistic | |||
| Mean (SD) | Range | Mean (SD) | Range | ||
| AGE (years) | 34.9 (9.1) | 25–50 | 32.3 (8.6) | 22–48 | t(38) = 0.7; p>.10 |
| EDUCATION LEVEL (years) | 13.3 (2.7) | 10–19 | 14.9 (2.2) | 10–18 | t(38) = −2.04; p = .05 |
| AGE OF ONSET (years) | 22.94 (3.38) | 16–28 | – | – | – |
| ILLNESS DURATION (years) | 11.25 (7.81) | 2–24 | – | – | – |
| ANTIPSYCHOTIC DOSE* (CPZ equivalent) | 656.7 (385.4) | 50–1200 | – | – | – |
| PANSS | |||||
| Positive | 16.3 (5.7) | 7–27 | – | – | – |
| Negative | 16.1 (6.7) | 7–30 | – | – | – |
| General | 34.5 (11.7) | 19–60 | – | – | – |
| Total | 66.8 (20.1) | 41–102 | – | – | – |
| WAIS-III IQ | |||||
| Estimated full scale IQ | 93.9 (11.8) | 75–115 | 105.8 (12.9) | 84–128 | t(38) = 3.0; p<.01 |
| HORMONE MEASUREMENTS | |||||
| Testosterone (nmol/L) | 13.1 (5.4) | 6.4–26 | 14.6 (5.6) | 2.5–29.4 | t(38) = −0.9; p>.10 |
| Estrodial (pmol/L) | 139.1 (31.5) | 88–192 | 136.3 (37.7) | <73–250 | t(38) = 1.0; p>.10 |
| Prolactin (mlU/ml) | 283.3 (287.4) | 18–1130 | 165.4 (108.9) | 64–581 | t(38) = 1.6; p>.10 |
| DIAGNOSIS | Schizophrenia | ||||
| paranoid subtype n = 7 | |||||
| undifferentiated subtype n = 3 | |||||
| residual subtype n = 2 | |||||
| disorganized subtype n = 1 | |||||
| schizoaffective disorder | |||||
| depressed subtype n = 4 | |||||
| bipolar subtype n = 1 | |||||
Means are listed, with standard deviations in parentheses.
Hormone Assays
Serum testosterone, estradiol and prolactin levels did not differ between men with schizophrenia and healthy men (see Table 1). There were no strong significant correlations among hormone levels (testosterone, estradiol and prolactin), age, symptom severity or antipsychotic medication (CPZ equivalent daily dosage) in the men with schizophrenia. There was also no strong significant correlation between testosterone and prolactin level in either group (all p’s>.10, data not shown).
Emotional Go/no-go Task Performance
A summary of behavioural results is presented in Table 2. Regarding RT, there was a significant main effect of condition, indicating slower reaction times in the inhibit negative condition (responding to neutral stimuli), F(1,38) = 48.1, p<.001, and a main effect of group, indicating that men with schizophrenia were slower overall, F(1,38) = 4.2, p<.05. The group by condition interaction for RT was not significant, F(1,38) = 1.3, p>.10. Regarding accuracy, there was a trend towards a significant main effect of condition, indicating decreased accuracy in the inhibit negative condition (responding to neutral stimuli), F(1,38) = 3.5, p = .07. A main effect of group indicated that men with schizophrenia were less accurate compared to healthy men, F(1,38) = 7.8, p<.01. The interaction between group and condition for accuracy was not significant, F<1. Error analysis revealed no significant main effect of condition or significant interaction for false alarms, both F<1. Men with schizophrenia tended to make more false alarm errors compared to healthy men, F(1,38) = 3.2, p = .08. There was trend towards a significant main effect of condition on the number of omissions, F(1,38) = 3.4, p = .07, with more omissions in the inhibit negative condition (responding to neutral stimuli). Men with schizophrenia made significantly more omission errors than the healthy men, F(1,38) = 9.1, p<.01. There was no significant interaction between group and condition, F<1. In men with schizophrenia, there was a strong trend for higher serum testosterone levels to be associated with greater accuracy in the inhibit negative condition (responding to neutral stimuli), r = .46, p = .06. There was no correlation between testosterone level and performance in the healthy men, r = .07, p>.10.
Table 2. Group means and standard deviations for behavioural performance parameters on the emotional Go/No-Go task.
| Patients | Controls | |||
| Inhibit negative- respond to neutral | Inhibit Neutral- respond to negative | Inhibit negative- respond to neutral | Inhibit Neutral- respond to negative | |
| RT [mean (SD), in ms] | 1029 (187) | 890 (152) | 967 (167) | 774 (114) |
| Accuracy [mean (SD), % correct] | 68 (15) | 71 (16) | 79 (13) | 83 (16) |
| False alarm errors [mean (SD), No. of errors] | 7 (6) | 7 (4) | 5 (4) | 5 (5) |
| Omissions [mean (SD), No. of errors] | 6 (4) | 5 (5) | 4 (2) | 2 (3) |
Note: Reaction times refer to correct responses only. Accuracy reflects both responses to targets and withheld responses to distracters. False alarm errors indicate responses to distracters. Omissions indicate withheld responses to targets.
fMRI Results
Main task effects: inhibiting responses to negative versus neutral words
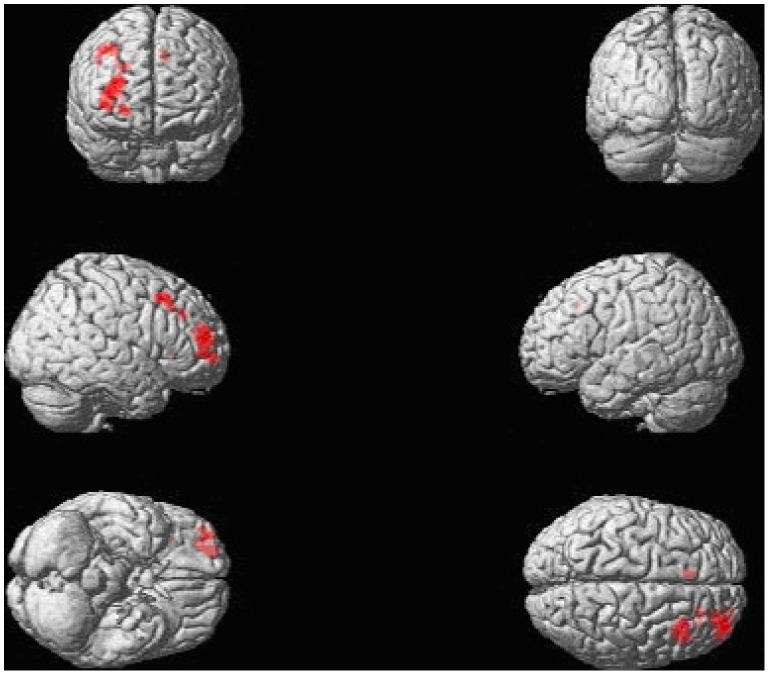
Table 3 presents an overview of the ROI analyses results. In the healthy males, we found a highly significant BOLD response increase in the right middle frontal gyrus ROI for the inhibit negative versus neutral contrast. Figure 1 displays a whole-brain rendered image for the contrast inhibit negative versus neutral in the healthy men, showing distinct activation of the middle frontal gyrus. A similar, but non-significant effect (after FDR correction) was observed in the left middle frontal gyrus ROI. In the men with schizophrenia, we observed a non-significant increase in BOLD response in the right middle frontal gyrus ROI (after FDR correction). Detailed whole brain results replicating previous findings [26] are presented in Table S1 and Figure S1.
Table 3. Results from the ROI analyses: (1) the main effect of the emotional go/no-go task, contrasting the inhibition of responses to negative stimuli with the inhibition of responses to neutral stimuli (“inhibit negative vs. neutral”) and (2) for the regression analysis of serum testosterone levels on BOLD response for the same contrast.
| Effect/contrast | Group | Region-of-Interest | Hemisphere | Parameter estimate | T-value | P-value |
| Main task effect | Healthy men | Middle frontal | Right | 0.12 | 3.84 | 0.000474* |
| for the contrast | gyrus | Left | 0.07 | 2.15 | 0.021658 | |
| “inhibit | Insula | Right | 0.05 | 0.94 | 0.178567 | |
| negative vs. | Left | −0.00 | −0.00 | 0.501115 | ||
| neutral” | Posterior | Right | −0.06 | −0.91 | 0.812465 | |
| cingulate | Left | −0.08 | −1.19 | 0.876216 | ||
| Precuneus | Right | 0.00 | 0.00 | 0.501371 | ||
| Left | −0.02 | −0.36 | 0.637576 | |||
| Men with | Middle frontal | Right | 0.07 | 2.08 | 0.026604 | |
| schizophrenia | gyrus | Left | 0.07 | 2.08 | 0.026604 | |
| Insula | Right | 0.03 | 0.67 | 0.256781 | ||
| Left | −0.02 | −0.47 | 0.677971 | |||
| Posterior | Right | 0.08 | 1.43 | 0.085147 | ||
| cingulate | Left | 0.07 | 1.34 | 0.099512 | ||
| Precuneus | Right | 0.06 | 1.12 | 0.138302 | ||
| Left | 0.05 | 0.94 | 0.180713 | |||
| Regression with | Healthy men | Middle frontal | Right | 0.01 | 0.94 | 0.178352 |
| testosterone as | gyrus | Left | 0.00 | 0.67 | 0.254801 | |
| a predictor of | Insula | Right | 0.00 | −0.07 | 0.525823 | |
| BOLD response | Left | 0.00 | 0.09 | 0.464033 | ||
| for the contrast | Posterior | Right | −0.01 | −0.75 | 0.770139 | |
| “inhibit | cingulate | Left | −0.01 | −0.65 | 0.737152 | |
| negative vs. | Precuneus | Right | 0.00 | 0.25 | 0.403758 | |
| neutral” | Left | 0.00 | 0.12 | 0.450925 | ||
| Men with | Middle frontal | Right | 0.02 | 3.76 | 0.000863* | |
| schizophrenia | gyrus | Left | 0.02 | 3.34 | 0.002059* | |
| Insula | Right | 0.01 | 1.11 | 0.141933 | ||
| Left | 0.02 | 2.37 | 0.015376* | |||
| Posterior | Right | 0.00 | 0.11 | 0.457676 | ||
| cingulate | Left | 0.00 | 0.28 | 0.392480 | ||
| Precuneus | Right | 0.02 | 1.69 | 0.055591 | ||
| Left | 0.02 | 2.00 | 0.031633 |
Separate analyses were conducted in the men with schizophrenia and the healthy men.
Note: *Indicates the effect survived FDR correction for multiple comparisons.
Figure 1. A whole-brain rendered image in the healthy men, showing significantly increased BOLD response when contrasting the inhibit negative and the inhibit neutral task conditions, p = .001 uncorrected with a minimum voxel extent k≥18.
The healthy men showed a network of increased activation that overlapped with previous findings (Vercammen et al., 2012, Journal of Psychiatry and Neuroscience,37(6): 379–388). We applied the statistical criterion employed previously with this paradigm (Vercammen et al., 2012, Journal of Psychiatric Research), based on a double threshold approach. A simulation script was used to determine cluster threshold (cluster_threshold_beta.m retrieved from https://www2.bc.edu/~slotnics/scripts.htm), with the following parameters: acquisition matrix (80×80), original voxel dimensions (3×3×3), number of slices (32), full width half maximum (FWHM) set to 0, resampled voxel resolution (2×2×2), mask (none), corrected p-value (.05), voxel based p-value (.001), iterations (1000).
Association between testosterone level and task-related activation
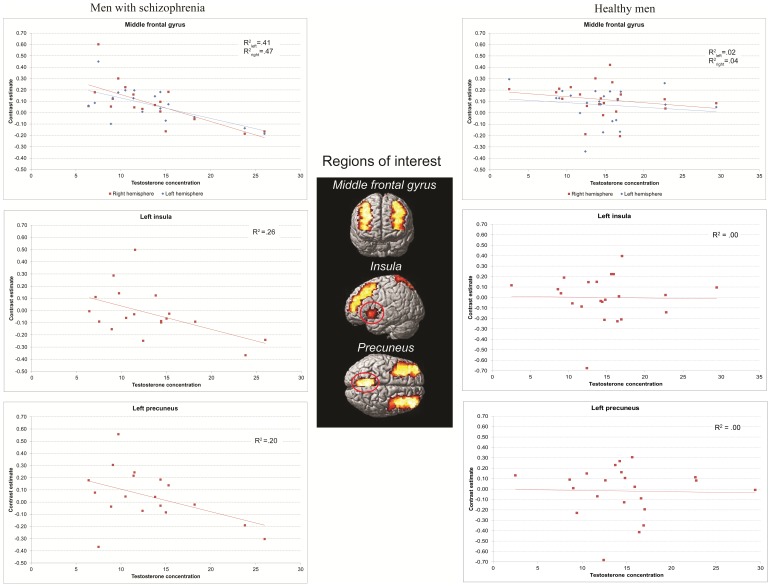
The regression analysis in the men with schizophrenia revealed a significant inverse correlation between testosterone levels and BOLD response in the bilateral middle frontal gyrus ROIs and the left insula ROIs for the inhibit negative versus neutral contrast (see Figure 2 and Table 3). A similar, but less robust correlation (not surviving FDR correction) was found for the left precuneus ROI. No significant associations were found for the remaining ROIs or the control region.
Figure 2. Results of a regression analysis in the men with schizophrenia and healthy men showing scatter plots of significant inverse correlations between testosterone levels and BOLD response in the bilateral middle frontal gyrus ROIs and the left insula ROIs for the inhibit negative versus neutral contrast in men with schizophrenia and no relationship in healthy men.
Although we found no correlation between serum prolactin and testosterone levels in either healthy men or men with schizophrenia (p>.10), due to the potential testosterone-inhibiting effect of prolactin, we ran additional analyses excluding participants with raised prolactin levels. Excluding the patient (n = 1) with relatively raised prolactin levels revealed findings consistent with our results reported above: a highly significant negative association between testosterone level and activation of the middle frontal gyrus ROIs (t = 3.08, p = .004 and t = 2.72, p = .008 for the right and left hemisphere, respectively), while the left insula and precuneus ROIs showed trend level effects (t = 1.59, p = .07 and t = 1.56, p = .07, respectively).
As estrogen effects have been demonstrated on neural responses during this task and it is known that estrogen and testosterone may interact, we conducted an additional analysis controlling for estrodial level. After adding estradiol as a nuisance covariate to the regression model with testosterone the negative association between serum testosterone level and activation of the middle frontal gyrus ROIs remained highly significant (t = 3.61, p = .001 and t = 3.03, p = .004, for the right and left hemisphere, respectively). The negative association between testosterone level and activation of the left insula ROI also remained significant (t = 2.15, p = .024), although it did not survive FDR correction.
There were no positive correlations between testosterone and brain activation in men with schizophrenia. None of the ROIs demonstrated an association (positive or negative) with serum testosterone level in healthy men.
Association among regional brain activation, task performance and clinical characteristics
In men with schizophrenia, there were strong, significant inverse correlations between task accuracy during the inhibit negative condition and activation of the right middle frontal gyrus ROI (r = −.51, p = .031) and the bilateral precuneus ROIs (r = −.50, p = .03 and r = −.47, p = .05). There were no significant correlations between brain activation and symptom severity or medication dose (all p’s>.10). In the healthy men, there were no strong, significant correlations with task performance. A detailed table of results is presented in Table S2.
Discussion
Although there has been increasing interest in the role of sex steroids on emotion and cognition in neuropsychiatric disorders, to our knowledge this is the first study examining the relationship between circulating testosterone in men with schizophrenia and brain activation during a task integrating cognitive and emotional processing. We used an emotional go/no-go paradigm to investigate the neural substrate of emotion-cognition interaction, which is known to be affected in schizophrenia, as well as being sensitive to hormonal influences [25], [27]. Our study revealed an inverse association between serum testosterone and BOLD response of the bilateral middle frontal cortex and the left insula during inhibition of responses to negative emotional words in men with schizophrenia. There was no significant relationship between testosterone and brain activation in the healthy men. Interestingly, while higher testosterone levels were associated with reduced brain activity testosterone levels were also related to better task performance in men with schizophrenia, suggesting that increased normal levels of testosterone may have beneficial effects in terms of neural processing efficiency in men with schizophrenia. The absence of a difference in circulating testosterone and prolactin levels between men with schizophrenia and healthy men suggests that the observed effects of testosterone on the brain activation are specific to hormonal-neuronal interactions and they are not based on potential abnormalities in the available blood hormone levels. These results support and extend our recent findings [10] demonstrating that high-normal testosterone levels may be of cognitive benefit to men with schizophrenia and suggest that these cognitive benefits may occur through subtle changes in cortical processing.
Emotional Go/no-go Performance and its Relationship to Testosterone Level
A consistent body of work has provided evidence of an interaction between “cold” cognitive and “hot” emotional processes. Affect can interfere with cognitive processes, including executive control and response inhibition [36], and conversely, executive control processes are used to regulate and manipulate the experience and expression of emotion [37]. The emotional go/no-go task assesses both the integrity of inhibition circuitry and potential perturbation by emotion processing, which is particularly relevant to disorders characterized by aberrant sensitivity to affective triggers such as schizophrenia [38]. Firstly, we found that across both participant groups the experimental manipulation of emotional content produced the expected behavioural effect: RT times were longer and accuracy was reduced when distracters were negative (inhibit responses to negative/respond to neutral stimuli). This can be explained by the fact that negative stimuli attract attention due to their increased salience, produce stronger response tendencies and retard inhibition, as has been observed in the emotional Stroop effect [39]. Secondly, men with schizophrenia showed an overall task performance deficit, suggesting a general impairment in response control, which is in agreement with recent reports [40]. Interestingly, in men with schizophrenia, performance in the inhibit negative condition tended to be enhanced in those with relatively higher endogenous testosterone. This finding appears to be in line with evidence from human and animal research implicating low testosterone in low mood and anxiety [41]–[44]. Attentional bias to negatively valanced information is associated with negative emotionality [45] and is common in neuropsychiatric disorders [46]. This bias tends to hamper the inhibition of responses to negative stimuli. Our findings suggest that higher endogenous testosterone levels may reduce this attentional bias to negative stimuli in schizophrenia, thereby facilitating the inhibition of automatic response tendencies to negative stimuli.
Task-related Activation and its Relationship to Testosterone in Schizophrenia
Healthy men showed the expected pattern of activation: inhibiting responses to negative stimuli versus neutral stimuli was associated with robust increases in activation of the middle frontal gyrus ROI. On the other hand, men with schizophrenia demonstrated modest activation changes when inhibiting responses to negative versus neutral stimuli. The middle frontal gyrus has an established role in executive control and emotion/cognition interaction [47], [48]. Relatively reduced responsiveness in schizophrenia is in accord with previous findings from our lab [26]. Regression analysis revealed that BOLD response in the prefrontal ROI was significantly and inversely associated with serum testosterone level in men with schizophrenia, but not in healthy men. An inverse relationship with testosterone was also observed in the left insula and precuneus, which play a part in self-referential and interoceptive processing and its integration with perceived emotional salience [49], [50]. Our findings are thus consistent with a modulatory impact of sex steroid signalling on the neural substrates of cognitive control in the context of emotion processing in schizophrenia. The direction of the association indicated that at higher normal levels of circulating testosterone, BOLD response was relatively reduced while inhibiting responses to negative stimuli. Interestingly, improved performance on the inhibit negative task condition tended to be associated with higher testosterone as well as reduced activation of the testosterone-sensitive regions (middle frontal gyrus and precuneus). These associations may suggest that – under certain circumstances – testosterone can produce more efficient processing in integral components of the neural network subserving emotion-guided actions and produce more adaptive behavioural output. At first glance, this finding may seem contradictory to the observation of reduced task-related activation in men with schizophrenia, as reduced activity has previously been interpreted as evidence of deficient processing in schizophrenia. We propose that when testosterone level is not taken into account, inter-individual variability in men with schizophrenia may result in less consistent activation patterns when assessed at the group level. However, within the group of men with schizophrenia, slight variations within the normal range of testosterone levels do appear to account for some of the systematic variability in the neural response to these specific task demands. These findings provide novel intriguing evidence of the possibility that sex steroids may be important modulators of task-related brain function in schizophrenia. More work is required to fully clarify the exact relationship between brain function, behavioural responses and endogenous hormones and the degree to which activation patterns associated with hormonal variation are in fact “abnormal”.
Our data indicate that the relationship between sex steroids and brain activation differs between men with schizophrenia and healthy men and that key structures dedicated to attentional direction and cognitive control over emotion processing are involved. There is a possibility that a more complex, non-linear relationship exists in healthy men. However, the group differences observed in this study (Figure 1) suggest that within the normal range, small variations in testosterone levels do not greatly impact emotional and cognitive processing in healthy men, whereas normal variation in testosterone levels can influence brain activity and behaviour in schizophrenia.
Limitations and Future Directions
The need to apply a strong, hypothesis driven approach to detect a significant relationship between testosterone and brain activity in specific regions may reflect the fact that the effect size of the comparison between two task conditions that differ only in terms of the emotional valance of the stimuli appear to be relatively small. The use of antipsychotics in the men with schizophrenia must be considered carefully as a potential confound as prolactin-elevating antipsychotics have been shown to influence testosterone levels [51]. However, in our sample, only 6 men with schizophrenia were receiving prolactin raising antipsychotics and the overall prolactin level did not differ significantly between schizophrenia and control groups. While many other factors appear to influence circulating testosterone levels in males (e.g., ethnicity, BMI, smoking, marital status, fatherhood, seasonality, and in men with schizophrenia: antipsychotics), our samples were well matched on age and ethnicity and we found no significant group differences in testosterone or prolactin levels, suggesting that these variables are unlikely to account for our findings.
We measured circulating total testosterone which is an indirect measure of free testosterone. Most circulating testosterone is bound to sex hormone binding protein which leaves only a small proportion to enter the cells and bind to receptors. However, free testosterone correlates well with total testosterone and both have been used as an index of bioavailable testosterone [52]. These novel findings open up an avenue for further research to explore the extent of testosterone-induced modulation of the neural response during cognitive and/or affective challenges in schizophrenia. Future studies may consider assessing the impact of sex hormone binding globulin and other circulating hormones such as corticosteroid, thyroid hormones and estrogen on brain activity in schizophrenia.
Conclusion
To our knowledge the current study is the first to link circulating testosterone levels to functional neural correlates of cognitive-emotional processing in men with schizophrenia. Within the context of normal-range circulating hormone levels, we observed an inverse relationship between testosterone and fMRI BOLD response in task-specific prefrontal brain regions during emotion-guided response inhibition. The fact that higher endogenous testosterone was associated with improved behavioural performance and a reduction in brain activation, suggests a role of sex steroid signalling in enhancing neural efficiency during negative emotional processing in schizophrenia.
Supporting Information
Thresholded T-map on sagittal sections, for the contrast inhibit negative>inhibit neutral, with a less conservative p-value = .01. Regions showing increased activation in the healthy men are indicated in yellow, regions showing increased activation in the men with schizophrenia are indicated in red. The figure demonstrates that there is some overlap in activation between the two groups, but that the pattern is less robust and shows a less contiguous spatial distribution in the men with schizophrenia as compared to the healthy men.
(TIF)
Overview of clusters showing significantly increased BOLD response when contrasting the inhibit negative and the inhibit neutral task conditions, p = .001 uncorrected; min. voxel extent k ≥18. We chose to apply the same statistical criterion as we employed in an earlier publication on this paradigm (Vercammen et al., 2012, Journal of Psychiatric Research), based on a double threshold approach. A simulation script was used to determine cluster threshold (cluster_threshold_beta.m retrieved from https://www2.bc.edu/~slotnics/scripts.htm), with the following parameters acquisition matrix (80×80), original voxel dimensions (3×3×3), number of slices (32), full width half maximum (FWHM) set to 0, resampled voxel resolution (2×2×2), mask (none), corrected p-value (.05), voxel based p-value (.001), iterations (1000). The healthy men showed a network of increased activation in the inhibit negative condition compared to the inhibit neutral condition, that overlapped with previous findings from our group (Vercammen et al., 2012, Journal of Psychiatry and Neuroscience,37(6): 379–388). The men with schizophrenia did not show significant activation changes at the same significance level. Lowering the statistical threshold did reveal a number of activation clusters in the patient group.
(DOC)
A table displaying the correlations between activation of the bilateral ROIs for the contrast inhibit negative>inhibit neutral, and clinical characteristics in the sample of men with schizophrenia. Significant correlations are indicated in bold.
(DOCX)
Acknowledgments
We would like to thank Loretta Moore and Nicholas Vella for assistance with recruitment and collecting neuropsychological and symptom data.
Funding Statement
This work was supported by the University of New South Wales School of Psychiatry; National Health and Medical Research Council Project grant number 568807; Neuroscience Research Australia; and the Australian Schizophrenia Research Bank, which is supported by the National Health and Medical Research Council of Australia; the Pratt Foundation; Ramsay Health Care; and the Viertal Charitable Foundation. This work was also supported by the Schizophrenia Research Institute (utilising funding from the New South Wales Ministry of Health and the Macquarie Group). CSW is a recipient of a National Health and Medical Research Council (Australia) Senior Research Fellowship (1021970). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Abel KM, Drake R, Goldstein JM (2010) Sex differences in schizophrenia. Int Rev Psychiatry 22: 417–428. [DOI] [PubMed] [Google Scholar]
- 2. Kulkarni J, Hayes E, Gavrilidis E (2012) Hormones and schizophrenia. Curr Opin Psychiatry 25: 89–95. [DOI] [PubMed] [Google Scholar]
- 3. Fernandez-Egea E, Garcia-Rizo C, Miller B, Parellada E, Justicia A, et al. (2011) Testosterone in newly diagnosed, antipsychotic-naive men with nonaffective psychosis: a test of the accelerated aging hypothesis. Psychosom Med 73: 643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Howes OD, Wheeler MJ, Pilowsky LS, Landau S, Murray RM, et al. (2007) Sexual function and gonadal hormones in patients taking antipsychotic treatment for schizophrenia or schizoaffective disorder. J Clin Psychiatry 68: 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huber TJ, Tettenborn C, Leifke E, Emrich HM (2005) Sex hormones in psychotic men. Psychoneuroendocrinology 30: 111–114. [DOI] [PubMed] [Google Scholar]
- 6. van Rijn S, Aleman A, de Sonneville L, Sprong M, Ziermans T, et al. (2011) Neuroendocrine markers of high risk for psychosis: salivary testosterone in adolescent boys with prodromal symptoms. Psychol Med 41: 1815–1822. [DOI] [PubMed] [Google Scholar]
- 7. Akhondzadeh S, Rezaei F, Larijani B, Nejatisafa AA, Kashani L, et al. (2006) Correlation between testosterone, gonadotropins and prolactin and severity of negative symptoms in male patients with chronic schizophrenia. Schizophr Res 84: 405–410. [DOI] [PubMed] [Google Scholar]
- 8. Goyal RO, Sagar R, Ammini AC, Khurana ML, Alias AG (2004) Negative correlation between negative symptoms of schizophrenia and testosterone levels. Ann N Y Acad Sci 1032: 291–294. [DOI] [PubMed] [Google Scholar]
- 9. Ko YH, Jung SW, Joe SH, Lee CH, Jung HG, et al. (2007) Association between serum testosterone levels and the severity of negative symptoms in male patients with chronic schizophrenia. Psychoneuroendocrinology 32: 385–391. [DOI] [PubMed] [Google Scholar]
- 10.Moore L, Kyaw M, Vercammen A, Lenroot R, Kulkarni J, et al.. (2013) Serum testosterone levels are related to cognitive function in men with schizophrenia. Psychoneuroendocrinology. [DOI] [PubMed]
- 11. Shirayama Y, Hashimoto K, Suzuki Y, Higuchi T (2002) Correlation of plasma neurosteroid levels to the severity of negative symptoms in male patients with schizophrenia. Schizophr Res 58: 69–74. [DOI] [PubMed] [Google Scholar]
- 12. Ko YH, Lew YM, Jung SW, Joe SH, Lee CH, et al. (2008) Short-term testosterone augmentation in male schizophrenics: a randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol 28: 375–383. [DOI] [PubMed] [Google Scholar]
- 13. van Honk J, Tuiten A, Verbaten R, van den Hout M, Koppeschaar H, et al. (1999) Correlations among salivary testosterone, mood, and selective attention to threat in humans. Horm Behav 36: 17–24. [DOI] [PubMed] [Google Scholar]
- 14.Mazur A, Booth A (1998) Testosterone and dominance in men. Behav Brain Sci 21: 353–363; discussion 363–397. [PubMed]
- 15. Archer J (2006) Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci Biobehav Rev 30: 319–345. [DOI] [PubMed] [Google Scholar]
- 16. Eisenegger C, Naef M, Snozzi R, Heinrichs M, Fehr E (2010) Prejudice and truth about the effect of testosterone on human bargaining behaviour. Nature 463: 356–359. [DOI] [PubMed] [Google Scholar]
- 17. Cherrier MM, Matsumoto AM, Amory JK, Johnson M, Craft S, et al. (2007) Characterization of verbal and spatial memory changes from moderate to supraphysiological increases in serum testosterone in healthy older men. Psychoneuroendocrinology 32: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Derntl B, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, et al. (2009) Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology 34: 687–693. [DOI] [PubMed] [Google Scholar]
- 19. Mehta PH, Beer J (2010) Neural mechanisms of the testosterone-aggression relation: the role of orbitofrontal cortex. J Cogn Neurosci 22: 2357–2368. [DOI] [PubMed] [Google Scholar]
- 20. van Wingen GA, Zylicz SA, Pieters S, Mattern C, Verkes RJ, et al. (2009) Testosterone increases amygdala reactivity in middle-aged women to a young adulthood level. Neuropsychopharmacology 34: 539–547. [DOI] [PubMed] [Google Scholar]
- 21. Volman I, Toni I, Verhagen L, Roelofs K (2011) Endogenous testosterone modulates prefrontal-amygdala connectivity during social emotional behavior. Cereb Cortex 21: 2282–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mendrek A, Lakis N, Jimenez J (2011) Associations of sex steroid hormones with cerebral activations during mental rotation in men and women with schizophrenia. Psychoneuroendocrinology 36: 1422–1426. [DOI] [PubMed] [Google Scholar]
- 23. Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, et al. (2000) Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry 57: 907–913. [DOI] [PubMed] [Google Scholar]
- 24. Kohler CG, Martin EA (2006) Emotional processing in schizophrenia. Cogn Neuropsychiatry 11: 250–271. [DOI] [PubMed] [Google Scholar]
- 25. Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ (2000) Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport 11: 1739–1744. [DOI] [PubMed] [Google Scholar]
- 26. Vercammen A, Morris R, Green MJ, Lenroot R, Kulkarni J, et al. (2012) Reduced neural activity of the prefrontal cognitive control circuitry during response inhibition to negative words in people with schizophrenia. J Psychiatry Neurosci 37: 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Amin Z, Epperson CN, Constable RT, Canli T (2006) Effects of estrogen variation on neural correlates of emotional response inhibition. Neuroimage 32: 457–464. [DOI] [PubMed] [Google Scholar]
- 28. Calev A, Edelist S (1993) Affect and memory in schizophrenia: negative emotion words are forgotten less rapidly than other words by long-hospitalized schizophrenics. Psychopathology 26: 229–235. [DOI] [PubMed] [Google Scholar]
- 29.First MB, Spitzer RL, Gibbon M, Williams JBW (2007) Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P). New York: Biometric Research Department, New York State Psyciatric Institute.
- 30. Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276. [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D (1997) Wechsler Adult Intelligence Scale. San Antonio: The psychological corporation.
- 32.Bradley MM, Lang PJ (1999) norms for English Words (ANEW): Instruction manual and affective ratings. Florida: University of Florida.
- 33. Goldstein M, Brendel G, Tuescher O, Pan H, Epstein J, et al. (2007) Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: an emotional linguistic go/no-go fMRI study. Neuroimage 36: 1026–1040. [DOI] [PubMed] [Google Scholar]
- 34. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, et al. (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- 35.Brett M, Anton J-L, Valabregue R, Poline J-B (2002) Region of interest analysis using an SPM toolbox. the 8th International Conference on Functional Mapping of the Human Brain,. Sendai, Japan.
- 36. Gray JR (2001) Emotional modulation of cognitive control: approach-withdrawal states double-dissociate spatial from verbal two-back task performance. J Exp Psychol Gen 130: 436–452. [DOI] [PubMed] [Google Scholar]
- 37. Gyurak A, Goodkind MS, Kramer JH, Miller BL, Levenson RW (2012) Executive functions and the down-regulation and up-regulation of emotion. Cogn Emot 26: 103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chiu PH, Holmes AJ, Pizzagalli DA (2008) Dissociable recruitment of rostral anterior cingulate and inferior frontal cortex in emotional response inhibition. Neuroimage 42: 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bentall RP, Kaney S (1989) Content specific information processing and persecutory delusions: an investigation using the emotional Stroop test. Br J Med Psychol 62 (Pt 4): 355–364. [DOI] [PubMed] [Google Scholar]
- 40. Gopin CB, Burdick KE, Derosse P, Goldberg TE, Malhotra AK (2011) Emotional modulation of response inhibition in stable patients with bipolar I disorder: a comparison with healthy and schizophrenia subjects. Bipolar Disord 13: 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aikey JL, Nyby JG, Anmuth DM, James PJ (2002) Testosterone rapidly reduces anxiety in male house mice (Mus musculus). Horm Behav 42: 448–460. [DOI] [PubMed] [Google Scholar]
- 42. Hermans EJ, Putman P, Baas JM, Koppeschaar HP, van Honk J (2006) A single administration of testosterone reduces fear-potentiated startle in humans. Biol Psychiatry 59: 872–874. [DOI] [PubMed] [Google Scholar]
- 43. Sankar JS, Hampson E (2012) Testosterone levels and androgen receptor gene polymorphism predict specific symptoms of depression in young men. Gend Med 9: 232–243. [DOI] [PubMed] [Google Scholar]
- 44. Aydogan U, Aydogdu A, Akbulut H, Sonmez A, Yuksel S, et al. (2012) Increased frequency of anxiety, depression, quality of life and sexual life in young hypogonadotropic hypogonadal males and impacts of testosterone replacement therapy on these conditions. Endocr J 59: 1099–1105. [DOI] [PubMed] [Google Scholar]
- 45. Leppanen JM (2006) Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry 19: 34–39. [DOI] [PubMed] [Google Scholar]
- 46. Mathews A, MacLeod C (1994) Cognitive approaches to emotion and emotional disorders. Annu Rev Psychol 45: 25–50. [DOI] [PubMed] [Google Scholar]
- 47. Grimm S, Weigand A, Kazzer P, Jacobs AM, Bajbouj M (2012) Neural mechanisms underlying the integration of emotion and working memory. Neuroimage 61: 1188–1194. [DOI] [PubMed] [Google Scholar]
- 48. Jasinska AJ, Yasuda M, Rhodes RE, Wang C, Polk TA (2012) Task difficulty modulates the impact of emotional stimuli on neural response in cognitive-control regions. Front Psychol 3: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129: 564–583. [DOI] [PubMed] [Google Scholar]
- 50. Taylor KS, Seminowicz DA, Davis KD (2009) Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 30: 2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Montgomery J, Winterbottom E, Jessani M, Kohegyi E, Fulmer J, et al. (2004) Prevalence of hyperprolactinemia in schizophrenia: association with typical and atypical antipsychotic treatment. J Clin Psychiatry 65: 1491–1498. [DOI] [PubMed] [Google Scholar]
- 52. Vermeulen A, Verdonck L, Kaufman JM (1999) A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84: 3666–3672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Thresholded T-map on sagittal sections, for the contrast inhibit negative>inhibit neutral, with a less conservative p-value = .01. Regions showing increased activation in the healthy men are indicated in yellow, regions showing increased activation in the men with schizophrenia are indicated in red. The figure demonstrates that there is some overlap in activation between the two groups, but that the pattern is less robust and shows a less contiguous spatial distribution in the men with schizophrenia as compared to the healthy men.
(TIF)
Overview of clusters showing significantly increased BOLD response when contrasting the inhibit negative and the inhibit neutral task conditions, p = .001 uncorrected; min. voxel extent k ≥18. We chose to apply the same statistical criterion as we employed in an earlier publication on this paradigm (Vercammen et al., 2012, Journal of Psychiatric Research), based on a double threshold approach. A simulation script was used to determine cluster threshold (cluster_threshold_beta.m retrieved from https://www2.bc.edu/~slotnics/scripts.htm), with the following parameters acquisition matrix (80×80), original voxel dimensions (3×3×3), number of slices (32), full width half maximum (FWHM) set to 0, resampled voxel resolution (2×2×2), mask (none), corrected p-value (.05), voxel based p-value (.001), iterations (1000). The healthy men showed a network of increased activation in the inhibit negative condition compared to the inhibit neutral condition, that overlapped with previous findings from our group (Vercammen et al., 2012, Journal of Psychiatry and Neuroscience,37(6): 379–388). The men with schizophrenia did not show significant activation changes at the same significance level. Lowering the statistical threshold did reveal a number of activation clusters in the patient group.
(DOC)
A table displaying the correlations between activation of the bilateral ROIs for the contrast inhibit negative>inhibit neutral, and clinical characteristics in the sample of men with schizophrenia. Significant correlations are indicated in bold.
(DOCX)