Abstract
Gene therapy is one of the most promising fields for developing new treatments for the advanced stages of ischemic and monogenetic, particularly autosomal or X-linked recessive, cardiomyopathies. The remarkable ongoing efforts in advancing various targets have largely been inspired by the results that have been achieved in several notable gene therapy trials, such as the hemophilia B and Leber's congenital amaurosis. Rate-limiting problems preventing successful clinical application in the cardiac disease area, however, are primarily attributable to inefficient gene transfer, host responses, and the lack of sustainable therapeutic transgene expression. It is arguable that these problems are directly correlated with the choice of vector, dose level, and associated cardiac delivery approach as a whole treatment system. Essentially, a delicate balance exists in maximizing gene transfer required for efficacy while remaining within safety limits. Therefore, the development of safe, effective, and clinically applicable gene delivery techniques for selected nonviral and viral vectors will certainly be invaluable in obtaining future regulatory approvals. The choice of gene transfer vector, dose level, and the delivery system are likely to be critical determinants of therapeutic efficacy. It is here that the interactions between vector uptake and trafficking, delivery route means, and the host's physical limits must be considered synergistically for a successful treatment course.
Science never solves a problem without creating ten more.
—George Bernard Shaw
Introduction
Successful cardiac gene therapy hinges on the development of a viable delivery strategy to transfer therapeutic genes to both diseased and, in some cases, healthy but at-risk myocardium. The critical unresolved problem to date is developing methods to transduce a sufficient fraction of myocytes to establish efficacy within safety limits. Initially, researchers did not invest substantial effort in improving methods of delivery since it was postulated that simple intramuscular cardiac injections would be sufficient. Also, the field in general was biased toward the development organ-tropic vectors, since these in theory would address the problem of achieving cardiac cell target specificity while reducing the risks of transgene-mediated immune responses.
Recently, however, interest has migrated toward the scientific aspects of delivery systems engineered specifically for the heart. This change in perspective represents a shift from a strategy solely focused on manipulating vector tropism through natural selection and vector engineering. This re-focus has been motivated by three factors: (1) inadequate cardiac-specific gene transfer performance as assessed by qPCR and qRT-PCR in various large animal and clinical investigations; (2) equivocal efficacy data as assessed by quantitative indices of myocardial performance in clinical trials; and (3) a stringent regulatory pathway for gene therapies, whereby significant vector re-engineering introduces significant delays in the regulatory pathway to clinical approval. Regardless of tropism, the risk of host immune reactions to the vector capsid is inherently a function of the delivery strategy, since the upper dose limit is frequently challenged when the desired outcome is not obtained. Thus, preventing systemic exposure is paramount and restricting vector to the heart is an important safety consideration.
A successful cardiac gene therapy program would include the optimal synergistic combination of (1) the correct transgene for the intended clinical aim (e.g., angiogenesis, improving contractility, reversal of remodeling, inhibition of apoptosis, inhibition of fibrosis, myocyte regeneration, or correction of a specific autosomal or X-linked recessive defect; (2) viral or nonviral vector with mechanistic transfer attributes and molecular trafficking considerations for sufficient transduction of a sufficient quantity of regional or globally targeted myocytes to achieve a therapeutic effect; (3) a safe clinically appropriate delivery route; and (4) the minimally effective dose as a function of (1), (2), and (3). Application of this strategy is predicted to reduce the risk of invoking an adaptive immune response, enhance therapeutic efficacy, and minimize adverse events at the time of delivery in the clinical setting. Therefore, the cardiac gene therapy treatment plan must be viewed as a whole system rather than a sum of independent elements. Unfortunately, these issues are not typically explored systematically in basic preclinical investigations in this research space, resulting in a significant translational gap. In this review we outline the key aspects of viral versus nonviral vectors and their transport/trafficking processes, clinically available cardiac gene delivery options, and selected examples of previous studies classified by vector type. We conclude with a critique of the field to date and address the need for a systematic approach where each of these factors should be carefully considered for any intended application of cardiovascular gene therapy.
Nonviral Versus Viral Vectors: Uptake and Trafficking Mechanisms
There are fundamental mechanistic differences between nonviral and viral vector therapeutic approaches with respect to gene transfer at the cellular level. These differences are largely independent of the administration route, assuming adequate delivery to the interstitium of the heart. Each specific vector type has advantages and disadvantages depending on the cardiac application and accompanying host response considerations. Recombinant vectors, whether viral or nonviral, must migrate through various biological barriers in one or more compartments to subsequently transfect target cells. In addition to cellular uptake, more challenges exist for successful transduction. The cell's response to the vector after uptake can vary significantly and reflects differing rates of protein, DNA, and RNA synthesis effecting therapeutic efficiency. These mechanisms are complex and relatively unpredictable a priori in the cardiomyopathic environment. It is important to note that most preclinical data are collected in animal model systems lacking the comorbidities found in actual patients (diabetes, chronic kidney disease, peripheral vascular disease, coronary artery disease) that can dramatically modulate therapeutic efficacy and decrease the relevance of encouraging preclinical data when translated to the clinic.
Interactions between the vector and host cardiac environment can be simply modeled in two distinct phases: (1) physical interactions and migration through one or more biological compartments (i.e., those encountered during or shortly after initial delivery), and (2) molecular trafficking interactions after cellular uptake of vector. Here we review the key delivery considerations for viral and nonviral vector selection with respect to their fundamental uptake and nuclear trafficking mechanisms.
Nonviral vector systems
Naked plasmid DNA (pDNA) and nonviral engineered vectors have traditionally been used as research tools to elucidate the fundamental mechanisms of gene expression. With several key advancements over time, however, these vectors are well positioned for clinical translation. Because of safety concerns with the host immune responses, nonviral vectors continue to be attractive alternatives to viral vectors in a number of cardiac disease trials. The main benefits of nonviral gene therapy targeting the cardiovascular system are (1) very low risk for an adaptive immune response, (2) low toxicity, (3) ease of production, (4) relative simplicity, and (5) increased flexibility in applying recombinant techniques for enhancing cardiac specificity with promoters and also incorporating larger expression cassettes.
Extra- and intracellular trafficking of nonviral vectors
A concise summary highlighting key steps for nonviral vector-mediated gene expression can be divided in two series of steps, one set describing transfer to the interstitial compartment to reach the targeted myocytes and the second describing trafficking in the extra- and intracellular compartments. The key transfer steps in the first series depicted in Fig. 1A are
FIG. 1.
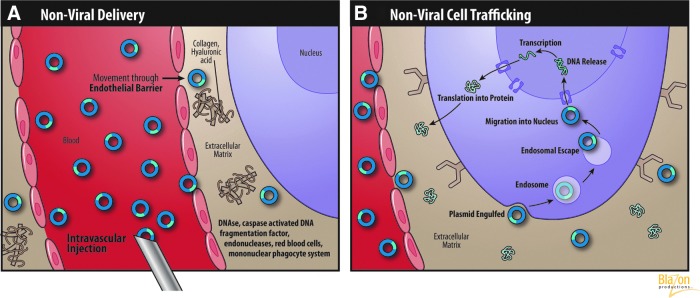
(A) Nonviral vector migration and transfer barriers. (B) Nonviral vector extra- and intracellular trafficking. (Reprinted with permission from Blazon Productions.)
Migration through the blood vessel compartment
Transfer from the vessel compartment through the endothelial barrier to reach the myocytes in the interstitial compartment
Extracellular matrix (ECM) navigation in the interstitial environment
Uptake via cell membrane interactions
In the first critical step of migration, a plethora of in vivo studies have identified nonviral vector degradation agents in the blood compartment. These blood elements are considered major impediments from the gene delivery perspective and must be considered. Most of these factors are located in the serum, and some examples include DNAses, caspase-activated DNA fragmentation factors, and endonucleases. Red blood cells and mononuclear phagocyte systems (macrophages) are also involved in DNA degradation during programmed cell death (Nagata, 2005). Approximately 50% of DNA liposome complexes bind to components in the blood. The majority of these interactions are with erythrocytes and occur within only 1 min after intravenous injection in mice. Moreover, these DNA molecules are readily cleared from the circulation, which is correlated with transfection efficiency (Sakurai et al., 2001). Furthermore, Kupffer cells in the liver and macrophages in the spleen ensure clearance of DNA particles from the blood (Al-Dosari and Gao, 2009). The interaction with serum proteins is another significant barrier for pDNA transfer. It has been shown that cells of the reticulo-endothelial system rapidly clear large amounts of DNA–liposome complexes coated with serum proteins (albumin) (Li et al., 1998). The next barrier is the vascular endothelium in the capillary wall. The permeability properties of the endothelium are regulated by cellular interactions, the basement membrane, and supporting matrix. It is important to note that the endothelial pores or spaces between the surface cell lining are less than 10–15 nm in diameter at rest. This imposes a physical rate limiting size constraint for passage (i.e., at rest without driving forces most vectors are too large). After transmigration across the endothelial barriers, the vector is subsequently exposed to various critical extra- and intracellular components depicted in Fig. 1B and are represented by the following.
The extracellular matrix
To reach the plasma membrane of targeted cells, the nonviral vector must navigate through the ECM, which is composed of extracellular fluid and various supporting protein structures. This process is regulated by the amount of ECM components such as tissue collagen and hyaluronic acid. Thus, to improve pDNA diffusion through the ECM, investigators have employed hyaluronidase and collagenase (Escoffre et al., 2010) to negate their binding effects. Mouse muscles pretreated with bovine hyaluronidase produce reportedly substantially higher levels of plasmid expression with an average of 18% of fibers successfully transfected (McMahon et al., 2001). Protecting pDNA from degradation in the ECM can be accomplished using chemical formulations such as poloxamers or through inhibition of endogenous DNAses (Escoffre et al., 2010).
Cell membrane binding and intracellular trafficking
The second series of steps are illustrated in the cellular compartment of Fig. 1B. After overcoming the ECM, nonviral vectors must be taken up by cells via receptor and/or nonspecific binding mechanisms. To achieve sufficient uptake in vivo even with plasmids containing cell-specific promoters, very high vector doses are required (Felgner, 1997). This inefficiency demands the addition of cell-specific ligands or antibodies. A large number of such uptake enhancement systems have been developed (Medina-Kauwe et al., 2005). Nucleic acids typically enter cells through endocytosis, phagocytosis, or micropinocytosis. Most of the macromolecules enter cells by clathrin-mediated endocytosis. Cell receptor binding triggers the local accumulation of clathrin on the cytoplasmic surface of the plasma membrane (Mukherjee et al. 1997). Then, rapid uncoating takes place and the clathrin vesicles fuse with early endosomes (Takei and Haucke, 2001). The cellular endosome is one of the difficult biological obstacles inhibiting transfer. Studies on endosome penetration and escape mechanisms have allowed nonviral vector transfer to improve, but they may never reach the efficiency of their viral counterparts. One of these mechanisms involves the use of fusion peptide or lipid components to rupture the endosome membrane (Li et al., 2004). Another uses polyethylenimine-mediated DNA transfer, which leads to osmotic swelling and rupture of endosomes and plasmid penetration into the cytoplasm (Akinc et al., 2005). The cytoplasmic space is composed of microfilament and microtubule systems constituting the cytoskeleton as well as a protein solution that also can damage and/or engulf vector. Another important consideration is the ability of the vectors to freely diffuse throughout the cell cytoplasm before nuclear transport. The cytosol is the part of the cytoplasm that is not contained in organelles. The mobility of microinjected fluorescein-labeled DNA fragments was measured in cytoplasm in several key studies. The authors here found that the ratio of the plasmid diffusion coefficient in cytoplasm to water decreased from 0.19 for 100 bp to 0.06 for 250 bp, which confirmed the dependence of cytoplasm diffusion on DNA size (Lukacs et al., 2000). The slow DNA diffusion in cytoplasm can be explained by molecular crowding or the binding of nucleic acids with cytosolic proteins. In addition, some of the nucleases, such as DNAase I and II, can be released from intracellular organelles and play a role in metabolic instability of pDNA (Lechardeur et al., 2005). The last physical barrier to pDNA just before transduction is the nuclear envelope. Nuclear pore complexes form channels through the nuclear envelope and regulate the transport of ions, molecules smaller than 50 kDa, or nucleic acids of up to 300 bp (Laskey, 1998). Larger molecules such as pDNA (2–10 MDa) in quiescent cells require a mechanism of active transport mediated through a specific targeting signal—the nuclear localization sequence (Wente, 2000). For dividing cells, DNA molecules enter the nucleus preferentially via the process of disassembly of the envelope during mitotic cell division (Dean et al., 2005). Whether the DNA delivered by nonviral vectors is released in the nucleus is not completely clear. The plasmid-based transgene stays in the nucleus in an episomal form without integration into the host genome.
Viral vector systems
Extra- and intracellular trafficking of viral vectors
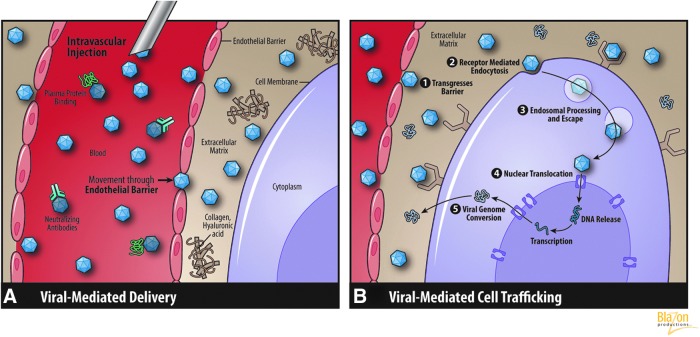
Compared with nonviral vectors, viruses have evolutionary advantages in their extra- and intracellular interactions. These advantages are largely explained by the fact that viruses can alter their capsid protein recognition by cell surface receptors. The basic physical interactions through biological barriers are generally similar to nonviral vectors used for cardiac gene therapy, but an important distinction is that any virus can be subjected to a host neutralizing antibody response, which is a very specific antibody that not only negates the vector effectiveness but could elicit a potent host immune response (Fig. 2A). These limitations present significant issues in clinical trials, whereby (1) repeat administration after a phase I trial (safety) may limit the ability of patients to undergo subsequent therapeutic trials or clinical application of the therapy, and (2) in the first-in-man CUPID trial of gene therapy for heart failure, up to 60% of patients were found seropositive for adeno-associated virus (AAV)-specific antibodies, excluding them from participation.
FIG. 2.
(A) Viral vector migration and transfer barriers. (B) Viral vector extra- and intracellular trafficking. (Reprinted with permission Blazon Productions.)
Despite this immune barrier viral vectors must circumvent, once in the interstitial compartment their performance is excellent. Their key trafficking steps are as follows and are depicted in Fig. 2B:
Binding to myocyte cell surface receptors. This is probably the most important factor affecting viral capture. For example, in AAV serotypes 2 and 3, heparin sulfate proteoglycan has been identified as a primary attachment receptor (Opie et al., 2003). For other serotypes, attachment receptors and coreceptors are less well defined (Ding et al., 2005). For adenovirus, the intracellular pathway is initiated with the Coxsackie or adenovirus receptor (CAR) receptor (Bergelson et al., 1997).
Receptor-mediated endocytosis. Endocytosis of the virus by the host cell occurs in distinct membrane compartments, called clathrin-coated pits, which can be internalized to form clathrin-coated vesicles (Maxfield and McGraw, 2004). Internalization of adenovirus also requires free cholesterol in the plasma membrane (Worgall et al., 2000).
Endosomal processing and escape. After endocytosis, viruses are compartmentalized into early endosomes, which mature into late endosomes that are degraded by fusion with lysosomes and secretory vesicles. These late endosomes recycle the material back to the plasma membrane. Acidification is another critical event in viral transduction. The lowered pH results in lysis of the endosome membrane and escape of the capsid into the cytosol (Maxfield and McGraw, 2004).
Nuclear translocation. After escaping into the cytosol, the pathway continues with trafficking to the nucleus. This process may be facilitated by ATP-dependent molecular motors (Leopold et al., 2000; Seisenberger et al., 2001). It is believed that AAV and adenovirus capsids must uncouple from the translocation machinery and associate with the nucleus. At the same time, viral nuclear transport appears to be slow, but agents such as proteasome inhibitors can promote this movement (Duan et al., 2000).
The final post-nuclear stage for AAV includes virion uncoating and viral genome conversion from a single-stranded to a double-stranded DNA (Ding et al., 2005; Nonnenmacher and Weber, 2012). Self-complementary or double-stranded vectors have been developed circumventing this step, often resulting in an order of magnitude or more improvement in transduction efficiency.
Cardiac Vector Delivery Options
Apart from the most frequently selected intramyocardial and antegrade intracoronary infusion methods featured in current clinical trials, a number of alternative vector delivery strategies have emerged (Katz et al., 2011). These novel approaches have the potential to reach the clinic in the near future. Some key examples of these include
Surgical and advanced percutaneous catheter-based infusion-mediated transvascular perfusion approaches
Direct myocardial delivery using mechanical and physical approaches
Image-guided injections featuring state-of-the-art cardiac mapping technology
Here we review each category and highlight key studies and their associated results subcategorized by vector type.
Percutaneous catheter-based infusion approaches
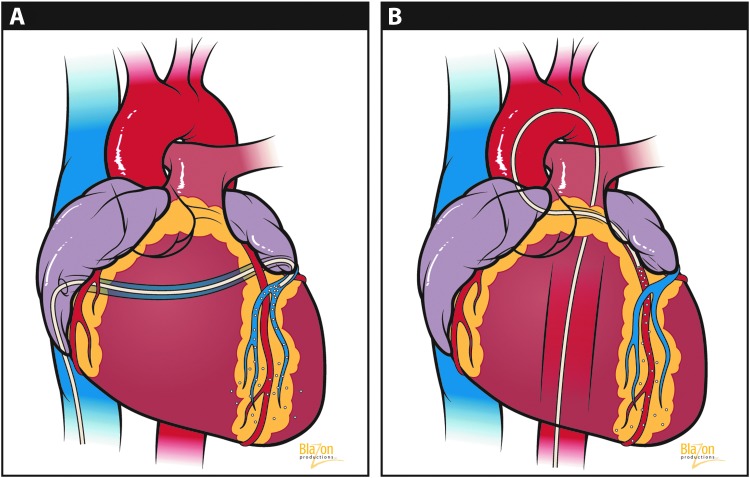
Interventional approaches include the following: (1) selective and nonselective antegrade (i.e., arterial side infusion) delivery (Fig. 3A, and (2) retrograde (i.e., venous side infusion) into the coronary sinus or anterior intraventricular vein (Fig. 3B). Both techniques can also be modified to include the option of concomitant arterial/venous vessel occlusions achieved with balloon. These approaches seek to maximize vector transfer across the endothelial barrier by increasing the capillary vessel–interstitial transport pressure gradients.
FIG. 3.
(A) Retrograde venous infusion. (B) Antegrade intracoronary infusion. (Reprinted with permission from Blazon Productions.)
Despite some remarkable success in preclinical models, most of these modified infusion methods are unlikely to be used clinically for patients with advanced cardiomyopathies. In many cases, global rather than regional gene delivery would be ideal, and these techniques generally allow for only regional myocardial gene delivery. Furthermore, regulatory concerns may be raised in patients with advanced coronary artery disease where vessels are diseased and interventions are not free of risk.
Adenovirus
A single intracoronary infusion of adenovirus resulted in measurable recombinant DNA expression in rabbit myocardium and collateral organs for 2 weeks. Surprisingly, no inflammatory response or myocardial necrosis was observed after the infusions (Barr et al., 1994). Delivery of Ad/beta galactosidase to rabbit hearts by intracoronary perfusion illustrated that transfection efficiency depends on the following critical parameters: coronary flow rate, the adenovirus dose, contact time with coronary circulation, and the composition of the perfusate. A single pass of Ad solution through the heart caused transduction of only 0.8% myocytes. However, with recirculation for 60 min, up to 40% of myocytes were transduced (Donahue et al., 1997). Subsequently, the basic principles for enhancing intravascular adenoviral gene transfer were formulated: (1) Increased vector residence time inside the coronary vessels; (2) transient coronary arterial or venous interruption increases transduction; (3) delivery with high-pressure infusion provides better efficiency; (4) retroinfusion through the coronary sinus has greater potential because of more favorable anatomy; (5) the use of numerous pharmacological agents can increase endothelial permeability; and (6) a washing procedure after gene transfer significantly reduces viral dissemination to collateral organs (Donahue et al., 1998; Hajjar et al., 1998; Boekstegers et al., 2000; Logeart et al., 2001; Wright et al., 2001; Bridges et al., 2002; Griscelli et al., 2003).
Myocardial overexpression of beta2-adrenergic receptors has been shown to enhance contractility in transgenic mice and reverse signaling abnormalities found in failing cardiomyocytes (Maurice et al., 1999; Shah et al., 2000). Aortic-banded rats infected with Ad.SERCA2a had significant improvement in left-ventricular systolic pressure, +dP/dt, −dP/dt, and rate of isovolumic relaxation—normalized to levels comparable to sham-operated rats (Miyamoto et al., 2000). Acute myocardial ischemia was attenuated after Ad-mediated βARKct delivery into the rabbit left ventricle (Tevaearai et al., 2005). Cardiac adenoviral S100A1 gene delivery restored diminished intracellular Ca2+ transients and sarcoplasmic reticulum Ca2+ load and also normalized myocardial contractile function in postinfarct rat hearts (Most et al., 2004).
Adeno-associated virus
It is worth noting that AAV vectors have largely supplanted adenoviruses although using many of the promising gene transfer methods and transgenes that were previously validated. In early studies, selective coronary catheterization with antegrade infusion of AAV/LacZ into pigs' circumflex artery resulted in successful transfer and expression of a reporter gene for at least 6 months after infusion without toxicity and inflammation (Kaplitt et al., 1996). Quantification of fluorescence after direct intracoronary delivery of AAV indicated up to 12% gene transfer in the region of infusion (Kaspar et al., 2005). Harvested mouse hearts after perfusion with AAV/LacZ for 15 min were transplanted and revascularized. Eight weeks later, the level of transduction was stable with ∼50% of cardiomyocytes continuing to express beta-gal (Svensson et al., 1999). Unfortunately, these promising results with respect to intravascular gene transfer efficiency in murine and rodent species have not translated to larger animals.
Intracoronary delivery of AAV encoding phospholamban in cardiomyopathic hamsters suppressed progressive dilated cardiomyopathy (Hoshijima et al., 2002). Left ventricular (LV) indirect intracoronary delivery with aortic and pulmonary artery clamping revealed up to 32% GFP expression to a similar degree at both 1 and 12 months after injection (Kaspar et al., 2005). Selective retrograde infusion of AAV6 into the anterior cardiac vein increased reporter gene expression in the targeted left anterior descending artery territory in the porcine heart (Raake et al., 2008). Robust and long-term βARKct expression was found after AAV6-mediated retrograde delivery with significant amelioration of LV hemodynamics and contractile function in heart failure pigs (Raake et al., 2013) although a quantitative assessment of gene transfer efficiency and cardiac delivery specificity was not provided, making a pharmacokinetic analysis impossible. We would argue that such detailed (qPCR and qRT-PCR) information will be important to optimize safe and dose-appropriate clinical translation.
Another transvascular delivery method for AAV is the use of extracorporeal circulation in the cardiac surgery setting. This system, molecular cardiac surgery with recirculating delivery, allows for isolation of the cardiac circulation from the systemic circulation and significantly extends vector residence time in coronary vasculature. Using this technique, it was possible, for the first time, to achieve AAV-mediated gene expression in the majority of myocytes in the left ventricle in a large animal model in situ (White et al., 2011). It is important to note that this is the only delivery system to date to demonstrate substantial evidence of cardiac specificity. Taken together, the available evidence confirms that in the in vivo setting, the primary challenge for viral vector-mediated transfer is successful transfer across the endothelial barrier. The study by White et al. (2011) demonstrates that a sustained vector concentration recirculating at a high-pressure gradient, ideally venous-to-arterial flow with cardiac-specific delivery, appears to be necessary to maximize vector-mediated gene transfer while limiting systemic exposure.
Lentivirus
Lentiviruses have more complex genomes that facilitate trafficking through the cellular and nuclear membrane without disruption and integration into nondividing cells such as myocytes with long-term expression and relative lack of antigenicity (Di Pasquale et al., 2012). The applicability of lentiviruses for the efficient transduction of postmitotic beating rat myocytes, cardiac fibroblasts, and well-differentiated myofibers has been shown previously in several studies (Sakoda et al., 1999).
In an efficacy study, a lentiviral vector containing the SERCA2 gene was infused into a rat heart by hypothermic intracoronary delivery 2 weeks after myocardial infarction. The transduction efficiency was approximately 40%. Six months after transduction, hemodynamic measurements revealed protection from adverse LV remodeling and an improved survival rate (Niwano et al., 2008).
Direct myocardial delivery using mechanical and physical approaches
Direct myocardial gene delivery is attractive for two main reasons: specific cardiac regions can be targeted, and it is possible to achieve a highly localized vector concentration. Nonetheless, these approaches may be associated with substantial systemic exposure through inadvertent penetration of myocardial vessels and spillage or overflow outside of the interstitial compartment (White et al., 2011).
Gene expression with naked DNA in vivo was first demonstrated in mouse myofibers after direct muscle injection (Wolff et al., 1990). However, subsequent studies found that the efficiency of gene transfer to heart tissue by direct injection is relatively low and variable in different species. Outside of the physical limitations of needle injection, this limitation is also largely explained by the low transfection efficiency of complexes of pDNA through the cell membrane. Therefore, many physical delivery methods have been proposed to overcome this barrier and improve gene transfer. Many of these systems can also be applied with viral vectors.
Needle injection
The simplest and most frequently used method for cardiac gene delivery is needle injection. Several groups have demonstrated the feasibility of this technique for delivering pDNA to the heart (Lin et al., 1990; Acsadi et al., 1991; Buttrick et al., 1992; von Harsdorf et al., 1993). However, a key disadvantage includes acute inflammatory responses secondary to needle injury. Furthermore, the resultant transgene expression is not uniform and limited to the immediate vicinity of injection sites and may not be sufficient to achieve global distribution as may be required for autosomal or X-linked recessive cardiomyopathies where the transduction of a majority of myocytes in diffuse global distribution may be required for maximal efficacy. This approach may be applicable for regional strategies (e.g., angiogenesis in the borderzone after myocardial infarction).
Nonviral vectors
It was shown for the first time in 1990 (Lin et al., 1990) that the programming of the β-galactosidase gene in cardiac myocytes was achieved for up to 3–4 weeks after intraventricular injection of pDNA vector. The key point established here was that reporter gene activity can typically be detected on the first day and remains relatively stable for up to 2 weeks after pDNA injection. Another key observation in a similar study was that the number of rats expressing the marker genes was reduced to 30% between 38 and 60 days postinjection (Buttrick et al., 1992). Successive studies have demonstrated that the transduction efficiency of plasmid vectors in the cardiac system is low and marked by insufficient expression levels of recombinant protein. Moreover, gene expression was restricted to within a few millimeters of the injection sites. This is a problem for many cardiac diseases since a large number of myocytes may need to be transduced for efficacy.
After these early studies, researchers started looking for tools to extend the cardiac expression profile of pDNA (Fishman et al., 1994). A key advancement was discovered in utilizing synthetic compounds in which DNA complexes are formed in particle structures capable of transferring genes into cells. Some of these widely used examples are (1) synthetic DNA composed of an expression cassette inserted into a plasmid and complexed with a cationic polymer (polyplex); (2) cationic lipid (lipoplex or liposome); and (3) a combination of (1) and (2) resulting in lipopolyplexes.
In terms of in vivo cardiac performance, the most effective nonviral vectors have been developed from liposomes (Lechardeur et al., 2005). Liposomes have some advantages delivering genes to cells: (1) they are very cost effective; (2) they protect DNA from nuclease-mediated degradation; (3) they can carry large DNA sequences; (4) they do not typically cause toxicity; and (5) they can be engineered to target specific cells (Kamimura et al., 2011). Efficient transfection of the heart was found 10 days after injection of DNA–liposome complexes in mice (Stewart et al., 1992). In a separate study, successful transfection with liposome complexes encoding hepatocyte growth factor after injection into the apex of infarcted hearts was demonstrated in the rat model (Aoki et al., 2000).
Adenovirus
The first studies to assess gene transfer into the myocardium via intramuscular delivery with viral vectors appeared 2–3 years after pDNA application (Stratford-Perricaudet et al., 1992; Guzman et al., 1993; Kass-Eisler et al., 1993). Almost right away, there were advantages and limitations of viral vectors discovered, most of which are relevant today: (1) the efficiency of adenovirus-mediated gene transfer is superior (∼140,000 times) to that of pDNA injection (Guzman et al., 1993; French et al., 1994); (2) the amount of recombinant protein increases with the amount of virus (French et al., 1994); (3) no marker gene activity can be detected farther than 5 mm from the injection site (French et al., 1994); and (4) adenovirus induces a prominent inflammatory response and has a transient nature of expression (Guzman et al., 1993; Kass-Eisler et al., 1993; French et al., 1994; Muhlhauser et al., 1996). It has been shown that intramyocardial injection of adenoviral vector carrying VEGF (Ad/VEGF) achieves localized expression for maximal at 7 days at a distance of 1.5 cm from the site of injection after a single vector administration in dogs (Magovern et al., 1996). Angiograms obtained 28 days after intramyocardial injection of Ad/VEGF demonstrated improved collateral perfusion in regions of vector administration (Lee et al., 2000). This gene delivery strategy involving direct myocardial administration of Ad/VEGF has been shown to be capable of “biological revascularization” of ischemic myocardium in an established porcine model (Mack et al., 1998; Patel et al., 1999). Interestingly, the area of Ad/beta galactosidase activity in the noninfarcted hearts was significantly higher (28%) than that in infarcted tissues (3.4%). Moreover, an inflammatory response consisting of mononuclear cell infiltration was much less intense 7 days after injection in noninfarcted control rat hearts than in infarcted hearts (Leor et al., 1996).
Adeno-associated virus
Early studies showed the ability to genetically modify rat myocardial cells after AAV/β-galactosidase after direct injection (Kaplitt et al., 1996). It was later demonstrated that efficient transduction in vivo after intramyocardial injection could be achieved in mice with an AAV vector. Hearts harvested after 8 weeks revealed stable expression in a large number of cardiomyocytes without inflammation. This was in contrast to Ad/LacZ-injected hearts, which displayed transient expression that was undetectable after 4 weeks and had intense inflammation and necrosis (Svensson et al., 1999). AAV encoding a LacZ reporter gene was injected into mouse hearts via a transdiaphragmatic approach. Uniform LacZ expression was revealed at 1 year postinjection (Woo et al., 2005). A direct comparison of intramyocardial injection of AAV9 with other serotypes was performed in the rat heart. Authors demonstrated that in this species and via this route AAV9 was the most cardiotropic serotype with rapid onset, and is relatively stable for 1 year (Bish et al., 2008). When compared with tail vein injection, Inagaki et al. (2006) were able to achieve global cardiac distribution at an ∼5-fold lower dose. In another study at 12 and 24 weeks, AAV1, 6, and 8 demonstrated the highest efficiency for transducing rat hearts (Palomeque et al., 2007). An interesting study in Rhesus macaques showed that transendocardial delivery of AAV6 is more cardiac-specific than AAV8 and 9 (Gao et al., 2011). Intramyocardial βARKct delivery via AAV6 into the already failing myocardium results in robust long-term expression in the LV of rats with heart failure. Moreover, at 3 months after gene transfer, significantly increased ejection fraction and dP/dt were found, whereas LV end systolic diameter was decreased (Rengo et al., 2009).
Lentivirus
In another study, persistent gene transfer was shown up to 10 weeks after intramyocardial injection of a lentiviral GFP vector (Fleury et al., 2003). In a mouse model of Fabry disease with LV hypertrophy, it was demonstrated that LV intraventricular injection of lentivirus-mediated α-galactosidase can correct the enzymatic deficiency and lipid accumulation (Yoshimitsu et al., 2006). In a different study, hearts transduced with a vector based on a lentivirus pseudotype HIV-1 after direct injection showed levels of transgene expression comparable to that achieved by adenovirus vectors (Zhao et al., 2002).
Microinjection
Microinjection directly delivers DNA into the nucleus and compared with standard injection results in a much higher level of gene expression (Capecchi, 1980). Despite this advantage, the entire methodology is impractical for in vivo cardiac applications because of scale.
Gene gun particle bombardment
Particle bombardment (e.g., gene gun or ballistic DNA transfer) is a technique whose principle is based on the bombardment of micrometer-sized heavy metal particles coated with therapeutic pDNA. This method utilizes pressurized medical gas that accelerates the metallic DNA complexes to a high velocity. The transfer to the target cells is achieved by means of direct penetration through the plasma membrane (Uchida et al., 2002). It was shown that using this technology with DNA-coated gold particles in a beating rat heart resulted in the detection of GFP expression for up to 3 weeks (Matsuno et al., 2003). In another rodent study demonstrating the method's cardiac specificity, DNA was detected only in the bombarded hearts but not in collateral organs. However, a problem with penetration throughout the thickness of the myocardium was noted, as only the surface layer of cardiomyocytes was transfected. The bombardment approach could not reach the endocardium and did not affect survival in a separate efficacy study (Nishizaki et al., 2000).
Liquid jet injection
The effectiveness of gene transfer was also observed with jet injection. This technique uses high-pressure gas to eject liquid containing a therapeutic molecule through a nozzle orifice in a sharp, focused liquid jet. The resultant impact force of the jet generates pores in the cell membrane and/or distributes product in the interstitial compartment for subsequent uptake. A rabbit study has shown that luciferase gene activity after jet injection was 50-fold higher than that after needle injection but lower than particle bombardment (Ren et al., 2002). The pressurized velocity profile of the liquid jet within safe limits of application contributes to the gene distribution pattern as well as depth of penetration. Other key variables are the diameter of the jet and injected volume, which also play key roles in the penetration depth (Arora et al., 2007).
Electroporation
The application of short-duration, high-intensity electric pulses in combination with pDNA injection enhances local cell membrane permeability and increases pDNA uptake (Satkauskas et al., 2002; Wells, 2004; Katz et al., 2013). Fluorescent images of GFP expression in embryonic chick hearts 48 hr after electroporation depicted an increased expression profile percentage by 48–50% compared with control hearts (Harrison et al., 1998). The combination of electroporation with intracardiac transplant graft injection of DNA–dendrimer complexes in mice improved gene expression by several orders of magnitude (Wang et al., 2001). Electroporation-mediated gene transfer to the beating rat hearts indicated that LV contractility function (LV dP/dt and dLVP) was altered only in the group with the maximum pulse regimen and the effect normalized after 20 min. Additional safety indicators included cardiac enzyme biomarkers, which also remained in the normal range (Ayuni et al., 2010). Delivery of a plasmid expressing VEGF to the porcine heart through electroporation showed that this technique can increase expression fivefold (Marshall et al., 2010), appears safe, and holds promise as a therapeutic approach for the treatment of heart disease (Hargrave et al., 2013).
Sonoporation
Sonoporation is another physical method that enhances cell membrane permeability via the application of ultrasound in the presence of microbubbles. The method involves the attachment of genes to gas-filled microbubbles, which are then mechanically destroyed within the target tissue by ultrasound. The transfection efficiency depends on the acoustic pressure, pulse duration, and the time of cells' exposure to ultrasound (Duvshani-Eshet et al., 2006; Newman and Bettinger, 2007). In one study, the rat heart treated with microbubbles containing CMV-luciferase plasmids demonstrated cardiac-specific transgene expression (3,096–4,730 RLU/mg/min) compared with control hearts. Time-point evaluation showed highest expression in the first 2 days (Bekeredjian et al., 2003). Electrocardiographic and echocardiographic tools were used to examine the effects of sonoporation on the murine heart. The results showed the appearance of transient arrhythmias and nonsignificant changes in ejection fraction and fractional shortening. Authors demonstrated heart-specific transfection with sonoporation-based gene delivery predominantly detected at the subendocardial layer of the myocardium (Tsunoda et al., 2005).
In another study, injection into the rat heart with naked plasmid encoding HGF during sonoporation 2 hr after acute myocardial infarction revealed that after 3 weeks the scar size, compared with control animals, was significantly reduced (16% vs. 40%). Furthermore, the progression of LV remodeling based on geometric and functional parameters was successfully prevented in the sonoporation group (Kondo et al., 2004).
Laser irradiation
Lasers provide an alternative energy source that in theory could improve pDNA uptake. The mechanism attempts to essentially bypass the rate-limiting cell membrane step. The permeability of the cell membrane is enhanced at the site of the beam impact by a local thermal effect (Mehier-Humbert and Guy, 2005). Evaluation of transgene transfection after intramyocardial injection of pDNA encoding VEGF in the setting of transmyocardial laser was performed in a pig model. Expression was detected in 56–60% of transmyocardial laser-transfected sites but in only 10–20% without the applied laser. In one efficacy study, there was a complete reversal of wall motion abnormalities compared with ischemic controls (Sayeed-Shah et al., 1998). This was confirmed later in a porcine model of chronic myocardial ischemia (Heilmann et al., 2003). However, in another similar large animal study with catheter-based transendocardial delivery, laser irradiation did not augment luciferase activity in ischemic and adjacent zones (Fuchs et al., 2001).
Magnetic field-enhanced transfection
Magnetofection is based on the principle of transferring paramagnetic nanoparticles containing pDNA into tissues under the influence of strong magnetic fields. The magnetic nanoparticles are typically made of iron oxide and coated with cationic lipids or polymers to complex with DNA through electrostatic interactions (Scherer et al., 2002). The main advantage evaluated in one murine study was the possibility of systemic administration of magnetic nanobead–DNA complexes to the heart under external magnetic control. Compared with the control group, these particles had a 36–85-fold impact on raising transfection efficiency. However, a key problem was that marker gene expression was limited to the nontarget endothelial cells (Li et al., 2008), limiting applicability to cardiac applications requiring transduction of myocytes nested behind tight endothelial junctions.
Image-guided injections
Minimally invasive percutaneous catheterization to achieve intramyocardial vector injection can be achieved either from direct epicardial or from endocardial injection and is an excellent choice for the most severely diseased patients. Comparative studies featuring microsphere retention investigating the difference between endocardial and epicardial injections have been conducted. It was shown that both the site of injection and the therapeutic volume influence myocardial retention. Better results were achieved with endomyocardial delivery and lower ranges of injectable volumes (Grossman et al., 2002).
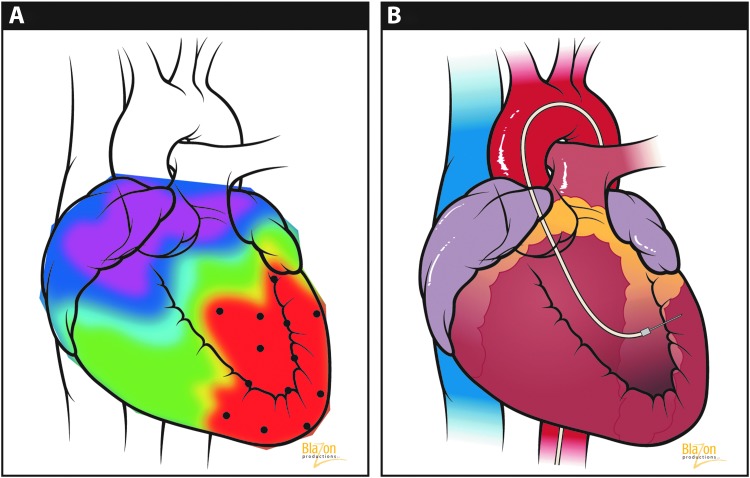
A substantial improvement of this technique's application has been demonstrated with the integration of an image guidance mapping system. The most popular system in place today for image-guided injection is the NOGA® system. The system applies electromagnetic field sensors to combine and analyze information from percutaneous intracardiac electrocardiograms and can provide estimated myocardial viability in real time. Three-dimensional electromechanical maps with this approach of the LV help to visibly differentiate between infarcted from normal tissue (Kornowski et al., 1998). An electromechanical mapping system (Fig. 4A) identifies and distinguishes critical ischemic (red), at risk border and healthy target zones before injection. Once these sites are identified, image-guided injections follow (Fig. 4B) and the therapeutic treatment plan can thus be optimized.
FIG. 4.
(A) NOGA® Electromechanical Mapping of the heart. Red area indicates advanced diseased myocardium and marks targeted injection sites. (B) Endocardial vector delivery at precise marked injection sites from the NOGA system represented by the black dots. (Blazon Productions. Reprinted with permission from Blazon Productions.)
Using NOGA guidance, vector-mediated transgenes can be selectively delivered into designated myocardial sites. In one study featuring NOGA, the evaluation of VEGF121 transfer in a porcine model showed no difference using a transendocardial or transepicardial delivery approach (Kornowski et al., 2000). A total of 13 patients with chronic stable angina were treated through direct epicardial injection of plasmid VEGF165 with the identification of ischemic myocardium with electromechanical mapping guidance. The results constitute evidence of perfusion augmentation and reduction in hibernating myocardium size (Vale et al., 2000).
In another particular mapping application, the feasibility of percutaneous intramyocardial administration of a radiocontrast agent to all regions of the left ventricle utilizing fluoroscopic guidance was confirmed upon postmortem examination (Sanborn et al., 2001). Another study demonstrating fluoroscopy-guided intramyocardial injection in the porcine heart revealed that plasmids encoding chloramphenicol acetyltransferase gene expression could be identified in 39 out of 48 injections sites. Moreover, no animals presented with signs of cardiac tamponade (Gwon et al., 2001).
An alternative to the NOGA are modified versions of clinically available cardiac imaging modalities, including echocardiography and magnetic resonance imaging (MRI). Echocardiography can provide imaging of heart structures in addition to providing an assessment of regional wall motion, valvular regurgitation, and pericardial effusion. Another key advantage with echo is the ability to monitor vector leakage in real time, which is not possible with fluoroscopy. Intracardiac echocardiography predicted the injection sites correctly in 87–100% of the available sites and achieved gene expression in 95% of injection sites in a porcine model (Park et al., 2001). The relative disadvantage of echo is the limited single-plane image and simultaneous requirement of fluoroscopy for catheter guidance.
Ubiquitous in clinical practice, 3D echo with contrast guidance can provide real-time feedback on the localization and retention of injectate, and is a valuable therapeutic modality for enhancing gene delivery (Baklanov et al., 2005). Ultrasound-targeted microbubble destruction provides a noninvasive method to deliver naked DNA to the heart with the potential for repeated target application. It was shown that 72% of the pDNA binds to perflutren lipid microbubbles. Transient endothelial injury induced by microbubbles facilitates plasmid/VEGF165 delivery with enhanced cardiac function after myocardial infarction in mice (Fujii et al., 2009).
Cardiovascular MRI offers the ability to optimize delivery. Moreover, catheter position could easily be delineated in relation to endocardial/epicardial surfaces and also distinguish between papillary and valvular structures. In one study that featured MRI-guided needle injections, a total of 53 injections were attempted, 43 of which were successful within the LV wall of the porcine heart (Lederman et al., 2002). The major disadvantages of MRI guidance are time, cost, and limited application in patient populations with various implanted cardiac devices.
Conclusions
The selection of (1) the appropriate transgene to modulate the selected molecular target, (2) vector type for any given transgene, (3) delivery approach, (4) dose, and (5) supporting clinical armamentarium as a whole system is the greatest predictor of successful cardiac gene therapy. For ischemic cardiomyopathy, the goal is to restore as much function as possible in a debilitated, diseased heart marked with fibrosis and/or ischemic damage. Given that further decline in LV function and progressive dilatation are the typical sequelae once a sufficient proportion of the myocardium is damaged, myocytes remote from the primary focus of damage may need to be transduced if the goal is to reverse maladaptive remodeling and to improve contractile function. A summary of various approaches in maximizing vector transfer to the heart is depicted in Fig. 5. It is important to note that each of these applied strategies, whether pharmacologic or device inspired, aims to increase transfer but is counterbalanced by safety.
FIG. 5.
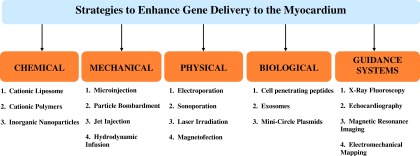
Strategies to enhance viral vector delivery to the myocardium.
Overall, the main goal of cardiac gene therapy for late-stage cardiomyopathy is to extend survival by rescuing as many failing or destined to fail myocytes as possible. Vector selection for this purpose must be matched with this clinical aim. To date, the AAV vector is the best option in terms of safety and efficacy having advanced through phase II trials with AAV1.SERCA2a (MYDICAR). AAV provides safe long-term expression, whereby the SERCA2a gene and other candidates in development (e.g., βARKct, S100A1) drive pump improvement through enhancing critical signaling and calcium handling energetic mechanisms. Adenovirus can achieve similar levels of gene transfer but is limited by the immune response because of interaction with antigen presenting cells and subsequent CD8+ activation against capsid proteins/transgene. Even though it appears that the viral vectors will render nonviral vectors obsolete for this application, there is one major area where the nonviral vectors have a huge advantage for two problems with AAV realized in the trials: (1) there is no opportunity for re-administration because of antibodies; (2) up to 60% of patients in the CUPID trial were excluded because of preexisting AAV antibodies. Here, nonviral vectors may both permit more aggressive multiple treatment regimen and provide a solution for patients excluded because of the presence of neutralizing antibodies against AAV.
The development and selection of a cardiac vector delivery system is paramount in the clinic. This problem cannot be overemphasized as it is very likely that standard intracoronary infusion with AAV vector in the clinical setting does not yet achieve the desired goal. In fact, when reviewing the published data featuring quantitative gene transfer analysis via PCR, >100-fold more expression in collateral organs such as the liver versus the heart has been documented using intracoronary delivery. Advanced interventional and surgical delivery options are under development to address this problem. In cases where a defined regional area is affected, minimally invasive guided needle injections and/or another direct alternative may be optimal. Two delivery strategies could be implemented whereby one would use a lower-dose regimen in healthy at-risk areas of myocardium globally and a localized direct strategy to effectively administer a high dose in severely affected zones.
Summarizing, the continual development of viral vectors, nonviral vectors, and their delivery system approaches will no doubt improve the options available in the clinic. Unfortunately, most preclinical investigations have not provided sufficient detailed data regarding delivery efficiency (geographic distribution of vector genomes/cell by qPCR and geographic distribution of gene expression by qRT-PCR, both in the heart and in collateral organs) to allow for a pharmacokinetic analysis to be undertaken. We must move beyond showing “representative” photographs of gene delivery and move toward reproducible, blinded, randomized quantitative assessments of delivery efficiency, expression, geographical distribution, and therapeutic efficacy. Only then can safe and effective clinical translation of these promising therapies become a reality.
Acknowledgments
We thank Anne Olson for the excellent illustrations in this article. We also thank the Gene Therapy Resource Program of the National Heart, Lung and Blood Institute. This work was supported by a grant from the National Heart, Lung and Blood Institute, NIH 1-R01-HL083078-01A2 (Charles R. Bridges PI).
Author Disclosure Statement
No competing financial interests exist.
References
- Acsadi G. Jiao S.S. Jani A., et al. Direct gene transfer and expression into rat heart in vivo. N. Biol. 1991;3:71–81. [PubMed] [Google Scholar]
- Akinc A. Thomas M. Klibanov A.M. Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- Al-Dosari M.S. Gao X. Non-viral gene delivery: principle, limitations, and recent progress. AAPS J. 2009;11:671–681. doi: 10.1208/s12248-009-9143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki M. Morishita R. Taniyama Y., et al. Angiogenesis induced by hepatocyte growth factor in non-infarcted myocardium and infarcted myocardium: up-regulation of essential transcription factor for angiogenesis, ets. Gene Ther. 2000;7:417–427. doi: 10.1038/sj.gt.3301104. [DOI] [PubMed] [Google Scholar]
- Arora A. Hakim I. Baxter J., et al. Needle-free delivery of macromolecules across the skin by nanoliter-volume pulsed microjets. Proc. Natl. Acad. Sci. USA. 2007;104:4255–4260. doi: 10.1073/pnas.0700182104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuni E.L. Gazdhar A. Giraud M.N., et al. In vivo electroporation mediated gene delivery to the beating heart. PLoS One. 2010;5:e14467. doi: 10.1371/journal.pone.0014467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baklanov D.V. De Muinck E.D. Simons M., et al. Live 3D echo guidance of catheter-based endomyocardial injection. Catheter. Cardiovasc. Interv. 2005;65:340–345. doi: 10.1002/ccd.20379. [DOI] [PubMed] [Google Scholar]
- Barr E. Carroll J. Kalynych A.M., et al. Efficient catheter-mediated gene transfer into the heart using replication-defective adenovirus. Gene Ther. 1994;1:51–58. [PubMed] [Google Scholar]
- Bekeredjian R. Chen S. Frenkel P.A., et al. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation. 2003;108:1022–1026. doi: 10.1161/01.CIR.0000084535.35435.AE. [DOI] [PubMed] [Google Scholar]
- Bergelson J.M. Cunningham J.A. Droguett G., et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Bish L.T. Morine K. Sleeper M.M., et al. Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum. Gene Ther. 2008;19:1359–1368. doi: 10.1089/hum.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekstegers P. Von Degenfeld G. Giehrl W., et al. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins. Gene Ther. 2000;7:232–240. doi: 10.1038/sj.gt.3301079. [DOI] [PubMed] [Google Scholar]
- Bridges C.R. Burkman J.M. Malekan R., et al. Global cardiac-specific transgene expression using cardiopulmonary bypass with cardiac isolation. Ann. Thorac. Surg. 2002;73:1939–1946. doi: 10.1016/s0003-4975(02)03509-9. [DOI] [PubMed] [Google Scholar]
- Buttrick P.M. Kass A. Kitsis R.N., et al. Behavior of genes directly injected into the rat heart in vivo. Circ. Res. 1992;70:193–198. doi: 10.1161/01.res.70.1.193. [DOI] [PubMed] [Google Scholar]
- Capecchi M.R. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980;22:479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- Dean D.A. Strong D.D. Zimmer W.E. Nuclear entry of non-viral vectors. Gene Ther. 2005;12:881–890. doi: 10.1038/sj.gt.3302534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W. Zhang L. Yan Z. Engelhardt J.F. Intracellular trafficking of adeno-associated viral vectors. Gene Ther. 2005;12:873–880. doi: 10.1038/sj.gt.3302527. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E. Latronico M.V. Jotti G.S. Condorelli G. Lentiviral vectors and cardiovascular diseases: a genetic tool for manipulating cardiomyocyte differentiation and function. Gene Ther. 2012;19:642–648. doi: 10.1038/gt.2012.19. [DOI] [PubMed] [Google Scholar]
- Donahue J.K. Kikkawa K. Johns D.C., et al. Ultrarapid, highly efficient viral gene transfer to the heart. Proc. Natl. Acad. Sci. USA. 1997;94:4664–4668. doi: 10.1073/pnas.94.9.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue J.K. Kikkawa K. Thomas A.D., et al. Acceleration of widespread adenoviral gene transfer to intact rabbit hearts by coronary perfusion with low calcium and serotonin. Gene Ther. 1998;5:630–634. doi: 10.1038/sj.gt.3300649. [DOI] [PubMed] [Google Scholar]
- Duan D. Yue Y. Yan Z., et al. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J. Clin. Invest. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvshani-Eshet M. Baruch L. Kesselman E., et al. Therapeutic ultrasound-mediated DNA to cell and nucleus: bioeffects revealed by confocal and atomic force microscopy. Gene Ther. 2006;13:163–172. doi: 10.1038/sj.gt.3302642. [DOI] [PubMed] [Google Scholar]
- Escoffre J.M. Teissie J. Rols M.P. Gene transfer: how can the biological barriers be overcome? J. Membr. Biol. 2010;236:61–74. doi: 10.1007/s00232-010-9275-0. [DOI] [PubMed] [Google Scholar]
- Felgner P.L. Non-viral strategies for gene therapy. Sci. Am. 1997;276:102–106. doi: 10.1038/scientificamerican0697-102. [DOI] [PubMed] [Google Scholar]
- Fishman G.I. Kaplan M.L. Buttrick P.M. Tetracycline-regulated cardiac gene expression in vivo. J. Clin. Invest. 1994;93:1864–1868. doi: 10.1172/JCI117174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury S. Simeoni E. Zuppinger C., et al. Multiply attenuated, self-inactivating lentiviral vectors efficiently deliver and express genes for extended periods of time in adult rat cardiomyocytes in vivo. Circulation. 2003;107:2375–2382. doi: 10.1161/01.CIR.0000065598.46411.EF. [DOI] [PubMed] [Google Scholar]
- French B.A. Mazur W. Geske R.S. Bolli R. Direct in vivo gene transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation. 1994;90:2414–2424. doi: 10.1161/01.cir.90.5.2414. [DOI] [PubMed] [Google Scholar]
- Fuchs S. Baffour R. Shou M., et al. Could plasmid-mediated gene transfer into the myocardium be augmented by left ventricular guided laser myocardial injury? Catheter. Cardiovasc. Interv. 2001;54:533–538. doi: 10.1002/ccd.1328. [DOI] [PubMed] [Google Scholar]
- Fujii H. Sun Z. Li S.H., et al. Ultrasound-targeted gene delivery induces angiogenesis after a myocardial infarction in mice. JACC Cardiovasc. Imaging. 2009;2:869–879. doi: 10.1016/j.jcmg.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Gao G. Bish L.T. Sleeper M.M., et al. Transendocardial delivery of AAV6 results in highly efficient and global cardiac gene transfer in rhesus macaques. Hum. Gene Ther. 2011;22:979–984. doi: 10.1089/hum.2011.042. [DOI] [PubMed] [Google Scholar]
- Griscelli F. Belli E. Opolon P., et al. Adenovirus-mediated gene transfer to the transplanted piglet heart after intracoronary injection. J. Gene Med. 2003;5:109–119. doi: 10.1002/jgm.322. [DOI] [PubMed] [Google Scholar]
- Grossman P.M. Han Z. Palasis M., et al. Incomplete retention after direct myocardial injection. Catheter. Cardiovasc. Interv. 2002;55:392–397. doi: 10.1002/ccd.10136. [DOI] [PubMed] [Google Scholar]
- Guzman R.J. Lemarchand P. Crystal R.G., et al. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ. Res. 1993;73:1202–1207. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- Gwon H.C. Jeong J.O. Kim H.J., et al. The feasibility and safety of fluoroscopy-guided percutaneous intramyocardial gene injection in porcine heart. Int. J. Cardiol. 2001;79:77–88. doi: 10.1016/s0167-5273(01)00410-7. [DOI] [PubMed] [Google Scholar]
- Hajjar R.J. Schmidt U. Matsui T., et al. Modulation of ventricular function through gene transfer in vivo. Proc. Natl. Acad. Sci. USA. 1998;95:5251–5256. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrave B. Downey H. Strange R., Jr., et al. Electroporation-mediated gene transfer directly to the swine heart. Gene. Ther. 2013;20:151–157. doi: 10.1038/gt.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R.L. Byrne B.J. Tung L. Electroporation-mediated gene transfer in cardiac tissue. FEBS Lett. 1998;435:1–5. doi: 10.1016/s0014-5793(98)00987-9. [DOI] [PubMed] [Google Scholar]
- Heilmann C.A. Attmann T. Von Samson P., et al. Transmyocardial laser revascularization combined with vascular endothelial growth factor 121 (VEGF121) gene therapy for chronic myocardial ischemia—do the effects really add up? Eur. J. Cardiothorac. Surg. 2003;23:74–80. doi: 10.1016/s1010-7940(02)00718-2. [DOI] [PubMed] [Google Scholar]
- Hoshijima M. Ikeda Y. Iwanaga Y., et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat. Med. 2002;8:864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- Inagaki K. Fuess S. Storm T.A., et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura K. Suda T. Zhang G. Liu D. Advances in gene delivery systems. Pharmaceut. Med. 2011;25:293–306. doi: 10.2165/11594020-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt M.G. Xiao X. Samulski R.J., et al. Long-term gene transfer in porcine myocardium after coronary infusion of an adeno-associated virus vector. Ann. Thorac. Surg. 1996;62:1669–1676. doi: 10.1016/s0003-4975(96)00946-0. [DOI] [PubMed] [Google Scholar]
- Kaspar B.K. Roth D.M. Lai N.C., et al. Myocardial gene transfer and long-term expression following intracoronary delivery of adeno-associated virus. J. Gene Med. 2005;7:316–324. doi: 10.1002/jgm.665. [DOI] [PubMed] [Google Scholar]
- Kass-Eisler A. Falck-Pedersen E. Alvira M., et al. Quantitative determination of adenovirus-mediated gene delivery to rat cardiac myocytes in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 1993;90:11498–11502. doi: 10.1073/pnas.90.24.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M.G. Swain J.D. Tomasulo C.E., et al. Current strategies for myocardial gene delivery. J. Mol. Cell. Cardiol. 2011;50:766–776. doi: 10.1016/j.yjmcc.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M.G. Fargnoli A.S. Bridges C.R. Myocardial gene transfer: routes and devices for regulation of transgene expression by modulation of cellular permeability. Hum. Gene Ther. 2013;24:375–392. doi: 10.1089/hum.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo I. Ohmori K. Oshita A., et al. Treatment of acute myocardial infarction by hepatocyte growth factor gene transfer: the first demonstration of myocardial transfer of a “functional” gene using ultrasonic microbubble destruction. J. Am. Coll. Cardiol. 2004;44:644–653. doi: 10.1016/j.jacc.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Kornowski R. Hong M.K. Leon M.B. Comparison between left ventricular electromechanical mapping and radionuclide perfusion imaging for detection of myocardial viability. Circulation. 1998;98:1837–1841. doi: 10.1161/01.cir.98.18.1837. [DOI] [PubMed] [Google Scholar]
- Kornowski R. Leon M.B. Fuchs S., et al. Electromagnetic guidance for catheter-based transendocardial injection: a platform for intramyocardial angiogenesis therapy. Results in normal and ischemic porcine models. J. Am. Coll. Cardiol. 2000;35:1031–1039. doi: 10.1016/s0735-1097(99)00642-7. [DOI] [PubMed] [Google Scholar]
- Laskey R.A. CIBA Medal Lecture. Regulatory roles of the nuclear membrane. Biochem. Soc. Trans. 1998;26:561–567. doi: 10.1042/bst0260561. [DOI] [PubMed] [Google Scholar]
- Lechardeur D. Verkman A.S. Lukacs G.L. Intracellular routing of plasmid DNA during non-viral gene transfer. Adv. Drug Deliv. Rev. 2005;57:755–767. doi: 10.1016/j.addr.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Lederman R.J. Guttman M.A. Peters D.C., et al. Catheter-based endomyocardial injection with real-time magnetic resonance imaging. Circulation. 2002;105:1282–1284. doi: 10.1161/01.CIR.0000012425.71261.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L.Y. Patel S.R. Hackett N.R., et al. Focal angiogen therapy using intramyocardial delivery of an adenovirus vector coding for vascular endothelial growth factor 121. Ann. Thorac. Surg. 2000;69:14–23. doi: 10.1016/s0003-4975(99)01102-9. discussion 23–24. [DOI] [PubMed] [Google Scholar]
- Leopold P.L. Kreitzer G. Miyazawa N., et al. Dynein- and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum. Gene Ther. 2000;11:151–165. doi: 10.1089/10430340050016238. [DOI] [PubMed] [Google Scholar]
- Leor J. Quinones M.J. Patterson M., et al. Adenovirus-mediated gene transfer into infarcted myocardium: feasibility, timing, and location of expression. J. Mol. Cell. Cardiol. 1996;28:2057–2067. doi: 10.1006/jmcc.1996.0199. [DOI] [PubMed] [Google Scholar]
- Li S. Rizzo M.A. Bhattacharya S. Huang L. Characterization of cationic lipid-protamine-DNA (LPD) complexes for intravenous gene delivery. Gene Ther. 1998;5:930–937. doi: 10.1038/sj.gt.3300683. [DOI] [PubMed] [Google Scholar]
- Li W. Nicol F. Szoka F.C., Jr. GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv. Drug Deliv. Rev. 2004;56:967–985. doi: 10.1016/j.addr.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Li W. Ma N. Ong L.L., et al. Enhanced thoracic gene delivery by magnetic nanobead-mediated vector. J. Gene Med. 2008;10:897–909. doi: 10.1002/jgm.1208. [DOI] [PubMed] [Google Scholar]
- Lin H. Parmacek M.S. Morle G., et al. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990;82:2217–2221. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- Logeart D. Hatem S.N. Heimburger M., et al. How to optimize in vivo gene transfer to cardiac myocytes: mechanical or pharmacological procedures? Hum. Gene Ther. 2001;12:1601–1610. doi: 10.1089/10430340152528101. [DOI] [PubMed] [Google Scholar]
- Lukacs G.L. Haggie P. Seksek O., et al. Size-dependent DNA mobility in cytoplasm and nucleus. J. Biol. Chem. 2000;275:1625–1629. doi: 10.1074/jbc.275.3.1625. [DOI] [PubMed] [Google Scholar]
- Mack C.A. Patel S.R. Schwarz E.A., et al. Biologic bypass with the use of adenovirus-mediated gene transfer of the complementary deoxyribonucleic acid for vascular endothelial growth factor 121 improves myocardial perfusion and function in the ischemic porcine heart. J. Thorac. Cardiovasc. Surg. 1998;115:168–176. doi: 10.1016/s0022-5223(98)70455-6. discussion 176–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magovern C.J. Mack C.A. Zhang J., et al. Direct in vivo gene transfer to canine myocardium using a replication-deficient adenovirus vector. Ann. Thorac. Surg. 1996;62:425–433. discussion 433–434. [PubMed] [Google Scholar]
- Marshall W.G., Jr. Boone B.A. Burgos J.D., et al. Electroporation-mediated delivery of a naked DNA plasmid expressing VEGF to the porcine heart enhances protein expression. Gene Ther. 2010;17:419–423. doi: 10.1038/gt.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno Y. Iwata H. Umeda Y., et al. Non-viral gene gun mediated transfer into the beating heart. ASAIO J. 2003;49:641–644. doi: 10.1097/01.mat.0000093746.63497.ae. [DOI] [PubMed] [Google Scholar]
- Maurice J.P. Hata J.A. Shah A.S., et al. Enhancement of cardiac function after adenoviral-mediated in vivo intracoronary beta2-adrenergic receptor gene delivery. J. Clin. Invest. 1999;104:21–29. doi: 10.1172/JCI6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F.R. Mcgraw T.E. Endocytic recycling. Nat. Rev. Mol. Cell. Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Mcmahon J.M. Signori E. Wells K.E., et al. Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase—increased expression with reduced muscle damage. Gene Ther. 2001;8:1264–1270. doi: 10.1038/sj.gt.3301522. [DOI] [PubMed] [Google Scholar]
- Medina-Kauwe L.K. Xie J. Hamm-Alvarez S. Intracellular trafficking of non-viral vectors. Gene Ther. 2005;12:1734–1751. doi: 10.1038/sj.gt.3302592. [DOI] [PubMed] [Google Scholar]
- Mehier-Humbert S. Guy R.H. Physical methods for gene transfer: improving the kinetics of gene delivery into cells. Adv. Drug Deliv. Rev. 2005;57:733–753. doi: 10.1016/j.addr.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Miyamoto M.I. Del Monte F. Schmidt U., et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc. Natl. Acad. Sci. USA. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most P. Pleger S.T. Volkers M., et al. Cardiac adenoviral S100A1 gene delivery rescues failing myocardium. J. Clin. Invest. 2004;114:1550–1563. doi: 10.1172/JCI21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlhauser J. Jones M. Yamada I., et al. Safety and efficacy of in vivo gene transfer into the porcine heart with replication-deficient, recombinant adenovirus vectors. Gene Ther. 1996;3:145–153. [PubMed] [Google Scholar]
- Mukherjee S. Ghosh R.N. Maxfield F.R. Endocytosis. Physiol. Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Nagata S. DNA degradation in development and programmed cell death. Annu. Rev. Immunol. 2005;23:853–875. doi: 10.1146/annurev.immunol.23.021704.115811. [DOI] [PubMed] [Google Scholar]
- Newman C.M. Bettinger T. Gene therapy progress and prospects: ultrasound for gene transfer. Gene Ther. 2007;14:465–475. doi: 10.1038/sj.gt.3302925. [DOI] [PubMed] [Google Scholar]
- Nishizaki K. Mazda O. Dohi Y., et al. In vivo gene gun-mediated transduction into rat heart with Epstein-Barr virus-based episomal vectors. Ann. Thorac. Surg. 2000;70:1332–1337. doi: 10.1016/s0003-4975(00)01708-2. [DOI] [PubMed] [Google Scholar]
- Niwano K. Arai M. Koitabashi N., et al. Lentiviral vector-mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol. Ther. 2008;16:1026–1032. doi: 10.1038/mt.2008.61. [DOI] [PubMed] [Google Scholar]
- Nonnenmacher M. Weber T. Intracellular transport of recombinant adeno-associated virus vectors. Gene Ther. 2012;19:649–658. doi: 10.1038/gt.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie S.R. Warrington K.H., Jr. Agbandje-Mckenna M., et al. Identification of amino acid residues in the capsid proteins of adeno-associated virus type 2 that contribute to heparan sulfate proteoglycan binding. J. Virol. 2003;77:6995–7006. doi: 10.1128/JVI.77.12.6995-7006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomeque J. Chemaly E.R. Colosi P., et al. Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo. Gene Ther. 2007;14:989–997. doi: 10.1038/sj.gt.3302895. [DOI] [PubMed] [Google Scholar]
- Park S.W. Gwon H.C. Jeong J.O., et al. Intracardiac echocardiographic guidance and monitoring during percutaneous endomyocardial gene injection in porcine heart. Hum. Gene Ther. 2001;12:893–903. doi: 10.1089/104303401750195863. [DOI] [PubMed] [Google Scholar]
- Patel S.R. Lee L.Y. Mack C.A., et al. Safety of direct myocardial administration of an adenovirus vector encoding vascular endothelial growth factor 121. Hum. Gene Ther. 1999;10:1331–1348. doi: 10.1089/10430349950018012. [DOI] [PubMed] [Google Scholar]
- Raake P.W. Hinkel R. Muller S., et al. Cardio-specific long-term gene expression in a porcine model after selective pressure-regulated retroinfusion of adeno-associated viral (AAV) vectors. Gene Ther. 2008;15:12–17. doi: 10.1038/sj.gt.3303035. [DOI] [PubMed] [Google Scholar]
- Raake P.W. Schlegel P. Ksienzyk J., et al. AAV6.betaARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur. Heart J. 2013;34:1437–1447. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S. Li M. Smith J.M., et al. Low-volume jet injection for intradermal immunization in rabbits. BMC Biotechnol. 2002;2:10. doi: 10.1186/1472-6750-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengo G. Lymperopoulos A. Zincarelli C., et al. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoda T. Kasahara N. Hamamori Y. Kedes L. A high-titer lentiviral production system mediates efficient transduction of differentiated cells including beating cardiac myocytes. J. Mol. Cell. Cardiol. 1999;31:2037–2047. doi: 10.1006/jmcc.1999.1035. [DOI] [PubMed] [Google Scholar]
- Sakurai F. Nishioka T. Saito H., et al. Interaction between DNA-cationic liposome complexes and erythrocytes is an important factor in systemic gene transfer via the intravenous route in mice: the role of the neutral helper lipid. Gene Ther. 2001;8:677–686. doi: 10.1038/sj.gt.3301460. [DOI] [PubMed] [Google Scholar]
- Sanborn T.A. Hackett N.R. Lee L.Y., et al. Percutaneous endocardial transfer and expression of genes to the myocardium utilizing fluoroscopic guidance. Catheter. Cardiovasc. Interv. 2001;52:260–266. doi: 10.1002/1522-726x(200102)52:2<260::aid-ccd1061>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Satkauskas S. Bureau M.F. Puc M., et al. Mechanisms of in vivo DNA electrotransfer: respective contributions of cell electropermeabilization and DNA electrophoresis. Mol. Ther. 2002;5:133–140. doi: 10.1006/mthe.2002.0526. [DOI] [PubMed] [Google Scholar]
- Sayeed-Shah U. Mann M.J. Martin J., et al. Complete reversal of ischemic wall motion abnormalities by combined use of gene therapy with transmyocardial laser revascularization. J. Thorac. Cardiovasc. Surg. 1998;116:763–769. doi: 10.1016/S0022-5223(98)00440-1. [DOI] [PubMed] [Google Scholar]
- Scherer F. Anton M. Schillinger U., et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- Seisenberger G. Ried M.U. Endress T., et al. Real-time single-molecule imaging of the infection pathway of an adeno-associated virus. Science. 2001;294:1929–1932. doi: 10.1126/science.1064103. [DOI] [PubMed] [Google Scholar]
- Shah A.S. Lilly R.E. Kypson A.P., et al. Intracoronary adenovirus-mediated delivery and overexpression of the beta(2)-adrenergic receptor in the heart: prospects for molecular ventricular assistance. Circulation. 2000;101:408–414. doi: 10.1161/01.cir.101.4.408. [DOI] [PubMed] [Google Scholar]
- Stewart M.J. Plautz G.E. Del Buono L., et al. Gene transfer in vivo with DNA-liposome complexes: safety and acute toxicity in mice. Hum. Gene Ther. 1992;3:267–275. doi: 10.1089/hum.1992.3.3-267. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L.D. Makeh I. Perricaudet M. Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J. Clin. Invest. 1992;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson E.C. Marshall D.J. Woodard K., et al. Efficient and stable transduction of cardiomyocytes after intramyocardial injection or intracoronary perfusion with recombinant adeno-associated virus vectors. Circulation. 1999;99:201–205. doi: 10.1161/01.cir.99.2.201. [DOI] [PubMed] [Google Scholar]
- Takei K. Haucke V. Clathrin-mediated endocytosis: membrane factors pull the trigger. Trends Cell. Biol. 2001;11:385–391. doi: 10.1016/s0962-8924(01)02082-7. [DOI] [PubMed] [Google Scholar]
- Tevaearai H.T. Walton G.B. Keys J.R., et al. Acute ischemic cardiac dysfunction is attenuated via gene transfer of a peptide inhibitor of the beta-adrenergic receptor kinase (betaARK1) J. Gene Med. 2005;7:1172–1177. doi: 10.1002/jgm.770. [DOI] [PubMed] [Google Scholar]
- Tsunoda S. Mazda O. Oda Y., et al. Sonoporation using microbubble BR14 promotes pDNA/siRNA transduction to murine heart. Biochem. Biophys. Res. Commun. 2005;336:118–127. doi: 10.1016/j.bbrc.2005.08.052. [DOI] [PubMed] [Google Scholar]
- Uchida M. Natsume H. Kobayashi D., et al. Effects of particle size, helium gas pressure and microparticle dose on the plasma concentration of indomethacin after bombardment of indomethacin-loaded poly-L-lactic acid microspheres using a Helios gun system. Biol. Pharm. Bull. 2002;25:690–693. doi: 10.1248/bpb.25.690. [DOI] [PubMed] [Google Scholar]
- Vale P.R. Losordo D.W. Milliken C.E., et al. Left ventricular electromechanical mapping to assess efficacy of phVEGF(165) gene transfer for therapeutic angiogenesis in chronic myocardial ischemia. Circulation. 2000;102:965–974. doi: 10.1161/01.cir.102.9.965. [DOI] [PubMed] [Google Scholar]
- Von Harsdorf R. Schott R.J. Shen Y.T., et al. Gene injection into canine myocardium as a useful model for studying gene expression in the heart of large mammals. Circ. Res. 1993;72:688–695. doi: 10.1161/01.res.72.3.688. [DOI] [PubMed] [Google Scholar]
- Wang Y. Bai Y. Price C., et al. Combination of electroporation and DNA/dendrimer complexes enhances gene transfer into murine cardiac transplants. Am. J. Transplant. 2001;1:334–338. doi: 10.1034/j.1600-6143.2001.10408.x. [DOI] [PubMed] [Google Scholar]
- Wells D.J. Gene therapy progress and prospects: electroporation and other physical methods. Gene Ther. 2004;11:1363–1369. doi: 10.1038/sj.gt.3302337. [DOI] [PubMed] [Google Scholar]
- Wente S.R. Gatekeepers of the nucleus. Science. 2000;288:1374–1377. doi: 10.1126/science.288.5470.1374. [DOI] [PubMed] [Google Scholar]
- White J.D. Thesier D.M. Swain J.B., et al. Myocardial gene delivery using molecular cardiac surgery with recombinant adeno-associated virus vectors in vivo. Gene Ther. 2011;18:546–552. doi: 10.1038/gt.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J.A. Malone R.W. Williams P., et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Woo Y.J. Zhang J.C. Taylor M.D., et al. One year transgene expression with adeno-associated virus cardiac gene transfer. Int. J. Cardiol. 2005;100:421–426. doi: 10.1016/j.ijcard.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Worgall S. Worgall T.S. Kostarelos K., et al. Free cholesterol enhances adenoviral vector gene transfer and expression in CAR-deficient cells. Mol. Ther. 2000;1:39–48. doi: 10.1006/mthe.1999.0013. [DOI] [PubMed] [Google Scholar]
- Wright M.J. Wightman L.M. Latchman D.S. Marber M.S. In vivo myocardial gene transfer: optimization and evaluation of intracoronary gene delivery in vivo. Gene Ther. 2001;8:1833–1839. doi: 10.1038/sj.gt.3301614. [DOI] [PubMed] [Google Scholar]
- Yoshimitsu M. Higuchi K. Dawood F., et al. Correction of cardiac abnormalities in fabry mice by direct intraventricular injection of a recombinant lentiviral vector that engineers expression of alpha-galactosidase A. Circ. J. 2006;70:1503–1508. doi: 10.1253/circj.70.1503. [DOI] [PubMed] [Google Scholar]
- Zhao J. Pettigrew G.J. Thomas J., et al. Lentiviral vectors for delivery of genes into neonatal and adult ventricular cardiac myocytes in vitro and in vivo. Basic Res. Cardiol. 2002;97:348–358. doi: 10.1007/s00395-002-0360-0. [DOI] [PubMed] [Google Scholar]