Abstract
Purpose of review
This review summarizes recent preclinical and human studies evaluating allergen-specific immunotherapy via the transcutaneous route, and provides a rationale for the application of modified allergens with reduced allergenicity. Furthermore, it covers approaches to generate hypoallergenic conjugates for specific dendritic cell targeting.
Recent findings
Efficacy and safety of specific immunotherapy by application of allergens to the skin have been demonstrated in both animal models as well as clinical trials. However, localized adverse events have been reported, and delivery of antigens via barrier-disrupted skin has been linked to the induction of unwanted T helper 2-biased immune responses and allergic sensitization. Coupling of carbohydrates to allergens has been shown to induce formation of nanoparticles, which can specifically target dendritic cells and potentiate immune responses, and by masking B-cell epitopes, can render the molecules hypoallergenic.
Summary
Due to its abundance of immunocompetent cells, the skin represents an attractive target tissue for novel and enhanced immunotherapeutic approaches. However, in order to avoid adverse events and therapy-induced sensitizations, transcutaneous immunotherapy requires the use of formulations with reduced allergenic potential. Combining novel hypoallergenic conjugates with painless transcutaneous immunization techniques may provide an efficient and patient-friendly alternative to the standard specific immunotherapy practices.
Keywords: allergen conjugates, carbohydrates, C-type lectin receptors, dendritic cells, transcutaneous immunotherapy
INTRODUCTION
Despite the proven clinical efficacy of standard allergen-specific immunotherapy (SIT) performed by subcutaneous injection (SCIT) or sublingual application (SLIT), only a small percentage of allergic patients decide to undergo this laborious and time-consuming procedure, and dropout rates are considerable [1]. Alternative approaches aiming at enhanced efficacy to reduce treatment duration and number of interventions, while simultaneously providing a high-safety profile, are under intense investigation. Among these, intranodal [2,3] and cutaneous administration of allergens represent the most promising routes. This article provides an overview on experimental approaches for allergen immunotherapy via the cutaneous route (see Table 1[4,5▪,6,7,8▪,9–13,14▪,15]). Furthermore, modifications of allergens for targeting specific receptors on antigen-presenting cells (APC), their implications for shaping the immune response, and the generation of hypoallergenic allergen conjugates are discussed.
Table 1.
Cutaneous allergen-specific immunotherapy approaches
| Treatment | Species | Allergen | Outcome | Reference |
| Tape stripping + patch | Human | Grass pollen extract | Decreased scores in nasal provocation tests; improvement in subjective symptom scores | Senti et al. [4] |
| Tape stripping + patch | Human | Grass pollen extract | Improvement in subjective symptom scores (dose-dependent) | Senti et al. [5▪] |
| ? + patch | Human (children) | Grass pollen extract | Reduction in antihistamine uptake; reduction in symptom scores | Agostinis et al. [6] |
| Intact skin + epidermal delivery system | Human (children) | Cow’ s milk | Improvement of cumulative tolerated dose in oral challenges | Dupont et al. [7] |
| Laserporated skin | Mouse | Recombinant Phl p 5 | Reduction of airway hyperresponsiveness; reduction of leukocyte infiltrate; systemic downregulation of TH1/TH2/TH17 responses; increased IgG2a and Treg cells | Bach et al. [8▪] |
| Laserporated skin | Mouse | Recombinant Phl p 5 + CpG Oligo 1826 | Reduction of IgE; increased IgG2a; reduction of cellular lung infiltrate | Hessenberger et al. [9] |
| Intact skin + epidermal delivery system | Mouse | Ovalbumin, pollen, house-dust mite, peanut protein extract | Increased IgG2a; decreased IgE/IgG2a ratio; decreased bronchial hyperresponsiveness; decreased BAL fluid and serum TH2 cytokines; decreased BAL fluid lymphocytes and eosinophils | Mondoulet et al. [10,11] |
| Intact skin + epidermal delivery system | Mouse | Ovalbumin | Decreased local and systemic responses; induction of regulatory responses | Dioszeghy et al. [12] |
| Intact skin + epidermal delivery system | Mouse | Peanut protein extract | Systemic downregulation of TH2 response; reduction of IgE; reduction of gastro-intestinal lesions; increased IgG2a and Foxp3 mRNA in esophageal mucosa | Mondoulet et al. [13,14▪] |
| Skin pretreatment with body sponge + patch | Mouse | Ovalbumin (plasmids formulated as mannosylated polyethylenimine nanoparticles) | Balanced systemic TH1/TH2 response; decreased nasal symptoms | Garaczi et al. [15] |
TRANSCUTANEOUS IMMUNOTHERAPY
The most obvious advantages of the skin over other target tissues are its easy accessibility, abundance of immunologically competent cell types, efficient drainage by the lymphatic system, and, with regard to its superficial layers, lack of vascularization [16]. Allergen-specific immunotherapy via the skin was originally introduced by Blamoutier et al. [17] as early as 1959 by application of an allergen extract to an area at the volar forearm, which had been pretreated by scratching with a needle. Recently, two different methods to enhance uptake of allergen by skin have been evaluated in clinical trials: skin barrier disruption by adhesive tape stripping, and increasing skin permeability via hydration by placing an occlusive chamber onto intact skin [5▪,7]. In the initial study evaluating epicutaneous immunotherapy on barrier-disrupted skin, patients received a total of 12 patches, containing a grass pollen extract in vaseline, for 48 h at weekly intervals, prior to and during the pollen season. The skin area at the upper arm or shoulder was tape stripped six times before patch application. According to the authors, this pretreatment enhanced uptake of the extract, due to partial removal of the cornified skin layer, and activated keratinocytes to produce pro-inflammatory cytokines thus promoting migration of Langerhans cells from the skin to the draining lymph nodes. Treated patients showed decreased scores in the first and second year after therapy (nasal provocation tests); however, the effect was not significant compared with a placebo group [4]. For a larger follow-up study, extracts were applied in aqueous solution, and treatment time as well as total number of treatments were reduced, while allergen dosage was enhanced. Symptom amelioration was comparable with the initial study, but a decline in local adverse reactions during the therapeutic schedule could be observed. The authors suggest development of peripheral T-cell tolerance as the underlying immunological mechanism of the therapeutic efficacy of epicutaneous immunotherapy [5▪].
Box 1.
no caption available
Another approach for transcutaneous immunotherapy utilized the Viaskin epidermal delivery system, which originally was developed for allergy diagnosis. Its applicability for epicutaneous immunotherapy was first tested in mouse models [10,11], and subsequently in a clinical study [7] with children allergic against cow's milk. This method utilizes an occlusive chamber on intact skin for 48 h, thus triggering perspiration and generating moisture, which both hydrates the superficial skin layer as well as dissolves the lyophilized allergens loaded onto a central polyethylene membrane. In contrast to tape stripping, allergens applied using this delivery system in a mouse model were not passively taken up but actively transported by skin resident dendritic cells to local lymph nodes. After repeated application, an increase in allergen-specific regulatory responses was observed [12]. A significant reduction of systemic T helper 2 type responses and esophageal eosinophilia could be detected after treatment of peanut-sensitized mice via intact skin followed by oral allergen exposure. In contrast, treatment via tape-stripped skin even promoted T helper 2 responses and localized eosinophilic infiltration [13,14▪].
In another mouse model, the skin at the site of antigen application was prepared with a sponge-like device to partly remove the stratum corneum and to activate Langerhans cells, followed by the application of a DNA vaccine encoding the model allergen ovalbumin, formulated as mannosylated polyethylenimine nanoparticles (DermAll). The results indicated a therapeutic effect, including a decrease in nasal symptoms and a modulation of the systemic T helper 2 type response [15].
Laser poration may provide an effective and easy-to-standardize alternative technique to disrupt the skin barrier for immunotherapy. We could recently demonstrate therapeutic efficacy in an asthma mouse model by application of an adjuvant-free preparation of recombinant grass pollen allergen via laser-generated micropores. In comparison to conventional SCIT, therapy via the transcutaneous route demonstrated equal efficacy, but showed reduced adverse effects [8▪].
Besides the promising outcomes of the aforementioned preclinical and clinical studies of specific immunotherapy via the cutaneous route, barrier-disrupted skin has also been described to represent a major route for allergic sensitization [18]. Most likely, a complex network of interactions between skin resident and associated cell types including keratinocytes [19], gamma-delta T cells [20], Langerhans cells [21▪], dendritic cells [22], and mast cells [23] accounts for the allergy-promoting potential of antigen delivery via the skin.
As the dermis is rich in mast cells, combining high-allergen doses with efficient barrier disruption techniques is also likely to induce localized or even systemic side-effects. Indeed, such adverse events including pruritus, erythema, wheals, and eczema, mainly being restricted to the site of application, have been described [5▪,7]. In rare cases even systemic allergic reactions were reported, comparable to side-effects known from standard SIT, which required treatment and led to study exclusion [5▪].
Therefore, neither natural allergen extracts, as currently used for SIT, nor ‘wild type’ recombinant allergens will fulfill the safety criteria for future transcutaneous approaches. In the next section, we will discuss experimental approaches that address these issues.
NOVEL ALLERGEN CONJUGATES FOR TRANSCUTANEOUS IMMUNOTHERAPY
A vaccine for transcutaneous immunotherapy of allergic diseases has to meet the following requirements:
a delivery system which enables to bypass the skin barrier;
addition of adjuvants to suppress de-novo TH2 priming;
in-vivo targeting of skin resident DCs to enhance vaccine potency and thus to shorten duration of therapy;
usage of hypoallergenic allergen derivatives to avoid cross-linking of preexisting IgE on mast cells.
The first two aspects have been addressed in recent publications [9,24,25]; therefore, the following paragraphs will focus on strategies concerning points (3) and (4).
Targeting of dendritic cells
Antigens can be delivered to dendritic cells by either passive uptake or via binding to specific receptors. Passive targeting of dendritic cells has been achieved by particulate antigen formulations such as polymeric beads or virus-like particles [26]. Similarly, pH-sensitive nanoparticles and hydrogels have been successfully employed for delivering antigen to dendritic cells [27,28]. Active targeting of dendritic cells has been shown using antibodies or antibody fragments against dendritic cell receptors such as MHCII, CD36, CD11c [29–31], and C-type lectin receptors (CLRs) [32]. The latter not only facilitate antigen uptake and delivery of the vaccine to dendritic cells, but also function as inducers or modulators of immune responses. Compared with antibodies, exogenous ligands of CLRs have a much lower binding affinity; however, this can be overcome by targeting CLRs with multivalent ligands such as glyconanoparticles, glycodendrimers, and glycoliposomes [32].
Shaping the immune response by C-type lectin receptor targeting
Targeting antigen to specific receptors on dendritic cells is not only an efficient means to increase antigen uptake and enhance immunity but can also be used to shape the immune response to individual needs. Specific antibody conjugates addressing different surface molecules on dendritic cells have been intensely studied to elucidate the role of different dendritic cell subsets and CLRs in modulating immunity. Several studies have demonstrated that the activation status of APCs plays a dominant role, for example, immunization with antigen–antibody conjugates under inflammatory conditions leads to TH1 responses, independently whether the antigen is targeted to the CLR DEC-205 [33], Langerin, Clec9A [34], or the Ig superfamily member Treml4 [35]. Notably in this context, activated B-cells can also take up antigen via DEC-205 and act as potent T-cell activators [36▪▪]. In contrast, immunization under non-inflammatory (steady state) conditions results in tolerance induction. Langerin+ migratory dendritic cells, but not lymph node resident dendritic cells, have been identified as major Treg inducers, independently of the source of the dendritic cell (skin vs. lung) or the targeted receptor [37▪▪].
Although subclass and activation status of dendritic cells seem to be dominant factors for T-cell activation and polarization, CLRs have a significant impact on immunity via their role as pattern recognition receptors (PRR). For example, the fungal receptor dectin-1 initiates TH1/TH17 immune responses [38] through a noncanonical caspase-8 inflammasome pathway [39], and similarly, Clec5a is crucial for inflammasome activation after viral infections [40,41]. The TH17-polarizing activity of the mycobacterial adjuvant cord factor (trehalose-6,6′-dimycolate) is highly dependent on the CLR Mincle (Clec 4e) [42] and its homologue MCL (Clec4d) [43]. Additionally, CLRs also act as modulators of immune polarization together with other innate immune receptors such as toll-like receptors (TLR). Stimulation of DC-SIGN with mannose-expressing pathogens, for example, modulates the TLR4 signaling pathway and enhances the expression of IL-10, IL-12 and IL-6, while fucose-expressing pathogens enhance TH2 immunity by downregulating IL-12 [44]. Signaling through the macrophage galactose (MGL) CLR synergizes with TLR2 in enhancing IL-10 and TNF-α expression [45]. Besides acting as a direct inducer of inflammasome activation, dectin-1 inhibits TLR9 signaling via SOCS1, thereby downregulating IL-12 and promoting TH17 responses [46].
Role of C-type lectin receptors in allergenicity
CLRs, like other PRRs, have coevolved with pathogens to provide immunity against viruses, bacteria, parasites and fungi. Consequently, CLRs specific for conserved carbohydrates on the surface of different pathogens can trigger different types of immune responses, such as TH1 for clearance of intracellular bacteria and viruses, TH17 for antifungal responses, and TH2 for providing protection against extracellular bacteria and parasites [47]. In recent years, evidence has accumulated indicating that CLRs also play an important role in the uptake of allergens and promotion of allergic immune responses. The mannose receptor and DC-SIGN have been identified as important receptors for glycosylated allergens such as Der p 1, Der p 2, Ara h 1, Bla g 2, Fel d 1, and Can f 1 [48–51]. Uptake of allergen via the mannose receptor seems to amplify TH2 type responses by suppressing pro-inflammatory signals and inducing CCL22 and CCL17, two chemokines which attract CCR4 positive TH2 cells [52]. However, mannose receptor ligation can also act synergistically with TLR4 activation to induce TNF-α secretion [53]. Moreover, depending on the mouse strain, TLR4 and mannose receptor activation was shown to be crucial for the priming of TH1 promoting M1 macrophages during fungal infection [54]. Interestingly, mannosylated allergens can also be taken up by DC-SIGN favoring TH1 immune polarization [51]. These data indicate that a complex interplay between CLRs, TLRs, and genetic factors influences the outcome of allergen uptake and presentation to T cells. Vaccination strategies utilizing CLR-mediated targeting of allergens for specific immunotherapy therefore likely will require activation of several innate receptors [55] to induce robust immunity with a clear polarization profile.
Carbohydrate-coupled allergens as novel therapeutics for specific immunotherapy
There are very few examples of targeting allergens to APCs through CLRs in the literature; however, recent data suggest that carbohydrate-coupled allergens are attractive candidates for SIT (Fig. 1). Coupling of the major cat allergen Fel d 1 to 2 μm sepharose beads (CBP-Fel d 1) was shown to enhance antigen uptake by dendritic cells [57]. In a mouse model for cat allergy, subcutaneous injection of CBP-Fel d 1 microparticles generated strong IgG1 and IgG2a antibody responses while suppressing airway inflammation and inducing specific T-cell anergy. The particles exhibited a longer lasting depot effect than alum thus preventing systemic antigen spreading. Fel d 1-loaded dendritic cells and Langerhans cells, but not macrophages, were detected in draining lymph nodes and spleen suggesting a critical role for these cells in antigen processing and presentation [58]. However, the observed therapeutic effect was mainly associated with antigen persistence at the site of immunization rather than with specific CLR targeting of dendritic cell subsets.
FIGURE 1.
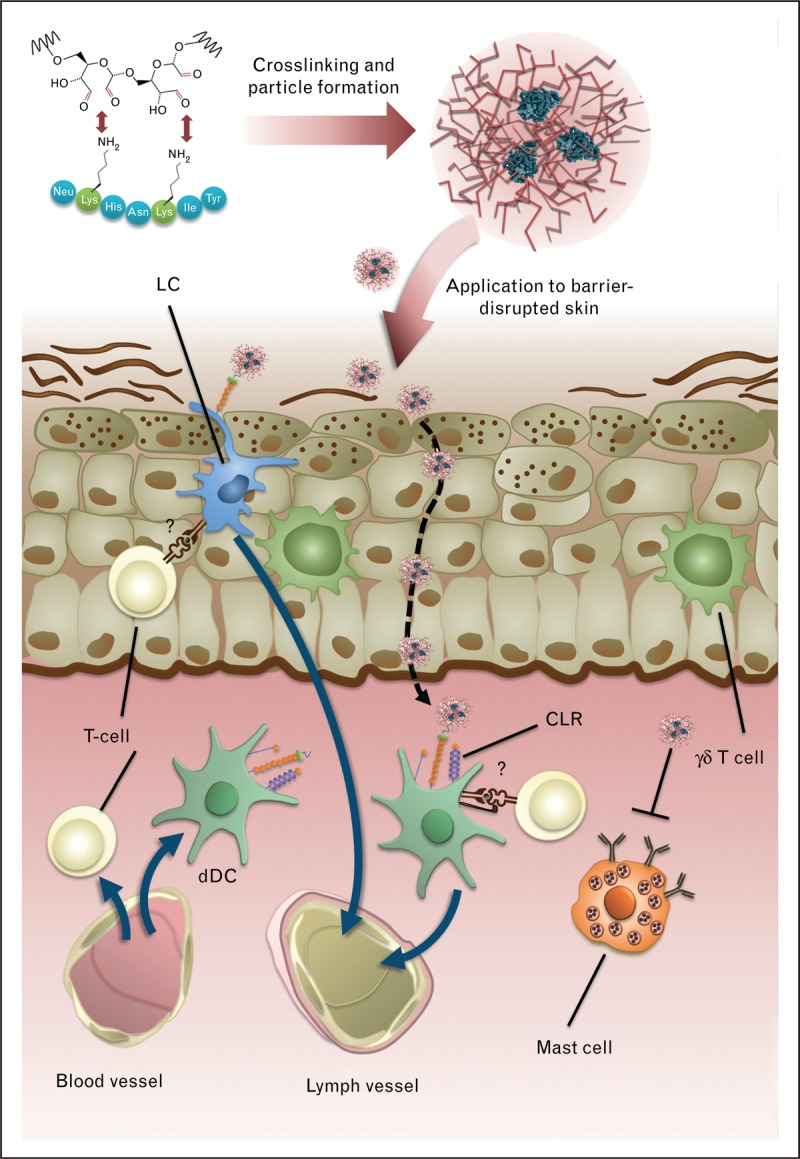
Concept of transcutaneous immunotherapy with carbohydrate-formulated allergen nanoparticles. Carbohydrates (e.g. mannan) are activated by mild periodate treatment and coupled to primary amines of allergens by reductive amination, thereby cross-linking the allergens and forming neoglycoconjugate nanoparticles with shielded IgE epitopes. Such nanoparticles are applied to barrier-disrupted skin where they are taken up by epidermal Langerhans cells (LC) via C-type lectin recteptors (CLR) or diffuse into the dermal compartment, in which they can be captured by dermal dendritic cells (dDC). It is unclear whether and to what extent they directly activate skin resident T cells. LCs and dDCs migrate to skin-draining lymph nodes in which they can promote antiallergic T cell responses. The carbohydrate moiety of the nanoparticles shields the allergen from interaction with receptor-bound IgE on dermal mast cells, thereby avoiding local side-effects. Notably, certain epidermal cells, such as keratinocytes or γδ T cells, may promote TH2-licensing of dendritic cells upon barrier disruption. Stimulation of dendritic cells by suitable C-type lectin receptors and/or other innate receptors can be utilized to suppress innate TH2 induction in the upper skin layers. Modified with permission from [24] and [56▪].
Another interesting dendritic cell-targeting approach focused on the model allergen ovalbumin (OVA) coupled to chitosan in a therapeutic mouse model mimicking SLIT. The conjugate induced enhanced antigen uptake, processing and presentation by bone marrow derived dendritic cells as well as oral APCs. Furthermore, the therapeutic effect, that is, reduction of airway hyperresponsiveness, lung inflammation and eosinophilia, was clearly associated with antigen-specific tolerance [59]. A recent study demonstrated that both, soluble OVA as well as particulate OVA-chitosan, are taken up by the same APC populations, but the uptake was significantly increased with OVA-chitosan nanoparticles. Interestingly, in this model, CD11b+CD11c−MHC-II+ oral macrophage-like cells rather than dendritic cells captured the majority of the antigen, and were shown to induce Foxp3+ Tregs in draining lymph nodes [60].
Our group has recently explored carbohydrate-mediated targeting of allergens to dendritic cells, and its influence on immunogenicity and allergenicity. Protein–carbohydrate complexes of papain and OVA were generated, and their allergenic potential (the capacity to cross-link surface bound IgE), dendritic cell-targeting in vivo and in vitro, and their immunogenicity were assessed [56▪]. We found that coupling carbohydrates to the protein surface can mask B-cell epitopes, thus making the protein ‘hypoallergenic’ (decreased binding and crosslinking of IgE antibodies directed against the native allergen). Interestingly, coupling of either mannan, dextran, or maltodextrin reduced the allergenic potential of papain, but not OVA [56▪]. An explanation for this effect could be the drastically reduced coupling efficiency for OVA at the used carbohydrate/protein ratio, probably leading to less effective shielding of B-cell epitopes. Building on these data, we were able to modulate the allergenic potential of the major birch pollen allergen Bet v 1 by using different protein to carbohydrate ratios (Fig. 2). This clearly indicates that hypoallergenicity of neoglycoconjugates depends on the carbohydrate, the allergen, and also the chemistry employed for the coupling reaction. Finally, we have shown that mannan–protein conjugates were efficiently captured by dendritic cells, both in vitro as well as in vivo (Fig. 3a). Moreover, intradermal immunization of mannan-neoglycoconjugates elicited elevated IgG titers (Fig. 3b) while suppressing de-novo IgE induction, compared with soluble antigen (Fig. 3c) [56▪].
FIGURE 2.
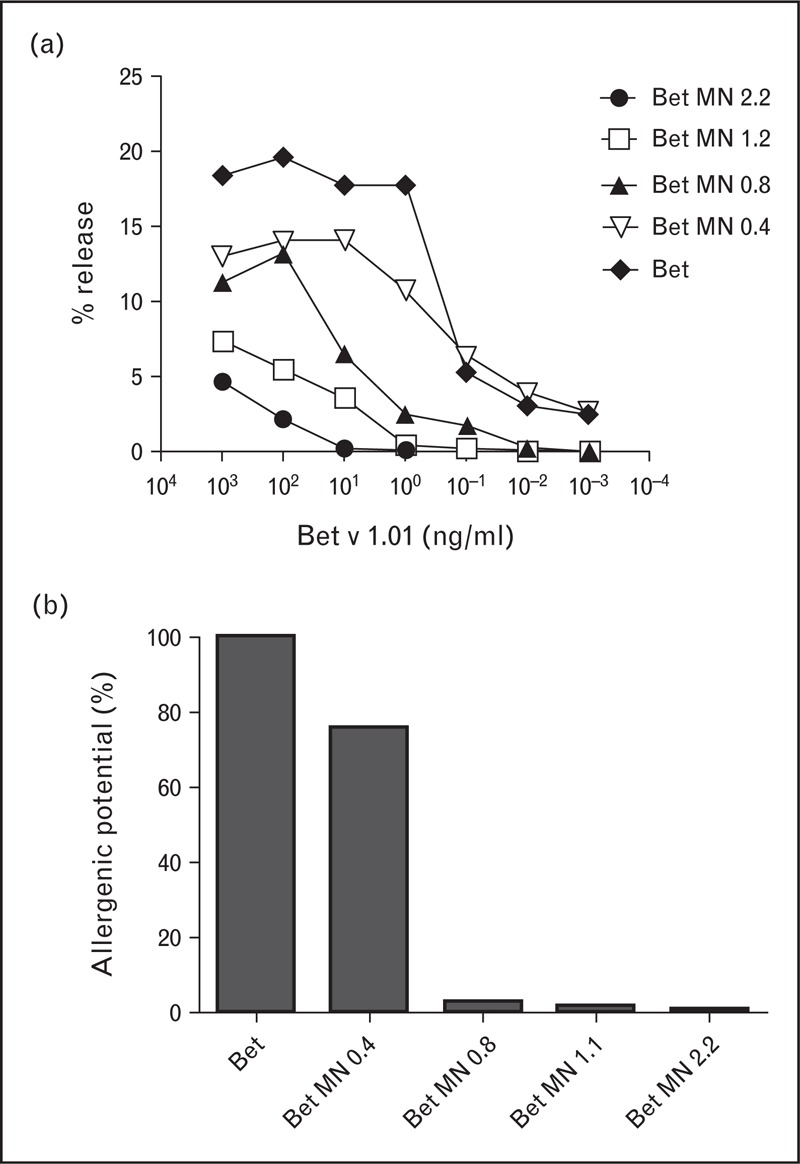
Modulating allergenicity of Bet v 1 through carbohydrate coupling. a) IgE-sensitized rat basophil leukemia (RBL) cells were stimulated with increasing concentrations of Bet v 1 coupled to increasing amounts of mannan (mannan:protein w/w ratios of 0.4–2.2). b) Allergenic potential in terms of the IgE cross-linking capacity compared to the wild-type protein, based on the 50%-release log-phase of the Gaussian release curve (EC50 value).
FIGURE 3.
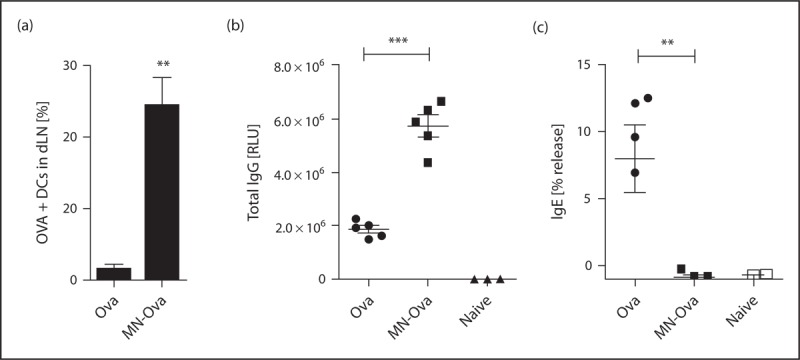
In-vivo uptake and immunogenicity of mannan-coupled ovalbumin (OVA). a) Percentage of dendritic cells in draining lymph nodes of BALB/c mice, which have taken up i.d. injected OVA or MN-OVA. OVA specific total IgG (b) and IgE (c) after two i.d. injections. IgG was measured by luminometric ELISA and IgE was quantitated by RBL release assay. RLU, relative light units. ∗∗P < 0.01, ∗∗∗P < 0.001, T-Test. Modified with permission from [56▪].
CONCLUSION
Transcutaneous immunotherapy of allergy is an attractive alternative to current therapeutic interventions, provided that the skin-inherent TH2-polarizing milieu and the potential for local and systemic side effects can be overcome. Hypoallergenic neoglycoconjugates have the potential to fulfill these requirements by specific targeting of dendritic cells, and modulating the immune profile. However, further innate immune receptors may have to be addressed in order to generate a robust and well tolerated immunotherapy platform. Such novel therapies would be ideally suited for application in children, and for treatment of food allergies (especially peanut allergy), which cannot be treated via the subcutaneous or sublingual route due to safety concerns.
Acknowledgements
This work was supported by the Austrian Science Fund (FWF), projects P21125 and W1213.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Footnotes
Correspondence to Josef Thalhamer, University of Salzburg, Department of Molecular Biology, Division of Allergy and Immunology, Hellbrunnerstrasse 34, 5020 Salzburg, Austria. Tel: +43 662 8044 5737; e-mail: Josef.Thalhamer@sbg.ac.at
REFERENCES
- 1.Kiel MA, Roder E, Gerth van Wijk R, et al. Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol 2013; 132:353–360.e2 [DOI] [PubMed] [Google Scholar]
- 2.Hylander T, Latif L, Petersson-Westin U, et al. Intralymphatic allergen-specific immunotherapy: an effective and safe alternative treatment route for pollen-induced allergic rhinitis. J Allergy Clin Immunol 2013; 131:412–420 [DOI] [PubMed] [Google Scholar]
- 3.Senti G, Crameri R, Kuster D, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol 2012; 129:1290–1296 [DOI] [PubMed] [Google Scholar]
- 4.Senti G, Graf N, Haug S, et al. Epicutaneous allergen administration as a novel method of allergen-specific immunotherapy. J Allergy Clin Immunol 2009; 124:997–1002 [DOI] [PubMed] [Google Scholar]
- 5▪.Senti G, von Moos S, Tay F, et al. Epicutaneous allergen-specific immunotherapy ameliorates grass pollen-induced rhinoconjunctivitis: A double-blind, placebo-controlled dose escalation study. J Allergy Clin Immunol 2012; 129:128–135 [DOI] [PubMed] [Google Scholar]; Follow-up study demonstrating efficacy of epicutaneous allergen-specific immunotherapy against grass pollen in a dose-dependent manner, showing symptom relief after only six applications.
- 6.Agostinis F, Forti S, Di Berardino F. Grass transcutaneous immunotherapy in children with seasonal rhinocon junctivitis. Allergy 2010; 65:404–411 [DOI] [PubMed] [Google Scholar]
- 7.Dupont C, Kalach N, Soulaines P, et al. Cow's milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J Allergy Clin Immunol 2010; 125:1165–1167 [DOI] [PubMed] [Google Scholar]
- 8▪.Bach D, Weiss R, Hessenberger M, et al. Transcutaneous immunotherapy via laser-generated micropores efficiently alleviates allergic asthma in Phl p 5-sensitized mice. Allergy 2012; 67:1365–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]; Transcutaneous immunization via laser-generated micropores shows similar efficiency as SCIT in a mouse model of allergic asthma, but induces fewer therapy induced side-effects.
- 9.Hessenberger M, Weiss R, Weinberger EE, et al. Transcutaneous delivery of CpG-adjuvanted allergen via laser-generated micropores. Vaccine 2013; 31:3427–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondoulet L, Dioszeghy V, Ligouis M, et al. Epicutaneous immunotherapy on intact skin using a new delivery system in a murine model of allergy. Clin Exp Allergy 2010; 40:659–667 [DOI] [PubMed] [Google Scholar]
- 11.Mondoulet L, Dioszeghy V, Vanoirbeek JA, et al. Epicutaneous immunotherapy using a new epicutaneous delivery system in mice sensitized to peanuts. Int Arch Allergy Immunol 2011; 154:299–309 [DOI] [PubMed] [Google Scholar]
- 12.Dioszeghy V, Mondoulet L, Dhelft V, et al. Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J Immunol 2011; 186:5629–5637 [DOI] [PubMed] [Google Scholar]
- 13.Mondoulet L, Dioszeghy V, Larcher T, et al. Epicutaneous immunotherapy (EPIT) blocks the allergic esophago-gastro-enteropathy induced by sustained oral exposure to peanuts in sensitized mice. PLoS ONE 2012; 7:e31967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪.Mondoulet L, Dioszeghy V, Puteaux E, et al. Intact skin and not stripped skin is crucial for the safety and efficacy of peanut epicutaneous immunotherapy (EPIT) in mice. Clin Transl Allergy 2012; 2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that in mice epicutaneous immunotherapy with peanut allergen on intact skin induces FoxP3 + T cells while application to tape-stripped skin reinforces TH2 immune responses.
- 15.Garaczi E, Szabo K, Francziszti L, et al. DermAll nanomedicine for allergen-specific immunotherapy. Nanomedicine 2013; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Combadiere B, Liard C. Transcutaneous intradermal vaccination. Hum Vaccin 2011; 7:811–827 [DOI] [PubMed] [Google Scholar]
- 17.Blamoutier P, Blamoutier J, Guibert L. Treatment of pollinosis with pollen extracts by the method of cutaneous quadrille ruling. Presse Med 1959; 67:2299–2301 [PubMed] [Google Scholar]
- 18.De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol 2012; 132:949–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutowska-Owsiak D, Ogg GS. The epidermis as an adjuvant. J Invest Dermatol 2012; 132:940–948 [DOI] [PubMed] [Google Scholar]
- 20.Strid J, Sobolev O, Zafirova B, et al. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science 2011; 334:1293–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪.Nakajima S, Igyarto BZ, Honda T, et al. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J Allergy Clin Immunol 2012; 129:1048–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]; Epicutaneous sensitization is dependent on epidermal, but not dermal Langerin + cells expressing the TSLP receptor.
- 22.Oyoshi MK, Larson RP, Ziegler SF, et al. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol 2010; 126:976–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otsuka A, Kubo M, Honda T, et al. Requirement of interaction between mast cells and skin dendritic cells to establish contact hypersensitivity. PLoS ONE 2011; 6:e25538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheiblhofer S, Thalhamer J, Weiss R. Laser microporation of the skin: prospects for painless application of protective and therapeutic vaccines. Expert Opin Drug Deliv 2013; 10:761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weldon WC, Zarnitsyn VG, Esser ES, et al. Effect of adjuvants on responses to skin immunization by microneedles coated with influenza subunit vaccine. PLoS ONE 2012; 7:e41501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manolova V, Flace A, Bauer M, et al. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol 2008; 38:1404–1413 [DOI] [PubMed] [Google Scholar]
- 27.Ruff LE, Mahmoud EA, Sankaranarayanan J, et al. Antigen-loaded pH-sensitive hydrogel microparticles are taken up by dendritic cells with no requirement for targeting antibodies. Integr Biol (Camb) 2013; 5:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baleeiro RB, Wiesmuller KH, Reiter Y, et al. Topical vaccination with functionalized particles targeting dendritic cells. J Invest Dermatol 2013; 133:1933–1941 [DOI] [PubMed] [Google Scholar]
- 29.Ejaz A, Ammann CG, Werner R, et al. Targeting viral antigens to CD11c on dendritic cells induces retrovirus-specific T cell responses. PLoS One 2012; 7:e45102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caminschi I, Shortman K. Boosting antibody responses by targeting antigens to dendritic cells. Trends Immunol 2012; 33:71–77 [DOI] [PubMed] [Google Scholar]
- 31.Caminschi I, Maraskovsky E, Heath WR. Targeting dendritic cells in vivo for cancer therapy. Front Immunol 2012; 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lepenies B, Lee J, Sonkaria S. Targeting C-type lectin receptors with multivalent carbohydrate ligands. Adv Drug Deliv Rev 2013; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Do Y, Didierlaurent AM, Ryu S, et al. Induction of pulmonary mucosal immune responses with a protein vaccine targeted to the DEC-205/CD205 receptor. Vaccine 2012; 30:6359–6367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Idoyaga J, Lubkin A, Fiorese C, et al. Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin DEC205, and Clec9A. Proc Natl Acad Sci U S A 2011; 108:2384–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hemmi H, Zaidi N, Wang B, et al. Treml4, an Ig superfamily member, mediates presentation of several antigens to T cells in vivo, including protective immunity to HER2 protein. J Immunol 2012; 188:1147–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪▪.Leung CS, Maurer MA, Meixlsperger S, et al. Robust T-cell stimulation by Epstein-Barr virus-transformed B cells after antigen targeting to DEC-205. Blood 2013; 121:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]; DEC205 is expressed not only on dendritic cells but also on activated B cells. The efficiency of DEC205 targeted vaccines is in part dependent on antigen presentation by B cells.
- 37▪▪.Idoyaga J, Fiorese C, Zbytnuik L, et al. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest 2013; 123:844–854 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that migratory dendritic cells have a superior ability to generate Treg in vivo, providing a rationale for the superiority of epicutaneous or transcutaneous immunotherapy compared with SCIT.
- 38.Gringhuis SI, den Dunnen J, Litjens M, et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol 2009; 10:203–213 [DOI] [PubMed] [Google Scholar]
- 39.Gringhuis SI, Kaptein TM, Wevers BA, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol 2012; 13:246–254 [DOI] [PubMed] [Google Scholar]
- 40.Chen ST, Liu RS, Wu MF, et al. CLEC5A regulates Japanese encephalitis virus-induced neuroinflammation and lethality. PLoS Pathog 2012; 8:e1002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu MF, Chen ST, Yang AH, et al. CLEC5A is critical for dengue virus-induced inflammasome activation in human macrophages. Blood 2013; 121:95–106 [DOI] [PubMed] [Google Scholar]
- 42.Shenderov K, Barber DL, Mayer-Barber KD, et al. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. J Immunol 2013; 190:5722–5730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyake Y, Toyonaga K, Mori D, et al. C-type lectin MCL is an FcRgamma-coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity 2013; 38:1050–1062 [DOI] [PubMed] [Google Scholar]
- 44.Gringhuis SI, den Dunnen J, Litjens M, et al. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis HIV-1 and Helicobacter pylori. Nat Immunol 2009; 10:1081–1088 [DOI] [PubMed] [Google Scholar]
- 45.van Vliet SJ, Bay S, Vuist IM, et al. MGL signaling augments TLR2-mediated responses for enhanced IL-10 and TNF-alpha secretion. J Leukoc Biol 2013; 94:315–323 [DOI] [PubMed] [Google Scholar]
- 46.Eberle ME, Dalpke AH. Dectin-1 stimulation induces suppressor of cytokine signaling 1, thereby modulating TLR signaling and T cell responses. J Immunol 2012; 188:5644–5654 [DOI] [PubMed] [Google Scholar]
- 47.van den Berg LM, Gringhuis SI, Geijtenbeek TB. An evolutionary perspective on C-type lectins in infection and immunity. Ann N Y Acad Sci 2012; 1253:149–158 [DOI] [PubMed] [Google Scholar]
- 48.Royer PJ, Emara M, Yang C, et al. The mannose receptor mediates the uptake of diverse native allergens by dendritic cells and determines allergen-induced T cell polarization through modulation of IDO activity. J Immunol 2010; 185:1522–1531 [DOI] [PubMed] [Google Scholar]
- 49.Emara M, Royer PJ, Abbas Z, et al. Recognition of the major cat allergen Fel d 1 through the cysteine-rich domain of the mannose receptor determines its allergenicity. J Biol Chem 2011; 286:13033–13040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsu SC, Chen CH, Tsai SH, et al. Functional interaction of common allergens and a C-type lectin receptor, dendritic cell-specific ICAM3-grabbing nonintegrin (DC-SIGN), on human dendritic cells. J Biol Chem 2010; 285:7903–7910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emara M, Royer PJ, Mahdavi J, et al. Retagging identifies dendritic cell-specific intercellular adhesion molecule-3 (ICAM3)-grabbing nonintegrin (DC-SIGN) protein as a novel receptor for a major allergen from house dust mite. J Biol Chem 2012; 287:5756–5763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chieppa M, Bianchi G, Doni A, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol 2003; 171:4552–4560 [DOI] [PubMed] [Google Scholar]
- 53.Nakaira-Takahagi E, Golim MA, Bannwart CF, et al. Interactions between TLR2, TLR4, and mannose receptors with gp43 from Paracoccidioides brasiliensis induce cytokine production by human monocytes. Med Mycol 2011; 49:694–703 [DOI] [PubMed] [Google Scholar]
- 54.Feriotti C, Loures FV, Frank de Araujo E, et al. Mannosyl-recognizing receptors induce an M1-like phenotype in macrophages of susceptible mice but an M2-like phenotype in mice resistant to a fungal infection. PLoS ONE 2013; 8:e54845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kasturi SP, Skountzou I, Albrecht RA, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011; 470:543–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56▪.Weinberger EE, Himly M, Myschik J, et al. Generation of hypoallergenic neoglycoconjugates for dendritic cell targeted vaccination: a novel tool for specific immunotherapy. J Control Release 2013; 165:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study descibes the generation of nanoparticles by cross-linking allergens with carbohydrates. These particles demonstrated reduced allergenicity and enhanced dendritic cell targeting and immunogenicity making them promising candidates for transcutaneous immunotherapy.
- 57.Andersson TN, Ekman GJ, Gronlund H, et al. A novel adjuvant-allergen complex, CBP-rFel d 1, induces up-regulation of CD86 expression and enhances cytokine release by human dendritic cells in vitro. Immunology 2004; 113:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thunberg S, Neimert-Andersson T, Cheng Q, et al. Prolonged antigen-exposure with carbohydrate particle based vaccination prevents allergic immune responses in sensitized mice. Allergy 2009; 64:919–926 [DOI] [PubMed] [Google Scholar]
- 59.Saint-Lu N, Tourdot S, Razafindratsita A, et al. Targeting the allergen to oral dendritic cells with mucoadhesive chitosan particles enhances tolerance induction. Allergy 2009; 64:1003–1013 [DOI] [PubMed] [Google Scholar]
- 60.Mascarell L, Saint-Lu N, Moussu H, et al. Oral macrophage-like cells play a key role in tolerance induction following sublingual immunotherapy of asthmatic mice. Mucosal Immunol 2011; 4:638–647 [DOI] [PubMed] [Google Scholar]


