Abstract
Background:
Modern ventriculoperitoneal shunts (VPS) are programmable, which enables clinicians to adjust valve-pressure according to their patients’ individual needs. The aim of this retrospective analysis is to evaluate indications for valve-pressure adjustments in idiopathic normal pressure hydrocephalus (iNPH).
Methods:
Patients operated between 2004 and 2011 diagnosed with iNPH were included. Kiefer-Scale was used to classify each patient. Follow-up exams were conducted 3, 6, and 12 months after shunt implantation and yearly thereafter. Initial valve-pressure was 100 or 70 mmH2O. Planned reductions of the valve-pressure to 70 and 50 mmH2O, respectively, were carried out and reactive adjustment of the valve-pressure to avoid over- and under-drainage were indicated.
Results:
A total of 52 patients were provided with a Medos-Hakim valveCodman® with a Miethke shunt-assistantAesculap® and 111 patients with a Miethke-proGAVAesculap®. 180 reductions of the valve-pressure took place (65% reactive, 35% planned). Most patients (89%) needed one or two adjustments of their valve-pressures for optimal results. In 41%, an improvement of the symptoms was observed. Gait disorder was improved most often after valve-pressure adjustments (32%). 18 times an elevation of valve-pressure was necessary because of headaches, vertigo, or the development of subdural hygroma. Optimal valve-pressure for most patients was around 50 mmH2O (36%).
Conclusion:
The goal of shunt therapy in iNPH should usually be valve-pressure settings between 30 and 70 mmH2O. Reactive adjustments of the valve-pressure are useful for therapy of over- and underdrainage symptoms. Planned reductions of the valve opening pressure are effective even if postoperative results are already satisfactory.
Keywords: Adjustments, gravitational valve, indications, iNPH, idiopathic normal pressure hydrocephalus, valve-pressure
INTRODUCTION
Patients with idiopathic normal pressure hydrocephalus (iNPH) suffer from a combination of gait disturbance, urinary incontinence, and cognitive decline.[19] At first, patients develop a short-term memory disorder, which leads to dementia eventually.[16] In addition to the main symptoms know as Hakim's triad, some patients experience headaches and vertigo but an enlargement of the ventricles in cerebral imaging is mandatory for the diagnosis iNPH.[15,23]
The implantation of ventriculoperitoneal shunts (VPS) is the therapy of choice for patients with iNPH.[4] During the last 15 years, our clinic has been confronted with iNPH patients more frequently.[20] Throughout this time, valve technology nationally and internationally has experienced crucial changes.[3] At first, gravitational-assisted, nonprogrammable valves (Miethke-Dual-SwitchAesculap®) were implanted and good clinical results achieved; 79% of operated patients showed satisfactory to excellent therapeutic outcomes 6-9 months postoperatively.[12] At the turn of the millennium, shunt technology evolved to gravitational-assisted, programmable valves (Miethke-proGAAesculap®) in which a gravitational-unit (shunt-assistant) is installed distally of the programmable differential-pressure-valve. This year a prospective, multi-center study showed the benefits of gravitational-assisted programmable valves, since they lead to the same favorable postoperative results but decrease the risks of overdrainage complications compared with shunt systems without gravitational units.[10] Therefore, these modern shunt systems are exclusively implanted to treat iNPH in our clinic.
In order to evaluate the indications for planned and reactive valve-pressure adjustments in patients with iNPH, we conducted this retrospective analysis of all patients, which received a programmable shunt system with gravitational unit in our department from 2004 to 2011.
MATERIALS AND METHODS
Patients with gait disturbance as the cardinal symptom of iNPH,[5,12] and if applicable, additional symptoms of Hakim's Triad, and an enlargement of the ventricles in cerebral imaging (Evans-Index ≥0.3) were diagnosed with cerebrospinal tap testing and intrathecal infusion test.[4] If the diagnosis of iNPH was verified, patients were offered a shunt-operation.
Clinical symptoms were classified preoperatively and during follow-up exams according to the Kiefer-Scale, which allows the scoring of 0-6 points for each symptom.[5] Thus, an increase of the Kiefer-Index correlates with a worsening of the clinical situation.[15]
Follow-up exams were used to adjust valve-pressures of the programmable units. Planned adjustments of valve-pressure were carried out based on a study protocol reducing the pressure from 100 to 70 mmH2O or from 70 to 50 mmH2O.[10] Moreover, investigators changed valve-pressures in reaction to the clinical development of each patient according to their individual experience (see results, reactive valve-pressure adjustments).
RESULTS
Of the 163 operated patients with iNPH, 52 were treated with a Codman-Medos programmable valveCodman® and a shunt-assistantMiethke, Aesculap®. The remaining 111 patients received a proGA valveMiethke, Aesculap®. During follow-ups, 180 reductions of valve-pressure were necessary. A total of 65% of these adjustments were reactions to the clinical course and 35% were planned adjustments. Median follow-up time was 42 months (6-162 months).
Reactive valve pressure adjustments
117 times, the reduction of valve-pressure was carried-out as a reaction to the clinical course of each patient. A worsening of gait disturbance was the reason for 47% of these adjustments, 22% took place because of an increase of urinary incontinence, 15% were carried out after an aggravation of vertigo and cognitive decline, and 14% were a result of an increase in headaches. Only 9% of reactive valve-pressure adjustments were necessary after an increase of Evans-Index. All in all, 49% of reactive valve-pressure adjustments took place because of a persistence of iNPH symptoms.
Reduction of valve-pressure
In most cases, one or two adjustments of valve-pressure were necessary to optimize clinical outcome [Figure 1]. Only 18 times, a third to fifth reduction of valve-pressure was indicated.
Figure 1.
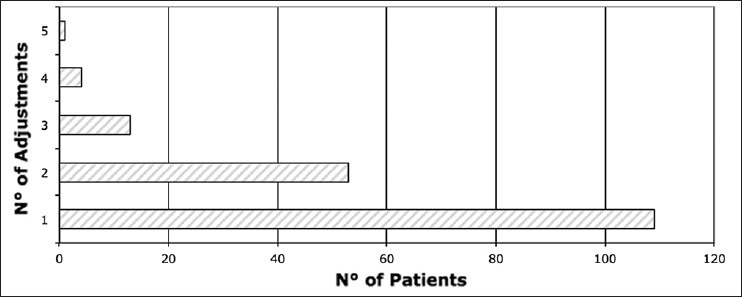
Number of valve-pressure adjustments per patient. In most cases (61%), one adjustment was needed. In 29% of cases, valves were adjusted twice, in 7% three times, in 2% four times, and in 0.6% (one patient) to optimize clinical outcome
The first adjustment to valve-pressure was in equal parts reactive (57 times) and planned (52 times). The following adjustments were mostly needed (reactive) because of a relapse of symptoms.
A clinical improvement according to Kiefer-Scale was seen after 41% of valve-pressure adjustments. Gait disturbance (33%), cognitive disorder (20%), vertigo (19%), headaches (15%), and urinary incontinence (13%) responded to the reduction of valve-pressure. A reduction of ventricle size (Evans-Index) was verified after only three (1.7%) of valve-pressure adjustments [Figure 2].
Figure 2.
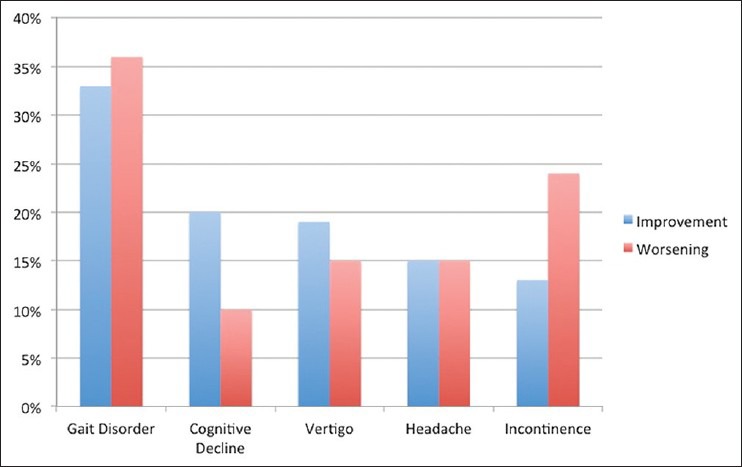
Clinical improvement and worsening of symptoms after valve-pressure adjustments. After 33% adjustments an improvement of gait, after 20% an improvement of cognitive abilities, after 19% a reduction of vertigo, after 15% fewer headaches, and after 13% an improvement of bladder control were seen. Gait disorder worsened most often after valve-pressure reduction (36%); cognitive decline (10%), vertigo and headaches (15%), and urinary incontinence (24%) showed an aggravation less often
An analysis of results for reactive and planned valve-pressure adjustments showed similar improvement rates (39% for reactive vs. 43% for planned adjustments).
Worsening of symptoms was observed after 21% of valve-pressure reductions. In 36% of these valve-pressure adjustments, a change for the worse of gait, in 24% of urinary incontinence, in 15% of vertigo and headaches, and in 10% of cognitive abilities was perceived [Figure 2].
Aggravation of symptoms also appeared equally after reactive and planned valve-pressure adjustments (19% reactive vs. 24% planned adjustments). Interestingly, 11 of 37 valve-pressure adjustments were observed more than 6 months after the adjustment due to lacking patient compliance.
Follow-up exams were carried out one month at the earliest but usually 3 months and then yearly after valve-pressure adjustment. Most clinical improvements were observed 3-6 months after valve-pressure adjustment. In 22 cases, an improvement of symptoms could be registered even more than 12 months after the last adjustment of valve-pressure.
Elevation of valve-pressure
Adjustments leading to an elevation of valve-pressure were necessary 18 times (reduction vs. elevation of valve-pressure ≅ 10:1) because of new or aggravated headaches, vertigo, and the formation of subdural hygroma in six cases each. Headaches and vertigo usually occurred simultaneously and after changing into the upright posture or after daily activities. An aggravation of gait disturbance, cognitive decline, and urinary incontinence gave reason for the elevation of valve-pressure in three, two, and one case, respectively. In 10 of these 18 cases, an elevation of valve-pressure to 70-90 mmH2O was needed. Six times the valves were adjusted ≥ 100 mmH2O and two times valve-pressure was elevated from 30 to 40 mmH2O. Each of these adjustments led to an improvement of symptoms, especially headaches, vertigo, and subdural hygroma were soothed after this treatment.
Optimized valve-pressure
The analysis of optimal valve-pressure, which leads to the lowest Kiefer-Index for the individual patients, showed the following results: Most patients (36%) were treated best with a valve-pressure of 50 mmH2O. A total of 26% needed an opening-pressure of 60-70 mmH2O and for 13% of the patients, optimal valve-pressure was >70 mmH2O. A valve-pressure of 30-40 mmH2O was needed for 21% of patients and an even lower pressure <30 mmH2O was used in only 4% of the patients [Figure 3].
Figure 3.
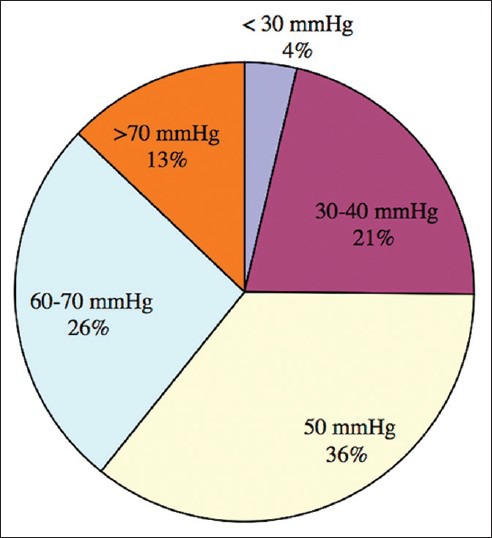
Optimized valve-pressure. A total of 83% patients were treated best with valve-pressures between 30 and 70 mmH2O
Complications
Complications making another operation necessary were recorded for 18 patients, which means a complication-rate of 11%. In six cases (4%), dysfunctions of the shunts were due to mechanical complications or dislocations of the abdominal catheter. A total of 4% developed subdural hygroma or hematoma, which could not be treated by elevating valve-pressure. Wound infections were seen in three patients (2%) and made a temporary externalization of the shunt system necessary. Two patients had an abdominal infection and one patient (<1%) had to be treated for ventriculitis by external drainage and intraventricular antibiotics.
DISCUSSION
Step-by-step reduction of valve-pressure
The initial setting of the programmable valves after implantation was 70-100 mmH2O. During follow-up exams, valve-pressure was adjusted according to plan and individual reactive needs. A slow reduction of valve-pressure decreases the risks for postoperative overdrainage complications since a respectable amount of cerebrospinal fluid (CSF) can be already lost during implantation of the shunt. Therefore, only six patients (4%) developed a subdural hematoma, which had to be operated eventually.
A prospective randomized study (SVASONA) showed that programmable valves with a shunt-assistant and an optimal valve-pressure of about 50 mmH2O reduce overdrainage complications without leading to underdrainage.[10] In addition, nonprogrammable valves with a fixed valve-pressure of 50 mmH2O do not allow treatment of patients who are treated best with valve-pressures above 60 mmH2O or below 40 mmH2O.
In this retrospective analysis, an elevation of valve-pressure was needed in 18 cases. 11 of these patients were finally treated with valve-pressures above 70 mmH2O. A direct approach without step-by-step reduction of valve-pressure would possibly lead to an increase of symptomatic overdrainage and to more cases in which an elevation of valve-pressure is necessary. All in all, a slow and step-by-step reduction of valve-pressure and an observation period of at least 3 months are recommended since our study shows that most beneficial effects are observed during this time period after valve-pressure adjustment. In this study, most clinical improvements were seen 3-6 months after valve-pressure adjustment. Adaptation to valve-pressure adjustments and consecutive changes in CSF dynamics are rather slow processes. In addition, for many of the patients, physiotherapy is recommended after discharge from hospital and during the further course of follow-up. Physiotherapy is indicated to relearn physiological gait and to decrease the patients’ fear of accidental downfalls and injuries. These ambulatory therapies might influence outcome after VPS implantation and could also be an explanation for improvements after this time-point of 3-6 months.
A wait-and-see attitude can also be justified for patients with shunts systems, which have already been adjusted twice. Using this cautious approach, it has to be considered though that the natural course of the disease and comorbidities such as cerebrovascular diseases can overshadow positive effects of treatment.[2,8,9] A review of unshunted patients suggested a fast deterioration of patients without surgery within 3 months after diagnosis.[21] Thus, worsening of symptoms is not only significantly influenced by the time-point of VPS implantation and thus the progression of the disease but also by simultaneously developing cardio-vascular and neurological conditions.
Optimal valve-pressure
The aim of valve-pressure adjustments should usually be a setting around 50 mmH2O for most patients. In this retrospective analysis, optimal valve-pressure was 30-70 mmH2O for 83% of patients and exactly 50 mmH2O in 36% of the cases. Thus, indications for planned reductions of valve-pressure are also based on the premises of optimal valve-pressure around 30-70 mmH2O.[13] Moreover, valve-pressure adjustments are based on the clinical experience of each investigator and time-points of reactive valve-pressure adjustments vary because clinical improvement can be observed more than 12 months after valve-pressure adjustment. Therefore, some clinicians might use the wait-and-see strategy in few cases. The concept of optimal valve-pressure also suggests that most valve-pressure adjustments are carried out during follow-up exams right after the implantation. Adjustments are less often necessary in timely distance to the operation for optimal valve-pressure is usually reached after one to two adjustments.
Reactive valve-pressure adjustments
Reactive Valve-Pressure Adjustments are reasonable in cases of under- and overdrainage symptoms. After 39% of reactive valve-pressure adjustments, clinical improvement was observed. A total of 117 reactive valve-pressure adjustments were necessary for the 163 patients of this study. This means that 117 operations due to underdrainage could be prevented because of programmable valves. In earlier times, 4% of patients with nonprogrammable valves needed revision operations because of underdrainage.[4] It is very probable though that underdrainage was only diagnosed in serious cases since the risks of the revision operation had to be weighed against the benefits of optimized valve-pressure. In patients with easily programmable valves, underdrainage is surely diagnosed much more often and reactive valve-pressure adjustments are indicated. Furthermore, the cause for reactive valve-pressure adjustments was most often (46%) an aggravation of gait disturbance, which emphasizes the role of gait disorder as cardinal symptom of iNPH.[16] The clinical course also showed an improvement of gait disorder most often (33%) after valve-pressure adjustment, which confirms earlier findings about improvement of iNPH symptoms.[16] After 21% of valve-pressure adjustments, symptoms were aggravated. This should not lead to the assumption that the preceding valve-pressure adjustments caused this clinical decline. It is well known that the results of shunt implantations are dependent on the time-point of operation and comorbidities of the patients.[9,11,13,14] In addition, literature shows a high coincidence of iNPH and other neurodegenerative diseases like Alzheimer's disease and Parkinsonism.[1,2,6] In summary, above all the progression of iNPH and the patients’ other diseases are responsible for the observed decline.
Planned valve-pressure adjustments
A total of 43% of the planned reductions of valve-pressure lead to further improvement of symptoms. After initial clinical improvement postoperatively with valve-pressures between 100 and 70 mmH2O, a phase of aggravation of symptoms can be observed once more. Thus, valve-pressure should be reduced. In the absence of this therapeutic regress, planned reductions of valve-pressure are reasonable and cause additional beneficial effects.
Evans-index
Clinical improvement is rarely accompanied by a decrease of Evans-Index. In a previous study, we showed that an improved neurological outcome in iNPH is associated with a minimal decrease of ventricular size or none at all.[14] These results confirm our analysis with only 1.7% cases with decreased Evans-Index after valve-pressure reduction. In contrast, an increase in ventricular size is suspicious for underdrainage or mechanical complications. If ventricular size persists or increases even further after reduction of valve-pressure, additional diagnostics in order to exclude shunt dysfunction are required. Therefore, in this study, 9% of valve-pressure reductions were due to an increase of Evans-Index.
Complications and outcome
Surgical therapy for iNPH is rare in complications and has good clinical success when strictly indicated. In this study, 11% of patients had to be operated because of complications. This complication rate is comparable with recent international data showing complication rates between 12% and 15%.[1,7] Clinical improvement is seen in 83-90% during the first year postoperatively[1,7,17] and 60% of operated patients show beneficial results even 5 years after shunt implantation. In addition, 5-7 years after operation, a success of treatment can still be verified even if revision operations were necessary.[18]
Conclusion for clinical practice
Optimal valve-pressure should be 30-70 mmH2O for most patients. Reactive valve-pressure adjustments are reasonable in cases of over- and underdrainage. Planned reductions of valve-pressure can even improve postoperative amended symptoms. Clinical improvement is seldomly accompanied by a decrease in ventricular size. Implantation of VPS in patients with iNPH is associated with few complications. In accordance with the current guidelines, we promote the usage of programmable valves with gravitational unit, which can prevent potential revision operations due to over- and underdrainage.[22]
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/1/140/119879
Disclaimer: The authors of this article have no conflicts of interest to disclose, and have adhered to SNI's policies regarding human/animal rights, and informed consent. Advertisers in SNI did not ask for, nor did they receive access to this article prior to publication.
Contributor Information
Leonie Gölz, Email: leonie.goelz@ukb.de.
Johannes Lemcke, Email: johannes.lemcke@ukb.de.
Ullrich Meier, Email: ullrich.meier@ukb.de.
REFERENCES
- 1.Akiguchi I, Ishii M, Watanabe Y, Watanabe T, Kawasaki T, Yagi H, et al. Shunt-responsive parkinsonism and reversible white matter lesions in patients with idiopathic NPH. J Neurol. 2008;255:1392–9. doi: 10.1007/s00415-008-0928-1. [DOI] [PubMed] [Google Scholar]
- 2.Bech-Azeddine R, Høgh P, Juhler M, Gjerris F, Waldemar G. Idiopathic normal-pressure hydrocephalus: Clinical comorbidity correlated with cerebral biopsy findings and outcome of cerebrospinal fluid shunting. J Neurol Neurosurg Psychiatry. 2007;78:157–61. doi: 10.1136/jnnp.2006.095117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto MA. Development of shunt technology especially for idiopathic normal pressure hydrocephalus. Brain Nerve. 2008;60:247–55. [PubMed] [Google Scholar]
- 4.Kahlon B, Sundbarg G, Rehncrona S. Comparison between the lumbar infusion and CSF tap tests to predict outcome after shunt surgery in suspected normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2002;73:721–6. doi: 10.1136/jnnp.73.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiefer M, Eymann R, Komenda Y, Steudel WI. Ein Graduierungssystem für den chronischen Hydrozephalus. A Grading System for Chronic Hydrocephalus. Zentralbl Neurochir. 2003;64:109–15. doi: 10.1055/s-2003-41881. [DOI] [PubMed] [Google Scholar]
- 6.Kiefer M, Meier U, Eymann R. Does idiopathic normal pressure hydrocephalus always mean a poor prognosis? Acta Neurochir Suppl. 2010;106:101–6. doi: 10.1007/978-3-211-98811-4_17. [DOI] [PubMed] [Google Scholar]
- 7.Klinge P, Hellström P, Tans J, Wikkelsø C On behalf of the European iNPH Multicentre Study Group. One-year outcome in the European multicentre study on iNPH. Acta Neurol Scand. 2012;126:145–53. doi: 10.1111/j.1600-0404.2012.01676.x. [DOI] [PubMed] [Google Scholar]
- 8.Klinge P, Marmarou A, Bergsneider M, Relkin N, Black PM. Outcome of shunting in idiopathic normal-pressure hydrocephalus and the value of outcome assessment in shunted patients. Neurosurgery. 2005;57(Suppl 3):S40–52. doi: 10.1227/01.neu.0000168187.01077.2f. [DOI] [PubMed] [Google Scholar]
- 9.Lemcke J, Meier U. Idiopathic normal pressure hydrocephalus (iNPH) and co-morbidity: An outcome analysis of 134 patients. Acta Neurochir Suppl. 2012;114:255–9. doi: 10.1007/978-3-7091-0956-4_50. [DOI] [PubMed] [Google Scholar]
- 10.Lemcke J, Meier U, Müller C, Fritsch MJ, Kehler U, Langer N, et al. Safety and efficacy of gravitational shunt valves in patients with idiopathic normal pressure hydrocephalus: A pragmatic, randomised, open label, multicentre trial (SVASONA) J Neurol Neurosurg Psychiatry. 2013;84:850–7. doi: 10.1136/jnnp-2012-303936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGirt MJ, Woodworth G, Coon AL, Thomas G, Williams MA, Rigamonti D. Diagnosis, treatment, and analysis of long-term outcomes in idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57:699–705. doi: 10.1093/neurosurgery/57.4.699. [DOI] [PubMed] [Google Scholar]
- 12.Meier U, Kiefer M, Sprung C. Evaluation of the Miethke dual- switch valve in patients with normal pressure hydrocephalus. Surg Neurol. 2004;61:119–27. doi: 10.1016/j.surneu.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Meier U, Lemcke J, Al-Zain F. Course of disease in patients with idiopathic normal pressure hydrocephalus (iNPH): A follow-up study 3, 4 and 5 years following shunt implantation. Acta Neurochir Suppl. 2008;102:125–7. doi: 10.1007/978-3-211-85578-2_25. [DOI] [PubMed] [Google Scholar]
- 14.Meier U, Mutze S. Is decreased ventricular size a correlate of positiv clinical outcome following shunt placement in 80 cases of normal pressure hydrocephalus? J Neurosurg. 2004;100:1036–40. doi: 10.3171/jns.2004.100.6.1036. [DOI] [PubMed] [Google Scholar]
- 15.Meier U, Zeilinger FS, Kintzel D. Signs, Symptoms and Course of Normal Pressure Hydrocephalus in Comparison with Cerebral Atrophy. Acta Neurochir (Wien) 1999;141:1039–48. doi: 10.1007/s007010050480. [DOI] [PubMed] [Google Scholar]
- 16.Miyoshi N, Kazui H, Ogino A, Ishikawa M, Miyake H, Tokunaga H, et al. Association between cognitive impairment and gait disturbance in patients with idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord. 2005;20:71–6. doi: 10.1159/000085858. [DOI] [PubMed] [Google Scholar]
- 17.Poca MA, Solana E, Martínez-Ricarte FR, Romero M, Gándara D, Sahuquillo J. Idiopathic normal pressure hydrocephalus: Results of a prospective cohort of 236 shunted patients. Acta Neurochir Suppl. 2012;114:247–53. doi: 10.1007/978-3-7091-0956-4_49. [DOI] [PubMed] [Google Scholar]
- 18.Pujari S, Kharkar S, Metellus P, Shuck J, Williams MA, Rigamonti D. Normal pressure hydrocephalus: Long-term outcome after shunt surgery. J Neurol Neurosurg Psychiatry. 2008;79:1282–6. doi: 10.1136/jnnp.2007.123620. [DOI] [PubMed] [Google Scholar]
- 19.Sakakibara R, Uchida Y, Ishii K, Kazui H, Hashimoto M, Ishikawa M, et al. Correlation of right frontal hypoperfusion and urinary dysfunction in iNPH: A SPECT study. Neurourol Urodyn. 2012;31:50–5. doi: 10.1002/nau.21222. [DOI] [PubMed] [Google Scholar]
- 20.Stranjalis G, Kalamatianos T, Koutsarnakis C, Loufardaki M, Stavrinou L, Sakas DE. Twelve-year hospital outcomes in patients with idiopathic hydrocephalus. Acta Neurochir Suppl. 2012;113:115–7. doi: 10.1007/978-3-7091-0923-6_23. [DOI] [PubMed] [Google Scholar]
- 21.Toma AK, Stapleton S, Papadopoulos MC, Kitchen ND, Watkins LD. Natural history of idiopathic normal-pressure hydrocephalus. Neurosurg Rev. 2011;34:433–9. doi: 10.1007/s10143-011-0316-7. [DOI] [PubMed] [Google Scholar]
- 22.Toma AK, Tarnaris A, Kitchen ND, Watkins LD. Use of the proGAV shunt valve in normal-pressure hydrocephalus. Neurosurgery. 2011;268(2 Suppl Operative):245–9. doi: 10.1227/NEU.0b013e318214a809. [DOI] [PubMed] [Google Scholar]
- 23.Zettl UK, Lehmitz R, Mix E. Klinische Liquordiagnostik. Walter de Gruyter. 2005;2:77–80. [Google Scholar]


