Abstract
Chloroquine is thought to exert its antimalarial effect by preventing the polymerization of toxic heme released during proteolysis of hemoglobin in the Plasmodium digestive vacuole. The mechanism of this blockade has not been established. We incubated cultured parasites with subinhibitory doses of [3H]chloroquine and [3H] quinidine. These [3H]quinoline compounds became associated with hemozoin as assessed by electron microscope autoradiography and subcellular fractionation. In vitro, binding of [3H]quinoline inhibitors to the hemozoin chain depended on the addition of heme substrate. These data counter previous conclusions regarding the lack of quinoline association with hemozoin, explain the exaggerated accumulation of quinolines in the plasmodium digestive vacuole, and suggest that a quinoline heme complex incorporates into the growing polymer to terminate chain extension, blocking further sequestration of toxic heme.
Full text
PDF
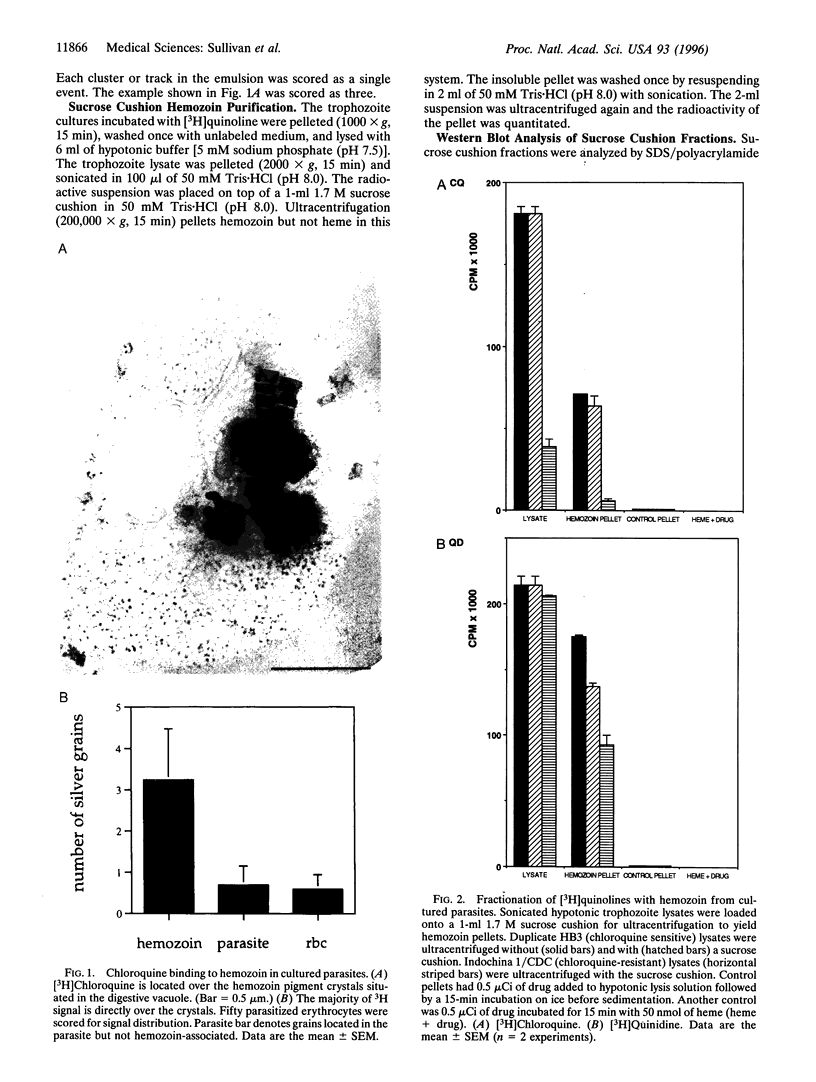
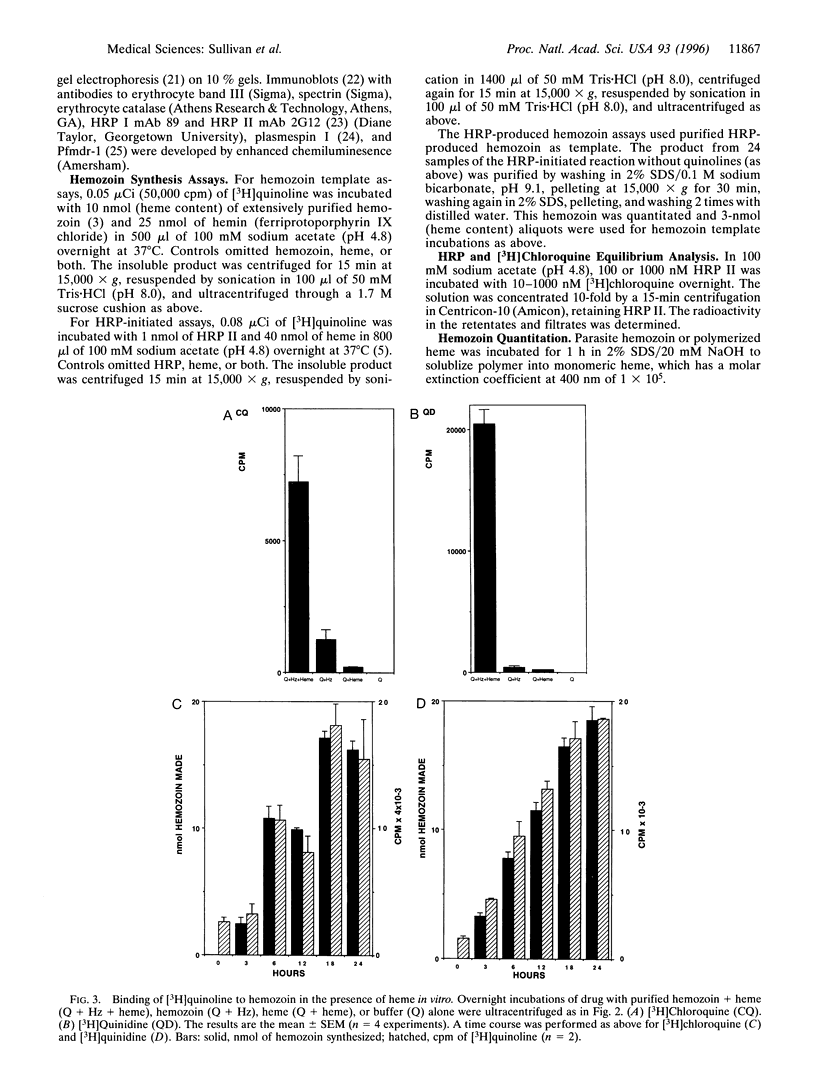
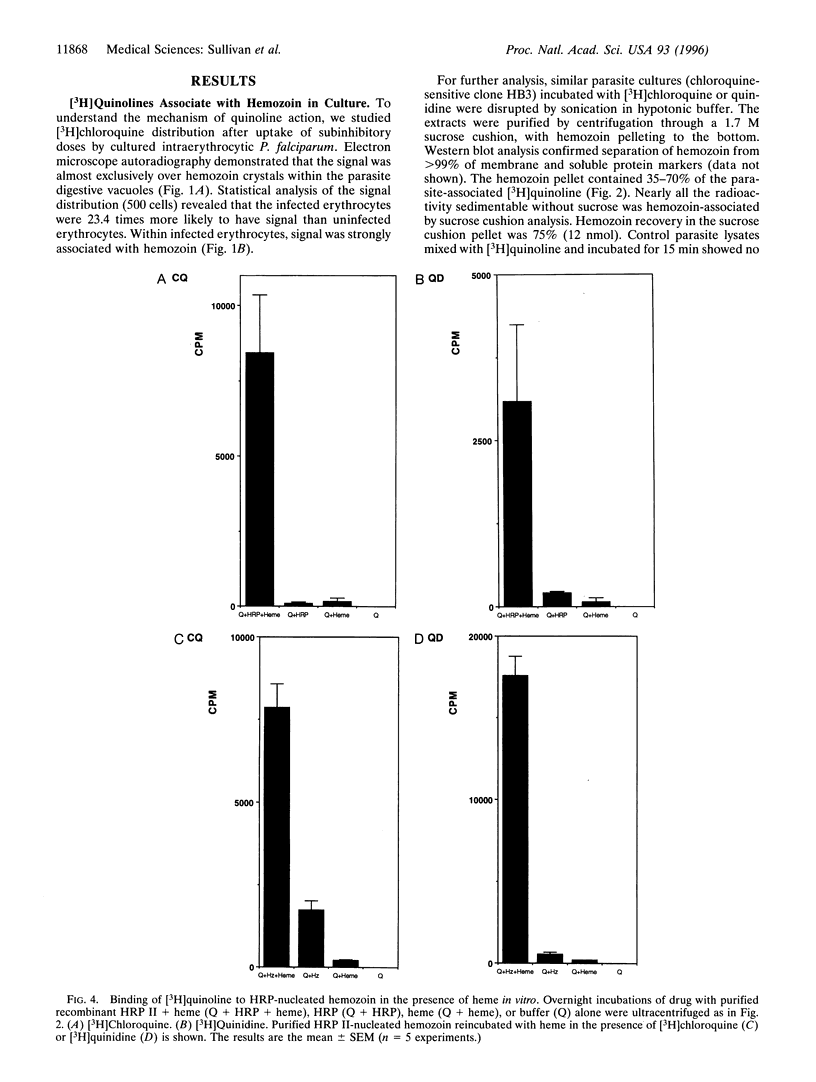


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M. High-resolution autoradiography of malarial parasites treated with 3 H-chloroquine. Am J Pathol. 1972 May;67(2):277–284. [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian D., Mohan Rao C., Panijpan B. The malaria parasite monitored by photoacoustic spectroscopy. Science. 1984 Feb 24;223(4638):828–830. doi: 10.1126/science.6695185. [DOI] [PubMed] [Google Scholar]
- Bendrat K., Berger B. J., Cerami A. Haem polymerization in malaria. Nature. 1995 Nov 9;378(6553):138–139. doi: 10.1038/378138a0. [DOI] [PubMed] [Google Scholar]
- COHEN S. N., PHIFER K. O., YIELDING K. L. COMPLEX FORMATION BETWEEN CHLOROQUINE AND FERRIHAEMIC ACID IN VITRO, AND ITS EFFECT ON THE ANTIMALARIAL ACTION OF CHLOROQUINE. Nature. 1964 May 23;202:805–806. doi: 10.1038/202805a0. [DOI] [PubMed] [Google Scholar]
- Chou A. C., Chevli R., Fitch C. D. Ferriprotoporphyrin IX fulfills the criteria for identification as the chloroquine receptor of malaria parasites. Biochemistry. 1980 Apr 15;19(8):1543–1549. doi: 10.1021/bi00549a600. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Karcz S., Galatis D., Culvenor J. G. A P-glycoprotein homologue of Plasmodium falciparum is localized on the digestive vacuole. J Cell Biol. 1991 Jun;113(5):1033–1042. doi: 10.1083/jcb.113.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn A., Stoffel R., Matile H., Bubendorf A., Ridley R. G. Malarial haemozoin/beta-haematin supports haem polymerization in the absence of protein. Nature. 1995 Mar 16;374(6519):269–271. doi: 10.1038/374269a0. [DOI] [PubMed] [Google Scholar]
- Egan T. J., Ross D. C., Adams P. A. Quinoline anti-malarial drugs inhibit spontaneous formation of beta-haematin (malaria pigment). FEBS Lett. 1994 Sep 19;352(1):54–57. doi: 10.1016/0014-5793(94)00921-x. [DOI] [PubMed] [Google Scholar]
- Fitch C. D., Chevli R. Sequestration of the chloroquine receptor in cell-free preparations of erythrocytes infected with Plasmodium berghei. Antimicrob Agents Chemother. 1981 Apr;19(4):589–592. doi: 10.1128/aac.19.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D., Kanjananggulpan P. The state of ferriprotoporphyrin IX in malaria pigment. J Biol Chem. 1987 Nov 15;262(32):15552–15555. [PubMed] [Google Scholar]
- Francis S. E., Gluzman I. Y., Oksman A., Knickerbocker A., Mueller R., Bryant M. L., Sherman D. R., Russell D. G., Goldberg D. E. Molecular characterization and inhibition of a Plasmodium falciparum aspartic hemoglobinase. EMBO J. 1994 Jan 15;13(2):306–317. doi: 10.1002/j.1460-2075.1994.tb06263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homewood C. A., Warhurst D. C., Peters W., Baggaley V. C. Lysosomes, pH and the anti-malarial action of chloroquine. Nature. 1972 Jan 7;235(5332):50–52. doi: 10.1038/235050a0. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Gluzman I. Y., Kyle D. E., Oduola A. M., Martin S. K., Milhous W. K., Schlesinger P. H. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science. 1987 Nov 27;238(4831):1283–1285. doi: 10.1126/science.3317830. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Macomber P. B., Sprinz H. Morphological effects of chloroquine on Plasmodium berghei in mice. Nature. 1967 May 27;214(5091):937–939. doi: 10.1038/214937a0. [DOI] [PubMed] [Google Scholar]
- Moreau S., Perly B., Chachaty C., Deleuze C. A nuclear magnetic resonance study of the interactions of antimalarial drugs with porphyrins. Biochim Biophys Acta. 1985 May 29;840(1):107–116. doi: 10.1016/0304-4165(85)90167-9. [DOI] [PubMed] [Google Scholar]
- Olliaro P. L., Goldberg D. E. The plasmodium digestive vacuole: metabolic headquarters and choice drug target. Parasitol Today. 1995 Aug;11(8):294–297. doi: 10.1016/0169-4758(95)80042-5. [DOI] [PubMed] [Google Scholar]
- Rock E. P., Marsh K., Saul A. J., Wellems T. E., Taylor D. W., Maloy W. L., Howard R. J. Comparative analysis of the Plasmodium falciparum histidine-rich proteins HRP-I, HRP-II and HRP-III in malaria parasites of diverse origin. Parasitology. 1987 Oct;95(Pt 2):209–227. doi: 10.1017/s0031182000057681. [DOI] [PubMed] [Google Scholar]
- Russell D. G. Immunoelectron microscopy of endosomal trafficking in macrophages infected with microbial pathogens. Methods Cell Biol. 1994;45:277–288. doi: 10.1016/s0091-679x(08)61857-9. [DOI] [PubMed] [Google Scholar]
- Slater A. F., Cerami A. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature. 1992 Jan 9;355(6356):167–169. doi: 10.1038/355167a0. [DOI] [PubMed] [Google Scholar]
- Slater A. F. Chloroquine: mechanism of drug action and resistance in Plasmodium falciparum. Pharmacol Ther. 1993 Feb-Mar;57(2-3):203–235. doi: 10.1016/0163-7258(93)90056-j. [DOI] [PubMed] [Google Scholar]
- Slater A. F., Swiggard W. J., Orton B. R., Flitter W. D., Goldberg D. E., Cerami A., Henderson G. B. An iron-carboxylate bond links the heme units of malaria pigment. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):325–329. doi: 10.1073/pnas.88.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. J., Jr, Gluzman I. Y., Goldberg D. E. Plasmodium hemozoin formation mediated by histidine-rich proteins. Science. 1996 Jan 12;271(5246):219–222. doi: 10.1126/science.271.5246.219. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Warhurst D. C., Gould S. The chemotherapy of rodent malaria XXXIII. The activity of chloroquine and related blood schizontocides and of some analogues in drug-induced pigment clumping. Ann Trop Med Parasitol. 1982 Jun;76(3):257–264. [PubMed] [Google Scholar]
- Yayon A., Cabantchik Z. I., Ginsburg H. Susceptibility of human malaria parasites to chloroquine is pH dependent. Proc Natl Acad Sci U S A. 1985 May;82(9):2784–2788. doi: 10.1073/pnas.82.9.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]



