Abstract
Background:
Traumatic brain injury (TBI) occurs in an estimated 80% of all pediatric trauma patients and is the leading cause of death and disability in the pediatric population. Decompressive craniectomy is a procedure used to decrease intracranial pressure by allowing the brain room to swell and therefore increase cerebral perfusion to the brain.
Methods:
This is a retrospective study done at St. Mary's Medical Center/Palm Beach Children's Hospital encompassing a 3 year 7 month period. All the pediatric patients who sustained a TBI and who were treated with a decompressive craniectomy were included. The patients’ outcomes were monitored and scored according to the Rancho Los Amigos Score at the time of discharge from the hospital and 6 months postdischarge.
Results:
A total of 379 pediatric patients with a diagnosis of TBI were admitted during this time. All these patients were treated according to the severity of their injury. A total of 49 pediatric patients required neurosurgical intervention and 7 of these patients met the criteria for a decompressive craniectomy. All seven patients returned home with favorable outcomes.
Conclusion:
This study supports the current literature that decompressive craniectomy is no longer an intervention used as a last resort but an effective first line treatment to be considered.
Keywords: Decompressive craniectomy, ICP monitoring, pediatric intracranial pressure, traumatic brain injury
INTRODUCTION
The 2012 Guidelines for Pediatric Head Injury state intracranial pressure (ICP) monitoring for patients with a traumatic brain injury (TBI) and a Glasgow Coma Scale (GCS) <8 is an option.[19] In addition, the 2012 Pediatric Head Injury Guidelines state “decompressive craniectomies with duraplasty, leaving the bone flap out, may be considered for pediatric patients with traumatic brain injury who are showing early signs of neurologic deterioration or herniation or are developing intracranial hypertension refractory to medical management during the early stages of treatment.”[19] According to these guidelines, ICP monitoring and decompressive craniectomies are listed as Options (level 3 evidence) not Recommendations (level 2 evidence) and not Guidelines (level 1 evidence). Small sample sizes of pediatric patients treated with decompressive craniectomy are seen throughout the literature with limited data and strength. The study done at St. Mary's Medical Center/Palm Beach Children's Hospital (SMMC/PBCH) is a case series of seven pediatric patients who underwent decompressive craniectomies for TBI and their outcomes were measured by the Rancho Los Amigos Score on discharge and 6 months postdischarge. Unfortunately due to the small sample size a comparison study of medical management only as compared with decompressive craniectomy could not be done.
BACKGROUND
TBI occurs in an estimated 80% of all pediatric trauma patients and is the leading cause of death and disability in the pediatric population.[3,26,27] Head injury is likely to occur in children and adolescents ranging from 0 to 19 years in approximately 200 per 100,000 population, according to the National Center for Health Statistics. These statistics include all head injuries that were either hospitalized or died (20,000 permanently disabled per year and 7000 deaths). Overall, children tend to have better outcomes than adults with the same injury score. However, recovery takes longer, usually from 6 months to years, whereas adults usually reach maximum recovery in approximately 6 months.[8,14,20] In the pediatric patient with a head injury, the mortality rate is 29% according to the National Center for Health Statistics.[21] Some trauma centers have reported head injuries accounting for 75-97% of pediatric trauma deaths.[21] Neurological deficits with delayed response times and short-term memory loss are reported in 10-20% of children with a GCS of 6-8 on admission, especially if the coma lasted for more than 3 weeks. Greater than 50% of children with GCS of 3-5 were reported as having permanent neurological deficits.[26] An increased number of TBI patients were identified in two pediatric age groups; one group consisted of 15-year-old males related to driving accidents and their sports involvement, the other group consisted of 0- to 1-year-old infants related to child abuse and falls.[2] Unfortunately child abuse is responsible for approximately 1300 severe or fatal head injuries per year.[3] Many neurosurgeons agree on the use of medical treatment of elevated ICP as a first line modality such as sedation, neuromuscular blockade, and various other medical measures to reduce ICP. However, not all neurosurgeons agree that a decompressive craniectomy is indicated or that a decompressive craniectomy should be performed. Should a decompressive craniectomy be performed as a last resort only to prevent brain herniation or perhaps implemented sooner to decrease the effects of an elevated ICP in the pediatric patient? Several studies have reported some evidence to suggest the latter results in better outcomes. Taylor et al. described a randomized trial of 13 out of 27 children who were treated with very early decompressive craniectomy in their treatment algorithm as opposed to the control group that was only treated medically. Impressively, 54% of the patients with decompression had a normal or mild disability outcome at 6 months, whereas only 14% of the patients treated medically had a normal or mild disability outcome at 6 months.[28] A similar study was conducted by Figaji et al., and although the sample size was small, with only five pediatric patients, all achieved a favorable outcome with early decompressive craniectomy.[7] This confirms the need for evaluating decompressive craniectomies in the pediatric population in a more aggressive fashion.
Assessing traumatic brain injury in pediatric patients
The assessment and reassessment of the pediatric patient is crucial and must be performed quickly and efficiently. Assessment of the airway, breathing, circulation, and neurological status should occur simultaneously. Monitoring neurological status for an impending increase in ICP is the major goal with the intention of minimizing brain injury and preventing brain herniation. Pupillary dysfunction, decrease level of consciousness, posturing or rigid extremities, bradycardia, hypertension, and irregular respirations are all signs of increased ICP that could rapidly result in brain herniation and death. Since children are difficult to assess neurologically, a pediatric GCS (PGCS) was created and evaluated for assessing the baseline of nonparticipatory children and preverbal children.[11] The PGCS is age based and can further facilitate the identification of patients at risk for increased ICP. Ultimately, computed tomography (CT) scan imaging is an important tool from the surgeon's perspective to help screen patient candidates for decompressive craniectomy. A CT scan also aids the surgeon in identifying the location and extent of craniectomy to be performed. In addition, a CT scan allows for further assessment of the brain injury to determine if additional duraplasty or resection will be required.
Anatomy
A child's head is anatomically different than an adult head with the child's head being larger in proportion to the body from a surface area standpoint. In children, neck muscles are less developed than an adult. The stability of a pediatric brain is dependent on age. Babies, infants, toddlers, and teenagers are each at different stages of skull and brain development.
A baby or infant, unlike an adult skull is thin and the sutures can be nonadherent. Therefore, fractures can extend into the cranial sutures causing them to be diastatic or split. In adults, the sutures are typically quite adherent and fractures will rarely displace a cranial suture. In babies and infants, the dura is more adherent to the skull and thus they are more prone to subdural hematomas (SDHs) rather than epidural hematomas (EDHs). However, when anatomical changes occur in toddlers and progress to teenage years, the adherence of the dura to the skull becomes looser. This population is more likely to sustain EDHs from skull fractures, especially those involving the temporal bone or middle meningeal artery. In elderly patients, due to atrophy, SDHs tend to be more common and thus EDHs are not as common.
The scalp surface area is larger and more vascular in children and therefore prone to significant blood loss. The pediatric brain is softer than the adult brain with a higher water content rendering the child more susceptible to acceleration–deceleration injuries and to the more common axonal or white-matter injury than in adults. The white matter is normally the axonal component of nerve cells conducting signal from one part of the brain to another. The myelin sheaths of the axons of a children's brain are not completely developed. Myelin is essential for axonal conduct and speed of signal, protecting the axon from tear due to thickness. Since the unmyelinated axonal portion is greater in children than in adults, these axons are more susceptible to shear injuries. Adults usually receive a contrecoup type injury due to the noncompliance of the skull.
Both child and adults vary on how well they tolerate increased ICP. Infants and babies with open sutures will tolerate an increased ICP better than an adult since the skull is not a closed vault. In adults with a thick solid skull the compartment does not expand. It is finite in volume and thus will not tolerate the increased ICP as well. This may preclude to early decompressive craniectomy in the older child and adult since they are less tolerant of an elevated ICP than an infant. Mortality from elevated ICP is lower in children in comparison to adults since they are less likely to herniate with increased ICP. However, neuropsychological sequelae are more common, affecting memory, cognition, communication skills, and psychosocial adaptation. According to the guidelines for the management of severe TBI, intracranial hypertension and poor outcome were correlated in several studies.[4]
Physiology
The Monroe–Kellie doctrine explains the principles of normal ICP. The cranial vault or intracranial compartment consists of three components, brain, cerebral blood volume (CBV), and cerebral spinal volume. Normal ICP (0-15) is comprised of 80% brain, 10% CBV, and 10% cerebral spinal fluid (CSF) volume. However, when insult occurs, compensatory mechanisms take place, first CSF is displaced, and second, hyperventilation may occur leading to vasoconstriction allowing blood displacement to take place in an effort to maintain normal ICP. The brain possesses a mechanism known as autoregulation, which allow constant blood flow in response to fluctuations in mean arterial pressure (MAP). Cerebral blood flow (CBF) is maintained at a MAP of 60-150 mmHg, and cerebral vasculature is either constricted or dilated through cerebral resistance depending on MAP. This is part of the autoregulatory mechanism, however, many other factors are known to affect this mechanism when MAP exceeds these ranges.
Since the brain has a limited ability to store energy, it depends on aerobic metabolism.[26] Cerebral metabolism is closely linked to CBF, which is suspected to involve vasodilators, adenosine, and free radicals. Seizures and fever, which are commonly seen in TBI patients, are also known to increase metabolic activity, which leads to an increase in CBF. The partial pressure of oxygen and carbon dioxide acts on the vascular smooth muscle causing vasodilatation and constriction even when autoregulation is lost. These mechanisms occur in order to maintain normal pressures within the cranial vault. When these mechanisms can no longer maintain normal ICP secondary injury ensues. It should be noted that CBF and CBV are not interchangeable. CBV which represents the volume of blood in the brain vasculature is the major contributor to the ICP. Cerebral edema causes an elevated ICP, which results in a lower cerebral perfusion pressure (CPP). Cerebral edema alters normal blood flow and vessel caliber, thus decreasing oxygen and essential nutrients to the brain. CBF is decreased when CBV is increased and the pressure gradient across the compartment is decreased.
Pathology
Primary trauma occurs at the time of impact either from the brain hitting against the facial bones, or the skull or some foreign object penetrating the brain. This is referred to as closed and open head injuries, respectively. Once the primary injury is sustained, a secondary brain injury may develop hours to days in response to a complex cascade of systemic and intracranial events that occurred from the initial injury.[5] Secondary brain injury is a complication of the initial trauma due to hypotension, alterations in CBF, inflammation, and cellular metabolism, which leads to ischemia and hypoxia, resulting in more neuronal damage and cell death.[18] In some studies, brain hypoxia, defined as the partial pressure of oxygen in brain tissue (Pbto2) <15 mmHg, has been identified as being the major cause of secondary cerebral damage after a severe head injury irrespective of ICP, CPP, and injury severity score.[22]
There are three types of primary cranial injuries: Skull fractures, focal brain injuries, and diffuse brain injuries. Skull fractures can be linear, depressed, or basilar. Focal brain injuries can consist of contusions or hematomas/hemorrhages. Diffuse brain injuries can be concussion, diffuse axonal injury (DAI), diffuse brain edema, or diffuse hypoxic/ischemic injury. Linear skull fractures heal well in both children and adults and rarely require surgical intervention. Skull fracture with depression greater than the thickness of the skull may require surgical intervention. Basilar skull fractures are most commonly seen at the temporal and orbital surface of the frontal bone and can lead to CSF otorrhea or rhinorrhea, respectively.
All focal brain injuries are well identified radiographically. This includes contusions caused by coup or contrecoup mechanisms. This is essentially bruising of the brain parenchyma itself. Hematomas can also be focal, this includes intraparenchymal hemorrhage (IPH), subarachnoid hemorrhage (SAH), SDH, and EDH. IPH is the propagation of a cerebral contusion that bleeds and turns into a cavity within the brain tissue filled with blood. SAH is a light coating of blood around the sulci and fissures of the outer edge of the brain. SDH is caused by the tearing of bridging veins usually outside the arachnoid membrane and accumulates blood outside the brain. EDH, which is commonly caused by the tearing of the middle meningeal artery, is blood accumulating outside of the dura. Diffuse brain injuries include concussion which is typically a jarring of brain cells or axons that is typically temporary and is rarely seen on imaging. DAI falls in the same scale of injury, but it is due to acceleration/deceleration forces that affect only white matter causing focal petechial contusions to various areas of the white matter. Often DAI cannot be detected on CT or magnetic resonance imaging (MRI), and diagnosis must be made based on clinical examination. Diffuse brain edema is rare but more common in children. This is essentially a diffuse reaction of brain cells (usually white/and gray matter) in response directly to the trauma. Hypoxic/ischemic injury of the brain after trauma is due to lack of oxygen, blood, or other important nutrients to brain cells for a critical but temporary period of time. This may appear similar to diffuse brain edema on imaging but is a separate etiology physiologically.
Management
Moderate to severe head injuries are admitted to the hospital, however, concussion in children is common and may be monitored at home. Toddlers usually sustain bumps and bruises to their heads from an array of mechanisms ranging from falls off the bed, countertops, stairs, etc., to direct impact from child abuse and motor vehicle crashes. Sometimes it is difficult to assess a younger child for a concussion because younger children may not be able to express feelings of nausea or headache. Acute signs and symptoms of a concussion include: Restlessness or irritability, crying with inability to console, and vomiting. Latter signs may include change in sleep patterns and poor attention span, which require further evaluation form either a neuropsychologist or other specialists. When in doubt, a child should always be referred to the emergency room for evaluation. Regardless of the type of injury once the patient is stable, the CT scan of the brain is the gold standard to evaluate head injury along with an hourly neurological assessment.
Severe head injuries will require assessment and treatment of airway, breathing, and circulation. Once these are identified and stabilized, and the CT is positive, many direct measures for monitoring the brain can be utilized. Both Pbto2 and ICP are frequently monitored at the bedside in many pediatric neuro trauma centers. The Licox monitor is a parenchymal brain probe that can measure local tissue oxygenation and local tissue temperature but does not remove CSF. The Camino ventriculostomy is a ventricular monitor that measures deep ICP and simultaneously can be used to remove CSF to treat elevated ICP, which is not available by the Licox. Decompressive craniectomy refers to the surgical removal of the skull in order to allow a greater volume within the intracranial compartment. The aim of a decompressive craniectomy is to lower the ICP by allowing more room for the brain to swell. Generally, most of the decompressive craniectomy types are dependent on the pathology, usually occurring unilaterally, on the same side of the pathologic lesion: SDH, EDH, cerebral contusion, depressed skull fracture, etc., It has also been documented that bifrontal decompressions can be used if the pathology is diffuse or bifrontal in nature. Another type of decompression that is rarely used in the traumatic situation is the suboccipital decompression. The use of the procedure has recently come under controversy due to the recent study, Decompressive Craniectomy in Patients with Severe Traumatic Brain Injury (DECRA) published in the New England Journal of Medicine.[17] DECRA was the first study to examine the use of decompressive craniectomy in a prospective, randomized, multi-center trial. Many criticisms of the study realized its limits, and conclusions should not be made from the study. There are many retrospective papers that demonstrate the benefit from decompressive craniectomies in the appropriate situation.[1,7,10,12,15,16,17,23,24,25,27,28] The upcoming randomized evaluation of surgery with craniectomy for uncontrollable elevation of ICP (Rescue ICP) trial, which is still recruiting subjects, will hopefully shed more light on the controversy.[13] SMMC and the conjoined PBCH is a Level II trauma center located in West Palm Beach, Florida. It is also a state designated Pediatric Trauma Center as well as a Brain and Spinal Cord Acute Care Center. At SMMC/PBCH, every TBI pediatric patient with a GCS < 8 is assessed rapidly via a neurosurgeon after the CT of the head is obtained. As per the pediatric head injury guidelines from 2012, ICP monitoring for patients with GCS < 8 is an option.[19] In addition, as per the pediatric head injury guidelines from 2012, “decompressive craniectomies with duraplasty, leaving the bone flap out, may be considered for pediatric patients with traumatic brain injury who were showing early signs of neurologic deterioration or herniation or are developing intracranial hypertension refractory to medical management during the early stages of treatment.”[19] According to these guidelines, ICP monitoring and decompressive craniectomies are listed as Options (level 3 evidence) not Recommendation (level 2 evidence), and not Guidelines (level 1 evidence). Small sample sizes of pediatric patients treated with decompressive craniectomy are seen throughout the literature with limited data and strength. The neurosurgeon on call decides whether to insert an ICP parenchymal monitor or a ventriculostomy drain with an ICP monitor. Recent studies have shown that patients being treated with ICP monitoring have a lower mortality rate than those patients without an ICP monitor in place.[6] If the ICP is greater than 20 torr for more than 10 minutes, protocol is established for maximizing medical management of ICP including sedation such as lorazepam or midazolam, analgesics such as fentanyl or morphine, paralytics such as vecuronium, and diuretics such as mannitol. Additional medications used to treat elevated ICP beyond positioning techniques include 3% sodium chloride drips or boluses. If this does not work to reduce ICP, protocol dictates pentobarbital coma with continuous EEG until burst suppression is achieved. The discussion regarding decompressive craniectomy can vary at any point along this algorithm. Usually it is completed when all medical management is exhausted or expectations are that medical management will be futile. The ICP monitoring through a ventriculostomy device can not only predict worsening intracranial pathology by calculating CPP, but can also be used therapeutically to drain CSF. The ICP monitor not only plays a significant role in managing cerebral perfusion but also aids in determining the need for decompressive craniectomy in the pediatric patient.
At SMMC/PBCH, the intracranial monitor and ventriculostomy drain is handled with strict aseptic technique. The dressing is changed every 48 hours with Bacitracin ointment and a dry dressing. Any evidence of infection at the site or blood or cloudy drainage is reported to the physician. Every 5 days a 3-5 cc specimen of CSF is obtained. The sample is obtained from the port closest to the ventriculostomy insertion site (proximal port), and is obtained by spontaneous or dependent drainage only, never aspirated. The sample is sent to the laboratory for cell count with differential, glucose, protein, gram stain, culture and sensitivity, and fungal smear and culture. Hourly readings are recorded when the port remains closed for 5 minutes for accuracy, otherwise the port may remain open for CSF drainage if ICP is greater than 20 torr for more than 15 minutes.
The goal of TBI management is to minimize secondary injury by controlling ICP, hypoxia, and hypotension. Reducing the volume of CSF is a valuable treatment aimed at decreasing an elevated ICP. Supporting the arterial pressure, and decreasing the ICP, lends to a suitable CPP. Maintaining the CPP is an essential constituent for obtaining adequate oxygen delivery and CBF. The brain, because of its minimal ability to store energy must maintain adequate blood flow. Many treatment modalities are aimed at an attempt to decrease ICP, increase CPP, and increase PbtO2, in order to reduce the effects of secondary brain injury, and increase survival. However, when all these modalities fail, a decompressive craniectomy may be indicated and necessary to save a life. At SMMC/PBCH there have been remarkable outcomes in pediatric patients who were treated with decompressive craniectomies.
Pain, fever, agitation, or seizures can increase ICP. To combat these potential issues, analgesics, sedation, and antiepileptic medications are used accordingly. If these treatment modalities are not effective to reduce ICP, or maintain CPP, then neuromuscular blockade or drug-induced coma may be considered. If neuromuscular blockade is used, sedation must be continuous and the patient is monitored carefully. When the ICP remains > 20 torr despite neuromuscular blockade, a pentobarbital coma induction is considered. When a pentobarbital coma is initiated at SMMC/PBCH, the use of mannitol, furosemide, morphine sulfate, and midazolam are discontinued. If seizures should develop then lorazepam or other anticonvulsant is provided until the seizure stops. Administration of packed red blood cells if hemoglobin is less than 10 g/dl, suctioning or adding PEEP if pO2 continues to fall, and maintaining the PCO2 between 33 and 37 torr by adjusting the ventilator rate and or tidal volume is considered. When the CPP is less than 50 torr, a norepinephrine, dopamine, epinephrine, or vasopressin drip is used to maintain the CPP 50-70 torr. A repeat CT scan of the brain is considered with any change or deterioration in the patient condition. The neurosurgeon may consider a decompressive craniectomy for refractory intracranial hypertension nonresponsive to medical intervention or any increased space occupying lesion.
At SMMC/PBCH, the clinical procedures and guidelines for ventriculostomy and ICP monitors are listed below. All items are individualized for each patient's needs and at the discretion of the neurosurgeon.
Insert arterial line
Optimize head elevation 30-45 degrees while keeping the head in a neutral position.
Measure MAP, ICP, and CPP hourly (CPP=MAP-ICP)
Notify MD for CPP<60 torr for a child and<45 torr for an infant
Notify MD for ICP>20 torr for more than 15 minutes despite sedation
Sedation: (Versed) midazolam, morphine sulfate, or fentanyl
Neuromuscular blockade if sedation not effective
Mannitol, 3% saline, monitor BMP every 6 hours, notify MD for serum osmolality>310 or abnormal electrolytes
Pentobarbital coma evaluation
(Ativan) lorazepam for seizures
Maintain normothermia using tylenol, cool sponge bath or a cooling blanket and notify MD if any shivering develops
Packed red blood cells for Hgh <10
Ventilator settings as needed
Licox monitor
Decompressive craniectomy.
Case series
SMMC/PBCH treated 379 pediatric patients with a diagnosis of TBI from January 1, 2009 to July 31, 2012, a 3 year 7 month period. All of these patients were treated according to the severity of their injury. A total of 49 pediatric patients required neurosurgical intervention and 7 of these patients met the criteria for a decompressive craniectomy. The mechanism of injury included: Jumping on the bed and hitting his head on a dresser, falling out of the back of a truck, motor vehicle collision with ejection, falling out of a golf cart, school bus accident, pedestrian versus car, and car surfing. For the purpose of this study only two types of craniectomies were used, left frontal/temporal/parietal craniectomy and a bilateral frontal/temporal/parietal craniectomy, refer to “Table 1: Pediatric Patient Summary Chart.” Their GCS scores ranged from 3 to 13 on admission. Five of these patients had a decompressive craniectomy on admission. These five patients, (A, C, D, E, and F) were treated with either evacuation of a SDH or an EDH and the neurosurgeon was unable to replace the cranium secondary to cerebral edema. In addition to the craniectomy, these five patients also had a ventriculostomy drain and ICP monitor inserted at the time of surgery therefore the opening ICP reading was near or within normal range due to the fact that the cranium was open. Two patients, (B and G) were treated with a decompressive craniectomy the day after admission. Both had an ICP monitor with the ventriculostomy drain inserted on admission at the bedside, patient B had an opening pressure of 27 torr, who presented with a left frontal hemorrhagic contusion with acute SDH, and patient G had an opening pressure of 8 torr, who sustained a small right frontotemporal subdural with right frontal lobe contusion and intraparenchymal hemorrhage. Irrespective of the opening pressure and despite maximal medical management, the ICP on both patients progressed malignantly high and the decision was made to perform a decompressive craniectomy to control the ICP and cerebral edema. Two of the seven patients (E and G) had a bifrontal, temporal, parietal craniectomy, while the other five patients had a left frontal, temporal, parietal craniectomy. All seven patients survived the injury and upon discharge from the hospital were able to return home.
Table 1.
Pediatric patient summary chart
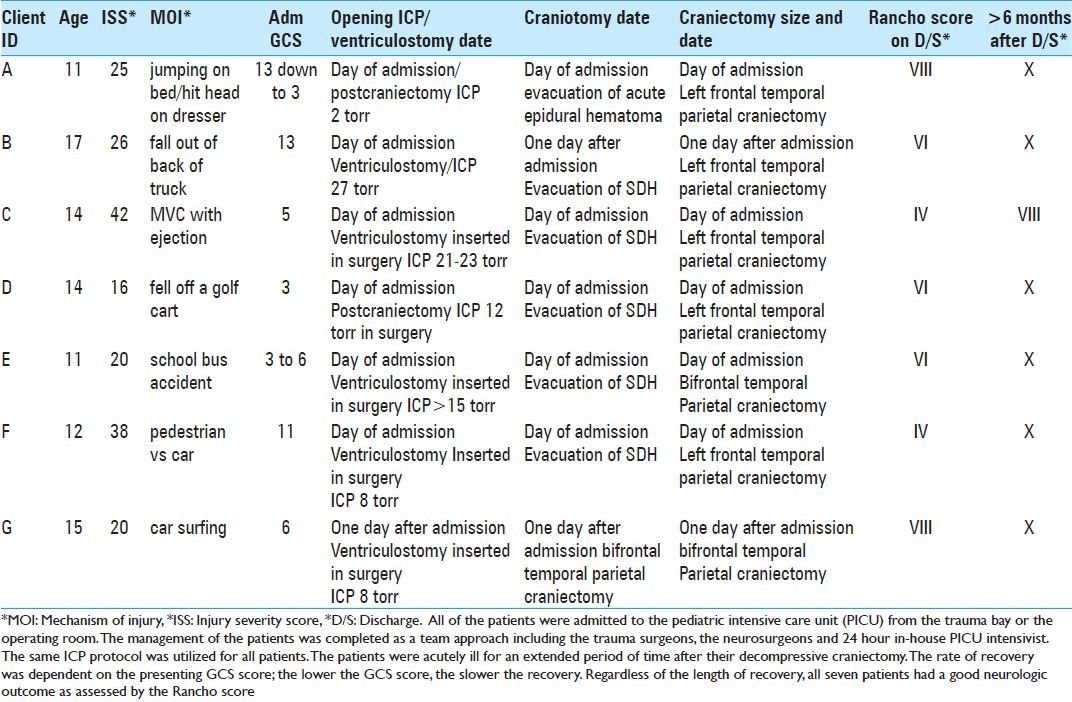
The Rancho Levels of Cognitive Functioning Scale “Table 2: Rancho Los Amigos Score” was used to determine outcome on discharge and at least 6 months after discharge. The neurosurgeon and the neuro nurse specialist evaluated the patients upon discharge and recorded the score. Once the patient was discharged, the patients followed up in the neurosurgeon's office for further cognitive functioning evaluation and discussion regarding reconstructive surgery. Typically at SMMC/PBCH, reconstructive cranioplasty is considered after 3-6 months depending on the patient's condition and the neurosurgeon's discretion. The neuro nurse specialist reviewed the notes and the Rancho score was determined. All of the patients in this study had their Rancho score determined in this manner except for one. This patient, identified as Patient C was injured in a motor vehicle accident and ejected. Patient C lived out of town and was unable to commute back to the area for follow up with the neurosurgeon affiliated with SMMC/PBCH trauma service. However, a long distant phone interview with the social worker over seeing his case was conducted by the neuro nurse specialist. After phone conversation and review of plan of care from office notes and the social worker, it was determined there were no physical deficits; however, the patient still required speech therapy for memory and cognitive deficits and a Rancho Score of VIII was determined. The patient's level of cognitive functioning is listed below. Refer to “Table 1: Pediatric Patient Summary Chart” and “Table 2: Rancho Los Amigos Score.”
Table 2.
Rancho los amigos score

All of the patients were admitted to the pediatric intensive care unit (PICU) from the trauma bay or the operating room. The management of the patients was completed as a team approach including the trauma surgeons, the neurosurgeons and 24 hour in-house PICU intensivist. The same ICP protocol was utilized for all patients. The patients were acutely ill for an extended period of time after their decompressive craniectomy. The rate of recovery was dependent on the presenting GCS score; the lower the GCS score, the slower the recovery. Regardless of the length of recovery, all seven patients had a good neurologic outcome as assessed by the Rancho score.
DISCUSSION
Pediatric patients who experience severe TBI require a quick and reliable method to reduce intracranial hypertension. Decompressive craniectomy is a surgical procedure in which part of the skull is removed in order to facilitate brain swelling and prevent brain compression and possible herniation. There are different surgical techniques involved when a decompressive craniectomy is used; however, most of the literature supports a large decompressive craniectomy technique.[12] Removing a large portion of the skull, for example, fronto-temporoparietal unilaterally or bilaterally allows for maximum swelling of the brain to occur. The large decompressive craniectomy technique can be used as an alternative to maximal conservative medical therapy instead of a last resort. In fact, a randomized study was done with two pediatric groups, one group was treated medically, while the other group had very early decompressive craniectomy; the results revealed that 14% of the medically treated patients versus 54% of the surgically treated patients had favorable outcomes.[28] Although the study at SMMM/PBCH is a case series versus a case controlled study, the results support that the pediatric patients treated with a decompressive craniectomy had favorable outcomes. According to the literature, a lack of conclusive evidence still prevails as to the efficacy of decompressive craniectomy in regard to patient outcome.[12] The literature supports that the pendulum has swung back toward decompressive craniectomy as an acceptable first line treatment as opposed to treating with maximal medical therapy.[28] Future research in the treatment, timing, short-term and long-term outcome, and risk of decompressive craniectomy would be a key issue for future investigation.
CONCLUSION
From the case series presented, one can conclude that early surgical intervention may offer the potential for excellent functional recovery. It is difficult to conclude, if these patients were treated with only maximal medical management for elevated ICP and not a craniectomy, would the results been the same? In addition, if these pediatric patients were not treated surgically at that time perhaps herniation and brain death would have occurred. As mentioned previously, the study by Taylor et al. was a randomized study done with two pediatric groups, one group was treated medically, while the other group had very early decompressive craniectomy, the results revealed that 54% of the surgically treated patients and only 14% of the medically treated patients had favorable outcomes.[28] Another study by Rutigliano et al. had 6 out of the 30 patients who were treated with decompressive craniectomies and demonstrated survival.[25] Figaji et al. had a similar case series with a sample size of five patients who all had favorable outcomes.[7] It is clear from these studies that surgical treatment for these patients should be considered an option.[7] Based on these studies and our case series, the positive outcomes of these young patients warrant the aggressive intervention of a decompressive craniectomy. Unfortunately all of these studies lack prospective data and therefore treatment of TBI remains controversial. International multi-center studies, such as the Recue ICP and STITCH trials, are underway and undoubtedly will lend further insight into the controversial areas of decompressive craniectomy and surgical management of traumatic intracerebral hemorrhage, respectively. Both studies will shed insight into the management of TBI but not necessarily in the pediatric population. One would imagine the pediatric brain, due to its neuroplasticity would recover better and the outcomes would be impressive as our case series demonstrated.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/1/128/119055
Disclaimer: The authors of this article have no conflicts of interest to disclose, and have adhered to SNI's policies regarding human/animal rights, and informed consent. Advertisers in SNI did not ask for, nor did they receive access to this article prior to publication.
Contributor Information
Neil Patel, Email: Npatel777@gmail.com.
Michael West, Email: Cut2fly@bellsouth.net.
Joanie Wurster, Email: Joan.Wurster@tenethealth.com.
Cassie Tillman, Email: Cassie.Tillman@tenethealth.com.
REFERENCES
- 1.Adamo M, Drazin D, Waldman JB. Decompressive craniectomy and postoperative complication management in infants and toddlers with severe traumatic brain injuries. J Neurosurg Pediatr. 2009;3:334–9. doi: 10.3171/2008.12.PEDS08310. [DOI] [PubMed] [Google Scholar]
- 2.Allard RH, van Merkesteyn JP, Baart JA. Child Abuse. Ned Tijdschr Tandheelkd. 2009;116:186–91. [PubMed] [Google Scholar]
- 3.Brain Injury Association of America. 2011. [Last accessed on 2013 Apr 2]. Available from: http://www.biausa.org/brain-injury-children.htm .
- 4.Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS. Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, et al. Guidelines for the management of severe traumatic brain injury, X: Brain oxygen monitoring and thresholds. J Neurotrauma. 2007;24(Suppl 1):565–70. doi: 10.1089/neu.2007.9986. [DOI] [PubMed] [Google Scholar]
- 5.Dutton R, McCunn M. Traumatic brain injury. Curr Opin Crit Care. 2003;9:503–9. doi: 10.1097/00075198-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Farahvar A, Gerber LM, Chiu YL, Carney N, Härtl R, Ghajar J. Increased mortality in patients with severe traumatic brain injury treated without intracranial pressure monitoring. J Neurosurg. 2012;117:729–34. doi: 10.3171/2012.7.JNS111816. [DOI] [PubMed] [Google Scholar]
- 7.Figaji AA, Fieggen AG, Peter JC. Early decompressive craniotomy in children with severe traumatic brain injury. Childs Nerv Syst. 2003;19:666–73. doi: 10.1007/s00381-003-0804-3. [DOI] [PubMed] [Google Scholar]
- 8.García García JJ, Manrique Martínez I, Trenchs Sainz de la Maza V, Suárez Suárez A, Martín de la Rosa L, Travería Casanova FJ, et al. Registry of mild craniocerebral trauma: Multicentre study from the Spanish Association of Pediatric emergencies. An Pediatr (Barc) 2009;71:31–7. doi: 10.1016/j.anpedi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Hagen C, Malkmus D, Durham P. Communication Disorders Service, Rancho Los Amigos Hospital, 1972. Revised 11/15/74 by Malkmus D, M.A., and Stenderup K, O.T.R. Revised scale 1997 by Hagen C [Google Scholar]
- 10.Hejazi N, Witzmann A, Fae P. Unilateral decompressive craniectomy for children with severe brain injury. Report of seven cases and review of the relevant literature. Eur J Pediatr. 2002;161:99–104. doi: 10.1007/s00431-001-0864-x. [DOI] [PubMed] [Google Scholar]
- 11.Holmes JF, Palchak MJ, MacFarlane T, Kupperman N. Performance of the pediatric glasgow coma scale in children with blunt head trauma. Acad Emerg Med. 2005;12:814–9. doi: 10.1197/j.aem.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Hung X, Wen L. Technical considerations in decompressive craniectomy in the treatment of traumatic brain injury. Int J Med Serv. 2010;7:385–90. doi: 10.7150/ijms.7.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutchinson PJ, Corteen E, Czosnyka M, Mendelow AD, Menon DK, Mitchell P, et al. Decompressive craniectomy in traumatic brain injury: The randomized multicenter RESCUEicp study (www.RESCUEicp.com. ) Acta Neurochir Suppl. 2006;96:17–20. doi: 10.1007/3-211-30714-1_4. [DOI] [PubMed] [Google Scholar]
- 14.Iranmanesh F. Outcome of head trauma in children. Indian J Pediatr. 2009;76:929–31. doi: 10.1007/s12098-009-0143-9. [DOI] [PubMed] [Google Scholar]
- 15.Jagannathan J, Okonkwo DO, Dumont AS, Ahmed H, Bahari A, Prevedello DM, et al. Outcome following decompressive craniectomy in children with severe traumatic brain injury: A 10-year single-center experience with long-term follow up. J Neurosurg. 2007;106(Suppl 4):268–75. doi: 10.3171/ped.2007.106.4.268. [DOI] [PubMed] [Google Scholar]
- 16.Kan P, Amini A, Hansen K, White GL, Jr, Brockmeyer DL, Walker ML, et al. Outcomes after decompressive craniectomy for severe traumatic brain injury in children. J Neurosurg. 2006;105(Suppl 5):337–42. doi: 10.3171/ped.2006.105.5.337. [DOI] [PubMed] [Google Scholar]
- 17.Keen JR, Colohan AR. State of the art in the treatment of cerebral trauma: Part I and part II. Contemp Neurosurg. 2012;34:25–6. [Google Scholar]
- 18.Knapp JM. Hyperosmolar therapy in the treatment of severe head injury in children: Mannitol and hypertonic saline. AACN Clin Issues. 2005;16:199–211. doi: 10.1097/00044067-200504000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Kochanek PM, Carney N, Adelson PD, Ashwal S, Bell MJ, Bratton S, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, childre and adolescents – second edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 20.Mackerle A, Gal P. Unusual penetrating head injury in children: Personal experiences and review of the literature. Childs Nerv Syst. 2009;25:909–13. doi: 10.1007/s00381-009-0901-z. [DOI] [PubMed] [Google Scholar]
- 21.National Center for Health Statistics April 1. 2010. [Last accessed on 2013 Apr 16]. Available from: http://www.cdc.gov/traumaticbraininjury/tbi_report_to_congress.html .
- 22.Oddo M, Levine JM, Mackenzie L, Frangos S, Feihl F, Kasner SE, et al. Brain hypoxia is associated with short-term outcome after severe traumatic brain injury independently of intracranial hypertenson and low cerebral perfusion pressure. Neurosurgery. 2011;69:1037–45. doi: 10.1227/NEU.0b013e3182287ca7. [DOI] [PubMed] [Google Scholar]
- 23.Polin RS, Shaffrey ME, Bogaev CA, Tisdale N, Germanson T, Bocchicchio B, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84–92. doi: 10.1097/00006123-199707000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Ruf B, Heckmann M, Schroth I, Hugens-Penzel M, Reiss I, Borkhardt A, et al. Early decompressive craniectomy and duraplasty for refractory intracranial hypertension in children: Results of a pilot study. Crit Care. 2003;7:R133–8. doi: 10.1186/cc2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutigliano D, Egnor MR, Priebe CJ, McCormack JE, Strong N, Scriven RJ, et al. Decompressive Craniectomy in pediatric patients with traumatic brain injury with intractable elevated intracranial pressure. J Pediatr Surg. 2006;41:83–7. doi: 10.1016/j.jpedsurg.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Stock A. Corden TE, editor. Pediatric Head Trauma. Medscape drugs, disease and procedures. 2011. [Last accessed on 2013 Sep 9]. Available from: http://emedicine.medscape.com/article/907273overview .
- 27.Suarez PE, Serrano Gonzalez A, Perez Diaz C, Garcia Salido A, Martinez de Azagra Garde A, Casado Flores J. Decompressive craniectomy in 14 children with severe head injury: Clinical results with long-term follow-up and review of literature. J Trauma. 2011;71:133–40. doi: 10.1097/TA.0b013e318211071f. [DOI] [PubMed] [Google Scholar]
- 28.Taylor A, Butt W, Rosenfeld J, Shann F, Ditchfield M, Lewis E, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial pressure. Childs Nerv Syst. 2001;17:154–62. doi: 10.1007/s003810000410. [DOI] [PubMed] [Google Scholar]


