Abstract
Bozepinib [(RS)-2,6-dichloro-9-[1-(p-nitrobenzenesulfonyl)-1,2,3,5-tetrahydro-4,1-benzoxazepin-3-yl]-9H-purine] is a potent antitumor compound that is able to induce apoptosis in breast cancer cells. In the present study, we show that bozepinib also has antitumor activity in colon cancer cells, showing 50% inhibitory concentration (IC50) values lower than those described for breast cancer cells and suggesting great potential of this synthetic drug in the treatment of cancer. We identified that the double-stranded RNA-dependent protein kinase (PKR) is a target of bozepinib, being upregulated and activated by the drug. However, p53 was not affected by bozepinib, and was not necessary for induction of apoptosis in either breast or colon cancer cells. In addition, the efficacy of bozepinib was improved when combined with the interferon-alpha (IFNα) cytokine, which enhanced bozepinib-induced apoptosis with involvement of protein kinase PKR. Moreover, we report here, for the first time, that in combined therapy, IFNα induces a clear process of autophagosome formation, and prior treatment with chloroquine, an autophagy inhibitor, is able to significantly reduce IFNα/bozepinib-induced cell death. Finally, we observed that a minor population of caspase 3-deficient MCF-7 cells persisted during long-term treatment with lower doses of bozepinib and the bozepinib/IFNα combination. Curiously, this population showed β-galactosidase activity and a percentage of cells arrested in S phase, that was more evident in cells treated with the bozepinib/IFNα combination than in cells treated with bozepinib or IFNα alone. Considering the resistance of some cancer cells to conventional chemotherapy, combinations enhancing the diversity of the cell death outcome might succeed in delivering more effective and less toxic chemotherapy.
Keywords: seven-member heterocycles, purines, IC50, interferon cytokine, cell death, breast and colon cancer cells
Introduction
The mortality to incidence ratio in cancer patients is extremely high, positioning cancer as a major cause of death worldwide.1 Chemotherapy has a role as either strategic treatment for locally advanced disease or palliative treatment for metastatic tumors. However, clinical use of chemotherapy is still unsatisfactory due to limited response rates, a small survival benefit, and a poor prognosis. Therefore, more effective and safer anticancer drugs are urgently needed. In this sense, combination therapies that enhance efficacy or permit administration of reduced doses have been successfully used in a broad variety of therapeutic applications.
Previous studies have demonstrated the potent antiproliferative activity of pyrimidine and purine benzo-fused seven-membered O,N-acetals in human breast and colon cancer cell lines in the micromolar range.2–4 Bozepinib shows a 50% inhibitory concentration (IC50) of 0.166 μM against the MDA-MB-231 human breast adenocarcinoma cell line. Moreover, this compound is able to selectively induce high levels of apoptosis in tumor cells and shows no acute toxicity in mice.5
Many chemotherapeutic drugs eradicate cancer cells by inducing apoptosis, and regardless of their primary targets, many are similar in terms of the cellular response to the apoptosis induced.6 However, many tumors have a seriously compromised apoptosis pathway, and new drugs inducing other cytotoxic effects must be explored in order to evade chemoresistance. Therefore, although apoptosis has been considered as the typical mechanism for cell death, accumulating evidence suggests that alternative cell death pathways play a role in the tumor response to chemotherapy.7,8 A potential mechanism of caspase-independent cell death is autophagy, which is defined as controlled lysosomal degradation of macromolecules and organelles. Autophagy was initially identified as a cell survival mechanism to protect against nutrient deprivation;9 however, in certain conditions, it results in a form of cell death now described as type II programmed cell death, which is being targeted for novel therapeutic strategies in cancer.10
Senescence was first described as a state of irreversible growth arrest that normal human fibroblasts enter into at the end of their replicative lifespan.11 By restricting cell proliferation and thereby impeding the accumulation of mutations, senescence acts as an important tumor suppression mechanism. Further, senescence induced by aberrant activation of oncogenes, oxidative stress, or DNA damage prevents proliferation of cells at risk of malignant transformation. Therefore, senescence offers an attractive therapeutic option if it can be induced in tumor cells.12
Interferons (IFNs) are agents with antiviral, antiproliferative, and immunomodulatory properties. Interferon-alpha (IFNα), a pleiotropic cytokine that regulates more than 100 genes, is used in the treatment of hematologic malignancies and solid tumors.13,14 Although IFNs are effective as single agents in certain clinical pathologic entities, increasing experience with these cytokines suggests that their greatest therapeutic potential may be realized when they are used in combination with other biological response modifiers and cytotoxic or antiviral agents.15 The apoptosis event has been well characterized for several combinations with IFNα; however, other mechanisms involved in the antitumor effectiveness of such combinations have not been explored. In fact, numerous studies in vitro and in vivo, including clinical trials, have used different IFN combinations with favorable outcomes.16–19 The double-stranded RNA-dependent protein kinase (PKR), induced by IFN type I, was initially identified as an innate immune antiviral protein.20,21 Since then, PKR has been linked to normal cell growth and differentiation, inflammation, cytokine signaling, and apoptosis, and is involved in the antiviral and antitumor activity of IFNs cytokines.21 It has been recently suggested the major role of PKR in the induction of apoptosis by several chemotherapeutic drugs such as etoposide, doxorubicin and 5-fluorouracil,22–24 with a potential clinical use in the future.
The aims of this work were to investigate the mechanisms by which bozepinib induces apoptosis in breast and colon cancer cells and to explore the activation of the proapoptotic proteins, tumor suppressor p53 and IFN-induced kinase PKR. In order to improve the antitumor efficacy of bozepinib, we analyzed the synergistic effect of a bozepinib/IFNα combination in breast and colon cancer cells and explored the mechanisms involved in the effectiveness of this combination. Our results show that PKR, but not p53, is involved in the apoptosis induced by bozepinib used alone and in combination with IFNα. In addition, we determined that bozepinib is able to induce other antitumor effects, including senescence and autophagy, which are strongly improved by using the IFNα combination.
Materials and methods
Cells and reagents
A human breast MCF-7 cell line (ECACC: 86012803) and human colon cancer RKO (ATCC: CRL-2577) and HCT-116 (ECACC: 91091005) cell lines were provided by the Cell Bank of the University of Granada (Granada, Spain). PKR+/+ and PKR−/− mouse embryonic fibroblasts25 and human colon HCT-116 p53+/+ and p53−/− cells26 were kindly provided by M Esteban (National Center of Biotechnology, Madrid, Spain) and B Vogelstein (Johns Hopkins Oncology Center, Baltimore, MD, USA), respectively. PKR was knocked down by RNA interference using shRNA-PKR as described previously.24 The cells were maintained in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 1% nonessential amino acid solution. Exponentially growing cells were used for all experiments. Bozepinib (Figure 1A) was synthesized as previously described,4 dissolved in dimethyl sulfoxide, and stored at −20°C. For each experiment, the stock solutions were further diluted in medium to obtain the desired concentrations. Human IFNα2b (Intron A®) was obtained from Schering-Plough (Union, NJ, USA) and mouse IFNα was sourced from Peprotech (Rocky Hill, NJ, USA). Z-VAD-FMK, a pan-caspase inhibitor, was provided by Santa Cruz Biotechnology (Santa Cruz, CA, USA) and chloroquine was obtained from Sigma-Aldrich (St Louis, MO, USA).
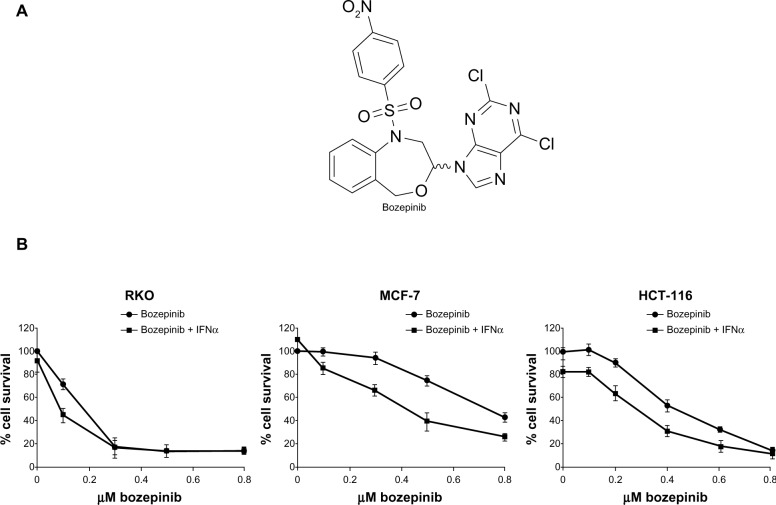
Figure 1.
Cytotoxic effect of bozepinib and combined bozepinib/IFNα therapy. (A) Chemical structure of bozepinib. (B) MCF-7, HCT-116, and RKO cell lines treated with increasing amounts of bozepinib alone (circle) or in combination with 50 IU/mL IFNα (square) for 6 days as described in the Materials and methods section. Cell lines have been defined in material and methods section. The curve for cell survival is represented as a percentage compared to mock-treated cells. Values shown represent the mean of triplicate determinations calculated from a single experiment. Experiments were repeated at least three times.
Abbreviation: IFNα, interferon-alpha.
Cell survival assay
The effect of bozepinib on cell viability was assessed using the sulforhodamine-B colorimetric assay. Aliquots of cell suspension (5 × 103 cells/well) were seeded onto 12-well plates and incubated for 24 hours. The cells were then treated with different concentrations of bozepinib in culture medium. Three days later, the wells were aspirated, fresh medium and treatment was added, and the cells were maintained for a further 3 days. Thereafter, the cells were processed as previously described,27 using a Titertek Multiscan apparatus (Flow Laboratories, Irvine, UK) at 492 nm. We evaluated the linearity of the sulforhodamine-B assay with the cell number for each cell stock before each cell growth experiment. The IC50 values were calculated by linear interpolation from semilogarithmic dose-response curves. To analyze the synergistic effect of addition of IFNα, cell viability was assayed as described above, treating cells with different concentrations of bozepinib in combination with IFNα (50 IU/mL). All experiments were plated in triplicate wells and carried out at least twice.
Apoptosis analysis
Cells were plated in six-well plates and maintained in an incubator overnight. The cells were then treated for 48 hours with bozepinib alone or in combination with IFNα (500 IU/mL). IFNα was added 8 hours before treatment with bozepinib. After 48 hours, the cells were trypsinized and analyzed using an Annexin V-fluorescein isothiocyanate detection kit (eBioscience Inc., San Diego, CA, USA). The samples were immediately processed using a FACSAria III flow cytometer (Becton Dickinson, BD Biosciences, Franklin Lakes, NJ, USA) from the service of the Scientific Instrumental Center (University of Granada).
Cell viability assay based on the metabolic cell activity
Cells in the exponential growth phase were plated on 96-well plates (5 × 103 cells/well) and maintained in the incubator overnight. On the following day, the cells were treated with dimethyl sulfoxide (control), 5 μM bozepinib, 500 IU/mL IFNα, or both concentrations of the bozepinib/IFNα combination. The cells were treated, or not, 2 hours before with 25 μM of the pan-caspase inhibitor Z-VAD-FMK or 20 μM chloroquine. After 48 hours, cell viability was measured using a sensitive colorimetric assay, ie, the Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan), following the manufacturer’s instructions. The Cell Counting Kit-8 is based on use of tetrazolium salt that is reduced to formazan dye in the presence of living cells. The microplate was read using a 450 nm filter.
Cell cycle analysis
Cells in the exponential growth phase were plated on six-well plates (5 × 104 cells/well) and maintained in the incubator overnight. On the following day, the cells were treated with dimethyl sulfoxide (control), 5 µM bozepinib, 500 IU/mL IFNα, or with both concentrations of the bozepinib/IFNα combination for 7 days. The cells were harvested, washed twice with phosphate-buffered saline, and fixed in 70% (vol/vol) cold ethanol for up to 1 week. Next, the cells were centrifuged, and the pellet was washed once with phosphate-buffered saline and resuspended in 250 μL of propidium iodide solution (100 μL/mL RNAse, 40 μL/mL propidium iodide in phosphate-buffered saline) for 30 minutes in the dark at 37°C. The samples were immediately analyzed using a FACScan flow cytometer from the Scientific Instrumental Centre (University of Granada).
Western blot analysis
The cells were plated on six-well plates in their respective medium. After treatment, the medium was removed and the cells were lysed in Laemmli buffer. The protein sample was subjected to electrophoresis, transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA, USA), and blocked in phosphate-buffered saline containing 5% nonfat dry milk for 1 hour at room temperature. Primary antibodies used included a polyclonal antibody to total human PKR (Santa Cruz Biotechnology), a polyclonal antibody to phospho-PKR (Thr 451, Sigma-Aldrich), a polyclonal antibody to phospho-eIF2α (Ser 51, Invitrogen, Carlsbad, CA, USA), a polyclonal antibody to phospho-p53 (Ser 15, 92845, Cell Signaling Technology, Beverly, MA, USA), and a monoclonal antibody to β-actin (Sigma-Aldrich, A2228). Secondary antibodies used included anti-rabbit immunoglobulin (Ig)G peroxidase conjugate (Sigma-Aldrich, A0545) and anti-mouse IgG peroxidase conjugate (Sigma-Aldrich, A9044). Bands were visualized using an enhanced chemiluminescent system (Amersham Pharmacia Biotech, Little Chalfont, UK) and a Kodak detector image.
Autophagy-related assay
The cells were plated on cover slips and transfected with pCMV-GFP-LC3 plasmid and pCMV-GFP control plasmid using Lipofectamine™ 2000 (Invitrogen). At 24 hours post-transfection, the cells were mock-treated (using a similar volume of dimethyl sulfoxide) or treated with 5 µM bozepinib, 500 IU/mL IFNα, or both concentrations of the bozepinib/IFNα combination over 48 hours. After treatment, the cells were washed with phosphate-buffered saline and fixed with 4% paraformaldehyde. Images were obtained using a Radiance 2100 confocal laser microscope (Bio-Rad).
Transmission electron microscopy
The cells were mock-treated (using a similar volume of dimethyl sulfoxide) or treated with 5 μM bozepinib, 500 IU/mL IFNα, or both concentrations of the bozepinib/IFNα combination over 48 hours. After treatment, the cells were washed three times with phosphate-buffered saline and then fixed with 0.5 mL of ice-cold glutaraldehyde (2.5% in 0.1 mol/L cacodylate buffer, pH 7.4) at 4ºC overnight. After washing, the cells were fixed in 1% OsO4 and embedded in Poly/Bed® resin (Polysciences Inc., Warrington, PA, USA). The ultrathin sections were doubly stained with uranyl acetate and lead citrate and analyzed by high resolution transmission electron microscopy (CM20; Philips, Eindhoven, the Netherlands).
Beta-galactosidase staining
Cells were plated on six-well plates in their respective medium. After 7 days of treatment, the medium was removed and the cells were fixed and stained using the Senescence β-Galactosidase Staining Kit (Cell Signaling Technology) according to the manufacturer’s protocol following overnight incubation at 37°C in pH 6.0 buffer. Senescent cells were observed by positive staining of a blue color and were photographed under a 10 × objective.
Statistical analysis
All data are presented as the mean ± standard deviation. Differences between groups were analyzed for statistical significance using the two-tailed Student’s t-test. P<0.05 was accepted as the statistical significance level.
Results
Interferon enhances cytotoxicity of bozepinib in colon and breast cancer cells by increasing apoptotic cell death
We have previously described the antitumor effect of bozepinib in an MCF-7 breast cancer cell line,4,5 and the antitumor and antiproliferative effects of IFNα are well characterized in cancer cells.21,24 In order to analyze if bozepinib also has a cytotoxic effect on colon cancer cells, we determined the IC50 values in several cancer cell lines. HCT-116 and RKO colon cancer cell lines were more sensitive to the cytotoxic effect of bozepinib, showing lower IC50 values than the MCF-7 breast cancer cell line (Table 1). Moreover, we investigated whether addition of a low dose (50 IU/mL) of IFNα was able to improve the cytotoxic effect of bozepinib. This low dose by itself was not able to induce a significant antiproliferative effect in the RKO and MCF-7 cancer cell lines, but slightly affected the viability of HCT-116 cells (Figure 1B). However, both compounds synergistically induced death of the cancer cell lines analyzed (Figure 1B), and consequently, the IC50 for bozepinib was reduced when combined with IFNα (Table 1).
Table 1.
Antiproliferative effects of bozepinib and bozepinip + IFNα on several cell lines
| Cell line | IC50 (μM)a
|
|
|---|---|---|
| Bozepinib | Bozepinib + IFNα | |
| RKO | 0.13 ± 0.01 | 0.09 ± 0.01 |
| MCF-7 | 0.78 ± 0.06 | 0.44 ± 0.03 |
| HCT-116 | 0.48 ± 0.08 | 0.31 ± 0.02 |
| MEFsPKR+/+ | 1.12 ± 0.18 | 0.89 ± 0.08 |
| MEFsPKR−/− | 1.74 ± 0.28 | 1.77 ± 0.18 |
Notes:
All experiments were conducted in triplicate and gave similar results. The data are the mean ± standard error of the mean of three independent determinations. IC50 was determined after 6 days of treatment.
Abbreviations: IFNα, interferon-alpha; IC50, 50% inhibitory concentration.
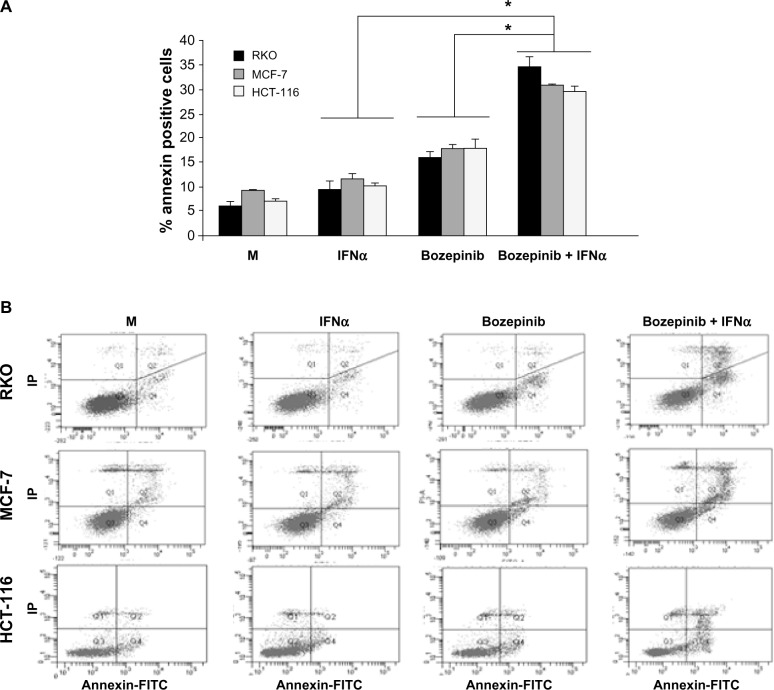
In order to determine if the effectiveness of the bozepinib/IFNα combination is due in part to an improvement in the apoptosis phenomenon, we treated MCF-7, HCT-116, and RKO cell lines with bozepinib alone or in combination with 500 IU/mL IFNα. As shown in Figure 2, the apoptosis induced by bozepinib at 48 hours was significantly increased when IFNα was added in all the cell lines analyzed (Figure 2).
Figure 2.
Apoptosis is enhanced by combination of bozepinib and IFNα. MCF-7, HCT-116, and RKO cell lines were mock-treated or treated with 5 µM bozepinib, 500 IU/mL IFNα, or the bozepinib/IFNα combination for 48 hours. Treated cells were then trypsinized and analyzed by flow cytometry using an Annexin V-fluorescein isothiocyanate detection kit. (A) Data are expressed as the mean ± standard error of the mean of three independent experiments. *P<0.05 (t-test). (B) Representative images from flow cytometry analysis.
Abbreviations: FITC, fluorescein isothiocyanate; IFNα, interferon-alpha; M, mock treated cells.
PKR but not p53 is involved in bozepinib-induced apoptosis and effectiveness of bozepinib/IFNα
We first analyzed PKR phosphorylation and its natural substrate, eIF2α, in the MCF-7 and HCT-116 cell lines. Treatment with bozepinib induced PKR and eIF2α phosphorylation in both tumor cell lines. Importantly, it was also observed that there was an increase in PKR levels after treatment with bozepinib that was more evident in the HCT-116 cell line, in which the basal PKR level (total and phosphorylated) was lower than that in MCF-7 cells. In contrast, levels of p53 and phospho-p53 were not affected during treatment with bozepinib in either cell line (Figure 3A).
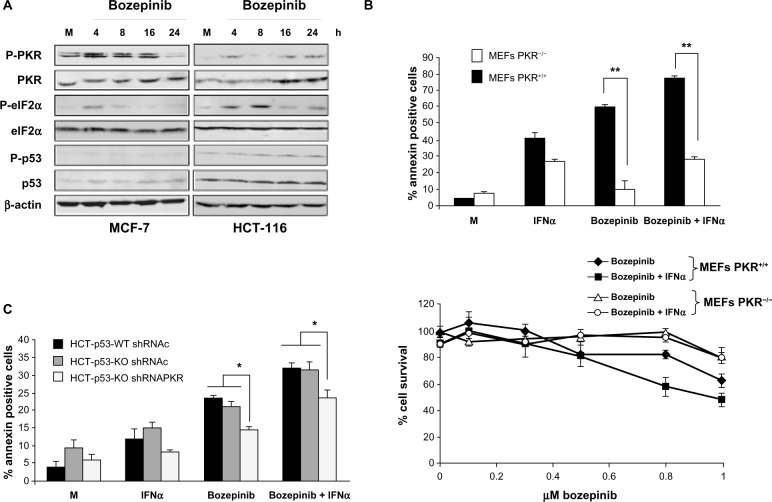
Figure 3.
PKR and p53 activation during bozepinib treatment and its involvement in apoptosis and cell viability on bozepinib, IFNα, and the bozepinib/IFNα combination. (A) MCF-7 and HCT-116 cell lines were mock-treated or treated with 5 μM bozepinib for 4, 8, 16, and 24 hours. Total proteins were extracted for immunoblot analysis using anti-phospho PKR, anti-whole PKR, anti-phospho eIF2α, anti-whole eIF2α, anti-phospho p53, anti-whole p53, and anti-β-actin antibodies. (B) PKR+/+ and PKR−/− mouse embryonic fibroblasts were mock-treated or treated with 2.5 μM bozepinib, 500 IU/mL IFNα, or the bozepinib/IFNα combination over 48 hours. **P<0.01, by t-test (upper panel). Subsequently, the cells were trypsinized and analyzed by flow cytometry for Annexin V positive determination. Cells were treated with increasing amounts of bozepinib alone or in combination with 50 IU/mL of mouse IFNα over 6 days as described in the Materials and methods section. The curve for cell survival was represented as the percentage compared to mock-treated cells. Values shown represent the mean of triplicate determinations calculated from a single experiment. Experiments were repeated at least three times (lower panel). (C) A wild-type HCT-116 p53 cell line and an HCT-116 p53 knockout cell line expressing short hairpin RNAs targeting PKR or expressing a control short hairpin RNA were mock-treated or treated with 5 μM bozepinib, 500 IU/mL human IFNα, or a combination of bozepinib/IFNα for 48 hours. *P<0.05 (t-test).
Abbreviations: IFNα, interferon-alpha; MEFs, mouse embryonic fibroblasts; shRNAc, control short hairpin RNA; PKR, RNA-dependent protein kinase; h, hours; M, mock treated cells; shRNAPKR, PKR short hairpin RNA.
Next, we analyzed the contribution of PKR and p53 to the apoptosis induced by bozepinib. Further, because PKR is an IFN-induced protein that is involved in most of the antiviral and antitumor effects of this cytokine,21 we also analyzed the effect of absence of PKR on the effectiveness of the bozepinib/IFNα combination. Bozepinib and IFNα were independently able to induce important levels of apoptosis in PKR+/+ mouse embryonic fibroblasts that was enhanced when the two compounds were combined. However, low levels of apoptosis were induced in the absence of the PKR protein in PKR−/− mouse embryonic fibroblasts, even when bozepinib and IFNα were combined (Figure 3B, upper panel).
To demonstrate the role of PKR in the cytotoxic effect of bozepinib and the bozepinib/IFNα combination, we also analyzed cell survival in PKR+/+ and PKR−/− mouse embryonic fibroblasts treated with increasing amounts of bozepinib, and the cytotoxic effect of bozepinib was found to be higher in PKR+/+ mouse embryonic fibroblasts in comparison with PKR−/− mouse embryonic fibroblasts (Figure 3B, lower panel, and Table 1). Further, our results showed that whereas viability was significantly reduced in PKR+/+ mouse embryonic fibroblasts when IFNα was added to bozepinib, cell viability was not affected by the bozepinib/IFNα combination in PKR−/− mouse embryonic fibroblasts.
Although p53 modification after treatment with bozepinib was not detected by Western blotting (Figure 3A), we went on to analyze the effect of bozepinib, IFNα, and the bozepinib/IFNα combination in the presence or absence of p53 protein in wild-type HCT-116 and HCT-116-p53 knockout cells (Figure 3C). Levels of apoptosis after the treatments were similar in both cell lines; however, when PKR was knocked down by RNA interference using shRNA-PKR, apoptosis was significantly reduced (Figure 3C). Taken together, these results suggest that, in part, PKR but not p53 contributes to the response of cancer cells to the bozepinib and bozepinib/IFNα combination.
Autophagy is involved in the synergistic effect of bozepinib/IFNα
We analyzed the ability of bozepinib to induce autophagy as well as regulation of this process by IFNα in the MCF-7 cell line, which is deficient in caspase 3 activation.28 Despite the low level of endogenous LC3 protein, LC3-II levels were weakly detected 48 hours after treatment with bozepinib and were more evident when IFNα was added (Figure 4A). Confocal microscopy was used to analyze the redistribution of LC3 protein into the autophagosomes of MCF-7 cells transfected with the pCMV-GFP-LC3 vector and the pCMV-GFP control vector, and the cells were then mock-treated or treated with bozepinib, IFNα, or the bozepinib/IFNα combination. As shown in Figure 4B, 48 hours post-treatment, the mock-treated cells displayed diffuse staining. However, a speckled fluorescent staining pattern was detected in almost all cells analyzed after treatment with bozepinib/IFNα, indicating redistribution of LC3 to autophagosomes. The speckled fluorescent stain was less pronounced after treatment with bozepinib alone or IFNα alone, and was detected in less than half of the cells analyzed (Figure 4B). Cells expressing the control vector pCMV-GFP displayed diffuse staining, even in the presence of treatment with bozepinib/IFNα (Figure 4B).
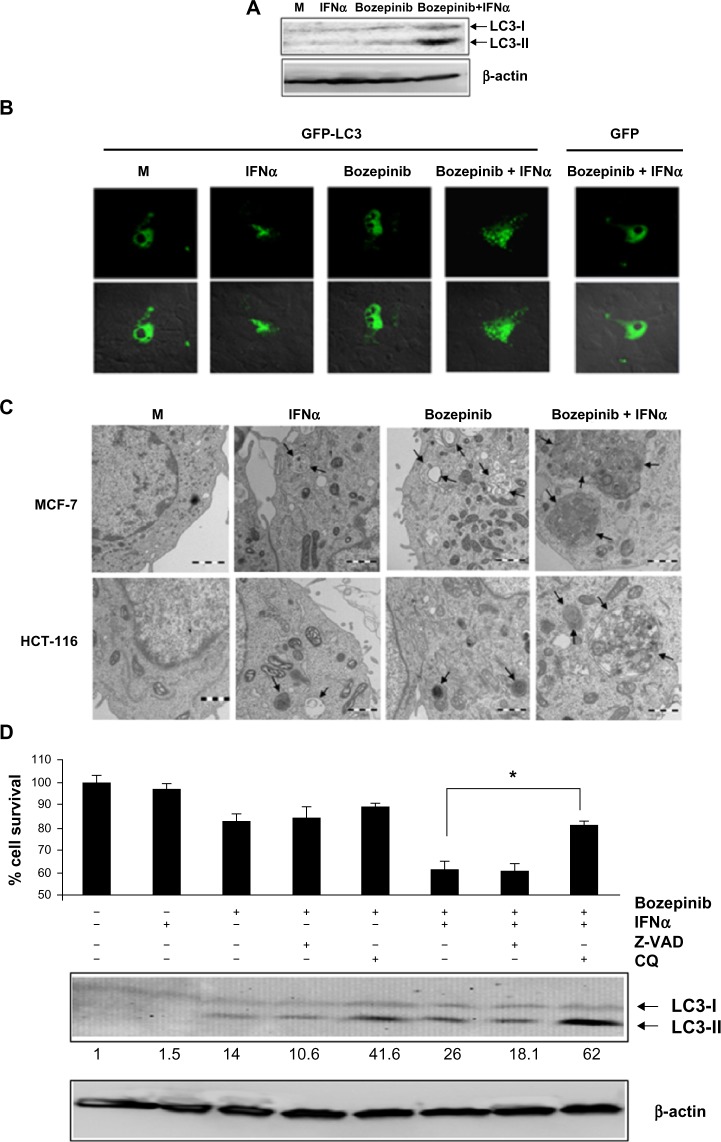
Figure 4.
Bozepinib induced LC3-autophagosome formation that was strongly enhanced when combined with IFNα. (A) MCF-7 cells were mock-treated or treated with 5 µM bozepinib, 500 IU/mL human IFNα, or a combination of bozepinib/IFNα for 48 hours. Total proteins were extracted for immunoblot analysis using anti-LC3 and anti-β-actin antibodies. (B) MCF-7 cells were plated on cover slips supported in six-well plates and transfected with 5 μg of GFP-LC3 or GFP-control plasmids as described in the Materials and methods section. After 24 hours, the cells were treated with 5 µM bozepinib, 500 IU/mL human IFNα, or a combination of bozepinib/IFNα for 48 hours. Cells were fixed and visualized using a Radiance 2000 confocal microscope. (C) MCF-7 and HCT-116 cells were mock-treated or treated with 5 µM bozepinib, 500 IU/mL human IFNα, or a combination of bozepinib/IFNα for 48 hours. Cells were fixed and prepared for visualization by transmission electron microscopy as described in the Materials and methods section. Transmission electron microscopy images show that the treated cells included typical autophagolysosomes (arrows) containing organelles and lamellar structures. (D) MCF-7 cells were treated with 20 μM of chloroquine or 25 μM of Z-VAD inhibitors 2 hours before 5 μM bozepinib, 500 IU/mL IFNα, or a combination of bozepinib/IFNα. After 48 hours, the cells were treated with a Cell Counting Kit-8, measured at 450 nm optical density and represented as described in the Materials and methods section. Total proteins were extracted for immunoblot analysis using anti-LC3 and anti-β-actin antibodies. *P<0.05 (t-test). Western blot signals were quantified using Image J software, and relative β-actin-normalized values were assigned in reference to nontreated cells (value 1).
Abbreviations: CQ, chloroquine; IFNα, interferon-alpha; M, mock treated cells.
Moreover, we analyzed cell morphology using transmission electron microscopy 48 hours post-treatment. The most spectacular morphologic effects were observed when IFNα was combined with bozepinib. As shown in Figure 4C, autophagic vacuoles surrounded by a double-layered membrane and containing cytoplasmic constituents were observed after treatment with bozepinib/IFNα. Similar to the MCF-7 cell line, colon cancer HCT-116 cells showed autophagic vacuoles after the treatments, and this was also observed in the transmission electron microscopy images (Figure 4C).
In order to analyze involvement of the autophagy process induced by the bozepinib/IFNα combination in cell viability, MCF-7 cells were treated with the autophagy inhibitor chloroquine and the caspase-3 inhibitor Z-VAD 2 hours before treatment with bozepinib, IFNα, or bozepinib/IFNα (Figure 4D). Whereas Z-VAD did not affect cell death induced by either bozepinib alone or the bozepinib/IFNα combination, viability after bozepinib/IFNα treatment was significantly higher in cells pretreated with chloroquine. Treatment with chloroquine caused accumulation of LC3-II, that has been suggested to be the result of chloroquine-induced inhibition of fusion between autophagosome and lysosomes,29,30 and was more evident after treatment with bozepinib/IFNα (Figure 4D).
These results indicate that bozepinib is able to induce the autophagy process in cancer cells that is clearly evidenced when it is combined with the IFNα cytokine, and suggest the contribution of autophagy to the cell death induced by the combination of bozepinib/IFNα.
IFNα enhances ability of bozepinib to induce lysosomal senescence-associated ß-galactosidase activity
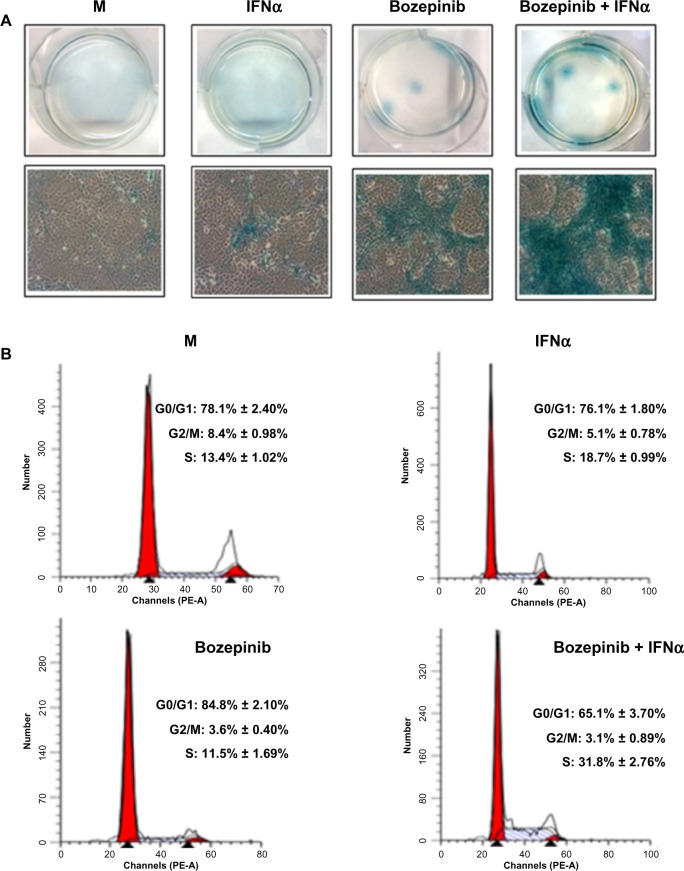
During long-term treatment with bozepinib and the bozepinib/IFNα combination at low doses, we observed that a minority population remained in all the cell lines analyzed, but was more evident in the MCF-7 cell line. In order to characterize this population, we investigated ß-galactosidase activity and the cell cycle in MCF-7 cells. ß-galactosidase activity was detected in the residual surviving population after 7 days of treatment with bozepinib, as shown in Figure 5A. However, although this population was minor after 7 days of treatment with bozepinib/IFNα, ß-galactosidase activity was more evident. Moreover, the percentage of cells arrested in S phase after treatment with bozepinib/IFNα was around 30%, whereas the mock-treated cells and those treated with bozepinib alone showed 11%–13% in S phase (Figure 5B) after 7 days of treatment. Therefore, both bozepinib and IFNα were able to induce senescence in the residual surviving population, and this was more evident when bozepinib and IFNα were used in combination.
Figure 5.
IFNα enhanced the ability of bozepinib to induce ß-galactosidase activity. MCF-7 cells were mock-treated or treated with 2.5 μM of bozepinib, 500 IU/mL human IFNα, or a combination of bozepinib/IFNα over 7 days. (A) Cells were fixed and stained using the Senescence β-Galactosidase Staining Kit as described in the Materials and methods section and photographed under a 10 × objective with a microscope (Leica) using visible light (lower panel) and the corresponding six wells were photographed under a 1 × objective using a standard camera (upper panel). (B) Cells were fixed and analyzed by flow cytometry after staining with propidium iodide. Values represent the mean of triplicate determinations calculated from a single experiment. Experiments were repeated at least three times.
Abbreviations: IFNα, interferon-alpha; M, mock treated cells.
Discussion
Purine derivatives have shown potent antitumor activity and represent a new generation of anticancer drugs.4 We have previously reported that bozepinib has an IC50 value ten times smaller than that of 5-fluorouracil in MCF-7 breast cancer cells. Moreover, bozepinib induces a considerable level of cell death by apoptosis via a mechanism that is still unknown. Bozepinib does not trigger acute toxicity in mice after 2 weeks of treatment.5 In the present study, we demonstrated that bozepinib also has antitumor activity in colon cancer cells, with IC50 values lower than those described for breast cancer cells (Figure 1 and Table 1), suggesting great potential of this synthetic drug in the treatment of cancer. In order to identify the molecular targets involved in bozepinib-mediated apoptosis, we analyzed the induction and activation of the proapoptotic proteins, PKR and tumor suppressor p53. PKR but not p53 was markedly induced and activated in breast and colon cancer cell lines during treatment with bozepinib, thereby triggering phosphorylation of eIF2α. It is well established that eIF2α phosphorylation is correlated with translational block and consequently leads to inhibition of protein synthesis, providing the cell with an opportunity to make adaptive responses to stress that could finally trigger cell death by apoptosis.31 Analysis of the level of PKR messenger (m)RNA during treatment with bozepinib suggested that upregulation of the PKR protein was not due to a transcriptional phenomenon (data not shown), similar to what we have described for 5-fluorouracil.24 It is widely known that p53 is critical for the apoptotic response to agents that damage DNA and cause cytotoxicity, such as 5-fluorouracil, etoposide, paclitaxel, and cisplatin.32 However, loss of p53 function is frequently involved in the resistance of tumors to chemotherapeutic agents. Moreover, apoptosis can also occur in mutant p53 cell lines in response to some of these chemotherapeutic drugs, suggesting that more targets are involved in induction of apoptosis in response to chemotherapy.24,33,34 Recently, it has been shown that the PKR protein plays an important role in induction of apoptosis by doxorubicin, etoposide, and 5-fluorouracil, with both p53 and PKR being necessary for cancer cell death by apoptosis in response to chemotherapy.22–24 Curiously, this study provides the first evidence that PKR but not p53 is involved in induction of apoptosis by an antitumor purine derivative (Figure 3). In fact, the levels of apoptosis induced by bozepinib are similar in HCT-116 colon cancer cells regardless of the presence or absence of p53 protein (Figure 3C). However, the absence or downregulation of PKR expression in mouse embryonic fibroblast knockout cells or in human colon cancer cells expressing PKR interference significantly decreased the apoptosis induced by bozepinib (Figure 3). Since p53 is mutated in more than 50% of tumors, drugs inducing apoptosis through molecular targets different from p53 are of great clinical interest.
Drug combinations in cancer therapy that enhance efficacy have had great success in a variety of therapeutic applications.16 Our results show that bozepinib and IFNα act synergistically to suppress the viability of breast and colon cancer cells to a greater extent than when either agent is used alone, reducing cell viability by more than 20% in all cell lines analyzed (Figure 1B). Several studies have demonstrated that IFNα enhances the chemosensitivity of cancer cells to a number of drugs, mainly via improvement of apoptosis. It has recently been reported that a combination of 9-cis-retinoic acid and IFNα induces marked antiproliferative and proapoptotic effects in cancer cells by modulation of critical targets, such as p27 Kip1 and p21 WAF1/Cip1 proteins.16 Moreover, several in vitro and in vivo studies have demonstrated the effectiveness of a combination of IFNα and 5-fluorouracil,35 where p27 Kip1, Fas/FasL, and TNF-related apoptosis-inducing ligand (TRAIL) have been found to be involved in enhancement of apoptosis. In addition, we have identified PKR protein as an interesting molecular target that is key to the effectiveness of the 5-fluorouracil/IFNα combination.24 The present study shows that one of the mechanisms by which IFNα improves the cytotoxic effect of bozepinib involves enhancement of apoptosis, and that the synergistic apoptotic effect induced by the bozepinib/IFNα combination is affected by the absence or downregulation of PKR protein (Figure 3). In fact, the cytotoxic effect of bozepinib was higher in PKR+/+ mouse embryonic fibroblasts in comparison with PKR−/− mouse embryonic fibroblasts, and cell viability was significantly reduced when IFNα was combined with bozepinib in PKR+/+ mouse embryonic fibroblasts. In contrast, cell viability was not affected by the bozepinib/IFNα combination in PKR−/− mouse embryonic fibroblasts. These data suggest that PKR, in part, contributes to the effectiveness of the bozepinib/IFNα combination, and therefore we hypothesize that its deregulation in tumors could affect the response of patients to combined therapies.
Given that most cancer cells show low levels of active caspases or mutations that inactivate the effectors of apoptosis,36 antitumor drugs inducing additional or alternative mechanisms of cell death are of great interest. It has been suggested that autophagy could constitute an alternative cell death pathway in cells with a disrupted apoptotic path way.10 In this sense, MCF-7 cells are a good model system to study drug-induced cell death by autophagy due to their defective caspase activation.37,38 Moreover, effects other than apoptosis induced by combined IFNα antitumor therapies have not yet been explored. In our study, bozepinib was able to induce autophagosomes, as shown by the conversion of LC3-I to LC 3-II (Figure 4A), relocalization of the GFP-LC3 protein (Figure 4B), and electron microscopic images (Figure 4C). Surprisingly, addition of IFNα clearly increased autophagosome levels in MCF-7 cells (Figure 4). Moreover, previous treatment with a low dose of chloroquine was able to significantly reduce the cell death induced by bozepinib/IFNα (Figure 4D). Similar as described for rottlerin and etoposide,38,39 autophagy leads to cell death in response to bozepinib/IFNα treatment. Consistent with the inability of MCF-7 cells to induce activation of caspase-3,28 pretreatment with the pan-caspase inhibitor Z-VAD did not affect the cell viability seen after the treatments (Figure 4D). Although it is known that autophagy is required for the production of IFNα by plasmacytoid dendritic cells during viral infection,40 and it has been recently shown that type I IFN induces autophagic trafficking of viral proteins of hepatitis C virus,41 the role of IFNα in the autophagy process is still unclear and knowledge is restricted to its antiviral function. Our results show, for the first time, evidence that IFNα is involved in the autophagy process in combination with an antitumor agent. The mechanism of action involved in this process needs to be investigated further, and might have important therapeutic implications.
Finally, we observed that during long-term treatment with even low doses of bozepinib and the bozepinib/IFNα combination, a minority population showing β-galactosidase activity persisted in MCF-7 cells, being once again more evident in surviving cells treated with the bozepinib/IFNα combination (Figure 5A). Moreover, this population showed a high percentage of cells arrested in S phase in comparison with cells treated or not with bozepinib or IFNα separately (Figure 5B). Because tumors often develop resistance to apoptosis induced by anticancer treatment, induction of senescence in tumor cells could be an alternative approach to cancer therapy, and be especially effective in the treatment of cancer cells in which apoptotic pathways are disabled.12 Although the exact mechanism by which IFNα regulates senescence is still under investigation, it has been suggested that IFNα downregulates telomerase activity along with inhibition of growth in Daudi lymphoma cells.42 It has also been suggested that overexpression of two IFN regulatory transcription factors (IRF5 and IRF7) is able to induce a senescence-related phenotype in immortal cells.43 More recently, early evidence has been reported showing that a combination of IFNα and a chemotherapeutic agent, vinblastine, triggers senescence; however, the authors showed this effect in endothelial cells in the context of angiogenesis within the tumor.44 Our results show that IFNα enhances the senescence provoked in tumor cells by bozepinib, suggesting that this cytokine could act directly in this process when combined with other antitumor drugs.
Conclusion
The development of novel anticancer drugs that are more effective and have fewer side effects in patients is an important research topic in cancer, and understanding the mechanisms involved in the antitumor effects of new compounds is necessary for their clinical application. Bozepinib is a potent antitumor agent that is able to induce apoptosis in breast and colon cancer cells. In this study, we have demonstrated that PKR but not p53 is involved in the apoptosis induced by bozepinib, which has encouraged us to explore targets for new compounds with high antitumor activity and enabling effectiveness at low doses. Given that p53 is mutated in more than 50% of tumors, drugs inducing apoptosis through molecular targets different to p53 are of great clinical interest. Moreover, our results highlight the benefit of combination chemotherapy using natural cytokines, such as IFNα, which can potentiate the apoptosis induced by chemotherapy. IFNα also enhances autophagy and senescence, which are processes suggested to be of great importance, especially in tumor cells that show resistance to conventional chemotherapy. Our study increases our knowledge about the synergistic effect induced by IFN, and supports the need to explore new combinations with potent antitumor agents such as bozepinib, which can enhance the diversity of cell death outcomes, leading to more effective and less toxic chemotherapy.
Acknowledgments
We gratefully acknowledge Jaime Lazuen for providing excellent technical assistance with the cytometry studies and Manuela Expósito for statistical assistance. We also thank staff from the Experimental Surgery Research Unit, and Pablo Bueno for support with equipment. This work was supported in part by grants from the Instituto de Salud Carlos III (Fondo de Investigación Sanitaria FEDER funds, CP08/0063, PI10/02295, and PI10/00592).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Díaz-Gavilán M, Conejo-García A, Cruz-López O, et al. Synthesis and anticancer activity of (R,S)-9-(2,3-dihydro-1,4-benzoxathiin-3-ylmethyl)-9H-purines. ChemMedChem. 2008;3(1):127–135. doi: 10.1002/cmdc.200700180. [DOI] [PubMed] [Google Scholar]
- 3.Díaz-Gavilán M, Gómez-Vidal JA, Entrena A, Gallo MA, Espinosa A, Campos JM. Study of the factors that control the ratio of the products between 5-fluorouracil, uracil, and tetrahydrobenzoxazepine O,O-acetals bearing electron-withdrawing groups on the nitrogen atom. J Org Chem. 2006;71(3):1043–1054. doi: 10.1021/jo052167m. [DOI] [PubMed] [Google Scholar]
- 4.Núñez MC, Díaz-Gavilán M, Conejo-García A, et al. Design, synthesis and anticancer activity against the MCF-7 cell line of benzo-fused 1,4-dihetero seven- and six-membered tethered pyrimidines and purines. Curr Med Chem. 2008;15(25):2614–2631. doi: 10.2174/092986708785909021. [DOI] [PubMed] [Google Scholar]
- 5.López-Cara LC, Conejo-García A, Marchal JA, et al. New (RS)-benzoxazepin-purines with antitumour activity: the chiral switch from (RS)-2,6-dichloro-9-[1-(p-nitrobenzenesulfonyl)-1,2,3,5-tetrahydro-4,1-benzoxazepin-3-yl]-9H-purine. Eur J Med Chem. 2011;46(1):249–258. doi: 10.1016/j.ejmech.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3(11):E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 7.de Bruin EC, Medema JP. Apoptosis and non-apoptotic deaths in cancer development and treatment response. Cancer Treat Rev. 2008;34(8):737–749. doi: 10.1016/j.ctrv.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Mansilla S, Llovera L, Portugal J. Chemotherapeutic targeting of cell death pathways. Anticancer Agents Med Chem. 2012;12(3):226–238. doi: 10.2174/187152012800228805. [DOI] [PubMed] [Google Scholar]
- 9.White E, Karp C, Strohecker AM, Guo Y, Mathew R. Role of autophagy in suppression of inflammation and cancer. Curr Opin Cell Biol. 2010;22(2):212–217. doi: 10.1016/j.ceb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalby KN, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6(3):322–329. doi: 10.4161/auto.6.3.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25(3):585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 12.Kong Y, Cui H, Ramkumar C, Zhang H. Regulation of senescence in cancer and aging. J Aging Res. 2011;2011:963172. doi: 10.4061/2011/963172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasselbalch HC. Interferon alpha2 in the treatment of hematological malignancies. Status and perspectives. Curr Drug Targets. 2011;12(3):387–391. doi: 10.2174/138945011794815220. [DOI] [PubMed] [Google Scholar]
- 14.Tarhini AA, Gogas H, Kirkwood JM. IFN-alpha in the treatment of melanoma. J Immunol. 2012;189(8):3789–3793. doi: 10.4049/jimmunol.1290060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkwood J. Cancer immunotherapy: the interferon-alpha experience. Semin Oncol. 2002;29(3 Suppl 7):18–26. doi: 10.1053/sonc.2002.33078. [DOI] [PubMed] [Google Scholar]
- 16.Dal Col J, Mastorci K, Fae DA, et al. Retinoic acid/alpha-interferon combination inhibits growth and promotes apoptosis in mantle cell lymphoma through Akt-dependent modulation of critical targets. Cancer Res. 2012;72(7):1825–1835. doi: 10.1158/0008-5472.CAN-11-2505. [DOI] [PubMed] [Google Scholar]
- 17.Tarhini AA, Cherian J, Moschos SJ, et al. Safety and efficacy of c ombination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J Clin Oncol. 2012;30(3):322–328. doi: 10.1200/JCO.2011.37.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonsson B, Hjorth-Hansen H, Bjerrum OW, Porkka K. Interferon alpha for treatment of chronic myeloid leukemia. Curr Drug Targets. 2011;12(3):420–428. doi: 10.2174/138945011794815301. [DOI] [PubMed] [Google Scholar]
- 19.Negrier S, Gravis G, Perol D, et al. Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncol. 2011;12(7):673–680. doi: 10.1016/S1470-2045(11)70124-3. [DOI] [PubMed] [Google Scholar]
- 20.Esteban M, Kerr IM. The synthesis of encephalomyocarditis virus polypeptides in infected L-cells and cell-free systems. Eur J Biochem. 1974;45(2):567–576. doi: 10.1111/j.1432-1033.1974.tb03583.x. [DOI] [PubMed] [Google Scholar]
- 21.García MA, Gil J, Ventoso I, et al. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev. 2006;70(4):1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon CH, Lee ES, Lim DS, Bae YS. PKR, a p53 target gene, plays a crucial role in the tumor-suppressor function of p53. Proc Natl Acad Sci U S A. 2009;106(19):7852–7857. doi: 10.1073/pnas.0812148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peidis P, Papadakis AI, Muaddi H, Richard S, Koromilas AE. Doxorubicin bypasses the cytoprotective effects of eIF2alpha phosphorylation and promotes PKR-mediated cell death. Cell Death Differ. 2011;18(1):145–154. doi: 10.1038/cdd.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García MA, Carrasco E, Aguilera M, et al. The chemotherapeutic drug 5-fluorouracil promotes PKR-mediated apoptosis in a p53-independent manner in colon and breast cancer cells. PLoS One. 2011;6(8):e23887. doi: 10.1371/journal.pone.0023887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang YL, Reis LF, Pavlovic J, et al. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14(24):6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bunz F, Hwang PM, Torrance C, et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104(3):263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villalobos C, García-Sancho J. Capacitative Ca2+ entry contributes to the Ca2+ influx induced by thyrotropin-releasing hormone (TRH) in GH3 pituitary cells. Pflugers Arch. 1995;430(6):923–935. doi: 10.1007/BF01837406. [DOI] [PubMed] [Google Scholar]
- 28.Yang XH, Sladek TL, Liu X, Butler BR, Froelich CJ, Thor AD. Reconstitution of caspase 3 sensitizes MCF-7 breast cancer cells to doxorubicin- and etoposide-induced apoptosis. Cancer Res. 2001;61(1):348–354. [PubMed] [Google Scholar]
- 29.Yoon YH, Cho KS, Hwang JJ, Lee SJ, Choi JA, Koh JY. Induction of lysosomal dilatation, arrested autophagy, and cell death by chloroquine in cultured ARPE-19 cells. Invest Ophthalmol Vis Sci. 2010;51(11):6030–6037. doi: 10.1167/iovs.10-5278. [DOI] [PubMed] [Google Scholar]
- 30.Geng Y, Kohli L, Klocke BJ, Roth KA. Chloroquine-induced autophagic vacuole accumulation and cell death in glioma cells is p53 independent. Neuro Oncol. 2010;12(5):473–481. doi: 10.1093/neuonc/nop048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol. 2005;6(4):318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 32.Martínez-Rivera M, Siddik ZH. Resistance and gain-of-resistance phenotypes in cancers harboring wild-type p53. Biochem Pharmacol. 2012;83(8):1049–1062. doi: 10.1016/j.bcp.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konstantakou EG, Voutsinas GE, Karkoulis PK, Aravantinos G, Margaritis LH, Stravopodis DJ. Human bladder cancer cells undergo cisplatin-induced apoptosis that is associated with p53-dependent and p53-independent responses. Int J Oncol. 2009;35(2):401–416. [PubMed] [Google Scholar]
- 34.Backus HH, Wouters D, Ferreira CG, et al. Thymidylate synthase inhibition triggers apoptosis via caspases-8 and -9 in both wild-type and mutant p53 colon cancer cell lines. Eur J Cancer. 2003;39(9):1310–1317. doi: 10.1016/s0959-8049(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura M, Nagano H, Sakon M, et al. Role of the Fas/FasL pathway in combination therapy with interferon-alpha and fluorouracil against hepatocellular carcinoma in vitro. J Hepatol. 2007;46(1):77–88. doi: 10.1016/j.jhep.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 36.Philchenkov A, Zavelevich M, Kroczak TJ, Los M. Caspases and cancer: mechanisms of inactivation and new treatment modalities. Exp Oncol. 2004;26(2):82–97. [PubMed] [Google Scholar]
- 37.Janicke RU, Ng P, Sprengart ML, Porter AG. Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J Biol Chem. 1998;273(25):15540–15545. doi: 10.1074/jbc.273.25.15540. [DOI] [PubMed] [Google Scholar]
- 38.Akar U, Chaves-Reyez A, Barria M, et al. Silencing of Bcl-2 expression by small interfering RNA induces autophagic cell death in MCF-7 breast cancer cells. Autophagy. 2008;4(5):669–679. doi: 10.4161/auto.6083. [DOI] [PubMed] [Google Scholar]
- 39.Xue L, Fletcher GC, Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci. 1999;14(3):180–198. doi: 10.1006/mcne.1999.0780. [DOI] [PubMed] [Google Scholar]
- 40.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315(5817):1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 41.Desai MM, Gong B, Chan T, et al. Differential, type I interferon-mediated autophagic trafficking of hepatitis C virus proteins in mouse liver. Gastroenterology. 2011;141(2):674–685. doi: 10.1053/j.gastro.2011.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akiyama M, Iwase S, Horiguchi-Yamada J, et al. Interferon-alpha repressed telomerase along with G1-accumulation of Daudi cells. Cancer Lett. 1999;142(1):23–30. doi: 10.1016/s0304-3835(99)00109-3. [DOI] [PubMed] [Google Scholar]
- 43.Li Q, Tang L, Roberts PC, et al. Interferon regulatory factors IRF5 and IRF7 inhibit growth and induce senescence in immortal Li-Fraumeni fibroblasts. Mol Cancer Res. 2008;6(5):770–784. doi: 10.1158/1541-7786.MCR-07-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Upreti M, Koonce NA, Hennings L, Chambers TC, Griffin RJ. Pegylated IFN-alpha sensitizes melanoma cells to chemotherapy and causes premature senescence in endothelial cells by IRF-1 mediated signaling. Cell Death Dis. 2010;1:e67. doi: 10.1038/cddis.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]