Abstract
Objective:
To compare changes over 48 weeks in bone mineral density (BMD) between participants randomized to lopinavir/ritonavir (LPV/r) + raltegravir (RAL) or LPV/r + 2–3 nucleoside/nucleotide reverse transcriptase inhibitors (N(t)RTIs) as second line therapy.
Design:
48-week open-label sub-study of the Second Line trial conducted in South Africa, India, Thailand, Malaysia and Argentina.
Methods:
Dual energy X-ray absorptiometry scans of proximal femur and lumbar spine were performed at baseline and week 48. Linear regression was used to compare means of differences between arms. McNemars test compared osteopenia and osteoporosis. Associations between percentage BMD changes and baseline variables were assessed by multivariate linear regression.
Results:
Two hundred and ten participants were randomized. Analyses were adjusted for sex, BMI and smoking status. Mean (95% CI) proximal femur BMD% reduced over 48 weeks by −5.2% (−6.7 to −3.8%) in the LPV/r+2-3N(t)RTIs arm and by −2.9% (−4.3 to −1.5%) in the LPV/r+RAL arm (P = 0.0001). Lumbar spine BMD reduced by −4.2% (−5.7 to −2.7%) in the LPV/r+2-3N(t)RTIs arm and by −2.0% (−3.5 to −0.6%) in the LPV/r+RAL arm (P = 0.0006). The incidence of osteopenia (7.6%) and osteoporosis (2.0%) assessed over 48 weeks were similar between arms. Reduced BMD over 48 weeks was significantly associated with longer duration of tenofovir on study [% change (SE) −1.58 (0.38) femur, −1.65 (0.38) spine, P = 0.0001] and low baseline BMI [% change (SE) 0.5 (0.13) femur, 0.17 (0.07) spine; P < 0.01].
Conclusion:
An N(t)RTI-sparing antiretroviral regimen of LPV/r and raltegravir as second line therapy is associated with less bone loss than a LPV/r regimen containing N(t)RTIs.
Keywords: antiretroviral therapy, bone mineral density, HIV, lopinavir, raltegravir, second line therapy, tenofovir
Introduction
Continued morbidity from non-AIDS illnesses is a concern in the management of HIV-infected patients despite the success in controlling virus replication. Low bone mineral density (BMD) and osteoporosis are prevalent in HIV-infected patients with some evidence suggesting that more osteoporosis-related fractures may be experienced in HIV-infected patients than the general population [1,2]. Osteopenia occurs in up to 50% of HIV patients and osteoporosis up to 15% [3]. Cross-sectional studies suggest this prevalence is associated with both HIV infection itself and a greater prevalence of traditional risk factors for low BMD; such as smoking, low BMI and steroid use [1]. However, studies in combination antiretroviral treatment (cART) naive patients initiating therapy have demonstrated loss of BMD over the first 48–96 weeks of treatment [4–8], with greater loss experienced by those initiating nucleoside/nucleotide reverse transcriptase inhibitors (N(t)RTIs) [5,6], most notably with tenofovir (TDF) [9]. In fact reduced BMD appears to be one of the few toxicities that worsens with cART treatment [10]. In an evaluation of 4640 HIV participants in 26 randomized ACTG trials the incidence of fractures was higher in the first 2 years after cART initiation than in subsequent years [11]. Little is known about the effects of exposure to HIV integrase inhibitors and BMD. There is some evidence that raltegravir (RAL) may increase BMD in the hip and spine over 48 weeks compared with TDF [12] or protease inhibitors [13]. However, these two studies were conducted on a small number of participants (n = 37 and 74, respectively) and only one was a randomized study [13].
The Second Line study provided a unique opportunity to examine if a N(t)RTI-sparing cART regimen containing RAL and lopinavir/ritonavir (LPV/r) results in less BMD loss than combinations containing N(t)RTIs in HIV-infected patients.
Methods
The Second Line study is a 96-week, multinational trial of participants virologically failing first-line therapy randomized 1 : 1 to currently recommended second line treatment (2-3N(t)RTI + LPV/r) or RAL (400 mg b.i.d.) + LPV/r (400/100 mg b.i.d. or 800 mg/200 mg q.i.d.). Eligible participants were HIV-1 positive adults (aged ≥16 years) who had received first-line cART comprised of a nonnucloeside reverse transcriptase inhibitor (NNRTI)+2N(t)RTIs for at least 24 weeks with no change within 12 weeks prior to screening; evidence of virological failure defined by two consecutive (≥7 days apart) plasma HIV RNA viral load more than 500 copies per ml; no previous exposure to protease inhibitors and/or integrase inhibitors. The study was approved by each site's Ethics Committee and was registered at Clinicaltrials.gov (NCT00931463). The cohort median (IQR) age was 38.5 (32.4–44.4) years, 55% male, 42% Asian and 36% African, 73% heterosexual transmission, with an estimated median duration of HIV infection of six (3.6–8.7) years. The primary 48-week results of the Second Line study have been described [14].
Of the 37 sites that participated in the Second Line study eight sites from five countries (South Africa, India, Malaysia, Thailand and Argentina) participated in the body composition sub-study (clinicaltrials.gov identifier: NCT01513122). These sites had access to a dual energy X-ray absorptiometry (DXA) scanner and recruitment was open to all participants screened at these sites between July 2010 and July 2011. The sub-study was approved by each site's Ethics committee's and all participants gave written, informed consent. DXA scans were performed at baseline and week 48 on either Lunar (India n = 48, Malaysia n = 13, Argentina n = 8, Thailand n = 22) or Hologic (Thailand n = 26, South Africa n = 94) DXA scanners. To assess BMD the proximal femur and lumbar spine (L2-L4) were measured as per a standard protocol provided to all sites. We did not use phantoms for quality assurance and scans were not subjected to central interpretation.
The primary objective was to determine the treatment arm difference in mean change from baseline BMD (%) at the proximal femur and lumbar spine to 48 weeks. We hypothesized that participants randomized to LPV/r+RAL would demonstrate smaller reductions in BMD over the 48 weeks compared with LPV/r+2-3N(t)RTIs. The secondary objectives included comparison between treatment arms for percentage of participants with osteopenia (T-score between −1.0 and −2.5) and osteoporosis (T-score less than −2.5), mean percentage change from baseline in Z-score and T-score, and to explore the relationship between lumbar spine and proximal femur BMD and baseline clinical demographics and cART. The T-score is the number of SDs below the average for a young adult at peak bone density. The Z-score is the number of SDs below an average person of the same age.
The baseline covariates that were included in the multivariate model as predictors of percentage change in proximal femur and lumbar spine BMD were age, sex, ethnicity, BMI, smoking, blood pressure; HIV and ART markers (randomized treatment arm, CD4+ lymphocyte counts, plasma HIVRNA, prior and on-study use of TDF, duration of TDF use); body composition (total body fat and lean mass); and bone-specific variables (prior hypogonadism and physical activity).
Statistical analysis
Analyses included available data from all participants who consented to the sub-study, who underwent randomization, received at least one dose of study medication and who completed both a week 0 and 48 DXA scan. Results were considered statistically significant at a two-sided α = 0.05. A sample size of 100 per randomized treatment arm was required to achieve 80% power to detect a mean difference of 1.7% change in BMD.
Linear regression was used to compare means of differences (baseline to week 48) between randomized arms. McNemars test for paired proportions was used to compare the categorized BMD variables. The association between BMD changes and the baseline variables was assessed by linear regression, any variable with P < 0.1 in univariate was included in the multivariate model. Backward stepwise methods were used to build the multivariate model. If parameters were found to be not normally distributed then the equivalent nonparametric test was used.
Results
The patient disposition is outlined in Figure 1. Six hundred and ninety-nine participants were screened for the parent Second Line study, of whom 236 consented to the bone sub-study. Two hundred and eleven participants were eligible and randomized into the sub-study and 210 made up the analysis population. Ninety-seven participants reached week 48 in the r/LPV+2-3N(t)RTI arm and 107 in the r/LPV+RAL arm.
Fig. 1.

Patient disposition of Second Line bone sub-study.
The baseline characteristics of the sub-study cohort are described in Table 1. The median age of the sub-study cohort was 38.8 years, 48% were male, 51% were Asian and 43% were African. Participant's median duration on cART at randomiszation was 3.4 years, 34% were on zidovudine, 48% on stavudine and 17% on TDF at baseline. The prevalence of osteopenia at the proximal femur was 20% and osteoporosis 2%, whereas at the lumbar spine prevalence of osteopenia was 31% and osteoporosis was 5%. At baseline, there were imbalances between the two treatment arms for sex, BMI and smoking status. All analyses were adjusted for the imbalances in these covariates.
Table 1.
Baseline characteristics.
| r/LPV+ 2-3N(t)RTI (n = 102) | r/LPV + RAL (n = 108) | Total (n = 210) | |
| Age (years) | 38.6 (34.2–44.1) | 38.9 (32.6–44.4) | 38.8 (32.9–44.2) |
| Sex, male | 55 (53.9) | 45 (41.7) | 100 (47.6) |
| Ethnicity | |||
| Caucasian | 4 (3.9) | 3 (2.8) | 7 (3.3) |
| Asian | 53 (52.0) | 55 (50.9) | 108 (51.4) |
| Hispanic | 1 (1.0) | 2 (1.9) | 3 (1.4) |
| African | 44 (43.1) | 47 (43.5) | 91 (43.3) |
| Unknown | 0 | 1 (0.9) | 1 (0.5)) |
| BMI | |||
| <18.5 | 18 (17.6) | 13 (12.0) | 31 (14.8) |
| 18.5 to <20 | 48 (47.1) | 59 (54.6) | 107 (51.0) |
| 20 to <30 | 26 (25.5) | 24 (22.2) | 50 (23.8) |
| 30 to <35 | 7 (6.9) | 8 (7.4) | 15 (7.1) |
| ≥35 | 3 (2.9) | 4 (3.7) | 7 (3.3) |
| HIV RNA log (copies/ml) | 4.3 (3.8–4.9) | 4.1 (3.4–4.6) | 4.1 (3.5–4.7) |
| CD4+ T lymphocytes (cells/μl) | 185 (80–296) | 218 (117–315) | 202 (104–307) |
| Hepatitis C antibody (positive) | 4 (3.9) | 5 (4.6) | 9 (4.3) |
| Hip/waist ratio | 1.2 (1.1–1.2) | 1.1 (1.1–1.2) | 1.2 (1.1–1.2) |
| Lumbar spine BMD (g/cm2) | 1.1 (0.9–1.2) | 1.0 (1.0–1.2) | 1.1 (1.0–1.2) |
| TDF prior to study | 1.0 (1.0–1.0) | 1.0 (0.9–1.2) | 1.0 (1.0–1.2) |
| No TDF prior to study | 1.1 (0.9–1.2) | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) |
| Lumbar spine T score | −0.7 (−1.5–0.1) | −0.7 (−1.4–0.2) | −0.7 (−1.5–0.2) |
| Lumbar spine Z score | −0.6 (−1.3–0.2) | −0.5 (−1.4–0.2) | −0.5 (−1.4–0.2) |
| Proximal femur BMD (g/cm2) | 1.0 (0.9–1.1) | 0.9 (0.9–1.0) | 1.0 (0.9–1.0) |
| Proximal femur T score | −0.3 (−0.8–0.4) | −0.3 (−1.0–0.6) | −0.3 (−0.9–0.5) |
| Proximal femur Z score | −0.2 (−0.6–0.7) | −0.1 (−0.8–0.7) | −0.1 (−0.7–0.7) |
| Proximal femur osteopenia | 18 (19.4) | 21 (20.0) | 39 (19.7) |
| Proximal femur osteoporosis | 0 (0.0) | 3 (2.9) | 3 (1.5) |
| Lumbar spine osteopenia | 34 (36.6) | 28 (26.7) | 62 (31.3) |
| Lumbar spine osteoporosis | 5 (5.4) | 5 (4.8) | 10 (5.0) |
| Smoking | |||
| Current | 22 (21.6) | 14 (13.0) | 36 (17.1) |
| Recently (within 12 months) | 1 (1.0) | 2 (1.9) | 3 (1.4) |
| Past | 16 (15.7) | 16 (14.8) | 32 (15.2) |
| Never | 63 (61.8) | 76 (70.4) | 139 (66.2) |
| Alcohol consumption | |||
| 0–2 drinks per day | 97 (95.1) | 104 (96.3) | 201 (95.7) |
| ≥2 drinks per day | 5 (4.9) | 4 (3.7) | 9 (4.3) |
| Days of exercise | |||
| Brisk walk past 7days | 5.0 (3.0–7.0) | 7.0 (5.0–7.0) | 6.0 (3.0–7.0) |
| Moderate activity past 7 days | 3.0 (1.0–6.0) | 3.0 (0.0–5.0) | 3.0 (0.0–5.0) |
| Vigorous activity past 7 days | 1.0 (0.0–3.0) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) |
| History of diabetes (yes) | 1 (1.0) | 3 (2.8) | 4 (1.9) |
| Family history of diabetes (yes) | 20 (19.6) | 25 (23.1) | 45 (21.4) |
| History nontraumatic fractures (yes) | 3 (2.9) | 0 | 3 (1.4) |
| Parental history of hip fracture (yes) | 2 (2.0) | 2 (1.9) | 4 (1.9) |
| cART duration | 2.9 (1.8–5.9) | 3.7 (2.1–6.0) | 3.4 (2.0–6.0) |
| On TDF (yes) | 20 (19.6) | 16 (14.8) | 36 (17.1) |
| On d4T (yes) | 50 (49.0) | 51 (47.2) | 101 (48.1) |
| On ZDV (yes) | 32 (31.4) | 40 (37.0) | 72 (34.3) |
Data are median (IQR) or n (%). BMD, bone mineral density; cART, combined antiretroviral therapy; d4T, stavudine; N(t)RTI, nucleoside/nucleotide reverse transcriptase inhibitor; r/LPV, ritonavir-boosted lopinavir; RAL, raltegravir; TDF, tenofovir; ZDV, zidovudine.
The primary endpoint is outlined in Figure 2. The mean (95% CI) proximal femur BMD (%) significantly reduced over the 48 weeks by −5.2% (−6.7 to −3.8%) in the LPV/r+2-3N(t)RTIs arm and by −2.9% (−4.3 to −1.5%) in the LPV/r+RAL arm, with a mean difference of −2.4% (−3.5 to −1.2; P = 0.0001). The mean (95% CI) lumbar spine BMD (%) significantly reduced by −4.2% (−5.7 to −2.7%) in the LPV/r+2-3N(t)RTIs arm and by −2.0% (−3.5 to – 0.6%) in the LPV/r+RAL arm, with a mean difference of −2.1% (−3.3 to −0.9; P = 0.0006). These results demonstrate that across two different anatomical regions BMD reduced in both treatment arms over 48 weeks, but reduced significantly less in recipients of r/LPV+RAL compared with r/LPV+2-3N(t)tRTI arm.
Fig. 2.
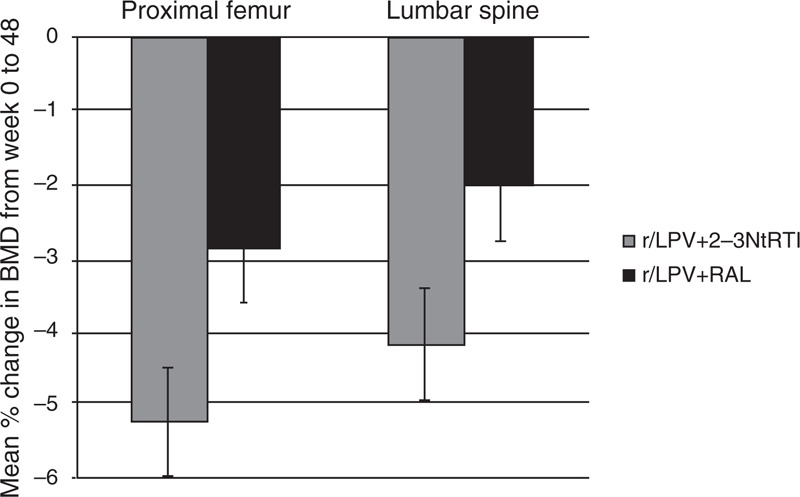
Mean percentage change (SE) from week 0 to 48 in proximal femur and lumbar spine bone mineral density (BMD) by treatment arm.
The change over 48 weeks in T and Z scores at both the proximal femur and lumbar spine were significantly different between the two treatment arms. The mean difference (r/LPV+2-3N(t)RTIs versus r/LPV+RAL) in proximal femur for Z score was −0.18 (−0.3 to −0.19, P = 0.003) and for T score was −0.20 (−0.3 to −0.1, P = 0.0002). The mean difference (r/LPV+2-3N(t)tRTIs versus r/LPV+RAL) in lumbar spine femur for Z score was −0.18 (−0.3 to −0.1, P = 0.006) and for T score was −0.21 (−0.3 to −0.1, P = 0.0001). These results confirm the hypothesis that BMD reduction was less in the r/LPV+RAL arm compared with r/LPV+2-3NtRTI arm.
The prevalence of osteopenia (−2.5 <T score<1.0) at baseline in both treatment groups was 26% across the two anatomical regions and the prevalence of osteoporosis (T score ≤ −2.5) was 3%. The incidence of low BMD (Z score < −2), osteopenia and osteoporosis at week 48 was not different between the two treatment arms (Table 2). Only two (1%) participants experienced a bone fracture, one in the N(t)RTI arm and one in the RAL arm.
Table 2.
Low bone mineral density, osteopenia, and osteoporosis.
| N | New cases at week 48 n (%) | Odds ratio (95% CI)a | P-value | |
| Low proximal femur BMD (Z score < −2) | ||||
| LPV/r + 2–3N(t)RTI | 94 | 2 (2.1) | 0.47 (0.04, 5.7) | 0.5507 |
| r/LPV + RAL | 106 | 1 (0.9) | ||
| Low lumbar spine BMD (Z score <−2) | ||||
| LPV/r + 2-3N(t)RTI | 94 | 3 (3.2) | 2.8 (0.7, 10.7) | 0.1442 |
| r/LPV + RAL | 107 | 9 (8.4) | ||
| Proximal femur osteopenia (T score between −1 and −2.5) | ||||
| LPV/r + 2-3N(t)RTI | 93 | 7 (7.5) | 1.1 (0.4, 3.2) | 0.8619 |
| r/LPV + RAL | 105 | 8 (7.6) | ||
| Lumbar spine osteopenia (T score between −1 and −2.5) | ||||
| LPV/r + 2-3N(t)RTI | 93 | 8 (8.6) | 0.8 (0.3, 2.2) | 0.6487 |
| r/LPV + RAL | 105 | 8 (7.6) | ||
| Proximal femur osteoporosis (T score <–2.5) | ||||
| LPV/r + 2-3N(t)RTI | 93 | 3 (3.2) | 0.4 (0.03, 4.3) | 0.4259 |
| r/LPV + RAL | 105 | 1 (1.0) | ||
| Lumbar spine osteoporosis (T scoe <–2.5) | ||||
| LPV/r + 2-3N(t)RTI | 93 | 5 (5.4) | 0.8 (0.2, 3.2) | 0.7215 |
| r/LPV + RAL | 105 | 4 (3.8) | ||
aAdjusted for baseline imbalances in sex, BMI and smoking; BMD, bone mineral density; N(t)RTI, nucleoside/nucleotide reverse transcriptase inhibitor; r/LPV, ritonavir boosted lopinavir; RAL, raltegravir.
The multivariate regression analysis results are summarized in Table 3. The covariates that predicted a change in BMD in both the lumbar spine and proximal femur were higher baseline BMI and longer duration of TDF on study. Having a higher baseline BMI predicted a greater BMD at week 48, for every 1 kg/m2 increase in BMI the femur BMD increased by 0.5% and spine BMD by 0.17% (P < 0.01). Participants prescribed TDF for longer throughout the 48 weeks of the study had reduced BMD at week 48, for every 1 year of TDF use on study the femur BMD reduced by −1.58% and spine BMD by −1.65% (P = 0.001).
Table 3.
Baseline predictors of percentage change in bone mineral density over 48 weeks included in the multivariate regression model.
| Risk Factor (n = 201) | Proximal femur | Lumbar spine | |||||||
| N | % Change in BMD (SE) | 95% CI | Pr> t | Overall P-value | % Change in BMD (SE) | 95% CI | Pr> t | Overall P-value | |
| Randomization arm | |||||||||
| r/LPV + 2-3N(t)RTIa | 94 | ||||||||
| r/LPV + RAL | 107 | −0.74 (1.21) | (−3.1, 1.6) | 0.54 | 0.54 | −0.58 (1.18) | (−2.9, 1.8) | 0.63 | 0.63 |
| Age | 201 | −0.04 (0.04) | (−0.1, 0.0) | 0.34 | 0.34 | −0.02 (0.04) | (−0.1, 0.1) | 0.66 | 0.66 |
| Sex | |||||||||
| Malea | 94 | ||||||||
| Female | 107 | 0.58 (0.82) | (−1.0, 2.2) | 0.48 | 0.48 | −0.82 (0.75) | (−2.3, 0.7) | 0.27 | 0.27 |
| Race | |||||||||
| Caucasiana | 6 | ||||||||
| Asian | 103 | −1.67 (1.82) | (−5.3, 1.9) | 0.36 | −3.13 (1.85) | (−6.8, 0.5) | 0.09 | ||
| Hispanic | 3 | −0.71 (3.01) | (−6.7, 5.2) | 0.81 | 4.09 (3.05) | (−1.9, 10.1) | 0.18 | ||
| African heritage | 88 | 0.92 (1.83) | (−4.5, 2.7) | 0.62 | 0.66 | −2.65 (1.85) | (−6.3, 1.0) | 0.45 | 0.01 |
| BMI (kg/m2) | 201 | 0.50 (0.13) | (0.3, 0.8) | 0.0001 | 0.0001 | 0.17 (0.07) | (0.0, 0.3) | 0.01 | 0.01 |
| Smoking | |||||||||
| Currentlya | 34 | ||||||||
| Recently | 3 | −3.06 (2.61) | (−8.2, 2.1) | 0.36 | 1.05 (2.59) | (−4.1, 6.2) | 0.69 | ||
| Past | 32 | −0.71 (3.01) | (−4.7, −0.4) | 0.81 | −1.90 (1.10) | (−4.1, 0.3) | 0.09 | ||
| Never | 132 | −0.92 (1.83) | (−3.8, −0.2) | 0.62 | 0.09 | −0.68 (0.90) | (−2.5, 1.1) | 0.45 | 0.31 |
| Duration TDF prior to study (years) | 201 | Not included in multivariate as univariate >0.1 | 0.33 (0.74) | (−1.1, 1.8) | 0.66 | 0.66 | |||
| Duration TDF on study (years) | 198 | −1.58 (0.38) | (−2.3, −0.8) | 0.0001 | 0.0001 | −1.65 (0.38) | (−2.4, −0.9) | 0.0001 | 0.0001 |
| CD4+ lymphocytes (cells/μl) | 201 | 0.00 (0.00) | (0.0, 0.) | 0.62 | 0.62 | Not included in multivariate as univariate >0.1 | |||
| CD4+ nadir (cells/μl) | |||||||||
| <50 | 60 | Not included in multivariate as univariate >0.1 | −4.39 (1.46) | (−7.6, −1.5) | 0.0031 | ||||
| 50–99 | 49 | −4.77 (1.47) | (−7.7, −1.9) | 0.0014 | |||||
| 100–199 | 57 | −3.00 (1.46) | (−6.5, −0.7) | 0.0149 | |||||
| 200–299 | 26 | −3.53 (1.57) | (−6.6, −0.4) | 0.0254 | |||||
| ≥300a | 9 | 0.0230 | |||||||
| Total body fat (kg) | 201 | −0.18 (0.07) | (−0.3, 0.03) | 0.02 | 0.02 | −0.10 (0.07) | (−0.2, 0.04) | 0.16 | 0.16 |
| Total lean mass (kg) | 201 | 0.04 (0.06) | (−0.1, 0.2) | 0.57 | 0.57 | Not included in multivariate as univariate >0.1 | |||
| Vigorous physical activity (days) | 200 | −0.42 (0.16) | (−0.7, −0.1) | 0.01 | 0.01 | Not included in multivariate as univariate >0.1 | |||
aReference group; % Change in BMD (SE): probability (standard error) co-efficient; r/LPV: ritonavir boosted lopinavir; N(t)RTI: nucleoside/nucleotide reverse transcriptase inhibitor; RAL: raltegravir; TDF: tenofovir.
Discussion
The effects of raltegravir on BMD in people living with HIV have not been well defined. The bone sub-study of the Second Line clinical trial was conducted on 210 HIV-positive participants recruited from a range of middle-income countries in which there is a paucity of BMD data. The data generated in this sub-study of participants with confirmed virological failure of an NNRTI+2N(t)RTI cART regimen, demonstrate that effective second line therapy with either WHO standard of care [ritonavir-boosted protease inhibitor (r/PI) + 2-3 N(t)RTIs] or the nucleoside sparing regimen (r/LPV + RAL) reduced BMD over 48 weeks. However, the raltegravir arm was associated with less BMD loss than the N(t)RTI arm. The covariates predicting a greater loss of BMD in both the lumbar spine and proximal femur over the 48 weeks were low baseline BMI and longer duration of TDF on study. In our regression analyses, we were unable to detect an independent contribution from treatment arm on BMD change. As such, use of raltegravir provides an option to avoid the demonstrated risks to bone health arising from use of tenofovir. These findings are important as the use of integrase inhibitors and tenofovir are becoming more common in middle to low-income countries.
Previous studies examining the effect of raltegravir on bone metabolism are limited. The SPIRAL-LIP study was a small study conducted on 74 virologically controlled participants randomized to either continue with their r/PI regimen (n = 35) or switch their r/PI to raltegravir (n = 39) while continuing their N(t)RTIs [13]. After 48 weeks, they reported no change in BMD or T scores in the r/PI arm but a significant improvement in total BMD, hip BMD and hip T score, with a significant difference between treatment groups. The SPIRAL-LIP study did not demonstrate any relationship between TDF use and changes in BMD or T score [13]. Another smaller, nonrandomized study [12] confirmed these findings in a cohort of HIV-controlled osteopenic or osteoporotic HIV participants (n = 37) that switched from TDF+r/PI to RAL+r/PI. After 48 weeks, this cohort experienced a significant increase in spine and hip BMD as well as a significant reduction in bone turnover markers [12]. One final small nonrandomized study on 30 HIV, treatment-naive African–Americans given RAL + TDF/FTC for 2 years demonstrated a significant reduction in total BMD of the same magnitude demonstrated in the 1 year of this Second Line sub-study (0.02 g/cm2) [15].
A reduction of between 0.02 and 0.05 g/cm2 or 2–5% BMD over 48 weeks in lumbar spine and proximal femur in this cohort is larger than previously reported cART-experienced cohorts. Over 48 weeks in the STEAL study a reduction of 0.01 g/cm2 BMD at the lumbar spine and proximal femur was reported [16], in participants who were virologically controlled but switched to either abacavir/lamivudine or tenofovir/emtricitabine at baseline. In addition the SMART study reported a 0.8% loss of femur BMD and 0.4% loss of spine BMD per year in participants continuously ART treated and the majority were virologically controlled at baseline [10]. Interestingly, the duration of cART at baseline in the STEAL study and SMART study was greater compared with the Second Line study, 6 years duration in STEAL and SMART compared with 4 years (median 3.4) second line. Therefore, in our Second Line study, the virologically failing participants experienced an overall decrease in BMD twice as great as other cART-experienced cohorts. Whether or not this is due to virological control at baseline is unknown. Interestingly, changing treatment in virologically failing HIV patients appears to have similar magnitudes of BMD loss as initiating treatment in naive patients [15]. Long-term follow-up of the Second Line study would be required to examine the magnitude of BMD change further, however these data suggest that if you continue N(t)RTIs into second line cART then you are likely to see continuing bone mineral loss.
Evidence suggests that the prevalence of osteoporosis in HIV-infected individuals may be more than three times greater compared with HIV-uninfected controls [3]. The prevalence of osteoporosis in HIV has been reported to be between 3 and 21% [17–19]. Osteopenia is more common, affecting about 30–50% of HIV-positive individuals [20,21]. Our data support these previous estimates of bone disease in HIV populations, 20–31% osteopenia and 2–5% osteoporosis at baseline. This demonstrates that in middle-income countries where treatment options are limited, similar rates of bone disease are found compared with HIV populations from developed countries. The reason for such a high prevalence of bone disease in HIV is of concern and most probably multifactorial. The influence of HIV disease, social factors and cART all contribute. Studies have confirmed that BMD is significantly lower in HIV positive patients over a 1-year period compared with HIV-negative patients, independent of cART [22], though in our multivariate analysis neither HIV duration or HIV disease category were significant predictors of low BMD. In addition, smoking and alcohol intake are known lifestyle factors more common in the HIV-infected population and have previously been documented as associated with reduced BMD [1]. However, in the Second Line study cohort neither smoking nor alcohol was significant predictor of low BMD in the multivariate regression analysis. Another lifestyle factor known to affect BMD is a low BMI. In this analysis a low BMI at baseline was associated with a low BMD over 48 weeks, which has been demonstrated previously [23–25]. Ultimately, these differences in BMD did not significantly affect clinically significant bone disease in this cohort as only two participants experienced fractures. The reason for this may be the relatively short time frame of 48 weeks. A reduction in BMD would precede the event of a fracture, and therefore longer term follow-up may be required.
Previous studies have demonstrated bone loss being greater during the first year of treatment with TDF-containing regimens compared with abacavir-containing regimens [5,26–28]. To support these findings, evidence of greater bone turnover has been reported in TDF regimens [16,27,29]. Cumulative exposure to TDF has also been shown to predict increased risk of osteoporotic fracture [30]. Our finding that TDF was associated with lower BMD outcomes over 48 weeks support these previous data. Mechanisms for drug-induced bone loss are not fully elucidated but likely are multifactorial. One explanation for the affect of TDF on bone is via bone remodelling by renal mechanisms with a resultant loss of phosphorus [31]. Other cART implicated with greater bone loss are protease inhibitors [4] and thymidine analogues [6]; however, both these studies were conducted on very small cohorts.
Fewer studies have looked at rates of actual bone fracture among people with HIV. Between the years 2000 and 2008, 4% of HIV-positive patients in the United States HIV Outpatient Study experienced bone fractures; a significantly higher rate than that estimated in the general US population [32]. Another study reported the relative risk of fracture to be 2.87 per 100 persons in a HIV population compared with 1.77 in non-HIV persons in a study on more than 8000 HIV-infected patients and 2 million non-HIV-infected patients [33]. A meta-analysis of 26 randomized clinical trials including 4640 HIV-positive participants reported a fracture rate of 0.4/100 person-years [11], and in a multivariate analysis of their antiretroviral naive participants they reported an increase in fracture associated with smoking and glucocorticoids use, but not exposure to ART [11]. In this Second Line sub-study, only two participants (1%) experienced a bone fracture over the 48 weeks of follow-up (one participant in the LPV/r+2-3N(t)RTIs arm and one in the LPV/r+RAL arm). This lower proportion of fractures could be explained by the relatively short duration of the study and smaller number compared with the previous reported cohorts. Longer term follow-up of the Second Line study will provide greater clarification of clinical bone disease in this cohort.
The measurement of BMD is not routine in middle to low-income settings due to high cost. Therefore the data available on the incidence and prevalence of clinical bone disease in HIV in these populations is very limited. The Second Line bone sub-study was conducted largely in Asian [51%] (India, Thailand and Malaysia) and African [43%] (South Africa) populations. There has only been one previous cross-sectional study of BMD in HIV-infected participants in India that demonstrated cART and low BMI were associated with a lower BMD [34]. One other study conducted bi-annual DXA scans in a cohort of 500 sero-negative participants in Peru, Thailand, San Francisco and South Africa and Brazil treated with TDF/FTC and reported BMD loss at the hip and spine with a TDF-containing regimen and 1% population with fractures over 72 weeks [35]. Overall, the data reported in this sub-study cohort are consistent with the limited data previously presented in similar populations. In addition, the data in these middle-income HIV populations are similar to that reported in developed countries. What is novel is that the incidence of newly acquired bone disease over a 48-week period has never previously been reported. In this Second Line cohort the incidence of up to 9% of newly identified cases of osteopenia and osteoporosis over such a short period is a concern among a relatively young population.
In this unique HIV population, osteopenia and osteoporosis were common. The loss of BMD was less in participants treated with raltegravir and greater in those exposed to tenofovir throughout the study. These data confirm that bone disease is a significant comorbidity in the ongoing long-term management of HIV in middle-income countries.
Acknowledgements
Thank you to the participants of the Second Line bone sub-study.
Second Line bone sub-study sub-committee contributions: A.M. designed the concept and analysis plan. She also oversaw the conduct of the study, including all data acquisition. A.M. drafted the article. C.M. performed all statistical analyses, reviewed the analysis plan and article. P.M. conceived the study, designed the concept and reviewed the analysis plan and article. J.H. designed the concept and reviewed the analysis plan and article. S.E. conceived the study, designed the concept and reviewed the analysis plan and article. W.B. was member of the Protocol Steering Committee, which developed and oversaw the protocol, reviewed all data and analyses, and reviewed the article. P.P. was member of the Protocol Steering Committee, which developed and oversaw the protocol, reviewed all data and analyses, and reviewed the article. S.F. was a member of the Protocol Steering Committee, which developed and oversaw the protocol, reviewed all data and analyses, and reviewed the article. D.C. was a member of the Protocol Steering Committee, which developed and oversaw the protocol, reviewed all data and analyses, and reviewed the article. M.B. conceived the study, designed the concept and reviewed the analysis plan and article. M.B. also oversaw conduct of the study, including all data acquisition.
Second Line bone sub-study investigators: Dr Nagalingeswaran Kumarasamy, Dr Sharne Foulkes, Prof Robin Wood, Dr Ploenchan Chetchotisakd, Prof Praphan Phanuphak, Dr Lerato Mohapi, Dr Adeeba Kamarulzaman, Dr Oscar Messina.
Second Line study team: D.C., S.E., M.B., A.H., N.E., H.H., M.A., S.H., N.B.-J., C.M., J.A., A.R., R.R., S.K., K.P., M.L., C.A., M.V., S.P, HIV Immunovirology Laboratory, St. Vincent's Hospital for Applied Medical Research.
Funding: This work was supported by The Kirby Institute, which is funded by the Australian Government Department of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government. The Kirby Institute is affiliated with the Faculty of Medicine, University of New South Wales.
Funding for the Second Line study was provided by The Kirby Institute, Merck & Co. Inc, Abbott Laboratories, NHMRC and amfAR.
Conflicts of interest
A.M., C.M., W.B., S.F. and P.P. declare there are no conflicts of interest. M.B. was paid to prepare and present educational materials for Boehringer-Ingelheim, Gilead, Janssen-Cilag and Merck Sharpe and Dohme. J.H.'s institution has received funding for investigator initiated research, service on advisory boards, lectures and conference sponsorship from Janssen-Cilag, Gilead Sciences, Merck Sharpe and Dohme and ViiV Healthcare. S.E. has received research funding from Abbott, Gilead, Pfizer, Merck Sharpe and Dohme and ViiV Healthcare. D.C. has received AbbVie and Merck Sharpe and Dohme grants, consultant and speaker fees. P.M. has received support in honoraria, research grants, lecture sponsorships and advisory boards from Abbott, Merck Sharpe and Dohme, Bristol Myers Squibb, Pfizer, Gilead, Glaxo-Smith Kline, Janssen-Cilag, and ViiV Healthcare.
Footnotes
Correspondence to Allison Martin, The Kirby Institute, University of New South Wales, Sydney, NSW 2052, Australia. Tel: +61 2 9385 0900; fax: +61 2 9385 0910; e-mail: secondline@kirby.unsw.edu.au
References
- 1.Daar ES, Smith KY, Powderly WG. Long- term complications of HIV and antiretroviral therapy. Clin Care Opt 2012; 1–18 [Google Scholar]
- 2.Sharma A, Tian F, Yin MT, Keller MJ, Cohen M, Tien PC. Association of regional body composition with bone mineral density in HIV-infected and uninfected women: women's interagency HIV study. J Acquir Immune Defic Syndr 2012; 61:469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 2006; 20:2165. [DOI] [PubMed] [Google Scholar]
- 4.Duvivier C, Kolta S, Assoumou L, Ghosn J, Rozenberg S, Murphy RL, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS 2009; 27:817–824 [DOI] [PubMed] [Google Scholar]
- 5.McComsey GA, Kitch D, Daar ES, Tierney C, Jahed NC, Tebas P, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis 2011; 203:1791–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Vonderen MG, Lips P, van Agtmael MA, Hassink EA, Brinkman K, Geerlings SE, et al. First line zidovudine/lamivudine/lopinavir/ritonavir leads to greater bone loss compared to nevirapine/lopinavir/ritonavir. AIDS 2009; 23:1367–1376 [DOI] [PubMed] [Google Scholar]
- 7.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr 2009; 51:554–561 [DOI] [PubMed] [Google Scholar]
- 8.Hansen A, Obel N, Nielsen H, Pedersen C, Gerstoft J. Bone mineral density changes in protease inhibitor-sparing vs. nucleoside reverse transcriptase inhibitor-sparing highly active antiretroviral therapy: data from a randomized trial. HIV Med 2010; 12:157–165 [DOI] [PubMed] [Google Scholar]
- 9.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients. JAMA 2004; 292:191–201 [DOI] [PubMed] [Google Scholar]
- 10.Grund B, Peng G, Gibert CL, Hoy JF, Isaksson RL, Shlay JC, et al. Continuous antiretroviral therapy decreases bone mineral density. AIDS 2009; 23:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin MT, Kendall MA, Wu X, Tassiopoulos K, Hochberg M, Huang JS, et al. Fractures afterantiretroviral initiation: an analysis of the ACTG longitudinal linked randomized trial (ALLRT) study. AIDS 2012; 26:2175–218422951635 [Google Scholar]
- 12.Bloch M, Tong W, Hoy J, Richardson R, Baker D, Carr A. Improved low bone mineral density and bone turnover markers with switch from tenofovir to raltegravir in virologically suppressed HIV-1+ adults at 48 weeks: the TROP study. Conference on Retroviruses and Opportunistic Infections, Washington 2012 [Google Scholar]
- 13.Curran A, Martinez E, Saumoy M, del Rio L, Crespo M, Larrousse M, et al. Body composition changes after switching from protease inhibitors to raltegravir: SPIRAL-LIP substudy. AIDS 2012; 26:475. [DOI] [PubMed] [Google Scholar]
- 14.SECOND-LINE study group Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, noninferiority study. Lancet 2013; 381:2091–2099 [DOI] [PubMed] [Google Scholar]
- 15.Wohl DA, Young L, Hyslop WB, Blevins S, Ragan D, Walsh K, et al. Effects of raltegravir (RAL) combined with tenofovir (TDF) and emtricitabine (FTC) on body shape, bone density and lipids in HIV+ african-americans initiating therapy: metabolic outcomes of the UNC-REAL study. XIX International AIDS Conferencexs Washington, DC 2012 [Google Scholar]
- 16.Haskelberg H, Hoy JF, Amin J, Ebeling PR, Emery S, Carr A. Changes in bone turnover and bone loss in HIV-infected patients changing treatment to tenofovir-emtricitabine or abacavir-lamivudine. PloS one 2012; 7:e38377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tebas P, Powderly WG, Claxton S, Marin D, Tantisiriwat W, Teitelbaum SL, et al. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS 2000; 14:F63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carr A, Miller J, Eisman JA, Cooper DA. Osteopenia in HIV-infected men: association with asymptomatic lactic acidemia and lower weight preantiretroviral therapy. AIDS 2001; 15:703–709 [DOI] [PubMed] [Google Scholar]
- 19.Nolan D, Upton R, McKinnon E, John M, James I, Adler B, et al. Stable or increasing bone mineral density in HIV-infected patients treated with nelfinavir or indinavir. AIDS 2001; 15:1275–1280 [DOI] [PubMed] [Google Scholar]
- 20.Tomažič J, Ul K, Volčanšek G, Gorenšek S, Pfeifer M, Karner P, et al. Prevalence and risk factors for osteopenia/osteoporosis in an HIV-infected male population. Wiener Klinische Wochenschrift 2007; 119:639–646 [DOI] [PubMed] [Google Scholar]
- 21.Cazanave C, Dupon M, Lavignolle-Aurillac V, Barthe N, Lawson-Ayayi S, Mehsen N, et al. Reduced bone mineral density in HIV-infected patients: prevalence and associated factors. AIDS 2008; 22:395. [DOI] [PubMed] [Google Scholar]
- 22.Bruera D, Luna N, David DO, Bergoglio LM, Zamudio J. Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS 2003; 17:1917–1923 [DOI] [PubMed] [Google Scholar]
- 23.Dolan SE, Carpenter S, Grinspoon S. Effects of weight, body composition, and testosterone on bone mineral density in HIV-infected women. J Acquir Immune Defic Syndr 2007; 45:161–167 [DOI] [PubMed] [Google Scholar]
- 24.Mallon PW. HIV and bone mineral density. Curr Opin Infect Dis 2010; 23:1. [DOI] [PubMed] [Google Scholar]
- 25.Bolland MJ, Grey AB, Gamble GD, Reid IR. Low body weight mediates the relationship between HIV infection and low bone mineral density: a meta-analysis. J Clin Endocrinol Metab 2007; 92:4522–4528 [DOI] [PubMed] [Google Scholar]
- 26.Martin A, Bloch M, Amin J, Baker D, Cooper DA, Emery S, et al. Simplification of antiretroviral therapy with tenofovir-emtricitabine or abacavir-lamivudine: a randomized, 96-week trial. Clin Infect Dis 2009; 49:1591–1601 [DOI] [PubMed] [Google Scholar]
- 27.Stellbrink HJ, Orkin C, Arribas JR, Compston J, Gerstoft J, Van Wijngaerden E, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis 2010; 51:963–972 [DOI] [PubMed] [Google Scholar]
- 28.Jacobson DL, Spiegelman D, Knox TK, Wilson IB. Evolution and predictors of change in total bone mineral density over time in HIV-infected men and women in the nutrition for healthy living study. J Acquir Immune Defic Syndr 2008; 49:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calmy A, Fux CA, Norris R, Vallier N, Delhumeau C, Samaras K, et al. Low bone mineral density, renal dysfunction, and fracture risk in HIV infection: a cross-sectional study. J Infect Dis 2009; 200:1746–1754 [DOI] [PubMed] [Google Scholar]
- 30.Bedimo R, Maalouf NM, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS 2012; 26:825. [DOI] [PubMed] [Google Scholar]
- 31.Fux CA, Rauch A, Simcock M, Bucher HC, Hirschel B, Opravil M, et al. Short communication Tenofovir use is associated with an increase in serum alkaline phosphatase in the Swiss HIV Cohort Study. Antivir=Ther 2008; 13:1077–1082 [PubMed] [Google Scholar]
- 32.Young B, Dao CN, Buchacz K, Baker R, Brooks JT. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000–2006. Clin Infect Dis 2011; 52:1061–1068 [DOI] [PubMed] [Google Scholar]
- 33.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large US healthcare system. J Clin Endocrinol Metab 2008; 93:3499–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paul TV, Asha HS, Thomas N, Seshadri MS, Rupali P, Abraham OC, et al. Hypovitaminosis D and bone mineral density in human immunodeficiency virus-infected men from India, with or without antiretroviral therapy. Endocrine Pract 2010; 16:547–553 [DOI] [PubMed] [Google Scholar]
- 35.Mulligan K, Glidden D, Gonzales P, Ramirez-Cardich ME, Liu AY, Namwongprom S, et al. Effects of emtricitabine/tenofovir on bone mineral density in seronegative men from 4 continents: DEXA results of the global iPrEx study. 18th Conference on Retroviruses and Opportunistic Infections, Boston, 2011. 2011 [Google Scholar]


