Abstract
Purpose of review
Long-acting antiretroviral (ARV) drugs may improve adherence to therapy and extend opportunities for therapeutic or prophylactic intervention to underserved patient populations. This review focuses on recent advances in the development of small molecule long-acting injectable ARV agents.
Recent findings
The need for combination ART and physicochemical and dosing limitations of current ARV drugs impede attempts to redevelop them as long-acting injectable formulations. However, the intrinsic properties of rilpivirine, a nonnucleoside reverse transcriptase inhibitor, and GSK1265744, an HIV-1 integrase strand transfer inhibitor, have enabled crystalline nanoparticle formulations to progress to clinical trials.
Summary
Investigational long-acting injectable nanoformulations of rilpivirine and GSK1265744 are clinical-stage development candidates. Complementary pharmacologic properties of both agents – different mechanisms of action, resistance profiles, metabolic pathways, lack of drug interactions and low daily oral doses – offer the potential for combination use. Phase I studies of the pharmacokinetics and safety of each long-acting formulation alone and in combination indicate that a monthly dosing regimen is possible for HIV treatment. An ongoing phase IIb trial of oral GSK1265744 and oral rilpivirine is evaluating this two-drug regimen for maintenance of virologic suppression; results will inform future studies using the injectable formulations. Additional preclinical and clinical studies indicate a potential use of each agent for HIV pre-exposure prophylaxis.
Keywords: GSK1265744, HIV-1, long-acting injectable antiretroviral, nanoformulation, rilpivirine, TMC278 LA
INTRODUCTION
Remarkable progress has been made in the global effort to defeat HIV infection with introduction and widespread scale-up of antiretroviral (ARV) treatment and prevention measures [1]. Combination therapy with HAART has significantly improved AIDS-related morbidity and mortality, extending the expected lifespan of patients with HIV. However, several factors contribute to the continuing issue of treatment failure and drug resistance, among them suboptimal drug efficacy and/or variable pharmacokinetics, inadequate compliance to lifelong therapy, pre-existing drug resistance and acute or chronic drug toxicities.
More recently, the use of ARV drugs for pre-exposure prophylaxis (PrEP) in high-risk populations has been validated through multiple clinical trials and led to regulatory approval of tenofovir/emtricitabine (TDF/FTC) for this indication in the USA [2–5]. Unfortunately, randomized clinical trials of TFD/FTC as PrEP have shown variable rates of efficacy, with low rates of protection correlated with drug nonadherence [6,7]. PrEP modalities that do not require coitally dependent ARV delivery or avoid daily ARV use may represent an effective alternative for HIV prevention.
Long-acting injectable ARV agents, capable of being administered on a monthly or less frequent basis, have the potential to improve adherence to therapy and extend opportunities for therapeutic or prophylactic intervention to underserved patient populations. This review will focus on recent advances in the development of small molecule long-acting injectable ARV agents with emphasis on two clinical-stage investigational agents, the HIV integrase strand transfer inhibitor (INSTI) GSK1265744 and the nonnucleoside reverse transcriptase inhibitor (NNRTI) rilpivirine (RPV; TMC278LA).
Box 1.
no caption available
DESIRED ATTRIBUTES OF LONG-ACTING ANTIRETROVIRALS FOR INJECTION
Long-acting injectable formulations of pharmacologic agents are well recognized as successful approaches for chronic indications such as contraception [8] and for certain psychiatric disorders [9,10]. These approaches, however, have not been employed for HIV therapy due to the physicochemical and dosing limitations of current ARV agents. The cornerstone of HIV therapy is the combination of appropriate ARV agents in a single regimen consisting of drugs with multiple mechanisms of action, a high genetic barrier to resistance, limited drug-drug interactions, minimal acute and chronic toxicity, and offering future treatment sequencing options. Optimally, a HAART regimen using long-acting drugs needs to be composed entirely of injectable drugs, have comparable efficacy with existing oral HAART, and should include the following characteristics: antiviral potency and pharmacokinetic characteristics allowing infrequent dosing (e.g., monthly) at a practical injection volume; no or minimal incremental toxicity related to method of administration; alternative oral formulations to facilitate treatment initiation and discontinuation; and physicochemical properties that enable formulation of sterile, injectable drug products with desirable stability, storage and administration attributes.
The majority of approved ARV agents is not well suited for redevelopment as long-acting injectable products using conventional pharmaceutical manufacturing approaches. In large part, this is due to insufficient antiviral potency, perhaps one or two orders of magnitude in scale, resulting in impractical monthly dosing requirements under an assumption of equivalent therapeutic drug exposures when compared with the daily, or more frequently administered, oral versions of the drugs in question. It is possible that novel and emerging technologies such as polymer-coated nanoparticle ARV drugs, in some instances also formulated with a targeting ligand such as folic acid to facilitate nanoparticle uptake in monocytes and macrophages, may address some limitations of current ARV drugs [11,12▪]. If successful, such targeting may enhance drug delivery to important tissues, reduce ARV dosing requirements and facilitate drug penetration into lymphoid and central nervous system sites. Intracellular nanoparticle ARV drug reservoirs within endosomes of monocytes and macrophages might supplement the release of drug from depot injection sites and further improve overall drug pharmacokinetics and pharmacodynamics [13▪].
Although not a focus of this review, biological approaches to HIV therapy take advantage of the longer intrinsic half-life of antibodies to allow for reduced dosing frequency. Ibalizumab (formerly TNX-355 and Hu5A8) is an anti-CD4 monoclonal antibody that disrupts viral entry and has demonstrated antiviral activity in early clinical studies. Ibalizumab is formulated as an intravenous infusion or subcutaneous injection and has demonstrated pharmacokinetics allowing for weekly or biweekly dosing intervals for the treatment of HIV [14].
PRESENT APPROACHES
Taking advantage of intrinsic drug properties and with the use of crystalline nanoparticle formulation methods, the poorly water-soluble ARV drugs RPV and GSK1265744 have been progressed into clinical trials. Both drugs have relatively low daily dosing requirements following oral administration, RPV 25 mg per day and GSK1265744 ≤30 mg per day (ViiV Healthcare, unpublished data), raising the potential for translation to a practical monthly dose. The two drugs have different metabolic pathways and no pharmacokinetic interaction [15]. Both RPV and GSK1265744 share physicochemical properties that permit formulation of sterile, injectable nanosuspensions. The ongoing clinical development program of these formulations has as a main objective the development of a long-acting, two-drug ARV regimen that can durably maintain HIV-1 control in virologically suppressed patients who elect to transition onto an injectable long-acting regimen. As with other long-acting depot injection medications, it is desirable to employ an oral lead-in phase with each drug to establish individual patient safety, tolerability and efficacy prior to transition to the long-acting formulations. The investigation of each agent as a potential ARV monotherapy for PrEP is also under clinical study.
GSK1265744
GSK1265744 is an HIV-1 INSTI and analogue of the inhibitor dolutegravir ([16,17]; ViiV Healthcare, unpublished data). The free acid of GSK1265744 has very low water solubility (<10 μg/ml). It is under clinical evaluation as both oral and long-acting injectable formulations. The drug is a subnanomolar inhibitor of HIV-1 integrase-catalyzed viral cDNA strand transfer with an in-vitro half maximal inhibitory concentration (IC50) of 0.22 and 0.34 nmol/l against HIV-1 BAL and NL432 in peripheral blood mononuclear cells, respectively. GSK1265744 is highly protein bound to serum albumin, resulting in a protein-adjusted 90% inhibitory concentration (PA-IC90) of 166 ng/ml. It has a plasma half-life of approximately 40 h following oral administration, allowing for once-daily dosing.
In double-blind, placebo-controlled proof-of-concept studies of GSK1265744 monotherapy in HIV-1-infected individuals receiving 5 or 30 mg once daily for 10 days, the drug significantly reduced plasma HIV-1 RNA from baseline to day 11 versus placebo (P < 0.001); mean decrease was 2.2–2.3 log10 copies/ml, respectively [18,19]. GSK1265744 was generally well tolerated, with no clinically relevant trends in laboratory values, vital signs or electrocardiograms.
A phase IIb, randomized, oral dose-ranging study is ongoing [20]. A total of 243 HIV-infected subjects were randomized to a blinded dose of GSK1265744 (10, 30 or 60 mg per day) or a control arm of efavirenz; all groups receive an investigator-selected backbone regimen of abacavir/lamivudine or TDF/FTC. After 24 weeks of triple therapy, patients randomized to GSK1265744 who achieved a plasma HIV-1 RNA level of less than 50 copies/ml enter a maintenance phase and modify their regimen to oral GSK1265744 and oral RPV 25 mg per day for an additional 72 weeks. Thus, the study evaluates a two-drug, two-class regimen of oral GSK1265744 and oral RPV for maintenance of virologic suppression. Study results will inform future trials using the long-acting injectable formulations.
The long-acting injectable nanosuspension of GSK1265744 is composed of crystalline active drug, milled to a median particle size of 200 nm, along with surfactant, polymer, tonicity agent and water for injection (ViiV Healthcare, unpublished data). The nanoparticles are essentially 100% active drug in contrast to matrix nanoparticles consisting of drug encased within a polymer or lipid matrix. This enables higher drug loading into the formulation (200 mg/ml) and a reduced injection volume.
Two phase I studies have reported the tolerability, safety and pharmacokinetics of GSK1265744 long-acting injection [19,21]. In a randomized, placebo-controlled, double-blinded, single-dose study, 56 HIV-uninfected adults received GSK1265744 as a gluteal intramuscular injection of 100, 200, 400 or 800 mg or an subcutaneous abdominal injection of 100, 200 or 400 mg or placebo injection vehicle. Following single intramuscular or subcutaneous injection, plasma drug concentrations increased rapidly over the first week, with sustained mean plasma concentrations above the PA–IC90 for approximately 24 weeks or longer for doses of at least 200 mg, as shown in Fig. 1.
FIGURE 1.
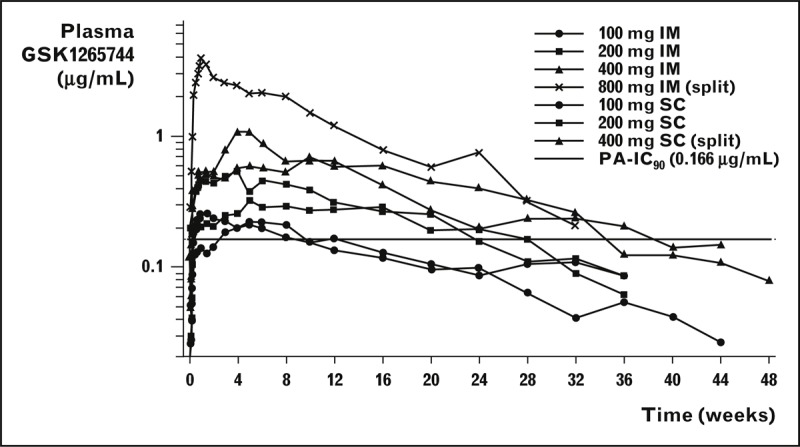
Mean plasma levels of GSK1265744 over time following single-dose administration of long-acting formulation [19]. GSK1265744 (200 mg/ml) was administered as a single IM (gluteal) or SC (abdominal) injection or equally divided IM or SC injection. Each cohort comprised eight healthy adult patients (six active/two placebo). The dashed line represents the plasma PA-IC90 value for GSK1265744 against wild-type HIV-1 of 0.166 μg/ml.
GSK1265744 was detected in plasma to 48 weeks. Mean absorption-limited apparent terminal-phase half-life ranged from 21 to 50 days as compared to approximately 40 h following single-dose oral administration. This longer apparent half-life following injection is a result of low solubility of the nanoparticle and inherent low tissue perfusion, prompting a slow absorption rate of drug from the injectable formulation with no change in the plasma elimination half-life of the dissolved active drug. When the injectable formulation was administered as two equally divided (split dosing) injections (Cohort 4, 400 mg × 2 intramuscular and Cohort 7, 200 mg × 2 subcutaneous), total drug release was increased so that the maximum observed plasma concentration (Cmax) was greater than dose proportional and there was a more pronounced decay in drug concentrations over time. However, overall extent of exposure [area under the curve (AUC) from time zero to infinity] did not change. The most common adverse event was a self-limited injection site reaction (ISR). ISRs were generally mild, with pain and erythema at the injection site reported as the most common findings. There were no systemic serious or grade 3 to 4 adverse events.
Two additional cohorts were included to evaluate drug distribution into rectal and cervicovaginal tissue to gain insight into the potential application of GSK1265744 for PrEP [22]. Sixteen male and female patients were randomized to two cohorts (4 males and 4 females per cohort) and received GSK1265744 400 mg intramuscular as a single injection or two 200-mg intramuscular gluteal injections. There were no placebo injections. Serial plasma sampling and rectal (males only) and cervicovaginal tissue biopsies were collected at Weeks 2 and 8 (single injection group) or Weeks 4 and 12 (split injection group). Plasma pharmacokinetic parameters of AUC0-Week4, AUC0-Week12 and Cmax were approximately two-fold higher and time to maximum plasma concentration occurred earlier following split administration as compared to the single injection, indicating split dosing increased the rate of absorption. Similar AUC0–∞ values between groups suggest that the extent of absorption was not affected by splitting the dose. GSK1265744 was detected in all tissues evaluated, with an apparent higher distribution in cervicovaginal tissue compared with rectal tissue. Median individual tissue:plasma drug concentration ratios ranged from 16 to 25% in the female genital tract and were 8% in rectal tissue in men. Median concentrations in vaginal tissue approach the plasma PA-IC90 of 166 ng/ml. Although observed GSK1265744 plasma concentrations following a single dose of 400 mg were, as predicted, lower than the estimated target therapeutic range, these results indicate therapeutic-range GSK1265744 plasma concentrations should produce tissue drug concentrations exceeding the PA-IC90 upon repeat administration and support its further investigation for PrEP.
In a second phase I study, HIV-negative adults received a 14-day lead-in of oral GSK1265744 30 mg per day to assess safety and tolerability before administration of the long-acting injection [21]. Patients were randomized into one of four cohorts: all received GSK1265744 800 mg intramuscular (as 400 mg × 2) as a loading dose followed by three monthly doses of 200 mg subcutaneous (Cohort 1); 200 mg intramuscular (Cohort 2); 400 mg intramuscular (Cohort 3); or after 3 months, a single further injection of 800 mg intramuscular (Cohort 4). Cohorts 2 and 3 also received gluteal intramuscular doses of TMC278 LA at months 3 (1200 mg) and 4 (900 or 600 mg, respectively). Forty-seven patients enrolled, with 40 receiving at least one long-acting injection; 38 completed all planned injections. Seven patients discontinued the oral lead-in phase, six for non-drug-related reasons and one for an adverse event (dizziness following first 30-mg dose). All dose cohorts achieved therapeutically relevant plasma concentrations within 3 days of the initial injection with prolonged exposure in excess of PA-IC90 for both drugs. RPV plasma concentrations following TMC278 LA dosing were comparable to steady-state concentrations observed from phase III studies of oral RPV dosed at 25 mg per day. Pharmacokinetic data suggest a once-monthly, synchronous intramuscular dosing schedule can be devised using clinically practicable dosing volumes and allowing a time window of several weeks for re-dosing to permit a degree of pharmacologic forgiveness. Co-administration was generally safe and well tolerated, with grade 1 ISRs being the most-commonly reported adverse events. GSK1265744 subcutaneous administration was associated with a greater number of ISR events per patient than observed after intramuscular administration with either GSK1265744 or TMC278 LA. Although mild-to-moderate ISRs occurred in a majority of study participants, the overall tolerability profile supports evaluation in longer-term clinical studies.
Finally, to further explore the potential of GSK1265744 for PrEP, a nonhuman primate intrarectal simian-HIV (SHIV) challenge study was performed [23]. Eight male rhesus macaques were dosed with GSK1265744 long-acting (50 mg/kg intramuscular) at two time points, 1 week prior to the first virus exposure and 4 weeks later. An additional eight male macaques were untreated and served as placebo controls. All animals were challenged intrarectally each week with SHIV162p3 [50 × tissue culture infectious dose 50 (TCID50)] for up to eight exposures. Infection status was monitored on a weekly basis. All eight placebo macaques became infected after a median of two rectal exposures (range 1–7) while none of the eight GSK1265744-treated animals had detectable systemic viremia 10 weeks after the last virus challenge. In protected animals, GSK1265744 plasma concentrations throughout the period of virus challenge were comparable to clinically relevant exposures in humans.
TMC278 LA — LONG-ACTING FORMULATION OF RILPIVIRINE
TMC278 LA injection is the investigational nanosuspension formulation of RPV. The oral tablet formulation of RPV was approved in 2011, in combination with other ARV drugs, for treatment of patients with HIV-1 infection who are ARV-naive with an HIV-1 RNA level of 100 000 copies/ml or less at the start of therapy. In addition, a fixed-dose combination of RPV/TDF/TFC is approved for the same indication.
Baert et al.[24▪] have published on the development of TMC278 LA nanosuspension injection. RPV free base is a stable crystalline polymorphic drug substance with very low water solubility (<0.1 mg/ml). Rilpivirine drug crystals are nanosized by a continuous wet milling process and suspended in aqueous vehicle with hydrophilic surfactant. In preclinical proof-of-concept studies, single injections of nano-formulated drug gave sustained release of RPV in plasma over 3 months in dogs and 3 weeks in mice. Further preclinical investigation of TMC278 LA 200 nmol/l injectable nanosuspension in rats and dogs included plasma pharmacokinetics, injection site concentrations and disposition to lymphoid tissues [25▪▪]. Rilpivirine plasma concentrations confirmed sustained and dose-proportional release lasting more than 2 months in rats and more than 6 months in dogs. Absolute bioavailability approached 100%, indicating complete release from the injection site. In dog, drug concentrations in the lymph nodes draining the intramuscular injection site exceeded plasma concentrations by over 100-fold 1 month after administration, while concentrations in lymphoid tissues decreased to 3-fold to 6-fold of the plasma concentrations beyond 3 months.
A subsequent phase I study of TMC278 LA 300 mg/ml nanosuspension evaluated the pharmacokinetics and safety of single gluteal intramuscular or abdominal subcutaneous injections in 51 HIV-negative adult patients at doses of 200, 400 and 600 mg, or vehicle (placebo) [26]. An additional nine patients received a single 400-mg injection into deltoid muscle. Consistent with the preclinical experience, rilpivirine was slowly released from the injection site into plasma, with drug concentrations of more than 10 ng/ml for 12–26 weeks. Dose proportionality and similar plasma concentration–time profiles were observed for both subcutaneous and gluteal intramuscular injections. ISRs consisted of redness, bruising, pain and to a lesser extent, induration, and were more frequent with active drug injections versus placebo. There was a greater frequency of ISRs with subcutaneous as compared to intramuscular administration. Among intramuscular injections, gluteal injections were better tolerated than deltoid injections. There were no serious adverse events or grade 3 or 4 adverse events.
Jackson et al.[27] reported results of a phase I clinical study of TMC278 LA pharmacokinetics in plasma, female genital tract and male rectum in HIV-negative adults. The investigators enrolled 20 women per dose group and evaluated single doses of 300, 600 and 1200 mg, while a sub-study enrolled six men who each received a single 600-mg dose. Blood and genital/rectal fluids were sampled to 84 days, and tissue biopsies were taken at three time points during the initial 28 days. All doses gave prolonged plasma and female genital tract and male rectum drug exposure. Among females, RPV concentrations in genital tract fluid were generally comparable to plasma drug concentrations, while vaginal tissue drug concentrations were 50% or more of the corresponding plasma measurement. In male patients, rectal fluid to blood plasma drug concentration ratios were 28 and 7% at Cmax and last observed concentration (Day 84), respectively. However, drug concentrations in rectal tissue were markedly higher and generally similar to blood plasma at study days 7 and 14. In total, these results support further evaluation of TMC278 LA as a potential PrEP strategy.
SPECIFIC DEVELOPMENT AND USE CONSIDERATIONS
There are several important considerations to highlight with respect to development of a long-acting injectable ARV regimen of GSK1265744 and TMC278 LA. Demonstration of safety and efficacy of an infrequently dosed ARV regimen delivered by a novel mode of administration must also be coupled with substantial evidence of the adequacy of a two-drug maintenance regimen to durably suppress viral replication. As rilpivirine is currently indicated for use in ARV-naive patients who have an HIV-1 RNA level of 100 000 copies/ml or less at the start of therapy, induction regimens will need to be tailored to the specifics of the clinical situation. Induction regimens that contain both oral GSK1265744 and oral rilpivirine are potentially attractive as such a strategy allows for a multiweek, individual patient-level assessment of tolerability, safety and efficacy prior to initial administration of the long-acting injectable formulations. Given prolonged systemic exposure and the inability to withdraw drug in the event of toxicity, a period of oral induction therapy or a lead-in period, perhaps of 1-month duration, is advisable. The latter refers to situations in which a patient is switching from suppressive oral ARV therapy with other drugs to a long-acting injectable ARV regimen of GSK1265744 and TMC278 LA. Additional strategies will need to be employed in HIV-1 positive patients discontinuing long-acting therapy, to prevent the evolution of resistance during a diminishing pharmacokinetic tail. Long-acting agents for the treatment of HIV must be compatible with existing oral HAART options, to allow for the transition to oral HAART during this tail. Both GSK1265744 and TMC278 LA have limited drug–drug interactions with existing ART classes.
CONCLUSION
The therapeutic potential of long-acting injectable ARV formulations for HIV therapy has not been evaluated due to an inability to formulate appropriate combination HAART using current ARV agents. Long-acting nanoparticle injectable formulations of the NNRTI RPV and INSTI GSK1265744 have enabled preliminary investigation of a once-monthly, two-drug regimen for maintenance of virologic suppression. Important considerations for successful clinical use must be addressed during development and include identification of appropriate oral induction or oral lead-in strategies for therapy initiation as well as additional strategies to assure HIV-1 infected patients discontinuing therapy do not risk emergence or evolution of drug resistance during a diminishing pharmacokinetic tail.
Acknowledgements
All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. The authors wish to acknowledge the following individual for editorial assistance during the development of this manuscript: Gina Uhlenbrauck.
Conflicts of interest
W.R.S. is an employee of GlaxoSmithKline, owns stock/stock options, and receives support for travel to meetings for the study or other purposes from GlaxoSmithKline. D.A.M. is an employee of GlaxoSmithKline and owns stock/stock options. J.C.P., Jr, is an employee of ViiV Healthcare and owns stock/stock options.
Source of funding: All authors are employees of GlaxoSmithKline or ViiV Healthcare.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Footnotes
Correspondence to William Spreen, PharmD, GlaxoSmithKline, 5 Moore Drive, Research Triangle Park, NC 27709, USA. Tel: +1 919 483 1393; fax: +1 919 315 5263; e-mail: william.r.spreen@gsk.com
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global report: UNAIDS report on the global AIDS epidemic 2012. Geneva, Switzerland:UNAIDS; 2012 [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. for the iPrEx Study Team Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, et al. for the Partners PrEP Study Team Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. for the TDF2 Study Group Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–434 [DOI] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration Press release: FDA approves first drug for reducing the risk of sexually acquired HIV infection. Silver Spring, MD: US Food and Drug Administration; 16 July 2012. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312210.htm [Accessed on 24 July 2013] [Google Scholar]
- 6.Van Damme L, Corneli A, Ahmed K, et al. for the FEM-PrEP Study Group Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrazzo J, Ramjee G, Nair G, et al. Preexposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE study (MTN 003) [Abstract no. 26LB]. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA 2013 [Google Scholar]
- 8.Westhoff C. Depot-medroxyprogesterone acetate injection (Depo-Provera®): a highly effective contraceptive option with proven long-term safety. Contraception 2003; 68:75–87 [DOI] [PubMed] [Google Scholar]
- 9.Adams CE, Fenton MKP, Quraishi S, David AS. Systematic meta-review of depot antipsychotic drugs for people with schizophrenia. B J Psychiatry 2001; 179:290–299 [DOI] [PubMed] [Google Scholar]
- 10.Schooler NR. Relapse and rehospitalization: comparing oral and depot antipsychotics. J Clin Psychiatry 2003; 64 Suppl 16:14–17 [PubMed] [Google Scholar]
- 11.Nowacek A, Miller S, McMillan R, et al. NanoART synthesis, characterization, uptake, release and toxicology for human monocyte-macrophage drug delivery. Nanomedicine (Lond) 2009; 4:903–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪.Puligujja P, et al. Macrophage folate receptor-targeted antiretroviral therapy facilitates drug entry, retention, antiretroviral activities and biodistribution for reduction of human immunodeficiency virus infections. Nanomedicine 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors describe the manufacture and in-vitro testing of polymer-coated ARV nanoparticles as a method to enhance macrophage uptake, retention and antiviral activity.
- 13▪.Gautam N, Roy U, Balkundi S, et al. Preclinical pharmacokinetics and tissue distribution of long-acting nanoformulated antiretroviral therapy. Antimicrob Agents Chemother 2013; 57:3110–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]; Nanoformulated atazanavir and ritonavir are prepared and evaluated in mice and monkey; results demonstrate blood and tissue drug concentrations are enhanced and prolonged compared with native drug.
- 14.Bruno CJ, Jacobson JM. Ibalizumab: an anti-CD4 monoclonal antibody for the treatment of HIV-1 infection. J Antimicrob Chemother 2010; 65:1839–1841 [DOI] [PubMed] [Google Scholar]
- 15.Ford S, Gould E, Chen S, et al. Lack of pharmacokinetic (PK) interaction between rilpivirine and the integrase inhibitors, dolutegravir and S/GSK1265744 [Abstract no. A-1249]. 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Underwood M, St Clair M, Johns B, et al. S/GSK1265744: a next generation integrase inhibitor (INI) with activity against raltegravir-resistant clinical isolates [Abstract no. MOAA0103]. 28th International AIDS Conference; Vienna, Austria 2010 [Google Scholar]
- 17.Yoshinaga T, Kobayashi M, Seki T, et al. Antiviral characteristics of S/GSK1265744, an HIV integrase inhibitor (INI) dosed by oral or long-acting parenteral injection [Abstract no. H-550]. 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA; 2012 [Google Scholar]
- 18.Min S, DeJesus E, McCurdy L, et al. Pharmacokinetics (PK) and safety in healthy and HIV-infected subjects and short-term antiviral efficacy of S/GSK1265744, a next generation once daily HIV integrase inhibitor [Abstract no. H-1228]. 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA; 2009 [Google Scholar]
- 19.Spreen W, Ford S, Chen S, et al. Pharmacokinetics, safety and tolerability of the HIV integrase inhibitor S/GSK1265744 long acting parenteral nanosuspension following single dose administration to healthy adults [Abstract no. TUPE040]. 19th International AIDS Conference; Washington, DC; 2012 [Google Scholar]
- 20.ViiV Healthcare Dose ranging study of GSK1265744 plus nucleoside reverse transcriptase inhibitors for induction of human immunodeficiency virus-1 (HIV-1) virologic suppression followed by virologic suppression maintenance by GSK1265744 plus rilpivirine. Bethesda, MD: National Library of Medicine; 2012. http://clinicaltrials.gov/ct2/show/NCT01641809 [Accessed on 24 July 2013] [Google Scholar]
- 21.Spreen W, Williams P, Margolis D, et al. First study of repeat dose co-administration of GSK1265744 and TMC278 long-acting parenteral nanosuspensions: pharmacokinetics, safety and tolerability in healthy adults [Abstract no. WEAB0103]. 7th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; Kuala Lumpur, Malaysia; 2013 [Google Scholar]
- 22.Ford S, Margolis D, Chen S, et al. Plasma and tissue GSK1265744 pharmacokinetics following long-acting parenteral administration in healthy male and female subjects [Abstract no. O_02]. 14th International Workshop on Clinical Pharmacology of HIV Therapy; Amsterdam, the Netherlands; 2013. [Google Scholar]
- 23.Andrews C, Gettie A, Russell-Lodrigue K, et al. Long-acting parenteral formulation of GSK1265744 protects macaques against repeated intrarectal challenges with SHIV [Abstract no. 24LB]. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA; 2013 [Google Scholar]
- 24▪.Baert L, van ’t Klooster G, Dries W, et al. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur J Pharm Biopharm 2009; 72:502–508 [DOI] [PubMed] [Google Scholar]; Authors describe development and preclinical proof of concept studies of injectable nanoformulation of rilpivirine.
- 25▪▪.Van ’t Klooster G, Hoeben E, Borghys H, et al. Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrob Agents Chemother 2010; 54:2042–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]; Preclinical experiments in rats and dogs demonstrate a rilpivirine 200 nanometer particle size injectable suspension provides drug release for up to 6 months. Drug concentrations in lymphoid tissues exceeded plasma concentrations suggesting macrophage uptake of nanoparticles and a secondary depot source of drug release. In addition, first human pharmacokinetic study of TMC278 long-acting injection is reported.
- 26.Verloes R, van ’t Klooster G, Baert L, et al. TMC278 long acting - a parenteral nanosuspension formulation that provides sustained clinically relevant plasma concentrations in HIV-negative volunteers. 17th International AIDS Conference; Mexico City, Mexico; 2008 [Google Scholar]
- 27.Jackson A, Else L, Tjia J, et al. Rilpivirine-LA formulation: pharmacokinetics in plasma, genital tract in HIV-females and rectum in males [Abstract no. 35]. 19th Conference on Retroviruses and Opportunistic Infections; Seattle WA; 2012 [Google Scholar]


