Abstract
A variety of structural abnormalities have been described in post traumatic stress disorder (PTSD), but only a few studies have focused on cortical thickness alterations in recent onset PTSD. In this study, we adopted surface-based morphometry (SBM), which enables an exploration of global structural changes throughout the brain, in order to compare cortical thickness alterations in recent onset PTSD patients, trauma-exposed subjects but without PTSD, and normal controls. Moreover, we used region of interest (ROI) partial correlation analysis to evaluate the correlation among PTSD symptom severity and significant changes of cortical thickness. The widespread cortical thickness reduction relative to the normal controls were found in bilateral inferior and superior parietal lobes, frontal lobes, hippocampus, cingulate cortex, and right lateral occipital lobes in trauma survivors, whereas cortical thickness was only increased in left calcarine cortex in PTSD group. The average cortical thickness of hippocampus and cingulate cortex decreased by 10.75% and 9.09% in PTSD, 3.48% and 2.86% in non PTSD. We further demonstrated that the cortical thicknesses of bilateral ACC and PCC, superior frontal lobes, and hippocampus are negatively correlated with CAPS scores in all trauma survivors. Our study results suggest that stress widens cortical thinning regions and causes more serious effect in recent onset PTSD than non PTSD. It also shows that the cortical thinning in recent onset PTSD predicts the symptom severity.
Keywords: Recent onset PTSD, Cortical thickness, Surface-based morphometry
Highlights
-
•
PTSD caused by rare, severe disaster (69 miners trapped in 1.4 km underground for 75 h).
-
•
The surface-based morphometry is based on such serious, sustained and acute trauma.
-
•
The comparisons are among healthy, survivors with, and without recent onset PTSD.
-
•
Hippocampus and cingulate thickness in PTSD decreased 3 times than non PTSD.
-
•
The altered regions in PTSD group are negatively correlated with CAPS scores.
1. Introduction
Neuroimaging studies have identified a number of functional and structural alterations in recent onset post-traumatic stress disorder (PTSD). Structural neuroimaging studies about recent onset PTSD focused primarily on gray matter volume alterations based on voxel based morphometry (VBM) and manual based hippocampal and amygdale volumetry. These studies have shown that recent onset PTSD is associated with smaller gray matter volume of limbic structures (Chen et al., 2006; Corbo et al., 2005; Wignall et al., 2004; Zhang et al., 2011). However, there are inconsistencies in previous study results (Bonne et al., 2001; Jatzko et al., 2006). Compared to the normal controls, decreased gray matter volumes or densities were identified in left or right hippocampus, left parahippocampal gyrus, bilateral calcarine cortex, left or right anterior cingulate cortex (ACC), bilateral or left insular cortex in recent onset PTSD subjects (Chen et al., 2006; Corbo et al., 2005; Zhang et al., 2011). Volumetric studies identified the smaller right hippocampus volume and whole brain volume (Wignall et al., 2004), while the longitudinal volumetric study did not detect any hippocampus volume changes (Bonne et al., 2003). As mentioned in our previous study, numerous confounding factors can be possible reasons for the difference of VBM study results, such as a different study method or trauma type or trauma duration time, or subtle cerebral cortex impairment (Landre et al., 2010; Zhang et al., 2011).
Cortical thickness is a reflection of the size, density, and arrangement of neurons, glial cells and nerve fibers (Narr et al., 2005). It is a parameter relatively invariant to brain size during mammalian evolution, as well as the content of, the neuropile (Keep and Jones, 1990). Although cellular characteristics cannot be quantified directly in imaging data, cortical thickness was thought to reflect cytoarchitectural abnormalities more closely than cortical volume does (Keep and Jones, 1990; Thompson et al., 2003).
VBM analyses are particularly sensitive to the degree of smoothing, differences in registration, and choice of normalization template (Bookstein, 2001; Jones et al., 2005; Park et al., 2004). Thus, the surface-based morphometry (SBM) analysis approach has been put forward as an alternative method for probing into the cortical gray matter changes, which also allows for the contributions of gray matter thickness and regional surface area to be defined independently. The FreeSurfer software is a feasible method to precisely quantify and characterize the cortical thickness in different brain regions (Fischl and Dale, 2000; Makris et al., 2006; van der Kouwe et al., 2008), and has been used in PTSD studies (Dickie et al., 2012; Geuze et al., 2008; Hunter et al., 2011; Kuhn et al., 2011; Landre et al., 2010; Liu et al., 2012; Lyoo et al., 2011; Woodward et al., 2009a).
In previous studies, imaging data support the neurocircuitry model of PTSD that emphasizes the functional relational relationship between the amygdale, ACC and hippocampus. However, most of previous studies focused on chronic PTSD and non PTSD cortical changes after trauma, and cortical thinning were found in parahippocampal gyrus, superior temporal cortex, and lateral orbital frontal cortex (Woodward et al., 2009b). ACC was a steady predication for PTSD recovery (Dickie et al., 2012), and cortical thickening after trauma exposure (Lyoo et al., 2011). However, there are many confounding factors may influence the results of chronic PTSD, such as substance use/abuse, trauma exposure time and trauma type. To reveal the subtle alteration in recent onset PTSD, trauma survivors from a single trauma event, with homogeneity in demographic variables and traumatic type, intensity, and duration of exposure, and avoided most comorbidity factors, may offer a distinct advantage in examining psychological consequences of PTSD and stress on brain structure.
The purpose of this study is to detect the pathogenesis of recent onset PTSD and stress by utilizing the SBM method. Based on the results of previous studies, we hypothesize that there may be explicit regional cortical thickness differences between the recent onset PTSD and trauma exposed non PTSD as compared with the healthy controls, and that the regional cortical thickness abnormalities may be associated with the severity of PTSD symptom.
2. Methods
2.1. Subjects
On July 29, 2007, a severe coalmine-flood disaster occurred at Zhijian Coalmine in Shanxian County in Henan Province. Sixty-nine male miners were rescued after a 75 hour ordeal in the darkness. Three months after the disaster, we attempted to contact all the 69 miners rescued. Forty-eight of them (69.6%) were successfully found and were willing to participate in the study. Six months later, 17 of these 48 coal mine disaster survivors met DSM-IV (Anon., 1994) diagnostic criteria for PTSD. 10 survivors and 5 survivors' wives with PTSD, 10 survivors without PTSD, and 25 normal controls were enrolled in the MRI study. The study protocol was approved by the Medical Ethical Committee of Xijing Hospital of the Fourth Military Medical University. Written voluntary and informed consent was obtained from all participants before the study. Each psychiatrist was trained to expertly use the DSM-IV and the Structured Clinical Interview for DSM-IV (SCID) (Spitzer et al., 1996) to diagnose PTSD. None of the subjects met diagnostic criteria for major depression, schizophrenia or bipolar disorder. The identified PTSD subjects had never received psychiatric treatment for their condition. Moreover, none of the subjects had a history of treatment with psychotropic drugs or of substance abuse. All subjects were free of metallic implants, neurological disorders and major medical conditions. The severity of their symptoms was assessed with the Clinician-Administered PTSD Scale (CAPS). The elapsed time between the traumatic event and the MRI scan ranged from 187 to 190 days.
2.2. Magnetic resonance imaging acquisition
All MRI scans were performed on a 3.0 T MR scanner (MAGNETOM Trio, Siemens AG, Erlangen, Germany) located in the Department of Radiology, Xijing Hospital. A high-resolution three-dimensional magnetization prepared rapid acquisition gradient echo (MPRAGE) T1-weighted sequence was used to acquire MR images of the whole brain (176 sagittal slices). The other MR imaging parameters applied in this study were as follows: TR = 1900 ms, TE = 2.26 ms, TI = 900 ms, flip angle = 9°, acquisition matrix = 256 × 256, field of view = 220 × 220 mm2 and 1.00 mm slice thickness with no inter-slice gap and isotropic resolution 1.0 × 1.0 × 1.0 mm3.
2.3. MR image processing
Cortical reconstruction and parcellation were performed with the FreeSurfer V4 package (v4.0.2) image analysis suite (Fischl and Dale, 2000), which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). This method is fully described (Du et al., 2007) and has been validated via both histological and manual measurements (Bischoff-Grethe et al., 2007; Pantazis et al., 2010). For cortical analysis, the gray matter–white matter interface of each hemisphere was segmented, tessellated, corrected for topological errors and inflated in order to unfold the cortical surface. Each individual hemispheric surface was then registered to a surface-based template using its sulco-gyral pattern to allow inter-subject analysis (Fischl and Dale, 2000). Cortical thickness was calculated from both intensity gradients across tissue class and continuity information, as the closest distance between the gray/white matter boundary and the gray/cerebro spinal fluid interface at each vertex of the tessellated surface (Fischl and Dale, 2000). Automated topology correction was performed as necessary (Fischl et al., 2001). We inspected each dataset at multiple points within the processing stream. We verified the Talairach transform, the accuracy of the skull strip, the accuracy of the white matter and pial surface segmentation, and finally inspected the cortical surface reconstruction for topological defects. Error correction was performed as necessary. Cortical thickness was measured as the distance between the gray/white matter boundary and the pial surface at each point on the cortical mantle. The data for each participant was resampled to an average participant and surface smoothing was performed using the ‘qcache’ command and a 10 mm full-width half-maximum Gaussian kernel, prior to statistical analysis. In this study, we get the cortical thickness values from the statistical output of FreeSurfer cortical parcellation, called “aparc.a2005s.stats”, which uses the Desikan–Killiany atlas, and has 75 regions.
Once the processing stream was satisfactorily completed, the QDEC (Query, Design, Estimate, Contrast) which is a FreeSurfer application intended to aid researchers in performing group averaging and inference on the morphometry data (cortical surface and volume), was used to explore cortical thickness differences between groups and the relation of cortical thickness to the cognitive performance variables(CAPS scores). Statistical parametric maps of the entire cortical mantle were generated to show group differences.
2.4. Statistical analysis
2.4.1. Cortical thickness analysis
A general linear model (GLM) was estimated at each vertex across the cortical surface, with cortical thickness as dependent variable, diagnosis and sex as categorical predictors, and age as continuous predictor. This procedure allows for a generation of statistical parametric maps to be plotted.
The maps show the distribution of p values for pair-wise comparisons between the diagnostic categories, as defined by the following contrasts: (1) PTSD versus the normal controls; (2) PTSD versus non-PTSD subjects; (3) non-PTSD subjects versus the normal controls. To correct multiple comparisons, p-maps were subject to an expected False Discovery Rate (FDR) correction of p < 0.05.
2.5. Correlation analysis
2.5.1. Correlations between ROI thickness and CAPS
The mean cortical thickness value of regions with significant cortical thinning was extracted from all trauma survivors. The mean regional cortical thickness and CAPS values were imported into the SPSS17.0 software (SPSS Inc., Chicago, IL) for correlation analysis, with age and gender being taken as covariate.
2.5.2. Cortical thickness change rates
To reduce the impact of individual differences, we measured the PTSD cortical thickness change rate in both PTSD and non PTSD, as cortical thickness change rate = (mean normal control − mean PTSD) × 100 / normal control cortical thickness. The same equation was also used for non PTSD cortical thickness change rate.
3. Results
3.1. Demographics
All subjects are right-handed, and there are no significant differences between groups in gender and age. All subjects in this study came from the same community and did not differ significantly in socioeconomic status (Table 1). As expected, the PTSD group had higher scores on the CAPS than the non-PTSD group (PTSD group: 84.25 ± 14.29; non-PTSD group: 33.15 ± 20.12) [F = 34.75; df = 23, p = 0.000].
Table 1.
Demographic characteristics of recent onset PTSD, non PTSD and normal controls.
| Variable | PTSD (mean + SD) |
Non-PTSD (mean + SD) |
Control (mean + SD) |
|---|---|---|---|
| Age | 41.6 ± 5.93 | 34.3 ± 7.37 | 36.6 ± 3.25 |
| Sex (male/female) | 10/5 | 10/0 | 22/3 |
| Marital status (Ma/Unma) | 14/1 | 10/0 | 23/2 |
| Smoked in previous 1 year | 11/4 | 8/2 | 10/15 |
| The length of being a miner | 4.7 ± 1.3 | 6.2 ± 1.1 | – |
| The length of education | 6.67 ± 2.06 | 7.25 ± 2.38 | 17 ± 2.14 |
| BMI | 26.34 ± 1.93 | 25.47 ± 1.55 | 25.12 ± 1.23 |
| CAPS | 84.25 ± 14.29 | 33.15 ± 20.12 | – |
| Global cortical thickness | 2.713 ± 0.109 | 2.721 ± 0.133 | 2.726 ± 0.193 |
PTSD, post-traumatic stress disorder; SD, standard deviation; CAPS, Clinician-Administered PTSD Scale; BMI, Body Mass Index; Ma, married; Unma, unmarried.
3.2. Cortical thickness
Global thickness measurements were examined by pair-wise comparisons, with age and gender as independent variables. There were no significant differences between groups in terms of whole brain average cortical thickness. But the cortex was significantly (FDR < 0.05) altered both in PTSD and non-PTSD, compared to the healthy controls (Table 2).
Table 2.
Cortical thickness in recent onset PTSD, non-PTSD and normal control.
| Cortical area | Mean (SD) in mm |
PTSD vs. non-PTSD |
PTSD vs. Healthy |
|||||
|---|---|---|---|---|---|---|---|---|
| Side | PTSD (n = 15) |
Non-PTSD (n = 10) |
Healthy (n = 25) |
T value | P value | T value | P value | |
| Cortical thickness decreasing (PTSD < control) | ||||||||
| Superior frontal gyrus | Left | 2.389 (0.128) | 2.422 (0.125) | 2.609 (0.251) | − 3.615 | 0.022 | − 4.835 | 0.008 |
| Right | 2.411 (0.247) | 2.477 (0.148) | 2.543 (0.268) | − 2.669 | 0.036 | − 4.203 | 0.013 | |
| Rostral middle frontal gyrus | Left | 2.147 (0.220) | 2.157 (0.196) | 2.252 (0.181) | − 1.644 | NS | − 1.863 | NS |
| Right | 2.199 (0.217) | 2.228 (0.234) | 2.331 (0.195) | − 2.293 | 0.042 | − 3.301 | 0.032 | |
| Hippocampal gyrus | Left | 2.291 (0.208) | 2.457 (0.215) | 2.549 (0.169) | − 3.202 | 0.028 | − 4.141 | 0.014 |
| Right | 2.361 (0.141) | 2.523 (0.224) | 2.610 (0.145) | − 3.914 | 0.019 | − 4.635 | 0.009 | |
| Inferior parietal lobes | Left | 2.678 (0.175) | 2.826 (0.186) | 2.922 (0.201) | − 2.845 | 0.033 | − 4.843 | 0.007 |
| Right | 2.692 (0.177) | 2.728 (0.182) | 2.812 (0.159) | − 2.323 | 0.041 | − 2.684 | 0.035 | |
| Superior parietal lobes | Left | 2.254 (0.144) | 2.318 (0.145) | 2.368 (0.137) | − 1.093 | NS | − 2.963 | 0.033 |
| Right | 2.322 (0.109) | 2.421 (0.157) | 2.468 (0.127) | − 1.527 | NS | − 3.307 | 0.032 | |
| Lateral occipital gyrus | Left | 2.815 (0.284) | 2.874 (0.267) | 2.941 (0.297) | − 1.368 | NS | − 1.788 | NS |
| Right | 2.554 (0.285) | 2.649 (0.253) | 2.757 (0.313) | − 3.048 | 0.032 | − 2.534 | 0.040 | |
| Anterior cingulate cortex (ACC) | Left | 2.721 (0.224) | 2.961 (0.219) | 3.080 (0.344) | − 3.755 | 0.028 | − 3.409 | 0.029 |
| Right | 2.628 (0.335) | 2.882 (0.224) | 2.972 (0.348) | − 3.643 | 0.021 | − 4.083 | 0.017 | |
| Posterior cingulate cortex (PCC) | Left | 2.601 (0.113) | 2.756 (0.141) | 2.771 (0.189) | − 3.286 | 0.029 | − 3.309 | 0.031 |
| Right | 2.607 (0.157) | 2.715 (0.115) | 2.799 (0.168) | − 3.048 | 0.032 | − 3.443 | 0.025 | |
| Cortical thickness thickening (PTSD > control) | ||||||||
| Calcarine gyrus | Left | 2.536 (0.301) | 2.517 (0.388) | 2.461 (0.253) | 2.058 | 0.047 | 4.426 | 0.011 |
| Right | 2.586 (0.240) | 2.563 (0.333) | 2.517 (0.254) | 1.579 | NS | 1.692 | NS | |
PTSD, post-traumatic stress disorder; SD, standard deviation. Results significant at false discovery rate threshold 0.05; NS: not significant.
3.3. Cortical thickness analysis in groups
3.3.1. PTSD vs. healthy control
3.3.1.1. PTSD < healthy control
Cortical thickness was significantly decreased in PTSD compared with the healthy control subjects in multiple regions across the cerebral cortex. In the left hemisphere, the cortical thinning regions were in the superior frontal gyrus, hippocampal gyrus, inferior parietal lobes, anterior cingulate cortex and posterior cingulate cortex. In the right hemisphere, the thinning regions were in the superior frontal gyrus, rostral middle frontal gyrus, hippocampal gyrus, superior parietal lobes, inferior parietal lobes, lateral occipital gyrus, anterior cingulate cortex, and posterior cingulate cortex (Fig. 1).
Fig. 1.
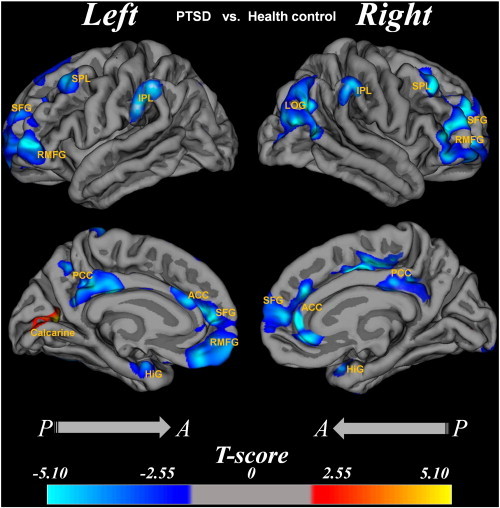
Cortical thickness difference between the PTSD patients and the normal controls.
Region abbreviations from FreeSurfer model: SFG, superior frontal gyrus; HiG, hippocampal gyrus; IPL, inferior parietal lobes; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; SFG, superior frontal gyrus; RMFG, rostral middle frontal gyrus; LOG, lateral occipital gyrus. The T value color was overlaid on the reconstruction image, and the color bar was shown at the bottom. T values in voxelwise statistical maps were calculated by a general linear model of cortical thickness at each voxel covarying for age and gender. Indicated regions in yellow illustrate where the cortices of PTSD patients are thicker than that of the normal controls at corrected p < 0.05 (FDR corrected), while the indicated region in blue illustrates where the cortices of PTSD patients are thinner than that of the normal controls at corrected p < 0.05 (FDR corrected). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3.1.2. PTSD > healthy control
There was cortical thickening only in the left calcarine cortex (Fig. 1).
3.3.2. PTSD vs. non-PTSD
3.3.2.1. PTSD < non-PTSD
In the left hemisphere, the cortical thinning regions were in the superior frontal lobes, hippocampus, the ACC and PCC. In the right hemisphere, the cortical thinning regions were in the lateral occipital gyrus, rostral middle frontal gyrus, hippocampus, the ACC and PCC.
3.3.2.2. PTSD > non-PTSD
There was cortical thickening only in the left calcarine cortex (Fig. 2).
Fig. 2.
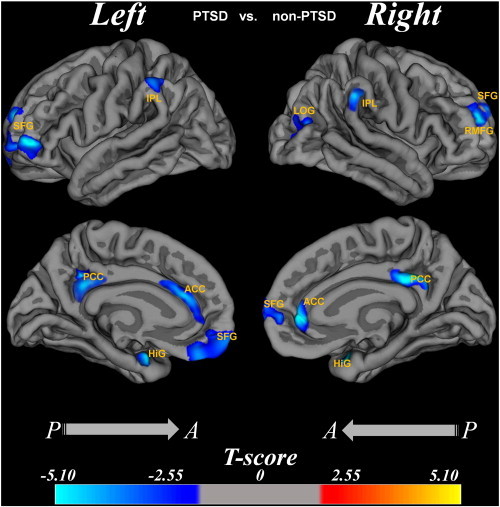
The cortical thickness difference between PTSD group and non-PTSD group.
Region abbreviations from FreeSurfer model: SFG, superior frontal gyrus; HiG, hippocampal gyrus; IPL, inferior parietal lobes; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; SFG, superior frontal gyrus; RMFG, rostral middle frontal gyrus; LOG, lateral occipital gyrus. The T value color was overlaid on the reconstruction image, and the color bar was shown at the bottom. The age and gender effects were removed by regression.
3.3.3. Non-PTSD vs. healthy control
The cortical thickness did not show significant difference between the two groups. There were no significant findings for this contrast.
3.3.4. ROI analysis
3.3.4.1. Correlations between regional cortical thickness and CAPS
There were negative correlations of cortical thickness with CAPS in the cingulated cortex, inferior parietal cortex, superior frontal cortex and hippocampus (as shown in Table 3, Fig. 3).
Table 3.
Correlations of CAPS scores with regional cortical thickness.
| Regions | Peak coordinates (X, Y, Z) |
Pearson r value | P value | Thickness at peak voxel | Mean thickness of ROI (SD) mm |
|---|---|---|---|---|---|
| Right hemisphere | |||||
| Inferior parietal lobes | (− 13,− 79,18) | − 0.524 | 0.007 | 2.675 | 2.717 (0.18) |
| Superior frontal lobes | (− 10,91,− 23) | − 0.484 | 0.014 | 2.384 | 2.395 (0.21) |
| Hippocampal gyrus | (2,− 24,− 48) | − 0.422 | 0.035 | 2.289 | 2.298 (0.13) |
| ACC | (35,73,− 7) | − 0.473 | 0.017 | 2.725 | 2.811 (0.31) |
| PCC | (31,− 74,− 14) | − 0.455 | 0.024 | 2.667 | 2.713 (0.24) |
| Left hemisphere | |||||
| Inferior parietal lobes | (21,− 65,18) | − 0.407 | 0.043 | 2.593 | 2.613 (0.13) |
| Superior frontal lobes | (− 6,51,47) | − 0.490 | 0.013 | 2.457 | 2.499 (0.28) |
| Hippocampal gyrus | (0,− 16,− 52) | − 0.482 | 0.015 | 2.381 | 2.393 (0.22) |
| ACC | (− 34,41,21) | − 0.472 | 0.017 | 2.629 | 2.631 (0.24) |
| PCC | (4,− 64,− 14) | − 0.413 | 0.039 | 2.651 | 2.681 (0.19) |
CAPS, Clinician-Administered PTSD Scale. Results significant at false discovery rate threshold p < 0.05; PCC, posterior cingulate cortex; ACC, Anterior cingulate cortex; x, y, and z coordinates were located the peak vertex by Qdec surface coordinates.
Fig. 3.
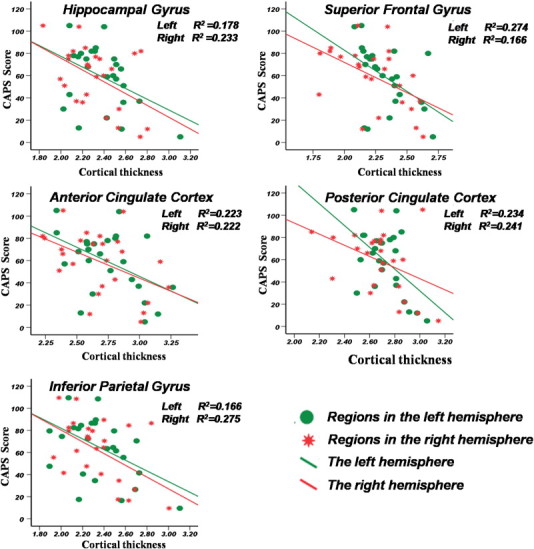
Correlations between CAPS and regions thickness analysis.
X-axis represents the mean cortical thickness where the cortex with significant change. Y-axis represents the CAPS score value, the Pearson's correlation analysis was used, the r2 value was shown in the top right corner. The green circles represent the absolute regions values in the left hemisphere. The red stars represent the absolute regions values in the right hemisphere. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In ROI analysis, compared with the healthy controls, the average cortical thickness of the hippocampus and the cingulate cortex decreased by 10.75% and 9.09% in PTSD, by 3.48% and 2.86% in non PTSD. The detail showed that the left part of the hippocampus decreased by 10.13% in PTSD and by 3.61% in non PTSD; the right part of the hippocampus decreased by 11.34% in PTSD and by 3.35% in non PTSD; the left part of the anterior cingulate cortex decreased by 11.76% in PTSD and by 3.89% in non PTSD; the right part of the anterior cingulate cortex decreased by 11.57% in PTSD and by 3.03% in non PTSD; the left part of posterior cingulate cortex decreased by 6.11% in PTSD and 1.51% in non PTSD; the right part of the posterior cingulate cortex decreased by 6.95% in PTSD and by 3.03% in non PTSD (and the details list in Table 2).
4. Discussion
The main findings of the present study are: 1) There is widespread regional cortical thinning in recent onset PTSD patients relative to non PTSD subjects and the normal controls. The region of cortical thinning in recent PTSD patients is much larger than that of non PTSD subjects. The cortical thinning region in PTSD is primarily in the bilateral hippocampus, ACC and PCC, left inferior and right superior parietal lobes, left superior frontal lobe, and right superior rostral middle frontal gyrus. The cortical thickening in recent onset PTSD patients is only in the left calcarine cortex. 2) There is no significant cortical thickness difference between non-PTSD and the normal control. The mean global cortical thickness of the three groups did not show significant difference. 3) In ROI analysis, the cortical thickness of the bilateral hippocampus, the cingulate cortex and frontal gyrus negatively correlated with the CAPS score in all trauma victims. 4) The cortical thinning rate of the bilateral hippocampus and cingulate cortex in recent onset PTSD patients is much larger than that in non-PTSD subjects. These results imply that severe stress has profoundly selective effects on the medial-frontal cerebral cortex, and cortical thinning in those regions may be associated with the development of recent onset PTSD and PTSD symptom severity.
The decreased gray matter volume and density in different regions were found by the VBM method (Bonne et al., 2008; Jatzko et al., 2006; Nardo et al., 2010; Zhang et al., 2011), but the VBM method does not have the specificity for the highly convoluted structures of the cortex (Makris et al., 2006). Surface-based morphometry is a highly reliable MRI-derived neuroanatomical measurement developed recently, which overcomes the shortcomings of volume-based analysis, and is unaffected by voxel geometry (Wonderlick et al., 2009). The multiple-plane surface-based analysis keeps the advantage of surface-based analysis over volume-based analysis (De Leebeeck et al., 2007), and can accurately show the signs of cortical morphological compensatory plasticity in recent onset PTSD (Wang et al., 2010).
The cortical thinning observed in the present study is partially consistent with the observations in previous studies about cortical thickness in recent onset or chronic PTSD (Geuze et al., 2008; Liu et al., 2012; Rauch et al., 2003; Woodward et al., 2009b), but is different from the observations in some previous studies (Landre et al., 2010; Lyoo et al., 2011). This may be due to the following reasons: first, in previous studies, the victim suffered from different traumas, such as combat related trauma (Geuze et al., 2008; Woodward et al., 2009b), sexual abuse (Landre et al., 2010) and subway disaster (Lyoo et al., 2011). The different trauma types and trauma duration time may have different effects on the subjects' brain structures, and thus may cause the heterogeneity of study results. Second, in previous studies, the comparisons are between PTSD and non PTSD (Geuze et al., 2008; Liu et al., 2012; Woodward et al., 2009b), or between PTSD and the normal controls (Landre et al., 2010; Lyoo et al., 2011), which may neglect or mix the stress effects with PTSD effect on brain structure. There is evidence to show that gray matter volume of the limbic region has decreased in traumatized non PTSD subjects (Ganzel et al., 2008) which provide evidence that the stress is associated with structural changes of cerebral cortex. Third, most previous studies were about chronic PTSD (Geuze et al., 2008; Landre et al., 2010; Lyoo et al., 2011; Woodward et al., 2009b). Their results reflected the pathogenesis of chronic PTSD, and the increased cortical thickness was found in recovery from chronic PTSD (Lyoo et al., 2011), possibly causing the inconsistency in the study results. In the present study, the subjects share the same traumatic event, avoiding the above-mentioned confounding factors, and the cortical thickness was measured 6 months after trauma, so it is very likely to ascertain that the cortical thickness reductions in the current study were associated with recent onset PTSD caused by a single prolonged trauma.
The hippocampus has been shown to play a role in contextual fear conditioning, and context modulation of extinction retention (Corcoran and Maren, 2001), and it is crucially involved in the encoding, consolidation and retrieval of declarative memories (Bekes, 2010). Dysfunction of the hippocampus is thought to be a central mechanism in the development of PTSD (Karl et al., 2006; Liberzon and Sripada, 2008; Wignall et al., 2004). Smaller bilateral hippocampal volume relative to the normal controls was also found in non PTSD subjects (Etkin and Wager, 2007), suggesting that the stress also has an effect on the hippocampus. Similar results were found in both recent onset PTSD (Zhang et al., 2011) and chronic PTSD (Bonne et al., 2008), and they were also verified in animal study (Golub et al., 2011). In the current study, we found bilateral hippocampus cortical thickness reductions in recent onset PTSD that is different from previous studies (Geuze et al., 2008; Liu et al., 2012; Woodward et al., 2009b), and may be associated with severe stress or recent onset PTSD. The cortical thinning rate of the bilateral hippocampus in recent onset PTSD patients is much larger than that in non-PTSD subjects. These results imply that severe stress has profoundly selective effects on the hippocampus cortex, and may be associated with PTSD symptom severity.
The present study shows that the bilateral cingulate cortex including ACC and PCC is thinning in recent PTSD, that is partially consistent with the results of meta-analyses of voxel-based morphometry studies of PTSD (Karl et al., 2006; Rauch et al., 2003). However, for the first time, we report cortical thinning in bilateral PCC in recent onset PTSD. The ACC plays a key role in frontolimbic networks associated with emotional regulating and cognitive functions (Paus, 2001). The cortical thickness of ACC correlates with PTSD symptom improvement (Dickie et al., 2012), implying that the functional recovery of PTSD correlates with ACC cortical thickness. PCC is also related to a series of higher cognitive functions involved in PTSD, such as episodic and autobiographical memory, and self-referential processing (Liberzon and Sripada, 2008). Moreover, it has been suggested that PCC could be implicated in coping with physical threats, and plays a role in the processing of distressing material. Recently, fMRI studies have shown that spontaneous activity in the default network during rest is altered in PTSD (Bluhm et al., 2009; Wu et al., 2011). Thus, the PCC cortical thinning in the present study may suggest dysfunction in the processing of distressing material in severe trauma-exposed victims with and without recent onset PTSD. The average cortical thickness of the cingulate cortex decreased almost 3 times in recent onset PTSD than in non-PTSD. This result implies that severe stress has profoundly selective effects on the bilateral cingulate cortex.
In the present study, we find bilateral cortical thinning in the superior and inferior parietal and superior frontal lobe. The frontal lobe associates with the function of learning and memory, and the parietal lobe has extensive connections with all these brain regions (Liberzon and Sripada, 2008). The structural alteration observed in the inferior frontal lobe is related to fear inhibition, while the medial frontal cortex is related to conditional fear elimination (Jelicic and Merckelbach, 2004). Previous fMRI study also provided evidence that traumatic emotional reactions have a primary impact on the prefrontal systems engaged by the conscious processing of fear signals (Williams et al., 2006). Moreover, PTSD patients had abnormal frontal and parietal activity during working memory (Etkin and Wager, 2007), and a lower cerebral perfusion in the right parietal cortex (Chung et al., 2006; Schuff et al., 2011). Some authors suggest that apparent memory deficits in PTSD may due to impairment in attention and concentration in the frontal lobe, rather than in hippocampus dysfunction (Rossi et al., 2006).
There was cortical thinning in the right lateral occipital gyrus, and thickening in the left calcarine cortex in recent onset PTSD. These regions are involved in visual information processing. It has been proved that the visual cortex has morphological compensatory plasticity in acquired strong visual stimulation (Noppeney et al., 2005). The fire disaster (Bronner et al., 2008; Chen et al., 2006) and mine-related trauma have strong visual stimulations (Zhang et al., 2011), which may also result in visual-cortex thinning. This result may associate with the type of trauma. The survivors in the current study were trapped in a total darkness environment for 75 h, thus cortical thickness increase may reflect the compensatory effect of the visual cortex. In addition, lateral occipital gyrus may be involved in the higher-order cognitive functions (Cavanna and Trimble, 2006), the cortical thinning of lateral occipital gyrus may result cognitive impairment.
We also find that CAPS is significantly correlated with the cortical thickness of the region and that the cortical thickness had significant changes, such as the inferior parietal and superior frontal lobes and the limbic system. Furthermore, the different regions have different scales of relevance. This is consistent with previous studies (Bryant et al., 2008; Liu et al., 2012), and may be related to the loss of neurons, as the duration of stress may be positively correlated with the degree of dendritic atrophy (Bonne et al., 2008).
We acknowledge that the present study has several limitations that will be addressed in future study. First the sample size of the current study is small, thus statistical issues may obscure the subtle changes of cortical thickness. Second, non PTSD subjects in our study are all male, although the related study did not find a gender effect on hippocampus volume, especially between PTSD and trauma-exposed controls (Nardo et al., 2010). It would be better to include female subjects in the non PTSD group. Third, the average years in education of our study subjects are much lower than that of the previous studies. There is a report that lower pre-trauma intelligence increases the risk of developing PTSD symptoms (McFarlane, 1997) which may have an impact on the current study results. Moreover, we get the cortical thickness values from the older version of the software package (v4.0.2), which has shown that the calculations, given the same input, can vary up to 15% between different versions of the software in recent research (Gronenschild et al., 2012). In order to investigate the regionally subtle cortical thickness alterations, we would process the data by FreeSurfer version later than V5.2.0 in future research.
In conclusion, the current study provides evidence that recent onset PTSD has a distinct pattern of cortical thinning in the limbic system, the frontal and parietal regions, and that may associate with pathophysiology of recent onset PTSD caused by a single prolonged trauma exposure. The severe stress still has an effect on the cerebral cortex in the same region as recent onset PTSD but at a different scale in non PTSD. Furthermore, cortical alterations of limbic structural, parietal and frontal cortex correlate with current PTSD symptom severity. These findings suggest that the severe stress and recent onset PTSD pathogenesis is not a regional specific but a different scale in cortical thinning. The cortical thinning region is much larger than that found in VBM study, suggesting that the cortical thickness may be a more sensitive biomarker than gray matter volume in exploring pathophysiological change of recent onset PTSD.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (81171278) and National Key Technology R&D Program in the 11th Five-year Plan of China (2007 BA 117B02). We would like to thank Zhenyu Zhou (GE Healthcare) for the assistance with the manuscript. The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Anon. Guidelines for psychiatric practice in public sector psychiatric inpatient facilities. Committee on State and Community Psychiatric Systems of the Council on Psychiatric Services. American Psychiatric Association. Am. J. Psychiatry. 1994;151:797–798. doi: 10.1176/ajp.151.5.797. [DOI] [PubMed] [Google Scholar]
- Bekes V. The etiological models of posttraumatic stress disorder. Psychiatr. Hung. 2010;25:133–141. [PubMed] [Google Scholar]
- Bischoff-Grethe A., Ozyurt I.B., Busa E., Quinn B.T., Fennema-Notestine C., Clark C.P., Morris S., Bondi M.W., Jernigan T.L., Dale A.M., Brown G.G., Fischl B. A technique for the deidentification of structural brain MR images. Hum. Brain Mapp. 2007;28:892–903. doi: 10.1002/hbm.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R.L., Williamson P.C., Osuch E.A., Frewen P.A., Stevens T.K., Boksman K., Neufeld R.W., Theberge J., Lanius R.A. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J. Psychiatry Neurosci. 2009;34:187–194. [PMC free article] [PubMed] [Google Scholar]
- Bonne O., Brandes D., Gilboa A., Gomori J.M., Shenton M.E., Pitman R.K., Shalev A.Y. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am. J. Psychiatry. 2001;158:1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne O., Brandes D., Segman R., Pitman R.K., Yehuda R., Shalev A.Y. Prospective evaluation of plasma cortisol in recent trauma survivors with posttraumatic stress disorder. Psychiatry Res. 2003;119:171–175. doi: 10.1016/s0165-1781(03)00098-2. [DOI] [PubMed] [Google Scholar]
- Bonne O., Vythilingam M., Inagaki M., Wood S., Neumeister A., Nugent A.C., Snow J., Luckenbaugh D.A., Bain E.E., Drevets W.C., Charney D.S. Reduced posterior hippocampal volume in posttraumatic stress disorder. J. Clin. Psychiatry. 2008;69:1087–1091. doi: 10.4088/jcp.v69n0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein F.L. “Voxel-based morphometry” should not be used with imperfectly registered images. NeuroImage. 2001;14:1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Bronner M.B., Knoester H., Bos A.P., Last B.F., Grootenhuis M.A. Posttraumatic stress disorder (PTSD) in children after paediatric intensive care treatment compared to children who survived a major fire disaster. Child Adolesc. Psychiatry Ment. Health. 2008;2:9. doi: 10.1186/1753-2000-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R.A., Felmingham K., Whitford T.J., Kemp A., Hughes G., Peduto A., Williams L.M. Rostral anterior cingulate volume predicts treatment response to cognitive-behavioural therapy for posttraumatic stress disorder. J. Psychiatry Neurosci. 2008;33:142–146. [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen S., Xia W., Li L., Liu J., He Z., Zhang Z., Yan L., Zhang J., Hu D. Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometric study. Psychiatry Res. 2006;146:65–72. doi: 10.1016/j.pscychresns.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Chung Y.A., Kim S.H., Chung S.K., Chae J.-H., Yang D.W., Sohn H.S., Jeong J. Alterations in cerebral perfusion in posttraumatic stress disorder patients without re-exposure to accident-related stimuli. Clin. Neurophysiol. 2006;117:637–642. doi: 10.1016/j.clinph.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Corbo V., Clement M.H., Armony J.L., Pruessner J.C., Brunet A. Size versus shape differences: contrasting voxel-based and volumetric analyses of the anterior cingulate cortex in individuals with acute posttraumatic stress disorder. Biol. Psychiatry. 2005;58:119–124. doi: 10.1016/j.biopsych.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Corcoran K.A., Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J. Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leebeeck A., Kumar L.K., de Lange V., Sinton D., Gordon R., Brolo A.G. On-chip surface-based detection with nanohole arrays. Anal. Chem. 2007;79:4094–4100. doi: 10.1021/ac070001a. [DOI] [PubMed] [Google Scholar]
- Dickie E.W., Brunet A., Akerib V., Armony J.L. Anterior cingulate cortical thickness is a stable predictor of recovery from post-traumatic stress disorder. Psychol. Med. 2012;1–9 doi: 10.1017/S0033291712001328. [DOI] [PubMed] [Google Scholar]
- Du A.T., Schuff N., Kramer J.H., Rosen H.J., Gorno-Tempini M.L., Rankin K., Miller B.L., Weiner M.W. Different regional patterns of cortical thinning in Alzheimer's disease and frontotemporal dementia. Brain. 2007;130:1159–1166. doi: 10.1093/brain/awm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Liu A., Dale A.M. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Ganzel B.L., Kim P., Glover G.H., Temple E. Resilience after 9/11: multimodal neuroimaging evidence for stress-related change in the healthy adult brain. NeuroImage. 2008;40:788–795. doi: 10.1016/j.neuroimage.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E., Westenberg H.G., Heinecke A., de Kloet C.S., Goebel R., Vermetten E. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. NeuroImage. 2008;41:675–681. doi: 10.1016/j.neuroimage.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Golub Y., Kaltwasser S.F., Mauch C.P., Herrmann L., Schmidt U., Holsboer F., Czisch M., Wotjak C.T. Reduced hippocampus volume in the mouse model of Posttraumatic Stress Disorder. J. Psychiatr. Res. 2011;45:650–659. doi: 10.1016/j.jpsychires.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Gronenschild E.H., Habets P., Jacobs H.I., Mengelers R., Rozendaal N., van Os J., Marcelis M. The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS One. 2012;7:e38234. doi: 10.1371/journal.pone.0038234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter M., Villarreal G., McHaffie G.R., Jimenez B., Smith A.K., Calais L.A., Hanlon F., Thoma R.J., Canive J.M. Lateralized abnormalities in auditory M50 sensory gating and cortical thickness of the superior temporal gyrus in post-traumatic stress disorder: preliminary results. Psychiatry Res. 2011;191:138–144. doi: 10.1016/j.pscychresns.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatzko A., Rothenhofer S., Schmitt A., Gaser C., Demirakca T., Weber-Fahr W., Wessa M., Magnotta V., Braus D.F. Hippocampal volume in chronic posttraumatic stress disorder (PTSD): MRI study using two different evaluation methods. J. Affect. Disord. 2006;94:121–126. doi: 10.1016/j.jad.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Jelicic M., Merckelbach H. Traumatic stress, brain changes, and memory deficits: a critical note. J. Nerv. Ment. Dis. 2004;192:548–553. doi: 10.1097/01.nmd.0000135494.20416.59. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Symms M.R., Cercignani M., Howard R.J. The effect of filter size on VBM analyses of DT-MRI data. NeuroImage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Karl A., Schaefer M., Malta L.S., Dorfel D., Rohleder N., Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci. Biobehav. Rev. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Keep R.F., Jones H.C. Cortical microvessels during brain development: a morphometric study in the rat. Microvasc. Res. 1990;40:412–426. doi: 10.1016/0026-2862(90)90036-q. [DOI] [PubMed] [Google Scholar]
- Kuhn S., Schubert F., Gallinat J. Structural correlates of trait anxiety: reduced thickness in medial orbitofrontal cortex accompanied by volume increase in nucleus accumbens. J. Affect. Disord. 2011;134:315–319. doi: 10.1016/j.jad.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Landre L., Destrieux C., Baudry M., Barantin L., Cottier J.P., Martineau J., Hommet C., Isingrini M., Belzung C., Gaillard P., Camus V., El Hage W. Preserved subcortical volumes and cortical thickness in women with sexual abuse-related PTSD. Psychiatry Res. 2010;183:181–186. doi: 10.1016/j.pscychresns.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Liberzon I., Sripada C.S. The functional neuroanatomy of PTSD: a critical review. Prog. Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li Y.J., Luo E.P., Lu H.B., Yin H. Cortical thinning in patients with recent onset post-traumatic stress disorder after a single prolonged trauma exposure. PLoS One. 2012;7:e39025. doi: 10.1371/journal.pone.0039025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo I.K., Kim J.E., Yoon S.J., Hwang J., Bae S., Kim D.J. The neurobiological role of the dorsolateral prefrontal cortex in recovery from trauma. Longitudinal brain imaging study among survivors of the South Korean subway disaster. Arch. Gen. Psychiatry. 2011;68:701–713. doi: 10.1001/archgenpsychiatry.2011.70. [DOI] [PubMed] [Google Scholar]
- Makris N., Kaiser J., Haselgrove C., Seidman L.J., Biederman J., Boriel D., Valera E.M., Papadimitriou G.M., Fischl B., Caviness V.S., Jr., Kennedy D.N. Human cerebral cortex: a system for the integration of volume- and surface-based representations. NeuroImage. 2006;33:139–153. doi: 10.1016/j.neuroimage.2006.04.220. [DOI] [PubMed] [Google Scholar]
- McFarlane A.C. Post-traumatic stress disorder: the importance of clinical objectivity and systematic research. Med. J. Aust. 1997;166:88–90. doi: 10.5694/j.1326-5377.1997.tb138729.x. [DOI] [PubMed] [Google Scholar]
- Nardo D., Hogberg G., Looi J.C., Larsson S., Hallstrom T., Pagani M. Gray matter density in limbic and paralimbic cortices is associated with trauma load and EMDR outcome in PTSD patients. J. Psychiatr. Res. 2010;44:477–485. doi: 10.1016/j.jpsychires.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Narr K.L., Toga A.W., Szeszko P., Thompson P.M., Woods R.P., Robinson D., Sevy S., Wang Y., Schrock K., Bilder R.M. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol. Psychiatry. 2005;58:32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Noppeney U., Friston K.J., Ashburner J., Frackowiak R., Price C.J. Early visual deprivation induces structural plasticity in gray and white matter. Curr. Biol. 2005;15:R488–490. doi: 10.1016/j.cub.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Pantazis D., Joshi A., Jiang J., Shattuck D.W., Bernstein L.E., Damasio H., Leahy R.M. Comparison of landmark-based and automatic methods for cortical surface registration. NeuroImage. 2010;49:2479–2493. doi: 10.1016/j.neuroimage.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.J., Levitt J., Shenton M.E., Salisbury D.F., Kubicki M., Kikinis R., Jolesz F.A., McCarley R.W. An MRI study of spatial probability brain map differences between first-episode schizophrenia and normal controls. NeuroImage. 2004;22:1231–1246. doi: 10.1016/j.neuroimage.2004.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Rauch S.L., Shin L.M., Segal E., Pitman R.K., Carson M.A., McMullin K., Whalen P.J., Makris N. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14:913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- Rossi S., Cappa S.F., Ulivelli M., De Capua A., Bartalini S., Rossini P.M. rTMS for PTSD: induced merciful oblivion or elimination of abnormal hypermnesia? Behav. Neurol. 2006;17:195–199. doi: 10.1155/2006/793256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N., Zhang Y., Zhan W., Lenoci M., Ching C., Boreta L., Mueller S.G., Wang Z., Marmar C.R., Weiner M.W., Neylan T.C. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: an MRI study. NeuroImage. 2011;54(Suppl. 1):S62–68. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer C., Freyberger H.J., Kessler C. Hysteria, dissociation and conversion. A review of concepts, classification and diagnostic instruments. Psychiatr. Prax. 1996;23:63–68. [PubMed] [Google Scholar]
- Thompson P.M., Hayashi K.M., de Zubicaray G., Janke A.L., Rose S.E., Semple J., Herman D., Hong M.S., Dittmer S.S., Doddrell D.M., Toga A.W. Dynamics of gray matter loss in Alzheimer's disease. J. Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kouwe A.J., Benner T., Salat D.H., Fischl B. Brain morphometry with multiecho MPRAGE. NeuroImage. 2008;40:559–569. doi: 10.1016/j.neuroimage.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Garfinkel S.N., King A.P., Angstadt M., Dennis M.J., Xie H., Welsh R.C., Tamburrino M.B., Liberzon I. A multiple-plane approach to measure the structural properties of functionally active regions in the human cortex. NeuroImage. 2010;49:3075–3085. doi: 10.1016/j.neuroimage.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wignall E.L., Dickson J.M., Vaughan P., Farrow T.F., Wilkinson I.D., Hunter M.D., Woodruff P.W. Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biol. Psychiatry. 2004;56:832–836. doi: 10.1016/j.biopsych.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Williams L.M., Kemp A.H., Felmingham K., Barton M., Olivieri G., Peduto A., Gordon E., Bryant R.A. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. NeuroImage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Wonderlick J.S., Ziegler D.A., Hosseini-Varnamkhasti P., Locascio J.J., Bakkour A., van der Kouwe A., Triantafyllou C., Corkin S., Dickerson B.C. Reliability of MRI-derived cortical and subcortical morphometric measures: effects of pulse sequence, voxel geometry, and parallel imaging. NeuroImage. 2009;44:1324–1333. doi: 10.1016/j.neuroimage.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward S.H., Kaloupek D.G., Grande L.J., Stegman W.K., Kutter C.J., Leskin L., Prestel R., Schaer M., Reiss A.L., Eliez S. Hippocampal volume and declarative memory function in combat-related PTSD. J. Int. Neuropsychol. Soc. 2009;15:830–839. doi: 10.1017/S1355617709990476. [DOI] [PubMed] [Google Scholar]
- Woodward S.H., Schaer M., Kaloupek D.G., Cediel L., Eliez S. Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Arch. Gen. Psychiatry. 2009;66:1373–1382. doi: 10.1001/archgenpsychiatry.2009.160. [DOI] [PubMed] [Google Scholar]
- Wu R.Z., Zhang J.R., Qiu C.J., Meng Y.J., Zhu H.R., Gong Q.Y., Huang X.Q., Zhang W. Study on resting-state default mode network in patients with posttraumatic stress disorder after the earthquake. Sichuan Da Xue Xue Bao Yi Xue Ban. 2011;42:397–400. [PubMed] [Google Scholar]
- Zhang J., Tan Q., Yin H., Zhang X., Huan Y., Tang L., Wang H., Xu J., Li L. Decreased gray matter volume in the left hippocampus and bilateral calcarine cortex in coal mine flood disaster survivors with recent onset PTSD. Psychiatry Res. 2011;192:84–90. doi: 10.1016/j.pscychresns.2010.09.001. [DOI] [PubMed] [Google Scholar]


