Abstract
Background:
Decompressive craniectomies (DC) mandate future cranioplasties, accounting for the large array of biomaterials for this purpose. Polymethylmethacrylate (PMMA) is a very reliable thermoplastic that can be prefabricated or even molded intraoperatively to create an adequate prosthesis. Preformed PMMA implants made by hand have been superseded by newer 3-D printed implants, but this is accompanied by higher costs and timing issues, apart from having limited availability in developing and third-world countries.
Methods:
A total of 26 patients were operated over a span of 11 years. A total of 26 custom hand-made PMMA prostheses were fabricated using original bone flaps with the aid of a prosthodontist, in a process that took approximately 70 minutes for each implant. The result was an exact duplication of the patient's bone flap.
Results:
Of the 26 patients who underwent cranioplasty, the majority of patients were males, with a mean age of 39.2 years and traumatic brain injury as main indication for DC. After a mean interval of 2.4 months, all 26 patients underwent a cranioplasty and prosthesis placement. Only two patients (7.6%) suffered from direct cranioplasty-related complications after a median follow-up of 10.4 months. Median Glasgow Outcome Scale scores improved significantly from 3 to 4 after cranioplasty (P = 0.008).
Conclusion:
Prefabrication of custom PMMA prostheses by hand when original bone flaps are available is an excellent alternative to newer 3-D printing techniques, because it is relatively cheaper, less time consuming, and offers excellent results in terms of anatomical reconstruction and improvement of neurological function in long-term follow-ups.
Keywords: Cranioplasty, decompressive craniectomy, prosthesis, polymethylmethacrylate
INTRODUCTION
Cranioplasty is defined as the surgical repair of acquired defects or congenital deformities of the cranium. It is performed mainly for anatomical reconstruction, brain protection, and cosmetics, but evidence has shown that there is also improvement of brain physiology and patient self-esteem.[11,20,23,27,37] The most common harms leading to cranioplasty include: Decompressive craniectomies (DC), tumor infiltration of calvarial bones, complications of previous cranioplasties and congenital deformities.[11,19]
Nowadays, DCs are the most common neurosurgical procedures requiring a future cranioplasty and they are usually performed after severe head trauma or severe cerebrovascular events. Performing a cranioplasty and thus reconstructing the skull after a DC poses a challenge to neurosurgeons, plastic reconstructive surgeons, and maxillofacial surgeons since the procedure often comprises very large skull defects, postoperative infections (due to foreign material implantation), subdural or epidural accumulations, seizures, postoperative hemorrhage, cerebrospinal fluid (CSF) leaks, and/or neurological deficits.[6,17]
Materials utilized for cranial reconstruction include bone, auto/allografts, distinct biomaterials, and even osteoinductive growth factors.[29] One of the most popular alloplastic materials utilized for this purpose is polymethylmethacrylate (PMMA), a conventional transparent thermoplastic first utilized for cranioplasty in the 1940s, during and after World War II.[13,38] PMMA has the notable advantage that it can be molded intraoperatively or prefabricated into the shape of the cranial defect.
Prefabrication of PMMA prostheses by hand has been used since the 1970s employing various procedures,[12,22,30,40] but these methods appear to have been shadowed by newer computer-aided design and computer-aided manufacturing (CAD/CAM) techniques, which basically consist of using imaging from the patients’ cranial defect and prefabricating the PMMA prosthesis using a 3-D printer.[7,8,10,16,18,24,32] Unfortunately, the use of these techniques becomes a challenge for developing and third-world countries, in which limited economic and logistical resources do not allow for the extensive use of such technology. This rationale defends the need for safe and alternative techniques for cranial reconstruction, especially when the patient's bone flap is available.
The purposes of this paper are to describe a technique to fabricate PMMA prostheses by hand using original bone flaps, and at the same time describe the surgical outcomes of this procedure. We also intend to demonstrate that these prostheses are a relatively nonexpensive, cosmetically and functionally acceptable alternative to newer 3-D printers.
MATERIALS AND METHODS
Between the years 2002 and 2013, a total of 26 patients underwent DC and subsequent cranioplasty with preformed PMMA prostheses made by hand. Patients in whom own bone flaps were not available were excluded. Patients’ records were thoroughly reviewed for data extraction. Patient age, sex, indication for initial DC, size of DC, interval time between DC and cranioplasty, duration of surgery, postoperative complications, follow up time and Glasgow Outcome Scale scores (GOSs) at the time of cranioplasty and at last follow-up visit were reviewed.
At the time of initial DC, the removed bone flaps were thoroughly irrigated with saline solution, cleaned from tissue debris and stored in a conventional refrigerator at the hospital laboratory. The custom PMMA prostheses were fabricated with the aid of a prosthodontist.
Prosthesis fabrication
The bone flap was inspected and the burr holes filled with methylmethacrylate (MMA; Codman Cranioplastic, Type 1-Slow Set, Johnson and Johnson, Raynham, MA) in a power-to-liquid ratio of 2:1. Thin areas of the bony flap were augmented with MMA to increase thickness to 2-3 mm. The C-Silicone (Speedex Putty, Coltène/Whaledent, Altstatten, Switzerland) was put over the working table in a sufficient amount to cover the internal surface of the bony flap, and was then mixed with the Universal Activator (Speedex Universal Activator, Coltène/Whaledent, Altstatten, Switzerland) for 2 minutes. The internal surface of the bony flap was covered with petroleum jelly body lotion and placed over the mixture and an impression was made in 5 minutes (the C-Silicone should not surpass the lateral borders of the bony flap). The same process was repeated for the external surface of the bony flap [Figure 1a]. Once both surfaces were impressed, the mold was opened and covered with petroleum jelly over both surfaces [Figure 1b]. The internal aspect of the mold was slowly filled with MMA, avoiding spillage as much as possible [Figure 1c]. Once a fair amount of internal surface was covered, the external mold was placed over the MMA for several seconds to give shape to the external surface. This process took about 40 minutes. Finally, the PMMA prosthesis was removed from the C-Silicone mold and excess protrusions at the margins were trimmed with a rongeur. The result is an exact duplication of the patient's bone flap [Figure 1d].
Figure 1.
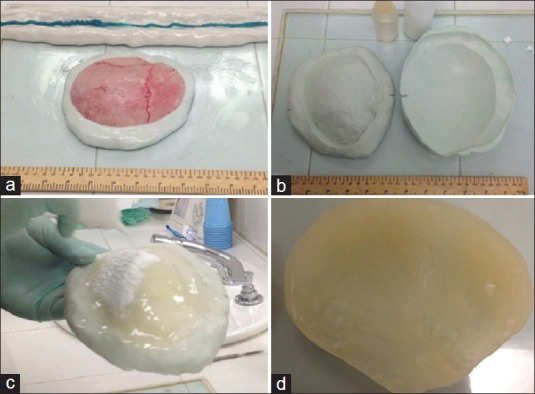
Key steps in the PMMA prosthesis fabrication
Surgical technique for prosthesis placement
The PMMA prosthesis is sterilized with plasma prior to surgery. Under general anesthesia, the scalp is reopened and dissected from the dura. The defect's borders are freed from any adjacent tissue. The temporal muscle is dissected and freed from the skin flap. To reattach it, several small holes (3 mm) are made on the PMMA prosthesis, and fixed with Nylon sutures [Figure 2]. The prosthesis is put in place and secured utilizing miniplates (The first five prostheses placed were secured with CranioFix® Titanium Clamps). A subcutaneous drain is put in place for 24 hours, and the scalp is closed in a conventional fashion.
Figure 2.
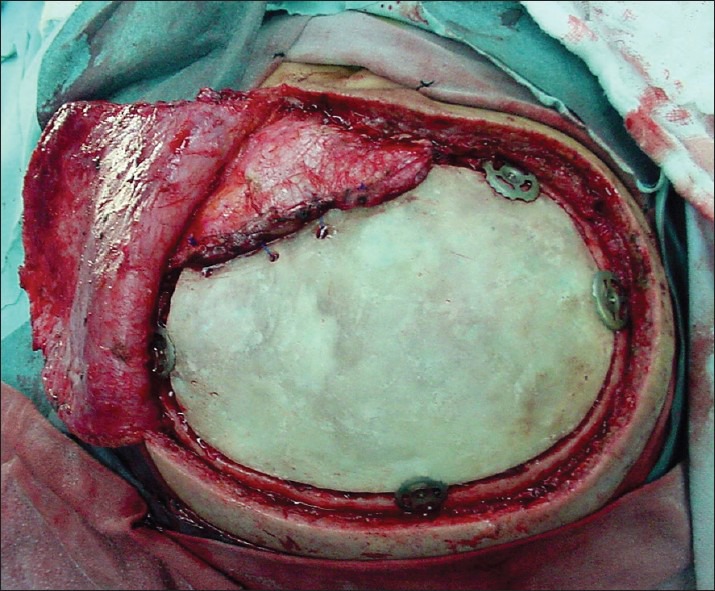
Reattachment of the temporalis muscle to the PMMA prosthesis. Several holes are made on the prosthesis and the muscle is secured utilizing nylon sutures. Our first cases involved fixing of the PMMA prosthesis with titanium clamps
Statistical analysis
The Mann–Whitney U Test was used to compare median GOSs. The results were considered significant when the P value was < 0.05. Descriptive results are presented as the mean ± SD when applicable. All data were analyzed using the statistical analysis add-on for Microsoft Excel for Mac (Microsoft, 2011).
RESULTS
A total of 26 PMMA prostheses were fabricated and consequently a total of 26 cranioplasties were performed between the years 2002 and 2013 [Table 1]. Mean patient age at cranioplasty was 39.2 ± 20.1 years with a range of 6.8-76.7 years. The majority of patients were males (65.4%) and the most common indication for initial DC was traumatic brain injury in 69.2% of cases, followed by hemorrhagic cerebrovascular events (spontaneous subarachnoid hemorrhage) in 19.2% of cases. All DCs were one-sided frontoparietal-temporal, and 92.4% of them were larger than 12 cm on the longest longitudinal measurement.
Table 1.
Summary of findings of 26 patients who underwent decompressive craniectomy and subsequent cranial reconstruction with prefabricated custom hand-made PMMA prostheses
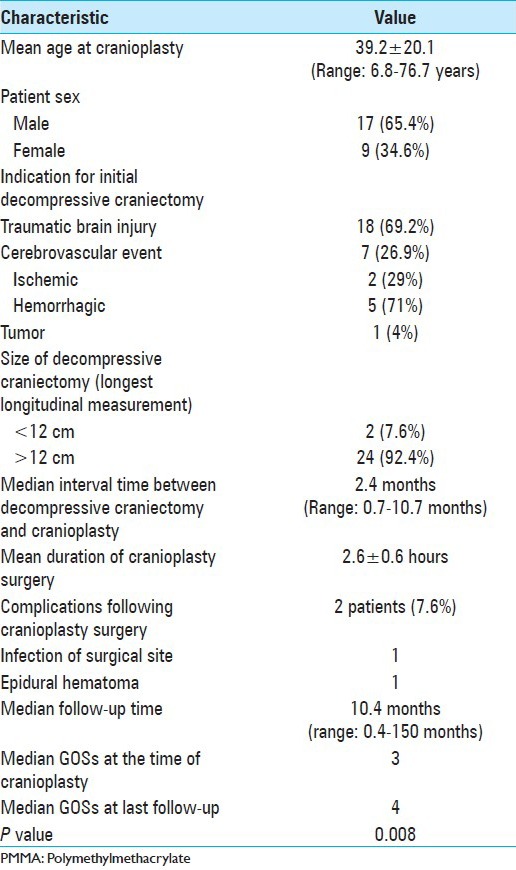
The median time between the initial DC and cranioplasty was 2.4 months, and during this time the prostheses were fabricated. Mean fabrication time for each prosthesis with this technique was 70 minutes, with a cost of US$800. Mean surgical time for prosthesis placement was 2.6 ± 0.6 hours. After a median follow-up time of 10.4 months, only two patients (7.6%) presented with cranioplasty-related complications: One epidural hematoma and one surgical infection. The first patient presented with the hematoma 2 days after surgery and required drainage; there was no need for prosthesis removal. The second patient presented with surgical infection in the form of an epidural collection 4 months after cranioplasty, which required debridement, lavage, and PMMA prosthesis removal. Two months later, the defect was reconstructed with a titanium mesh. Fortunately, both patients did well on follow-up visits. Although not a direct cranioplasty complication, another two patients (7.6%) presented with temporal muscle atrophy, which is a widely known complication, not only of DCs[5,31] but also in many cranial operations involving that region.
We measured the GOSs of patients at the time of cranioplasty and found a median score of 3. This score improved significantly after the cranioplasty and was found to be 4 at last follow-up (P = 0.008).
Patient satisfaction was assessed on follow-up visits. Out of the 26 patients, 24 (92.4%) felt comfortable with their aesthetic result, and did not inquire about a second operation for cosmetic improvement [Figure 3]. Only the two patients who suffered from temporal muscle atrophy requested an additional surgical intervention for cosmetic improvement, which involved filling of the defect with silicone.
Figure 3.
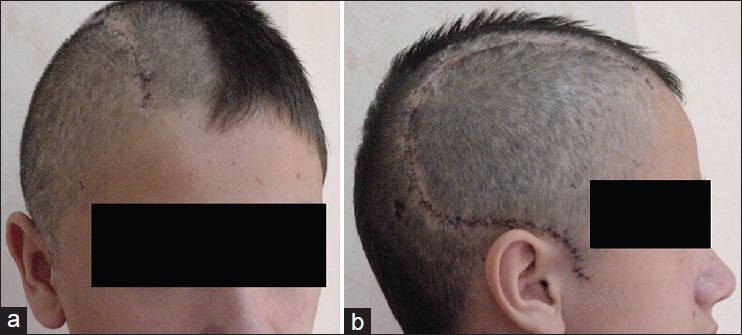
Postoperative result. (a) Right oblique view; (b) Right lateral view
DISCUSSION
The cranioplasty technique is itself an art, and the persistence of neurosurgical procedures and trauma has nourished this expanding field. Early cranioplasties date back to 3000 BC,[35] and the challenge of not just “filling a hole” but creating an adequate topographical substitute for a cranial defect has led to a numerous array of biomaterials including bone autografts, allografts, and xenographs; metals such as aluminum, gold, silver, and titanium; celluloids, PMMA, polyethylene, silicon, and many others.[2,35]
Autogenous bone has been historically preferred over alloplastic materials to reconstruct the cranium, due to allegedly better mechanical, biologic, and immunologic properties.[26,34] Autogenous bone is available in the form of the original patient's bone flap or through bone harvesting. The latter technique frequently requires additional surgeries for harvesting and has associated morbidities, and its use has decreased substantially over the years. In contrast, when attempting to use the original bone flap, the neurosurgeon faces the challenge of not always having the complete flap available due to the initial traumatic event (complex skull fractures, gunshot injuries, etc.). When the flap is in fact available, the challenge now is how to keep it “alive” during the waiting period. The most used method is freezing the bone and storing it in a bone bank, a process that keeps the bone matrix architecture, but causes tissues to nonetheless “die.” Freezing the bone results in partial resorption, especially in large craniectomies and sometimes requires an additional surgery for correction.[28] Another method still used today is to store the craniectomy flap in the fatty tissue of the abdomen. However, handling a bone flap in the abdomen becomes a challenge when other abdominal surgeries such as gastrostomies and ventriculo-peritoneal shunt placements are needed. Additionally, this method entails an additional surgical wound, abdomen scar and potential infection, jeopardizing the bone's viability.
When autogenous bone is not available, alloplastic materials are required. It is agreed that the ideal implant material should have the following characteristics: It must fit the cranial defect and achieve complete closure, be biocompatible, inert, nonthermal conducting, radio-transparent, nonmagnetic, lightweight, rigid, simple to shape, easily applicable, and inexpensive.[3,7] Nowadays, both titanium and PMMA are the most widely used alloplastic materials.[26,28] However, titanium is more expensive[26] and harder to manufacture than PMMA.[34]
PMMA has the advantages of being inert, radio-transparent, nonmagnetic, simple to shape, relatively inexpensive and with adequate mechanical properties.[1,14,15] As mentioned earlier, PMMA implants can be prefabricated or molded intraoperatively. Prefabrication is technically simpler, and it has the advantage over intraoperative molding of reduced surgical time, blood loss and infection rate; satisfaction of aesthetic result is also greater.[24]
Prefabrication of PMMA implants can be done in two ways: By hand or with CAD/CAM techniques. Hand-fabrication is cheaper and less time-consuming than using 3-D computed tomography data from a patient and a 3-D printer to fabricate the prosthesis,[10,24] but the latter method has gained popularity over the last years because it does not require the original bone flap and has yielded outstanding fitting and cosmetic results. When CAD/CAM methods are not available due to economic, logistical, or preferential matters, hand-fabrication of the PMMA prosthesis is done with techniques depending on whether or not the original bone flap is available. If it is not available, there exist methods such as the use of plaster applied to the patient's head as a “negative” impression and ultimately creating an acrylic flap,[25] preforming of the prosthesis in the dental laboratory using a mold of the patient's bony defect as a model,[9,21] doing a primary replication of the bone flap in wax and then using this pattern to fabricate the definitive prosthesis,[12,30,36] and others.[22,33] When the bone flap is available, such as in this series of patients, one can fabricate an exact PMMA copy of the bone flap using impression materials as a mold.[40]
The technique described in this paper uses C-silicone, which is a very easy-to-use and moldable impression material. Total manufacturing time was less than 2 hours, and the prosthesis had a cost of US$800. Three-dimensional printed prostheses, in contrast, take more than 8 hours to manufacture[24] and have a cost of US$1000-5000.[10] At the time of cranioplasty, all prostheses fabricated with our method were perfectly fitting, and a satisfactory aesthetic result was achieved in the majority of cases.
One of the most interesting benefits of cranioplasty is the improvement of neurologic function, which was proven on this paper with the significant improvement of the GOSs from 3 to 4 (P = 0.008) from the time of cranioplasty to last follow-up visit. The improvement of neurologic function is attributed to changes in brain physiology, particularly improvements on cerebral blood flow, cerebrovascular reserve capacity, and even cerebral glucose metabolism.[11,20,23,27,37]
Complications related to PMMA implants present in 9.2-23% of patients, with infection being the most common and feared with a rate of 9.2-19%, because most of the time it requires reoperation and implant removal.[4,6,21,26] Other complications include postoperative hematoma, chronic pain, scalp erosion, and migration of the implant.[21] Of note, our complication rate has been of only 7.6%, with infection being of only 3.8%.
Timing of cranioplasty is still controversial, with evidence showing either a slight decrease in infection rate when performed early (within 3 months of craniectomy) or no particular advantage of early versus delayed surgical repair.[6,39] We endorse early cranioplasty, trying to diminish infection rates as much as possible.
The drawbacks of the method described in this paper are that (1) The original bone flap must be available (in the case of skull fractures, large bone fragments can still be joined together for prosthesis fabrication); (2) PMMA is not incorporated into and vascularized by contiguous bone (compared with autologous bone); and (3) It initially requires the aid of a posthodontist or technician, but the technique is easy to learn and can be carried out by neurosurgeons alone.
In most cases when a DC is done, the large bone flap is available and it is up to the neurosurgeon to decide what to do with it. In larger tertiary-care hospitals or first-world countries, bone flaps may be disposed of in favor of using CAD/CAM techniques for prosthesis fabrication. In other settings, the bone may be stored in a freezer for later use or used to fabricate an exact duplication with the technique described herein.
CONCLUSION
Hand-fabrication of PMMA prostheses is an excellent alternative to CAD/CAM prostheses when original bone flaps are available. The use of impression materials to duplicate the patient's bone flap is a cheap and safe way to reconstruct the cranium when time and cost are an important limitation. Infection, being the most feared complication of allograft implants, has a very low rate with the technique described in this paper. The benefits of cranioplasty on neurologic function are also validated. This technique has proven to be safe and has yielded excellent results.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/1/136/119535
Disclaimer: The authors of this article have no conflicts of interest to disclose, and have adhered to SNI's policies regarding human/animal rights, and informed consent. Advertisers in SNI did not ask for, nor did they receive access to this article prior to publication.
Contributor Information
Enrique Caro-Osorio, Email: ecaro@itesm.mx.
Rafael De la Garza-Ramos, Email: rafdelag@gmail.com.
Sergio R. Martínez-Sánchez, Email: sergiorene@itesm.mx.
Félix Olazarán-Salinas, Email: felixolazaran@gmail.com.
REFERENCES
- 1.Akan M, Karaca M, Eker G, Karanfil H, Akoz T. Is polymethylmethacrylate reliable and practical in full-thickness cranial defect reconstructions? J Craniofac Surg. 2011;22:1236–9. doi: 10.1097/SCS.0b013e31821c0f34. [DOI] [PubMed] [Google Scholar]
- 2.Aydin S, Kucukyuruk B, Abuzayed B, Aydin S, Sanus GZ. Cranioplasty: Review of materials and techniques. J Neurosci Rural Pract. 2011;2:162–7. doi: 10.4103/0976-3147.83584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blake DP. The use of synthetics in cranioplasty: A clinical review. Mil Med. 1994;159:466–9. [PubMed] [Google Scholar]
- 4.Blum KS, Schneider SJ, Rosenthal AD. Methyl methacrylate cranioplasty in children: Long-term results. Pediatr Neurosurg. 1997;26:33–5. doi: 10.1159/000121158. [DOI] [PubMed] [Google Scholar]
- 5.Bowles AP., Jr Reconstruction of the temporalis muscle for pterional and cranio-orbital craniotomies. Surg Neurol. 1999;52:524–9. doi: 10.1016/s0090-3019(99)00112-3. [DOI] [PubMed] [Google Scholar]
- 6.Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010;112:1120–4. doi: 10.3171/2009.6.JNS09133. [DOI] [PubMed] [Google Scholar]
- 7.Chiarini L, Figurelli S, Pollastri G, Torcia E, Ferrari F, Albanese M, et al. Cranioplasty using acrylic material: A new technical procedure. J Craniomaxillofac Surg. 2004;32:5–9. doi: 10.1016/j.jcms.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Chrzan R, Urbanik A, Karbowski K, Moskala M, Polak J, Pyrich M. Cranioplasty prosthesis manufacturing based on reverse engineering technology. Med Sci Monit. 2012;18:MT1–6. doi: 10.12659/MSM.882186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper PR, Schechter B, Jacobs GB, Rubin RC, Wille RL. A pre-formed methyl methacrylate cranioplasty. Surg Neurol. 1977;8:219–21. [PubMed] [Google Scholar]
- 10.Dean D, Min KJ, Bond A. Computer aided design of large-format prefabricated cranial plates. J Craniofac Surg. 2003;14:819–32. doi: 10.1097/00001665-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Dujovny M, Aviles A, Agner C, Fernandez P, Charbel FT. Cranioplasty: Cosmetic or therapeutic? Surg Neurol. 1997;47:238–41. doi: 10.1016/s0090-3019(96)00013-4. [DOI] [PubMed] [Google Scholar]
- 12.Dumbrigue HB, Arcuri MR, LaVelle WE, Ceynar KJ. Fabrication procedure for cranial prostheses. J Prosthet Dent. 1998;79:229–31. doi: 10.1016/s0022-3913(98)70222-7. [DOI] [PubMed] [Google Scholar]
- 13.Elkins CW, Cameron JE. Cranioplasty with acrylic plates. J Neurosurg. 1946;3:199–205. doi: 10.3171/jns.1946.3.3.0199. [DOI] [PubMed] [Google Scholar]
- 14.Eppley BL. Biomechanical testing of alloplastic PMMA cranioplasty materials. J Craniofac Surg. 2005;16:140–3. doi: 10.1097/00001665-200501000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Gasparini G, Boniello R, Moro A, Tamburrini G, Di Rocco C, Pelo S. Cranial reshaping using methyl methacrylate: Technical note. J Craniofac Surg. 2009;20:184–90. doi: 10.1097/SCS.0b013e318191ced4. [DOI] [PubMed] [Google Scholar]
- 16.Gerber N, Stieglitz L, Peterhans M, Nolte LP, Raabe A, Weber S. Using rapid prototyping molds to create patient specific polymethylmethacrylate implants in cranioplasty. Conf Proc IEEE Eng Med Biol Soc 2010. 2010:3357–60. doi: 10.1109/IEMBS.2010.5627903. [DOI] [PubMed] [Google Scholar]
- 17.Godil SS, Shamim MS, Enam SA, Qidwai U, Qadeer M, Sobani ZA. Cranial reconstruction after decompressive craniectomy: Prediction of complications using fuzzy logic. J Craniofac Surg. 2011;22:1307–11. doi: 10.1097/SCS.0b013e31821c6d37. [DOI] [PubMed] [Google Scholar]
- 18.Goh RC, Chang CN, Lin CL, Lo LJ. Customised fabricated implants after previous failed cranioplasty. J Plast Reconstr Aesthet Surg. 2010;63:1479–84. doi: 10.1016/j.bjps.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Greene AK, Mulliken JB, Proctor MR, Rogers GF. Pediatric cranioplasty using particulate calvarial bone graft. Plast Reconstr Surg. 2008;122:563–71. doi: 10.1097/PRS.0b013e31817d61c1. [DOI] [PubMed] [Google Scholar]
- 20.Isago T, Nozaki M, Kikuchi Y, Honda T, Nakazawa H. Sinking skin flap syndrome: A case of improved cerebral blood flow after cranioplasty. Ann Plast Surg. 2004;53:288–92. doi: 10.1097/01.sap.0000106433.89983.72. [DOI] [PubMed] [Google Scholar]
- 21.Jaberi J, Gambrell K, Tiwana P, Madden C, Finn R. Long-term clinical outcome analysis of poly-methyl-methacrylate cranioplasty for large skull defects. J Oral Maxillofac Surg. 2013;71:e81–8. doi: 10.1016/j.joms.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Jordan RD, White JT, Schupper N. Technique for cranioplasty prosthesis fabrication. J Prosthet Dent. 1978;40:230–3. doi: 10.1016/0022-3913(78)90022-7. [DOI] [PubMed] [Google Scholar]
- 23.Kuo JR, Wang CC, Chio CC, Cheng TJ. Neurological improvement after cranioplasty-analysis by transcranial doppler ultrasonography. J Clin Neurosci. 2004;11:486–9. doi: 10.1016/j.jocn.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Lee SC, Wu CT, Lee ST, Chen PJ. Cranioplasty using polymethyl methacrylate prostheses. J Clin Neurosci. 2009;16:56–63. doi: 10.1016/j.jocn.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Maniscalco JE, Garcia-Bengochea F. Cranioplasty: A method of prefabricating alloplastic plates. Surg Neurol. 1974;2:339–41. [PubMed] [Google Scholar]
- 26.Marchac D, Greensmith A. Long-term experience with methylmethacrylate cranioplasty in craniofacial surgery. J Plast Reconstr Aesthet Surg. 2008;61:744–53. doi: 10.1016/j.bjps.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 27.Millard DR, Jr, Yates BM. Practical variations of cranioplasty. Am J Surg. 1964;107:802–9. doi: 10.1016/0002-9610(64)90165-5. [DOI] [PubMed] [Google Scholar]
- 28.Moreira-Gonzalez A, Jackson IT, Miyawaki T, Barakat K, DiNick V. Clinical outcome in cranioplasty: Critical review in long-term follow-up. J Craniofac Surg. 2003;14:144–53. doi: 10.1097/00001665-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Neovius E, Engstrand T. Craniofacial reconstruction with bone and biomaterials: Review over the last 11 years. J Plast Reconstr Aesthet Surg. 2010;63:1615–23. doi: 10.1016/j.bjps.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Origitano TC, Izquierdo R, Scannicchio LB. Reconstructing complex cranial defects with a preformed cranial prosthesis. Skull Base Surg. 1995;5:109–16. doi: 10.1055/s-2008-1058941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raza SM, Thai QA, Pradilla G, Tamargo RJ. Frontozygomatic titanium cranioplasty in frontosphenotemporal (“pterional”) craniotomy. Neurosurgery. 2008;62(3 Suppl 1):262–5. doi: 10.1227/01.neu.0000317402.46583.c7. [DOI] [PubMed] [Google Scholar]
- 32.Rotaru H, Stan H, Florian IS, Schumacher R, Park YT, Kim SG, et al. Cranioplasty with custom-made implants: Analyzing the cases of 10 patients. J Oral Maxillofac Surg. 2012;70:e169–76. doi: 10.1016/j.joms.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 33.Sabin H, Karvounis P. The neurosurgeon-dentist team in cranioplasty. J Am Dent Assoc. 1969;79:1183–8. doi: 10.14219/jada.archive.1969.0069. [DOI] [PubMed] [Google Scholar]
- 34.Sahoo N, Roy ID, Desai AP, Gupta V. Comparative evaluation of autogenous calvarial bone graft and alloplastic materials for secondary reconstruction of cranial defects. J Craniofac Surg. 2010;21:79–82. doi: 10.1097/SCS.0b013e3181c3ba58. [DOI] [PubMed] [Google Scholar]
- 35.Sanan A, Haines SJ. Repairing holes in the head: A history of cranioplasty. Neurosurgery. 1997;40:588–603. doi: 10.1097/00006123-199703000-00033. [DOI] [PubMed] [Google Scholar]
- 36.van Gool AV. Preformed polymethylmethacrylate cranioplasties: Report of 45 cases. J Maxillofac Surg. 1985;13:2–8. doi: 10.1016/s0301-0503(85)80005-9. [DOI] [PubMed] [Google Scholar]
- 37.Winkler PA, Stummer W, Linke R, Krishnan KG, Tatsch K. Influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. J Neurosurg. 2000;93:53–61. doi: 10.3171/jns.2000.93.1.0053. [DOI] [PubMed] [Google Scholar]
- 38.Woolf JI, Walker AE. Cranioplasty: Collective review. Int Abs Surg. 1945;81:1–23. [Google Scholar]
- 39.Yadla S, Campbell PG, Chitale R, Maltenfort MG, Jabbour P, Sharan AD. Effect of early surgery, material, and method of flap preservation on cranioplasty infections: A systematic review. Neurosurgery. 2011;68:1124–9. doi: 10.1227/NEU.0b013e31820a5470. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto Y, Mendel E, Raffel C. Acrylic cranioplasty with alginate molding: Technical note. Neurosurgery. 1997;41:305–9. doi: 10.1097/00006123-199707000-00055. [DOI] [PubMed] [Google Scholar]


