Abstract
While much work has been conducted regarding the neurogenesis response to traumatic brain injury (TBI) in rodents, it remains largely unknown whether neurogenesis in adult human brain also responds to TBI in a similar manner. Here, we performed immunocytochemistry on 11 brain specimens from patients with traumatic brain injury, who underwent surgical intervention. We found that expression of neural stem/progenitor cell (NSC) protein markers, including DCX, TUC4, PSA-NCAM, SOX2 and NeuroD, was increased in the perilesional cortex of human brain after TBI compared to that of normal brain. Confocal images showed that these NSC proteins were expressed in one single cell. We also found that proliferative markers were expressed in NSC protein-positive cells after TBI, and the number of proliferative NSCs was significantly increased after TBI. Our data suggest that TBI may also induce neurogenesis in human brain.
Key words: brain trauma, human, injury, neurogenesis, stem cells
Introduction
Traumatic brain injury (TBI) is a serious public health problem that causes disability and significant health care expenditures for those affected. While the clinical management of traumatic brain injury has greatly improved with the development of standardized approaches to care, there are currently no medical treatment adjuncts that have been shown effective in improving mortality or limiting disability following injury, which emphasizes the need for new therapeutic developments.
Recent evidence showed that neurogenesis continues in the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) in adult mouse, rat, non-human primate, and human brain.1–5 Newly generated cells can differentiate into functional mature neurons and integrate into neuronal networks,6,7 including those involved in cognitive function.8–10 We and other investigators have shown that ischemic brain injury stimulates the proliferation of neural stem/progenitor cells (NSCs) located in the SVZ and SGZ of adult rodent brain. The resulting newborn cells migrate into damaged brain regions,11–13 where they differentiate into mature neuronal cells.11–13 Studies have reported that brain trauma also induces cell proliferation at the ipsilateral hippocampus and SVZ,14–17 which may persist for at least one year.15 An animal study shows that a significant increase in newly generated cells was observed not only in the hippocampus, but also in the traumatized cerebral cortex, white matter structures, and some contralateral regions.18 Newborn doublecortin (DCX)-positive neuroblasts in the SVZ emigrate toward cerebral cortex lesions.19 Double-labeled studies have documented that many of these proliferating cells appear to differentiate into various adult cells, including astrocytes, oligodendrocytes, and neurons,17,20–22 and that newly generated granule neurons are capable of extending projections along the hippocampal mossy fiber pathway in the acute post-traumatic period,23 suggesting that these newborn cells can differentiate into neural lineages. Taken together, these findings suggest that TBI induces neurogenesis in rodents. Although it remains unclear what relevance injury-induced neurogenesis may have in the recovery process following TBI, the demonstration of neurogenesis in damaged regions in adult brains and of the presence of proliferating cells with the ability to give rise to neurons in the damaged regions of brains after TBI have reinvigorated our hopes of rebuilding damaged tissues by endogenous neural cell replacement.
While much work has been conducted regarding the neurogenesis response to TBI in rodents, it remains largely unknown whether neurogenesis in adult human brain also responds to TBI in a similar manner. In the present study, we found that the expression of NSC protein markers, including DCX, TUC4, PSA-NCAM, SOX2, and NeuroD, was increased in the perilesional cortex of human brain after TBI compared to that of normal brain. Confocal images showed that these NSC proteins were expressed in one single cell. In addition, we found that proliferative markers were expressed in cells located in the perilesional regions after TBI. Double or triple immunocytochemistry showed that NSC-positive cells expressed proliferative protein and that these cells were significantly increased after TBI, suggesting that TBI may also induce neurogenesis in human brain as it does in the animal TBI model.
Methods
Human brain specimens
Eleven brain specimens from patients with traumatic brain injury were obtained from the First Affiliated Hospital, Wenzhou Medical College (China), undergoing surgical resection between 2007 and 2009. The patients ranged in age from 48 to 78 years, with a mean of 56.9 years (median 57). Patient information is summarized in Table 1. Control human brain specimens without clinical or postmortem evidence of neurological diseases (n=4; age from 41−55 years, with a mean of 48 years) were obtained from the Brain and Tissue Bank for Developmental Disorders of the National Institute of Child and Health and Human Development (University of Maryland, Baltimore, MD). All studies involving patients were conducted under the protocol approved by the Institutional Research Review Board at Marin General Hospital (California), and Wenzhou Medical College (China).
Table 1.
Clinical Features of Patients with TBI
| Case no. | Sex | Age (yr) | Location | Time of surgery (h) |
|---|---|---|---|---|
| 1 | M | 78 | Right frontotemporal | 5 |
| 2 | M | 77 | Right occipital | 6 |
| 3 | F | 61 | Left frontotemporal | 5 |
| 4 | M | 77 | Left occipitotemporal | 2 |
| 5 | M | 46 | Right frontotemporal | 7 |
| 6 | F | 58 | Right frontotemporoparietal | 14 |
| 7 | M | 61 | Right frontotemporoparietal | 8 |
| 8 | M | 60 | Right frontal | 16 |
| 9 | F | 48 | Left frontotemporoparietal | 6 |
| 10 | M | 60 | Left frontotemporal | 2 |
| 11 | F | 60 | Right frontal | 1 |
Immunohistochemistry
Brain tissue was post-fixed in paraformaldehyde for 24 h, incubated with 30% sucrose for 3 days, and embedded in paraffin; 6-μm sections were cut on a microtome and stored at room temperature. Sections were deparaffinized with xylene and rehydrated with ethanol. To obtain more efficient immunostaining, brain sections were subjected to an antigen retrieval procedure according to the manufacturer's instructions (Vector Laboratories, Burlingame, CA). After heating under pressure for 2 min, samples were washed extensively in PBS. Endogenous peroxidase activity was blocked by 30 min incubation at room temperature in 1% H2O2. After several washes with PBS, sections were incubated in blocking solution (2% goat serum, 0.1% Triton X-100, 1% bovine serum albumin in PBS) for 1 h at room temperature. Primary antibodies used were mouse monoclonal anti-Ki67 antigen (1:50; Novocastra, Newcastle upon Tyne, United Kingdom), rabbit anti-Ki67 antigen (1:100; Zymed, South San Francisco, CA), goat anti-minichromosome maintenance 2 (MCM2, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-cleaved caspase 3 (1:200; BD PharMingen, San Diego, CA), affinity-purified goat anti-DCX (1:200; Santa Cruz Biotechnology), mouse monoclonal anti-βIII-tubulin (TUJ1, 1:250; Covance, Berkeley, CA), rabbit polyclonal anti-TUC-4 (1:10,000; Chemicon International, Temecula, CA), mouse monoclonal anti-polysialylated neuronal cell adhesion molecule (PSA-NCAM, 1:500; Chemicon), affinity-purified goat polyclonal anti-Neuro D (1:100; Santa Cruz Biotechnology), and rabbit anti-SOX2 (1:200; Abcam, Cambridge, MA). The markers for neural precursor cells have been evidenced to cross-react with proteins of the appropriate molecular weight in human brain tissues.24 Primary antibodies were added in blocking solution and incubated with sections at 4°C overnight. Sections were then washed with 1x PBS and incubated with biotinylated goat anti-rabbit or anti-goat antibody (1:200) (for polyclonal antibodies) or biotinylated horse anti-mouse antibody (1:200, for monoclonal antibodies) for 1 h at room temperature. Avidin–biotin complex (Vector Elite, Vector Laboratories) and a diaminobenzidine or nickel solution (Vector Laboratories) were used to obtain a visible reaction product. Controls for immunohistochemistry included pre-absorption and co-incubation of the antibodies with the corresponding antigens. Sections were dehydrated, sealed, and coverslipped. A Nikon microscope and a Nikon digital color camera were used for examination and photography of the slides, respectively.
Double or triple immunostaining
Double or triple immunostaining was performed on brain sections as previously described.12 The primary antibodies used, in addition to those listed above, were rabbit anti-Musashi1 (1:500; Chemicon) and mouse monoclonal anti-NeuN (1:200; Chemicon). The secondary antibodies were Alexa Fluor 488-, 594-, or 647-conjugated donkey anti-mouse, anti-goat, or anti-rabbit IgG (1:200–500; Molecular Probes, Carlsbad, CA). Nuclei were counterstained with DAPI using prolong Gold antifade reagent (Molecular Probes). Fluorescence signals were detected using an LSM 510 NLO Confocal Scanning System mounted on an Axiovert 200 inverted microscope (Carl Zeiss, Jena, Germany) equipped with a two-photon Chameleon laser (Coherent), and images were acquired using LSM 510 Imaging Software (Carl Zeiss). Two-, three-, or four-color images were scanned using argon, 543 HeNe, 633 HeNe, and Chameleon (750–780 nm for DAPI) lasers. Selected images were viewed at high magnification, and 3-dimensional images were constructed using Imars software. Controls included omitting either the primary or secondary antibody or pre-absorbing the primary antibody.
Quantification of immunopositive cells
Immunopositive cells were counted in three to five different fields per section at an absolute magnification of 400X, chosen from the perilesional cortical area of maximal labeling using an eyepiece grid covering an area of 0.0625 mm2. Vessels and blood cells were excluded from analysis. The images were acquired using a Nikon Eclipse-800 microscope equipped with Nikon digital camera DXM1200. The number of immunopositive cells was counted manually in a blinded fashion. The final results were expressed as the number of immunopositive cells per field.
Statistical analysis
Quantitative results were expressed as the mean±SEM. The statistical significance of differences between means was evaluated using one-way analysis of variance (ANOVA). When ANOVA showed significant differences, pair-wise comparisons between means were tested by the Scheffé post-hoc test. P<0.05 was regarded as statistically significant.
Results
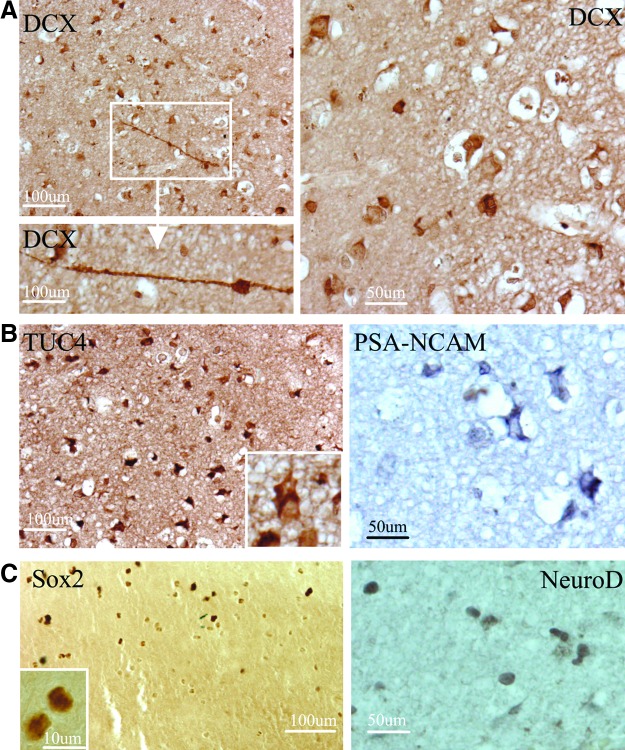
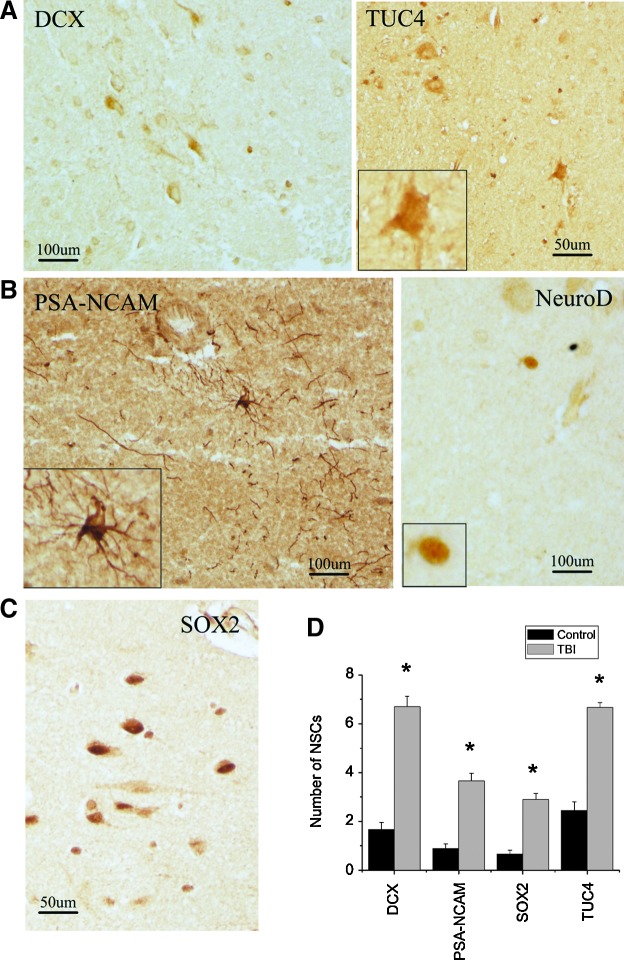
To determine whether NSCs in the adult human brain respond to TBI, we first performed immunocytochemistry for NSC protein markers in sections of cortical regions from 11 patients with TBI who underwent surgical intervention (Table 1). We found that DCX, a microtubule-associated protein expressed almost exclusively in immature neurons25 and currently a “gold standard” in measuring neurogenesis,26 was expressed in cells in the perilesional cortex of adult human brain after TBI. Some of the DCX-positive cells displayed morphological characteristics of migrating cells with a leading process and tail edge (Fig. 1A). TUC-4 (also known as TOAD-64 and CRMP-4), a protein expressed early in neuronal differentiation,27 and PSA-NCAM, a marker for immature neuronal-committed progenitors that are at more mature stages than DCX,28 were highly expressed in the cerebral cortex surrounding the damaged regions (Fig. 1B). In addition, we found that SOX2, a transcription factor expressed by self-renewing and multipotent stem cells of the embryonic neuroepithelium and considered a persistent marker for multipotential neural stem cells,29 as well as NeuroD, a basic helix loop helix transcription factor expressed in immature, differentiating neuronal progenitor cells,30,31 were found in the nuclei of cells in the cortical regions after TBI (Fig. 1C), suggesting that they might be neural progenitor cells. Since the tissue samples were from different cortex regions, we next asked whether there were regional differences in injury-induced neurogenesis. Of the 11 TBI patient samples, we found that the number of DCX-positive cells was between 3.66±0.577 (mean±SD) and 8.33±1.5; TUC4-positive cells were between 5.33±1.52 and 7.66±1.53; SOX2-positive cells were between 1.66±0.55 and 3.66±0.56; and PSA-NCA-positive cells were between 2.33±0.55 and 4.33±0.58. Interestingly, we also found that the DCX-, TUC-4-, PSA-NCA-, and SOX2-positive cells were present in the normal cortex (Fig. 2A–C); however, the number of these cells was significantly increased in the traumatic brain (p<0.05; Fig. 2D).
FIG. 1.
Expression of NSC proteins in perilesional cortical regions of adult human brain after TBI. (A) The early neuronal marker DCX is expressed in the cytoplasm of numerous cells in the perilesional regions of adult human brain after TBI. Some of DCX-positive cells display leading and tailing processes (right bottom panel). (B) TUC4 and PSA-NCAM are expressed in cytoplasm of cells located in the cortical regions. (C) Sox2 and NeuroD are expressed in nuclei of cells located in the cortical regions after TBI. Data shown are representative fields from at least three experiments per panel. Color image is available online at www.liebertpub.com/neu.
FIG. 2.
Expression of NSC proteins in normal front cortex in human. (A) DCX- and TUC4-positive cells are found in the normal cortex in human. (B) PSA-NCAM- and NeuroD-positive cells are observed in the normal cortex in human. (C) SOX2 is expressed in the cells located in the front cortex in human. (D) The number of NSC protein-positive cells in normal and traumatic brain. *p<0.05. Color image is available online at www.liebertpub.com/neu.
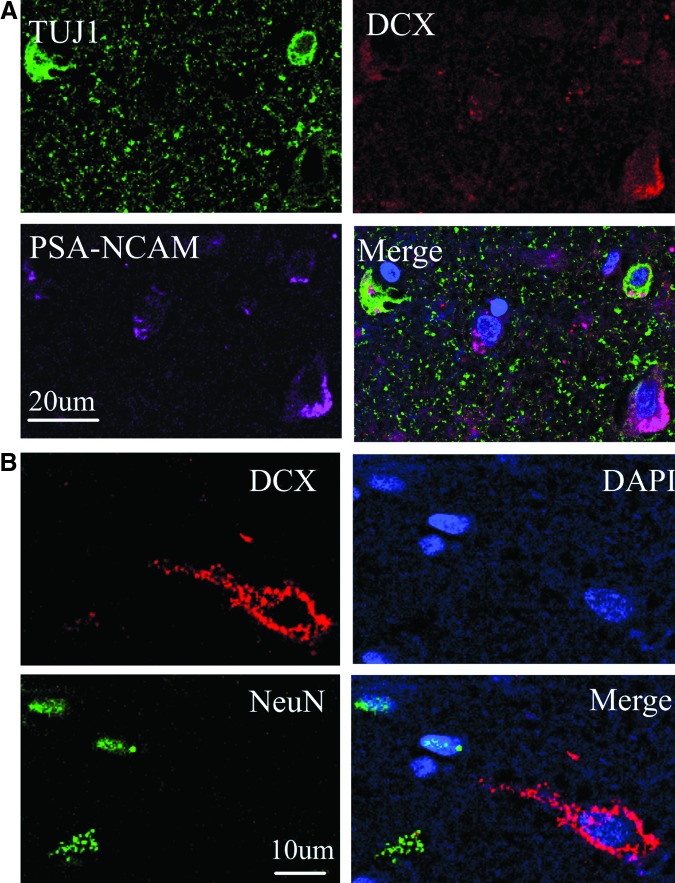
To date, there is no single protein marker for identifying NSCs. Thus, we next performed double or triple immunostaining to see whether these NSC proteins were expressed in one single cell after TBI. As shown in Figure 3, DCX-positive cells expressed PSA-NCAM and TUJ1 (β III tubulin), an early marker of neuronal lineage in vivo,32 but not neuronal nuclear antigen (NeuN), a mature neuronal marker33 (Fig. 2B), confirming their identity as dividing NSCs.
FIG. 3.
Co-expression of NSC proteins in perilesional regions of adult human brain after TBI. (A) Triple immunostaining shows that TUJ1 (green) is expressed in the DCX (red)- and PSA-NCAM (purple)-positive cells in the cortical regions of adult human brain after TBI. (B) The early neuronal marker DCX (red) is not co-localized with the mature neuronal marker NeuN (green). Nuclei are counterstained with DAPI. Color image is available online at www.liebertpub.com/neu.
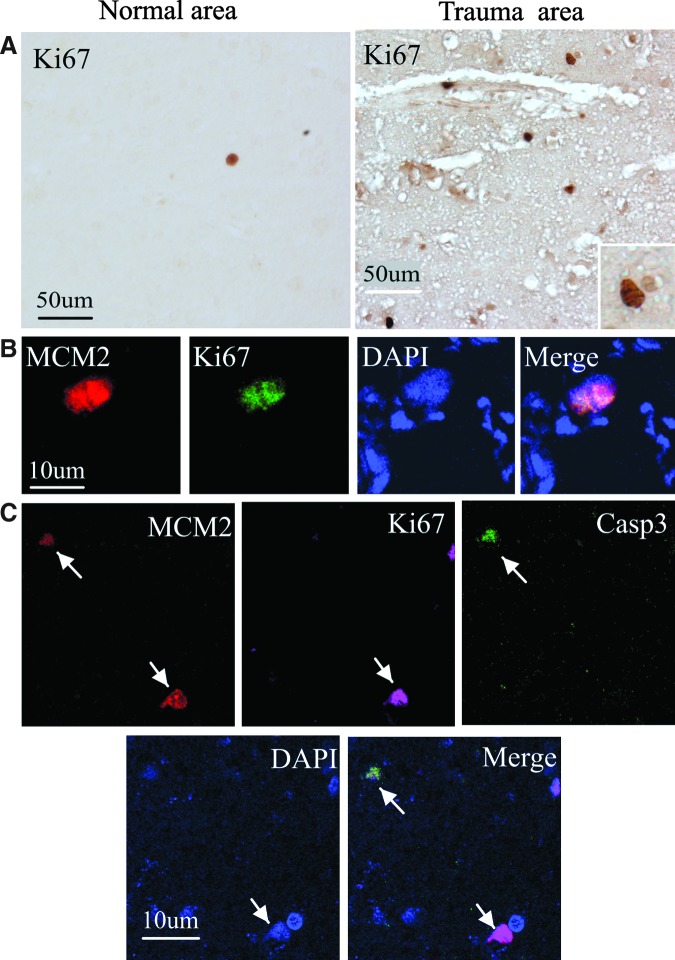
Self-renewal is one of the unique properties of stem cells. We next performed immunostaining using an antibody against the cell proliferation marker Ki67 antigen34 to determine if cells expressing NSC marker proteins seen after ICH showed evidence of a proliferative state. As shown in Figure 4A, Ki67-immunopositive cells were observed in cortical regions after TBI but were barely detectable in the normal cortex of adult human brain. Moreover, double-label immunostaining showed that these cells also expressed a new proliferation marker MCM2 (Fig. 4B). Only some Ki67-positive cells showed evidence of caspase-3 cleavage or abnormal nuclear morphology (Fig. 4C), which may represent dying cells attempting to enter the cell cycle.
FIG. 4.
Presence of dividing cells in the cortical regions of adult human brain after TBI. (A) Ki67-positive cells are barely detected in the normal regions of brain (left panel), but observed in the nuclei of cells in the cortical regions after TBI (right panel). Ki67-stained nucleus is shown at higher magnification (inset). (B) Ki67 (green) is co-localized with the cell proliferation marker MCM2 (red) in the nucleus of a cell. DAPI (blue) is used to counterstain all nuclei. (C) MCM2 (red) co-localizes with a cell-death marker, the 17- to 20-kDa cleavage product of caspase-3 (Casp3; green) in cells with misshapen nuclei (upper left), but not in cells with normal-appearing nuclei (lower right). Some of Ki67 (purple) co-localize with MCM2, but not with caspase-3. Nuclei are counterstained with DAPI. Color image is available online at www.liebertpub.com/neu.
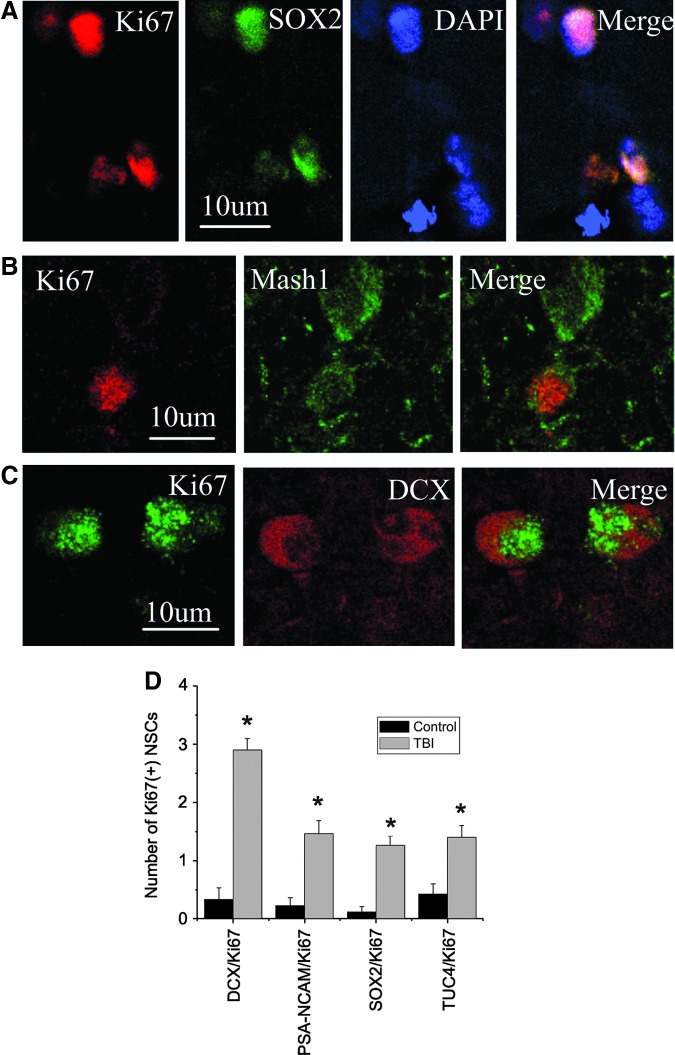
To determine if proliferative cells were of neural progenitor cells, we performed double immunocytochemistry using anti-proliferative protein marker and NSC marker proteins, which were recorded using two-photon laser scanning confocal microscopy and Imars software. As shown in Figure 5, cells that expressed Ki67 were also reactive for NSC markers such as SOX2, Musashi1, an evolutionally conserved marker for central nervous system progenitor cells including neural stem cells,35 and DCX, suggesting that most Ki67-positive cells in the cortical regions were NSCs; these cells were significantly induced after brain trauma (Fig. 5D; p<0.05).
FIG. 5.
Co-expression of neuronal lineage and cell proliferative protein markers in cortical region of adult human brain after TBI. (A) Cell proliferative marker Ki67 (red) is co-localized with SOX2-positive (green) nucleus. (B) Ki67 (red) is expressed in the nucleus of a cell with Mash1-positive (green) cytoplasm. (C) DCX (red) is expressed in the cytoplasm of a cell with Ki67-positive (red) nucleus. Nuclei are counterstained with DAPI (blue). (D) The number of Ki67-positive NSCs in normal and traumatic brain. *p<0.05. Color image is available online at www.liebertpub.com/neu.
Discussion
The main finding of this study is that the expression of NSC proteins and cell proliferative markers is increased in peri-damaged brain regions after TBI in humans compared to that of normal brain. Two-photon laser scanning confocal images show that expression of NSC and proliferative proteins are observed together in the same cell. Quantification analysis indicates that the number of proliferative NSCs is also significantly increased after TBI. Our data suggest that neurogenesis may be induced in human brain after TBI, and that the mature brain retains a degree of innate repair and regenerative potential to restore damaged neuronal populations through endogenous neurogenesis.
Many studies demonstrate that various brain injuries induce neurogenesis in a number of neurological disorders in humans, including Huntington's disease,36 ischemic stroke,37 Alzheimer's disease,38 epilepsy,39 and aneurysmal subarachnoid hemorrhage.40 Here, we further demonstrate that TBI may also induce neurogenesis in humans. To determine whether these newborn cells can replace damaged cells, the first question is whether these newly generated neurons are functional. Previous studies show that cognitive deficits are observed in animals and in humans after TBI.41,42 Interestingly, cognitive deficits can be significantly self-recovery following all but the most severe episodes of TBI, and the cognitive functions of juveniles recover to a greater extent than adult patients following TBI.41–43 The mechanisms underlying this phenomenon remain unclear, although recent studies suggest that the cognitive recovery that occurs following TBI is associated with the integration of newly generated neurons.42 The role of newborn cells in learning and memory has been tested using a range of hippocampus-dependent behaviors with mixed results. For example, studies show that trace eyeblink conditioning was affected in anti-proliferative drug-treated animals, and contextual fear conditioning was impaired following irradiation and genetic ablation of adult neurogenesis. In an effort to more precisely ablate specific populations of neural precursor cells, genetically engineered mice have become available that can regulate neurogenesis in a more direct and temporally controlled manner. We produced transgenic mice that express herpes simplex virus thymidine kinase (TK) under control of the DCX promoter. Treatment with the antiviral drug ganciclovir (GCV) depleted DCX-expressing and BrdU-labeled cells from the SVZ and dentate gyrus and abolished neurogenesis and associated neuromigration induced by focal cerebral ischemia. GCV treatment of DCX-TK transgenic, but not WT mice, also increased infarct size and exacerbated post-ischemic sensorimotor behavioral deficits. These findings suggest that injury-induced neurogenesis contributes to brain injury outcome.44
We found that neurogenesis mainly occurs in the peri-damaged brain regions after TBI in humans. However, it remains unclear whether these cells are born locally or from neurogenic regions. Normally, NSCs reside in the postnatal SVZ and the SGZ of the dentate gyrus. The newborn cells migrate into the olfactory bulb via the rostral migratory stream, while the SGZ of the dentate gyrus provides new granular neurons throughout life. Following TBI, NSCs in each of these areas become activated. Previous studies have shown that increased SVZ neurogenesis with subsequent migration of newborn neurons into the damaged region has been described in a rat model of intracerebral hemorrhage and focal ischemia model.2,12,45 Therefore, it is possible that newborn cells in the SVZ can migrate into the boundary zone of the injured cortex after severe TBI.46 However, little is known about cell migration after TBI. Recent studies show that stromal cell-derived factor-1α (SDF-1α) and its receptor, CXCR4, contribute to NSC migration after brain injury.47 Although SDF-1α expression did not increase after TBI, SDF-1α leaked from the injured area and diffused into the cortex after TBI.48 Little is known about which factors determine the magnitude and amplification of neurogenesis after TBI, but recent studies show that nerve growth factor and DCX expression correlates with improved outcome in children with severe TBI.49
Traumatic brain injury stimulates an increase in the proliferation of endogenous NSCs, particularly in the neocortex where it does not normally occur, suggesting that the adult brain has the inherent potential to restore populations lost to injury and that it may become possible to manipulate endogenous multipotent precursors in situ to replace lost or damaged neurons. Our data show that NSC-protein markers are found in the cells located in normal cortex, but these cells barely express proliferative markers such as Ki67, suggesting that, even if there are some NSCs in the normal cortex in humans, the majority are in quiescent. Several studies have shown that a variety of pharmacologic agents associated with increasing neurogenesis lead to improved outcomes after TBI. These include growth factors, which are important mediators for neurogenesis. Specifically, basic fibroblast growth factor (FGF-2) and epidermal growth factor (EGF) have been shown to be a potent mitogenic factor for NSCs both in vitro and in vivo. FGF-2 knockout mice exhibited diminished hippocampal DG neurogenesis in response to seizure or trauma, which can be reversed by administrating FGF-2.5 TBI-induced proliferative response in the SVZ and hippocampus is linked to increased EGF expression. Compared to vehicle-infused animals, FGF-2- or EGF-infused animals had significantly more BrdU-positive cells in the SVZ and hippocampus. In addition, animals treated with EGF or FGF-2 showed significant improvement in cognitive function.50,51 Vascular endothelial growth factor (VEGF) is an angiogenic protein with neurotrophic and neuroprotective effects. Recently, we found that VEGF also stimulate neurogenesis in vitro and in vivo. A study shows that VEGF significantly increases the number of proliferating cells in the SVZ and the perilesional cortex after TBI. Functional outcome was significantly better in mice treated with VEGF compared with vehicle-treated animals after TBI.52,53 Erythropoietin (EPO), a naturally occurring cytokine, is most widely recognized for its role in stimulating the maturation, differentiation, and survival of hematopoietic progenitor cells. Michael Chopp's group found that rhEPO administration significantly increases the number of BrdU-labeled cells in both the contralateral and ipsilateral DG and promotes restoration of spatial memory after TBI.54 Notably, these growth factors not only stimulate neurogenesis, but also have a neuroprotective role. It therefore becomes difficult to attribute improvements in behavior to their effects on neurogenesis only. In order to demonstrate that functional recovery requires newly generated cells derived from NSC activation, more cell-specific genetic assays should be performed in the future.
Acknowledgments
This work was partially supported by a Wenzhou Medical College 5010 grant, a National Institute of Health (NIH) grant (AG21980 and NS62414, to K.J.), and funds from the Foundation of Zhejiang Provincial Top Key Discipline of Surgery. Some of the human tissue were obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland (Baltimore, MD). The role of the NICHD Brain and Tissue Bank is to distribute tissue, and, therefore, it does not endorse the studies performed or the interpretation of results.
Author Disclosure Statement
The authors have no conflict of interests to report.
References
- 1.Eriksson P.S. Perfilieva E. Bjork-Eriksson T. Alborn A.M. Nordborg C. Peterson D.A. Gage F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 2.Jin K. Minami M. Lan J.Q. Mao X.O. Batteur S. Simon R.P. Greenberg D.A. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDermott K.W. Lantos P.L. Distribution and fine structural analysis of undifferentiated cells in the primate subependymal layer. J. Anat. 1991;178:45–63. [PMC free article] [PubMed] [Google Scholar]
- 4.Sanai N. Tramontin A.D. Quinones-Hinojosa A. Barbaro N.M. Gupta N. Kunwar S. Lawton M.T. McDermott M.W. Parsa A.T. Manuel-Garcia Verdugo J. Berger M.S. Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura S. Takagi Y. Harada J. Teramoto T. Thomas S.S. Waeber C. Bakowska J.C. Breakefield X.O. Moskowitz M.A. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc. Natl. Acad. Sci. USA. 2001b;98:5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song H.J. Stevens C.F. Gage F.H. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat. Neurosci. 2002;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- 7.van Praag H. Schinder A.F. Christie B.R. Toni N. Palmer T.D. Gage F.H. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saxe M.D. Battaglia F. Wang J.-W. Malleret G. David D.J. Monckton J.E. Garcia A.D.R. Sofroniew M.V. Kandel E.R. Santarelli L. Hen R. Drew M.R. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shors T.J. Miesegaes G. Beylin A. Zhao M. Rydel T. Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C.L. Zou Y. He W. Gage F.H. Evans R.M. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 11.Arvidsson A. Collin T. Kirik D. Kokaia Z. Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 12.Jin K. Sun Y. Xie L. Peel A. Mao X. O. Batteur S. Greenberg D.A. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol. Cell. Neurosci. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 13.Teramoto T. Qiu J. Plumier J.-C. Moskowitz M.A. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J. Clin. Invest. 2003;111:1125–1132. doi: 10.1172/JCI17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun H. Schafer K. Hollt V. BetaIII tubulin-expressing neurons reveal enhanced neurogenesis in hippocampal and cortical structures after a contusion trauma in rats. J. Neurotrauma. 2002;19:975–983. doi: 10.1089/089771502320317122. [DOI] [PubMed] [Google Scholar]
- 15.Chen X.H. Iwata A. Nonaka M. Browne K.D. Smith D.H. Neurogenesis and glial proliferation persist for at least one year in the subventricular zone following brain trauma in rats. J. Neurotrauma. 2003;20:623–631. doi: 10.1089/089771503322144545. [DOI] [PubMed] [Google Scholar]
- 16.Chirumamilla S. Sun D. Bullock M.R. Colello R.J. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J. Neurotrauma. 2002;19:693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- 17.Dash P.K. Mach S.A. Moore A.N. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J. Neurosci. Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Urrea C. Castellanos D.A. Sagen J. Tsoulfas P. Bramlett H.M. Dietrich W.D. Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor. Neurol. Neurosci. 2007;25:65–76. [PubMed] [Google Scholar]
- 19.Sundholm-Peters N.L. Yang H.K. Goings G.E. Walker A.S. Szele F.G. Subventricular zone neuroblasts emigrate toward cortical lesions. J. Neuropathol. Exp. Neurol. 2005;64:1089–1100. doi: 10.1097/01.jnen.0000190066.13312.8f. [DOI] [PubMed] [Google Scholar]
- 20.Lu D. Qu C. Goussev A. Jiang H. Lu C. Schallert T. Mahmood A. Chen J. Li Y. Chopp M. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J. Neurotrauma. 2007;24:1132–1146. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun D. Colello R.J. Daugherty W.P. Kwon T.H. McGinn M.J. Harvey H.B. Bullock M.R. Cell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injury. J. Neurotrauma. 2005;22:95–105. doi: 10.1089/neu.2005.22.95. [DOI] [PubMed] [Google Scholar]
- 22.Tatsumi K. Haga S. Matsuyoshi H. Inoue M. Manabe T. Makinodan M. Wanaka A. Characterization of cells with proliferative activity after a brain injury. Neurochem. Int. 2005;46:381–389. doi: 10.1016/j.neuint.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Emery D.L. Fulp C.T. Saatman K.E. Schutz C. Neugebauer E. McIntosh T.K. Newly born granule cells in the dentate gyrus rapidly extend axons into the hippocampal CA3 region following experimental brain injury. J. Neurotrauma. 2005;22:978–988. doi: 10.1089/neu.2005.22.978. [DOI] [PubMed] [Google Scholar]
- 24.Jin K. Peel A.L. Mao X.O. Xie L. Cottrell B.A. Henshall D.C. Greenberg D.A. Increased hippocampal neurogenesis in Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2004b;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francis F. Koulakoff A. Boucher D. Chafey P. Schaar B. Vinet M.C. Friocourt G. McDonnell N. Reiner O. Kahn A. McConnell S.K. Berwald-Netter Y. Denoulet P. Chelly J. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- 26.Couillard-Despres S. Winner B. Schaubeck S. Aigner R. Vroemen M. Weidner N. Bogdahn U. Winkler J. Kuhn H.G. Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- 27.Minturn J.E. Fryer H.J. Geschwind D.H. Hockfield S. TOAD-64, a gene expressed early in neuronal differentiation in the rat, is related to unc-33, a C. elegans gene involved in axon outgrowth. J. Neurosci. 1995;15:6757–6766. doi: 10.1523/JNEUROSCI.15-10-06757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doetsch F. Garcia-Verdugo J.M. Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komitova M. Eriksson P.S. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci. Lett. 2004;369:24–27. doi: 10.1016/j.neulet.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 30.Lee J.E. NeuroD and neurogenesis. Dev. Neurosci. 1997;19:27–32. doi: 10.1159/000111182. [DOI] [PubMed] [Google Scholar]
- 31.Lee J.E. Hollenberg S.M. Snider L. Turner D.L. Lipnick N. Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- 32.Fanarraga M.L. Avila J. Zabala J.C. Expression of unphosphorylated class III beta-tubulin isotype in neuroepithelial cells demonstrates neuroblast commitment and differentiation. Eur. J. Neurosci. 1999;11:516–527. [PubMed] [Google Scholar]
- 33.Mullen R.J. Buck C.R. Smith A.M. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 34.Gerdes J. Lemke H. Baisch H. Wacker H.H. Schwab U. Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 35.Kaneko Y. Sakakibara S. Imai T. Suzuki A. Nakamura Y. Sawamoto K. Ogawa Y. Toyama Y. Miyata T. Okano H. Musashi1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. Dev. Neurosci. 2000;22:139–153. doi: 10.1159/000017435. [DOI] [PubMed] [Google Scholar]
- 36.Curtis M.A. Penney E.B. Pearson A.G. van Roon-Mom W.M. Butterworth N.J. Dragunow M. Connor B. Faull R.L. Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc. Natl. Acad. Sci. USA. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin K. Wang X. Xie L. Mao X.O. Zhu W. Wang Y. Shen J. Mao Y. Banwait S. Greenberg D.A. Evidence for stroke-induced neurogenesis in the human brain. Proc. Natl. Acad. Sci. USA. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin K. Galvan V. Xie L. Mao X.O. Gorostiza O.F. Bredesen D.E. Greenberg D.A. Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APPSw,Ind) mice. Proc. Natl. Acad. Sci. USA. 2004a;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crespel A. Rigau V. Coubes P. Rousset M.C. de Bock F. Okano H. Baldy-Moulinier M. Bockaert J. Lerner-Natoli M. Increased number of neural progenitors in human temporal lobe epilepsy. Neurobiol. Dis. 2005;19:436–450. doi: 10.1016/j.nbd.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 40.Sgubin D. Aztiria E. Perin A. Longatti P. Leanza G. Activation of endogenous neural stem cells in the adult human brain following subarachnoid hemorrhage. J. Neurosci. Res. 2007;85:1647–1655. doi: 10.1002/jnr.21303. [DOI] [PubMed] [Google Scholar]
- 41.Anderson V.A. Catroppa C. Rosenfeld J. Haritou F. Morse S.A. Recovery of memory function following traumatic brain injury in pre-school children. Brain Inj. 2000;14:679–692. doi: 10.1080/026990500413704. [DOI] [PubMed] [Google Scholar]
- 42.Sun D. McGinn M.J. Zhou Z. Harvey H.B. Bullock M.R. Colello R.J. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp. Neurol. 2007;204:264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Ewing-Cobbs L. Barnes M.A. Fletcher J.M. Early brain injury in children: development and reorganization of cognitive function. Dev. Neuropsychol. 2003;24:669–704. doi: 10.1080/87565641.2003.9651915. [DOI] [PubMed] [Google Scholar]
- 44.Jin K. Wang X. Xie L. Mao X.O. Greenberg D.A. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc. Natl. Acad. Sci. USA. 2010;107:7993–7998. doi: 10.1073/pnas.1000154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masuda T. Isobe Y. Aihara N. Furuyama F. Misumi S. Kim T.S. Nishino H. Hida H. Increase in neurogenesis and neuroblast migration after a small intracerebral hemorrhage in rats. Neurosci. Lett. 2007;425:114–119. doi: 10.1016/j.neulet.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 46.Lu D. Mahmood A. Zhang R. Copp M. Upregulation of neurogenesis and reduction in functional deficits following administration of DEtA/NONOate, a nitric oxide donor, after traumatic brain injury in rats. J. Neurosurg. 2003;99:351–361. doi: 10.3171/jns.2003.99.2.0351. [DOI] [PubMed] [Google Scholar]
- 47.Imitola J. Raddassi K. Park K.I. Mueller F.-J. Nieto M. Teng Y.D. Frenkel D. Li J. Sidman R.L. Walsh C.A. Snyder E.Y. Khoury S.J. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1{alpha}/CXC chemokine receptor 4 pathway. Proc. Natl. Acad. Sci. USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Itoh T. Satou T. Ishida H. Nishida S. Tsubaki M. Hashimoto S. Ito H. The relationship between SDF-1alpha/CXCR4 and neural stem cells appearing in damaged area after traumatic brain injury in rats. Neurol. Res. 2009;31:90–102. doi: 10.1179/174313208X332995. [DOI] [PubMed] [Google Scholar]
- 49.Chiaretti A. Antonelli A. Genovese O. Pezzotti P. Rocco C.D. Viola L. Riccardi R. Nerve growth factor and doublecortin expression correlates with improved outcome in children with severe traumatic brain injury. J. Trauma. 2008;65:80–85. doi: 10.1097/TA.0b013e31805f7036. [DOI] [PubMed] [Google Scholar]
- 50.Sun D. Bullock M.R. McGinn M.J. Zhou Z. Altememi N. Hagood S. Hamm R. Colello R.J. Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp. Neurol. 2009;216:56–65. doi: 10.1016/j.expneurol.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun D. Bullock M.R. Altememi N. Zhou Z. Hagood S. Rolfe A. McGinn M.J. Hamm R. Colello R.J. The effect of epidermal growth factor in the injured brain after trauma in rats. J. Neurotrauma. 2010;27:923–938. doi: 10.1089/neu.2009.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee C. Agoston D.V. Vascular endothelial growth factor is involved in mediating increased de novo hippocampal neurogenesis in response to traumatic brain injury. J. Neurotrauma. 2010;27:541–553. doi: 10.1089/neu.2009.0905. [DOI] [PubMed] [Google Scholar]
- 53.Thau-Zuchman O. Shohami E. Alexandrovich A.G. Leker R.R. Vascular endothelial growth factor increases neurogenesis after traumatic brain injury. J. Cereb. Blood Flow. Metab. 2010;30:1008–1016. doi: 10.1038/jcbfm.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiong Y. Mahmood A. Chopp M. Emerging treatments for traumatic brain injury. Expert. Opin. Emerg. Drugs. 2009;14:67–84. doi: 10.1517/14728210902769601. [DOI] [PMC free article] [PubMed] [Google Scholar]